- 1Department of Reproductive Medicine, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 2Department of Neurology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 3Department of Neurology, The First Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 4Department of Infectious Diseases, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 5Neuromedical Technology Innovation Center of Hebei Province, Shijiazhuang, Hebei, China
Erythroblast Island Macrophages (EIMs) are specialized cells that play a crucial role in erythropoiesis by forming erythroblastic islands (EBIs) and supporting the maturation of erythroblasts. These macrophages express a variety of surface markers that mediate their interactions with erythroblasts and regulate erythropoiesis. Most studies on EMIs rely on flow cytometry analysis and selecting the correct surface markers is of great importance when conducting research. This review provides a brief overview of the surface markers expressed by EIMs, including α5-integrin, CD11b, CD16, CD163, CD169, CD206, CSF1R, EPOR, F4/80, Gr1, MerTK, PPARγ, Timd4, and VCAM1. We also discuss the heterogeneity of EIMs and subsets of EIMs, such as F4/80+VCAM-1+CD169+EPOR+ macrophages.
1 Introduction
Erythroblast island (EBI) macrophages (EIMs) play a critical role in the regulation of erythropoiesis, the process by which red blood cells are produced in the bone marrow.
EBIs are unique structure composed of a central macrophage surrounded by multiple developing erythroid cells. These specialized macrophages support the differentiation and maturation of erythroblasts (1, 2). The interaction between EIMs and erythroblasts is facilitated by a variety of surface protein expressed on the macrophages and erythroblasts. Depending on the species and the type of organ, the surface markers of EIMs used in flow cytometry can vary. In this review, we briefly summarize the major surface markers expressed on EIMs and their function in erythropoiesis, including α5-integrin, CD11b, CD16, CD163, CD169, CD206, CSF1R, EPOR, F4/80, Gr1, MerTK, PPARγ, and VCAM1 (2–7).
2 Surface markers of erythroblast island macrophages
2.1 F4/80
F4/80 is a glycoprotein expressed on the surface of macrophages, including EIMs, and serves as a general identifier for macrophage populations. F4/80 is expressed in mouse EIMs but not in human or rat EIMs (7). In mouse, F4/80 is expressed by EIMs in mouse bone marrow, fetal liver and spleen with stress erythropoiesis (2–7). >90% of native EIMs expressed F4/80, which are CD45+ and almost all of them express Vcam1, CD169, ER-HR3, Hmox1, Fpn, and Trf. These molecules are essential for cell adhesion, heme catabolism, iron export, and transport (2, 4, 5, 7).
Depleting F4/80+ macrophages using clodronate liposomes or other methods impairs erythropoiesis in both steady-state and stress conditions (2, 8). In hemolytic anemia or myeloablation, F4/80+ EIMs in the spleen support the proliferation and differentiation of stress erythroid progenitors (SEPs) (2, 4, 9, 10). It should be mentioned that F4/80−/− mice are fertile, and F4/80 is not necessary for the development and distribution of mouse tissue macrophage populations (11).
2.2 Vascular cell adhesion molecule-1
VCAM-1 is a type I transmembrane glycoprotein expressed on EIMs. VCAM-1 is expressed in human EIMs and in > 90% mouse EIMs, but not in rat (7, 12).
VCAM-1 mediates the attachment of erythroblasts to EIMs through its interaction with the α4β1 integrin on erythroblasts. This interaction is essential for the formation and maintenance of EBIs, providing a supportive microenvironment for erythropoiesis (13).
And VCAM-1-deficient mice exhibiting impaired erythropoiesis (14). Similarly, clodronate administration eliminated F4/80+VCAM1+ macrophages in the spleen and bone marrow and impaired erythroid expansion in the spleen as well as RBC and reticulocyte production in response to EPO supplementation (8). VCAM-1+ macrophages work in concert with BMP4-producing CD169+ splenic macrophages to enhance erythropoietic recovery, particularly under stress conditions such as hemolytic anemia. This interaction supports the rapid proliferation and differentiation of erythroid progenitors (2).
2.3 CD11b (Mac-1, αM integrin)
CD11b is a member of the integrin family of cell adhesion receptors and plays a significant role in cell adhesion, migration, and interaction with other cell types. It forms a heterodimer with CD18 to create the αMβ2 integrin (Mac-1), which is expressed on various immune cells, including macrophages.
Human EIMs express CD11b. Moderate CD11b in mouse fetal liver EIMs was detected (7), but minute in mouse bone marrow EIMs (3). Actually, CD11b+ cells participating in BM EBIs are maturing granulocytes, which constitute a predominant cell population in EBIs among erythroblasts and macrophages (3). In stress erythropoiesis, CD11b+Ly6G+F4/80- granulocytes are found associated at the periphery of 40%-60% EBIs (15).
Interestingly, in phenylhydrazine(PHZ)-induced stress erythropoiesis, peripheral monocytes (CD11b+Ly6C+F4/80+) migrate to mouse spleen and become pre-red pulp macrophage (pre-RPMs) (CD11b+Ly6C−F4/80+), red pulp macrophage (RPMs) (CD11bloLy6C−F4/80hi) at last. These monocyte-derived macrophages expand the murine stress erythropoietic niche during the recovery from anemia (10).
2.4 CD16 (FcγRIII)
CD16 is a low-affinity Fc receptor for IgG expressed on various immune cells, including natural killer (NK) cells, macrophages, neutrophils, and certain subsets of T cells. While CD16 is not traditionally associated with EIMs, recent studies have suggested its potential role in immune-mediated regulation of erythropoiesis.
