- 1Hematology and Bone Marrow Transplant Unit, Hospital Monsignor R. Dimiccoli, Barletta, Italy
- 2Hematology and Bone Marrow Transplant Unit, Hospital Cardinale G. Panico, Tricase, Lecce, Italy
The treatment paradigm for large B-cell lymphoma (LBCL) has undergone significant changes in recent years. Patients who fail to achieve a complete response (CR) after first-line therapy (1L) or relapse within 12 months are considered to have a poor prognosis. For these individuals, new therapeutic options, such as CAR-T cell therapy or bispecifics, have largely replaced traditional approaches, including chemotherapy, autologous hematopoietic stem cell transplantation (auto-HCT), and best supportive care. Accurate staging and evaluation of treatment response are critical, especially for patients achieving a partial response (PR) at the end of 1L. Patients with PR represent a distinct and less well-defined subgroup compared to those with stable or progressive disease or those achieving CR. These patients often have better outcomes than those with progressive disease (PD) or stable disease (SD), and their management remains less simple. Nowadays, prognostic classifications and treatment guidelines continue to evolve, offering new perspectives on how best to approach this subset. While immunotherapy with anti-CD19 CAR-T cells has become the standard of care for refractory LBCL, the role of salvage therapies may still be relevant for patients with PR who are not fully chemorefractory. This review underscores the importance of refining the definitions, prognostic assessments, and therapeutic strategies for patients with partial response, aiming to optimize outcomes in this challenging clinical context.
1 Introduction
Large B-cell lymphoma (DLBCL) is the most frequent form of the non-Hodgkin lymphoma (NHL) subtype, and it globally accounts for a third of all NHLs, ranging between 20% and 50% by country. LBCL incidence increases with age and is generally higher in men than in women. Like NHL incidence, LBCL incidence rose in the first half of the 20th century but has largely plateaued (1). Most cases of LBCL can be cured with the chemoimmunotherapy schedule based on rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or with R-POLA-CHP, a recent CHOP derivative with the substitution of vincristine with polatuzumab vedotin (2, 3). However, one third of newly diagnosed DLBCL patients are refractory to first-line therapy (1L) or experience a relapse; some of them can be cured with other therapies, but the majority of them succumb to the disease.
Until a few years ago, the standard of treatment for patients with relapsed/refractory (R/R) DLBCL was high-dose chemotherapy followed by autologous stem cell transplantation (ASCT) (4, 5). This approach was based on the premise that treatment resistance could be overcome by administering higher doses of chemotherapy. However, it has since become clear that ASCT in chemo-insensitive disease is largely futile, with a response to salvage therapy being a necessary prerequisite to proceed with transplantation.
Patients with primary refractory disease rarely respond to second-line therapies, with the SCHOLAR-1 meta-analysis showing response rates of 17% for PR and 3% for CR (6). Additionally, early relapse patients tend to have worse outcomes than those with late relapse.
In the last years, the therapeutic landscape for R/R LBCL has evolved, and the status of lymphoma at the time of receiving salvage therapy has become a crucial prognostic factor. Today, high-risk patients have access to new alternative options, no longer limited to clinical trials or best supportive care. One significant development is the emergence of chemo-free therapies, such as CAR-T cell therapy, immunoconjugate antibodies that have provided a promising alternative to chemotherapy (7). These new options have highlighted the importance of detecting the lack of chemosensitivity, as identifying this early could prevent the unnecessary and potentially harmful administration of further chemotherapy.
This shift in treatment paradigm has led to a change in how we classify and approach relapsed patients. Patients who relapse more than 1 year after initial chemotherapy are now considered chemosensitive and should be considered for ASCT if eligible. Conversely, those with a primary refractory disease or with a relapse within 1 year after the end of therapy are candidates for second-line CAR-T cell therapy if eligible, marking immunotherapy as the new standard of care for patients with refractory LBCL.
In this context, the benefit of ASCT appears to be diminished, particularly for patients with refractory or early relapsing disease, who are the majority of cases. In the post-rituximab era, indeed, we can consider the chemoresistance and the chemosensitivity during or at the end of first-line therapy the basis for decision-making, and consequently, we particularly focused on patients in partial remission (PR), the most difficult setting to define in this respect.
2 LBCL patients in PR in the CAR-T as a second-line option
Given the satisfactory responses to CAR-T cell therapy in patients with R/R LBCL after at least two lines of therapy, three prospective clinical trials were launched to evaluate CAR-T therapy as a second-line option for patients with primary refractory or early relapsed (within 12 months) LBCL: ZUMA-7 (8), TRANSFORM (9), and BELINDA (10) compared axicabtagene ciloleucel (axi-cel), lisocabtagene maraleucel (liso-cel), and tisagenlecleucel (tisa-cel), respectively, with salvage chemotherapy followed by ASCT.
Notably, ZUMA-7 demonstrated improved OS in CAR-T therapy in this high-risk population, leading to the inclusion of CAR-T as a second-line (2L) option in the National Comprehensive Cancer Network (NCCN) guidelines for patients with primary refractory disease or relapse within 12 months after completing 1L therapy. The primary endpoint for all three trials was event-free survival (EFS), although the definitions of EFS varied. All trials included disease progression and death as events, along with lack of complete or partial response (stable disease) at a designated time point. These time points differed, since they were longer in ZUMA-7 at 150 days compared to 9 weeks in TRANSFORM and 12 weeks in BELINDA. In these trials, refractory disease was defined as the absence of CR following 1L therapy, while relapsed disease was defined as CR followed by biopsy-proven disease recurrence within 12 months after the end of 1L therapy. It is important to emphasize that determining disease refractoriness is crucial for risk stratification in LBCL. As highlighted by Locke et al., patients with PR at the end of treatment (EOT) represent a distinct prognostic category with outcomes better than those of primary progressive disease (PPD) but worse than those of patients achieving CR (8). In CAR-T studies, patients with PR after 1L therapy are not always distinguished from refractory patients. In the TRANSFORM trial, 39% of patients in the liso-cel group and 49% in the SOC group had PR as the best response to 1L therapy. In ZUMA-7, the percentage of these patients was not specified, but they were all assigned to the SOC arm.
