- 1Laboratory of Molecular Immunology, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan
- 2Division of Collaboration and Education, International Institute for Zoonosis Control, Hokkaido University, Sapporo, Japan
- 3International Collaboration Unit, International Institute for Zoonosis Control, Hokkaido University, Sapporo, Japan
- 4Laboratory of Biomedical Science, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan
- 5Research Center for Food Safety, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan
Background: B-cell activating factor (BAFF) is crucial for B-cell survival, proliferation, and immunoglobulin secretion. However, its role in hematopoietic malignancies, particularly concerning B-cell differentiation stages, remains unclear. This study explored the involvement of BAFF in B-cell malignancy progression and treatment response.
Methods: Mouse malignant B-cell lineage cell lines A20 and MPC-11 OUAr cells were analyzed for responsiveness to BAFF both in vitro and in vivo. For an in vitro study, recombinant BAFF was examined for its capacities, rescuing those cells under drug-induced cytotoxicity. For an in vivo study, tumor progression induced by inoculation of the tumor cells was compared between wild-type and BAFF-knockout (BAFF-KO) mice. Transcriptomic analysis was conducted to assess immune responses and signaling pathways associated with BAFF-dependent tumor growth.
Results: MPC-11 OUAr cells exhibited characteristics of more differentiated B cells, with a capacity for IgG secretion and elevated expression of B-cell maturation antigen (BCMA). In vitro, recombinant BAFF reduced drug-induced cytotoxicity on A20 cells but had no apparent effect on MPC-11 OUAr cells. In vivo, BAFF-KO mice exhibited better survival when administered with MPC-11 OUAr cells than wild-type mice, whereas the opposite trend was observed when these mice were administered with A20 cells. Transcriptomic analyses revealed that wild-type mice bearing MPC-11 OUAr tumors exhibited elevated expression of genes linked to angiogenesis and the PI3K-Akt signaling pathway.
Conclusions: Our findings suggest variable impacts of BAFF on B-cell lineage malignant cells depending on their cell types and highlight the complex role of BAFF in hematopoietic malignancies. Even when BAFF serves as a promoter of B-cell lineage tumors, its roles may not be restricted to its direct effects on malignant cells but may involve indirect effects on other cells constituting the tumor microenvironment, leading to an environment favorable to the malignant cells. The differential impact of BAFF on lymphoma subtypes underscores the need for targeted therapeutic strategies modulating BAFF signaling in B-cell malignancy.
Introduction
B-cell activating factor (BAFF, also known as TNFSF13B) is a critical molecule in B-cell development (1–5). BAFF is expressed by monocytes, macrophages, dendritic cells, and lymphoid cells, including B cells and activated T cells (2, 4, 6, 7). BAFF induces B-cell proliferation and immunoglobulin secretion and is an important survival factor for immature, naive, and activated B cells (1, 4). BAFF exists either on the cell surface as a type II transmembrane protein or as a soluble form after cleavage by a protease called furin (4). There are three separate receptors for BAFF: BAFF receptor (BAFF-R), transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI), and B-cell maturation antigen (BCMA) (1, 8). BAFF-R, TACI, and BCMA are expressed at different stages of B-cell development (9). BAFF-R is highly expressed from transitional B cells to mature naive B cells (9). TACI is highly expressed from mature naive B cells to activated and memory B cells (9). BCMA is most highly expressed by plasmablasts and short- and long-lived plasma cells (8, 9). Malignant B-cell lineage cells often share immunophenotypic characteristics with their normal B-cell counterparts, reflecting the expansion of a dominant clone leading to the development of leukemia or lymphoma (10). The expression levels of the BAFF receptors are presumed to vary according to the differentiation stage of B cells, and BAFF signaling is thought to differ depending on the cancer type (11).
BAFF-knockout (BAFF-KO) and BAFF-transgenic (BAFF-Tg) mouse models provide valuable insights into the intricate relationship between BAFF and its immunological functions in the development of autoimmune and lymphoproliferative diseases, shedding light on potential therapeutic targets and mechanisms underlying immune system regulation. BAFF-KO mice show no apparent birth defects and grow to at least 6 to 8 months of age without unusual morbidity, and all major organs, including the thymus, spleen, and lymph node, are present, although the average spleen weights of BAFF-KO mice are significantly reduced (12, 13). BAFF-KO mice have significantly fewer marginal zone and follicular B cells than wild-type animals (13). The remaining B cells mostly exhibited characteristics of T1 transitional B cells (13). Although these cells were normal in number, there were almost no cells of a T2 phenotype (13, 14). BAFF-KO mice exhibit a deficiency in mature B cells and an impaired immune response, in contrast to BAFF-Tg mice, which, due to their heightened production of BAFF, display increased numbers of mature B cells and effector T cells (8, 15). There is a correlation between excess BAFF in BAFF-Tg mice and the development of an autoimmune disease, resembling SLE in humans (16). BAFF-Tg mice exhibit autoimmune-like manifestations, including the presence of high levels of rheumatoid factors, circulating immune complexes, anti-DNA autoantibodies, and immunoglobulin deposition in the kidneys (15). Interestingly, a small percentage (3%–5%) of BAFF-Tg mice spontaneously develop B-cell lymphoproliferative diseases as they age (17). The development of B-cell lymphoproliferative diseases in BAFF-Tg mice appears to be linked to the action of tumor necrosis factor (TNF), as introducing TNF deficiency into a BAFF-Tg background increases the incidence of B-cell lymphoma (18).
BAFF is believed to play a complex and context-dependent role in hematologic malignancies, influencing both disease progression and treatment outcomes. Therefore, investigating BAFF and its receptors is crucial for understanding the mechanisms underlying hematopoietic malignancies and may lead to the discovery of new therapeutic targets or enhancements to existing treatments. To explore this, this study aimed to examine whether BAFF responsiveness in various hematopoietic malignancies correlates with the differentiation stage of B cells. Here, two mouse lymphoma cell lines were tested for their survival/proliferation in the presence of BAFF, both in vitro and in vivo, to examine whether the effect of BAFF varies depending on the nature of the cells, including differentiation status and expression levels of BAFF receptors.
Materials and methods
Mouse cell lines
A20 was purchased from the ATCC, Manassas, USA. A20 is a BALB/c B-cell lymphoma line derived from a spontaneous reticulum cell neoplasm found in an old BALB/cAnN mouse (19). MPC-11 OUAr was obtained from the Japanese Cancer Research Resources Bank, which originated from ATCC TIB-15™ established from the Merwin plasma cell tumor-11 induced in a BALB/c mouse (20). A20 cells were cultured at 37°C and 5% CO2 in RPMI-1640 medium (Fujifilm WAKO, Osaka, Japan) containing 10% heat-inactivated fetal bovine serum (HI-FBS) (Thermo Fisher Scientific, Waltham, MA, USA), 100 U/mL of penicillin, and 100 µg/mL of streptomycin (WAKO). MPC-11 OUAr cells were maintained at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM; WAKO) containing 10% HI-FBS (Thermo), 100 U/mL of penicillin, and 100 µg/mL of streptomycin (WAKO).
