- Division of Hematologic Malignancies & Cellular Therapeutics, University of Kansas Medical Center, Kansas City, KS, United States
Recent advancements in Chimeric Antigen Receptor T cell (CAR T) therapies and manufacturing technologies have expanded treatment options for patients with hematologic malignancies and autoimmune diseases. This review synthesizes the findings from abstracts and oral presentations delivered at the 66th American Society of Hematology (ASH) Annual Meeting. The clinical trial data featured in this review highlights the safety and efficacy of next-generation CAR T constructs, the efficiency of emerging manufacturing technologies, and the expanded indications for CAR T cell therapeutics.
1 Introduction
As indications and applications for Chimeric Antigen Receptor T cell (CAR-T) therapies have continued to evolve, the number of trials underway has grown in an astonishing fashion. Given over 500 CAR-T related abstracts at the American Society of Hematology (ASH) 2024 we placed an emphasis on the abstracts and oral presentations most relevant to clinicians in community and academic settings based on author discretion, including both malignant and non-malignant conditions. We have excluded studies that include only animal or laboratory-based data.
2 Non-Hodgkin lymphoma
2.1 Aggressive B-cell lymphoma
2.1.1 Prospective studies
The most notable prospective studies presented in non-Hodgkin lymphoma explore novel methods of improving upon the current Food and Drug Administration (FDA) approved autologous Cluster of Differentiation 19 (CD19) targeting CAR-T therapies with allogeneic and healthy donor derived CAR-T cells (CTX112), novel targets ROR1 (OCT-808), BAFF-R (Pmb-CT101),or bispecific (IMPT-314) and trispecific targeting (CD19.20.22) to prevent immune escape, enhancing T-cell signaling with IL-2 (SYNCAR-001), and rapid manufacture directly from peripheral blood mononuclear cells (UF-Kure19). All of these methods have the potential to significantly improve outcomes in the non-Hodgkin lymphoma realm.
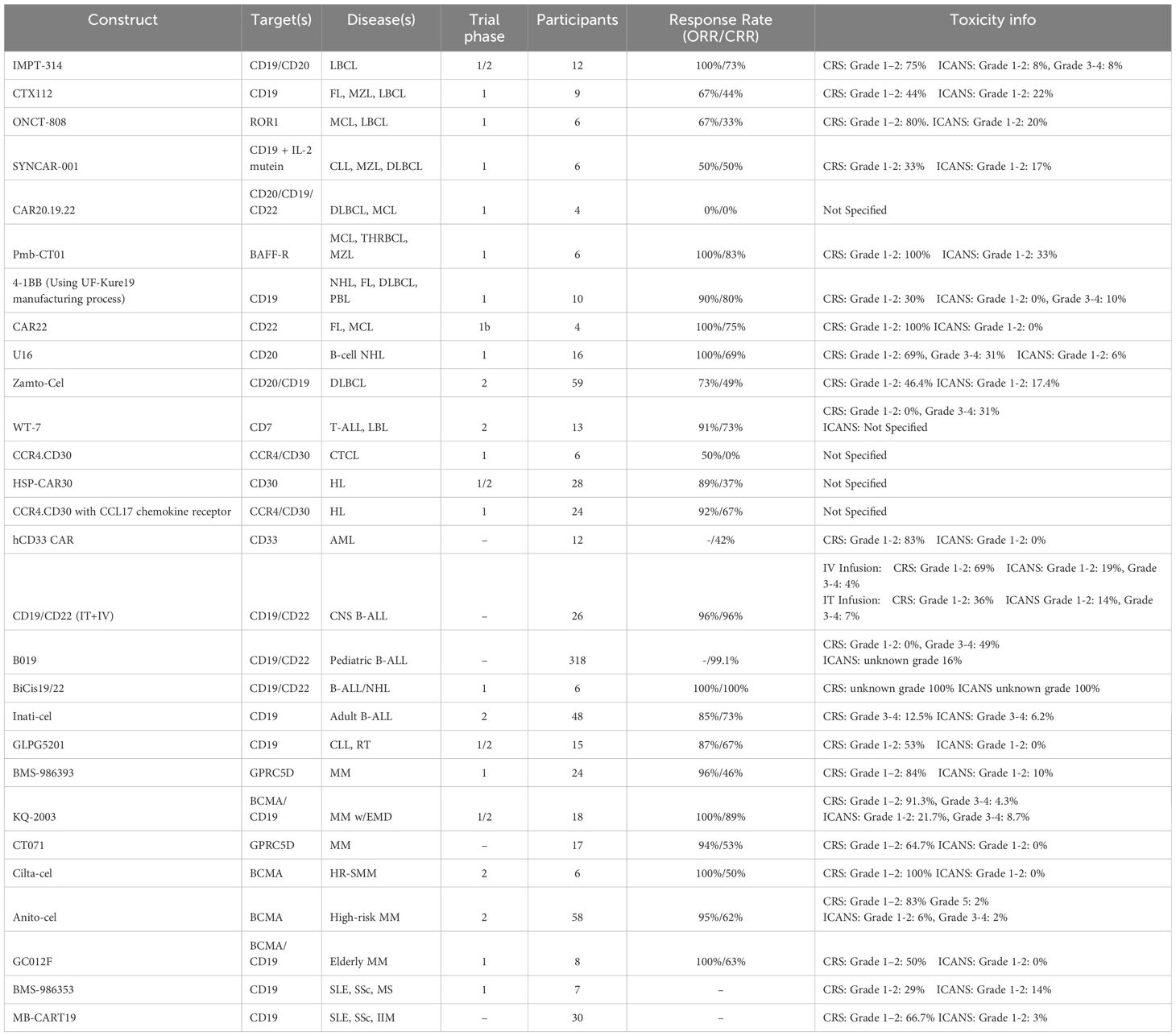
Rondecabtagene Autoleucel: Rondecabtagene autoleucel (IMPT-314) is an autologous bispecific CD19/CD20 Chimeric Antigen Receptor (CAR) product designed to improve cell persistence while decreasing CAR-T exhaustion and antigen escape (Table 1). Duali-T-1 is a phase 1/2 multi-center, open-label, single-arm trial which enrolled 12 patients with relapsed or refractory (R/R), CAR-T-naive large B-cell lymphoma (LBCL) after two or more lines of therapy (1). The median age of the cohort was 65 years (range 21-83), and 58% of the cohort had advanced stage disease at the onset of the trial. In phase 1, patients received a single dose of IMPT-314 at either dose level 1 (DL1) of 100 million CAR+ cells (n=10) or dose level 2 (DL2) of 300 million CAR+ cells (n=2). Objective response at day 28 was 100% (11/11), with one patient receiving the dose prior to the 28-day cutoff date, and a complete response (CR) was observed in 73% (8/11) of the cohort at three months. At the time of last follow-up (median 5.6 months), 73% (8/11) of the cohort remained in response. Adverse events (AE) included grade 1 or 2 cytokine release syndrome (CRS) in 75% (9/12) of the patients and immune effector cell-associated neurotoxicity syndrome (ICANS) in 17% (2/12) of patients. There were no infections or fatal adverse events recorded. The results from phase 1 of the Duali-T-1 trial were used to adjust the dosing for phase 2 which is currently ongoing.