In the ALYCANTE trial (11), a phase II study in which 62 transplant-ineligible patients were treated with axi-cel as 2L therapy, 16% of patients had PR as their best response to 1L therapy.
In the PILOT phase 2 trial (12), in which 61 patients not intended for ASCT received liso-cel, 25% of them were in PR as the best response to 1L therapy. Real-world data from the DESCAR-T registry (13, 14), a French nationwide registry of all patients treated with approved CAR-T therapies, showed that 26.2% of LBCL patients treated with axi-cel in 2L had PR as their disease status before CAR-T infusion. However, in the CAR-T SIE study (15), an Italian real-world multicenter observational study on CAR-T therapy for LBCL and mantle cell lymphoma (MCL), stratification of LBCL patients in PR after 1L therapy was not performed.
3 In which prognostic category should patients in partial remission be considered?
Historically, patients in PR after 1L therapy have often been grouped with relapsed LBCL patients in clinical trials (16), and the lack of chemosensitivity has been repeatedly identified as an adverse prognostic factor in LBCL patients (16, 17). In the current paradigm of treatment, the concept of chemosensitivity is crucial and should be determined based on response to 1L therapy in LBCL patients. LBCL cases that fail to respond adequately to 1L treatment or relapse early after initial immunochemotherapy are considered “primary refractory disease” and have poor outcomes. Definitions of primary refractory disease vary in the literature. Some definitions include failure to achieve PR or CR after 1L therapy, while other definitions encompass treatment failure or relapse within 12 months of completing immunochemotherapy (18). This definition has become more relevant following the results of three clinical trials—ZUMA-7, BELINDA, and TRANSFORM—which randomized primary refractory patients to receive CAR-T therapy versus SOC (salvage therapy + auto-HCT). Notably, both ZUMA-7 and TRANSFORM demonstrated improved OS in the CAR-T arm (19).
There is no consensus among lymphoma specialists regarding the definition of early treatment failure. Studies such as CORAL and the Center for International Blood and Marrow Transplant Research (CIBMTR) identified refractory disease or relapse within 12 months of diagnosis as unfavorable, while the ZUMA-7, BELINDA, and TRANSFORM trials, along with LY.12, defined high-risk patients as those with refractory disease or relapse within 12 months of completing frontline therapy. In order to clarify the definition of primary refractory LBCL, A.M. Bock et al. (20) proposed to categorize these patients into three groups:
● Stable or progressive disease (PD) during or by the end of 1L therapy, including transient interim PR or CR, and primary PD (PPD).
● PR as the best response at the end of treatment (EOT PR).
● Early relapse within 3, 3–6, or 6–12 months after achieving CR at the end of 1L therapy.
In their study, two cohorts were analyzed: 949 patients (MER) and 2,755 patients (LEO). Among these, 132 (13.9%; PPD = 40, EOT PR = 40, early relapse = 52) and 308 (11.3%; PPD = 145, EOT PR = 66, early relapse = 97) patients met the inclusion criteria for primary refractory disease, respectively. The 2-year OS rates were 30% for PPD, 50% for EOT PR, and 58% for early relapse, with PPD patients showing significantly worse outcomes compared to the other two groups. Based on these results, primary refractoriness in LBCL patients was defined as stable or PD during or by the EOT (PPD group). Patients with inadequate responses (EOT PR) and early relapse had similar outcomes and may be better grouped as early relapse.
4 The patient in partial remission in the current guidelines
The current criteria for assessing response to therapy in NHL are based on the Lugano classification of 2014 (21). A positron emission tomography (PET)-CT–based partial metabolic response in lymph nodes and extra lymphatic sites is defined as a Deauville score (DS) of 4 or 5, with reduced uptake compared to baseline and residual masses of any size. However, there is a distinction between such results at interim PET scans, where they indicate a responding disease, and at end-of-treatment PET scans, where they are considered residual disease. In the bone marrow, residual uptake greater than that in normal marrow but reduced compared to baseline is also considered significant. Persistent focal changes in the marrow within the context of a nodal response should prompt further investigation, such as MRI, biopsy, or interval scans. The evaluation of lymph nodes, residual masses, and bone marrow therefore involves a quantitative approach, primarily assessing FDG uptake reduction compared to baseline as a key parameter. According to Cheson et al. (21), a score of 4 or 5 at an interim PET scan suggests the presence of a chemotherapy-sensitive disease when uptake is lower from baseline, and it is classified as a partial metabolic response. At the end of treatment, residual metabolic disease with a score of 4 or 5 is considered a treatment failure, even when there is a reduction in uptake from baseline. A score of 4 or 5 with unchanged or increased intensity compared to baseline, or the presence of new foci compatible with lymphoma, indicates treatment failure at both interim and end-of-treatment assessments. In 2025, during the era of second-line CAR-T therapy for LBCL, updated guidelines were published for managing LBCL by the NCCN and the British Society of Hematology (BSH) (22). The NCCN guidelines categorize patients based on disease stage, distinguishing between stages I/II (excluding stage II with extensive mesenteric disease) and advanced stages, including stage II with extensive mesenteric disease, stage III, and stage IV. For patients with stage I/II disease, interim PET is performed after three to four cycles of R-CHOP, with three possible response outcomes: complete response, progressive disease, and partial response. Patients in partial response, defined by positive interim PET with a DS of 4, are recommended to undergo a new biopsy. If the biopsy is negative, the treatment pathway follows the complete response pathway. No specific recommendation is provided for cases with positive biopsies, but it is implicit that continuation with an additional two to three cycles of R-CHOP, with or without ISRT, would be necessary. For advanced stages, including stage II with extensive mesenteric disease, stage III, and stage IV, interim PET is conducted after two to four cycles of R-CHOP. While three response outcomes are possible, the guidelines consolidate complete and partial responses into the same pathway, with progressive disease in a separate pathway. Patients in partial response with a DS of 4 or 5 on interim PET continue therapy until six cycles of R-CHOP are completed. At restaging, if the DS is still 4 or 5, a new biopsy is performed. A positive biopsy at this stage confirms refractory disease (Table 1). For patients with a DS of 4 in advanced stages, brief interval restaging should be considered, as this result may represent either active disease or a post-treatment inflammatory response (Figures 1, 2). The BSH guidelines propose a PET-adapted approach specifically for patients aged 61–80 years with stage I/II disease, IPI 0, and no bulky disease. These patients undergo interim PET after two initial cycles of R-CHOP. If a complete metabolic response (CMR) is achieved with a DS of 1–3, treatment is completed with an additional two cycles of R-CHOP. For patients without CMR, defined by a DS of 4 or 5, four additional cycles of R-CHOP are administered, followed by radiotherapy consolidation. A change in treatment is recommended only for patients with no response or progressive disease on interim PET. At the end of treatment, patients with CMR on interim PET generally require only a CT scan for end-of-treatment imaging. For patients without CMR or those who did not undergo interim PET, an end-of-treatment PET-CT scan is recommended, typically 3 to 6 weeks after the final dose of antibody. Residual FDG-avid foci warrant biopsy wherever feasible. If biopsy is not possible and imaging findings remain inconclusive, a repeat PET scan after an interval of 8 to 12 weeks is advised (Figure 3).