Quantification of IgG in cell culture
A20 and MPC-11 OUAr cells were cultured in vitro, and both cells and culture supernatants were harvested. Cells were resuspended in RIPA buffer and sonicated using Sonifier 250 (Branson, Brookfield, USA). The protein concentrations of the cell lysates were determined by the DC protein assay (Bio-Rad, Hercules, USA). Quantification of IgG in cell lysate and culture supernatant was performed by using IgG (Total) Mouse Uncoated ELISA Kit (Thermo), according to the manufacturer’s instructions.
Cell viability assay
A20 and MPC-11 OUAr cells were plated into 96-well plates at a concentration of 2.0 × 105 cells/mL. Cytarabine (LKT Labs, Inc., Saint Paul, USA), methotrexate (MP Biomedicals, Inc., Irvine, USA), doxorubicin (Cell Signaling Technology, Danvers, USA), cyclophosphamide (TCI, Tokyo, Japan), prednisolone (WAKO), bortezomib (WAKO), thalidomide (Sigma-Aldrich, Burlington, USA), and dexamethasone (Sandoz AG, Basel, Switzerland) were diluted and added to the plates. A 50-ng/mL recombinant mouse IL-6 (rmIL-6) (R&D Systems #406-ML-005) and 100 ng/mL of recombinant mouse BAFF (rmBAFF) (R&D Systems #8876-BF-010, Minneapolis, USA) were added to the plate and then incubated for 72 h. The AlamarBlue Cell Viability Reagent (Invitrogen, Carlsbad, USA) was applied and incubated at 37°C for 4 h. Fluorescence intensity was measured using an excitation wavelength of 555 nm and an emission wavelength of 595 nm using a microplate reader (SpectraMax Paradigm, Molecular Devices, San Jose, CA, USA).
Mice
BALB/cA mice were purchased from CLEA Japan, Tokyo, Japan. BAFF-KO mice on a BALB/cA background were produced by the CRISPR/Cas offset-nicking method (21). All mice were maintained under specific pathogen-free conditions in a level 2 physical containment facility. The animal experiments were reviewed and approved by an institutional animal research committee (approval P22-090) and an institutional committee on genetically modified organisms (approval L22-024) at the Graduate School of Agricultural and Life Sciences, The University of Tokyo.
In vivo tumor challenges
A20 and MPC-11 OUAr cells were washed three times with PBS and resuspended in PBS. Mice were implanted subcutaneously at the dorsal base of the tail with 1 × 106 A20 or MPC-11 OUAr cells (n = 10 per group for wild-type and BAFF-KO mice, respectively). The volume of a subcutaneous tumor nodule was calculated as length × breadth × height/2. Mice were monitored daily for signs of morbidity and mortality and were housed according to institutional ethics and biosafety standards. For the preparation of samples for histological and gene expression analyses, mice bearing A20 cells were sacrificed 4 weeks after cell transplantation, while mice bearing MPC-11 OUAr cells were sacrificed 2 weeks post-transplantation, due to the different speeds of tumor growth.
Pathological examination of sections of paraffin-embedded mouse nodule
Pieces of nodules from tumor-implanted mice were fixed with 10% neutral buffered formalin and then embedded in paraffin. Sections were dewaxed and then boiled in antigen retrieval buffer (Tris-EDTA buffer, pH 9). The sections were probed with goat anti-mouse MRP14 (Santa Cruz, CA, USA) followed by the secondary antibody AlexaFluor 546-conjugated donkey anti-goat IgG (Thermo) for 1 h, and then counterstained with Hoechst 33342 (Dojindo, Kumamoto, Japan). Fluorescence observation was performed with Keyence BZ-X800 (Keyence, Osaka, Japan).
Quantitative reverse transcription-PCR
Total RNA was extracted from A20 and MPC-11 OUAr cells, as well as from nodules from tumor-implanted mice using TRIzol (Thermo). The concentration of total RNA was measured by NanoDrop™ OneC (Thermo). Real-time PCR assay was carried out using 0.5 µL of cDNA as the template, 10 µL of SYBR Select Master Mix (Thermo), and 0.25 µM of primers with the Applied Biosystems QuantStudio 5 Real-Time PCR System (Thermo). Data were analyzed by the 2−ΔCt method and normalized by Actb. Primer information is provided in Supplementary Table S1.
Transcriptome analysis
Total RNA was extracted from the A20 and MPC-11 OUAr cell culture in vitro, as well as nodules from tumor-implanted mice, and was sent to Macrogen Japan for RNA sequencing. For purification and library construction, next-generation sequencing technology was used for paired-end sequencing on these libraries based on the Illumina sequencing platform. The filtered high-quality sequences were compared to the reference genome of Mus musculus. According to the comparison results, the expression level of each gene was calculated, and the samples were analyzed for expression quantity, expression differences, functional enrichment of differentially expressed genes (DEGs), and protein network interaction. The conditions for screening DEGs were as follows: expression fold |log2FoldChange| > 1, significant P-value < 0.05 (22). The R language Pheatmap package was used to perform bidirectional clustering analysis on the union of differential genes and samples of all comparison groups (23). In some analyses, iDEP 2.01 was used (24). Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Set Enrichment Analysis (GSEA) were performed to identify signaling pathways with the most significant DEG enrichment (25, 26).
Quantification of BAFF
BAFF levels were quantified in cell lines, tumor nodules, and serum samples using the Mouse BAFF/BLyS/TNFSF13B DuoSet ELISA kit (R&D Systems) according to the manufacturer’s instructions. For cell lines, A20 and MPC-11 OUAr cells were collected and lysed in RIPA buffer followed by sonication. Nodule BAFF levels were assessed by homogenizing nodules in RIPA buffer. Serum BAFF levels were directly measured from mouse serum samples.