CD16 is only used in human EIMs study, and bone marrow CD14+CD16+CD163+EPOR+ cells immunophenotypically was defined as EBI central macrophages. In anemic patients with newly diagnosed multiple myeloma, CD14+CD16+CD163+EPOR+ macrophages have been reported to be decreased (16). But, the particular function of CD16 in EIMs is still need to be investigated in future.
2.5 CD163
CD163 is a scavenger receptor expressed on macrophages, including EIMs, and plays a significant role in erythropoiesis by regulating inflammation, clearing hemoglobin-haptoglobin complexes, and supporting the maturation of erythroblasts.
CD163 is expressed in human, rat (3, 5, 7, 16). Data for CD163 in mouse EIMs is slightly controversial. In Li’s study, ~35% mouse EIMs expressed CD163 (4), but Seu et al. showed that mouse EIMs do not express CD163 (3).
In Fabriek’s study, the authors identified a 13–amino acid motif (CD163p2) in CD163 functioning as a receptor for hemoglobin-haptoglobin (Hb-Hp) complexes and is believed to contribute to the clearance of free hemoglobin. The interaction of erythroblasts with the CD163p2 motif promotes erythroid expansion (17).
Interestingly, glucocorticoids can induce differentiation of monocytes towards macrophages that share functional and phenotypical aspects with erythroblastic island macrophages, in which the expression level of CD16, CD163, CD169, CD206 is increased (18).
2.6 CD169 (Siglec-1 or Sialoadhesin)
CD169 is a sialic acid-binding immunoglobulin-like lectin expressed on macrophages, including EIMs. It plays a critical role in erythropoiesis by mediating cell-cell interactions and providing a supportive niche for erythroblast maturation.
CD169 is expressed in human and >90% mice EIMs (7), but not in mouse fetal liver or in rat EIMs (3). Mouse EIMs usually co-express CD169 and VCAM1. The depletion of CD169+ macrophages using clodronate leads to a reduction in bone marrow erythroblasts, yet it does not result in peripheral blood anemia. Bone marrow and splenic EIMs play a critical role in recovery from hemolytic anemia and acute blood loss (8).
The CD169-CD43 interaction plays a role in erythroblastic island formation and erythroid differentiation. In vitro study has shown that blocking CD169 expression on bone marrow-derived macrophages disrupts the formation of erythroblastic islands. And CD43, expressed on erythroblasts, serves as the counter-receptor for CD169 (19).
2.7 CD206
CD206 (also known as the mannose receptor) is a C-type lectin receptor expressed on macrophages, including EIMs. It plays a significant role in erythropoiesis by facilitating the recognition and phagocytosis of pathogens, as well as regulating immune responses within the erythroblastic island niche.
CD206 was expressed in human EIMs and ~15% mouse EIMs (7). In human study, glucocorticoid stimulation directs monocyte differentiation to CD16+CD163+CD169+CXCR4+CD206+ macrophages, which share functional and phenotypical aspects with EIMs. These macrophages are motile and bind erythroblasts, which express TAM-receptor family members and phagocytose pyrenocytes (18). In mouse study, GM-CSF can significantly decrease the expression of CD206 on EIMs, impairing erythropoiesis. This indicates that CD206 plays a role in maintaining the proper function of EIMs and supporting efficient erythropoiesis (20).
2.8 Colony-stimulating factor 1 receptor, CD115
CSF1R is a tyrosine kinase receptor expressed on macrophages, including EIMs. The proliferation, differentiation, and survival of many tissue-resident macrophages rely on signaling through the macrophage CSF1R. This includes macrophages within hematopoietic tissues. In rats, homozygous loss-of-function mutations in Csf1r lead to a near-complete depletion of tissue-resident macrophages, which is associated with severe postnatal growth retardation and osteopetrosis. But no dysregulation of erythropoiesis or iron homeostasis was observed in CSF1R knockout rats. Notably, despite the loss of macrophages in the bone marrow, liver, and spleen, and the altered phenotype of the remaining macrophages, CSF1R knockout rats exhibited normal hematological parameters and showed no signs of iron deficiency or overload. These results suggest that CSF1R-dependent macrophages are not absolutely required for early-life steady-state erythropoiesis and iron homeostasis in rats (21).
2.9 MAEA (erythroblast macrophage protein)
EMP is a transmembrane protein expressed on both erythroblasts and EIMs. It plays a crucial role in the regulation of erythropoiesis by mediating the attachment of erythroblasts to macrophages and facilitating the enucleation process, which is essential for the maturation of erythroblasts into red blood cells.
Abnormal EMP expression or function can lead to significant impairments in erythropoiesis, resulting in anemia. EMP-null mice exhibit severe anemia due to defective erythroblast enucleation and impaired erythropoiesis. EMP mutant macrophages were small and immature and displayed membrane ruffles containing F-actin. These macrophages fail to establish significant contacts with erythroblast. And EMP-null macrophages fail to develop the extensive cytoplasmic projections characteristic of normal EIMs, suggesting that EMP is required for the proper development and function of EIMs (22). In addition, EIMs EMP-mediated association inhibits apoptosis of erythroblasts (23). Furthermore, EMP expression by EIMs, rather than erythroblasts, is required for the development of EBIs in the bone marrow (24). Interestingly, EMP deletion in spleen macrophages did not alter their numbers or functions (24).
2.10 Erythropoietin receptor
EPOR was expressed in human, mouse and rat EIMs, which plays a critical role in supporting erythropoiesis by mediating the effects of Erythropoietin (EPO) (4, 7, 25, 26). EBIs in mouse BM and fetal liver (FL) were predominantly formed by F4/80+Epor + macrophages. Human FL EBIs are also formed by EPOR+ macrophages, which co-express high level of Vcam1 and CD169 known to be involved in macrophage-erythroblast interaction and supporting erythropoiesis (4).
