- 1Hematology Department, London North West University Healthcare NHS Trust, London, United Kingdom
- 2Saint Petersburg State University Hospital, Saint Petersburg, Russia
- 3Acute Leukemia Advocates Network, Bern, Switzerland
- 4Novartis, Basel, Switzerland
- 5CLL Advocates Network, Bern, Switzerland
- 6CML Advocates Network, Bern, Switzerland
- 7School of Life and Medical Sciences, University of Hertfordshire, Hatfield, United Kingdom
Background: Leukaemia patients require comprehensive care, including treatment, hospital visits, and family support. An awareness of the impact of leukaemia on family members is crucial. The Acute Leukemia Advocates Network, Chronic Lymphoid Leukemia (CLL) Advocates Network, and Chronic Myeloid Leukemia (CML) Advocates Network assessed the impact of leukaemia on patients and their families.
Methods: A global anonymous online study ran from 18/09/21 to 07/01/2022, targeting leukaemia patients and their family members/partners. Demographic information, disease characteristics, treatment, and family members’ experience of caring for a relative with blood cancer were collected. Family members/partner assessed the disease impact on patients using the Hematological Malignancy-Patient Reported Outcome measure (HM-PRO) Part-A.
Results: 571 family members/partners (70.9% female, aged 55 years) responded. They represented patients with CML (32.0%), CLL (26.3%), acute myeloid leukaemia (AML) (19.3%) acute lymphoblastic leukaemia (17.5%), and other leukaemias (4.9%). The majority (89.1%) lived with the patient, and 69.7% accompanied them to clinics. Daily support included household chores (60%), shopping (51.5%), transportation (37.1%), finances (28.9%), personal care (23.3%) and childcare (16.8%). About one-third (29.6%) felt that the diagnosis was insensitively conveyed and 81.3% sought prognosis information. Approximately 64% searched for alternative treatments. Approximately 40% of family members/partners (prevalently sons/daughters) expressed an impact on general quality of life, particularly on emotional behaviour and eating/drinking habits. The impact was greatest in family members representing patients aged <18 years with ALL and AML compared to CLL and CML.
Conclusion: The burden on leukaemia patients’ family members is significant but often overlooked.
1 Introduction
Leukaemia comprises a group of haematological malignancies characterised by abnormal proliferation of hematopoietic stem cells in the bone marrow. In the United States (US) in 2021, leukaemia accounted for approximately 3.2% of all new cancer incidence and similar values for mortality (1). Predominant subtypes include acute myeloid leukaemia (AML), acute lymphoblastic leukaemia (ALL), chronic myeloid leukaemia (CML), and chronic lymphocytic leukaemia (CLL) (1). CLL is the most common subtype of leukaemia, with approximately 4.1 cases/100,000 adults, resulting in approximately 4,500 deaths per year in the US (2). AML is the second most common form of leukaemia, making up approximately 1.1% of cancer diagnoses, but around 1.9% of cancer deaths (3). AML most frequently occurs in adults, causing symptoms of bone marrow failure and organ infiltration (3). CML accounts for approximately 15% of all diagnoses of leukaemia in adults (1–2 cases/100,000 adults).
Prognosis varies by subtype and patient age. The five-year survival rate for children with ALL can exceed 85%, whereas for adults with AML, it is approximately 27% (4).
It is recognised that leukaemia significantly impacts patients’ quality of life (QoL) (5), leading to physical, emotional, and financial burden. However, significantly less attention has been given to the burden experienced by family members and caregivers, despite their crucial role in providing both practical and emotional support throughout the course of the disease (6–8). Family members are often responsible for daily caregiving tasks such as transportation, managing finances, and personal care, and are deeply affected by the patient’s psychological and physical state (9). Their perspectives are essential for delivering comprehensive, patient-centred care and for informing the development of effective support services.
To address this gap, the global network partnership of Acute Leukemia Advocates Network (ALAN), CLL Advocates Network (CLLAN) and the CML Advocates Network (CMLAN) (referred to thereafter as “network”), developed a multi-country survey aimed to assess the burden of leukaemia on patients and on their family members/partners.
Previous studies have used tools such as the Hematological Malignancy Patient-Reported Outcome (HM-PRO) measure to assess the QoL and the broader impact on patients, providing critical data to support comprehensive care strategies (10). While the HM-PRO has been also used to evaluate the impact of disease on patients with leukaemia (5, 8), the present study extends this approach by incorporating the perspectives of family members.
Therefore, the aim of this study was to explore and quantify the burden of leukaemia on family members and partners, including their caregiving roles, emotional strain, and their perceptions of patient well-being.
This paper presents findings from the family member/partner cohort of a global survey, with a particular focus on their caregiving roles, perceived disease burden, and their assessment of the patient’s QoL using the HM-PRO. Understanding these insights is critical for developing and optimizing care strategies that address both the needs of patients and caregivers.
2 Methods
2.1 Study design
A global anonymous online survey of 83 questions (some with sub questions) was conducted from 18 September 2021 to 07 January 2022 in 10 languages for participation by patients with leukaemia and their family members/partner, recruited through the Network via email, social media, and newsletters. Items included nonsensitive demographic information, disease characteristics, therapy, and family members’ experience (e.g. helping with: hospital visits, daily activity, household chores, personal care, transportation and managing finances) with their relative’s disease and treatment. In addition, the hematological malignancy PRO measure, HM-PRO, Part A (impact scale) was included and completed by family members/partners as proxies to assess the perceived impact of leukaemia on patients (10–13).
2.2 Clinical outcome assessment - HM-PRO
The HM-PRO is a validated patient-reported outcome (PRO) instrument designed to assess the impact (Part A) and signs and symptoms (Part B) of haematological malignancies (10–13). The scales have linear scoring systems ranging from 0 to 100, with higher scores representing greater (negative) impact on QoL and symptom burden. In order to determine the impact (no impact vs impact) a score of 0–7 represented no impact on QoL (i.e. a score of >7 represented impact on QoL) as previously described elsewhere (14). This analysis focuses on Part A across four domains capturing socio-demographics, disease and treatment profiles, and QoL. The four domains included physical behaviour (PB), emotional behaviour (EB), social well-being (SW), eating and drinking habits (ED). It explores whether age and years living with the disease are associated with HM-PRO scores in patients with acute and chronic leukaemia. The full study questionnaire included 200 items (some with sub-items) across 16 sections. According to FDA PRO Guidelines, establishing content validity is considered the most important step in the psychometric evaluation of a newly developed PRO instrument (15).The HM-PRO has undergone extensive psychometric testing, including content and construct validity, internal consistency (Cronbach’s alpha >0.8), and test–retest reliability. It was translated into 10 languages following ISPOR guidelines (16). This rigorous process included forward–backward translation, expert review, cognitive debriefing with patients, and pilot testing with native-speaking patients and caregivers to ensure conceptual clarity and cultural relevance (10). Translation certificates were issued by the MAPI Research Trust, confirming cross-cultural equivalence. Results obtained from Part A of the HM-PRO instrument are described in the present report.