CTX112: Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 editing was used to produce CTX112, a next-generation allogenic CAR-T cell therapy which improved upon the previous generation’s efficacy and functional persistence (2). Phase 1 of the NCT05643742 trial was conducted to identify dose limiting toxicities, determine overall and complete response rates (ORR/CRR), and gather pharmacokinetic data. Nine patients with follicular lymphoma (FL) (3), marginal zone lymphoma (MZL) (2), or LBCL (4) followed up for at least three months after receiving a dose ranging from 30 x 10^6 (DL1) to 300 x 10^6 (DL3) T cells. The results showed a ten-fold increase (133,701 vs.13,830 copies/μg*days) in functional persistence (after 14 days) when compared with the previous generation. Adverse events included CRS (grade 1 or 2) in 4/9 (44%) of the patients and ICANS (grade 1) in 2/9 (22%) of the patients. There were no instances of graft versus host disease (GvHD), hemophagocytic lymphohistiocytosis, or infections (grade 3 or higher), and the ORR and CRR were reported to be 6/9 (67%) and 4/9 (44%) respectively. One patient from the cohort has remained in complete remission for over a year after their infusion. Currently, dose optimization is being tested in a phase 2 clinical trial.
ONCT-808: ONCT-808 is a novel CAR-T cell therapy product which targets receptor tyrosine kinase-like orphan receptor 1 (ROR1), an oncoembyrotic pseudo-tyrosine kinase receptor present in many hematologic and solid malignancies (3). Results from phase 1 of a multicenter, open-label trial (NCT 05588440) showed early activity in a cohort of six patients with R/R B-cell lymphoma (mantle cell lymphoma (MCL) and LBCL). Patients were treated with 0.3 x10^6 cells (n=2), 1 x10^6 cells (n=3), and 3.0 x 10^6 cells (n=1). No dose limiting toxicities were reported in the first two dosing cohorts and 2 AEs included grade 1–2 CRS (4/5), grade 2 ICANS (1/5), grade 3 pneumonia (1/5), and grade 3–4 cytopenia (1/5). Notably the patient who received 3.0 x 10^6 cells was an 80 year-old male with high tumor burden LBCL and died due to complications of shock but had no evidence of lymphoma upon autopsy. At follow-up of one month two patients displayed a complete metabolic response and two others displayed a partial metabolic response. ONCT-808 CAR-T cells were present beyond 12 months after infusion. The primary endpoint of the ongoing phase 2 trial is assessing ORR at 0.3 and 1.0 x10^6 cells.
SYNCAR-001: Data from the first clinical trial using an orthogonal cytokine system to increase CAR-T cell persistence, function and proliferation was presented. SYNCAR-001 is an autologous CAR-T cell construct which expresses an IL-2 receptor selective for STK-009, a pegylated IL-2 mutein, ensuring that there is no off-CAR binding of IL-2 (4). Administration of the combined product, STK-009-001, was reported in six patients (3 CLL, 2 MZL, 1 DLBCL) during the NCT05665062 phase 1 trial. No CRS or ICANS above grade 2 were observed and four patients experienced no CRS or ICANS. A CRR was achieved by three patients (2 MZL, 1 DLBCL), but the CLL patients’ disease had progressed by day 28. Additionally, the STK-009 was shown to increase the fitness, expansion, and persistence of SYNCAR-001 without inducing IL-2 biomarkers.
CAR20.19.22: Pre-clinical trials demonstrated the potential effectiveness of CAR20.19.22, a trispecific duoCAR-T cell construct designed to target heterogenous B cell malignancies, but the results from the phase 1, single-center, prospective trial (NCT05094206) showed a 0% ORR at day 28 in a cohort of four patients (2 DLBCL, 2 MCL). This was the first human trial which featured a CAR-T product that utilized inducible co-stimulator (ICOS) signaling within targeting domains (5). More xenograft studies will be performed to elucidate the failure of this construct.
Pmb-CT01: Pmb-CT01 is a CAR-T cell construct that targets the B-cell activating factor receptor (BAFF-R). This receptor is critical for B cell function and survival, so tumor escape through downregulation is unlikely to occur. (6). A phase 1 clinical trial was conducted (NCT05370430) to assess the dose limiting toxicities and therapeutic efficacy of the construct. Of the six patients (4 MCL, 1 T cell/histiocyte rich large B-cell lymphoma (THRBCL), 1 MZL) five were evaluable and achieved a CRR lasting from 4–20 months. There were no dose limiting toxicities reported at either dose level (50 x10^6 cells (DL1), 200 x10^6 cells (DL2)). AEs included grade 1 CRS in all 5 patients and grade 1 ICANS in 2 patients. Pmb-CT01 shows potential to provide long lasting remission in patients with R/R B-cell malignancies.
UF-Kure19: UF-Kure19 is a novel CAR-T manufacturing platform that bypasses the need for ex vivo CAR-T expansion by producing CAR-T constructs directly from peripheral blood mononuclear cells (7). This platform allows for production of viable T cells in less than 24 hours and requires less machinery, reducing costs significantly. Limited accessibility to CAR-T therapy is an international hurdle to cancer treatment that advancements in the UF-Kure19 platform could potentially solve.
A single-arm, multi-center phase 1 study (NCT05400109) was conducted to assess the safety profile and efficacy of a second generation 4-1BB CAR manufactured using the UF-Kure19 platform (7). Ten patients with R/R CD19+ NHL (2 MCL), 4 FL, 3 DLBCL, and 1 plasmablastic lymphoma) were enrolled in the study. Each patient received a dose of 17.5 million cells and a CMR was noted in 80% (8/10) of the patients. AEs included CRS in 3 patients (1 grade 1, 2 grade 2), ICANS in one patient (grade 3) which resolved within 24 hours, and additional grade 3 hypoxia (n=1), neutropenia (n=8), leukopenia (n=6), lymphopenia (n=4), and a thromboembolic event (n=1). The high safety profile of UF-Kure19 is attributed to the higher proportion of naive and early memory T cells which demonstrate slower tumor-killing kinetics than other CAR-T products.
2.1.2 Retrospective studies
Real world outcomes remain paramount to the implementation and use of standard of care CAR-T therapies. The focus of these studies was on differences in efficacy and toxicity based upon second versus third line use and the specific commercially available CAR-T constructs to use. Many clinicians have postulated that earlier use of CAR-T would improve outcomes and reduce toxicity. With the shortcomings of cross-trial comparisons and no large, randomized head to head studies of the three available FDA approved CD19 CAR-T constructs in non-mantle cell B-NHL, retrospective real world data and match-adjusted indirect comparison of landmark trials was presented to compare constructs.
CAR-T Cell Therapy in Early Relapsed/Refractory Large B-Cell Lymphoma: Real World Analysis from the Cell Therapy Consortium: Following the recent Food and Drug Administation (FDA) approval of axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel (liso-cel) for second line therapy in patients with R/R LBCL, a retrospective study was conducted comparing patient outcomes when treated with CAR-T as second line (2L) versus third line (3L+) therapy (8). At 90 days post-infusion, there was an ORR of 70% and CRR of 57% in the 122 evaluable patients with no significant difference between patients receiving CAR T 2L versus 3L+. Additionally, there was no significant difference in 9-month overall survival between 2L and 3L+ patients. Furthermore, there was no significant difference in toxicity with similar incidence of all and high grade CRS and ICANS. The authors not the limited study size and limited follow up of a median of only 11.1 months.
Real-World Outcomes of Early Relapsed/Refractory Large B Cell Lymphoma Patients Treated with 2nd Line CAR-T Therapy: Another study conducted at the University of Pennsylvania retrospectively measured patient outcomes when axi-cel or liso-cel CAR-T was administered 2L in patients with R/R LBCL (9). In a group of 48 evaluable patients, progression free survival (PFS) was 46% and 12-month OS was 83% given a median follow-up of 13 months. In terms of toxicity, in multivariate analysis for development of CRS only receipt of liso-cel was predictive (OR 0.12, P=<0.01) and on multivariate analysis for ICANS receipt of bridging radiation and elevated LDH at time of lymphodepletion were predictive (OR 9.5, =-0.02 for both). Outcomes did not differ based on which CAR-T product was received. The authors note that both products appear to have similar efficacy in the real world, comparable to both ZUMA-7 and transform, with lower CRS with liso-cel.
