- 1Department of Pharmacy, The Ohio State University, Columbus, OH, United States
- 2Division of Hematology, The Ohio State University, Columbus, OH, United States
- 3The Ohio State University College of Pharmacy, Columbus, OH, United States
- 4Department of Medicine, Division of Hematology, Medical Oncology and Palliative Care, The University of Wisconsin Carbone Center, Madison, WI, United States
Venetoclax creates ongoing challenges when combined with posaconazole due to a known drug-drug interaction. Herein, we investigated the safety between venetoclax 100mg and 70mg when administered with posaconazole in acute myeloid leukemia in this single-center, retrospective comparative analysis. Primary safety endpoints were incidence/duration of cytopenias and incidence of tumor lysis syndrome during the first treatment cycle. A total of 113 patients received venetoclax 100mg while 32 patients received 70mg. Comparing venetoclax 100mg vs 70mg, no statistically significant differences were seen in grade 3 neutropenia (89.4% vs 84.4%, p=0.53), grade 4 neutropenia (88.5% vs 87.5%, p=1.0), median duration in days of grade 4 neutropenia (23 [range 1–105] vs 28 [range 1-81], p=0.35), grade 3/4 anemia (88.5% vs 84.4%, p=0.55), grade 3/4 thrombocytopenia (81.4% vs 87.5%, p=0.42), or tumor lysis syndrome (2.7% vs 6.3%, p=0.30). In adult patients with acute myeloid leukemia, a target dose of venetoclax 100mg with posaconazole may be a safe alternative. Further studies assessing dose optimization are warranted.
1 Introduction
Candidacy for intensive induction chemotherapy has often been correlated with prognosis in acute myeloid leukemia (AML), with historically limited treatment options remaining in the absence of targetable mutations for older adults and unfit patients (1). Fortunately, a new standard of care has propelled venetoclax (VEN) with hypomethylating agent (HMA) or low-dose cytarabine (LDAC) therapy to the forefront, demonstrating favorable response and better tolerability for these complex populations (2, 3). However, this regimen is not without risk and carries evolving intricacies in the management of toxicities and navigation of drug-drug interactions (4).
One noteworthy interaction involves posaconazole (POSA), a frequently used prophylactic triazole antifungal agent, which is preferred for its survival benefit observed in patients with AML exhibiting prolonged neutropenia (5). POSA introduces complexity due to its strong inhibition of the cytochrome P(CYP)450 3A4 pathway and the p-glycoprotein (p-gp) transporter, both of which are integral to VEN metabolism. As such, the best approach to dosing VEN with concomitant POSA is debatable. Dosing recommendations provided in the package insert offer some guidance, however the overall availability of pharmacokinetic (PK) data is limited. A frequently referenced in vivo PK study involving 12 patients recommended reducing VEN by at least 75% from the target 400mg when combined with POSA, which results in a dose of 100mg daily (6). Conversely, this differs from the current FDA-recommended dose of VEN 70mg when given with POSA.
Clinical experience has sustained the need for reevaluation of the proposed dose adjustment recommendations. In 2021, our institution adopted the practice to use VEN 100mg daily with POSA rather than the daily dose of 70mg recommended in the package insert. The rationale was based on the known PK of strong CYP3A4 inhibitors more generally, such as voriconazole, demonstrating inhibition comparable to POSA in other settings. However, overall conclusions on the optimal VEN dosing strategy with POSA remain mixed (7–10). As several of the oral agents now available for AML are significantly metabolized through the CYP3A4 pathway, the topic of how to safely administer preventative triazole antifungal agents that inhibit these metabolic enzymes continues to be relevant (11). Aside from drug-drug interactions, the shift to VEN 100mg also addresses the practicality of the dosing regimen with aim to reduce the multiple dosage strengths required to administer a dose of 70mg and the associated risk of medication-related errors. Although this change has occurred across some centers already, clinical data reviewing the safety and efficacy between these two doses of VEN is limited.
Thus, this study investigated the safety of VEN 100mg daily post-intervention as compared to the originally recommended 70mg daily when administered with POSA at our institution and aimed to provide a real-world clinical evaluation of the two dosing strategies.
