- 1Struttura Complessa. (SC) Farmacia Presidio Ospedaliero (PO) Cattinara-Maggiore, Azienda Sanitaria Universitaria Giuliano Isontina, Trieste, Italy
- 2Unità Clinica Operativa (UCO) Ematologia, Azienda Sanitaria Universitaria Giuliano Isontina, Trieste, Italy
- 3Dipartimento di Scienze Mediche, Chirurgiche e della Salute, Università degli Studi di Trieste, Trieste, Italy
Pharmaceutical expenditure is constantly growing, in parallel with the increasing incidence of cancers and drug development costs. Enrolling patients in clinical trials provides countless advantages both for patients and the healthcare system; the pharmacoeconomic point of view is usually the least to be considered, but its magnitude should not be underestimated. The aim of this retrospective study was to estimate the pharmaceutical costs “avoided” by enrolling patients with hematological diseases in interventional clinical trials in a single institution. For each patient, the Standard-of-Care (SOC) therapy, which he or she would have received outside the clinical trial, was identified based on international guidelines, local clinical practice and Italy’s criteria for eligibility. The sum of the costs for SOC drugs represents the potential avoided pharmaceutical expenditure. In a 5-year period, 124 patients were enrolled in 37 interventional clinical trials. Thanks to an academic clinical trial several patients were treated with gemtuzumab ozogamicin, avoiding around €270.000 of SOC therapy. Enrolling patients with Non-Hodgkin’s Lymphoma generated a total avoided pharmaceutical expenditure of €254.546, with an average annual saving of more than €50.000, avoiding the costs of drugs such as polatuzumab vedotin (more than €70.000 enrolling 2 patients), intravenous and subcutaneous rituximab, liposomal doxorubicin, bendamustine and lenalidomide. Our analysis demonstrates how enrolling patients with hematological diseases in clinical trials can alleviate the burden on the Hematology Department pharmaceutical budget, avoiding SOC drug expenditure and securing grants, reimbursements, and payments not included in our analysis.
Introduction
According to the World Health Organisation, the number of new cancers in Europe in 2022 was approximately 4.5 million. Hematological cancers such as non-Hodgkin’s lymphoma, leukemia, multiple myeloma and Hodgkin’s lymphoma ranked 7th, 12th, 16th and 24th respectively (1).
With the advances in medicine and pharmacotherapy, the costs of treatment for hematological malignancies have increased significantly (2).
These rising costs weigh heavily on a complex healthcare system with limited resources and an increasing number of patients in need of treatment.
This can be seen from the OsMed ‘National Report on Medicines use in Italy’ of 2023 produced by AIFA (Agenzia Italiana del Farmaco), where the Anatomical Therapeutic Chemical (ATC) Classification “L - Antineoplastic and Immunomodulating Agents” represents the first therapeutic category in terms of both public expenditure and average Daily Defined Dose (DDD) cost.
The expenditure trend shows a 5% increase compared to 2022, reaching €7.358 billion, 28,3% of the total expenditure of the Italian National Health Service (INHS). This trend seems to be steadily increasing despite a reduction in prices (-4,8%) and in the average DDD cost (-3,0%) (3). In order to ensure homogeneous and rapid access to potentially innovative therapies, in 2015 AIFA established the ‘Fund for Innovative Medicines’. Based on parameters such as therapeutic need, added therapeutic value compared to the current standard of care and the quality of evidence, certain drugs may enter this specific reimbursement fund (4).
By the end of 2024, AIFA considers 70 therapeutic indications of 53 active substances to be innovative. 31 therapeutic indications are non-oncological, 25 are for solid tumors oncology and 14 (20%) are for hematological pathologies, of which 4 are Chimeric Antigen Receptor T-cells (5).
In this context of increasing pharmaceutical expenditure, the Hematology Department of the Azienda Sanitaria Universitaria Giuliano Isontina covers a population of about 360.000 inhabitants and takes care of patients with both malignant and benign hematological diseases.
In 2022, the pharmaceutical expenditure of the Hematology Department was approximately €8 million, representing 17% of the pharmaceutical expenditure of the entire hospital.
