- 1The Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States
- 2Vanderbilt University School of Medicine, Nashville, TN, United States
- 3The Department of Physical Medicine and Rehabilitation, Vanderbilt University Medical Center, Nashville, TN, United States
Lytic bone disease in multiple myeloma (MM) patients is cited as a major source of pain in upwards of 90% of patients, with lesions believed to exist in at least 80% of patients. Lytic bone disease increases the risk of pathologic fractures substantially, most frequently noted in the vertebrae (thoracic, followed by lumbar and cervical spine), skull, pelvis, and ribs. Even with significant advancement in the treatment of MM, such as novel chemotherapy agents and bisphosphonates, chronic pain remains a critical threat to quality of life for patients with MM. Additional factors that threaten quality of life in this population include nerve compression and infiltration, providing ample evidence of the need for effective pain relief and palliative measures as part of the MM treatment plan. The field of interventional radiology (IR) is uniquely positioned to provide targeted supportive care for patients with MM via minimally invasive, image-guided procedures that deliver effective results. This literature review investigates the role of interventionalists in providing supportive care and treatment for MM patients, focusing on procedures such as vertebroplasty and kyphoplasty, radiofrequency ablation (RFA), microwave ablation, cryoablation, high-intensity focused ultrasound (HIFU), and radiation therapy. From an interventional pain perspective, we explore nerve blocks and nerve ablations in the context of MM. Figures are derived from procedures performed at our institution to illustrate these approaches.
Introduction
Multiple myeloma
Multiple myeloma (MM), a largely incurable plasma cell disorder, is expected to compromise nearly 2% of all new cancer cases and an estimated 36,000 new diagnoses in the United States in 2024. MM prevalence increases with age, with a median age of 69 at the time of diagnosis and 87.3% of new diagnoses occurring after age 55 (1, 2). As a result of abnormal monoclonal immunoglobulin proliferation, patients diagnosed with MM face complications such as anemia, renal toxicity, hypercalcemia, and lytic bone disease. These osteolytic lesions are cited as a major source of pain in upwards of 90% of patients and are believed to exist in at least 80% of patients, as evidenced by skeletal survey x-rays (3, 4).
Lytic bone disease results from overactivation of RANKL and dysregulation of the cells involved in bone remodeling, noted as induced apoptosis in osteocytes, increased osteoclast activity (bone resorption), and decreased osteoblast activity (bone formation) (3–5). With diminished bone integrity and interrupted bone remodeling, the risk of pathologic fractures increases substantially. Lytic lesions and ensuing skeletal complications contribute to pathologic fractures in over 60% of patients over the course of their disease, most frequently noted in the vertebrae (thoracic, followed by lumbar and cervical spine), skull, pelvis, and ribs (6–8). Even with significant advancement in the treatment of MM, such as novel chemotherapy agents and bisphosphonates, chronic pain remains a critical threat to quality of life for patients with MM (3, 8). Additional factors that threaten quality of life in this population include nerve compression and infiltration, providing ample evidence of the need for effective pain relief and palliative measures as part of the MM treatment plan.
Treatment of pain
Pain in MM is multifactorial. The periosteum, or outside of the bone, is heavily innervated with sensory nerves, specifically tropomyosin receptor kinase A sensory nerve fibers (A-delta) and C-fibers (3, 9, 10). This dense innervation contrasts sharply with the minimal innervation found in the bone cortex, suggesting that pain associated with pathologic fractures primarily originates from the periosteum. Following bone injury, the sprouting of sympathetic nerve fibers and ectopic primary sensory fibers along with the upregulation of inflammatory cytokines (such as TNFα, IL-6, and IL-1β) amplify this pain response (3, 9). It’s been hypothesized that minimally invasive procedures utilizing bone cement may alleviate significant pain for patients impacted by bone disease, both by stabilizing the fractured region and destroying nerve endings near the vertebral body (11). The field of interventional radiology (IR) is uniquely positioned to provide targeted supportive care for patients with MM via minimally invasive, image-guided procedures that deliver effective results. These interventions are often adjunctive to oncologic or orthopedic treatments and require shorter recovery time compared to traditional surgical techniques (12). This review investigates the role of interventionalists in providing supportive care and treatment for MM patients, focusing on procedures such as vertebroplasty and kyphoplasty, radiofrequency ablation (RFA), microwave ablation, cryoablation, high-intensity focused ultrasound (HIFU), and radiation therapy. From an interventional pain perspective, we explore nerve blocks and nerve ablations in the context of MM. Figures are derived from procedures performed at our institution to illustrate these approaches.
Lesion interventions
Vertebroplasty and kyphoplasty
Treatment of painful, pathologic vertebral fractures in patients with MM can include vertebroplasty and kyphoplasty, particularly when lytic lesions occur in regions susceptible to compressive forces. In vertebroplasty, bone cement made up of polymethylmethacrylate (PMMA) is injected directly into the fractured vertebral body. In comparison, kyphoplasty first comprises placement of an inflatable balloon into the vertebral body with creation of a potential space or cavity, prior to injecting PMMA or absorbable calcium phosphate (13) (Figures 1A, B, 2). The primary objective of both procedures is to consolidate and stabilize pathologic bone fractures to achieve measurable pain relief. Notably, kyphoplasty also aims to restore vertebral body height via balloon inflation (3, 13). The most common complication of kyphoplasty is cement leakage, noted in 18% of treated vertebral levels in a 2009 study involving 76 MM patients. Rare but serious sequelae of leakage include motor deficits from spinal cord compression or pulmonary embolism, reported in 2% to 13% of cases (13, 14).