Statistical analysis
Differences among groups were analyzed by unpaired t-test, one-way ANOVA followed by Bonferroni multiple comparisons test, or two-way ANOVA followed by Bonferroni multiple comparisons test with GraphPad Prism 10 software (GraphPad Software, Inc., CA, USA). In the group of mice inoculated with MPC-11 OUAr, mice that did not develop tumors by day 40 were considered to have failed tumor engraftment and were excluded from the analysis. Additionally, in the MPC-11 OUAr-inoculated group, mice were considered to have reached the humane endpoint when the longest dimension exceeded 2 cm, whereas in the A20 treatment group, reaching a longest dimension exceeding 1 cm was considered achieving the humane endpoint. The different endpoints were set because the time intervals from engraftment to each endpoint varied. Survival curves were then constructed based on the endpoint. Survival was evaluated by Kaplan–Meier (Gehan–Breslow–Wilcoxon test) from the first day of cancer cell transplantation until death. Significant differences were determined and designated with asterisks as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
Results
MPC-11 OUAr cells have more differentiated B-cell characteristics than A20 cells
A20 and MPC-11 OUAr cells were first examined for IgG expression by measuring IgG levels in the cell lysates and culture supernatants. IgG levels were significantly higher both in the cell lysate and culture supernatant of MPC-11 OUAr cells compared with A20 cells (Supplementary Figure S1A), and the ability of MPC-11 OUAr cells to secrete IgG indicated that the cells have a plasma cell-like phenotype. Next, gene expression levels of Tnfrsf13c (BAFF-R), Tnfrsf13b (TACI), and Tnfrsf17 (BCMA) were compared between A20 and MPC cells. Although no significant difference was observed between those cells in the expression of Tnfrsf13c, MPC-11 OUAr cells showed higher expression levels of Tnfrsf13b and Tnfrsf17 compared with A20 cells (Supplementary Figure S1B). BCMA is known as a marker for terminally differentiated B-lineage cells including plasma cells, and the markedly higher expression of Tnfrsf17 in MPC-11 OUAr cells, together with the ability of IgG secretion, indicates that MPC-11 OUAr cells have a plasma cell-like phenotype and are characterized by having more differentiated B-lineage cells than A20 cells.
BAFF rescues A20 cells from drug-induced stress in vitro
In a preliminary study of antitumor compounds, methotrexate, doxorubicin, bortezomib, prednisolone, and dexamethasone successfully induced the suppression of cell proliferation in both A20 and MPC-11 OUAr cells (Supplementary Table S2). Therefore, a rescue study on malignant cells by BAFF was performed in the presence of those compounds. Although a prominent promotion of cell proliferation in A20 or MPC-11 OUAr cells was not induced by either rmBAFF, rmIL-6, or their combination in the absence of antitumor compounds, a rescue effect was observed in those cells under the pressure of the compounds. Of note, a rescue effect of rmBAFF was prominent in A20 cells as improved cell proliferation was found in A20 cells cultured with rmBAFF in the presence of doxorubicin, bortezomib, prednisolone, or dexamethasone (Figure 1A). Furthermore, a synergistic rescue effect of rmBAFF and rmIL-6 to A20 cells was observed under drug pressure by prednisolone and dexamethasone (Figure 1A). In contrast, rmBAFF did not alleviate drug effects in MPC-11 OUAr cells, while rmIL-6 demonstrated a rescue effect on the lymphoma cells against the cytotoxicity induced by prednisolone and dexamethasone (Figure 1B).
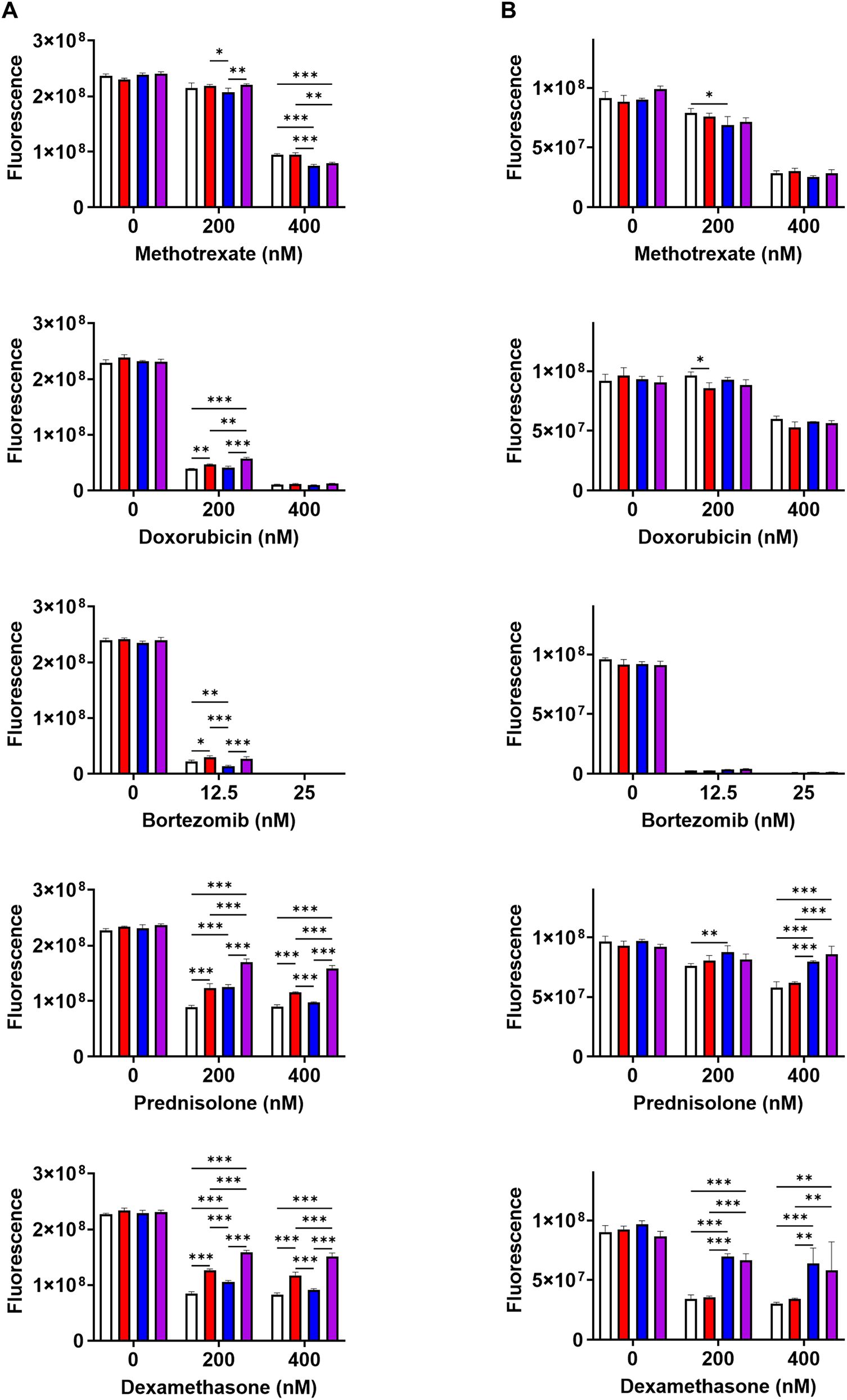
Figure 1. BAFF rescues A20 cells from drug-induced stress in vitro. Growth inhibition by drugs on A20 (A) and MPC-11 OUAr cells (B) was assessed using AlamarBlue assay. A20 and MPC-11 OUAr cells were cultured for 72 h in the absence/presence of individual drugs (methotrexate, doxorubicin, bortezomib, prednisolone, and dexamethasone), untreated (white bars) or treated with rmBAFF (100 ng/mL: red bars) or rmIL-6 (50 ng/mL: blue bars), or co-treated with rmBAFF and rmIL-6 (purple bars). *P < 0.05, **P < 0.01, ***P < 0.001 with two-way ANOVA with Bonferroni multiple comparisons.