2.3 Statistical analysis
Data are presented as number and % or median and interquartile range. Comparison between the frequency of family members/partners (categorical variables) responding to specifc questions was assessed by Chi-squared test. Comparison of individual domain scores between family members was performed by Kruskal Wallis one-way analysis of variance (ANOVA) followed by Bonferroni post hoc. Probability of type I error was set at 5% level i.e. p ≤ 0.05. All analysis was performed using SPSS software (version 22 for Windows, IBM, Armonk, NY).
3 Results
3.1 Characteristics of family members/partners
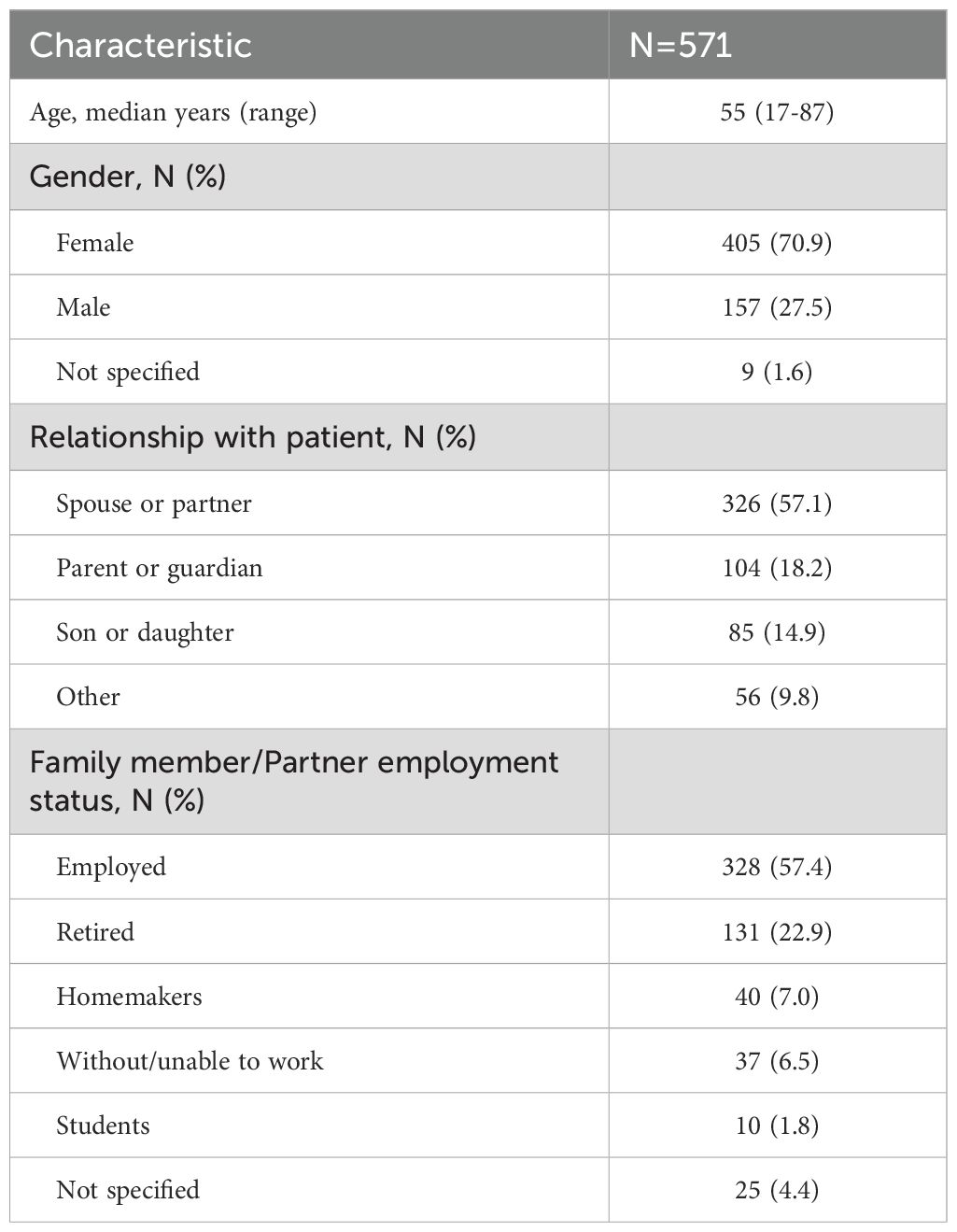
A total of 571 family members/partners (405 females, 70.9%) and median age of 55 years (range 17–87) responded to the survey (Table 1). Three hundred twenty-eight (57.4%) were employed, 131 (22.9%) retired, 40 (7.0%) homemakers, 10 (1.8%) students and the remaining without/unable to work or not specified.
Family members represented patients with CML (N=183, 32.0%), CLL (N=150, 26.3%), AML (N=110, 19.3%), ALL (N=100, 17.5%), and other leukaemias (N=28, 4.9%).
The majority (N=408, 89.1%) were living with the patient and most were relatives (N=326, 57.1%) spouse/partner, 104 (18.2%) parent or guardian, 85 (14.9%) son/daughter, 17 (3.0%) others.
3.2 Activities undertaken by family members/partners as part of their caring role
Almost three-quarters (N=398; 69.7%) of family members/partners accompanied patients to the clinic, of whom 233 (58.5%) were employed (Table 2). Besides a range of activities related to treatment and illness, the majority of family members/partners also provided emotional support (N=516; 90.4%). Most family members/partners (461, 80.7%) provided support for one or more daily activities: 344 (60.2%) household chores; 294 (51.5%) shopping; 266 (46.6%) accompanying on trips or appointments (not illness related); 212 (37.1%) transportation; 165 (29%) managing finances; 133 (23%) personal care; 96 (16.8%) looking after children (Table 3). Of note, of family members/partners providing support (N=461), 273 (59%) were currently employed.