80% of this amount was used for drugs belonging to ATC L. 65% was allocated to antineoplastic agents.
A recent analysis published by a private Italian Cancer Centre assessed the potential costs avoided through clinical trials in oncology and onco- hematology. The analysis shows that enrolling patients in interventional clinical trials with supplied drugs saved INHS more than 4 million € annually in Standard-of-Care (SOC) drugs (6).
The aim of this report is to evaluate the impact of enrolling patients in clinical trials for hematological diseases in terms of potential pharmaceutical cost-saving, thus not impacting on the Hematology Department’s pharmaceutical budget and the INHS.
Method
We conducted a retrospective, single-center analysis of patients enrolled in hematology interventional clinical trials between January 1, 2020 and December 31, 2024.
All patients with benign and malignant diseases treated according to experimental protocols were selected. Our Hematology Department enrolls patients both in profit-sponsored and non-profit Phase II and III trials.
The drugs they were treated with in the clinical trial were either approved and not yet approved in Italy.
Patients for whom SOC was the best supportive care, or for whom the clinician identified a drug under a compassionate use program as the therapeutic alternative, were excluded.
To determine the hypothetical SOC for each analyzed patient, we referenced international guidelines, local clinical practice, and AIFA’s eligibility criteria. This identified SOC is the therapy which the patient would have received outside the clinical trial.
The dosages of the theoretical SOC drugs were calculated based on the actual anthropometry of the patients. The cost of these drugs was then calculated by multiplying the number of SOC cycles by the dose in milligrams and the price per milligram. If a patient did not complete the experimental treatment, the number of SOC cycles was determined based on the cycles completed in the clinical trial.