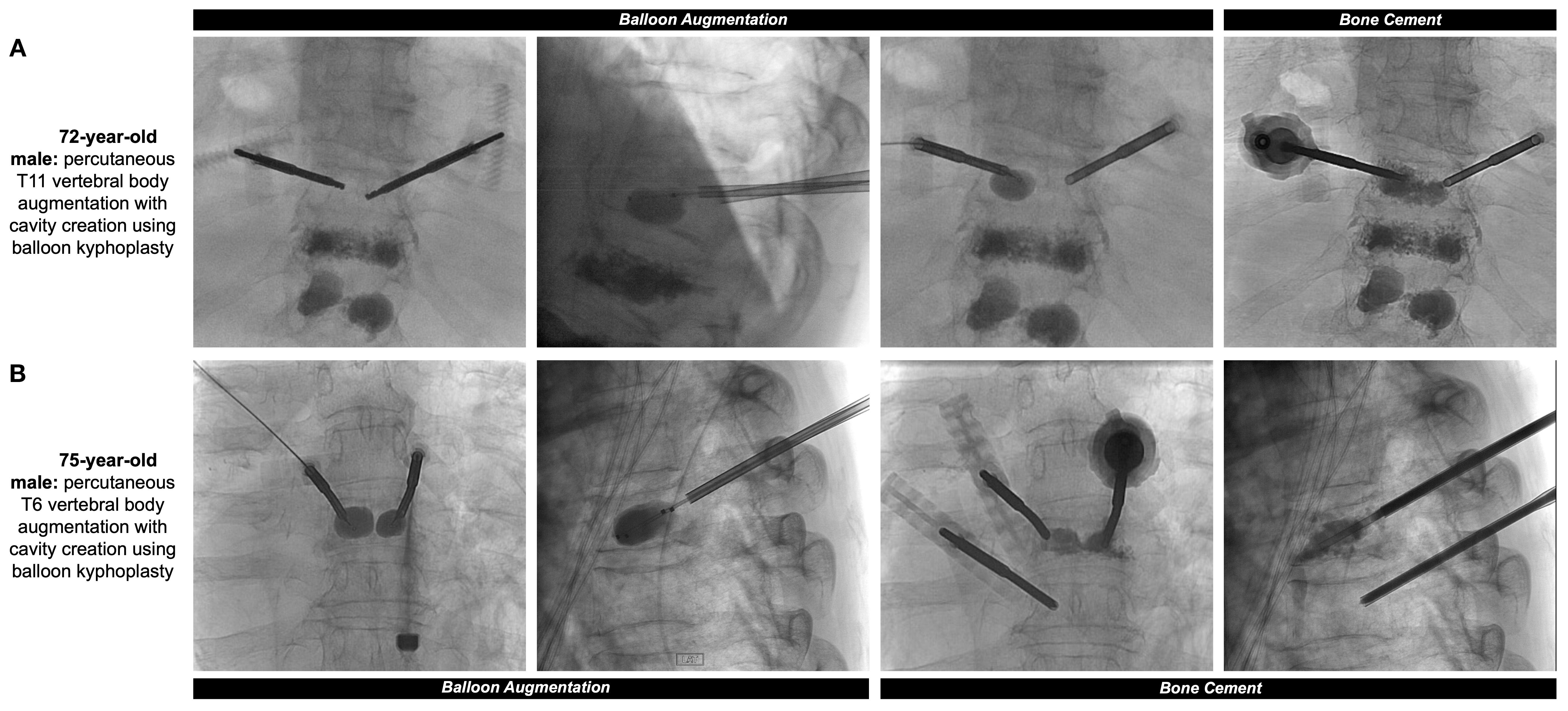
Figure 1. (A) 72-year-old male with a past medical history of MM with painful thoracic and lumbar spinal metastases and multilevel compression deformities. Two 11-gauge coaxial needles were introduced into the posterior third of the T11 vertebral body, followed by hand drills and bilateral 10mm augmentation balloons. PMMA was then injected into the T11 vertebral body with adequate filling demonstrated. Pre- and post-operative sagittal surveys are included in Figure 2. (B) 75-year-old male with a past medical history of MM with multiple compression fractures. The same process as the previous patient was repeated at the level of the T6 vertebral body, with small substitutions including 10-gauge coaxial needles and 15mm augmentation balloons.