Matching-Adjusted Indirect Comparison (MAIC) of Efficacy and Safety Outcomes for Lisocabtagene Maraleucel (liso-cel) Versus Axicabtagene Ciloleucel (axi-cel) and Tisagenlecleucel (tisa-cel) for the Treatment of Third-Line or Later (3L+) Relapsed or Refractory (R/R) Follicular Lymphoma (FL): Data from the registrational clinical trials comparing the safety and efficacy of axi-cel, liso-cel, and tisagenlecleucel (tisa-cel) CAR-T constructs was also presented (10). The study was conducted using patient data for liso-cel from TRANSCEND and aggregate data for axi-cel from ZUMA-5 and for tisa-cel from ELARA. Given a higher number of patients in TRANSCEND received bridging therapy compared to ZUMA-5, those receiving bridging were excluded in the comparison. The study showed that liso-cel demonstrated a higher CR with similar ORR, PFS and duration of response (DOR) rates compared with both axi-cel and tisa-cel in patients with R/R follicular lymphoma (FL). Liso-cel also demonstrated a more favorable safety profile in terms of ICANS and CRS.
Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicenter seamless design study: 5-year OS data was presented from the TRANSCEND (NCT02631044) study in which liso-cel demonstrated durable remission and high efficacy in patients with R/R LBCL (11). In a cohort of 345 patients the 5-year OS was 38.1%, and for those who achieved CR, the 5-year survival rate was 55.9% (12). These data demonstrate the potential for liso-cel therapy to be curative for patients achieving a CR following liso-cel. with R/R LCBL.
Real-World (RW) Outcomes of Lisocabtagene Maraleucel (liso-cel) in Patients (pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL) and Secondary Central Nervous System (sCNS) Involvement from the Center for International Blood and Marrow Transplant Research (CIBMTR) Registry: The registrational CAR-T trials all excluded secondary central nervous system (CNS) disease. Data from a retrospective study analyzing outcomes of patients with R/R LBCL and secondary CNS involvement were presented (13). All 36 patients were treated with liso-cel at a median follow-up of 12 months the ORR was 64%, CR rate was 53%, and the probability of PFS and OS at 12 months was 36% and 39%, respectively. This was a heavily treated population with a median of 3 previous lines of therapy and 9% with prior autologous transplant. These results demonstrate liso-cel’s potential in secondary CNS lymphoma, a disease with historically extremely poor outcomes.
2.2 Indolent/other B-cell lymphoma
In the indolent lymphoma space, phase I studies with novel CAR targets (CD20, CD22, and dual targeted CD19,CD20) were presented, along with four year follow-up for tisa-cel and five year follow-up for axi-cel.
CAR22: The FDA recently designated CAR22 as a breakthrough therapy (BTD) for patients with LBCL. In a phase 1b trial, CAR22-directed CAR-T cells were used to treat patients with R/R FL and MCL. The four evaluable patients showed an ORR of 100% at 28 days post-infusion and three of the four achieved CR. All the patients experienced CRS (grade 2 or less), but there were no instances of neurotoxicity reported (14).
Tisa-cel: The phase 2 ELARA trial was conducted to assess the efficacy and safety of tisa-cel CAR-T therapy in patients with R/R FL after two or more lines of therapy. The 4-year follow-up in a cohort of 94 evaluable patients showed 79.3% OS at 48 months, a median of 53.3 months PFS, 50.2% PFS at 48-months, and 66.1% PFS at 48-months for patients who achieved complete remission (15). Minimal residual disease (MRD) negativity was reported in 87.5% of patients at any point during the trial. There were no new safety signals reported in the phase 2 trial, however 6.2% of patients developed second primary malignancies.
Axi-cel: Five-year follow-up from the phase 2 ZUMA-5 trial analyzing the efficacy and safety of axi-cel in the treatment of R/R indolent NHL was presented. Median duration of response (DOR) for the cohort of 159 patients (127 FL, 31 MZL) was 60.4 months, and 53.4% of patients remained in response at 60 months (16). Median PFS was 62.2 months, with 50.4% of the patients reaching 60 months PFS. The 60-month OS was estimated to be 69.0%. More than half of the cohort required no additional therapy after the 60-month cut-off date. Notably, no new safety signals were reported and there were no lymphoma-specific deaths after the prior analysis. There was one progression event in an FL patient that occurred 24 months after therapy. This analysis demonstrates the curative potential of axi-cel therapy in patients with R/R indolent NHL.
U16: U16, a CAR-T construct that targets CD20, was used to treat R/R B-cell NHL patients who had previously received rituximab therapy. The trial included 16 patients, 9 of which underwent rituximab therapy within 3 months of U16 infusion (17). Results included 100% ORR, 69% CRR within 3 months, and a median PFS of 14.5 months. All patients experienced CRS (68.75% G1-2, 31.25% G3) and one patient experienced ICANS. The study noted that patients who had received rituximab within 3 months of infusion had a higher relapse rate (77.8% and 28.6%) and shorter PFS.
Zamto-Cel: Zamtocabtagene Autoleucel (Zamto-Cel) is an autologous tandem CD20-CD19-directed non-cryopreserved CAR-T cell therapy that is delivered as a fresh product with a 14-day vein to vein time. A phase 2 trial (DALY II USA) was conducted to assess the effectiveness and safety of zamto-cel in patients with R/R DLBCL (39). In a cohort of 59 evaluable patients this manufacturing process achieved a success rate of 91% with a 96% mean product viability and 29.4% transduction efficacy. In most patients a fresh product was used and there were no recorded deaths during manufacturing. The results included a 72.9% ORR, 49.2% CRR, 6 and 12-month PFS of 55% and 42% respectively, 72% OS at 12-months, and a median DOR of 11.4 months. AEs included CRS (2 grade 1) in 46.4% of patients, ICANS (2 grade 1) in 17.4% of patients, and one case of immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome (IEC-HS) which resolved within two days. The 14-day vein to vein manufacturing process of zamto-cel combined with its high efficacy and safety profiles make this therapy feasible for high-risk patients with R/R DLBCL.
2.3 T-cell lymphoma
While representing a relatively small proportion of NHL, T-cell lymphomas that do not respond to initial therapy have an extremely poor prognosis and the need for effective immune effector cell therapies in this space remains. We review a CD7 targeting allogeneic CAR-T construct for acute lymphoblastic leukemia/lymphoma and a CCR4-CD30 targeting CAR-T construct for cutaneous T-cell lymphomas.
WT-7: Patients with T cell malignancies experience poor prognoses and the current treatment options are sparse. Treatment using CAR-T therapeutics is challenging due to the fratricide of T cell targeting constructs, however WT-7 is an allogeneic CD7 targeting CAR-T product engineered using CRISPR/CAS9 that does not express CD7 or T-cell receptor alpha constant (TRAC) which has been shown to increase the longevity of the product. In phase 2 of the WU-CART-007–1001 trial, 13 patients with R/R T-cell acute lymphoblastic leukemia (T-ALL) and lymphoblastic lymphoma (LBL) were infused with WT-7 and the 11 evaluable patients achieved an ORR and CRR of 91% and 73% respectively (40). AEs included 31% of patients experiencing CRS (grade 3), infections, and one case of GvHD. Expansion of the product in peripheral blood continued until day 90 post-infusion. In the 6 patients achieving a CR, 83% achieved MRD negativity. Five patients received a consolidative allogeneic transplant. Clinical trials are currently enrolling for further evaluation of WT-7’s efficacy and safety.