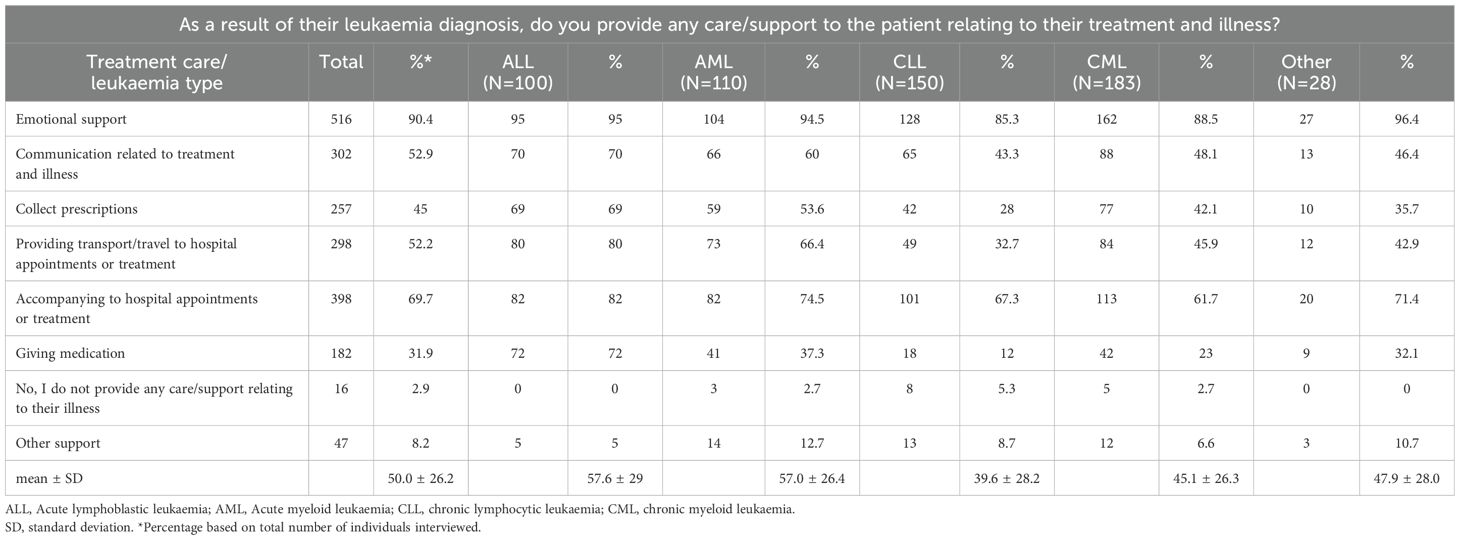
Table 2. Questions related to care/support to the patient relating to their treatment and illness stratified by leukaemia type.
We also explored whether the frequency of treatment/illness-related activities (Table 2) and daily activities in patients with different leukaemia subtypes (Table 3). This analysis revealed that family members/partners representing patients with ALL and AML were burdened with a range of different activities approximately 2-fold compared to patients with CLL or CML. The extent of emotional support provided by family members was consistently high (~ 90%), regardless of leukaemia type. In addition to leukaemia type, we also explored whether the extent of support in terms of activities related to illness/treatment and daily activities were representative across different age groups and gender of patients (Tables 4, 5). While no marked differences were observed with regard to patient gender (apart from a slightly higher need for childcare in male patients; 20.2% in males). 12.8% in females; Table 5), marked differences were observed in the extent of activity across different patient age groups. Family members/partners representing patients <18 years of age provided a higher degree of support (~ 62-80%) as reflected in the different activities undertaken (treatment/illness related as well as daily activities) compared to family members/partners representing older patients (18–64 and >64 years age groups; ~ 31-48%). Interestingly, the majority of family members/partners (82.5%) did not provide any care/support relating to everyday activities in patients aged 18–64 years and to a lesser extent in patients aged >64 years (27.7%) (Table 5).
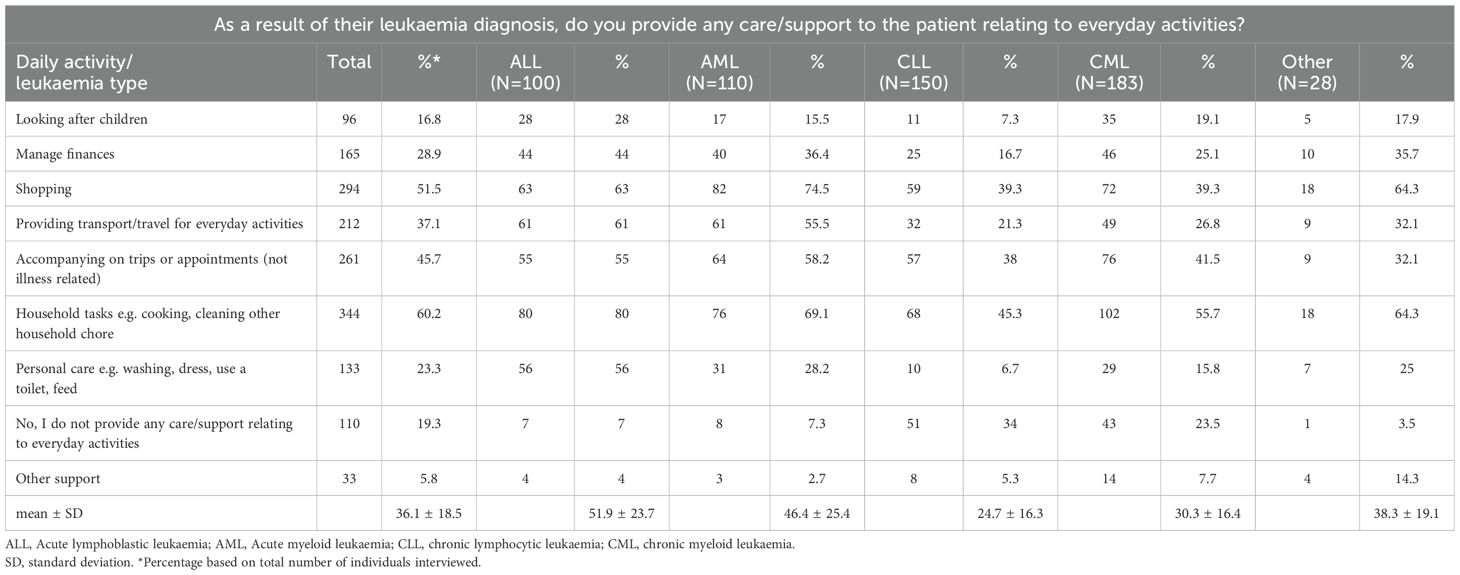
Table 3. Questions related to care/support to the patient relating to their everyday activities stratified by leukaemia type.