A sample of the calculations is available in the Supplementary Data.
The sum of the costs for SOC drugs thus represents potential of the pharmaceutical budget avoided expenditure, as patients were treated according to the clinical trial therapy with drugs provided by the sponsors.
Results
During the five-year period, 124 patients were enrolled in 37 interventional clinical trials, of which 23 were profit-sponsored and 14 academically-sponsored, with a peak of enrollment in 2023 (32 patients) (Supplementary Tables S1, S2 in the Supplementary Data).
Four patients were excluded from the analysis: one because the only possible therapy was best supportive care and 3 because they were candidates for a drug under compassionate use program as the hypothetical SOC.
The pathological area that enrolled the largest number of patients was non-Hodgkin’s lymphoma, which includes diffuse large B-cell lymphoma, marginal zone lymphoma, follicular lymphoma, and mantle cell lymphoma. A total of 74 patients were enrolled over 5 years, with a peak of 21 patients in 2022 (Figure 1).
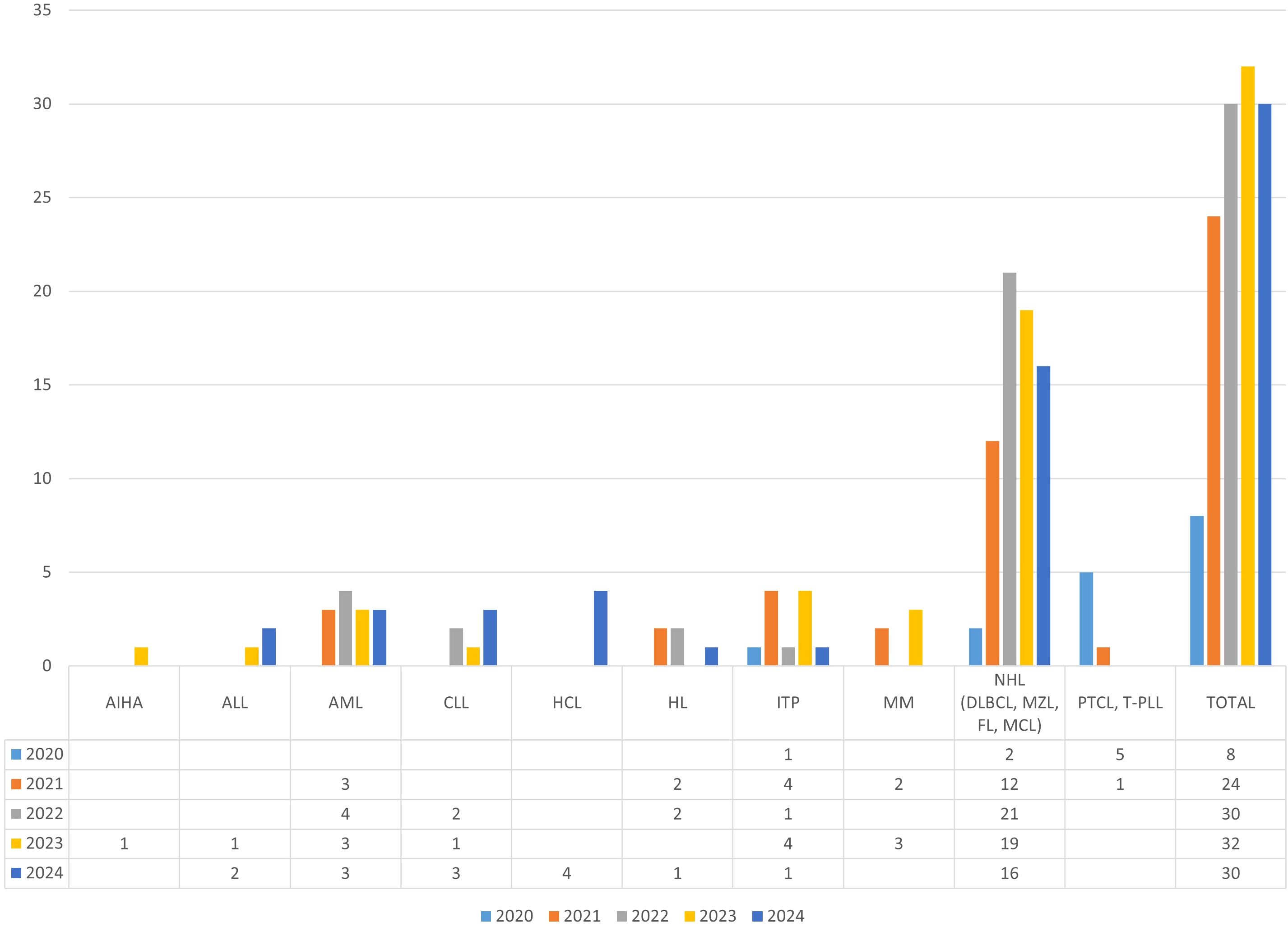
Figure 1. Patients per year per pathology group. AIHA, Autoimmune Hemolytic Anaemia; ALL, Acute Lymphoblastic Leukemia; AML, Acute Myeloid Leukemia; CLL, Chronic Lymphocytic L Leukemia; HCL, Hairy Cell Leukemia; HL, Hodgkin Lymphoma; ITP, Immune Thrombocytopenia; MM, Multiple Myeloma; NHL, Non-Hodgkin Lymphoma; DLBCL, Diffuse Large B-Cell Lymphoma; MZL, Marginal Zone Lymphoma; FL, Follicular Lymphoma; MCL, Mantle Cell Lymphoma; PTCL, Peripheral T-cell lymphoma; T-PLL, T-Cell Prolymphocytic Leukemia.
The avoided drug expenditure for SOC therapy over this five-year period was €754.556,72.