CCR4.CD30.: CCR4.CD30.CAR-T is a product that co-expresses CD30 and CCR4 targeting domains and was designed to treat patients with cutaneous T-cell lymphoma (CTCL). In a phase 1 trial, six patients with CD30+ CTCL (mycosis fungoides (MF), Sézary syndrome (SS), primary cutaneous ALCL (pcALCL), or lymphomatoid papulosis) were infused with this product in addition to a CD30 targeting T-cell construct (18). Results demonstrated a modest ORR of 50% and a CR of 0% however, these results are comparable with current CTCL therapies such as brentuximab vedotin (ALCANZA trial) and pembrolizumab (CITN-10). Notably, tumor biopsies revealed CCR4.CD30.CAR-T product within tumors of patients, while the CD30 product remained in the peripheral blood. Further trials are being conducted to increase the efficacy of this construct.
3 Hodgkin lymphoma
While the use of checkpoint inhibitor therapy and CD30 antibody-drug conjugates in combination with traditional cytotoxic chemotherapy has significantly improved long-term outcomes in classical Hodgkin disease, a significant unmet need in those with relapsed/refractory disease remains.
HSP-CAR30: Results were presented from a phase I/II clinical trial evaluating the efficacy and safety of HSP-CAR30, the first CD30 targeting CAR-T construct ever produced in Europe (19). 28 patients with checkpoint inhibitor and CD30 antibody-drug conjugate exposed R/R classical Hodgkin lymphoma (HL) were treated with HSP-CAR30 and the cohort experienced an ORR and CR of 89% and 37% respectively. At 12 months, PFS was 37%, with an OS rate of 69.8%. The mean DOR was 10 months, but in patients who experienced CR, DOR was 21.1 months. All grade CRS occurred in 39% of patients, with only one patient (9%) experiencing grade 3 CRS, one patient with grade 3 ICANS, and one patient with macrophage activation syndrome. At peak expansion, the T stem cell memory (TSCM) and T cell memory (TCM) cells were predominant suggesting their importance in providing the durable remission experienced by this cohort.
CCR4.CD30.CAR: Another CD30 targeting CAR-T product which co-expresses the CCL17 chemokine receptor, CCR4, was evaluated for enhanced tumor homing in a clinical trial (20). Reed Sternberg cells produce CCL17 which creates an inhibitory barrier for T-cells. CCR4 is the receptor for CCL17 and it has previously been demonstrated with CARs expression CCR4 are able to overcome the potentially deleterious impact of CCL17. Twenty-four patients with R/R HL were given the CCR4.CD30.CAR-T product which resulted in 16 patients achieving CR and six patients achieving a partial response. At a median follow-up of 29.6 months, the cohort had a median PFS of 6.4 months with a PFS of 9.3 months for patients in CR. CCL17, a biomarker for HL, levels in the plasma were reduced by 86% in these patients after 2 weeks which indicates an improved response rate when compared with a previously evaluated CD30.CAR-T product which did not express CCR4.
4 Acute leukemia
While great strides have been made treating B-Acute Lymphoblastic Leukemia (B-ALL) with CD19 directed CAR-T therapy, finding an appropriate target that is well preserved on the myeloid leukemic cells but not expressed on normal hematopoietic stem cells for treatment of Acute Myeloid Leukemia (AML) has remained elusive.
4.1 AML
hCD33 CAR: Currently there are no effective treatment options for patients with R/R acute myeloid leukemia (AML) who had received a hematopoietic stem cell transplant (HSCT) and CAR-T therapy has not produced promising results because of the heterogeneity of the disease. A study was conducted to assess the efficacy of CAR-T cell therapy in a cohort of 12 AML patients whose malignancies expressed CD33 (21). The humanized hCD33 CAR-T cells were transfused in singular doses ranging from 6.2 × 10^4 cells/kg to 6.15 × 10^5 cells/kg. A CRR of 41.67% (5/12) was reported for this cohort. There were no instances of ICANS and 10 instances of CRS (9 grade 1, 1 grade 2). Other AEs included liver damage, sepsis and infection. Follow-up (ranging from 4 months to 1 year) showed six of the patients had died from complications, five had survived and received another HSCT, and one had achieved complete remission without another HSCT.
4.2 B-ALL
The B-ALL trials presented in ASH focused on novel methods of improving upon the three FDA approved CD19 directed CAR-T products with a bicistronic CD19/22 target (B019, BiCis 19/22). Further study of the older population with R/R B-ALL was presented as well.
B019: A newly developed bicistronic CD19/CD22 CAR T-cell therapy (B019) was used to treat a cohort of 318 pediatric patients (age ≤ 18) with R/R B-ALL (22). The therapy demonstrated a 99.1% CRR, all of whom were MRD negative. Consolidative allogeneic stem cell transplant occurred in 14% of patients and was associated with improved outcomes with a 12 months EFS of 86% versus 71% (p=0.04) in those that underwent transplant versus those that did not. However, there was no difference in 12 month OS between those that underwent transplant and those that did not. Sixty-nine of the patients who relapsed underwent another infusion of the construct, 49 of which achieved CR again, experiencing remission for a range of 1.0 to 22.1 months. Grade 3–4 CRS developed in 155 patients resulting in two deaths throughout the trial, and neurotoxicity was reported in 52 patients. The durable remission achieved in this trial with minimal safety concerns demonstrates this construct’s potential effectiveness in treating children with R/R B-ALL.
BiCis19/22: A phase I trial was conducted to assess the safety and efficacy of another bicistronic CD19/22 CAR-T construct (BiCis19/22) in children, adolescents, and young adults with B-ALL or NHL (23). The CD19 portion is associated with a CD28 co-stimulatory receptor while the CD22 portion is associated with 41BB. Six patients (median age 25.5y [20.5-29.2y]) were infused with autologous CAR-T cells, all of which achieved CR within 28 days. Two patients are in CR 15 months after infusion without the use of any interval therapies and one was in early follow-up (57 days) at the time of publication. Dose-limiting toxicities including ICANS, CRS and IEC-HS were observed in all patients, but adjustments to dosing resolved these issues. The remarkable efficacy of BiCis19/22 demonstrates promise in dual antigen CAR-T therapies for patients with B-ALL and NHL.
Inati-cel: Inati-cel is a CD19-specific CAR-T construct with a distinct single-chain variable fragment (HI19α) that was recently approved for therapeutic use in China for adult patients with R/R B-ALL (24). A phase 2 trial was conducted with 48 patients which demonstrated an ORR and CRR of 85.4% and 72.9%, respectively. Median DOR was 20.7 months with a 2-year OS rate of 55.2%. The safety profile of the trial was excellent with low incidences of severe CRS and ICANS reported.
CD19 CAR in older patients >55 in CR: Adult patients with B-ALL experience abysmal outcomes partially due to the low tolerability of current pediatric inspired treatment regimens and the higher risk of consolidative allogeneic stem cell transplantation. A sing-arm, non-randomized, pilot study was conducted to assess the safety and efficacy of CD-19 targeted CAR-T therapy as a consolidative therapy in adults over 55 years of age who are currently experiencing CR (25). Thirteen patients with a median age of 66 were infused with the phase 2 recommended dose (200M CAR-T cells). The cells expanded in the blood and CSF and no instances of ICANS or CRS above G2 were reported. At a median of 175 days follow-up, 12 patients remained in remission with one Ph+ patient relapsing. All patients had normal cognitive function and mobility which suggests an excellent safety profile of this regimen. This pilot study concluded that CAR-T therapy could offer older patients with B-ALL a safe and effective method of treatment in the future.
4.3 Retrospective studies
Retrospective studies for CAR-T cell therapy in B-ALL highlight the safety and efficacy of brexu-cel in the over 60 population and explore the use of intrathecal CAR-T for B-ALL with CNS involvement.