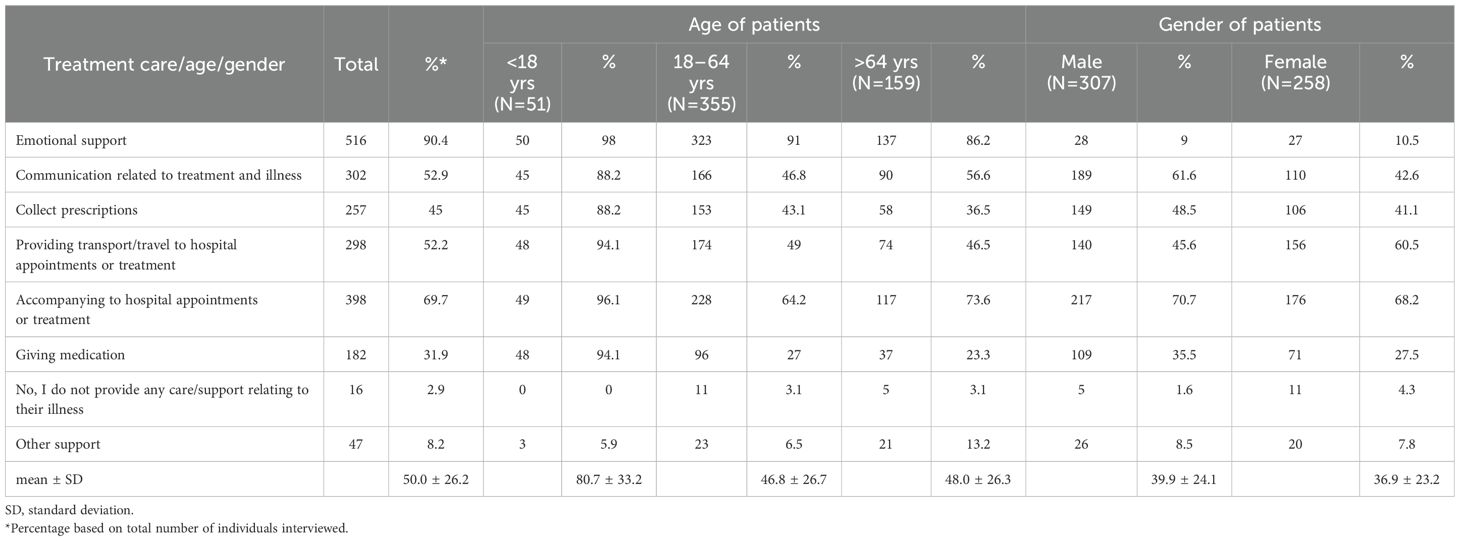
Table 4. Questions related to care/support to the patient relating to their treatment and illness stratified by patient age and gender.
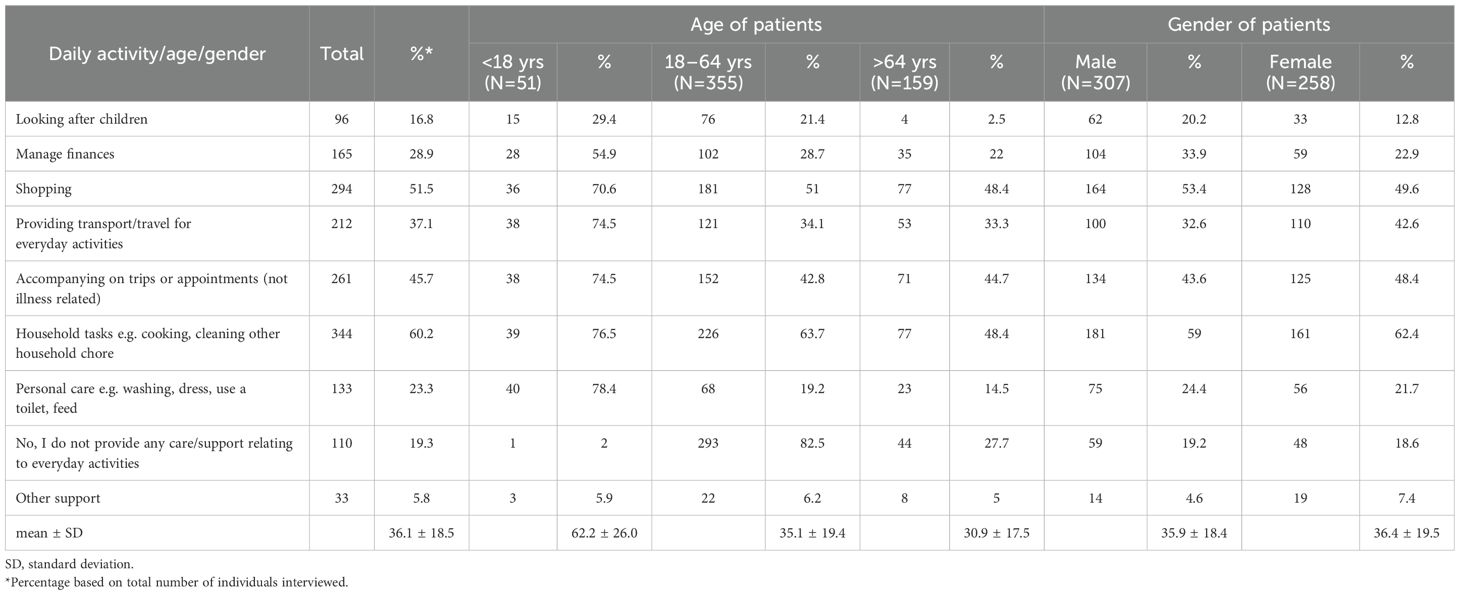
Table 5. Questions related to care/support to the patient relating to their everyday activities stratified by patient age and gender.