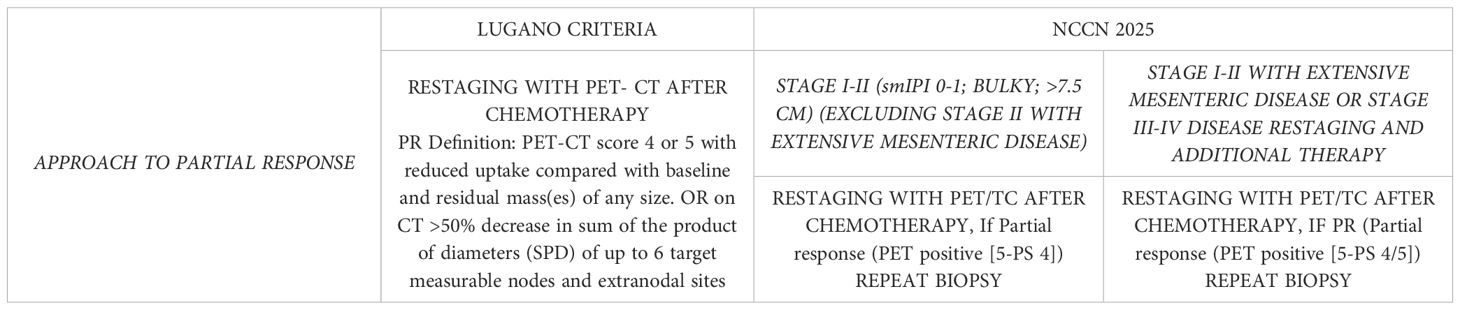
Table 1. According to the Lugano classification, reduction in FDG uptake and tumor size compared to baseline is enough to define PR, while according to the most recent guidelines (NCCN 2025), a histological re-evaluation is advisable.
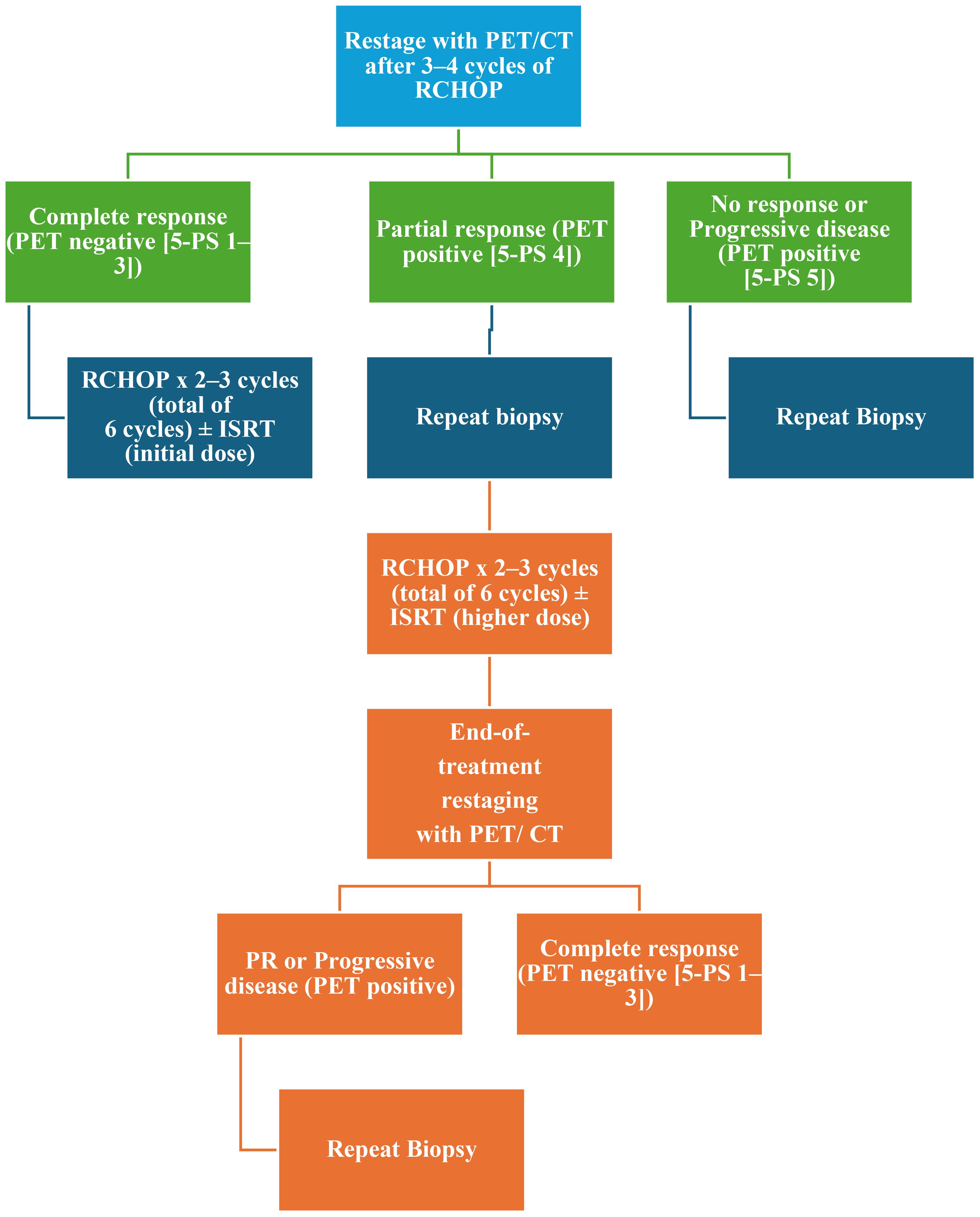
Figure 1. Management of stage I–II (smIPI 0–1; BULKY; ≥7.5 cm) (excluding stage II with extensive mesenteric disease) restaging and additional therapy according to NCCN 2025. NCCN, National Comprehensive Cancer Network; 5-PS, PET five-point