F4/80+Epor-eGFP+ macrophages could be increased under EPO induced stress erythropoiesis. EPO injection also increases surface expression of Vcam1 but not CD169 on the F4/80+Epor-eGFP+ macrophages (4). In addition, EPOR activation enhances the phagocytic activity of EIMs, facilitating the engulfment and digestion of extruded nuclei from erythroblasts. This process is crucial for the final stages of erythroblast maturation and maintaining hematopoietic homeostasis. In similar stress conditions, such as hemolytic anemia, EPOR signaling in splenic macrophages promotes the transition of SEPs from proliferation to differentiation. This function is essential for rapid erythropoietic recovery (25).
2.11 EKLF/KFL1
EKLF/KLF1 (Erythroid Krüppel-like Factor 1) is a transcription factor critical for definitive erythropoiesis, traditionally known for its role in erythroid lineage maturation. However, because of its essential function in a transient subset of fetal liver (FL) macrophages that support erythroblastic island (EBI) formation and function, we briefly describe the function of EKLF/KLF1 in EIMs, here.
FL macrophages exhibit a distinct transcriptional profile compared to adult splenic macrophages or erythroid cells, marked by EKLF-dependent genes involved in cell adhesion, vasculature development, and iron metabolism, critical for EBI niche function.
EKLF directly activates adhesion molecules (e.g., Vcam1 in macrophages and Icam4 in erythroblasts), facilitating erythroid-macrophage interactions necessary for EBI integrity. Loss of EKLF disrupts expression of genes critical for iron homeostasis (e.g., Epor), heme synthesis, and cell-cycle regulation, impairing erythroid maturation and enucleation.
EKLF expression in FL macrophages is transient, peaking at E12.5–E18.5 in mice, coinciding with definitive erythropoiesis. This temporal regulation ensures efficient red blood cell production during embryogenesis.
Single-cell RNA-Seq identifies EKLF+ macrophages as a distinct subset (clusters 4, 5, 7) with enriched expression of Adra2b, Add2, and Sptb, which serve as surface markers for isolating these niche-supportive macrophages. EKLF collaborates with Klf3, E2f1, E2f4, and Sp4 to form a transcriptional network regulating macrophage identity and erythroid support (6).
2.12 Gr1 (Ly-6G/Ly-6C)
Gr1 is a cell surface marker commonly used to identify granulocytes and certain subsets of monocytes/macrophages in mice. Ly-6G usually was used to gate out granulocytes and it was not expressed in EIMs (4, 7). Ly-6C was express in ∼69% mouse EIMs but not in human or rat EIMs (7). Study in Epor-eGFP+ mice showed ∼52% of the F4/80+/Epor-eGFP+ EBI macrophages express Ly-6C.
While Gr1 is not traditionally associated with EIMs, recent studies have highlighted its potential role in stress erythropoiesis, particularly under conditions of anemia or myelosuppression (27). For example, Ly-6C+ monocytes/macrophages are identified as a subset that can migrate to the spleen and differentiate into macrophages that form EBIs under stress conditions such as PHZ-induced hemolytic anemia. These Ly-6C+ monocyte-derived macrophages express markers associated with EIMs, such as CD169 and VCAM-1, and support the proliferation and differentiation of stress erythroid progenitors in the spleen (10).
2.13 α5-Integrin
α5-Integrin (also known as the α5β1 integrin complex) is an essential adhesion molecule expressed on EIMs that plays a critical role in the formation and function of EBIs, which are specialized microenvironments for erythropoiesis.
α5-Integrin, in combination with β1 integrin, forms a heterodimer that acts as a receptor for fibronectin and vitronectin, facilitating the adhesion of EIMs to the extracellular matrix (ECM) within the bone marrow niche. This interaction is essential for the stability and structural integrity of EBIs (5, 28). α5-Integrin likely functions in concert with other adhesion molecules expressed on EIMs, such as VCAM-1 and CD169, to maintain the structural and functional integrity of EBIs. These molecules form a complex adhesion network that ensures the close and stable interaction between EIMs and erythroblasts (13), which could be inhibited by peptides that block adhesion of ICAM-4 to αV integrins (5, 13). But, interestingly, deficiency of both α4 and α5 integrins in macrophages does not negatively influence stress erythropoiesis (29).
2.14 Peroxisome proliferator-activated receptor gamma
PPARγ is a nuclear receptor that plays a significant role in the function of EIMs PPARγ is involved in regulating macrophage polarization, phagocytosis, and the maintenance of erythropoietic homeostasis (30).
PAR2 deficiency causes a partially penetrant severe embryonic anemia. Erythroblastic island sizes and frequency of F4/80+ macrophages in fetal liver of PAR2−/− embryos were markedly reduced compared with PAR2+/− embryos. (30). Expression of EpoR was also reduced in PAR2−/− embryos relative to PAR2+/− embryos, but not changed in KLF-1 expression. Furthermore, tissue specific analysis shows fetal livers from macrophage PAR2-deficient mice also exhibited smaller erythroblastic islands. But Macrophage PAR2 is dispensable for steady-state postnatal erythropoiesis and hemolytic stress in adult animals (30).
Saffarzadeh et al. found similar result that the absence of PPARγ affects neonatal development and VCAM-1 expression of splenic iron-recycling red pulp macrophages (RPMs) and bone marrow erythroblastic island macrophages (EIMs). PPARγ and Spi-C collaborate in inducing transcriptional changes, including VCAM-1 and integrin αD expression (31).
EPO administration increases the phagocytic activity of peritoneal and splenic macrophages by upregulating PPARγ expression. Dying cell-released S1P activates macrophage EPO signaling, and promotes apoptotic cell clearance and immune tolerance (32). And PPARγ-dependent repression of Wnt signaling via prostaglandin production is critical for this process (25).