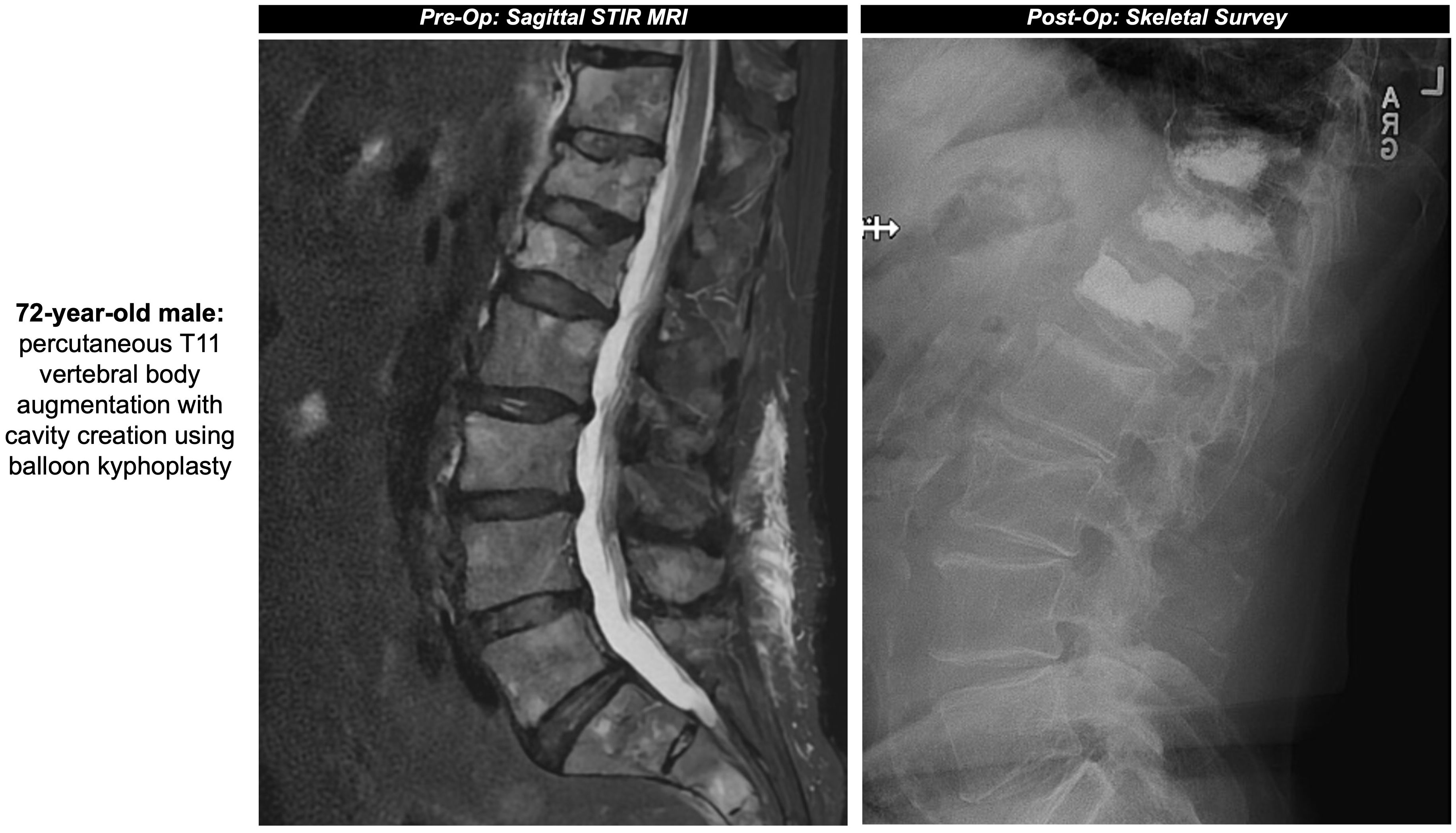
Figure 2. Pre- and post-operative sagittal surveys of the patient from Figure 1A.
A single-center cohort study reported that progression-free and overall-survival over a median follow-up of 51 months were significantly higher in MM patients who underwent surgical intervention (i.e. vertebroplasty/kyphoplasty) compared with those managed non-surgically, particularly for patients with more advanced disease according to the International Staging System (ISS II/III) (15). Likewise, while both procedures seem to offer similar reductions in pain and improvements in quality of life (16–18), kyphoplasty may provide improved post-procedure results and vertebral height increase compared to vertebroplasty (16, 19), though the clinical utility of this significance is yet to be determined (15).
It’s worth noting there is both heterogeneity of studies and a scarcity of randomized controlled trials directly comparing vertebroplasty and/or kyphoplasty for the treatment of vertebral fractures in patients with MM. Previous studies in broad populations with vertebral compression fractures reported comparable reductions in pain and disability between vertebroplasty and kyphoplasty (20), with both procedures providing greater pain relief than non-surgical management (21). In cohorts including MM patients, balloon kyphoplasty has been shown to be a safe and effective intervention for pain reduction compared to non-surgical management (22, 23), as is vertebroplasty with stable pain relief nearly 4 years after the procedure (24). Although these outcomes are promising, robust evidence supporting the effectiveness of kyphoplasty and vertebroplasty specifically in MM remains limited, highlighting the need for additional well-controlled studies targeting this patient population. Additionally, exploring the role of kyphoplasty and vertebroplasty as adjunctive or complementary therapies is warranted, especially in light of findings suggesting that vertebroplasty combined with chemotherapy may offer superior pain relief and 5-year survival rates compared to chemotherapy alone (25).
Lesion ablation
Ablation techniques, such as RFA, microwave ablation, and cryoablation, are often complementary approaches to cementoplasty to achieve pain relief in patients with MM due to hypothesized destruction of nerve fibers and reduction in the activity of “bone-chewing” osteoclasts (through microwave ablation and cementoplasty). These procedures provide a non-invasive, outpatient option with reduced morbidity and mortality compared to surgical measures (10, 26). However, given they do not inherently provide bone support, ablative procedures should be considered in concert with cementoplasty if the goal of treatment is to prevent additional pathologic fractures from weakened bone.
RFA is a thermal procedure thought to provide pain relief and reduction of inflammatory cytokines via frictional heating, between 60-100 °C, produced from applicators delivering alternating currents (8, 13, 27). RFA procedures increase the risk of adverse effects due to thermal energy, and thus care should be taken to ensure appropriate placement of probes, necessary grounding pads if using monopolar probes, and continuous monitoring of the ablation zone (between 0.5 to 4-5cm) during the ablation process (27, 28). Further, RFA generates heat and a magnetic field that may negatively interact with devices such as spinal cord stimulators when performed in proximity (29). Safety measures specific to each device should be employed when treating patients with potentially conflicting hardware.