scale; smIPI, stage-modified International Prognostic Index; RCHOP, cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine; PET, positron emission tomography; ISRT, involved site radiation therapy; PR, partial response.
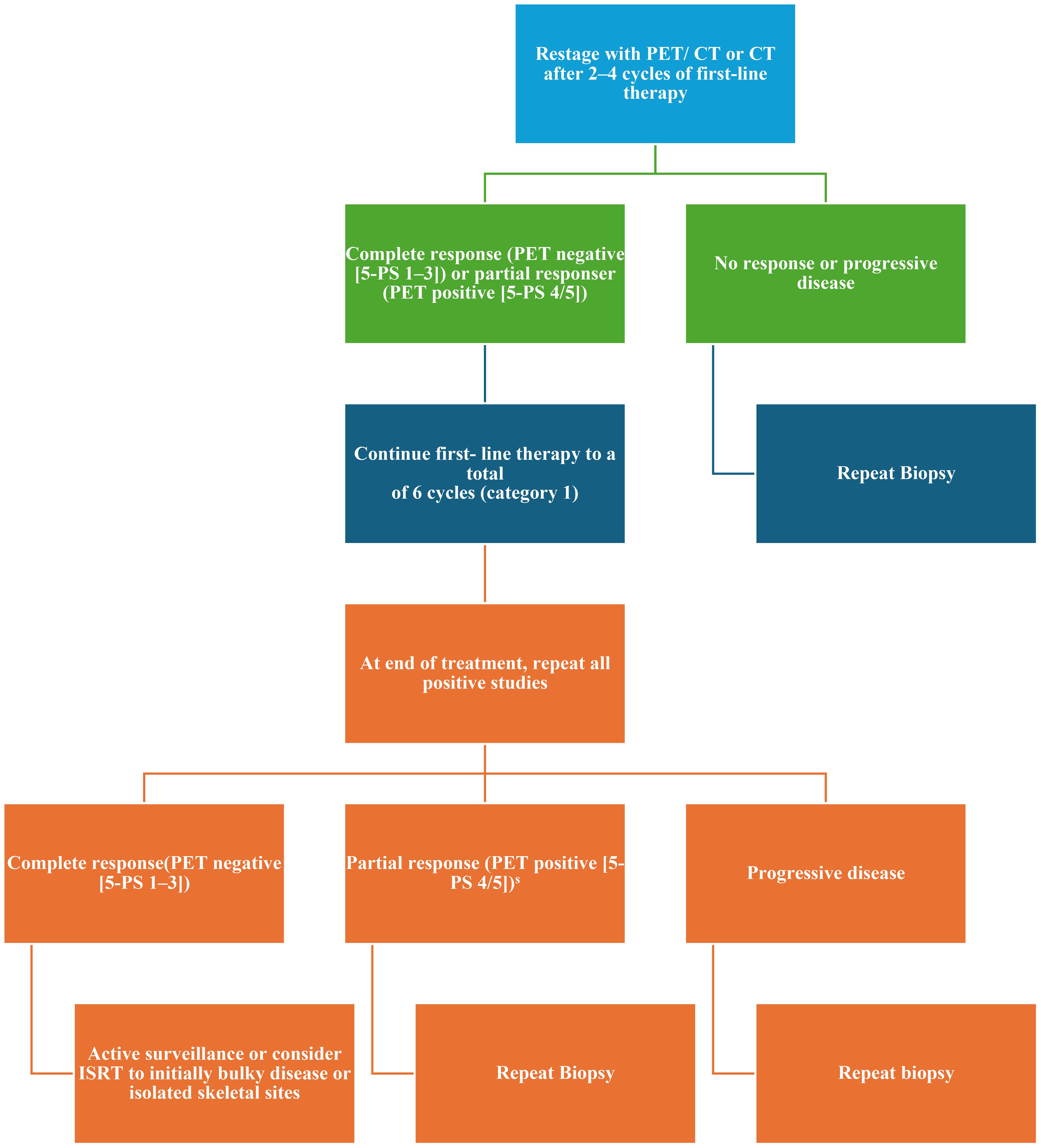
Figure 2. Management of stage I–II with extensive mesenteric disease or stage III–IV disease restaging and additional therapy according to NCCN 2025. NCCN, National Comprehensive Cancer Network; 5-PS, PET five-point scale; PET, positron emission tomography; ISRT, involved site radiation therapy; PR, partial response.