2.15 Mer tyrosine kinase
MerTK is a receptor tyrosine kinase expressed on EIMs that plays a critical role in the phagocytosis of extruded erythroblast nuclei and the maintenance of erythropoietic homeostasis (33). MerTK is expressed in human and >90% mouse EIMs (7). Dysfunction of MerTK leads to impaired erythropoiesis, characterized by the accumulation of nucleated erythrocytes and severe anemia (34).
F4/80+Epor-eGFP+ EIMs express MerTK and Timd4, which are required for nucleus engulfment (4). The pyrenocytes were engulfed by EIMs via a MerTK-protein S-dependent mechanism. Protein S appeared to function as a bridge between the pyrenocytes and macrophages by binding to PtdSer on the pyrenocytes and MerTK on the macrophages (35). This coordinated action ensures the efficient clearance of cellular debris and maintains hematopoietic homeostasis (36).
During stress erythropoiesis condition, such as hemolytic anemia, MerTK expression in EIMs is upregulated to enhance the phagocytic capacity, supporting the increased demand for erythropoiesis (4). Interestingly, GM-CSF treatment leads to decreased expression of Mertk, Axl, and Timd4 on mouse BM EBI macrophages as well as phagocytosis of senescent RBCs (20).
2.16 T-cell immunoglobulin and mucin domain-containing protein 4
Timd4 is a receptor expressed on macrophages, including EIMs, and plays a crucial role in the clearance of extruded erythroblast nuclei and the maintenance of erythropoietic homeostasis.Timd4 is expressed in human EIMs and >90% mouse EIMs (7).
Timd4 is involved in the recognition and engulfment of extruded erythroblast nuclei, which are expelled during the enucleation process of erythroblast maturation. These nuclei expose phosphatidylserine on their surface, which serves as an “eat me” signal recognized by Timd4 (4, 34). Efficient clearance of extruded nuclei by Timd4+ macrophages prevents the accumulation of cellular debris and the release of inflammatory signals, which could disrupt erythropoietic homeostasis (34).
EPO signaling can upregulate Timd4 expression on EIMs, enhancing their capacity to support erythropoiesis by promoting the clearance of extruded nuclei and maintaining a supportive microenvironment for erythroblast development (4). In contrast, GM-CSF treatment leads to decreased expression of Timd4, Mertk, and Axl on mouse BM EBI macrophages as well as phagocytosis of senescent RBCs (20).
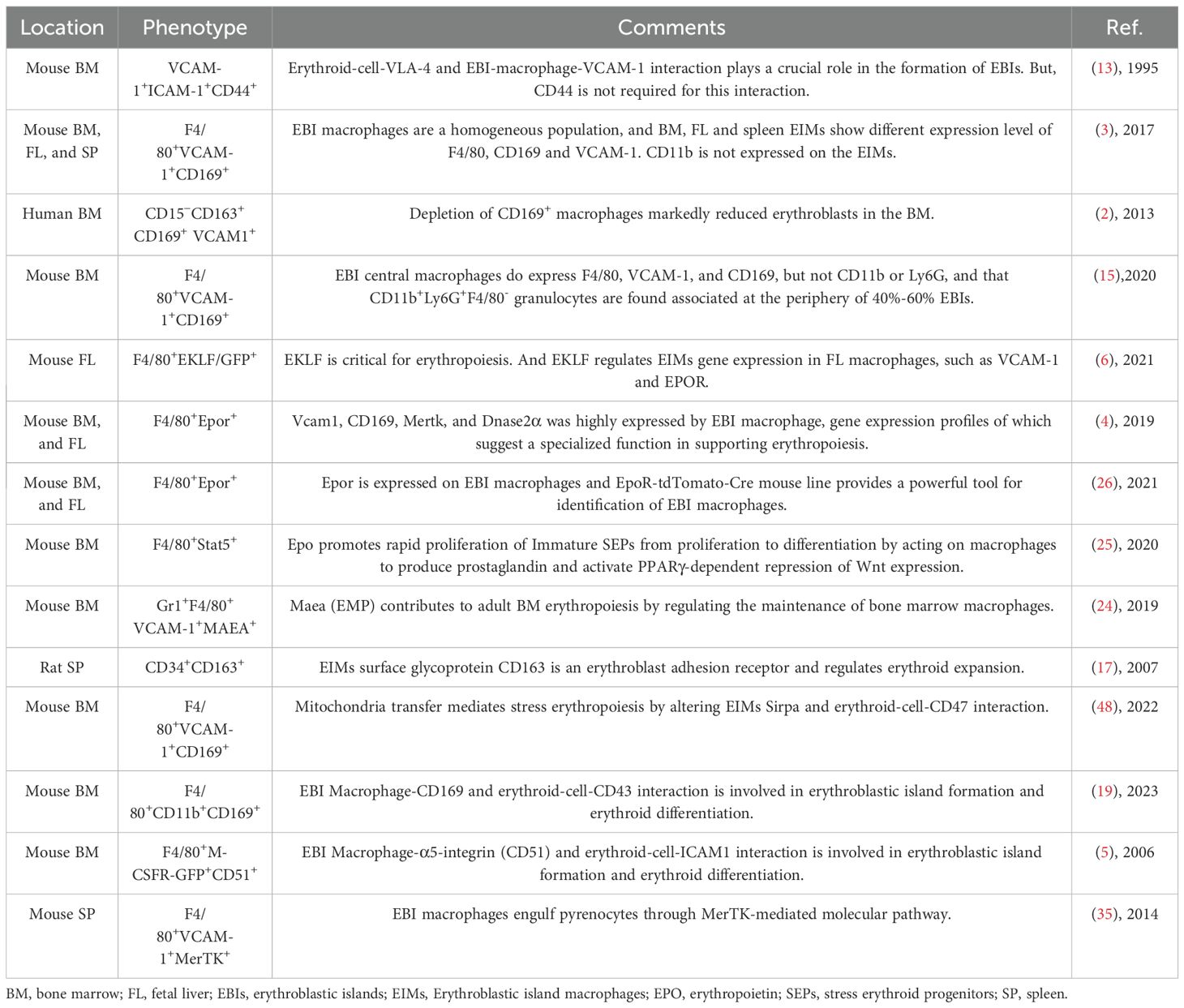
3 Summary of surface marker combinations for EIMs in flow cytometry
Below is a comparison of commonly used marker combinations for identifying EIMs, highlighting their applications, advantages, limitations, and supporting references:
3.1 General considerations
● Species differences: Mouse markers (e.g., F4/80, Gr1) lack direct human homologs; human studies rely on CD163, CD169, and VCAM1.