Brexu-cel in those over 60, ROCCA consortium: A retrospective study was conducted to assess the safety and efficacy of brexucabtagene autoleucel (brexu-cel) in older adult patients (≥60 years) with R/R B-ALL using data from the Real-World Outcomes Collaborative for CAR-T in ALL (ROCCA) consortium (26). This study analyzed the outcomes of 58 patients aged 60–69 and 15 aged 70 or above. There was no significant difference in OS when comparing these two age groups, but patients over 70 had lower PFS and experienced AEs at higher rates. Notably, the data determined that older groups of patients experienced similar ORR and CR rates to younger patients, allowing researchers to conclude that brexu-cel is safe and effective for older adults. However, future prospective clinical trials are needed to determine the optimal CAR-T therapy for older patients.
CD19/CD22 CAR: The effectiveness of intrathecal CAR-T therapy in patients with R/R CNS B-cell acute lymphoblastic leukemia (B-ALL) was retrospectively analyzed. Twenty-two patients with active disease and four in remission were given intrathecal (IT) and intravenous (IV) CD19/CD22 CAR T cells (27). Twenty-one of the 22 patients with active disease achieved CR in the bone marrow and CNS, and none of the four patients in remission experienced relapse. Adverse events after IV infusion included CRS (69% grade 1 or 2, 4% grade 3) and ICANS (19%). Similarly, the adverse events after IT infusion included CRS (31% grade 1 or 2, 4% grade 3) and ICANS (15%). At follow-up of 10 months, 20 patients remained in CR which demonstrates the potential efficacy of intrathecal CAR-T therapy in this high-risk patient population to control CNS disease. Additionally, the incidence of ICANS in this population was not prohibitive.
5 Chronic leukemia
5.1 CLL
We highlight a phase I/II trial investigating the use of the CD19 targeting GLPG5201 CAR-T construct in refractory CLL and Richter transformation (RT). To date, there is no standard of care for RT and there remains an unmet need in this population with very poor outcomes.
GLPG5201: EUPLAGIA-1 is a prospective phase I/II clinical trial investigating the safety and efficacy of GLPG5201, a CD19 targeting CAR T-cell construct, in patients with double-refractory chronic lymphocytic leukemia (CLL) and Richter transformation. This construct is rapidly manufactured (7-day vein-to-vein time), eliminating the need for cryopreservation, and preferentially selects memory phenotype T-cells (28). Fifteen patients (6 CLL, 9 RT) of median age 66 were enrolled in the clinical trial and after a median follow-up of 8.8 months, an ORR and CRR of 86.7% and 66.7% were reported. No instances of ICANS were reported and all instances of CRS were grade 2 or lower. This study demonstrated efficacy and a manageable tolerability profile in a patient population whose outcomes remain poor.
6 Multiple myeloma
Since the approval of two B-cell maturation antigen (BCMA) targeting CAR-T products the MM treatment paradigm has continued to evolve. We highlight trials involving novel targets of (G protein–coupled receptor class C group 5 member D (GPRC5D) and BCMA/CD19 dual target. We further present the trial of anito-cel, a BCMA construct with a novel D-domain binder and the use of cilta-cel for high risk smoldering myeloma.
BMS-986393: The efficacy of CAR T-cell therapeutics in treating MM is currently being investigated because of early resistance observed in patients treated with quadruplet induction and monoclonal antibodies. A phase 1 clinical trial was conducted to evaluate the safety and efficacy of BMS-986393, a CAR construct which targets GPRC5D, in treating patients with R/R MM (29). After one infusion, the 24 evaluable patients experienced an ORR and CRR of 96% and 46% after a median follow-up of 5.9 months. AEs were mild and 78% of the cohort was still in response at the time of follow-up. Longitudinal measurement of BCMA levels indicated deep tumor clearance, suggesting high efficacy in this patient population.
The QUINTESSENTIAL study is another ongoing clinical trial investigating the efficacy and safety of BMS-986393 treatment in patients with R/R MM (41). Phase 1 included 84 patients with at least 3 previous lines of therapy, 42% of which had high risk cytogenetics (del[17p], t[4;14], and/or t[14;16]), who received a single infusion of 25–450x10^6 CAR-T cells. Throughout the entire cohort, 94% of patients experienced some treatment-related (TR) AE which included CRS, ICANS, or other neurotoxicities (dizziness, ataxia, dysarthria or nystagmus). These results were considered manageable and were noted to be lower than some BCMA-related therapies. The study produced 79 efficacy-evaluable patients, 91% of whom maintained a response rate and 48% of whom maintained a complete response after a median follow-up of 14.6 months.
KQ-2003: A BCMA/CD19 dual-targeting CAR T construct (KQ-2003) was investigated in a prospective, multi-center, open label, dose escalation trial which enrolled 23 patients with R/R MM, 14 of whom had extramedullary disease (EMD). In the 18 evaluable patients, the ORR was 100% with a CRR of 88.9%, and notably, the EMD cohort achieved a PET-based ORR of 85.7% with a CRR of 64.3% (30). Median DOR was not reached, but 6-month PFS and OS rates were 86.5% and 86.2%, with 12-month PFS and OS rates reaching 75.3% and 86.2% respectively. AEs included CRS (21/23) and ICANS (5/23) with few patients experiencing grade 3/4 events. KQ-2003 appears promising, especially for patients with EMD MM, and is currently being investigated in more phase I/II trials.
CT071: CT071 is another CAR-T construct that targets GPRC5D and is manufactured using the CARcelerate platform which produces T cells in approximately 30 hours, greatly shortening vein-to-vein time (31). A cohort of 17 patients with R/R MM were infused with CT071 and achieved an ORR of 94.1% and a CRR of 52.9% with 15 patients achieving MRD negativity. AEs included CRS (grade 1/2) in 11 patients and other hematologic toxicities (grade 3/4) including lymphopenia (100%), leukopenia (88.2%), neutropenia (76.5%), thrombocytopenia (52.9%), and anemia (47.1%).
Cilta-cel for smoldering myeloma: The CAR-PRISM study is the first clinical trial to examine the effectiveness of CAR T-cells when treating patients with a premalignant condition who had received no prior induction therapy. The researchers hypothesized that patients with high-risk smoldering myeloma (HR-SMM), a precursor condition to MM, would respond favorably to CAR-T therapy due to their decreased tumor burden, genome complexity, and healthier immune systems (32). Ciltacabtagene Autoleucel (cilta-cel), a BCMA-directed CAR-T-cell therapy, was infused into 6 patients during the phase II study which yielded an ORR of 100% and a CRR of 50% at the 28-day post infusion mark with deepening responses over time. At a median follow up of 6 months (60 days to 1 year) all patients continue to have no evidence of progression. AEs included CRS (grade 1/2) and hematological toxicities due to lymphodepletion. Long term follow-up data to assess the strength of remission will be reported in the future. Results indicating durable response to cilta-cel therapy without induction therapy could potentially revolutionize MM treatment regimens.
Anito-cel: Preliminary results from the iMMagine-1 phase 2 trial were presented and show promising results for patients with high-risk R/R MM who had 3 or more previous lines of treatment (33). 58 patients were infused with anitocabtagene autoleucel (anito-cel, previously CART-ddBCMA), an anti-BCMA CAR-T-cell construct with a novel D-domain binder to reduce immunogeneicty, which was designed for high-risk MM patients. Early results indicated an ORR of 95% and a CRR, or stringent CRR, of 62% with 92% of patients achieving MRD negativity. Although OS and PFS have not been reached, estimates show an OS of 95% and a 6-month PFS of 90%. AEs included CRS in 84% of patients (83% grade 1/2), ICANS (8% grade 1/2), and hematologic toxicities were commonly reported. Durable efficacy was demonstrated in this high-risk cohort, notably in patients refractory to 3–5 lines of previous treatment, upon infusion of anito-cel. Further follow-up will be presented as the trial progresses.