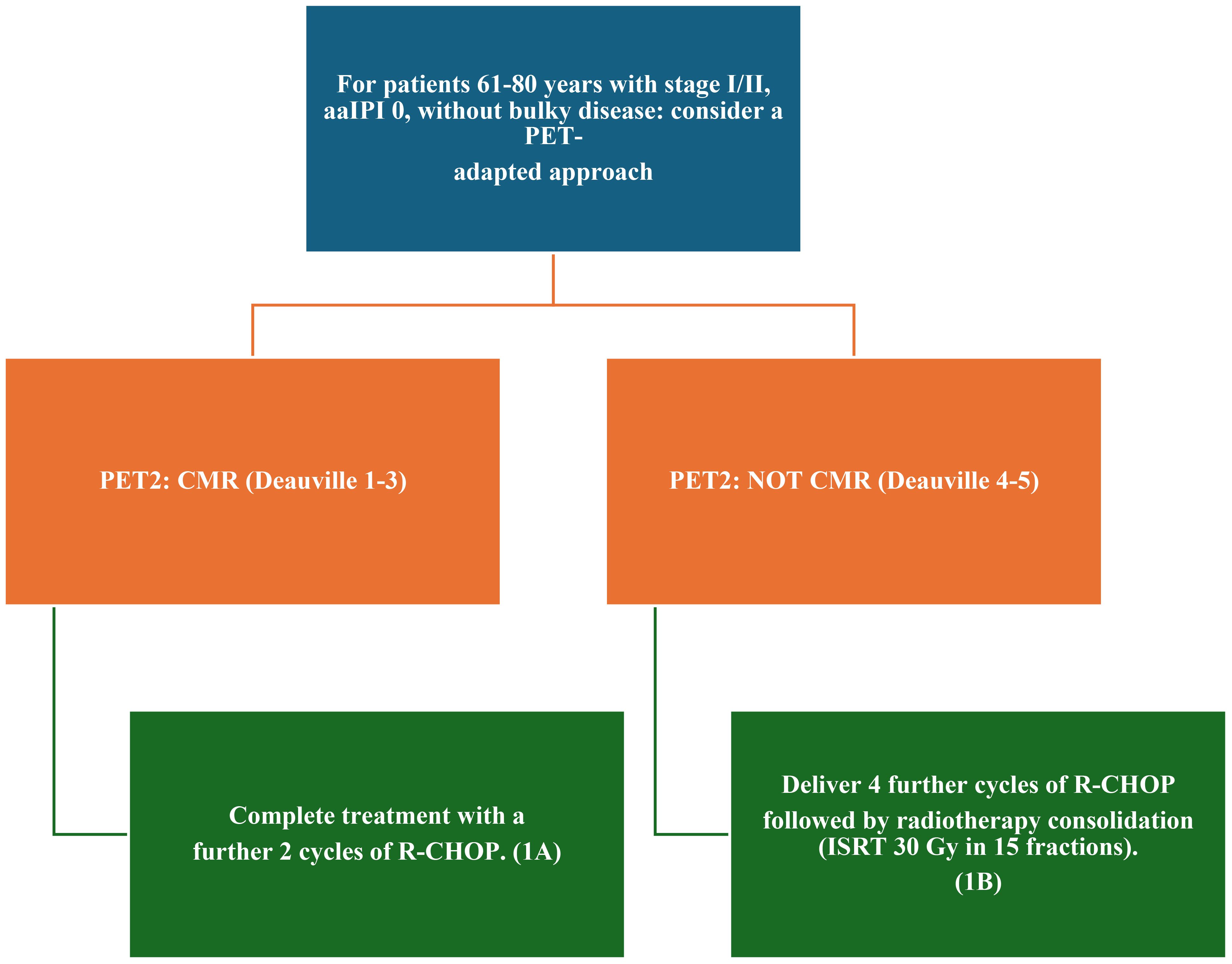
Figure 3. 2024 Recommendations for the management of stage I and II disease according to the British Society of Hematology. RCHOP, cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine; PET, positron emission tomography; ISRT, involved site radiation therapy; PR, partial response; aaIPI, age-adjusted International Prognostic Index; CMR, complete metabolic response.
5 Discussion
Patients with primary refractory disease rarely respond to second-line therapies, with the SCHOLAR-1 meta-analysis showing response rates of 17% for PR and 3% for CR (6). Additionally, early relapse patients tend to have worse outcomes than those with late relapse. Today, high-risk patients have access to new alternative treatment options, no longer limited to clinical trials or best supportive care.
One significant development is the emergence of chemo-free therapies, such as CAR-T cell therapy, immunoconjugate antibodies that have provided promising alternatives to chemotherapy (23). These new options have highlighted the importance of detecting the lack of chemosensitivity, as identifying this earlier could prevent the unnecessary and potentially harmful administration of further chemotherapy. This shift in treatment paradigm has led to a change in how we classify and approach relapsed patients. Patients who relapse more than 1 year after initial therapy are now considered chemosensitive and should be considered for ASCT if eligible. Conversely, those who relapse within a year after completing initial therapy are candidates for second-line CAR-T cell therapy if eligible, making immunotherapy the new standard of care for patients with refractory LBCL.
In this context, defining partial remission accurately at the right time in a patient’s clinical journey is critical. The 2025 guidelines from the NCCN and the BSH offer strategies for patients with LBCL in partial remission, particularly for stages I and II. According to the NCCN guidelines, patients in partial remission after 3–4 cycles of R-CHOP with a DS of 4 at interim PET should undergo biopsy to determine whether to pursue the CR or PR pathway. If biopsy results are negative, the CR pathway can be followed. The BSH guidelines, on the other hand, suggest that patients aged 61–80 years with DS 4 or 5 at interim PET (without comparing uptake to baseline) should proceed with four additional cycles of R-CHOP followed by radiotherapy, without performing a biopsy. For all the other patients, the negative predictive value for interim PET is approximately 80%, with studies showing that only a small percentage of PET-negative patients experience positive end-of-treatment PET scans.
