- 1Department of Internal Medicine, The New York Medical College Graduate Medical Education Program at St. Mary’s General Hospital and Saint Clare’s Health, Denville, NJ, United States
- 2Department of Hematology and Oncology, Saint Michael’s Medical Center, Newark, NJ, United States
Background: Extramedullary plasmacytoma (EMP) involving the gastrointestinal (GI) tract is a rare and serious manifestation of multiple myeloma (MM), often indicative of disease relapse and poor prognosis.
Case presentation: We report a case of a 64-year-old female with MM previously treated with cyclophosphamide, bortezomib, and dexamethasone (CyBorD) chemotherapy, autologous stem cell transplant, and denosumab, who presented four months post-transplant with melena. Esophagogastroduodenoscopy revealed a submucosal gastric nodule. Histopathology and flow cytometry confirmed monoclonal IgG kappa plasma cell infiltration, consistent with plasmacytoma. Bone marrow biopsy demonstrated remission with less than 3% plasma cells. The patient received local radiotherapy and was started on a daratumumab-based chemotherapy regimen for relapsed MM.
Conclusion: Secondary EMP involving the stomach is an exceedingly rare but important differential in MM patients presenting with GI bleeding. This case highlights the need for vigilance in monitoring for extramedullary relapse post-transplant, as early diagnosis and aggressive treatment may impact outcomes.
Background
Plasma cell tumors are immunoproliferative monoclonal diseases of the B-cell lineage that originate from malignant transformed plasma cells. Plasmacytoma and multiple myeloma (MM) constitute the two main categories of plasma cell tumors.
Plasmacytoma consists of two types: solitary plasmacytoma of bone and solitary extramedullary plasmacytoma (EMP). In the primary form of EMP, there is no clinical evidence of any other plasma cell tumor present. In contrast, the secondary type is characterized by the presence of another plasma cell neoplasm (PCN), often MM, which is confirmed at the time of the EMP diagnosis (1).
Secondary EMP develops due to the “bone marrow escape” of a subclone of plasma cells. This subclone migrates out of the bone marrow, infiltrating soft tissues and losing its dependence on the bone marrow microenvironment (2). Secondary EMP is often a sign of advanced MM or relapse, and, therefore, the distinction between primary and secondary EMP is crucial, as they are treated differently and have a different prognosis (3).