3.3 Perception regarding HCP communication of leukaemia diagnosis
Approximately 15% of family members/partners (88 of 571) believed that the diagnosis should have been communicated more sensitively. Only 31 family members/partners (5.4%) did not understand the diagnosis. Furthermore, of the 464 (81.3%) who desired to know the prognosis, 286 (61.6%) were informed by the physician, and of the 56 (19.6%) who did not desire to receive such information 10 (3.5%) received it (p<0.0001). Out of all family members/partners, 364 (64%) searched for information on alternative treatment options from other sources, although 324 (89%) believed that the patients were involved in treatment decisions, and 200 (54.9%) would have desired written information from the clinic. Most family members/partners were worried while awaiting clinical tests regardless of the explanations about results provided in about 75% (N=428) of the cases. Half of the family members/partners considered side effects to be of high impact, both physically and emotionally.
3.4 Perception of patients’ quality of life as measured by HM-PRO Part A
The impact of disease on patients was underestimated by family members/partner as described by the HM-PRO Part A scores completed by the family members (proxies) who answered the items based on their perceptions of the patients’ QoL (Figure 1A).
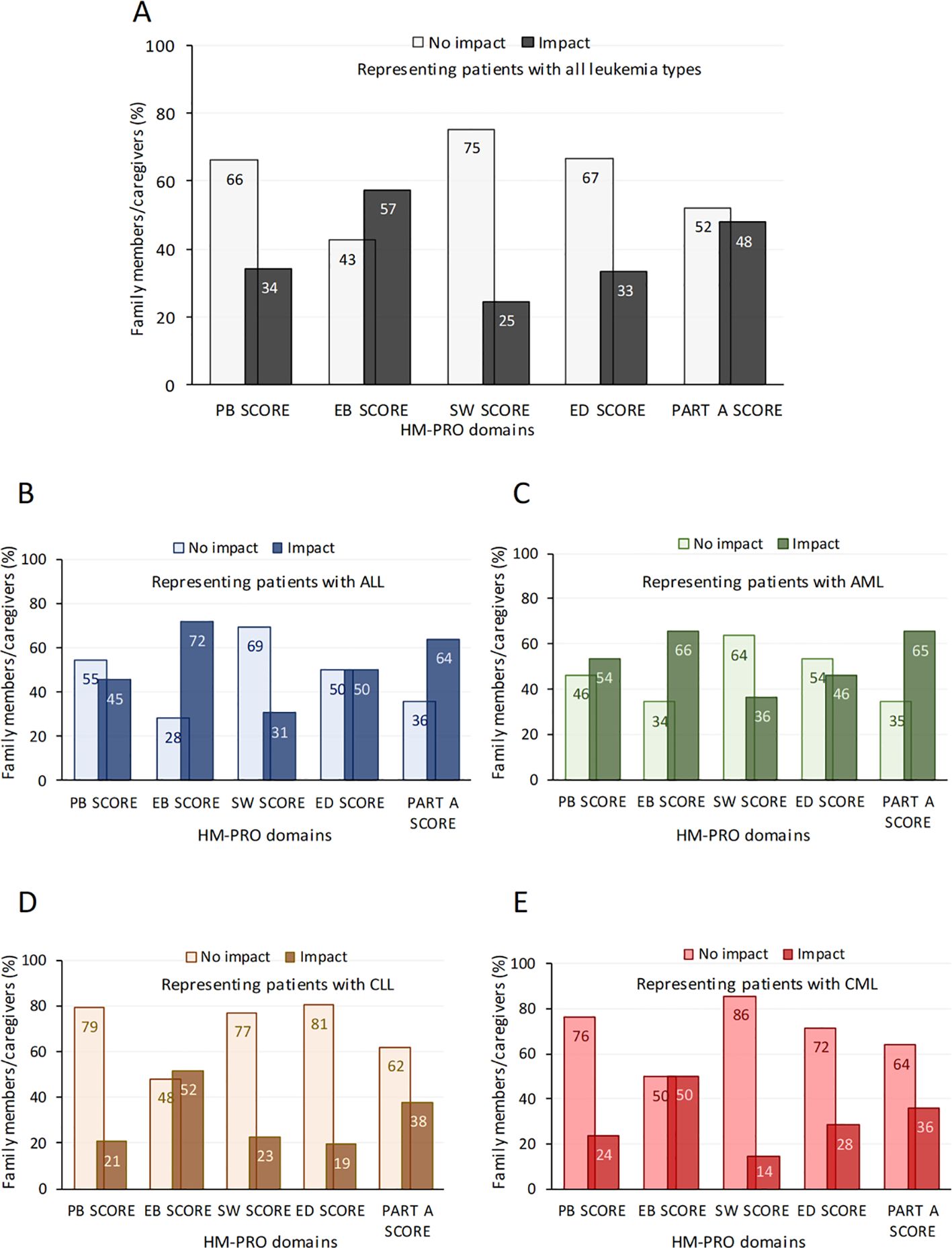
Figure 1. Perception of the impact of disease on patients by family members/partner as measured by the HM-PRO Part (A) The impact on QoL across 4 HM-PRO domains (and the Part A score) by caregivers/family members representing patients with all types of leukaemia (A) and also stratified by ALL (B), AML (C), CLL (D) and CML (E). Data are presented as % family members/caregivers perceived as having an impact or no impact of the disease on QoL. A score of 0–7 represented no impact on QoL (i.e. a score of >7 represented impact on QoL) as previously described elsewhere(14). ALL, Acute lymphoblastic leukaemia; AML, Acute myeloid leukaemia; CLL, chronic lymphocytic leukaemia; CML, chronic myeloid leukaemia; EB, emotional behaviour; ED, eating and drinking; PB, physical behaviour; SW, social and wellbeing; Part A (impact scale), HM-PRO, Hematological Malignancy Patient-Reported Outcome.
Considering all HM-PRO domains, approximately 40% (39.5 ± 13.5%) of family members/partners expressed an impact on general QoL (Figure 1A). Emotional behaviour (EB score) was reported as affecting 57% of individuals, with 25% of them reporting a moderate or a very large impact and 33% of family members/partners reported that eating/drinking habits (ED score) were affected by leukaemia (Figure 1A). The impact of disease on patients by family members/partner was perceived to be greater in patients with ALL and AML (Figures 1B, C) compared to patients with CLL or CML (Figures 1D, E) with specific domains such as physical behaviour (PB score), emotional behaviour (EB score), eating and drinking disturbance (ED score) and summary impact (Part-A) score domains affected to a greater extent. Supporting these observations, in fact, HM-PRO scores for all family members/partners were higher for emotional behaviour and eating/drinking habits (Table 6).

Table 6. Comparison of HM-PRO domain scores for different caregivers/family members representing patients with different types of leukaemia.