Similar to vertebroplasty and kyphoplasty, evidence from randomized controlled trials evaluating ablative techniques in patients with multiple myeloma remains limited. A systemic review and meta-analysis inclusive of MM patients found that RFA of spinal metastatic tumors in combination with vertebroplasty provides durable pain relief with 5% local tumor progression > 12 months after the combined procedure (30), though once again additional work is required to establish correlative findings in MM patient cohorts. In a randomized controlled trial investigating RFA prior to vertebroplasty in 36 MM patients, the introduction of ablation did not appear to significantly alter pain relief after vertebroplasty (31), while other studies have demonstrated improved pain relief in patients with bone metastases treated with RFA and vertebroplasty (32, 33).
RFA uses ionic agitation to produce fictional heating, while microwave ablation’s mechanism of action is via production of a non-ionizing electromagnetic microwave field. Microwave ablation can reach higher intra-lesional temperatures, ablate larger tumor volumes, and is less susceptible to the heat sink phenomenon compared to RFA, with both techniques requiring adequate preventative measures to reduce the risk of iatrogenic skin burns (12, 27). In a cohort of patients with spinal metastases (MM n = 10) who underwent combined microwave ablation and vertebroplasty, follow-up imaging 6 months after the procedure demonstrated no locoregional progression in nearly 97% of surviving patients (34). As recognized by the authors in this study, clinical evidence for microwave ablation in the spine is limited, though seems to provide durable pain relief and tumor control with minimal adverse effects.
Cryoablation, while perhaps more applicable for bone tumors, freezes bone lesions instead of applying heat like RFA and microwave ablation. Inert gases such as argon or helium are cycled through small cryoprobes to induce freeze-thaw cycles, with the tip of the cryoablation probe reaching upwards of -160 °C. Cellular ischemia and apoptosis are triggered when the surrounding tissue reaches -40 °C (12, 28). Compared to heat-based methods, cryoablation produces an anesthetic effect and can be visualized in real-time [e.g. ice ball formation] via CT or MRI, though the freeze-thaw cycles can take significant more time than heat-based methods (12, 27). In a group of 33 patients with axial loading skeletal metastases, over 80% of patients reported pain reduction 2 years after combined cryoablation and cementoplasty (35), though it’s important to consider the risk of post-cryoablation adverse events when selecting cryoablation over RFA and microwave ablation (36). Adverse events may include damage to the surrounding skin or inadvertent extension of the cryoablation ice ball outside of the specified treatment area, putting critical neural and vascular structures at undue risk of injury.
High-intensity focused ultrasound
High-intensity focused ultrasound (HIFU), like the name suggests, utilizes high-energy ultrasound which, when attenuated on target, heats the tissue above 80 °C. Beams between 1500 to 3000J are required to treat lesions localized to the periosteum, while higher requirements are needed to treat the cortex. A single-institution study on HIFU in patients with solid malignancies (MM iliac metastasis, n = 1) determined all bone metastases were palliated in symptoms following the procedure, with uptake reduction confirmed on PET-CT (37). Though effective, HIFU is limited by cortical depth, non-homogeneous ablation from singular sonification, and is better visualized in the context of MR-guided ultrasound (27). When applied in 13 patients with symptomatic bone metastases, MRI-guided HIFU provided durable pain relief in 11 patients, measured by both reductions in pain score and medication dosage for the treatment of pain relief (38). Even with promising results, there has yet to be a study specifically focused on the application of HIFU in the MM population, including defining its role in clinical practice and opportunities as an adjunctive therapy for palliation (37).
Radiation therapy
We would be remiss not to discuss radiation therapy, the gold standard treatment of bony metastases. However, radiation therapy has a high non-response rate of 20% with the greatest benefit achieved weeks after cessation of treatment. These factors, in combination with nearly 40% of patients failing to achieve pain relief after initial radiation therapy, provide ample opportunity for image-guided interventional techniques as discussed previously to help address these concerns (28, 39). Radiation therapy uses either x-ray or proton radiation [~30+ Gy, or smaller doses given over several weeks] to damage cancer cell DNA, either directly or indirectly via free radical generation, and induce cellular apoptosis. Unlike interventional procedures like vertebroplasty that may bolster vertebral stability, radiation therapy serves a palliative purpose for patients with MM, specifically to control large lesions and assist with bone-related pain. In a study on 442 patients with MM divided into “radiation therapy naïve” and “radiation therapy treated” cohorts, the most common indication for radiation therapy was for palliative pain purposes (42%) followed by pathologic fractures (28%) (40). Further, in a large institutional study on 239 patients with MM, the equivalent dose of radiation therapy did not impact risk of progression, with a 7.8% local progression risk per lesion at 1-year post-radiation therapy (41).
Interventionalists may see an increase in patients treated with both radiation therapy and image-guided ablative procedures, given the addition of cryoablation to radiation therapy regimens has been shown to significantly improve the complete response rate and self-rated quality of life in patients with painful bony metastases (42). Alternatively, prophylactic stabilization of lesions via vertebroplasty may help prevent pathologic fractures after radiation therapy (34). It remains underexplored whether other ablative techniques or cementoplasty are superior in isolation to radiation therapy for treatment of pathologic fractures and pain relief, despite image-guided procedures having reduced morbidity and procedural costs compared to the standard of care (26). A retrospective study on 73 MM patients found kyphoplasty was more effective than radiation therapy or systemic therapy alone in reducing pain, disability, and fracture incidence (43). The International Myeloma Working Group recommends systemic combination therapy and advocates sparing use of radiotherapy to preserve marrow reserve (44). In this context, vertebroplasty/kyphoplasty and percutaneous ablative modalities merit consideration as adjunctive options, though once again robust comparative evidence versus systemic therapy or radiotherapy is lacking.