The estimated prevalence of patients with MM and plasma cell involvement of the gastrointestinal (GI) tract at the time of diagnosis or during the disease is estimated to be 0.9% (4). In this case report, we will present a patient with MM who underwent a bone marrow transplant. When MM was in remission, the patient presented with an upper GI bleed and was diagnosed with plasmacytoma of the GI tract, indicating a relapse of MM.
Case presentation
A 64-year-old female presented to the hospital with the complaints of black stools for a week and anemia.
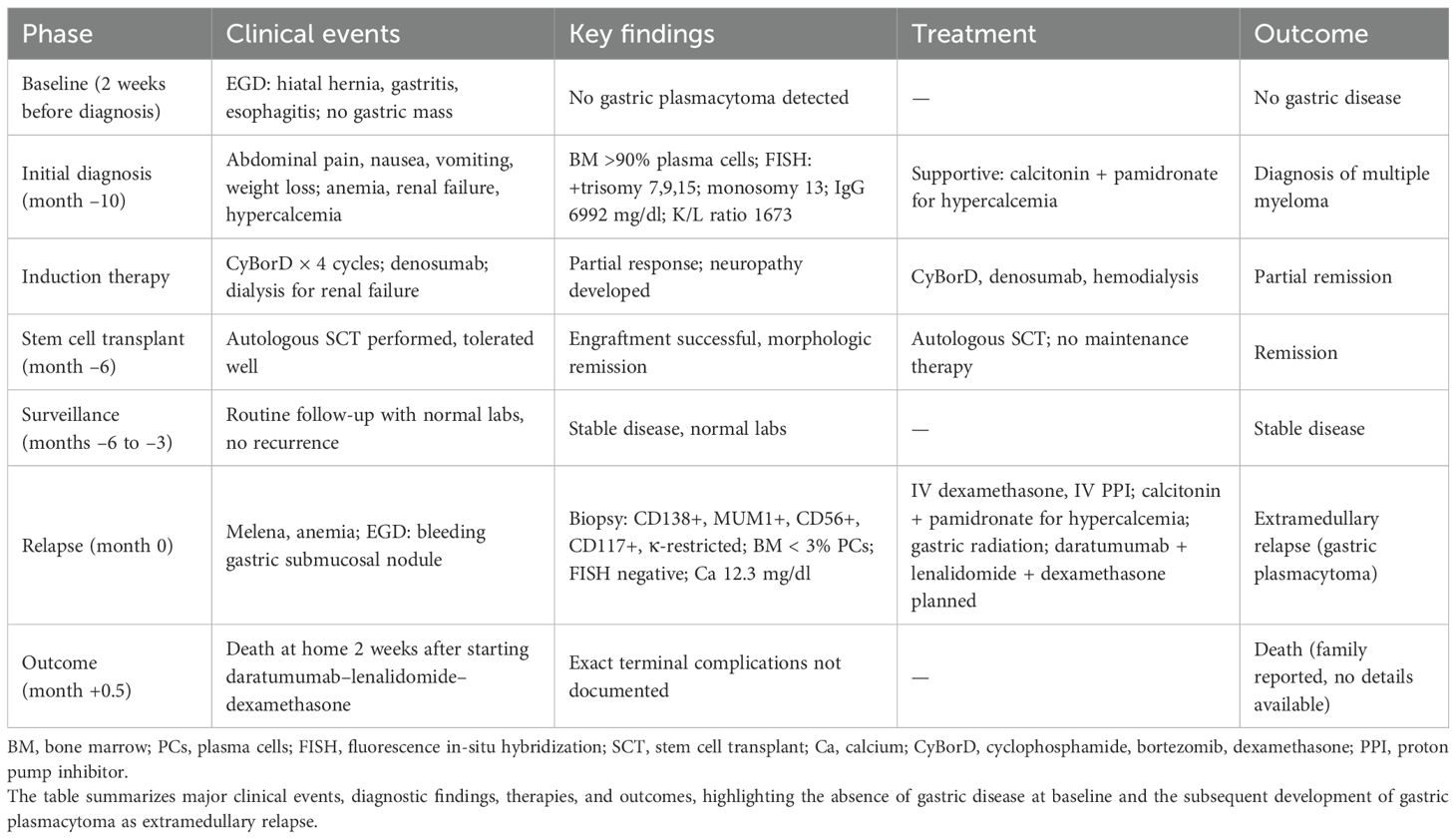
Ten months ago, the patient was admitted with abdominal pain accompanied by nausea and vomiting, which had been occurring intermittently for 3 months. Of note, an esophagogastroduodenoscopy (EGD) performed 2 weeks prior to the initial diagnosis of MM revealed only hiatal hernia, gastritis, and esophagitis, without evidence of gastric mass or plasmacytoma. The patient also experienced an unintentional weight loss of 20 lbs. over the past few months. A serum metabolic panel revealed elevated levels of calcium and phosphate, along with paraproteinemia. A complete blood count showed normocytic anemia. The patient also had worsened kidney function.
Given these findings, the diagnosis of MM was considered, and a skeletal survey was performed, which revealed multiple lytic lesions in the calvarium. The K/L ratio was 1673, the beta 2 macroglobulin level was 23.2, and the IgG level was 6992. A bone marrow biopsy was then conducted, demonstrating that more than 90% of the cells were plasma cells. Flow cytometry of the bone marrow biopsy confirmed the diagnosis by showing that monoclonal IgG kappa plasma cells comprised 70% of the total cells. FISH was positive for trisomy 7, 9, 15, and monosomy 13.
During the hospitalization, the patient’s kidney function did not improve, and the patient started on hemodialysis. Given the patient has renal failure and dependence on dialysis, the decision was made to use a CyBorD regimen with acyclovir prophylaxis. After receiving four cycles of combination therapy, she had neuropathy in her feet and fingers and continued receiving denosumab.
The patient underwent an autologous bone marrow transplant 6 months after the diagnosis and tolerated the infusion of stem cells without incidence. She was not placed on maintenance therapy following SCT. Restaging studies, including blood and urine electrophoresis with immunofixation and serum quantitative free light chains, were obtained at monthly intervals for 3 months, and no evidence of recurrence was observed.