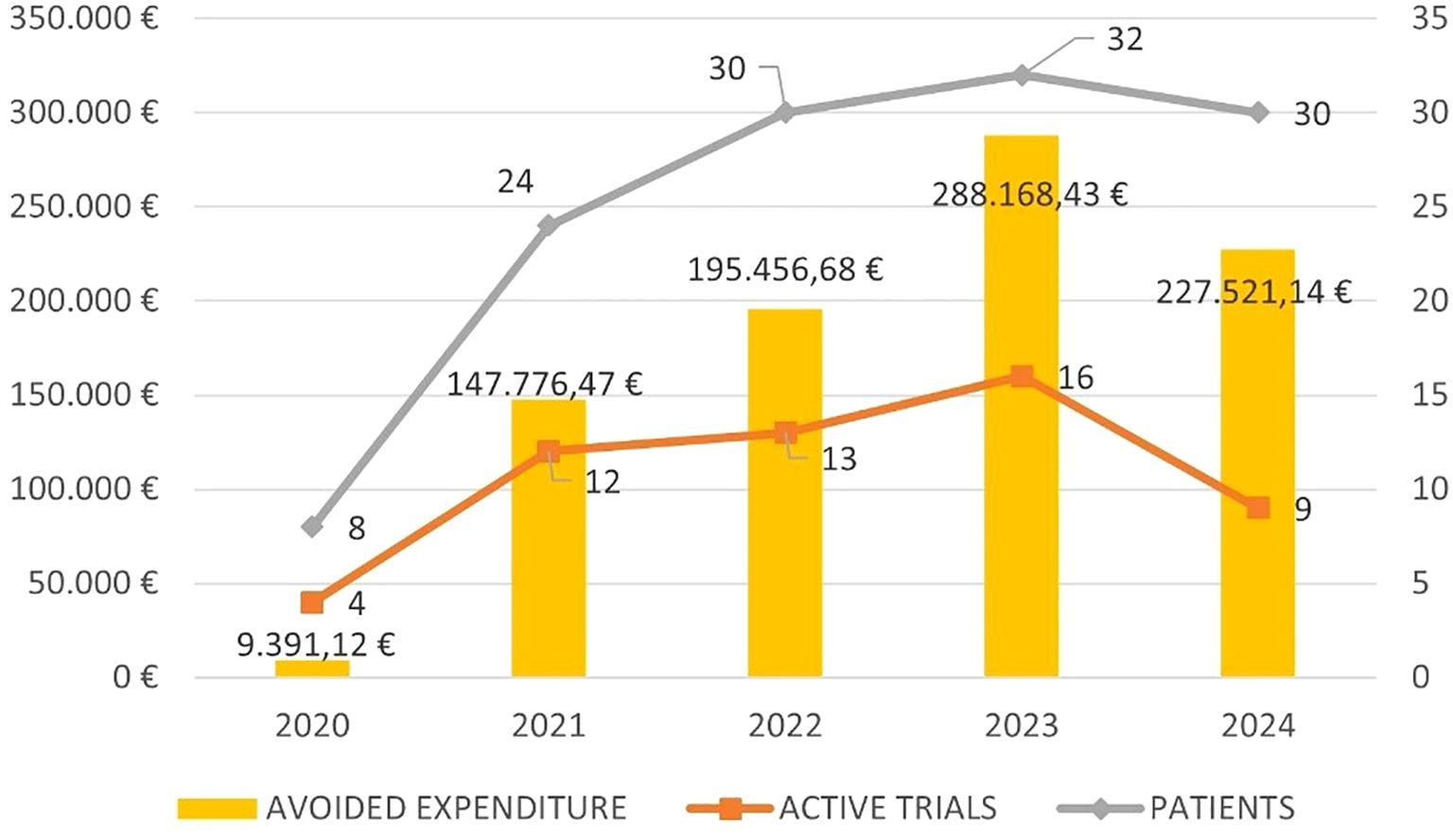
Figure 2 shows the five-year trend in avoided expenditure, the number of active trials and patients enrolled.
Table 1 details the avoided costs per active substance per year. The active substance for which a greater ‘saving’ was shown is gemtuzumab ozogamicin (GO), a toxin-conjugated anti-CD33 monoclonal antibody used for first-line acute myeloid leukemia. This drug was provided as part of an academic study protocol aimed at evaluating the impact of GO, in combination with standard chemotherapy, on minimal residual disease levels.