Stratifying HM-PRO scores by leukaemia subtype revealed significantly higher scores across all domains in patients with ALL and AML compared to patients with CLL or CML (Table 6).
Comparing domain scores between the different types of family members/partners, a statistically significant difference in the reporting of emotional behaviour (p=0.01), eating and drinking score (p<0.001) and summary impact (Part-A) (p<0.001) was found, in that sons/daughters of patients perceived the greatest impact (Table 6).
4 Discussion
Leukaemia imposes a significant burden not only on patients (5, 8, 17) but also on their families and caregivers. This impact extends beyond the direct physical and emotional toll on patients, influencing the psychological, social, and economic aspects of the lives of those who support them and are on duty 24/7. Previous studies have highlighted the multifaceted nature of this burden and its far-reaching consequences (18, 19).
The main findings from this real-life global survey highlights the impact of leukaemia on family member caregivers of the patient.
The psychological impact on family member/partner caregivers is profound and experience high levels of stress, anxiety, and depression (19, 20). In a cross-sectional study including Danish patients (N=375) and caregivers (n=140), caregivers of patients with haematological cancers undergoing active treatment face a high symptom burden, which significantly impacts their QoL, including sleep, psychological well-being, and emotional health (18).
The physical health of caregivers can also deteriorate due to stress and lack of self-care, leading to increased susceptibility to illnesses and chronic conditions. This would also lead to the content of the glass to be depleted and consequently nothing left to give, leading to burnout. Furthermore, the burden of leukaemia on family member/partner caregivers also includes the emotional toll of witnessing the suffering of a loved one (18–20). This in in line with our observations where approximately 40% of individuals reported a negative impact on patients’ QoL and specific HM-PRO domains such as emotional behaviour and eating and drinking habits. This proportion significantly increased across most domains in family member/partner caregivers representing patients with AML or ALL compared to those representing patients with CLL or CML.
These differences in HM-PRO domain scores reflect differences observed in activities by family members/partners related to treatment or illness and daily activities where a substantially higher frequency was observed when caring for patients with AML or ALL compared to patients with CLL or CML.
These observations were expected. The acute leukaemias (AML and ALL) are characterised by a rapid onset and aggressive progression, often requiring immediate and intensive treatment such as high-dose chemotherapy and stem cell transplants (3, 21–25). These treatments are also associated with severe side effects, prolonged hospital stays, and frequent medical visits, imposing substantial physical, emotional, and financial burdens on caregivers (1, 24, 26–28).
In contrast, chronic leukaemias (CLL and CML) typically progress more slowly and can often be managed with less intensive treatments, including oral medications (2, 29, 30).
Results from this survey also highlighted that when the leukaemia patient is under 18 years old, the caregiving burden further intensifies. Children and adolescents with AML or ALL require not only medical care but also emotional and developmental support. Parents or caregivers must manage their own emotional distress while ensuring the child’s psychological needs are met, often leading to high levels of stress and anxiety (31, 32).
The impact on QoL is significant, as caregivers must often reduce work hours or stop working entirely, leading to financial strain. Moreover, the emotional toll of witnessing a child undergo aggressive treatments and potential long-term effects can be devastating (19, 32–34).
Economically, the impact of leukaemia on families can be substantial (35). The financial burden is further exacerbated by lost income when family member caregivers reduce their working hours or leave their jobs to provide care. Indeed, up to one-third (N=196; 34.3%) of family members in the present study reported that their employment was affected and just under half (N=275; 48.2%) experienced financial impact as a result of the patient’s diagnosis of leukaemia. Contribution of the family member/partner caregivers to the economy of the respective community and the country is hugely underestimated. It is ironic that such group of a society should suffer financial hardship.
Psychologically, children of parents with leukaemia often experience heightened levels of stress, anxiety, and depression. It is well established that children living with a parent undergoing cancer treatment are at an increased risk of developing emotional and behavioural problems (36). Indeed, although representing a small proportion of the total population in our study (N=85; 14.9%), children of patients with leukaemia were observed to perceive the greatest impact of the diagnosis as reflected by significantly higher scores for emotional behaviour, eating and drinking score and summary impact (Part-A) domains. Children often suppress their emotions to avoid adding to the stress of the family, which can lead to internalised emotional distress (37). The need to maintain a semblance of normalcy and support their ill parent can cause children to neglect their own emotional needs (38).
Socially, children may experience a sense of isolation and disruption in their normal social activities and often withdraw from social interactions and extracurricular activities (39). This withdrawal is due to the increased responsibilities at home, such as taking care of siblings or household chores, and the emotional toll of their parent’s illness.
Academically, the impact can be substantial. Children with a parent suffering from cancer often show decreased academic performance (40). The stress and responsibilities at home can lead to difficulty concentrating in school, lower grades, and increased absenteeism. The need to be physically present at home, especially during critical phases of the parent’s treatment, can result in missed school days and falling behind academically.
The emotional toll of witnessing a parent’s suffering is another critical aspect. Children and adolescents often experience feelings of helplessness and frustration as they watch their parent go through treatment and its side effects (41).
The combination of acute treatment regimens and the age of the patient creates a complex caregiving environment whereby the immediate and long-term needs of both the patient and caregiver are intertwined.
We have previously evaluated the burden of disease on patients with different types of acute leukaemia (5, 8). The present findings provide an important adjunct to our understanding of the substantial impact of both acute and chronic leukaemia, highlighting the need for comprehensive support systems for these families.
Findings from this study identify several practical areas where targeted support could help caregivers more effectively. These include offering emotional and social support through counselling and peer groups to reduce stress and isolation, and providing educational tools to improve caregivers’ understanding of treatments and how to navigate care systems. Financial and workplace support, such as flexible job policies and financial aid, could help ease the economic difficulty associated with caregiving. Clinical care should also include regular checks on caregiver well-being and offer personalised resources. Paediatric caregivers, especially parents of children with leukaemia, may benefit from training and counselling and support tailored to their child’s developmental needs, along with help managing daily responsibilities. In addition, healthcare providers should be trained to communicate with greater empathy and offer clear, written information and follow-up meetings and discussions to help caregivers improve their understanding and cope better.