2 Materials and methods
2.1 Study design and description of participants
The purpose of this investigation was to describe the effects surrounding our institutional practice change with VEN dosing and POSA. This study was designed as a retrospective cohort review of adult patients aged 18 years or older with newly diagnosed (ND) or relapsed/refractory (R/R) AML treated with VEN 100mg or 70mg combined with HMA or LDAC plus concurrent POSA at The Ohio State University Comprehensive Cancer Center between November 2018 and February 2024. Patients were eligible if they received a minimum of seven days of VEN and at least one dose of POSA at the time of treatment initiation. Patients were excluded if they had a diagnosis of acute promyelocytic leukemia or myelodysplastic syndromes, were incarcerated, pregnant or enrolled on clinical trial. Patients were also excluded if they received VEN in combination with agents other than HMA or LDAC. The dosing of POSA followed standard guidelines per package insert.
2.2 Data collection and measurements
Primary safety composite endpoints were the incidence/duration of cytopenias and the incidence of tumor lysis syndrome (TLS) during the first cycle. This study placed emphasis on the first treatment cycle with the intent to characterize dosing at a point where drug toxicities and related complications were suspected to be highest. Secondary endpoints included combined complete remission (CR), CR with incomplete count recovery (CRi), and CR with partial hematologic recovery (CRh) within the first two cycles as well as overall survival (OS) and progression-free survival (PFS). Safety evaluations were restricted to cycle 1 (C1) to capture the heterogeneity seen in dosing in the initial treatment cycle and efficacy evaluations occurred up to the start of cycle 3 (C3) to allow time for a minimum of one bone marrow assessment to be performed.
2.3 Statistics
The comparison between VEN 100mg and 70mg was conducted using the chi-squared test or Fisher’s exact test for categorical variables, and the Wilcoxon rank sum test was used for continuous variables. PFS was defined as the time from treatment initiation to progression, relapse or death. OS was defined as the time from treatment initiation to death from any cause. Duration of response (DOR) was calculated from the date when CR/CRi/CRh was first achieved and defined as the length of time from the first documented response to relapse or death. All time-to-event outcomes, including PFS, OS, and DOR, were censored at the last date patients were known to be event-free and estimated using the Kaplan-Meier method.
3 Results
A total of 145 patients were included; 113 patients received VEN 100mg and 32 patients received 70mg. Baseline demographics were well-balanced between groups. Median age was 69.5 years, 55.9% were male, 60.0% had a performance status (Eastern Cooperative Oncology Group) of 1 at treatment initiation, and median follow-up was 13.2 months. Disease type was characterized as primary AML in 63.5%, ND in 69.0%, and complex karyotype in 28.5% of the total cohort. Disease risk, per European LeukemiaNet (ELN) 2022, was adverse in 64.9% and 68.8%, intermediate in 26.1% and 6.3%, and favorable in 9.0% and 25.0% (p=0.0081) of patients treated with VEN 100mg and 70mg, respectively. According to ELN 2024, more patients in both 100mg and 70mg groups were considered favorable (57.5% and 46.9%), respectively, but there was a higher proportion of adverse risk disease among patients treated with the lower dose (VEN 70mg 31.3% vs 100mg 18.6%, p=0.30) (Table 1). A greater percentage of patients with R/R vs ND disease expressed mutations in NPM1 (17.1% vs 12.8%, p=0.57), IDH1 (17.1% vs 5.1%, p=0.07), and IDH2 (17.1% vs 10.3%, p=0.36) genes, respectively.

Table 1. Patient demographics outlined according to patient- and disease-specific characteristics between treatment groups.
Most patients within the total cohort received azacitidine/VEN (71.0%). A minority received decitabine/VEN (28.3%), and one patient received LDAC/VEN. Patients were started on chemotherapy and POSA simultaneously in most cases (74.5%), consistent with institutional practice, and the patients already on POSA were often R/R cases transitioning from other treatments. Median duration in days of POSA was similar between 100mg and 70mg groups (74 [range 2-2227] vs 64 [range 7-613], p=0.53), respectively; however, POSA was discontinued more often in lower dosed VEN (70mg 31.2% vs 100mg 15.0%, p=0.04). Regarding POSA discontinuation, hepatotoxicity was reported as the reason for cessation in 8 out of 27 events (29.6%, p=0.77). Other common reasons for discontinuation included QTc prolongation (14.8%), drug interactions (3.7%), cytopenias (3.7%), and transition to hospice care or death (29.6%). There were no definitive cases of invasive fungal infections documented, but two cases of suspected fungal infections were reported (7.4%) leading to a change in empiric antifungal therapy.