For patients who achieve a CMR on interim PET (iPET2), BSH recommends a CT scan for end-of-treatment imaging. For those without CMR on iPET2, or those who did not undergo an iPET2, a PET-CT scan should be performed. Similarly, the NCCN guidelines recommend end-of-treatment PET for patients in stages I/II (PR pathway) and stages III/IV (CR/PR pathway). If DS is 4 or 5, a repeat biopsy should be performed, or clinical judgment should be used if biopsy is not possible. Both sets of guidelines emphasize the importance of waiting a few weeks to assess the response, with BSH recommending 8 weeks after the last dose of antibody, and the NCCN recommending a brief interval in cases with a DS of 4, as this may reflect post-treatment inflammation rather than active disease.
This timing is crucial, as there are significant barriers to the timely delivery of CAR-T therapy in clinical practice. Patients often undergo weeks or months of eligibility and fitness assessments, CAR-T manufacturing, and logistical planning. Some patients experience symptomatic, life-threatening progressive disease before receiving CAR-T and require urgent chemotherapy as bridging therapy. The time from decision to infusion, known as “brain-to-vein” time, is a critical factor. This period, along with pre-apheresis therapies that may affect the health or CAR-T cell manufacturing process, could impact outcomes (24). In fact, it may be more useful to focus on the “brain-to-vein” time as a better indicator of CAR-T treatment success, rather than the traditional “vein-to-vein” time.
Beyond the guidelines, there are still significant challenges as well as the setting of patients who arrive at transplant or CAR-T therapy after demonstrating a response (CR or PR) to salvage chemo-immunotherapy. May we consider them completely chemo-insensitive? May they do well with auto-HCT consolidation? Could this option at least be discussed with the patient?
Patients who relapse within the first year after the end of chemoimmunotherapy and achieve a CR with platinum-based salvage therapy can benefit from high-dose chemotherapy (HDT) and ASCT (25). Two studies showed that high-dose therapy and auto-HCT consolidation are curative for approximately 45% of patients with LBCL despite achieving only a PR after salvage therapy (26, 27). On the other hand, patients considered as CAR-T candidates often receive bridging chemotherapy before CAR-T therapy: some of them achieve a response, including CR. The three trials ZUMA-7, BELINDA, and TRANSFORM were not designed to address the management of LBCL patients in PR responsive to salvage therapies and excluded patients who received any second-line treatment. In these trials, differences in EFS and OS between CAR-T and ASCT were calculated from the time of randomization, not from the time of infusion of cell products This raises the question of whether it would have been more appropriate for CAR-T trials to treat all patients with salvage chemotherapy before randomizing them, as most patients with prior therapy exposure may have less potent CAR-T products.
One retrospective study evaluating patients with relapsed LBCL in PR after the last therapy at the time of ASCT or CAR-T, included in the CIBMTR registry database, demonstrated a lower relapse rate (40% vs. 53%) and improved 2-year OS (69% vs. 47%), respectively, in the ASCT group as compared to the CAR-T group. These results are relevant, but differences between the two cohorts in terms of previous lines of therapy (more than two lines: 33% ASCT vs. 67% CAR-T) and tumor burden (largest node > 5 cm: 29% ASCT vs. 41% CAR-T) should be noted. There is also a substantial difference between the salvage treatment used before ASCT, aimed to induce CR or, at least, a very good PR, and the bridging therapy before CAR-T, ranging from glucocorticoids as a single agent to brief chemoimmunotherapy (28).
In a single-center retrospective study, Strati et al. (29) showed that tumor burden, as measured with total metabolic tumor volume (TMTV), differentially affects the response to CAR-T and ASCT among LBCL patients who achieve PR. A total of 111 LBCL with R/R LBCL in PR after the last line of therapy were included in the study. After a propensity score matching applied for only 26 patients per group, the relapse/progression rate was 40% for patients who received CAR-T and 58% for those who received ASCT. Among patients with low TMTV, the relapse/progression rate was 43% for patients who received CAR-T compared with 61% for those who received ASCT. In contrast, among patients with high TMTV, the relapse/progression rate was similar between the two groups.
In a multivariate analysis that included TMTV, IPI, and the number of previous therapies, only TMTV maintained its association with relapse/progression rate. No change in relapse progression was observed among patients with PR that proceeded to ASCT based on TMTV, but it proportionally increased corresponding to TMTV among those who proceeded to receiving CAR-T; TMTV, however, seems to have a role in distinguishing different subsets of PR patients.
These results support the role of auto-HSCT for transplant-eligible patients and suggest that, for some patients, ASCT may be a reasonable first option, particularly if they have already responded to salvage chemotherapy. Other studies have shown that patients with chemosensitive disease, even those with primary refractory or early relapsed disease, can achieve durable disease control with ASCT consolidation.
In a study comparing ASCT and CAR-T in patients aged over 65 with chemosensitive R/R LBCL in PR after salvage chemotherapy (30, 31), the results showed similar PFS and OS at 1 year, with no significant difference between the two therapies. However, CAR-T therapy was associated with lower non-relapse mortality (NRM) compared to ASCT, particularly in high-risk subgroups. These findings support CAR-T as a viable option for older adults with chemosensitive disease and suggest that CAR-T may be preferable for fit older patients with relapse beyond 1 year.
However, it must be taken into account that all these retrospective studies have expected bias, and among these, the authors considered the definition of PR to be an important limitation: the interpretation of PR and diagnostic modality varied among institutions, especially in the non-clinical trial setting.