GC012F: Advanced age can often exclude patients with R/R MM from clinical trials, but researchers in China hypothesize that GC012F (AZD0120), a BCMA and CD19 dual-targeting CAR-T cell therapy developed using the novel FasTCAR-T platform, could still be effective in treating an elderly patient population (over 70 years old). In a phase 1 trial, 8 patients (median age 71.5) were infused with one dose of GC012F, and after a median follow-up of 7.1 months, the ORR and CRR were 100% and 62.5% respectively (42). All patients achieved MRD negativity and robust T cell expansion was reported in all patients. No ICANS or G2 or above CRS were reported. These results indicate a durable response and manageable safety profile in elderly patients, which suggests that age alone should not exclude patients from future CAR-T therapeutic clinical trials.
7 Non-malignant conditions
As experience and comfort with CAR-T therapy has matured, interest in its use in B-cell predominate autoimmune disorders has resulted in multiple constructs being studied for systemic lupus erythematosus (SLE), idiopathic inflammatory myopathies (IIMs), systemic sclerosis (SSc), multiple sclerosis (MS), Autoimmune hemolytic anemia (AIHA), and immune-mediated platelet transfusion refractoriness (iPTR).
BMS-986353: Recent studies have demonstrated that CD19 directed CAR-T-cell therapy could lead to responses in patients with autoimmune disorders including SLE, IIMs, SSc, and multiple MS. The BMS-986353 construct uses the same CAR used in the lisocabtagene maraleucel (liso-cel) product but is manufactured using the NEX-T™ process which reduces manufacturing time greatly (34). In a phase 1 trial, seven patients (5 SLE, 1 SSc, 1 relapsing-remitting MS [RRMS]) were tapered off autoimmune-directed therapies and then infused with one dose of BMS-986353. At a median follow-up of 167.5 days, disease activity scores (SLEDAI-2K, PGA) and disease biomarkers (dsDNA, complement) were significantly decreased, and every patient remained off autoimmune therapy without disease flare ups. CRS grade 1/2 was observed in two patients (1 SLE; 1 SSc), grade 1 ICANS was observed in one SSc patient, and transient lymphodepletion-related grade 3/4 cytopenia occurred in five patients (4 SLE, 1 MS). Notably, the construct’s expansion and B-cell depletion were comparable to the liso-cel product’s effect in DLBCL patients in the TRANSFORM trial (NCT03575351). Enrollment in this trial remains open, and more data will be presented in the future.
MB-CART19: MB-CART19 is another CD19 directed CAR that was investigated in a clinical trial of 30 patients with treatment-refractory autoimmune disorders (18 SLE, 7 SSc, 5 IIM). At a median follow-up of 12.5 months, all SLE patients achieved DORIS criteria remission, all IIM patients achieved a major response based on 2016 ACR/EULAR criteria, and no SSc patients’ interstitial lung disease had progressed (43). AEs included no severe toxicities, but CRS grade 1/2 (was observed in 66.7% of patients and grade 1 ICANS occurred in one patient. One IIM patient relapsed, but the symptoms were milder than before treatment. The other 29 patients remain off autoimmune therapies with no relapse. These results demonstrate the promising efficacy and safety profile of MB-CART19 in the treatment of refractory autoimmune disorders.
CD19 CAR for AIHA: AIHA is another condition hypothesized to improve upon CD19-directed CAR-T-cell instigated B-cell lymphodepletion (35). A clinical trial enrolling eight patients with refractory AIHA was conducted and resulted in the 7 evaluable patients achieving complete remission which lasted a median time of 6.3 months (range, 1.3-9.7 months). The median time to partial remission (PR) and CR were 15 and 57 days respectively. AEs included grade 1/2 CRS in all patients and one reported case of ICANS. B-cell aplasia lasted a median time of 85 days, after which single-cell V(D)J sequencing data demonstrated the B-cell lineage and plasmablasts had been reset. This unprecedented level of remission achieved in refractory AIHA patients is promising and future trials are underway to further assess the efficacy of CAR-T-cell therapeutics in the treatment of this patient population.
CD19 and BCMA CAR for iPTR: iPTR is hypothesized to result from the incomplete elimination of long-lived plasma cells and presents a serious concern for patients with hematological malignancies. B cell depletion using CD19 and BCMA directed CAR-T-cell constructs was investigated for efficacy in reducing iPTR in a cohort of 12 patients (36). These patients received one mixed dose of murine CD19 and BCMA CAR T-cells. Within 5-months of infusion, the mean fluorescence intensity (MFI) of anti-HLA antibodies was completely negative and has remained negative for the past 30.5 months. IPTR was completely corrected in these patients and all the adverse events, including CRS and cytopenia, were reversible and transient. These results indicate that CAR-T-cell therapy has the potential to treat antibody-mediated disease.
8 Administrative, access, manufacturing, and other
Inpatient versus outpatient CAR T: A retrospective study was conducted to assess the differences between patient outcomes when treated with CAR-T-cell therapy in an inpatient versus an outpatient setting (37). The Intermountain Health CAR-T-cell program’s scheduling and billing records were used to gather patient information and then two proportion pooled z-tests were performed to compare OS rates between patient settings. The analysis of 125 patients concluded that inpatient administration of CAR-T therapies yielded significantly lower rates of OS and CR at 1 year follow-up. The inpatient group also experienced higher rates of CRS and ICANS. The differences in outcomes and toxicities of patients treated in an inpatient setting implies a “walk in the door effect” which demonstrates that patients who have a healthier baseline (able to “walk in the door”) respond better to CAR-T therapeutics.
Lymphodepleting chemotherapy, CIBMTR: A retrospective study compared patient outcomes when given either fludarabine or bendamustine as a lymphodepleting agent prior to infusion of CD19 directed CAR-T-cell therapy (38). Center for International Blood and Marrow Transplant Research (CIBMTR) data from 2017 to 2023 revealed that both regimens resulted in similar OS, PFS and CR outcomes. However, DLBCL patients treated with fludarabine experienced higher rates of ORR, while patients treated with bendamustine had fewer adverse side effects (CRS, ICANS, and prolonged cytopenia). The study concluded that when deciding on a lymphodepleting regimen, clinicians should take into account the severity of the patient’s condition and the safety of the regimens in consideration.
9 Discussion
The 66th ASH Annual Meeting and Exposition featured many abstracts and oral presentations detailing recent innovations in CAR-T constructs and manufacturing technology that have led to improvements in efficacy and safety in patients with malignant and non-malignant hematologic diseases. Continued development of CAR-T immunotherapy has the potential to continue to revolutionize treatment for patients with relapsed and refractory hematological malignancies and could allow for creative treatment solutions for patients with a variety of autoimmune disorders. We excitedly await full-length publication of many of these presentations and subsequent regulatory approval of their use.