Comparing VEN 100mg vs 70mg in C1, no statistically significant differences were seen in grade 3 neutropenia (89.4% vs 84.4%, p=0.53), grade 4 neutropenia (88.5% vs 87.5%, p=1.00), median duration in days of grade 4 neutropenia (23 [range 1-105] vs 28 [range 1-81], p=0.35), grade 3/4 anemia (88.5% vs 84.4%, p=0.55), grade 3/4 thrombocytopenia (81.4% vs 87.5%, p=0.42), or TLS (2.7% vs 6.3%, p=0.30). Further characterizations of toxicity are described in Table 2.

Table 2. Toxicity events recorded through the first treatment cycle and subsequent treatment and/or dosing modifications implemented.
Depiction of VEN dosing was notable between groups. Most patients started C1 with the plan to receive 28 days of therapy. Of the 113 patients in the VEN 100mg group, nine patients were planned for 21 days, six patients planned for 14 days, and one patient planned for 7 days of VEN. However, for the 32 patients in the 70mg group, only two patients were set to receive less than 28 days of VEN from initiation. Of the total patients that reached cycle 2 (C2), most of the dose reductions to the VEN course in C2 occurred in the 100mg group, with twenty-six patients reduced to 21 days, thirteen patients reduced to 14 days, and two patients reduced to 7 days, compared to only three patients that were reduced to 21 days in the 70mg group. Chemotherapy dose reductions during C1, which were described as an unplanned decrease in either the intended dose or number of days of chemotherapy per cycle, were significantly more frequent in patients dosed with less VEN (70mg 37.5% vs 100mg 14.2%, p=0.0031), while dose delays, defined as chemotherapy that was ordered as intended but postponed in administration to later in the cycle, remained similar (100mg 9.7% vs 70mg 12.5%). Reasons for dose delay or reduction were most attributed to infection concerns. For patients who reached C2 and further reached C3, a delay in commencement of the cycle was noted in about half of the patients across groups (100mg 51.8% vs 70mg 64.0% and 100mg 51.0% vs 70mg 40.0%), for cycles 2 and 3 respectively.
At least one episode of febrile neutropenia occurred in 69 patients total, with similar incidence seen between VEN 100mg and 70mg (47.8% vs 46.9%, p=0.93). In the entire cohort, only 47 (32.4%) patients started treatment in the outpatient setting, among which 8 (17.0%) required hospitalization for infection management during C1: 3 in the VEN 100mg group and 5 in the VEN 70mg group, but there were no statistically significant differences between groups (100mg 9.4% vs 70mg 33.3%, p=0.09). Granulocyte-colony stimulating factor (GCSF) was administered in 15 patients total across the first three cycles, where all but one of these patients were treated with VEN 100mg.
Treatment response and outcomes are described in Table 3. Among evaluable patients who received VEN 100mg vs 70mg, combined CR/CRi/CRh by the beginning of C3 was achieved in 50.0% vs 40.0% (p=0.42), with a median duration of response in months (95% CI) of 10.3 (5.4-20.8) vs 11.7 (2.3-NR), (p=0.76), respectively. Comparison between ND and R/R patients revealed similar rates of response. However, median DOR in months was numerically longer in the R/R subgroup for both VEN 100mg and 70mg [R/R 20.8 (1.5-NR) vs ND 8.2 (3.9-NR)] and [R/R NR (2.3-NR ) vs ND 11.7 (3.8-NR)], respectively. Of the 24 patients who went on to receive a hematopoietic stem cell transplant following chemotherapy, most of these patients received VEN 100mg [23 (21.3%) 100mg vs 1 (3.1%) 70mg, p=0.02] with 16 patients achieving combined CR/CRi/CRh at time of transplant, and within the 100mg cohort a significantly higher number of patients with R/R (n=33) vs ND (n=75) disease underwent transplant (33.3% vs 16.0%, p=0.04). There were no significant differences in survival outcomes in months, including median PFS (5.7 vs 4.2, p=0.53) and OS (7.0 vs 6.2, p=0.48), as illustrated in Figures 1A, B, respectively.