Pain interventions
Nerve blocks
Patients with MM are often at a higher risk for complications with anesthesia, as graded by the American Society of Anesthesiologists (ASA) Physical Status Classification system, during fixation of pathologic fractures. This increased risk is due to factors such as renal dysfunction, hyper-viscosity syndrome, anemia, and hypercalcemia (45). For patients with MM who may be sensitive to alterations in their hemodynamic response, peripheral nerve blocks offer a safe alternative to general anesthesia while still providing adequate pain control (46). Nerve blocks have decreased systemic effects on hypotension and respiration compared to general anesthesia (47), and preserve mean arterial pressure and cardiac index when compared to central blocks via spinal anesthesia (48). Where non-steroidal anti-inflammatory drugs may be contraindicated to preserve renal function in the perioperative period, peripheral nerve blocks offer both intraoperative and post-operative pain relief despite few reports on nerve blocks in the MM patient population (49). In a case study on a 44-year-old ASA III MM patient with a fractured femur, a psoas compartment block and sciatic block were selected in favor of general anesthesia, due to the patient’s high levels of creatinine and urea, and central block, due to the high prothrombin and international normalized ratio. Adequate pain relief was achieved with no complications encountered during or after the procedure (45), providing evidence for the feasibility of nerve blocks over other anesthesia for select patients with MM. Interventionalists can thus play an important, though perhaps underutilized, role in providing pain relief via treatment of nerve plexuses or peripheral nerves through image-guided techniques (50).
Nerve ablation
Akin to the niche interventionalists can fill via nerve blockades, nerve ablation serves as an additional method for longer control of cancer-related pain. As described above, pain in systemic conditions like MM is multifactorial and related to the release of pro-inflammatory cytokines, nerve infiltration of bone marrow, and stimulation of afferent nerves (51). Nerve ablation causes degeneration of sensory fibers and accompanying myelin sheaths distal to the lesion, known as Wallerian degeneration, leading to a temporary halt of nerve signal transmission. This process can be achieved via either chemical or thermal ablation, with the latter including conventional RFA, though RFA may have faster recurrence and is more time consuming than chemical denervation via alcohol (52) (Figure 3). The temporary disruption and subsequent potential for peripheral axonal regeneration can be attributed to the preservation of Schwann cells, but the risk of recurrence may be diminished with a larger distance between the proximal axonal stump and distal fibers (52, 53). To achieve the greatest success with pain management, ablative techniques like RFA must target the bone-tumor interface for substantial destruction of nerve endings in addition to addressing the mass itself (54). However, research on nerve ablation in the MM patient population is limited, with some studies suggesting there is no significant difference between pain relief achieved via RFA from neural destruction and decreased cytokine production versus vertebroplasty with stabilized trabecular bone (31).
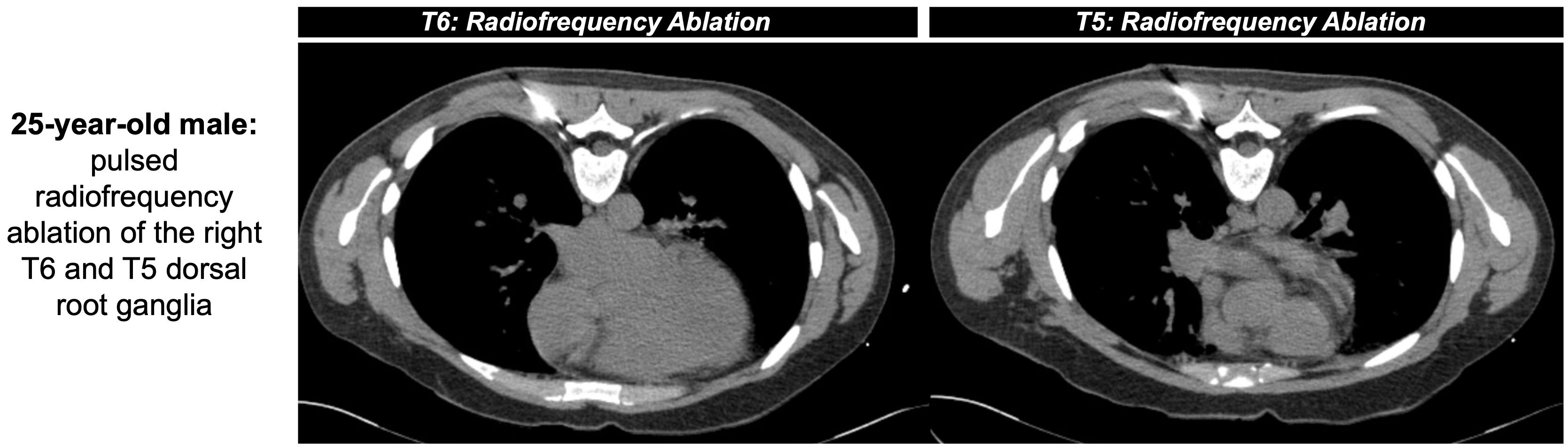
Figure 3. 25-year-old male with intractable herpatic neuralgia at the level of T6 presented for CT-guided RFA (shown as an example of nerve ablation non-specific to MM). Pulsed RFA was applied (42 °C for 2 minutes at a frequency of 2 hertz, 60 volts, impedance between 200 and 500 ohms) outside both the right T5 and T6 neural foramina. An additional lesion was created near T6 via conventional RFA (80 °C for 90 seconds) before removal of instruments with positive post-operative outcomes.