Author contributions
SD: Writing – original draft, Writing – review & editing. FL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Larson SM, Farooq U, Hu B, Latif T, Mensah FA, Kolli D, et al. First results of IMPT-314, an autologous bispecific CD19/CD20 chimeric antigen receptor (CAR) in enriched naive and central memory T cells, for the treatment of large B cell lymphoma (LBCL). Blood. (2024) 144:4824. doi: 10.1182/blood-2024-200697
2. Ghobadi A, McGuirk JP, Shaughnessy P, Tam CS, Allen M, Pan C, et al. CTX112, a next-generation allogeneic CRISPR-cas9 engineered CD19 CAR T cell with novel potency edits: data from phase 1 dose escalation study in patients with relapsed or refractory B-cell Malignancies. Blood. (2024) 144:4829. doi: 10.1182/blood-2024-203563
3. Wang M, Johnson PC, Yazji S, Katz Y, Pietrofeso A, Robinson J, et al. A phase 1/2 study of a ROR1-targeting CAR T cell therapy (ONCT-808) in adult patients with relapsed/refractory (R/R) aggressive B cell lymphomas (BCL). Blood. (2024) 144:1743. doi: 10.1182/blood-2024-200998
4. Palomba ML, Caimi PF, Mei M, Hernandez-Ilizaliturri F, Shouse G, Winter AM, et al. A phase 1 study to evaluate the safety and tolerability of a combination autologous CD19 CAR T cell therapy (SYNCAR-001) and orthogonal IL-2 (STK-009) in subjects with relapsed or refractory CD19 expressing hematologic Malignancies (NCT05665062). Blood. (2024) 144:3453. doi: 10.1182/blood-2024-202090
5. Shah NN, Szabo A, Kearl T, Schneider D, Longo W, Johnson BD, et al. Phase 1 study of trispecific anti-CD20, anti-CD19, anti-CD22 (CAR20.19.22) T cells for relapsed, refractory B-Cell Malignancies. Blood. (2024) 144:3448. doi: 10.1182/blood-2024-200899
6. Del Real MM, Baird JH, Chen L, Song JY, Wang X, Kambhampati S, et al. Favorable safety profile and durable responses to pmb-CT01 (BAFFR-CAR T cell) therapy in patients with B-cell lymphomas ineligible for or who failed CD19-targeted therapy, including CD19-negative disease. Blood. (2024) 144:3127. doi: 10.1182/blood-2024-198416
7. Deng C, Caimi PF, Farooq U, Idippily N, Giraudo MF, Reese JS, et al. David wald; phase I study results of UF-kure19, a CAR-T product manufactured in less than 1 day, in patients with relapsed/refractory non-hodgkin’s lymphoma. Blood. (2024) 144:94. doi: 10.1182/blood-2024-206596
8. Rojek AE, Ahmed N, Llobell MG, Ahmed S, Hu M, Mead M, et al. CAR T cell therapy in early relapsed/refractory large B-cell lymphoma: real world analysis from the cell therapy consortium. Blood. (2024) 144:4503. doi: 10.1182/blood-2024-198153
9. Schneider M, Chong EA, Barta SK, Nasta SD, Svoboda J, Carter J, et al. Real-world outcomes of early relapsed/refractory large B cell lymphoma patients treated with 2nd line CAR T therapy. Blood. (2024) 144:5123. doi: 10.1182/blood-2024-202736
10. Boardman AP, Reguera JL, Spin P, Wang P, Almuallem L, Ellis J, et al. Matching-adjusted indirect comparison (MAIC) of efficacy and safety outcomes for lisocabtagene maraleucel (liso-cel) versus axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) for the treatment of third-line or later (3L+) relapsed or refractory (R/R) follicular lymphoma (FL). Blood. (2024) 144:3028. doi: 10.1182/blood-2024-197948
11. Abramson JS, Palomba ML, Gordon LI, Lunning M, Wang M, Arnason JE, et al. Five-year survival of patients (pts) from transcend NHL 001 (TRANSCEND) supports curative potential of lisocabtagene maraleucel (liso-cel) in relapsed or refractory (R/R) large B-cell lymphoma (LBCL). Blood. (2024) 144:3125. doi: 10.1182/blood-2024-200204
12. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. (2020) 396:839–52. doi: 10.1016/S0140-6736(20)31366-0
13. Ahmed S, Kallam A, Frigault M, Hunter BD, Patel SS, Bernasconi D, et al. Real-World (RW) outcomes of lisocabtagene maraleucel (liso-cel) in patients (pts) with relapsed or refractory (R/R) large B-Cell lymphoma (LBCL) and secondary central nervous system (sCNS) involvement from the center for international blood and marrow transplant research (CIBMTR) registry. Blood. (2024) 144:472. doi: 10.1182/blood-2024-199702
14. Kramer AM, Srinagesh H, Su Y-J, Hamilton MP, Levine K, Migliore E, et al. CD22-directed CAR T-cell therapy achieves complete remission in CD19-directed CAR T-cell refractory follicular and mantle cell lymphoma. Blood. (2024) 144:2064. doi: 10.1182/blood-2024-198489
15. Thieblemont C, Dreyling M, Dickinson MJ, Martínez-Lopez J, Kolstad A, Butler J, et al. Clinical outcomes of patients with high-risk relapsed/refractory follicular lymphoma treated with tisagenlecleucel: phase 2 ELARA 4-year update. Blood. (2024) 144:3034. doi: 10.1182/blood-2024-201730
16. Neelapu SS, Chavez JC, Sehgal AR, Epperla N, Ulrickson M, Bachy E, et al. 5-year follow-up analysis from ZUMA-5: A phase 2 trial of axicabtagene ciloleucel (Axi-cel) in patients with relapsed/refractory indolent non-hodgkin lymphoma. Blood. (2024) 144:864. doi: 10.1182/blood-2024-194627
17. Cheng Q, Tan J, Liu R, Kang L, Zhu H, Zhang Y, et al. Long-Term Remission and Survival in Patients with Rituximab-Refractory/Relapsed B-Cell Non-Hodgkin Lymphoma after Treatment with U16 (CD20 CAR-T cells). Blood. (2024) 144:3447. doi: 10.1182/blood-2024-200516
18. Reef DK, Cheng CJA, Babinec C, Hoye K, West J, Morrison JK, et al. Phase 1 trial of CD30-directed, CCR4 co-expressing chimeric antigen receptor T-cells in patients with CD30+ Cutaneous T-cell lymphomas refractory to brentuximab vedotin. Blood. (2024) 144:3468. doi: 10.1182/blood-2024-201799
19. Gonzalez ACC, Escribà-Garcia L, Ujaldón-Miró C, Fernández PP, Montserrat-Torres R, Escudero-López E, et al. HSP-CAR30, an academic memory-enriched CART30, for the treatment of relapsed or refractory hodgkin lymphoma and CD30+ T cell lymphoma: clinical results of a phase I/II trial. Blood. (2024) 144:920. doi: 10.1182/blood-2024-210129
20. Grover N, Moore D, Ivanova A, Beaven AW, Dittus C, Cheng CJA, et al. CD30.CAR-T cells co-expressing the CCR4 chemokine receptor in relapsed/refractory hodgkin lymphoma. Blood. (2024) 144:919. doi: 10.1182/blood-2024-200932
21. Lin Y, Zhao D, Deng B, Liu D, Yan H, Li B, et al. The safety and efficacy of CD33 CAR-T therapy for RR AML after HSCT. Blood. (2024) 144:3467. doi: 10.1182/blood-2024-207659
22. Wan X, Li W, Cai J, Yang X, Yang L, Yang J, et al. Safety and efficacy of bicistronic CD19/CD22 CAR T cell therapy in childhood B cell acute lymphoblastic leukemia. Blood. (2024) 144:681. doi: 10.1182/blood-2024-204200
23. Duncan BB, Silbert SK, Rankin AW, Yates B, Little L, Foley T, et al. Initial experience with CD19/CD22 biCistronic CAR T-cells in children and young adults with recurrent or refractory B-cell Malignancies. Blood. (2024) 144:680. doi: 10.1182/blood-2024-198259