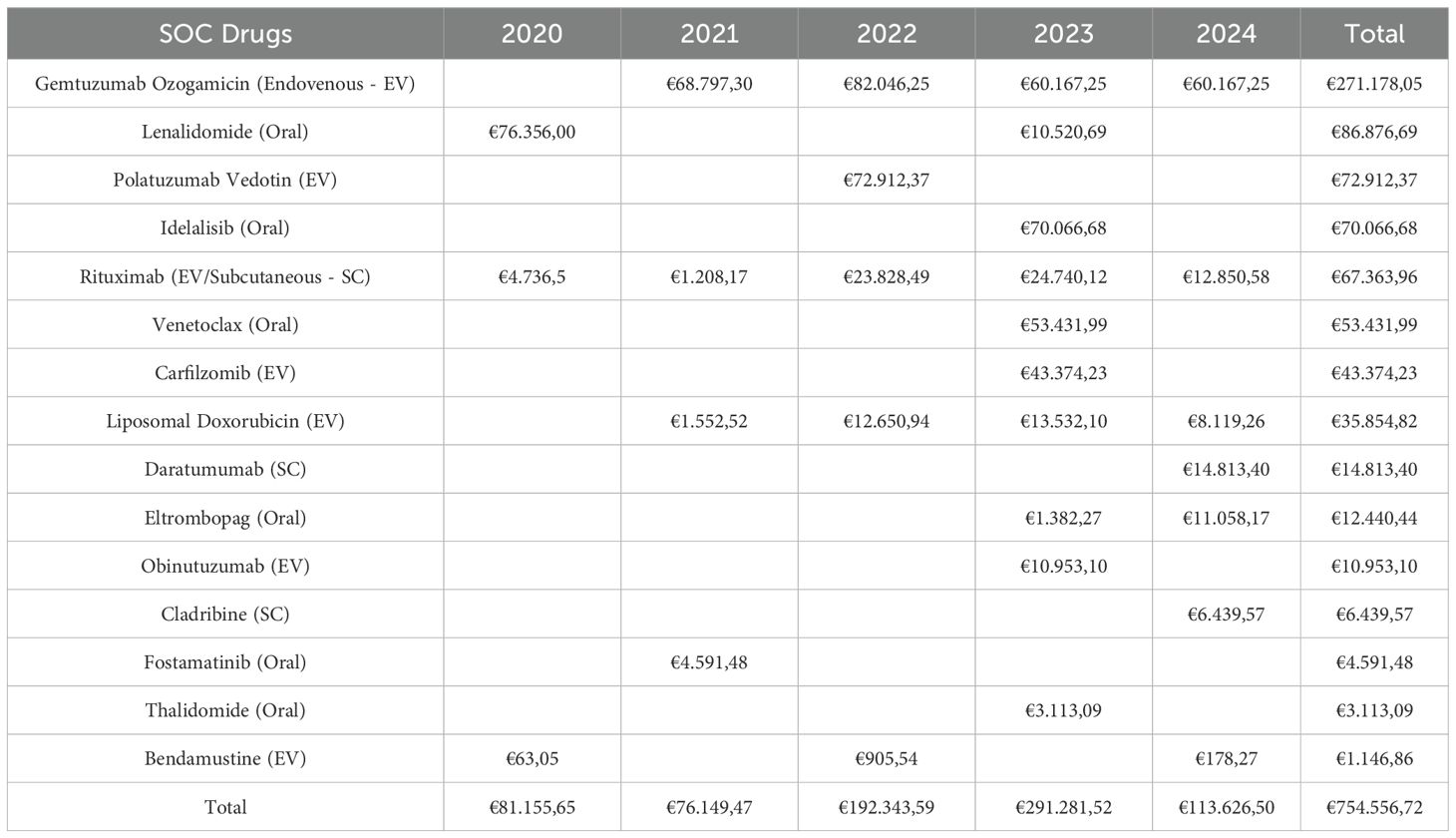
Table 1. Summary table of avoided costs per active substance per year, with subtotals per year and per molecule.
It should be highlighted that, in this instance, the SOC and the experimental protocol therapy align.
Enrolling 13 patients in 5 years in this trial generated an average non-expenditure for SOC drugs of almost €68.000/year.
Enrolling patients in multiple myeloma trials resulted in savings on lenalidomide and thalidomide, but also on daratumumab, which is the molecule with the second highest spending in Italian hospitals (€456,3 million) (3).