5 Study limitations
While this large and heterogenous cohort offers important strengths, several limitations need to be noted. First, the online and voluntary nature of recruitment may have introduced selection bias, attracting participants who were more engaged/committed, health awareness, or linked to advocacy groups. This may potentially impact the generalisability of findings to caregivers who are more isolated or have limited access to information technology resources. Second, the data were based on family members rating the patient’s QoL, rather than the patients providing their own input. While past research shows that caregivers can accurately report visible changes, their responses may still be influenced by their own emotions and views (42). This could create differences between what caregivers’ report and what patients actually experience, especially in emotional aspects. Future studies that include input from both patients and caregivers could help confirm and strengthen these findings.
6 Conclusions
The burden of leukaemia on family members/partner is often neglected, despite being of paramount importance when attempting to provide holistic care. The psychological, emotional and social challenges faced by adult children of parents diagnosed with leukaemia evidence the need for targeted support systems. Moreover, the impact was observed to be greatest in family members representing patients aged <18 years with ALL and AML compared to other leukaemia subtypes such as CLL and CML. Interventions such as counselling, support groups, patient organisations, and tailored community support services can help mitigate these effects and provide family members with the necessary resources to cope with the burden of caring. This study extends our understanding of the impact of leukaemia on the QoL of the affected patient to also the importance of recognizing and addressing the broader impact on families that support patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
EO: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. TI: Conceptualization, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. SN: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. ZP-W: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. NY: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. DC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. SS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors wish to thank the patients and their families who agreed to participate in this study. We also wish to thank Colin Gerard Egan (CE Medical Writing SRLS, Pisa, Italy) for editorial assistance.
Conflict of interest
Author ZP-W was employed by the company Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chennamadhavuni A, Lyengar V, Mukkamalla SKR, Shimanovsky A. “Leukemia.,”, in: StatPearls (2024). Treasure Island (FL: StatPearls Publishing. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK560490/ (Accessed June 4, 2024).
2. Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet. (2018) 391:1524–37. doi: 10.1016/S0140-6736(18)30422-7
3. Stubbins RJ, Francis A, Kuchenbauer F, Sanford D. Management of acute myeloid leukemia: A review for general practitioners in oncology. Curr Oncol. (2022) 29:6245–59. doi: 10.3390/curroncol29090491
4. Ossenkoppele G, Löwenberg B. How I treat the older patient with acute myeloid leukemia. Blood. (2015) 125:767–74. doi: 10.1182/blood-2014-08-551499
5. Pemberton-Whiteley Z, Nier S, Geissler J, Wintrich S, Verhoeven B, Christensen RO, et al. Understanding quality of life in patients with acute leukemia, a global survey. J Patient Cent Res Rev. (2023) 10:21–30. doi: 10.17294/2330-0698.1951
6. Golics CJ, Basra MKA, Salek MS, Finlay AY. The impact of patients’ chronic disease on family quality of life: an experience from 26 specialties. Int J Gen Med. (2013) 6:787–98. doi: 10.2147/IJGM.S45156
7. Shah R, Ali FM, Finlay AY, Salek MS. Family reported outcomes, an unmet need in the management of a patient’s disease: appraisal of the literature. Health Qual Life Outcomes. (2021) 19:194. doi: 10.1186/s12955-021-01819-4
8. Salek S, Nier S, Pemberton-Whiteley Z, Ionova T, Ianni G, Tripepi G, et al. Impact of leukemia subtype and demographics on patient quality of life in 76 countries: a cross-sectional study. Front Hematol. (2025) 3:1502166. doi: 10.3389/frhem.2024.1502166
9. Rezaei M, Keyvanloo Shahrestanaki S, Mohammadzadeh R, Aghili MS, Rajabi M, Abbasi M, et al. Caregiving consequences in cancer family caregivers: a narrative review of qualitative studies. Front Public Health. (2024) 12:1334842. doi: 10.3389/fpubh.2024.1334842
10. Goswami P, Oliva EN, Ionova T, Else R, Kell J, Fielding AK, et al. Development of a novel hematological Malignancy specific patient-reported outcome measure (HM-PRO): content validity. Front Pharmacol. (2020) 11:209. doi: 10.3389/fphar.2020.00209
11. Goswami P, Oliva EN, Ionova T, Else R, Kell J, Fielding AK, et al. Hematological Malignancy specific patient-reported outcome measure (HM-PRO): construct validity study. Front Pharmacol. (2020) 11:1308. doi: 10.3389/fphar.2020.01308
12. Goswami P, Oliva EN, Ionova T, Else R, Kell J, Fielding AK, et al. Paper and electronic versions of HM-PRO, a novel patient-reported outcome measure for hematology: an equivalence study. J Comp Eff Res. (2019) 8:523–33. doi: 10.2217/cer-2018-0108
13. Goswami P, Oliva EN, Ionova T, Salek S. Responsiveness and the minimal clinically important difference for HM-PRO in patients with hematological Malignancies. Blood. (2018) 132:2294. doi: 10.1182/blood-2018-99-117094
14. Goswami P, Oliva EN, Ionova T, Salek S. Translating the science of patient reported outcomes into practice: meaningfulness of HM-PRO scores in patients with hematological Malignancies. Blood. (2018) 132:4860. doi: 10.1182/blood-2018-99-117180
15. FDA. Core patient-reported outcomes in cancer clinical trials (2021). Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/core-patient-reported-outcomes-cancer-clinical-trials (Accessed January 15, 2024).
16. Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. (2005) 8:94–104. doi: 10.1111/j.1524-4733.2005.04054.x
17. Bosshard R, O’Reilly K, Ralston S, Chadda S, Cork D. Systematic reviews of economic burden and health-related quality of life in patients with acute myeloid leukemia. Cancer Treat Rev. (2018) 69:224–32. doi: 10.1016/j.ctrv.2018.07.005
18. Nielsen IH, Tolver A, Piil K, Kjeldsen L, Grønbæk K, Jarden M. Family caregiver quality of life and symptom burden in patients with hematological cancer: A Danish nationwide cross-sectional study. Eur J Oncol Nurs. (2024) 69:102538. doi: 10.1016/j.ejon.2024.102538
19. Grover S, Rina K, Malhotra P, Khadwal A. Caregiver burden in the patients of acute myeloblastic leukemia. Indian J Hematol Blood Transfus. (2019) 35:437–45. doi: 10.1007/s12288-018-1048-4
20. Jepsen LØ, Friis LS, Hansen DG, Marcher CW, Høybye MT. Living with outpatient management as spouse to intensively treated acute leukemia patients. PloS One. (2019) 14:e0216821. doi: 10.1371/journal.pone.0216821
21. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. (2015) 373:1136–52. doi: 10.1056/NEJMra1406184
22. Brown PA, Shah B, Advani A, Aoun P, Boyer MW, Burke PW, et al. Acute lymphoblastic leukemia, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:1079–109. doi: 10.6004/jnccn.2021.0042
23. Pui C-H, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. (2008) 371:1030–43. doi: 10.1016/S0140-6736(08)60457-2
24. Puckett Y, Chan O. “Acute lymphocytic leukemia.,”, in: StatPearls (2024). Treasure Island (FL: StatPearls Publishing. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK459149/ (Accessed July 25, 2024).