24. Ying W, Lv L, Song Y, Wei X, Zhou H, Liu Q, et al. Sustained remission and decreased severity of CAR T-cell related adverse events: an updated report on the pivotal study of inaticabtagene autoleucel (Inati-cel; CNCT19) treatment in adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-cell ALL) in China. Blood. (2024) 144:4196. doi: 10.1182/blood-2024-199983
25. Aldoss I, Wang X, Zhang J, Guan M, Espinosa R, Agrawal V, et al. CD19-CAR T cells as definitive consolidation for older adults with B-cell acute lymphoblastic leukemia in first complete remission: A pilot study. Blood. (2024) 144:966. doi: 10.1182/blood-2024-201784
26. Muhsen I, Advani AS, Roloff GW, Jeyakumar N, Miller K, Zhang A, et al. Efficacy and safety of brexucabtagene autoleucel for relapsed/Refractory B-Cell acute lymphoblastic leukemia in patients aged 60 and above. Blood. (2024) 144:523. doi: 10.1182/blood-2024-210682
27. Zhang Y, Jing P, Wu F, Ling Z, and Wang K. Safety and Feasibility of Intrathecal Infusion of CD19 and/or CD22 CAR T Cells in r/r B-ALL Patients with CNSL or High-Risk CNS Relapse. Blood. (2024) 144:3466. doi: 10.1182/blood-2024-201376
28. Ortiz-Maldonado V, Martínez-Cibrián N, Alserawan L, Betriu S, Triguero A, Blum S, et al. Euplagia-1: A phase 1/2 trial of GLPG5201, a fresh stem-like early memory CD19 CAR T-cell therapy with a 7-day vein-to-vein time, in patients with relapsed/refractory CLL and RT. Blood. (2024) 144:3452. doi: 10.1182/blood-2024-207546
29. Bal S, Htut M, Berdeja JG, Anderson LD, Rossi A, Gregory T, et al. BMS-986393, a G protein-coupled receptor class C group 5 member D (GPRC5D)-targeted CAR T cell therapy, in patients (pts) with relapsed/refractory (RR) multiple myeloma (MM) and 1–3 prior regimens: updated phase 1 safety and efficacy results. Blood. (2024) 144:2069. doi: 10.1182/blood-2024-203279
30. Jiang H, Li L, Chen K, Yang C, Ma R, Xie L, et al. A prospective investigator-initiated phase 1/2 study of BCMA/CD19 dual-targeting CAR T therapy in patients with relapsed/refractory multiple myeloma including those with extramedullary disease. Blood. (2024) 144:923. doi: 10.1182/blood-2024-201160
31. Du J, Jin L, Gu S, Lu J, He H, Ruan Q, et al. GPRC5D-targeted CAR T-cell therapy CT071 for the treatment of refractory/relapsed multiple myeloma. Blood. (2024) 144:3451. doi: 10.1182/blood-2024-204790
32. Nadeem O, Nikiforow S, DeBraganca K, Bosch-Vilaseca A, O’Donnell EK, Sperling AS, et al. Early safety and efficacy of CAR-T cell therapy in precursor myeloma: results of the CAR-PRISM study using ciltacabtagene autoleucel in high-Risk smoldering myeloma. Blood. (2024) 144:1027. doi: 10.1182/blood-2024-202676
33. Freeman CL, Dhakal B, Kaur G, Maziarz RT, Callander N, Sperling AS, et al. ; phase 2 registrational study of anitocabtagene autoleucel for the treatment of patients with relapsed and/or refractory multiple myeloma: preliminary results from the IMMagine-1 trial. Blood. (2024) 144:1031. doi: 10.1182/blood-2024-198499
34. Mueller F, Patel K, Reshef R, Cherry M, Nash R, Ayala E, et al. Tolerability, efficacy, pharmacokinetics, and pharmacodynamics of BMS-986353 (CC-97540), a CD19-directed chimeric antigen receptor (CAR) T cell therapy manufactured using a next-generation process, for severe, refractory autoimmune diseases: updated data from ongoing phase 1, multicenter, open-label studies. Blood. (2024) 144:2088. doi: 10.1182/blood-2024-194525
35. Li R, Zhang L, Pan H, Li W, Ma J, Tian L, et al. CD19 CAR T-cell therapy in refractory autoimmune hemolytic anemia. Blood. (2024) 144:682. doi: 10.1182/blood-2024-202980
36. Ma Y, Cui Q, Yang C, Cui W, Li M, Li Z, et al. Sequential infusion of anti-CD19 and anti-BCMA chimeric antigen receptor T-cells: A promising treatment strategy for refractory immune-mediated platelet transfusion refractoriness. Blood. (2024) 144:683. doi: 10.1182/blood-2024-209249
37. Hunter BD, Hoda D, Asch J, and Sharma P. The “Walk through the clinic door” Effect: differences in survival and toxicity based on site of CAR T administration in a default outpatient CAR T program. Blood. (2024) 144:1749. doi: 10.1182/blood-2024-208868
38. Ali A, Ahmed N, Kim S, Bye M, Mirza S, Sieg AG, et al. Real world comparison of efficacy and safety of fludarabine-Versus bendamustine-Based lymphodepleting chemotherapy for CD19 chimeric antigen receptor (CAR) T-Cell therapy in relapse/Refractory (r/r) large B-Cell lymphoma (LBCL). Blood. (2024) 144:71. doi: 10.1182/blood-2024-200269
39. Shah NN, Maziarz RT, Jacobson CA, Johnston PB, Abhyankar S, Isufi I, et al. Interim results from a phase 2 pivotal study (DALY II USA) of tandem CD20-CD19-Directed non-Cryopreserved CAR-T cells - zamtocabtagene autoleucel (Zamto-Cel) in patients with relapsed/Refractory diffuse large B cell lymphoma. Blood. (2024) 144:68. doi: 10.1182/blood-2024-208539
40. Ghobadi A, Aldoss I, Maude SL, Bhojwani D, Faramand R, Bajel A, et al. WU-CART-007 (WT-7), an allogeneic CAR T-cell targeting CD7 in relapsed/refractory (R/R) T-cell acute lymphoblastic leukemia/lymphoma (T-ALL/LBL): phase 2 results. Blood. (2024) 144:3450. doi: 10.1182/blood-2024-202005
41. Bal S, Anderson LD, Nadeem O, Berdeja JG, Rossi A, Gregory T, et al. Efficacy and safety with extended follow-up in a phase 1 study of BMS-986393, a G protein-coupled receptor class C group 5 member D (GPRC5D)-targeted CAR T cell therapy, in patients (pts) with heavily pretreated relapsed/refractory (RR) multiple myeloma (MM). Blood. (2024) 144:922. doi: 10.1182/blood-2024-201356
42. Du J, Qiang W, Lu J, Jia Y, He H, Liu J, et al. Autologous B cell maturation antigen (BCMA) and CD19 dual targeting fastcar-T cells (GC012F/AZD0120) as first-line therapy for elderly patients with newly diagnosed multiple myeloma patients. Blood. (2024) 144:2072. doi: 10.1182/blood-2024-204774
Keywords: leukemia, oncology, hematology, lymphoma, CAR T cancer therapy, immunotherapy
Citation: Davis S and Lutfi F (2025) Advances in Chimeric Antigen Receptor T-cell therapy for hematologic malignancies and autoimmune conditions: a review of the 2024 American Society of Hematology Annual Meeting. Front. Hematol. 4:1610794. doi: 10.3389/frhem.2025.1610794
Received: 14 April 2025; Accepted: 09 June 2025;
Published: 23 June 2025.
Edited by:
Amanda Olson, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Lukasz Chlewicki, Eli Lilly, United StatesStella Bouziana, King’s College Hospital NHS Foundation Trust, United Kingdom
Şule Haskoloğlu, Ankara University, Türkiye
Copyright © 2025 Davis and Lutfi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel Davis, c2FtZGF2aXMyMDIwQGdtYWlsLmNvbQ==
 Samuel Davis
Samuel Davis Forat Lutfi
Forat Lutfi