● Functional vs. phenotypic markers:
○ Adhesion molecules (VCAM-1, CD169, α5-Integrin, EMP) define structural niches.
○ Functional markers (Epor, MerTK, EMP, Timd4, PPARγ) link to, stress anemia, erythroblast maturation, iron recycling, nuclear extrusion or phagocytosis.
● Multiparametric panels: Combining ≥3 markers improves specificity but increases technical challenges.
3.2 Recommendations
● Steady-state erythropoiesis: Use F4/80+VCAM-1+CD169+ (mouse) or CD15−CD163+CD169+ (human).
● Stress conditions: Prioritize Ly-6C+F4/80+VCAM-1+ or F4/80+Epor+.
● Functional studies: Include MerTK, Timd4, or PPARγ to assess phagocytosis or metabolic roles.
3.2.1 F4/80+VCAM-1+CD169+
● Species/organ: Mouse BM, FL, and spleen.
● Function: Standard for identifying EIMs; CD169 and VCAM-1 mediate erythroblast adhesion.
○ Advantages: High specificity for EIMs in steady-state erythropoiesis. Widely validated across tissues.
○ Limitations: Heterogeneity in CD169 expression (e.g., absent in FL macrophages) (3, 4, 15).
3.2.2 CD15−CD163+CD169+VCAM1+
● Species/organ: Human BM.
● Function: Depletion of CD169+ macrophages reduces erythroblasts.
○ Advantages: Human-specific markers distinguish EIMs from other myeloid cells (CD15 exclusion).
○ Limitations: Requires multiple markers, increasing complexity. Limited data on functional heterogeneity (2).
3.2.3 F4/80+Epor+
● Species/organ: Mouse BM and FL.
● Function: Identifies EIMs with specialized roles in iron recycling and phagocytosis.
○ Advantages: Links EIMs to EPO signaling and stress erythropoiesis. High functional specificity (e.g., Hmox1, MerTK expression).
● Limitations:
○ Epor expression may vary under stress or disease. Requires transgenic models (e.g., EpoR-tdTomato-Cre mice) for validation (4, 26).
3.2.4 Gr-1(Ly-6C)+F4/80+VCAM-1+EMP+
● Species/organ: Mouse spleen during stress erythropoiesis.
● Function: Marks monocyte-derived macrophages supporting stress erythroid progenitors.
○ Advantages: Critical for studying stress erythropoiesis (e.g., anemia). Combines Gr1 (Ly6C/Ly6G) with adhesion molecules.
○ Limitations: Ly-6G+ cells also include granulocytes. Gr1+ macrophages are transient and context-dependent. Ly-6G+ cells overlap with granulocyte markers complicates analysis, and Ly-6C should be used instead (24).
3.2.5 F4/80+CD11b+CD169+
● Species/organ: Mouse BM.
● Function: Highlights CD169-CD43 interaction in EBI formation.
○ Advantages: Useful for studying erythroblast-macrophage adhesion.
○ Limitations: CD11b+ granulocytes are peripherally associated with EBIs, requiring careful gating (19).
For detailed protocols, refer to Tables 1, 2 for references, antibody clones and suppliers.