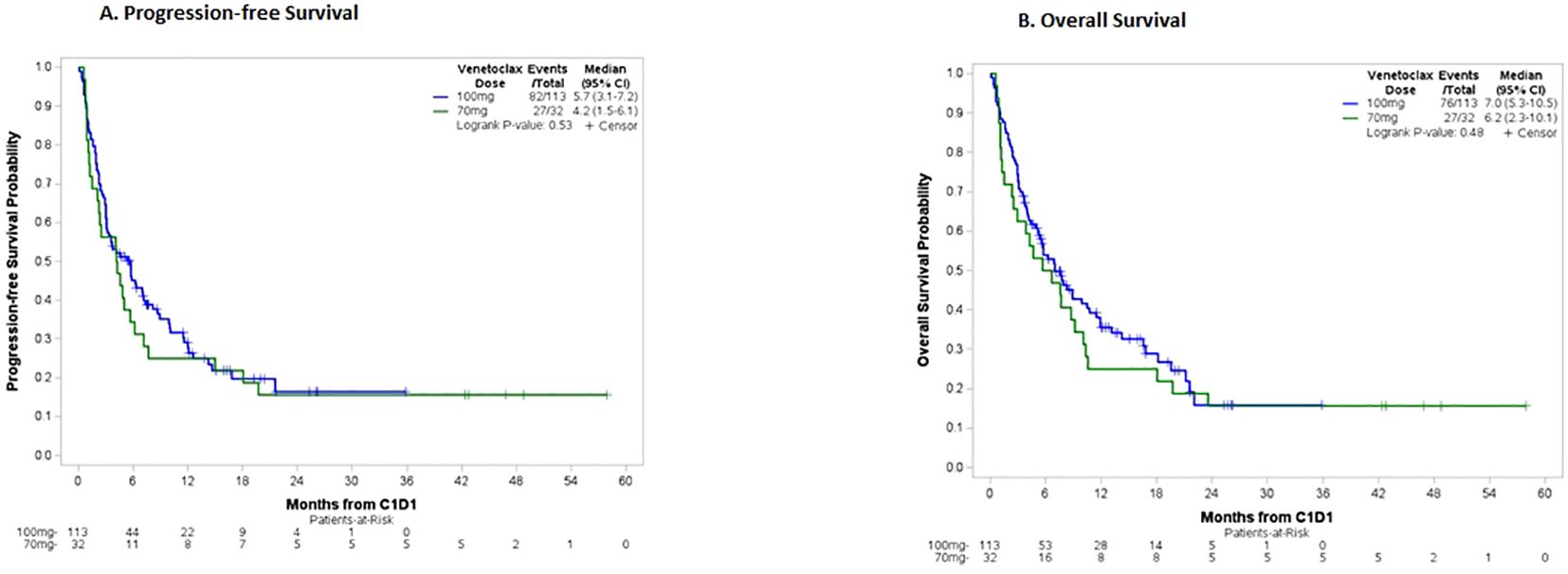
Figure 1. (A) Progression-free survival, total cohort. Patients in the 100mg group had a median PFS of 5.7 months vs 4.2 months in the 70mg group. (B) Overall survival, total cohort. Patients in the 100mg group had a median OS of 7.0 months vs 6.2 months in the 70mg group.
4 Discussion
When comparing VEN 100mg vs 70mg in ND and R/R AML, no statistically significant differences were seen in incidence and duration of cytopenias nor TLS during C1. These results along with existing unanswered questions may further support a reevaluation of current FDA-approved dose adjustments.
Optimal methods in dosing VEN to account for drug interactions remain of interest given the limited PK data published at the time of initial commercial availability. This curiosity sparked discussions surrounding institutional practice, which ultimately led to our internal comparative analysis. Medication safety was a significant driver in these discussions and the adjustment to a dose of 100mg at our center as VEN is currently only available in 10mg-, 50mg-, and 100mg- strength tablets, dramatically complicating the tablets needed to total 70mg when used with POSA as labeled. Furthermore, with VEN also having an indication in CLL that utilizes a different ramp-up schedule, confusion soon arose when the same manufacturer packaging had to be adapted to the diagnosis and treatment of AML. The results of this study suggest that VEN 100mg may be an effective and safe alternative to VEN 70mg when administered concurrently with POSA, which facilitates patient convenience and medication safety.
Some of our institutional experience with using VEN 100mg vs 70mg may outline the gradual trends seen in management of toxicities and supportive care. Our study focused on VEN dose adjustments during C1 to gain better understanding of initial management in patients newly started on therapy, with prolonged cytopenias seen in both groups. Most patients started out on a full course of 28 days regardless of a VEN target dose of 100mg or 70mg. For those that were started on a reduced regimen of less than 28 days for C1, all but two of these patients were in the VEN 100mg group. At the start of C2, many patients were reduced to 21 days of VEN or even further to 7 or 14 days, and these changes occurred more frequently for those that received VEN 100mg. This could be explained by the dosing trends observed parallel to the growing familiarity with VEN in subsequent cycles for patients in remission. As a result, this increased awareness may have led to a more rapid adjustment in duration for those that received 100mg, rather than simply due to patients receiving a higher starting dose.