Variable effects of host-derived BAFF in tumor development in vivo
To examine the effect of BAFF on tumor growth in vivo, either A20 or MPC-11 OUAr cells were inoculated subcutaneously into the base of the tail of wild-type and BAFF-KO mice, and tumor development was monitored. Subcutaneous inoculation of A20 cells included tumors in both wild-type and BAFF-KO mice, but lesion sizes were smaller in wild-type mice accompanied by a better survival rate compared with BAFF-KO mice (Figure 2A). On the other hand, MPC-11 OUAr inoculation brought an opposite trend; compared with wild-type mice, smaller lesions and a better survival rate were found in BAFF-KO mice (Figure 2B).

Figure 2. Variable effects of host-derived BAFF in tumor development in vivo. (A) Tumor volume and survival rates were assessed in wild-type and BAFF-KO mice injected with 1 × 106 A20 cells. (B) Tumor volume and survival rates were assessed in wild-type and BAFF-KO mice injected with 1 × 106 MPC-11 OUAr cells. The volume of a subcutaneous tumor nodule was calculated as length × breadth × height/3. Each line represents individual mouse data. Survival was evaluated by Kaplan–Meier from the first day of cancer cell transplantation until death. Mice were sacrificed when tumor length exceeded 1 cm for A20 cells or 2 cm for MPC-11 OUAr cells, or when a significant compromise to their quality of life was observed. *P < 0.05 with the Gehan–Breslow–Wilcoxon test.
Increase in BAFF levels in MPC-11 OUAr tumor-bearing wild-type mice
To explore the mechanisms for the different contributions of host-derived BAFF in tumor development, we next examined BAFF levels in tumors from wild-type and BAFF-KO mice. BAFF levels in tumors were equivalent between wild-type and BAFF-KO mice inoculated with A20 cells (Figure 3A). In contrast, BAFF levels in tumors by MPC-11 OUAr cells were higher in wild-type mice than in BAFF-KO mice (Figure 3A). Because BAFF levels were higher in tumors by A20 cells than those by MPC-11 OUAr cells in BAFF-KO mice, it was speculated that A20 cells have a higher expression of BAFF than MPC-11 OUAr cells. To confirm this, cell lysates of A20 and MPC-11 OUAr cells cultured in vitro were examined for BAFF levels, and the results showed a higher expression of BAFF in A20 cells (Figure 3B).
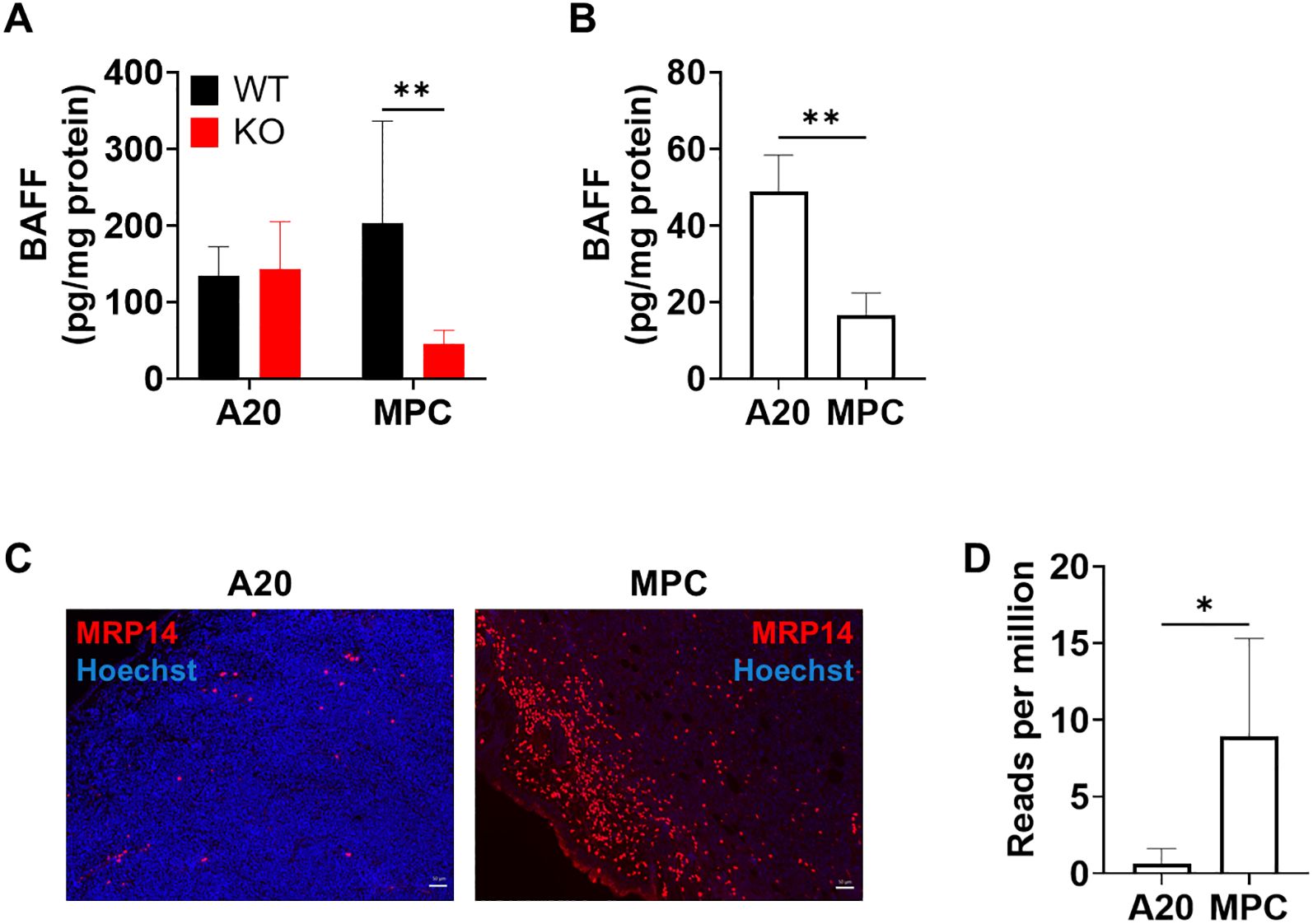
Figure 3. Increase in BAFF levels in the nodule of wild-type mice transplanted with MPC-11 OUAr cells. (A) BAFF levels in the nodule of wild-type and BAFF-KO mice injected with 1 × 106 A20 or MPC-11 OUAr cells were measured by sandwich ELISA. Samples were collected at the endpoint. (B) BAFF levels in cell lysates of A20 and MPC-11 OUAr cells were measured by sandwich ELISA. **P < 0.01 by unpaired t-test. (C) Nodule sections from wild-type mice bearing A20 (4 weeks post-transplantation) or MPC-11 OUAr (2 weeks post-transplantation) were stained with anti-MRP14 antibody. An accumulation of MRP14+ cells was observed in the nodules of MPC-11 OUAr-bearing mice, while it was scarce in A20-bearing mice. Bar = 50 μm. (D) Expression levels of S100a9 (coding MRP14) in tumors from wild-type mice bearing A20 (4 weeks post-transplantation) or MPC-11 OUAr (2 weeks post-transplantation). The results are extracted from transcriptome data and shown as reads of the target gene per million total reads.