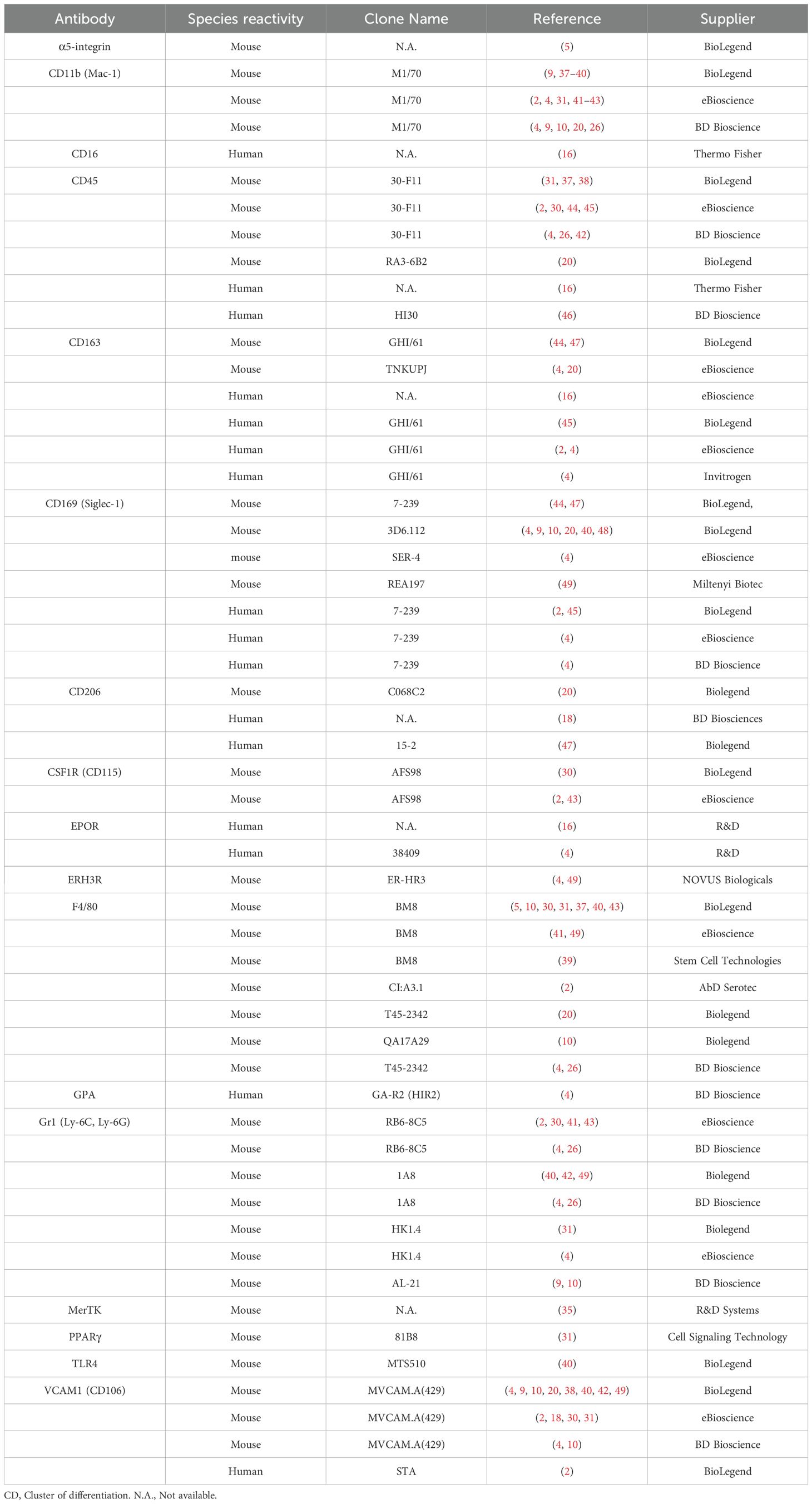
Table 1. Available monoclonal antibodies used in flow cytometric study of erythroblast island macrophage. Selection is based on molecules described in the text.
Author contributions
MF: Writing – original draft, Writing – review & editing. FM: Writing – original draft, Writing – review & editing. GF: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research is supported by Medical Science Research Project of Hebei, No20210152.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, et al. CD169⁺ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. (2013) 19:429–36. doi: 10.1038/nm.3057
3. Seu KG, Papoin J, Fessler R, Hom J, Huang G, Mohandas N, et al. Unraveling macrophage heterogeneity in erythroblastic islands. Front Immunol. (2017) 8:1140. doi: 10.3389/fimmu.2017.01140
4. Li W, Wang Y, Zhao H, Zhang H, Xu Y, Wang S, et al. Identification and transcriptome analysis of erythroblastic island macrophages. Blood. (2019) 134:480–91. doi: 10.1182/blood.2019000430
5. Lee G, Lo A, Short SA, Mankelow TJ, Spring F, Parsons SF, et al. Targeted gene deletion demonstrates that the cell adhesion molecule ICAM-4 is critical for erythroblastic island formation. Blood. (2006) 108:2064–71. doi: 10.1182/blood-2006-03-006759
6. Mukherjee K, Xue L, Planutis A, Gnanapragasam MN, Chess A, Bieker JJ. EKLF/KLF1 expression defines a unique macrophage subset during mouse erythropoiesis. Elife. (2021) 10. doi: 10.7554/eLife.61070
7. Li W, Wang Y, Chen L, An X. Erythroblast island macrophages: recent discovery and future perspectives. Blood Sci. (2019) 1:61–4. doi: 10.1097/BS9.0000000000000017
8. Ramos P, Casu C, Gardenghi S, Breda L, Crielaard BJ, Guy E, et al. Macrophages support pathological erythropoiesis in polycythemia vera and β-thalassemia. Nat Med. (2013) 19:437–45. doi: 10.1038/nm.3126
9. Liao C, Hardison RC, Kennett MJ, Carlson BA, Paulson RF, Prabhu KS. Selenoproteins regulate stress erythroid progenitors and spleen microenvironment during stress erythropoiesis. Blood. (2018) 131:2568–80. doi: 10.1182/blood-2017-08-800607
10. Liao C, Prabhu KS, Paulson RF. Monocyte-derived macrophages expand the murine stress erythropoietic niche during the recovery from anemia. Blood. (2018) 132:2580–93. doi: 10.1182/blood-2018-06-856831
11. Lin HH, Faunce DE, Stacey M, Terajewicz A, Nakamura T, Zhang-Hoover J, et al. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J Exp Med. (2005) 201:1615–25. doi: 10.1084/jem.20042307
12. Fujiwara H, Nishimura H, Irei I, Akiyama T, Hamazaki S, Wada H, et al. Human bone marrow VCAM-1 + macrophages provide a niche for reactive and neoplastic erythropoiesis. Kawasaki Medical Journal, Kawasaki Medical Society, 577 Matsushima Kurashiki, 701-0192, Japan (2017).