Four patients were enrolled in an academically-sponsored trial comparing cladribine to rituximab and vemurafenib in patients with newly diagnosed hairy cell leukemia. Regardless of the treatment arm, INHS did not spend more than €6.000 on cladribine for these patients, as the experimental and control arms were supplied by the Academic Promoter.
We enrolled 2 patients in a profit-sponsored trial for relapsed/refractory diffuse large B-cell lymphoma. By doing so, we avoided spending more than € 70.000 on polatuzumab vedotin.
Enrolling patients in clinical trials has resulted in ‘savings’ not only in the use of intravenous drugs but also for oral treatments such as idelalisib, venetoclax, eltrombopag, and fostamatinib.
Discussion
Clinical trials remain the cornerstone of clinical research, since they allow the development of more effective treatment strategies, as well as the access to novel drugs or treatment approaches that are expected to be more effective and possibly better tolerated than the SOC.
Several studies have documented that the outcome of patients treated in clinical trials is better than that of patients treated with SOC (7–10). Moreover, being treated in the context of a clinical trial may be the sole therapeutic option in several circumstances.
Besides the scientific significance, participating in a clinical trial also provides an opportunity for growth for the Hospital in terms of expertise of the healthcare team, the facilities and equipment required and adherence to Good Clinical Practice. Finally, the potential pharmaceutical savings should also be considered, as the experimental drugs are provided free of charge, thus patient’s treatment will not affect the budget of INHS.
The aim of this work was to demonstrate how enrolling patients in clinical trials can alleviate the burden on the INHS pharmaceutical budget. We showed that even in a single Institution, that covers a relatively small area, enrolling patients in clinical trials allowed us to save about €700.000 in a 5-years period. The majority of trials (59%) were phase 3, with sponsored trials being frequent (37%). It is therefore remarkable that, in our experience, GO was the drug that allowed us to ‘save’ the most and was provided in the context of an academic trial.
The amount of the ‘avoided costs’ has varied slightly over the years, with a mean of €173.662 saved per year. There may be several reasons for such variability including the availability of clinical trials, the duration of the enrollment period, the study design and the stringency of the inclusion criteria that may have influenced the number of patients enrolled. Moreover, based on how the saved costs were calculated, the response to treatment, which ultimately determines its duration, may also have contributed to this variability.
Finally, the actual cost of the drug may vary compared to the previous year, which means that the SOC cost for certain drugs has also changed over the period considered. This is the case of lenalidomide, whose price has significantly dropped since the end of 2022, when the molecule lost its patent.
It should also be mentioned that for commercially sponsored trials, this analysis does not consider any grant, payments or reimbursements; the aim of this report is to focus on the pharmaceutical expenditure only.
Other experiences published by oncology research and treatment institutes confirm that clinical trials are a therapeutic opportunity and a potential opportunity for cost savings (6, 11–13).
In conclusion, our retrospective analysis demonstrates the significant, although currently underestimated, financial benefit of enrolling patients in hematology clinical trials. These findings underscore the importance of continued investment in clinical trial infrastructure and personnel, not only to advance patient care and scientific knowledge, but also to contribute to the financial sustainability of the healthcare system. Such evidence may also encourage decision-makers to invest more in clinical trials, further incentivizing both institutional support and national funding for this crucial aspect of medical advancement.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SL: Formal Analysis, Data curation, Methodology, Writing – review & editing, Conceptualization, Writing – original draft. EL: Writing – review & editing, Data curation, Investigation, Conceptualization, Validation, Writing – original draft. MR: Writing – review & editing, Data curation. CP: Writing – review & editing. IC: Writing – review & editing. RP: Writing – review & editing. ML: Data curation, Writing – review & editing. ED: Writing – review & editing, Investigation. CR: Writing – review & editing. FZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2025.1613099/full#supplementary-material
References
1. Available online at: https://gco.iarc.fr/today/en (Accessed August 22, 2024).
2. Meropol NJ, Schrag D, Smith TJ, Mulvey TM, Langdon RM Jr, Blum D, et al. American Society of Clinical Oncology. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. (2009) 27:3868–74. doi: 10.1200/JCO.2009.23.1183
3. The Medicines Utilisation Monitoring Centre. National Report on Medicines use in Italy. Year 2023. Rome: Italian Medicines Agency (2024).