25. Vakiti A, Reynolds SB, Mewawalla P. “Acute myeloid leukemia.,”, in: StatPearls (2024). Treasure Island (FL: StatPearls Publishing. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK507875/ (Accessed July 25, 2024).
26. Crossnohere NL, Richardson DR, Reinhart C, O’Donoghue B, Love SM, Smith BD, et al. Side effects from acute myeloid leukemia treatment: results from a national survey. Curr Med Res Opin. (2019) 35:1965–70. doi: 10.1080/03007995.2019.1631149
27. Abrahão R, Huynh JC, Benjamin DJ, Li QW, Winestone LE, Muffly L, et al. Chronic medical conditions and late effects after acute myeloid leukaemia in adolescents and young adults: a population-based study. Int J Epidemiol. (2021) 50:663–74. doi: 10.1093/ije/dyaa184
28. Al-Mahayri ZN, AlAhmad MM, Ali BR. Long-term effects of pediatric acute lymphoblastic leukemia chemotherapy: can recent findings inform old strategies? Front Oncol. (2021) 11:710163. doi: 10.3389/fonc.2021.710163
29. Osman AE, Deininger MW. Chronic Myeloid Leukemia: Modern therapies, current challenges and future directions. Blood Rev. (2021) 49:100825. doi: 10.1016/j.blre.2021.100825
30. Kipps TJ, Stevenson FK, Wu CJ, Croce CM, Packham G, Wierda WG, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. (2017) 3:16096. doi: 10.1038/nrdp.2016.96
31. Lewandowska A. The needs of parents of children suffering from cancer—Continuation of research. Children (Basel). (2022) 9:144. doi: 10.3390/children9020144
32. Qiu M, Wu Y. Understanding the experience of family caregivers of patients with leukemia: a qualitative analysis of online blogs. Humanit Soc Sci Commun. (2024) 11:1–11. doi: 10.1057/s41599-024-02830-y
33. Cui P, Yang M, Hu H, Cheng C, Chen X, Shi J, et al. The impact of caregiver burden on quality of life in family caregivers of patients with advanced cancer: a moderated mediation analysis of the role of psychological distress and family resilience. BMC Public Health. (2024) 24:817. doi: 10.1186/s12889-024-18321-3
34. Irwanto, Ratwita M, Prihaningtyas RA, Mustakim MRD. Impact of caregiver’s psychological aspects towards quality of life of children with acute lymphoblastic leukemia (ALL). Asian Pac J Cancer Prev. (2020) 21:2683–8. doi: 10.31557/APJCP.2020.21.9.2683
35. Yucel E, Zhang S, Panjabi S. Health-related and economic burden among family caregivers of patients with acute myeloid leukemia or hematological Malignancies. Adv Ther. (2021) 38:5002–24. doi: 10.1007/s12325-021-01872-x
36. Inhestern L, Johannsen LM, Bergelt C. Families affected by parental cancer: quality of life, impact on children and psychosocial care needs. Front Psychiatry. (2021) 12:765327. doi: 10.3389/fpsyt.2021.765327
37. Compas BE, Worsham NL, Ey S, Howell DC. When mom or dad has cancer: II. Coping, cognitive appraisals, and psychological distress in children of cancer patients. Health Psychol. (1996) 15:167–75. doi: 10.1037//0278-6133.15.3.167
38. Morris JN, Martini A, Preen D. The well-being of children impacted by a parent with cancer: an integrative review. Support Care Cancer. (2016) 24:3235–51. doi: 10.1007/s00520-016-3214-2
39. Krattenmacher T, Kühne F, Halverscheid S, Wiegand-Grefe S, Bergelt C, Romer G, et al. A comparison of the emotional and behavioral problems of children of patients with cancer or a mental disorder and their association with parental quality of life. J Psychosom Res. (2014) 76:213–20. doi: 10.1016/j.jpsychores.2013.11.020
40. Thastum M, Watson M, Kienbacher C, Piha J, Steck B, Zachariae R, et al. Prevalence and predictors of emotional and behavioural functioning of children where a parent has cancer: a multinational study. Cancer. (2009) 115:4030–9. doi: 10.1002/cncr.24449
41. Faulkner RA, Davey M. Children and adolescents of cancer patients: The impact of cancer on the family. Am J Family Ther. (2002) 30:63–72. doi: 10.1080/019261802753455651
42. Wen F-H, Chen J-S, Chou W-C, Chang W-C, Shen WC, Hsieh C-H, et al. Family caregivers’ Subjective caregiving burden, quality of life, and depressive symptoms are associated with terminally ill cancer patients’ Distinct patterns of conjoint symptom distress and functional impairment in their last six months of life. J Pain Symptom Manage. (2019) 57:64–72. doi: 10.1016/j.jpainsymman.2018.09.009
Keywords: leukaemia, quality of life, HM-PRO, burden, caregiver
Citation: Oliva EN, Ionova T, Nier S, Pemberton-Whiteley Z, York N, Costello D and Salek S (2025) Disease and treatment burden in patients with leukaemia: family members/partner perspective. Front. Hematol. 4:1570055. doi: 10.3389/frhem.2025.1570055
Received: 02 February 2025; Accepted: 10 April 2025;
Published: 06 May 2025.
Edited by:
Bhavana Bhatnagar, West Virginia University, United StatesReviewed by:
Senthilnathan Palaniyandi, University of Missouri, United StatesGiovangiacinto Paterno, University of Rome Tor Vergata, Italy
Copyright © 2025 Oliva, Ionova, Nier, Pemberton-Whiteley, York, Costello and Salek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Esther Natalie Oliva, ZW5vbGl2YUBnbWFpbC5jb20=
 Esther Natalie Oliva
Esther Natalie Oliva Tatyana Ionova
Tatyana Ionova Samantha Nier
Samantha Nier Zack Pemberton-Whiteley3,4
Zack Pemberton-Whiteley3,4 Nick York
Nick York Sam Salek
Sam Salek