In a previous study, we demonstrated that MRP14+ inflammatory macrophages are the major producers of BAFF in Leishmania-infected tissues (7). Therefore, tumors were examined for the accumulation of MRP14+ cells in wild-type mice inoculated with either A20 or MPC-11 OUAr cells. In mice bearing A20 cells, MRP14+ cells were hardly observed (Figure 3C). In contrast, mice bearing MPC-11 OUAr cells showed an accumulation of MRP14+ cells in the tumor (Figure 3C). Upregulated expression of MRP14 (S100a9) in MPC-11 OUAr-bearing tumor was also confirmed by transcriptome analyses (Figure 3D). This finding indicates that the immune profile within the cancer microenvironment differs between MPC-11 OUAr and A20 and that the clustering pattern of MRP14-positive cells also varies between these models.
Analysis of differentially expressed genes in the transcriptome
To further explore differences in host immune responses for each cancer type and elucidate how host-derived BAFF contributes to the responses, transcriptome analyses were performed on tumors including A20 or MPC-11 OUAr cells in wild-type and BAFF-KO mice. The group composition included each cell line and nodules of wild-type and BAFF-KO, transplanted using each cell line, resulting in six groups. The numbers of DEGs between groups are shown in Figure 4A. Only two genes were differentially expressed among the three A20 groups, whereas 42 genes were differentially expressed among the three MPC-11 OUAr groups. Among the 42 genes differing in the MPC-11 OUAr groups, 38 had the highest expression in wild-type mice, followed by BAFF-KO mice and cell lines, and were linked to G protein-coupled receptor signaling pathway regulation, including Gpsm1, Itgb3, and Rgs18 (Supplementary Table S3). Together, the MPC-11 OUAr-derived tumor seemed to be more influenced by BAFF-dependent host immune responses than the A20-derived tumor. In fact, no significant difference in the local expression of BAFF was observed between the wild-type and BAFF-KO mice transplanted with A20.
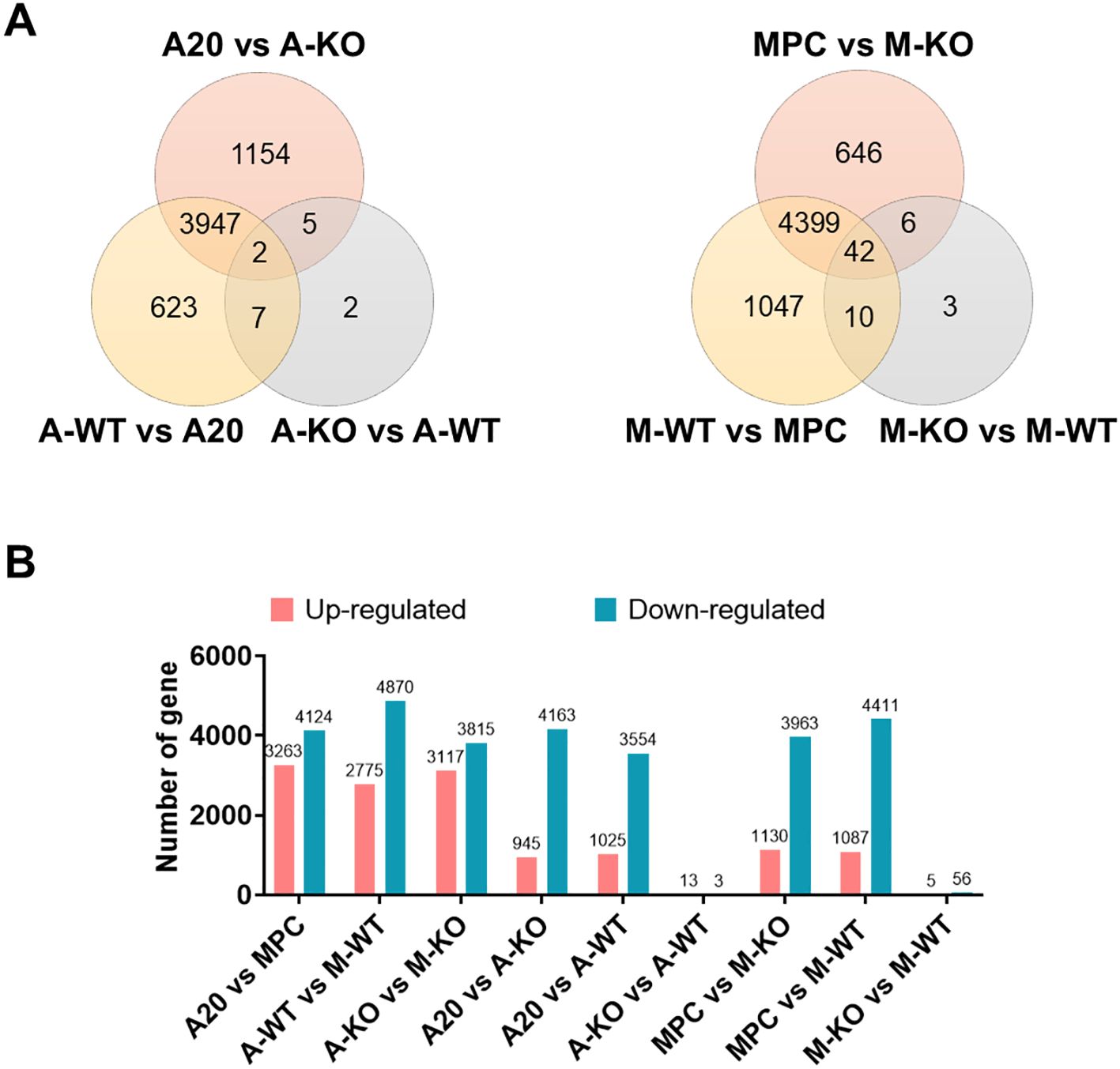
Figure 4. Host BAFF-dependent gene expressions in tumor-bearing mice. (A) Venn diagram of DEGs in the A20 or MPC-11 OUAr groups. For each tumor cell type, gene expression levels in in vitro-cultured cells, tumor in wild-type mice, and tumor in BAFF-KO mice were compared. (B) The number of DEGs in pairwise comparison. Red represents upregulated genes, and green represents downregulated genes. A20 = in vitro-cultured A20 cells, MPC = in vitro-cultured MPC-11 OUAr cells, A-WT = tumor in wild-type mice transplanted with A20 cells, M-WT = tumor in wild-type mice transplanted with MPC-11 OUAr cells, A-KO = tumor in BAFF-KO mice transplanted with A20 cells, M-KO = tumor in BAFF-KO mice transplanted with MPC-11 OUAr cells.