In conclusion, although CAR-T therapy became the new standard of treatment for patients who did not reach a CR after 1L and early relapse, ASCT could be considered as an important treatment option for patients who achieve a response to salvage chemotherapy. The decision between ASCT and CAR-T should be based on patient characteristics, including age, comorbidities, and response to prior therapies. As clinical practice continues to evolve, a personalized approach to treatment is critical, with careful consideration of the timing and type of therapy used to maximize patient outcomes.
6 Conclusion
As highlighted by the results of several retrospective studies, the definition of PR remains inconsistent across various clinical settings, despite ongoing efforts at classification and standardization, culminating in the Lugano classification of 2014. The different definition of PR is particularly crucial in the context of managing patients with R/R LBCL, especially when considering the treatment decisions for those in PR following frontline therapy. This challenge becomes even more significant when managing patients with refractory disease, where the clinical decision-making landscape has evolved substantially.
Recent guidelines, however, recommend repeating a biopsy in cases of suspected partial remission based on interim or end-of-treatment PET uptake and associated DS for advanced stages and after interim PET for stage I/II patients.
The current treatment paradigm for R/R LBCL patients considers the timing of recurrence (within 12 months or after 12 months), which influences whether the disease is considered chemosensitive. For patients who relapse within 12 months (early relapse), the prognosis is generally poor and the disease is often regarded as chemoresistant. However, for those in PR after first-line R-CHOP therapy, considered primary refractory, the management approach remains nuanced. While their prognosis aligns with that of these patients, some retrospective studies suggest that their disease may not be completely chemo-insensitive. This observation forms the basis for considering caution in routinely offering CAR-T treatment to all patients. For those under 65 years of age, salvage therapies followed by ASCT are recommended, particularly when timely access to CAR-T therapy is challenging.
The growing body of evidence suggests that a subset of patients in PR after salvage therapy could still benefit from ASCT, potentially leading to durable disease control. Nevertheless, to comprehensively address the management of LBCL in PR following salvage therapy, which was excluded in the design of the three major CAR-T trials, and to explore further the comparative effectiveness of ASCT versus CAR-T in patients with chemosensitive disease, the design of randomized trials could be a logical next step. This would help clarify the optimal therapeutic approach for this patient population and potentially refine existing treatment guidelines.
Author contributions
GT: Writing – original draft. EA: Writing – review & editing. CB: Writing – review & editing. VC: Writing – review & editing. GD: Writing – review & editing. CG: Writing – review & editing. ML: Writing – review & editing. DL: Writing – review & editing. SM: Writing – review & editing. RM: Writing – original draft, Writing – review & editing. TS: Writing – review & editing. VS: Writing – original draft, Writing – review & editing. VP: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
GT received a speaker honorarium from Takeda, Roche, Beigene, and AbbVie.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang SS. Epidemiology and etiology of diffuse large B-cell lymphoma. Semin Hematol. (2023) 60:255–66. doi: 10.1053/j.seminhematol.2023.11.004
2. Karsten IE, Shumilov E, Schmitz N, and Lenz G. Sequencing of therapy for patients with diffuse large B-cell lymphoma in the era of novel drugs. Br J Haematol. (2024) 205:2163–74. doi: 10.1111/bjh.19860
3. Tilly H, Morschhauser F, Sehn LH, Friedberg JW, Trnéný M, Sharman JP, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. New Engl J Med. (2022) 386:351–63. doi: 10.1056/NEJMoa2115304
4. Gisselbrecht C and Van Den Neste E. How I manage patients with relapsed/refractory diffuse large B cell lymphoma. Br J Haematol. (2018) 182:633–43. doi: 10.1111/bjh.15412
5. Sarkozy C and Sehn LH. Management of relapsed/refractory DLBCL. Best Pract Res Clin Haematol. (2018) 31:209–16. doi: 10.1016/J.BEHA.2018.07.014
6. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. (2017) 130:1800–8. doi: 10.1182/blood-2017-03-769620
7. Bock AM and Epperla N. Therapeutic landscape of primary refractory and relapsed diffuse large B-cell lymphoma: Recent advances and emerging therapies. J Hematol Oncol. (2025) 18:68. doi: 10.1186/s13045-025-01702-5
8. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. New Engl J Med. (2022) 386:640–54. doi: 10.1056/NEJMoa2116133
9. Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy, versus standard of care (SOC) with salvage chemotherapy (CT) followed by autologous stem cell transplantation (ASCT) as second-line (2L) treatment in patients (Pts) with relapsed or refractory (R/R) large B-cell lymphoma (LBCL): results from the randomized phase 3 transform study. Blood. (2021) 138:91–1. doi: 10.1182/blood-2021-147913
10. Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. New Engl J Med. (2022) 386:629–39. doi: 10.1056/NEJMoa2116596
11. Houot R, Bachy E, Cartron G, Gros F-X, Morschhauser F, Oberic L, et al. Axicabtagene ciloleucel as second-line therapy in large B cell lymphoma ineligible for autologous stem cell transplantation: a phase 2 trial. Nat Med. (2023) 29:2593–601. doi: 10.1038/s41591-023-02572-5