Future directions
Guidelines and recommendations
The IMWG released updated clinical practice guidelines in 2021 detailing the management of lytic bone disease in MM patients, including Zoledronic acid, Denosumab, and cementoplasty for compression fractures (55). The IMWG considers kyphoplasty to be a “grade A” recommendation and vertebroplasty to be a “grade C” recommendation. The updated guidelines do not include ablative procedures as a complementary approach to achieve pain relief in MM patients, nor do they discuss HIFU, nerve ablation, or nerve blocks in the MM patient population. Ablative procedures, including nerve ablation, can provide palliative pain control and even some tumor control, whether independent from or in concert with cementoplasty (34, 51, 56), though conflicting evidence of utility demands additional investigation before ablative procedures can be incorporated into clinical treatment guidelines (31). We provide evidence from our institution of ablative procedures tested in our MM patient population, with the hope of advancing our understanding of how ablative procedures may benefit patients in concert with vertebroplasty or kyphoplasty. We also support the IMWG’s recommendation to use radiation therapy sparingly, given additional kyphoplasty has been demonstrated to be more effective than additional radiation for pain relief (43, 44).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
AW: Writing – review & editing, Writing – original draft. SA: Writing – review & editing. MD: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. National Cancer Institute. Myeloma - Cancer Stat Facts. SEER. Available online at: https://seer.cancer.gov/statfacts/html/mulmy.html (Accessed September 17, 2025).
2. Qureshi A, Tariq MJ, Shah Z, Abu Zar M, Aslam S, Rafae A, et al. Evidence-based supportive care in multiple myeloma. J Community Hosp Intern Med Perspect. (2020) 10:313–7. doi: 10.1080/20009666.2020.1771124
3. Coluzzi F, Rolke R, and Mercadante S. Pain management in patients with multiple myeloma: An update. Cancers (Basel).MDPI AG. (2019) 11. doi: 10.3390/cancers11122037
4. Miceli TS, Gonsalves WI, and Buadi FK. Supportive care in multiple myeloma: Current practices and advances. Cancer Treat Res Commun. (2021) 29. doi: 10.1016/j.ctarc.2021.100476
5. Raje NS, Bhatta S, and Terpos E. Role of the RANK/RANKL pathway in multiple myeloma. Clin Cancer Res. (2019) 25:12–20. doi: 10.1158/1078-0432.CCR-18-1537
6. Vogel MN, Weisel K, Maksimovic O, Peters S, Brodoefel H, Claussen CD, et al. Pathologic fractures in patients with multiple myeloma undergoing bisphosphonate therapy: Incidence and correlation with course of disease. Am J roentgenol. (2009) 193:656–61. doi: 10.2214/AJR.08.2002
7. Melton LJ, Kyle RA, Achenbach SJ, Oberg AL, and Rajkumar SV. Fracture risk with multiple myeloma: A population-based study. J Bone Mineral Res. (2005) 20:487–93. doi: 10.1359/JBMR.041131
8. Thalambedu N, Kamran M, and Al-Hadidi S. The role of vertebral augmentation procedures in the management of multiple myeloma. Clin Hematol Int.Saabron Press. (2024) 6:51–8. doi: 10.46989/001c.92984
9. Nencini S and Ivanusic JJ. The physiology of bone pain. How much do we really know? Front Physiol. (2016) 7:157. doi: 10.3389/fphys.2016.00157
10. Deib G, Deldar B, Hui F, Barr JS, and Khan MA. Percutaneous microwave ablation and cementoplasty: Clinical utility in the treatment of painful extraspinal osseous metastatic disease and myeloma. Am J roentgenol. (2019) 212:1377–84. doi: 10.2214/AJR.18.20386
11. Chen LH, Hsieh MK, Niu CC, Fu TS, Lai PL, and Chen WJ. Percutaneous vertebroplasty for pathological vertebral compression fractures secondary to multiple myeloma. Arch Orthop Trauma Surg. (2012) 132:759–64. doi: 10.1007/s00402-012-1474-y
12. Ahmed O, Feinberg N, and Lea WB. Interventional techniques for the ablation and augmentation of extraspinal lytic bone metastases. Semin Intervent Radiol. (2019) 36:221–8. doi: 10.1055/s-0039-1693117
13. Souza F, Aguilera A, Chaitowitz I, and Subhawong TK. Diagnostic and interventional radiology considerations in metastatic bone disease. Oper Tech Orthop. (2021) 31. doi: 10.1016/j.oto.2021.100893
14. Huber FX, McArthur N, Tanner M, Gritzbach B, Schoierer O, Rothfischer W, et al. Kyphoplasty for patients with multiple myeloma is a safe surgical procedure: Results from a large patient cohort. Clin Lymphoma Myeloma. (2009) 9:375–80. doi: 10.3816/CLM.2009.n.073
15. Zhang F, Liu S, Zhou X, Wang W, Jia C, Wang Q, et al. Percutaneous vertebroplasty/kyphoplasty contributes to the improved outcome in patients with newly diagnosed multiple myeloma: A single center cohort study. J Bone Oncol. (2024) 47:100615. doi: 10.1016/j.jbo.2024.100615
16. Köse KC, Cebesoy O, Akan B, Altinel L, Dinçer D, and Yazar T. Functional results of vertebral augmentation techniques in pathological vertebral fractures of myelomatous patients. J Natl Med Assoc. (2006) 98:1654–8.