In BAFF-KO mice transplanted with A20 cells, 13 genes were upregulated compared to wild-type mice with A20 transplants (Figures 4B, Supplementary Figure S2A; Supplementary Tables S3, Supplementary Tables S4), including Acvr2b, which is part of the TGF-β family (27). In wild-type mice bearing MPC-11 OUAr cells, 56 genes were upregulated compared to BAFF-KO mice with MPC-11 OUAr (Figures 4B, Supplementary Figure S2B; Supplementary Tables S3, Supplementary Tables S5), including those involved in angiogenesis (e.g., Angpt2, Lgals3, Hmox1, Itgb3), which likely promote angiogenesis within the tumor microenvironment. Genes associated with the PI3K-Akt signaling pathway (e.g., Vwf, Angpt2, Itga2b, Itgb3) were also elevated. This pathway, known for its role in cell survival and proliferation, is often amplified or activated in cancers (28), likely driving cancer progression in these wild-type mice.
Discussion
Expression patterns of the three receptors for BAFF are reported to vary during stages of B-cell development (29). Consequently, it is currently not fully elucidated if B-cell lymphomas uniformly express all three receptors or if BAFF plays similar roles in tumor development derived from various lymphomas. In this study, we demonstrated distinct roles of BAFF in tumor development derived from A20 and MPC-11 OUAr cells. It was conceivable that MPC-11 OUAr cells have an advanced stage of B-cell differentiation compared with A20 cells according to a higher expression of BCMA and a capacity for IgG secretion.
In vitro survival assay results showed that responsiveness to BAFF varies depending on both drug and cell types. BAFF mitigated the cytotoxic effects of doxorubicin, bortezomib, prednisolone, and dexamethasone in A20 cells but not in MPC-11 OUAr cells, suggesting a selective protective role of BAFF that could contribute to therapeutic resistance in specific B-cell malignancies. The rescue effects of BAFF on A20 cells are more prominent under the pressure of bortezomib, prednisolone, and dexamethasone compared with methotrexate and doxorubicin, which target nucleic acid synthesis. This indicates that BAFF can support tumor cell survival through immune cell signaling but not for protecting the nuclei. BAFF primarily activates the NF-κB pathway and promotes the expression of anti-apoptotic factors, which likely suppress apoptosis induced by doxorubicin, bortezomib, and prednisolone in A20 cells. In contrast, methotrexate acts on the cell cycle and DNA synthesis, which are irrelevant to the BAFF signaling pathway, and this may be why a rescue effect by BAFF was not observed with this drug. The combination of IL-6 and BAFF significantly enhanced the survival of A20 cells but not MPC-11 OUAr cells, highlighting a potential synergistic interaction that may be relevant in the microenvironment. This suggests that the cytokines involved in cancer cell proliferation differ by cancer type. BAFF/BAFF-R signaling promotes the expression of anti-apoptotic molecules in B cells via the non-canonical NF-κB pathway (30). The activation through the non-canonical NF-κB pathway is unique to BAFF-R among BAFF receptors (31). The results show that BAFF rescues IL-6, activating JAK through its receptor and inducing the STAT3 signaling pathway, which promotes the expression of anti-apoptotic genes and aids in cell survival. Therefore, while some drugs showed a synergistic effect with BAFF, there were also patterns in which no synergistic effect was observed.
It was intriguing that the effects of BAFF on tumor cell growth were not in parallel in vitro and in vivo. Although minimal rescue effects by BAFF were observed on MPC-11 OUAr cells in vitro, tumor growth in mice subcutaneously administered with MPC-11 OUAr cells was more prominent in wild-type mice compared with BAFF-KO mice. Conversely, BAFF-KO mice were more sensitive to A20 cells than wild-type mice. These results suggest differences in the environments influencing tumor engraftment and growth between in vitro and in vivo conditions, with the tumor microenvironment playing a crucial role in tumor growth in vivo. In the mouse model transplanted with A20 cells, the role of host-derived BAFF did not appear significant to the tumor growth. Rather, BAFF seems to work for the suppression of A20 tumor growth in vivo. BAFF not only supports cancer cell survival but also plays a crucial role in maintaining normal humoral immunity (32, 33). BAFF plays an important role in the induction of immunoglobulin production by B cells and is essential for the formation of splenic germinal centers, the sites of antibody affinity maturation and memory B-cell formation (32). A study suggests that BAFF has complex roles in immune regulation that could impact tumor growth differently under certain conditions (34). In a previous report, a BAFF-overexpressing B16.F10 melanoma cell model was generated, revealing that BAFF-expressing tumors grow more slowly in vivo compared to control tumors (34). This research indicates that BAFF’s interaction with its receptors on specific B-cell populations affects cancer progression variably. BAFF-related pathways may help sustain immune responses that could indirectly counter cancer spread, especially when humoral immunity is engaged in antitumor activities. Local BAFF may exacerbate the condition, whereas systemic BAFF could enhance humoral immunity and exert an antitumor effect. Findings from the mouse model bearing A20 cells suggest that in cases where humoral immunity is active against cancer cells, blocking BAFF could potentially worsen the disease.