12. Sehgal A, Hoda D, Riedell PA, Ghosh N, Hamadani M, Hildebrandt GC, et al. Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): an open-label, phase 2 study. Lancet Oncol. (2022) 23:1066–77. doi: 10.1016/S1470-2045(22)00339-4
13. Brisou G, Cartron G, Bachy E, Thieblemont C, Castilla-Llorente C, Le Bras F, et al. Real world data of axicabtagene ciloleucel as second line therapy for patients with large B cell lymphoma: first results of a lysa study from the french descar-T registry. Blood. (2023) 142:5138. doi: 10.1182/BLOOD-2023-180241
14. Broussais F, Bay JO, Boissel N, Baruchel A, Arnulf B, Morschhauser F, et al. DESCAR-T, le registre national des patients traités par CAR-T Cells. Bull Cancer. (2021) 108:S143–54. doi: 10.1016/j.bulcan.2021.07.002
15. Stella F, Chiappella A, Casadei B, Bramanti S, Ljevar S, Chiusolo P, et al. A multicenter real-life prospective study of axicabtagene ciloleucel versus tisagenlecleucel toxicity and outcomes in large B-cell lymphomas. Blood Cancer Discov. (2024) 5:318–30. doi: 10.1158/2643-3230.BCD-24-0052
16. Rovira J, Valera A, Colomo L, Setoain X, Rodríguez S, Martínez-Trillos A, et al. Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Ann Hematol. (2015) 94:803–12. doi: 10.1007/s00277-014-2271-1
17. Villela L, López-Guillermo A, Montoto S, Rives S, Bosch F, Perales M, et al. Prognostic features and outcome in patients with diffuse large B-cell lymphoma who do not achieve a complete response to first-line regimens. Cancer. (2001) 91:1557–62. doi: 10.1002/1097-0142(20010415)91:8<1557::AID-CNCR1165>3.0.CO;2-4
18. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-hodgkin’s lymphomas. J Clin Oncol. (1999) 17:1244–4. doi: 10.1200/JCO.1999.17.4.1244
19. Bommier C, Lambert J, and Thieblemont C. Comparing apples and oranges: The ZUMA-7, TRANSFORM and BELINDA trials. Hematol Oncol. (2022) 40:1090–3. doi: 10.1002/hon.3001
20. Bock AM, Mwangi R, Wang Y, Khurana A, Maurer MJ, Ayers A, et al. Defining primary refractory large B-cell lymphoma. Blood Adv. (2024) 8:3402–15. doi: 10.1182/bloodadvances.2024012760
21. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: the lugano classification. J Clin Oncol. (2014) 32:3059–67. doi: 10.1200/JCO.2013.54.8800
22. Fox CP, Chaganti S, McIlroy G, Barrington SF, Burton C, Cwynarski K, et al. The management of newly diagnosed large B-cell lymphoma: A British Society for Haematology Guideline. Br J Haematol. (2024) 204:1178–92. doi: 10.1111/bjh.19273
23. García-Sancho AM, Cabero A, and Gutiérrez NC. Treatment of relapsed or refractory diffuse large B-cell lymphoma: new approved options. J Clin Med. (2023) 13:70. doi: 10.3390/jcm13010070
24. Lunning M. Autologous and allogeneic CAR T-cell therapies: spotlighting the “Brain-to-vein” Time. Clin Adv Hematol Oncol. (2022) 20:134–7.
25. Shargian L, Amit O, Bernstine H, Gurion R, Gafter-Gvili A, Rozovski U, et al. The role of additional chemotherapy prior to autologous HCT in patients with relapse/refractory DLBCL in partial remission—A retrospective multicenter study. Eur J Haematol. (2023) 110:149–56. doi: 10.1111/ejh.13884
26. Sauter CS, Matasar MJ, Meikle J, Schoder H, Ulaner GA, Migliacci JC, et al. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood. (2015) 125:2579–81. doi: 10.1182/blood-2014-10-606939
27. Shah NN, Ahn KW, Litovich C, He Y, Sauter CS, Fenske TS, et al. Is autologous transplant in relapsed DLBCL patients achieving only a PET+ PR appropriate in the CAR T-cell era? Blood. (2021) 137:1416–23. doi: 10.1182/blood.2020007939
28. Shadman M, Pasquini M, Ahn KW, Chen Y, Turtle CJ, Hematti P, et al. Autologous transplant vs chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission. Blood. (2022) 139:1330–9. doi: 10.1182/blood.2021013289
29. Strati P, Pasvolsky O, Feng L, Xu G, Tewari SO, Varghese J, et al. ASCT vs CART for patients with relapsed LBCL in PR: role of TMTV. Blood Adv. (2023) 7:2586–9. doi: 10.1182/bloodadvances.2022009622
30. Akhtar OS, Cao B, Wang X, Torka P, Al-Jumayli M, Locke FL, et al. CAR T-cell therapy has comparable efficacy with autologous transplantation in older adults with DLBCL in partial response. Blood Adv. (2023) 7:5937–40. doi: 10.1182/bloodadvances.2023010127
31. Westin JR, Locke FL, Dickinson M, Ghobadi A, Elsawy M, van Meerten T, et al. Safety and efficacy of axicabtagene ciloleucel versus standard of care in patients 65 years of age or older with relapsed/refractory large B-cell lymphoma. Clin Cancer Res. (2023) 29:1894–905. doi: 10.1158/1078-0432.CCR-22-3136
Keywords: DLBCL, partial response (PR), CAR-T, bispecific antibody, autologous stem cell transplantation (ASCT)
Citation: Tarantini G, Arcuti E, Buquicchio C, Carluccio V, De Santis G, Germano CR, Leo M, Loconte DC, Mallano S, Miccolis RM, Santeramo TM, Strafella V and Pavone V (2025) A focus on LBCL patients in partial remission in the CAR-T era. Front. Hematol. 4:1675099. doi: 10.3389/frhem.2025.1675099
Received: 28 July 2025; Accepted: 23 October 2025;
Published: 11 November 2025.
Edited by:
Guido Gini, Azienda Ospedaliero Universitaria Ospedali Riuniti, ItalyReviewed by:
Gerardo Musuraca, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyFrancesco D’Alò, Università Cattolica del Sacro Cuore di Roma, Italy
Copyright © 2025 Tarantini, Arcuti, Buquicchio, Carluccio, De Santis, Germano, Leo, Loconte, Mallano, Miccolis, Santeramo, Strafella and Pavone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Tarantini, Z2l1c2VwcGV0YXJhbnRpbmkwQGdtYWlsLmNvbQ==
 Giuseppe Tarantini
Giuseppe Tarantini Elena Arcuti1
Elena Arcuti1