17. Khan OA, Brinjikji W, and Kallmes DF. Vertebral augmentation in patients with multiple myeloma: A pooled analysis of published case series. Am J Neuroradiology. (2014) 35:207–10. doi: 10.3174/ajnr.A3622
18. Erdem E, Samant R, Malak SF, Culp WC, Brown A, Peterson L, et al. Vertebral augmentation in the treatment of pathologic compression fractures in 792 patients with multiple myeloma. Leukemia. (2013) 27:2391–3. doi: 10.1038/leu.2013.162
19. La Maida GA, Sala F, Callea G, Capitani D, and Singh S. Efficacy of unipedicular baloon kyphoplasty for treatment of multiple myeloma vertebral lesions. Asian Spine J. (2011) 5:162. doi: 10.4184/asj.2011.5.3.162
20. Evans AJ, Kip KE, Brinjikji W, Layton KF, Jensen ML, Gaughen JR, et al. Randomized controlled trial of vertebroplasty versus kyphoplasty in the treatment of vertebral compression fractures. J neurointerv Surg. (2016) 8:756–63. doi: 10.1136/neurintsurg-2015-011811
21. Papanastassiou ID, Phillips FM, Van Meirhaeghe J, Berenson JR, Andersson GBJ, Chung G, et al. Comparing effects of kyphoplasty, vertebroplasty, and non-surgical management in a systematic review of randomized and non-randomized controlled studies. Eur Spine J. (2012) 21:1826–43. doi: 10.1007/s00586-012-2314-z
22. Boonen S, Van Meirhaeghe J, Bastian L, Cummings SR, Ranstam J, Tillman JB, et al. Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J Bone Mineral Res. (2011) 26:1627–37. doi: 10.1002/jbmr.364
23. Berenson J, Pflugmacher R, Jarzem P, Zonder J, Schechtman K, Tillman JB, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. (2011) 12:225–35. doi: 10.1016/S1470-2045(11)70008-0
24. Holmes HE, Balian V, Kular S, Batty R, Connolly DJA, Chantry A, et al. Outcomes of percutaneous vertebroplasty in multiple myeloma: a tertiary neurosciences experience with long-term follow-up. Front Oncol. (2024) 14:1291055. doi: 10.3389/fonc.2024.1291055
25. Yang Z, Tan J, Xu Y, Sun H, Xie L, Zhao R, et al. Treatment of MM-associated spinal fracture with percutaneous vertebroplasty (PVP) and chemotherapy. Eur Spine J. (2012) 21:912–9. doi: 10.1007/s00586-011-2105-y
26. Dupuy DE and Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities—Part II. J Vasc Interventional Radiol. (2001) 12:1135–48. doi: 10.1016/S1051-0443(07)61670-4
27. Ryan A, Byrne C, Pusceddu C, Buy X, Tsoumakidou G, and Filippiadis D. CIRSE standards of practice on thermal ablation of bone tumours. Cardiovasc Intervent Radiol. (2022) 45:591–605. doi: 10.1007/s00270-022-03126-x
28. Sgalambro F, Zugaro L, Bruno F, Palumbo P, Salducca N, Zoccali C, et al. Interventional radiology in the management of metastases and bone tumors. J Clin Med.MDPI. (2022) 11. doi: 10.3390/jcm11123265
29. Abdullah N, Muir C, Eldrige JS, Pingree MJ, and Hagedorn JM. Peri-procedural management of implanted spinal cord stimulators in patients undergoing radiofrequency ablation: A case report and manufacturer-specific recommendations. Pain Practice. (2020) 20:405–11. doi: 10.1111/papr.12860
30. Chen AL, Sagoo NS, Vannabouathong C, Reddy Y, Deme S, Patibandla S, et al. Combination radiofrequency ablation and vertebral cement augmentation for spinal metastatic tumors: A systematic review and meta-analysis of safety and treatment outcomes. North Am Spine Soc J (NASSJ). (2024) 17:100317. doi: 10.1016/j.xnsj.2024.100317
31. Orgera G, Krokidis M, Matteoli M, Varano GM, La Verde G, David V, et al. Percutaneous vertebroplasty for pain management in patients with multiple myeloma: is radiofrequency ablation necessary? Cardiovasc Intervent Radiol. (2014) 37:203–10. doi: 10.1007/s00270-013-0624-0
32. Tian QH, Wu CG, Gu YF, He CJ, Li MH, and Cheng YD. Combination radiofrequency ablation and percutaneous osteoplasty for palliative treatment of painful extraspinal bone metastasis: A single-center experience. J Vasc Interventional Radiol. (2014) 25:1094–100. doi: 10.1016/j.jvir.2014.03.018
33. Wallace AN, Greenwood TJ, and Jennings JW. Radiofrequency ablation and vertebral augmentation for palliation of painful spinal metastases. J Neurooncol. (2015) 124:111–8. doi: 10.1007/s11060-015-1813-2
34. Khan MA, Deib G, Deldar B, Patel AM, and Barr JS. Efficacy and safety of percutaneous microwave ablation and cementoplasty in the treatment of painful spinal metastases and myeloma. Am J Neuroradiology. (2018) 39:1376–83. doi: 10.3174/ajnr.A5680
35. Castañeda Rodriguez WR and Callstrom MR. Effective pain palliation and prevention of fracture for axial-loading skeletal metastases using combined cryoablation and cementoplasty. Tech Vasc Interv Radiol. (2011) 14:160–9. doi: 10.1053/j.tvir.2011.02.008
36. Schmit GD, Kurup AN, Morris JM, Kumar SK, Schmitz JJ, Welch BT, et al. Percutaneous cryoablation of plasmacytomas: oncologic effectiveness and adverse events. J Vasc Interventional Radiol. (2023) 34:1303–10. doi: 10.1016/j.jvir.2023.04.013
37. Orgera G, Monfardini L, Della Vigna P, Zhang L, Bonomo G, Arnone P, et al. High-intensity focused ultrasound (HIFU) in patients with solid Malignancies: evaluation of feasibility, local tumour response and clinical results. Radiol Med. (2011) 116:734–48. doi: 10.1007/s11547-011-0634-4