13. Sadahira Y, Yoshino T, Monobe Y. Very late activation antigen 4-vascular cell adhesion molecule 1 interaction is involved in the formation of erythroblastic islands. J Exp Med. (1995) 181:411–5. doi: 10.1084/jem.181.1.411
14. Ulyanova T, Phelps SR, Papayannopoulou T. The macrophage contribution to stress erythropoiesis: when less is enough. Blood. (2016) 128:1756–65. doi: 10.1182/blood-2016-05-714527
15. Tay J, Bisht K, McGirr C, Millard SM, Pettit AR, Winkler IG, et al. Imaging flow cytometry reveals that granulocyte colony-stimulating factor treatment causes loss of erythroblastic islands in the mouse bone marrow. Exp Hematol. (2020) 82:33–42. doi: 10.1016/j.exphem.2020.02.003
16. Huang H, Yu PY, Wei C, Li YW, Liang LJ, Liu YZ, et al. Regulatory effect and mechanism of erythroblastic island macrophages on anemia in patients with newly diagnosed multiple myeloma. J Inflammation Res. (2023) 16:2585–94. doi: 10.2147/JIR.S413044
17. Fabriek BO, Polfliet MM, Vloet RP, van der Schors RC, Ligtenberg AJ, Weaver LK, et al. The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor. Blood. (2007) 109:5223–9. doi: 10.1182/blood-2006-08-036467
18. Heideveld E, Hampton-O'Neil LA, Cross SJ, van Alphen FPJ, van den Biggelaar M, Toye AM, et al. Glucocorticoids induce differentiation of monocytes towards macrophages that share functional and phenotypical aspects with erythroblastic island macrophages. Haematologica. (2018) 103:395–405. doi: 10.3324/haematol.2017.179341
19. Bai J, Fan F, Gao C, Li S, Li W, Wei T, et al. CD169-CD43 interaction is involved in erythroblastic island formation and erythroid differentiation. Haematologica. (2023) 108:2205–17. doi: 10.3324/haematol.2022.282192
20. Cao W, Fan W, Wang F, Zhang Y, Wu G, Shi X, et al. GM-CSF impairs erythropoiesis by disrupting erythroblastic island formation via macrophages. J Transl Med. (2022) 20:11. doi: 10.1186/s12967-021-03214-5
21. Roberts M, Carter-Cusack D, Huang S, Maxwell E, Summers KZ, Irvine KM, et al. Blood cell homeostasis is maintained in the absence of resident mononuclear phagocytes in fetal liver and bone marrow in CSF1R knockout rats. Blood. (2024) 144:1149–9. doi: 10.1182/blood-2024-194571
22. Soni S, Bala S, Gwynn B, Sahr KE, Peters LL, Hanspal M. Absence of erythroblast macrophage protein (Emp) leads to failure of erythroblast nuclear extrusion. J Biol Chem. (2006) 281:20181–9. doi: 10.1074/jbc.M603226200
23. Hanspal M, Smockova Y, Uong Q. Molecular identification and functional characterization of a novel protein that mediates the attachment of erythroblasts to macrophages. Blood. (1998) 92:2940–50. doi: 10.1182/blood.V92.8.2940
24. Wei Q, Boulais PE, Zhang D, Pinho S, Tanaka M, Frenette PS. Maea expressed by macrophages, but not erythroblasts, maintains postnatal murine bone marrow erythroblastic islands. Blood. (2019) 133:1222–32. doi: 10.1182/blood-2018-11-888180
25. Chen Y, Xiang J, Qian F, Diwakar BT, Ruan B, Hao S, et al. Epo receptor signaling in macrophages alters the splenic niche to promote erythroid differentiation. Blood. (2020) 136:235–46. doi: 10.1182/blood.2019003480
26. Zhang H, Wang S, Liu D, Gao C, Han Y, Guo X, et al. EpoR-tdTomato-Cre mice enable identification of EpoR expression in subsets of tissue macrophages and hematopoietic cells. Blood. (2021) 138:1986–97. doi: 10.1182/blood.2021011410
27. Jacobsen RN, Forristal CE, Raggatt LJ, Nowlan B, Barbier V, Kaur S, et al. Mobilization with granulocyte colony-stimulating factor blocks medullar erythropoiesis by depleting F4/80(+)VCAM1(+)CD169(+)ER-HR3(+)Ly6G(+) erythroid island macrophages in the mouse. Exp Hematol. (2014) 42:547–61.e4. doi: 10.1016/j.exphem.2014.03.009
28. Mankelow TJ, Spring FA, Parsons SF, Brady RL, Mohandas N, Chasis JA, et al. Identification of critical amino-acid residues on the erythroid intercellular adhesion molecule-4 (ICAM-4) mediating adhesion to alpha V integrins. Blood. (2004) 103:1503–8. doi: 10.1182/blood-2003-08-2792
29. Ulyanova T, Georgolopoulos G, Papayannopoulou T. Reappraising the role of α5 integrin and the microenvironmental support in stress erythropoiesis. Exp Hematol. (2020) 81:16–31.e4. doi: 10.1016/j.exphem.2019.12.004
30. Saffarzadeh M, Grunz K, Nguyen TS, Lee YK, Kitano M, Danckwardt S, et al. Macrophage protease-activated receptor 2 regulates fetal liver erythropoiesis in mice. Blood Adv. (2020) 4:5810–24. doi: 10.1182/bloodadvances.2020003299
31. Okreglicka K, Iten I, Pohlmeier L, Onder L, Feng Q, Kurrer M, et al. PPARγ is essential for the development of bone marrow erythroblastic island macrophages and splenic red pulp macrophages. J Exp Med. (2021) 218. doi: 10.1084/jem.20191314