The expression pattern of BAFF during normal B-cell differentiation is unclear, and most studies suggest that BAFF is not expressed by normal resting or activated B cells (35). Since MPC-11 OUAr cells do not produce BAFF, the increase in BAFF levels in the nodule of wild-type mice likely originated from host cells. MRP14+ cells, which accumulated in the nodule of mice inoculated with MPC-11 OUAr cells, may contribute to local BAFF production. In MPC-11 OUAr-transplanted mice, tumor progression worsened in wild-type mice compared to BAFF-KO mice, suggesting that host-derived BAFF-producing cells accumulating in the nodule could exacerbate the tumor in cases where cancer cells do not produce BAFF. In wild-type mice bearing nodules with MPC-11 OUAr cells, genes involved in angiogenesis and the PI3K-Akt signaling pathway were upregulated. A transcriptome analysis showed that the MPC-11 OUAr cells expressed VEGFA, which induces angiogenesis, and IL-10, which promotes the recruitment of suppressor cells such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells to the tumor site, at higher levels than A20 cells. This suggests that MPC-11 OUAr cells may also contribute to the recruitment of macrophages to the tumor site. Furthermore, HIF-1α was highly expressed in tumors of BALB/cA mice bearing MPC-11 OUAr compared to MPC cell lines. HIF-1α regulates the transcription of genes involved in processes such as angiogenesis (36). It is speculated that some of the MDSCs accumulating tumors differentiate into tumor-associated macrophages, leading to HIF-1α activation. This, in turn, may enhance the production of angiogenesis-related factors, including VEGF, thereby promoting tumor invasion and proliferation. The occurrence of angiogenesis may facilitate the recruitment of MRP14+ cells. Also, BAFF affects physiological T-cell activation through BAFF-R-mediated activation of the PI3K-Akt signaling pathway, which mirrors one of the pathological mechanisms of T-cell-mediated autoimmune diseases (37).
Conversely, BAFF detected in the nodules of BAFF-KO mice inoculated with A20 cells likely originated from the tumor cells. In this scenario, A20 cells produce BAFF, which may be involved in autocrine-driven proliferation. In the nodule of BAFF-KO mice transplanted with A20, Acvr2b, which is part of the TGF-β family, was upregulated compared to wild-type mice bearing A20 cells. TGF-β initially suppresses cancer by inhibiting cell proliferation but later promotes tumor progression by enhancing cell motility and epithelial-to-mesenchymal transition, leading to metastasis. This dual role is crucial in advancing cancer aggressiveness and invasiveness (27). This study suggests that BAFF contributes to cancer progression even in B-cell malignancies that do not produce BAFF autonomously. In environments where BAFF-producing cells are present, host-derived BAFF can exacerbate cancer in BAFF-negative cancer cells. Conversely, some cancer cells can autonomously produce BAFF, allowing them to survive and proliferate without host-derived BAFF. The effect of BAFF on cancer is thus determined by the interplay between the immune response, receptor presence, and the cancer cell’s BAFF-producing capability. Several models describe the tumor microenvironment: one dominated by lymphoma cells, another with minimal immune infiltration where lymphoma cells depend on microenvironmental interactions, and a third with a low proportion of lymphoma cells and a diverse immune cell presence (38, 39). The findings from this study highlight the complex and context-dependent roles of BAFF in hematological malignancies. BAFF plays a crucial role in B-cell development and survival, influencing both normal and malignant B-cell proliferation. This study underscores the significance of BAFF in B-cell malignancies by demonstrating its differential effects on various B-cell lymphoma cell lines and its impact on tumor growth in mouse models. In this study, we have used only one line of lymphoma (A20) and one line of plasmacytoma (MPC-11 OUAr) among several tumor lines with a BALB/c background available from cell banks, just as proof of concept for the distinct roles of BAFF in tumor development by different tumor cell types. In the future, by examining more tumor cell types with distinct characteristics, the resolution in understanding their roles will become much higher.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal study was approved by the Institutional Animal Research Committee of the Graduate School of Agricultural and Life Sciences, The University of Tokyo. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
NT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. JY: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. WF: Methodology, Writing – review & editing, Resources. CS: Methodology, Resources, Writing – review & editing. YG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was partly supported by KAKENHI (21H02722, 22H05057, 24K02271) from the Japan Society for the Promotion of Science.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2025.1626886/full#supplementary-material
References
1. Mackay F and Browning JL. BAFF: A fundamental survival factor for B cells. Nat Rev Immunol. (2002) 2:465–75. doi: 10.1038/nri844
2. Shu HB, Hu WH, and Johnson H. TALL - 1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol. (1999) 65:680–3. doi: 10.1002/jlb.65.5.680
3. Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. (2000) 404:995–9. doi: 10.1038/35010115
4. Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, et al. BLyS: Member of the tumor necrosis factor family and B lymphocyte stimulator. Science. (1979) 1999) 285:260–3. doi: 10.1126/science.285.5425.260
5. Mukhopadhyay A, Ni J, Zhai Y, Yu GL, and Aggarwal BB. Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-κB, and c-jun NH2- terminal kinase. J Biol Chem. (1999) 274:15978–81. doi: 10.1074/jbc.274.23.15978
6. Schneider P, Mackay F, Steiner V, Hofmann K, Bodmer JL, Holler N, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. (1999) 189:1747–56. doi: 10.1084/jem.189.11.1747
7. Nagai K, Fujii W, Yamagishi J, Sanjoba C, and Goto Y. Inflammatory CD11b+ Macrophages produce BAFF in spleen of mice infected with leishmania donovani. Pathogens. (2024) 13:232. doi: 10.3390/pathogens13030232