4. AIFA. The Medicines Utilisation Monitoring Centre. National Report on Medicines use in Italy. Year 2023. Rome: Italian Medicines Agency (2024). Available online at: https://www.aifa.gov.it/documents/20142/2594020/AIFA_Rapporto%20OsMed_2023.pdf.
5. Available online at: https://www.aifa.gov.it/farmaci-innovativi (Accessed January 20, 2025).
6. Gasperoni L, Cafaro A, Ferretti E, Di Iorio V, Nanni O, and Masini C. The role of clinical trials in the sustainability of the Italian national health service cancer drug expenditure. Eur J Hosp Pharm. (2023) 30:96–100. doi: 10.1136/ejhpharm-2022-003297
7. Duenas JAC, Sanchez PN, and Bonilla CE. Comparison of clinical outcomes among cancer patients treated in and out of clinical trials. BMC Cancer. (2023) 23:786. doi: 10.1186/s12885-023-11305-3
8. Engelbak Nielsen Z, Eriksson S, Schram Harsløf LB, Petri S, Helgesson G, Mangset M, et al. Are cancer patients better off if they participate in clinical trials? A mixed methods study. BMC Cancer. (2020) 20:401. doi: 10.1186/s12885-020-06916-z
9. Unger JM, Barlow WE, Martin DP, Ramsey SD, LeBlanc M, Etzioni M, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. (2014) 106(3):dju002. doi: 10.1093/jnci/dju002
10. Bouzalmate-Hajjaj A, Massó Guijarro P, Khan KS, Bueno-Cavanillas A, and Cano-Ibáñez N. Benefits of participation in clinical trials: an umbrella review. Int J Environ Res Public Health. (2022) 19:15368. doi: 10.3390/ijerph192215368
11. Liniker E, Harrison M, Weaver JM, Agrawal N, Chhabra A, Kingshott V, et al. Treatment costs associated with interventional cancer clinical trials conducted at a single UK institution over 2 years (2009-2010). Br J Cancer. (2013) 109:2051–7. doi: 10.1038/bjc.2013.495
12. Calvin-Lamas M, Portela-Pereira P, Rabuñal-Alvarez MT, Martinez-Breijo S, Martín-Herranz MI, and Gómez-Veiga F. Drug cost avoidance in prostate cancer clinical trials. Actas Urol Esp. (2015) 39:553–7. doi: 10.1016/j.acuro.2015.05.002
Keywords: hematology, costs, pharmacoeconomics, clinical trials, drugs, avoided pharmaceutical expenditure
Citation: Loiacono S, Lucchini E, Rossetti MC, Palmieri C, Cebulec I, Provasi R, Lena M, De Bellis E, Roni C and Zaja F (2025) The “added value” of enrolling hematological patients in clinical trials: a single-center experience. Front. Hematol. 4:1613099. doi: 10.3389/frhem.2025.1613099
Received: 16 April 2025; Accepted: 23 June 2025;
Published: 11 July 2025.
Edited by:
Valentina Giudice, University of Salerno, ItalyReviewed by:
Gabriela Oliveira, Federal University of the Southern Frontier, BrazilHartmut Link, Hematology Oncology Kaiserslautern, Germany
Copyright © 2025 Loiacono, Lucchini, Rossetti, Palmieri, Cebulec, Provasi, Lena, De Bellis, Roni and Zaja. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Loiacono, c3RlZmFuby5sb2lhY29ub0Bhc3VnaS5zYW5pdGEuZnZnLml0
†ORCID: Stefano Loiacono, orcid.org/0000-0001-9136-1351
Elisa Lucchini, orcid.org/0000-0002-4857-8325
Francesco Zaja, orcid.org/0000-0003-3995-8817
 Stefano Loiacono
Stefano Loiacono Elisa Lucchini
Elisa Lucchini Maria Chiara Rossetti
Maria Chiara Rossetti Clara Palmieri1
Clara Palmieri1 Francesco Zaja
Francesco Zaja