38. Catane R, Beck A, Inbar Y, Rabin T, Shabshin N, Hengst S, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases—preliminary clinical experience. Ann Oncol. (2007) 18:163–7. doi: 10.1093/annonc/mdl335
39. Chow E, Zeng L, Salvo N, Dennis K, Tsao M, and Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol. (2012) 24:112–24. doi: 10.1016/j.clon.2011.11.004
40. Talamo G, Dimaio C, Abbi KKS, Pandey MK, Malysz J, Creer MH, et al. Current role of radiation therapy for multiple myeloma. Front Oncol. (2015) 5:40. doi: 10.3389/fonc.2015.00040
41. Gao RW, Fleuranvil R, Harmsen WS, Greipp PT, Baughn LB, Jevremovic D, et al. Predictors of local control with palliative radiotherapy for multiple myeloma. Int J Radiat OncologyBiologyPhysics. (2023) 117:S108. doi: 10.1016/j.ijrobp.2023.06.070
42. Di Staso M, Gravina GL, Zugaro L, Bonfili P, Gregori L, Franzese P, et al. Treatment of solitary painful osseous metastases with radiotherapy, cryoablation or combined therapy: propensity matching analysis in 175 patients. PloS One. (2015) 10:e0129021. doi: 10.1371/journal.pone.0129021
43. Kasperk C, Haas A, Hillengass J, Weiss C, Neben K, Goldschmidt H, et al. Kyphoplasty in patients with multiple myeloma a retrospective comparative pilot study. J Surg Oncol. (2012) 105:679–86. doi: 10.1002/jso.22101
44. Kyriakou C, Molloy S, Vrionis F, Alberico R, Bastian L, Zonder JA, et al. The role of cement augmentation with percutaneous vertebroplasty and balloon kyphoplasty for the treatment of vertebral compression fractures in multiple myeloma: a consensus statement from the International Myeloma Working Group (IMWG). Blood Cancer J. (2019) 9:27. doi: 10.1038/s41408-019-0187-7
45. Binici O and Akyol F. Combined sciatic-psoas compartment nerve block in a patient with multiple myeloma. East J Med. (2016) 21(4):197–99. doi: 10.5505/ejm.2016.09719
46. Büttner B, Mansur A, Hinz J, Erlenwein J, Bauer M, and Bergmann I. Combination of general anesthesia and peripheral nerve block with low-dose ropivacaine reduces postoperative pain for several days after outpatient arthroscopy. Medicine. (2017) 96:e6046. doi: 10.1097/MD.0000000000006046
47. Mounet B, Choquet O, Swisser F, Biboulet P, Bernard N, Bringuier S, et al. Impact of multiple nerves blocks anaesthesia on intraoperative hypotension and mortality in hip fracture surgery intermediate-risk elderly patients: A propensity score-matched comparison with spinal and general anaesthesia. Anaesth Crit Care Pain Med. (2021) 40:100924. doi: 10.1016/j.accpm.2021.100924
48. Rani KR, Vaishnavi R, Vikas KN, and Ashok MS. Comparison of paravertebral block with conventional spinal anesthesia in patients undergoing unilateral inguinal hernia repair. Anesth Essays Res. (2020) 14:29. doi: 10.4103/aer.AER_19_20
49. Kalingarayar S, Nandhakumar A, and Thennavan A. Anaesthesia for fixation of repeated pathological fractures in a patient with multiple myeloma. Indian J Anaesth. (2017) 61:1009. doi: 10.4103/ija.IJA_467_17
50. Midia M and Dao D. The utility of peripheral nerve blocks in interventional radiology. Am J roentgenol. (2016) 207:718–30. doi: 10.2214/AJR.16.16643
51. Gharaei H, Imani F, and Vakily M. Radiofrequency thermal ablation in painful myeloma of the clavicle. Korean J Pain. (2014) 27:72–6. doi: 10.3344/kjp.2014.27.1.72
52. Choi EJ, Choi YM, Jang EJ, Kim JY, Kim TK, and Kim KH. Neural ablation and regeneration in pain practice. Korean J Pain. (2016) 29:3–11. doi: 10.3344/kjp.2016.29.1.3
53. Tran A, Reiter DA, Cruz AR, and Gonzalez FM. Genicular nerve ablation review using cooled-radiofrequency nerve ablation. Semin Intervent Radiol. (2022) 39:130–7. doi: 10.1055/s-0042-1745797
54. Callstrom MR and Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol. (2007) 10:120–31. doi: 10.1053/j.tvir.2007.09.003
55. Terpos E, Zamagni E, Lentzsch S, Drake MT, García-Sanz R, Abildgaard N, et al. Treatment of multiple myeloma-related bone disease: recommendations from the Bone Working Group of the International Myeloma Working Group. Lancet Oncol. (2021) 22:e119–30. doi: 10.1016/S1470-2045(20)30559-3
Keywords: multiple myeloma, interventional radiology (IR), kyphoplasty, ablation, vertebroplasty, HIFU
Citation: Witt A, Aujla S and Case MD (2025) The role of interventional radiology in pain management for multiple myeloma: a literature review. Front. Hematol. 4:1649720. doi: 10.3389/frhem.2025.1649720
Received: 18 June 2025; Accepted: 17 September 2025;
Published: 02 October 2025.
Edited by:
Douglas Sborov, The University of Utah, United StatesReviewed by:
Hathal Haddad, University of Tübingen, GermanyZiga Cizman, University of Utah Hospital, United States
Copyright © 2025 Witt, Aujla and Case. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meaghan Dendy Case, bWVhZ2hhbi5kZW5keS5jYXNlQHZ1bWMub3Jn
 Atlee Witt
Atlee Witt Saumya Aujla
Saumya Aujla Meaghan Dendy Case
Meaghan Dendy Case