8. Vincent FB, Morand EF, Schneider P, and MacKay F. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol. (2014) 10:365–73. doi: 10.1038/nrrheum.2014.33
9. Kumar G and Axtell RC. Dual role of B cells in multiple sclerosis. Int J Mol Sci. (2023) 24:2336. doi: 10.3390/ijms24032336
10. Milone MC, Xu J, Chen SJ, Collins MKA, Zhou J, Powell DJ, et al. Engineering-enhanced CAR T cells for improved cancer therapy. Nat Cancer. (2021) 2:780–93. doi: 10.1038/s43018-021-00241-5
11. Tagami N, Yuda J, and Goto Y. Current status of BAFF targeting immunotherapy in B-cell neoplasm. Int J Clin Oncol. (2024) 29:1676–83. doi: 10.1007/s10147-024-02611-2
12. Quah PS, Sutton V, Whitlock E, Figgett WA, Andrews DM, Fairfax KA, et al. The effects of B-cell–activating factor on the population size, maturation and function of murine natural killer cells. Immunol Cell Biol. (2022) 100:761–76. doi: 10.1111/imcb.12585
13. Schiemann B, Gommerman JLL, Vora K, Cachero TGG, Shutga-Morskaya S, Dobles M, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. (1979) 2001) 293:2111–4. doi: 10.1126/science.1061964
14. Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease: Impaired B cell maturation in mice lacking BLyS. Immunity. (2001) 15:289–302. doi: 10.1016/S1074-7613(01)00183-2
15. Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. (1999) 190:1697–710. doi: 10.1084/jem.190.11.1697
16. MacKay F and Schneider P. Cracking the BAFF code. Nat Rev Immunol. (2009) 9:491–502. doi: 10.1038/nri2572
17. Nocturne G, Ly B, Paoletti A, Pascaud J, Seror R, Nicco C, et al. Long-term exposure to monoclonal anti-TNF is associated with an increased risk of lymphoma in BAFF-transgenic mice. Clin Exp Immunol. (2021) 205:169–81. doi: 10.1111/cei.13602
18. Batten M, Fletcher C, Ng LG, Groom J, Wheway J, Laâbi Y, et al. TNF deficiency fails to protect BAFF transgenic mice against autoimmunity and reveals a predisposition to B cell lymphoma. J Immunol. (2004) 172:812–22. doi: 10.4049/jimmunol.172.2.812
19. Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, and Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. (1979) 122:549–54. doi: 10.4049/jimmunol.122.2.549
20. Laskov R and Scharff MD. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells: I. adaptation of the merwin plasma cell tumor-11 to ciylture, cloning, and characterization of gamma globulin siybunit. . J Exp Med. (1970) 131:515–41. doi: 10.1084/jem.131.3.515
21. Omachi S, Fujii W, Azuma N, Morimoto A, Sanjoba C, Matsumoto Y, et al. B-cell activating factor deficiency suppresses splenomegaly during Leishmania donovani infection. Biochem Biophys Res Commun. (2017) 489:528–33. doi: 10.1016/j.bbrc.2017.06.005
22. Anders S and Huber W. Differential expression analysis for sequence count data. Genome Biol. (2010) 11:R106. doi: 10.1186/gb-2010-11-10-r106
23. Zhang X, Chao P, Zhang L, Xu L, Cui X, Wang S, et al. Single-cell RNA and transcriptome sequencing profiles identify immune-associated key genes in the development of diabetic kidney disease. Front Immunol. (2023) 14:1030198. doi: 10.3389/fimmu.2023.1030198
24. Ge SX, Son EW, and Yao R. iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinf. (2018) 19:534. doi: 10.1186/s12859-018-2486-6
25. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U.S.A. (2005) 102:15545–50. doi: 10.1073/pnas.0506580102
26. Kanehisa M, Furumichi M, Tanabe M, Sato Y, and Morishima K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. (2017) 45:D353–D361. doi: 10.1093/nar/gkw1092
27. Xie F, Ling L, Van Dam H, Zhou F, and Zhang L. TGF-β signaling in cancer metastasis. Acta Biochim Biophys Sin (Shanghai). (2018) 50:121–32. doi: 10.1093/abbs/gmx123
28. Peng Y, Wang Y, Zhou C, Mei W, and Zeng C. PI3K/akt/mTOR pathway and its role in cancer therapeutics: are we making headway? Front Oncol. (2022) 12:819128. doi: 10.3389/fonc.2022.819128
29. Ng LG, Sutherland APR, Newton R, Qian F, Cachero TG, Scott ML, et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. (2004) 173:807–17. doi: 10.4049/jimmunol.173.2.807
30. Claudio E, Brown K, Park S, Wang H, and Siebenlist U. BAFF-induced NEMO-independent processing of NF-κB2 in maturing B cells. Nat Immunol. (2002) 3:958–65. doi: 10.1038/ni842
31. Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French DMM, et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-κB2. Immunity. (2002) 17:515–24. doi: 10.1016/S1074-7613(02)00425-9
32. Yan M, Marsters SA, Grewal IS, Wang H, Ashkenazi A, and Dixit VM. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat Immunol. (2000) 1:37–41. doi: 10.1038/76889
33. Yu G, Boone T, Delaney J, Hawkins N, Kelley M, Ramakrishnan M, et al. APRIL and TALL-I and receptor BCMA and TACI: System for regulating humoral immunity. Nat Immunol. (2000) 1:252–6. doi: 10.1038/79802
34. Liu W, Stachura P, Xu HC, Varaljai R, Shinde P, Ganesh NU, et al. BAFF attenuates immunosuppressive monocytes in the melanoma tumor microenvironment. Cancer Res. (2022) 82:264–77. doi: 10.1158/0008-5472.CAN-21-1171
35. Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: A mechanism for growth and survival. Blood. (2004) 103:689–94. doi: 10.1182/blood-2003-06-2043
36. Hirota K and Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. (2006) 59:15–26. doi: 10.1016/j.critrevonc.2005.12.003
37. Hu S, Wang R, Zhang M, Liu K, Tao J, Tai Y, et al. BAFF promotes T cell activation through the BAFF-BAFF-R-PI3K-Akt signaling pathway. Biomedicine Pharmacotherapy. (2019) 114:108796. doi: 10.1016/j.biopha.2019.108796
38. Scott DW and Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. (2014) 14:517–34. doi: 10.1038/nrc3774
Keywords: B cell, tumor, BAFF, mouse model, knockout (KO) mice
Citation: Tagami N, Yamagishi J, Fujii W, Sanjoba C and Goto Y (2025) Immune profile focusing on B-cell activating factor in B-cell malignancies. Front. Hematol. 4:1626886. doi: 10.3389/frhem.2025.1626886
Received: 12 May 2025; Accepted: 20 August 2025;
Published: 12 September 2025.
Edited by:
Rachel Maurie Gerstein, University of Massachusetts Medical School, United StatesReviewed by:
Hao Sun, Dana–Farber Cancer Institute, United StatesYasmine Lounici, Hospices Civils de Lyon, France
Copyright © 2025 Tagami, Yamagishi, Fujii, Sanjoba and Goto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasuyuki Goto, YXlnb3RvQGcuZWNjLnUtdG9reW8uYWMuanA=
 Nami Tagami1
Nami Tagami1 Junya Yamagishi
Junya Yamagishi Wataru Fujii
Wataru Fujii Chizu Sanjoba
Chizu Sanjoba Yasuyuki Goto
Yasuyuki Goto