Four months after the bone marrow transplant, the patient presented with black stools, which she had been experiencing for a week. A fecal occult blood test (FOBT) was positive, prompting an EGD to rule out GI bleeding. The EGD revealed a 10-mm submucosal nodule (Figure 1). Biopsies taken from the nodule demonstrated gastric oxyntic mucosa with significant infiltration of lymphoplasmacytic cells, consistent with a plasma cell neoplasm (Figure 2). Flow cytometry analysis showed monoclonal IgG kappa plasma cells, representing 0.7% of the total cell population. Immunohistochemistry confirmed plasma cell lineage, with atypical cells positive for CD138, MUM1, CD56, CD117, and kappa light chain, and negative for CD20, cyclin D1, lambda, and immunoglobulin heavy chains (IgG, IgA, IgM, and IgD). CD3 highlighted scattered background T cells. No significant lymphoid component was present, supporting a monoclonal plasma cell neoplasm.
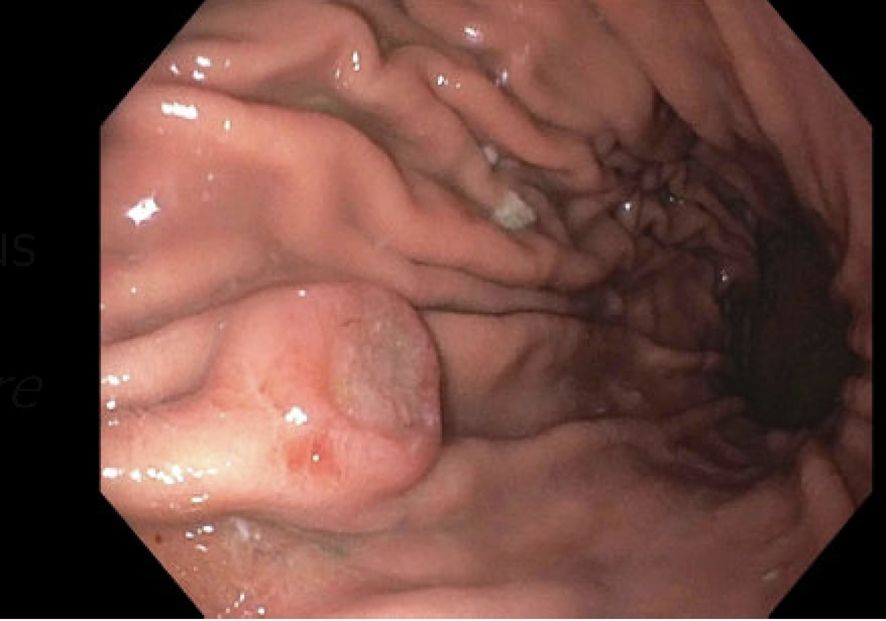
Figure 1. A medium-sized, ulcerated, non-circumferential mass was found in gastric body. Biopsies were taken and the mass was identified as plasmacytoma.
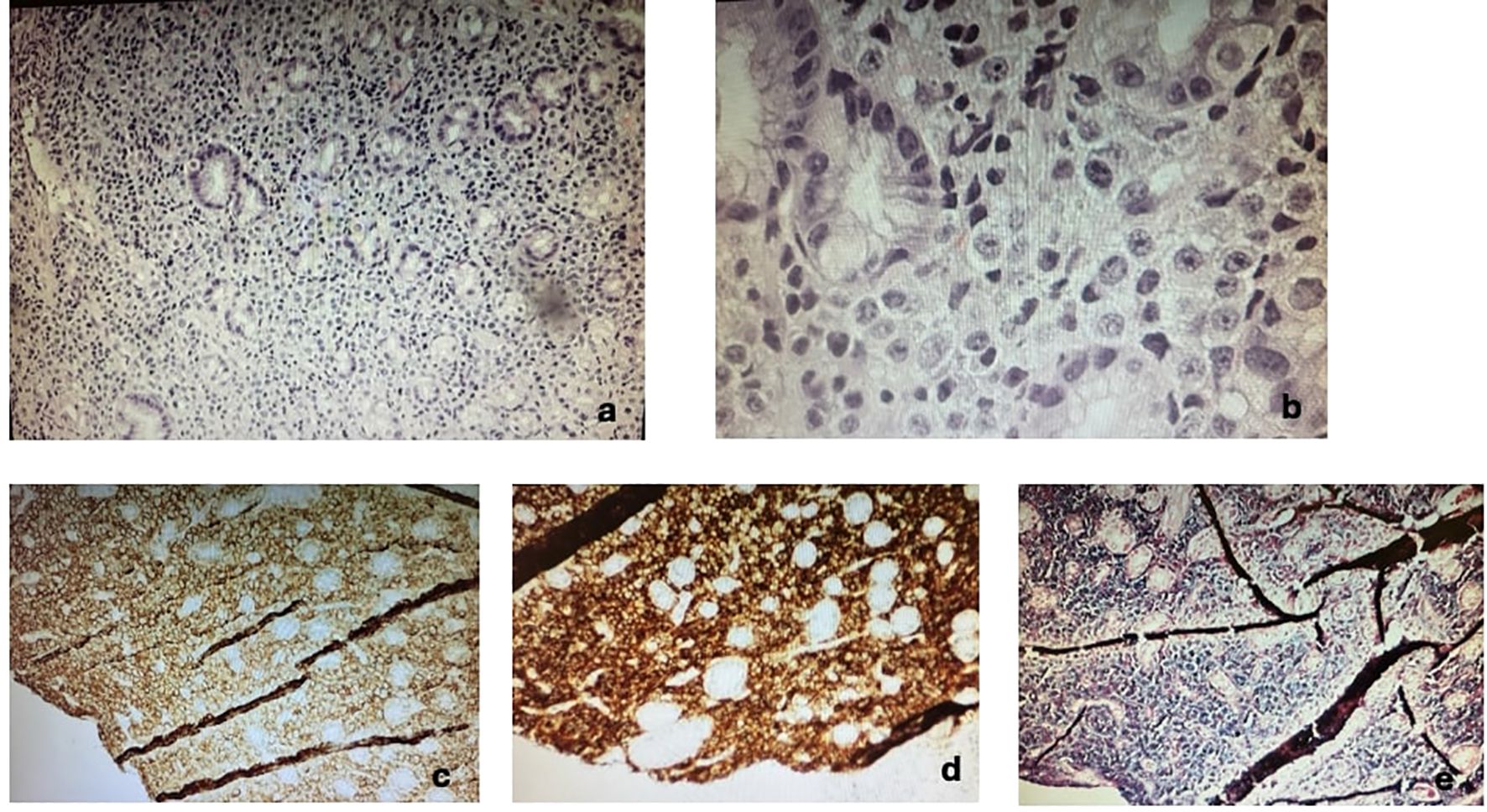
Figure 2. Immunohistochemistry of gastric lesion confirming plasmacytoma. (a) Low magnification H&E, (b) high magnification, (c) CD138+, (d) CD56+, (e) κ light chain+. Additional stains (not shown) demonstrated positivity for MUM1 and CD117, and negativity for CD20, cyclin D1, lambda, and immunoglobulin heavy chains (IgG, IgA, IgM, IgD).
A repeat bone marrow biopsy indicated normocellular bone marrow with maturing trilineage hematopoiesis and myeloid hyperplasia; the plasma cells were scattered and not increased, comprising less than 3%. FISH studies performed on the bone marrow at relapse were negative for cytogenetic abnormalities, whereas the gastric lesion itself was not tested by FISH or molecular assays. These findings were consistent with a relapse of MM as plasmacytoma, and the patient was started on IV dexamethasone 40 mg daily with an IV PPI drip. The patient also had a calcium level of 12.3 mg/dl and received calcitonin and pamidronate. The patient received a 4-day course of high-dose dexamethasone for relapsed MM. Radiation treatment was given for gastric plasmacytoma, and daratumumab-based chemotherapy regimen combined with bortezomib, lenalidomide, and dexamethasone (VRd) was planned. Following initiation of daratumumab, lenalidomide, and dexamethasone, the patient was discharged home. Two weeks later, she developed hypotension, tachycardia, and shortness of breath during outpatient dialysis and died shortly thereafter. No autopsy was performed. A clinical timeline summarizing the patient’s disease course from initial evaluation through relapse is shown in Table 1 and Figure 3.