Additionally, there has been greater standardization in timing of bone marrow procedures for patients on VEN/HMA regimens compared to initial use. Currently, most patients receive their first disease assessment bone marrow biopsy following C1, which can generate earlier dose adjustments at the time of the second cycle for patients achieving disease control with the first cycle. Dose reductions from the original 28-day cycle of VEN is an area that greatly requires further research. Current clinical trials are evaluating the ideal length of VEN during C1, such as 14 days of VEN for a 28-day cycle. Depending on these final conclusions, frontline dosing strategies for VEN-based regimens may further change (12).
Considering extended cytopenias were observed in both groups, further investigation is needed to assess whether differences in myelosuppression become evident after the first treatment cycle. Dose reductions mid-cycle were significantly higher with VEN 70mg for C1. Experience acquired in toxicity management over time may describe why dose reductions were seen more frequently with earlier use of lower VEN dosing before the conversion to 100mg was made. Growth factor support has also become increasingly frequent at our center in settings of AML treatment without evidence of active disease, with six patients who received GCSF as early as C1 in this study. Interestingly, 5 out of 6 of these patients received VEN 100mg, but this may be because GCSF use was not well established in the earlier years of VEN which was when a dose of 70mg was more likely to be used. The integration of GCSF at our center remains variable. An analysis of the VIALE-A and VIALE-C trials explored the use of post-remission GCSF and found an improvement in treatment delays between cycles without adverse impact on DOR or OS (13). These findings among future studies may lead to more consistent incorporation of GCSF support in VEN-containing regimens as well as streamline management of cytopenias moving forward.
Surprisingly, our study observed lower response rates relative to the pivotal VIALE-A study [CR/CRi (VEN 100mg vs 70mg, 50.0% vs 40.0%) vs (Dinardo, et al, 66.4% for patients receiving VEN + azacitidine)]. Median OS also appeared significantly shorter in our study (VEN 100mg vs 70mg, 7.0 vs 6.2 months) vs 14.7 months for patients receiving VEN + azacitidine on VIALE-A (2). This may be explained at least in part by cytogenetic and molecular risk stratification. In our study, 64.9% of patients in the VEN 100mg cohort and 69.0% in the VEN 70mg cohort had adverse baseline disease risk according to ELN 2022 guidelines. In the VIALE-A trial, only 36% of patients had adverse risk cytogenetics according to the NCCN guidelines for AML, version 2.2016. These two risk stratification systems differ in that the ELN guidelines incorporate both cytogenetic and molecular findings, but it is notable that the patients in our cohort appeared to have higher rates of adverse risk disease based on these models. Compared to ELN 2022 stratification, a higher percentage of patients in our study had favorable risk disease according to the ELN 2024 criteria for patients receiving lower intensity VEN-based regimens. However, the use of these classification tools is limited in this study given our mixed populations differing from the settings in which they were validated. Incorporation of this updated classification, along with newer tools, into clinical practice and research remains an evolving area of discussion.
In addition to newly diagnosed AML, our study captured patients treated with VEN and HMA or LDAC in R/R settings. These R/R patients appeared to perform better with numerically longer DOR, PFS, and OS compared to frontline treatment at both 100mg and 70mg target doses, although not statistically significant. The retrospective nature of this study affects our ability to make definitive conclusions; however, the etiology is likely multi-factorial. We observed higher transplant rates in the R/R setting, which was significantly different between the VEN 100mg vs 70mg cohorts. We also observed higher rates of NPM1 and IDH1/2 mutations in R/R patients treated with VEN 100mg, which was not statistically significant. This suggests that VEN-sensitive disease or transplant contributed to survival in the R/R cohort. Overall, there were no statistically significant differences in treatment settings between VEN 100mg and 70mg which could support that either dose may be appropriate regardless of line of therapy.
This study was associated with limitations, one of which being sample size with a total of 145 patients included, but a skewed distribution of patients receiving 100mg (n=113) vs 70mg (n=32) of VEN. Although there were a larger number of patients who received VEN 100mg, baseline demographics were well-balanced between groups. Treatment with VEN 100mg vs 70mg was ultimately driven by our institutional standard at the time. The heavier placement of patients on the VEN 100mg arm following our practice change also appears to correlate with the exponential increase in usage of VEN with it now recognized as the standard of care for intensive chemotherapy ineligible patients with AML. Similarly, attempts have been made to provide consistency among antifungal selection over the last several years at our center which could have introduced some treatment selection bias. Hesitancy with VEN 70mg and POSA may have influenced selection of other antifungal agents to avoid this level of interaction altogether. Of the 501 total patients screened for this study, 296 patients were excluded due to receipt of an antifungal other than POSA, with many of these patients treated in the earlier years of VEN. Another limitation identified, as with any oral medication review, is that adherence could not be verified outside of hospital or clinic administrations. Finally, setting toxicity evaluation to C1 only may be considered a limitation, but creates opportunities for future research. Evaluation beyond the first cycle in subsequent studies could provide additional insight into delayed toxicities and the trajectory of treatment schedules including dose reductions or delays.