32. Luo B, Gan W, Liu Z, Shen Z, Wang J, Shi R, et al. Erythropoeitin signaling in macrophages promotes dying cell clearance and immune tolerance. Immunity. (2016) 44:287–302. doi: 10.1016/j.immuni.2016.01.002
33. Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y, et al. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. (2001) 292:1546–9. doi: 10.1126/science.292.5521.1546
34. Yoshida H, Kawane K, Koike M, Mori Y, Uchiyama Y, Nagata S. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature. (2005) 437:754–8. doi: 10.1038/nature03964
35. Toda S, Segawa K, Nagata S. MerTK-mediated engulfment of pyrenocytes by central macrophages in erythroblastic islands. Blood. (2014) 123:3963–71. doi: 10.1182/blood-2014-01-547976
36. Toda S, Nishi C, Yanagihashi Y, Segawa K, Nagata S. Clearance of apoptotic cells and pyrenocytes. Curr Top Dev Biol. (2015) 114:267–95. doi: 10.1016/bs.ctdb.2015.07.017
37. Sood S, Alpsoy A, Jiao G, Dhiman A, King CS, Conjelko G, et al. Loss of Bicra/Gltscr1 leads to a defect in fetal liver macrophages responsible for erythrocyte maturation in mice. bioRxiv. (2024). doi: 10.1101/2024.10.17.618940
38. Ma JK, Su LD, Feng LL, Li JL, Pan L, Danzeng Q, et al. TFPI from erythroblasts drives heme production in central macrophages promoting erythropoiesis in polycythemia. Nat Commun. (2024) 15:3976. doi: 10.1038/s41467-024-48328-8
39. Perron-Deshaies G, St-Louis P, Romero H, Scorza T. Impact of erythropoietin production by erythroblastic island macrophages on homeostatic murine erythropoiesis. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21238930
40. Bisht K, Tay J, Wellburn RN, McGirr C, Fleming W, Nowlan B, et al. Bacterial lipopolysaccharides suppress erythroblastic islands and erythropoiesis in the bone marrow in an extrinsic and G- CSF-, IL-1-, and TNF-independent manner. Front Immunol. (2020) 11:583550. doi: 10.3389/fimmu.2020.583550
41. Doty RT, Fan X, Young DJ, Liang J, Singh K, Pakbaz Z, et al. Studies of a mosaic patient with DBA and chimeric mice reveal erythroid cell-extrinsic contributions to erythropoiesis. Blood. (2022) 139:3439–49. doi: 10.1182/blood.2021013507
42. Romano L, Seu KG, Papoin J, Muench DE, Konstantinidis D, Olsson A, et al. Erythroblastic islands foster granulopoiesis in parallel to terminal erythropoiesis. Blood. (2022) 140:1621–34. doi: 10.1182/blood.2022015724
43. Wei Q, Pinho S, Dong S, Pierce H, Li H, Nakahara F, et al. MAEA is an E3 ubiquitin ligase promoting autophagy and maintenance of haematopoietic stem cells. Nat Commun. (2021) 12:2522. doi: 10.1038/s41467-021-22749-1
44. Ventura T, Fidanza A, Wilson MC, Ferguson DCJ, Lewis PA, May A, et al. Proteomic analysis reveals a potential role for extracellular vesicles within the erythroblastic island niche. Front Mol Biosci. (2024) 11:1370933. doi: 10.3389/fmolb.2024.1370933
45. May A, Ventura T, Fidanza A, Volmer H, Taylor H, Romanò N, et al. Modelling the erythroblastic island niche of dyserythropoietic anaemia type IV patients using induced pluripotent stem cells. Front Cell Dev Biol. (2023) 11:1148013. doi: 10.3389/fcell.2023.1148013
46. Wang Y, Xiang X, Chen H, Zhou L, Chen S, Zhang G, et al. Intratumoral erythroblastic islands restrain anti-tumor immunity in hepatoblastoma. Cell Rep Med. (2023) 4:101044. doi: 10.1016/j.xcrm.2023.101044
47. Lopez-Yrigoyen M, Yang CT, Fidanza A, Cassetta L, Taylor AH, McCahill A, et al. Genetic programming of macrophages generates an in vitro model for the human erythroid island niche. Nat Commun. (2019) 10:881. doi: 10.1038/s41467-019-08705-0
48. Yang C, Yokomori R, Chua LH, Tan SH, Tan DQ, Miharada K, et al. Mitochondria transfer mediates stress erythropoiesis by altering the bioenergetic profiles of early erythroblasts through CD47. J Exp Med. (2022) 219. doi: 10.1084/jem.20220685
49. Hasan S, Johnson MC, Kini AR, Baldea AJ, Muthumalaiappan K. A shift in myeloid cell phenotype via down regulation of siglec-1 in island macrophages of bone marrow is associated with decreased late erythroblasts seen in anemia of critical illness. Front Med (Lausanne). (2019) 6:260. doi: 10.3389/fmed.2019.00260
Keywords: erythroblast island macrophages, surface markers, erythropoiesis, erythroblast island, VCAM-1, CD163, CD169, EPOR
Citation: Fang M, Mei F and Li G (2025) Erythroblast island macrophages and their surface markers. Front. Hematol. 4:1580621. doi: 10.3389/frhem.2025.1580621
Received: 20 February 2025; Accepted: 14 April 2025;
Published: 13 May 2025.
Edited by:
John Strouboulis, King’s College London, United KingdomReviewed by:
Laura Gutiérrez, University of Oviedo, SpainKaustav Mukherjee, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2025 Fang, Mei and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangrui Li, Z3JsaUBoZWJtdS5lZHUuY24=
 Mei Fang
Mei Fang Fengjun Mei
Fengjun Mei Guangrui Li
Guangrui Li