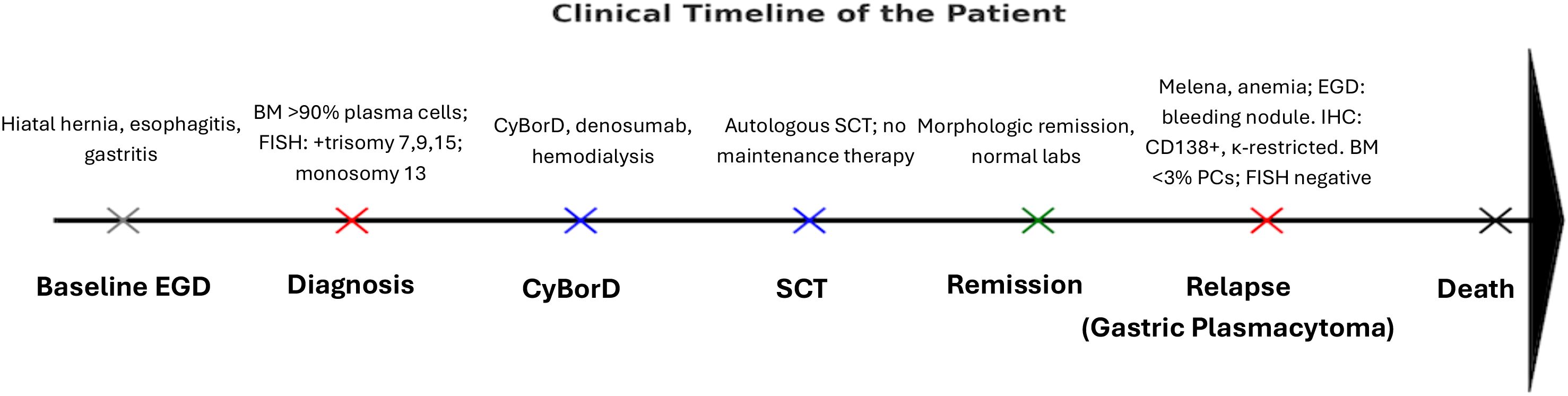
Figure 3. Schematic timeline of the patient’s clinical course. Gray = baseline negative EGD; red = disease events (diagnosis, relapse); blue = treatment milestones (CyBorD, SCT); green = remission; black = outcome (death). This illustrates the sequence from initial evaluation through gastric plasmacytoma relapse and death. BM, bone marrow; SCT, stem cell transplant; PCs, plasma cells; FISH, fluorescence in-situ hybridization; Ca, calcium; CyBorD, cyclophosphamide, bortezomib, dexamethasone.
Discussion
EMP involving the GI tract is an extremely rare manifestation of MM, with only 38 cases reported in the literature presenting with upper GI bleeding (5). Although EMP is a rare cause of GI bleeding, it should be considered in patients with MM.
Extramedullary disease manifesting in MM is classified into primary EMM and secondary EMM. Primary EMM refers to the presence of extramedullary disease at the time of diagnosis, while secondary EMM occurs when extramedullary disease develops during the relapse of MM. It is estimated that primary EMM affects up to 19% of patients with MM, while secondary EMM impacts up to 28% of patients at the time of relapse (4). Secondary EMP is associated with a poor prognosis compared to primary EMP (5), with median overall survival ranging from 6 to 12 months after diagnosis in several studies. The aggressive nature of secondary EMP underscores the importance of early detection and initiation of therapy to potentially prolong survival (6).
Early recognition of secondary extramedullary disease during MM surveillance is crucial, as it may present atypically and carries significant therapeutic and prognostic implications. Incorporating imaging modalities such as PET-CT in routine follow-up may aid in the earlier identification of extramedullary relapses (7).
EMM in the GI tract is considered a rare occurrence. In a study involving a database of 2,584 patients with MM, Talamo et al. found that only 0.9% exhibited plasma cell involvement of the GI tract as the cause of their disease (4). This included 3 patients with primary EMM and 21 with secondary EMM. The stomach is the most common site for extramedullary plasma cell involvement in the GI tract.