Many opportunities for consideration surrounding VEN dosing and antifungal prophylaxis in AML still exist. One such topic of investigation is whether a VEN ramp-up is needed when TLS risk appears to be low. One small retrospective study investigated this question and found no clinical or laboratory differences in TLS despite removing the ramp-up (14). Standard practice for TLS prevention includes a ramp-up dosing schedule for all patients and implementing prophylactic measures including a urate-lowering agent, intravenous fluids, and increased laboratory monitoring, as modeled in the key VEN studies (15). In our study, only one patient received VEN without a ramp-up due to relatively low concerns for TLS. The low incidence observed remains consistent with known patterns of VEN in AML, therefore, it could be reasonable to explore modifications to the ramp-up schedule and/or consider reserving for patients with higher risk factors only.
Given that the most common reason for POSA discontinuation in our study was hepatotoxicity, the question remains whether this should be the agent of choice initially. It is well established that triazole antifungals can adversely affect the liver, but the degree of hepatotoxicity may differ among agents (16). Our data revealed that POSA was discontinued more often in patients who received VEN 70mg. While the reason for discontinuation was mostly hepatic-related, the reason for this higher occurrence with 70mg is puzzling and believed to be unassociated with VEN exposure. Instead, this relationship could be speculated within the realm of earlier clinical practice with VEN and POSA where a more cautious approach may have been employed to manage toxicities from a newly approved therapy. Separately, another reason for POSA discontinuation in many cases was the transition to comfort measures which coincidentally was more prevalent in the VEN 70mg group. Finally, an atypical case of cytopenias where POSA was discontinued was later attributed to mitigation of VEN hematologic toxicity overall.
Of the eight cases of hepatoxicity that led to POSA discontinuation in our study, the suspected etiology was medication-induced resulting in isolated transaminitis in five patients, hyperbilirubinemia in two patients, and one patient with transaminitis possibly secondary to hepatic congestion in the setting of fluid overload. It is common practice to hold POSA for significant hepatotoxicity while etiology is investigated, which necessitates a change in the VEN dose. What remains unclear, however, is the threshold at which POSA should be held and the best course of action when there is no other explainable cause. Nonetheless, it is understood that antifungal prophylaxis remains vital in acute leukemia management especially during periods of neutropenia, but how this is accomplished may continue to have increasing variability as clinical challenges are encountered with VEN-containing regimens.
Early toxicities observed with VEN 70mg and POSA have consequently led to the exploration of lower initial doses of VEN and whether that can minimize the duration and severity of toxicities without compromising efficacy. A recently published prospective PK study of older patients with ND AML suggested that VEN 100mg dosing with POSA may lead to supratherapeutic concentrations and that reducing VEN even further to 50mg with POSA may produce similar concentrations to VEN 400mg in the absence of other drug interactions. Notably, VEN 50mg was the dose used with concurrent strong CYP3A4 inhibitors in the pivotal VIALE-A study, but using 50mg of VEN in combination with POSA is not common in current clinical practice. Furthermore, the investigators in this study experimented with VEN trough levels which could be offered as a surrogate for therapeutic drug monitoring (TDM) in the future, potentially reducing the gap between VEN dosing and metabolism given its interpatient variability (17).
Other studies have proposed a similar utility for TDM in VEN dosing which could be a promising solution to many of the current dosing concerns (18). For example, TDM could measure VEN in settings where one or more concurrent drug interactions exist which are often encountered in patients with multiple comorbidities. These same populations also happen to be those most often considered appropriate for nonintensive VEN-containing regimens. VEN dosing and adherence to the ramp-up schedule continues to support the need for multidisciplinary perspectives to ensure appropriate patient and provider education and comprehension in both inpatient and outpatient settings.