In symptomatic EMM in the GI tract, the clinical manifestations vary according to involved localization and may be roughly divided into three symptom groups: direct invasion of a specific organ causing hemorrhage, perforation, or hepatic failure; mechanical pressure due to mass effect causing GI obstruction; and production of malignant effusion (4). In our case, the GP first became symptomatic when it started hemorrhaging. Direct visualization of EMP through EGD and biopsy is the most common method for diagnosing EMP in the GI tract, especially when it leads to bleeding. However, this approach is not applicable for EMP located distal to the ligament of Treitz. In such cases, alternative diagnostic methods, such as laparoscopy or capsule endoscopy, are necessary if GI EMP is suspected (5).
One important differential diagnosis in this setting is a B-cell lymphoma with plasmacytic differentiation (e.g., marginal zone lymphoma) or EBV-associated plasmablastic lymphoma, especially given the plasmablastic morphology. However, the gastric lesion in our case was CD138+, MUM1+, CD56+, κ-restricted, and negative for CD20 and cyclin D1, without a significant lymphoid background, making a lymphoma diagnosis unlikely. While EBV studies were not performed, the immunophenotype and the patient’s known IgG-κ MM strongly support a diagnosis of EMP, and we acknowledge the absence of EBV testing as a limitation. Although FISH at relapse was performed only on the bone marrow and was negative, this does not exclude a clonal relationship. EMP often arise from subclones that differ genetically from the dominant marrow clone at diagnosis. Importantly, the gastric lesion showed an immunophenotype concordant with the patient’s original myeloma clone (CD138+, MUM1+, CD56+, CD117+, κ-restricted). Taken together with the clinical history, these findings support the interpretation of this lesion as an extramedullary relapse of multiple myeloma despite marrow remission, rather than a de-novo lymphoma. We acknowledge that clonality studies, such as IGH rearrangement or NGS on the gastric biopsy, were not performed, which is a limitation.
The decision for the treatment pathway arises from multidisciplinary team discussions, and it is usually in the form of local radiotherapy and surgery for cases of primary extramedullary plasma cell neoplasms and systemic chemotherapy for secondary neoplasms. Available data on treatment outcomes for EMP are almost entirely derived from retrospective studies (8). Some agents and combinations have shown some efficacy; however, as expected based on known prognoses, this is typically lower than for MM patients without extramedullary involvement. The lack of prospective studies makes it challenging to provide strong recommendations for any treatment approach. A recent expert consensus review has offered potential treatment strategies for consideration (9). For upfront treatment of EMP in transplant-ineligible patients, the addition of daratumumab to bortezomib, melphalan, and prednisone (VMP) or VRd was suggested (9, 10). In patients eligible for transplantation, intensive anti-myeloma and anti-lymphoma treatment regimens, such as bortezomib, thalidomide, and dexamethasone (VTD) or VRd in combination with stem cell transplant (SCT), are considered a theoretical option. For patients experiencing a relapse, suggested treatments are often based on lymphoma-like regimens, including PACE (cisplatin, doxorubicin, cyclophosphamide, etoposide), DCEP (dexamethasone, cyclophosphamide, etoposide, and cisplatin), or Dexa-BEAM (dexamethasone, carmustine, etoposide, cytarabine, melphalan) (9). However, the duration of response to these treatments is typically four months or less.
The current response criteria, including minimal residual disease assessment, should be applied to patients with EMP. The first evaluation of EMD—identified through PET/CT (considering size and metabolic uptake) or MRI—should take place three months after the initiation of treatment, with additional assessments at the discretion of the physician (9). It is recommended that both baseline and follow-up evaluations utilize the same imaging technique to minimize variability between different methods. To declare a complete remission, all evidence of EMP must have disappeared according to the proposed standardization for the metabolic complete remission definition (7). Additionally, both serum and urine M proteins should be undetectable by immunofixation. Bone marrow must test negative for minimal residual disease using flow cytometry to determine MRD negativity.