Upon retrospective review, our study found no difference in safety or efficacy endpoints between VEN 100mg and 70mg with concurrent POSA in patients with AML, suggesting VEN 100mg may be a viable alternative to current FDA labeling of 70mg, especially when logistical challenges and safety concerns among dosing administration are encountered during the first treatment cycle. This study also found no significant difference in response rates, PFS, or OS for patients receiving VEN 100mg vs 70mg in this context. However, optimal strategies in management of ongoing cytopenias as evidenced in both groups, including initial dose reductions beyond 70mg and dosing duration, remain unknown making future investigation necessary. Larger studies and additional PK analyses evaluating the effects of POSA with VEN and its efficacy and safety implications at multiple dose levels throughout subsequent cycles will help provide further understanding of these drug-drug interactions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ohio State University Institutional Review Board and Clinical Scientific Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Informed consent was waived by The Ohio State University Institutional Review Board because this study was designed as a retrospective chart review.
Author contributions
TF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. AH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – review & editing. YH: Data curation, Formal analysis, Methodology, Resources, Writing – review & editing. PK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – review & editing. AW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – review & editing. MF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – review & editing. KM: Data curation, Writing – review & editing. NH: Data curation, Investigation, Methodology, Resources, Writing – review & editing. GB: Resources, Writing – review & editing. JB: Resources, Writing – review & editing. BB: Resources, Writing – review & editing. UB:Resources, Writing – review & editing. AE: Resources, Writing – review & editing. NG: Resources, Writing – review & editing. SH: Resources, Writing – review & editing. KL: Resources, Writing – review & editing. SL: Conceptualization, Methodology, Writing – review & editing. ML: Resources, Writing – review & editing. AM: Resources, Writing – review & editing. AS: Resources, Writing – review & editing. JJ: Formal analysis, Investigation, Methodology, Resources, Writing – review & editing, Conceptualization. KS: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Writing – review & editing. KK: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
TF: Pfizer, Inc.: Consultancy; Bristol Myers Squibb, Inc.: Honoraria. AW: Jazz Pharmaceuticals: Consultancy. JB: Syndax Pharmaceuticals, Inc.: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Other: consulting fees; Astellas: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Other: consulting fees. UB: Ryvue: Other: IDMC; Incyte: Consultancy; Beigene: Membership on an entity’s Board of Directors or advisory committees; Daiichi Sankyo: Consultancy; Takeda: Other: IDMC; Novartis: Consultancy; Vincerx Pharma: Membership on an entity’s Board of Directors or advisory committees; Astellas: Consultancy; Agios: Membership on an entity’s Board of Directors or advisory committees; BMS: Consultancy; Genentech: Consultancy; Abbvie: Consultancy; Rigel: Consultancy; Sumitomo: Consultancy. A-KE: AstraZeneca US: Membership on an entity’s Board of Directors or advisory committees; OncLive: Honoraria; VJ HemeOnc: Honoraria; Dava Oncology: Honoraria; Karyopharm Therapeutics: Other: Spouse employment; GTC: Honoraria. AM: BMS: Membership on an entity’s Board of Directors or advisory committees; Novartis: Membership on an entity’s Board of Directors or advisory committees; Treadwell Therapeutics: Membership on an entity’s Board of Directors or advisory committees; Daiichi Saynko: Membership on an entity’s Board of Directors or advisory committees; Foghorn Therapeutics: Membership on an entity’s Board of Directors or advisory committees; Leukemia and Lymphoma Society Beat AML Study: Other: Senior Medical Director. JJ: Sobi, Inc.: Honoraria. KK: Rigel Pharmaceuticals, Inc.: Honoraria, Consultancy.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. National Cancer Institute. Acute myeloid leukemia treatment (PDQ)—health professional version(2025). Available online at: https://www.cancer.gov/types/leukemia/hp/adult-aml-treatment-pdq (Accessed March 3 2025).
2. Dinardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. (2020) 383:617–29. doi: 10.1056/NEJMoa2012971
3. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute myeloid leukemia. Version 4.2023. (2023).
4. Abbvie Inc. Venclexta (venetoclax). U.S. Food and Drug Administration website (2016). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208573s000lbl.pdf.
5. Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. (2007) 356:348–59. doi: 10.1056/NEJMoa061094
6. Agarwhal SK, DiNardo CD, Potluri J, Dunbar M, Kantarjian HM, Humerickhouse RA, et al. Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther. (2017) 39:359–67. doi: 10.1016/j.clinthera.2017.01.003
7. Rausch CR, DiNardo CD, Maiti A, Jammal N, Kadia TM, Marx KR, et al. Duration of cytopenias with concomitant venetoclax and azole antifungals in acute myeloid leukemia. Cancer. (2021) 127:2489–99. doi: 10.1002/cncr.33508
8. Jonas BA and Pollyea DA. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia. (2019) 33:2795–804. doi: 10.1038/s41375-019-0612-8
9. Sienkiewicz BM, Łapiński ŁChecktae, and Wiela-Hojeńska A. Comparison of clinical pharmacology of voriconazole and posaconazole. Contemp Oncol (Pozn). (2016) 20:365–73. doi: 10.5114/wo.2016.64594
10. Brüggemann RJ, Alffenaar JW, Blijlevens NM, Billaud EM, Kosterink JG, Verweij PE, et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. (2009) 48:1441–58. doi: 10.1086/598327
11. Stemler J, Koehler P, Maurer C, Müller C, and Cornely OA. Antifungal prophylaxis and novel drugs in acute myeloid leukemia: the midostaurin and posaconazole dilemma. Ann Hematol. (2020) 99:1429–40. doi: 10.1007/s00277-020-04107-1
12. Borate U, Huang Y, Zeidner JF, Swords RT, Koenig KL, Stein EM, et al. A randomized phase 2 trial of 28-day (Arm A) versus 14-day (Arm B) schedule of venetoclax + Azacitidine in newly diagnosed acute myeloid leukemia patients ≥ 60 years. Blood. (2024) 144:2907.2. doi: 10.1182/blood-2024-210967
13. DiNardo CD, Pratz KW, Panayiotidis P, Wei X, Vorobyev V, Illes A, et al. The impact of post-remission granulocyte colony-stimulating factor use in the phase 3 studies of venetoclax combination treatments in patients with newly diagnosed acute myeloid leukemia. Am J Hematol. (2024) 100:185–8. doi: 10.1002/ajh.27515
14. Beun Z, Thompson E, Zhan T, Keiffer G, Kasner M, and Wilde L. Tumor lysis syndrome in patients with AML initiated on venetoclax-based treatment without a dose ramp-up. Blood. (2023) 142:3720. doi: 10.1182/blood-2023-184610
15. Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. (2020) 135:2137–45. doi: 10.1182/blood.2020004856
16. O’Flynn R, Zhou YP, Waskin H, Leong R, and Straus W. Hepatic safety of the antifungal triazole agent posaconazole: characterization of adverse event reports in a manufacturer’s safety database. Expert Opin Drug Saf. (2022) 21:1113–20. doi: 10.1080/14740338.2022.2047177
17. Kawedia JD, Rausch CR, Liu X, Qiao W, DiNardo CD, Daver N, et al. Prospective pharmacokinetic evaluation of venetoclax in AML supports re-evaluation of recommended dose adjustments with azole antifungals. Am J Hematol. (2025) 100(4):740–3. doi: 10.1002/ajh.27613
Keywords: venetoclax, posaconazole, leukemia, dosing, interaction, myeloid
Citation: Freeman T, Habib A, Huang Y, Kumar PS, Waller A, Fleming M, McMillan K, Hartzler N, Behbehani G, Blachly JS, Blaser BW, Borate U, Eisfeld A-K, Grieselhuber NR, Handa S, Larkin K, Lee S, Long M, Mims A, Srisuwananukorn A, Jain J, Sahasrabudhe K and Koenig K (2025) Real-world experience of venetoclax target dosing with concomitant posaconazole in adult patients with acute myeloid leukemia. Front. Hematol. 4:1664425. doi: 10.3389/frhem.2025.1664425
Received: 11 July 2025; Accepted: 25 August 2025;
Published: 23 September 2025.
Edited by:
Francesco Di Raimondo, University of Catania, ItalyReviewed by:
Calogero Vetro, Bolzano Central Hospital, ItalyFabio Guolo, San Martino Hospital (IRCCS), Italy
Copyright © 2025 Freeman, Habib, Huang, Kumar, Waller, Fleming, McMillan, Hartzler, Behbehani, Blachly, Blaser, Borate, Eisfeld, Grieselhuber, Handa, Larkin, Lee, Long, Mims, Srisuwananukorn, Jain, Sahasrabudhe and Koenig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tracelyn Freeman, VHJhY2VseW4uRnJlZW1hbkBvc3VtYy5lZHU=
†These authors have contributed equally to this work and share last authorship
 Tracelyn Freeman
Tracelyn Freeman Alma Habib2
Alma Habib2 Ying Huang
Ying Huang Pooja S. Kumar
Pooja S. Kumar Gregory Behbehani
Gregory Behbehani Ann-Kathrin Eisfeld
Ann-Kathrin Eisfeld Nicole R. Grieselhuber
Nicole R. Grieselhuber Alice Mims
Alice Mims