Prospective data from patients with clearly defined EMP is essential for accurately evaluating treatment outcomes. Conducting adequately powered trials to assess outcomes in this rare group of patients poses a significant challenge. In such situations, including these patients in clinical trials for subgroup analysis of treatment effects, based on pre-defined hypotheses, may yield the most reliable evidence. Recent trials of novel MM treatments indicate that patients with EMD are increasingly being studied in greater detail, which is a positive trend that should be encouraged.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CD: Writing – review & editing, Investigation, Writing – original draft. FA: Writing – review & editing, Writing – original draft, Methodology. SB: Writing – review & editing, Writing – original draft. AM: Writing – review & editing, Visualization, Writing – original draft. HS: Writing – review & editing, Methodology, Supervision, Conceptualization, Visualization. GG: Writing – original draft, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alexiou C, Kau RJ, Dietzfelbinger H, Kremer M, Spiess JC, Schratzenstaller B, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. (1999) 85:2305–14. doi: 10.1002/(SICI)1097-0142(19990601)85:11<2305::AID-CNCR2>3.0.CO;2-3
2. Usmani SZ, Heuck C, Mitchell A, Szymonifka J, Nair B, Hoering A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematological. (2012) 97.11:1761. doi: 10.3324/haematol.2012.065698
3. Sevcikova S, Minarik J, Stork M, Jelinek T, Pour L, and Hajek R. Extramedullary disease in multiple myeloma - controversies and future directions. Blood Rev. (2019) 36:32–9. doi: 10.1016/j.blre.2019.04.002
4. Talamo G, Cavallo F, Zangari M, Barlogie B, Lee CK, Pineda-Roman M, et al. Clinical and biological features of multiple myeloma involving the gastrointestinal system. Haematologica. (2006) 91:964–7.
5. Iosif E, Rees C, Beeslaar S, Shamali A, Lauro R, and Kyriakides C. Gastrointestinal bleeding as initial presentation of extramedullary plasma cell neoplasms: A case report and review of the literature. World J Gastrointest Endosc. (2019) 11:308–21. doi: 10.4253/wjge.v11.i4.308
6. Rosiñol L, Cibeira MT, Bladé J, Esteve J, Aymerich M, Rozman M, et al. Extramedullary multiple myeloma escapes the marrow microenvironment and demonstrates poor prognosis. Haematologica. (2004) 89:851–6.
7. Zamagni E, Nanni C, Dozza L, Carlier T, Bailly C, Tacchetti P, et al. Standardization of 18F-FDG-PET/CT according to Deauville criteria for metabolic complete response definition in newly diagnosed multiple myeloma. J Clin Oncol. (2021) 39:116–25. doi: 10.1200/JCO.20.00386
8. Bladé J, Beksac M, Caers J, Jurczyszyn A, von Lilienfeld-Toal M, Moreau P, et al. Extramedullary disease in multiple myeloma: a systematic literature review. Blood Cancer J. (2022) 12:45. doi: 10.1038/s41408-022-00643-3
9. Rosiñol L, Beksac M, Zamagni E, Van de Donk NWC., Anderson KC, Badros A, et al. Expert review on soft-tissue plasmacytomas in multiple myeloma: definition, disease assessment and treatment considerations. Br J Haematol. (2021) 194:496–507. doi: 10.1111/bjh.17338
10. Beksac M, Tuglular T, Gay F, Mina R, Katodritou E, Unal A, et al. P872: efficacy of daratumumab plus bortezomib, cyclophosphamide and dexamethasone in patients with multiple myeloma presenting with extramedullary disease: a european myeloma network study (emn19). Hemasphere. (2023) 7:e994593a. doi: 10.1097/01.HS9.0000970392.99459.3a
Keywords: multiple myeloma, extramedullary plasmacytoma, gastric plasmacytoma, relapse, autologous stem cell transplant
Citation: Dirican CD, Ajayi F, Al Mardini A, Bijoy S, Shaaban H and Guron G (2025) Case Report: Extramedullary plasmacytoma of the stomach: a rare manifestation of relapsed multiple myeloma. Front. Hematol. 4:1655966. doi: 10.3389/frhem.2025.1655966
Received: 29 June 2025; Accepted: 22 September 2025;
Published: 10 October 2025.
Edited by:
Bhavana Bhatnagar, West Virginia University, United StatesReviewed by:
Srinivas Devarakonda, The Ohio State University, United StatesSaja Asakrah, Emory University, United States
Copyright © 2025 Dirican, Ajayi, Al Mardini, Bijoy, Shaaban and Guron. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Canan D. Dirican, Y2FuYW5kaWxheWRpcmljYW5AZ21haWwuY29t
 Canan D. Dirican
Canan D. Dirican Folasade Ajayi2
Folasade Ajayi2