- 1Department of Neurological Rehabilitation, Ningbo Rehabilitation Hospital, Ningbo, China
- 2Faculty of Rehabilitation, Gannan Medical University, Ganzhou, China
- 3Faculty of Marine, Ningbo University, Ningbo, China
- 4Faculty of Health Service and Health, Ningbo College of Health Sciences, Ningbo, China
Objective: This study aimed to explore how varying levels of interactive motor-cognitive dual task difficulty affect brain activation, functional connectivity (FC), and behavioral performance in healthy adults using functional near-infrared spectroscopy (fNIRS).
Methods: We recruited 28 healthy participants to perform interactive motor-cognitive dual task at three difficulty levels: easy task (ET), medium task (MT), and difficult task (DT). The tasks involved walking while simultaneously engaging in cognitive challenges. A continuous-wave fNIRS system was used to collect fNIRS data during the task, focusing on 10 regions of interest (ROIs): left/right prefrontal cortex (LPFC/RPFC), left/right dorsolateral prefrontal cortex (DLPFC/DRPFC), left/right premotor cortex (LPMC/RPMC), left/right sensorimotor cortex (LSC/RSC), and left/right motor cortex (LMC/RMC). Simultaneously, the subjects’ gait data during walking were collected using an Inertial Measurement Unit (IMU) sensor, and their cognitive performance was recorded by the researchers.
Results: Statistical analysis revealed statistically significant differences in the mean HbO levels among the three groups for the DRPFC, LPMC/RPMC, RSC, and LMC/RMC regions. Additionally, significant differences were found in the activation of channels 3, 18, 24, 25, 28, and 29 across the three groups. The group-averaged FC in the DT (0.61 ± 0.21) was significantly higher than that in the ET (0.46 ± 0.21, P = 0.023). ROI-to-ROI FC analysis showed significant differences among the three groups in the LSC∼RPMC, RPMC∼RSC, and RSC∼RMC connections. The lateralization index (LI) ranged from 0.10 to 0.35, indicating a predominant right-brain lateralization during the interactive motor-cognitive dual task. Additionally, compared to the MT, both speed and stride length, as well as cognitive performance, were lower during the DT.
Conclusion: We found that increased task difficulty heightened activation in the premotor and motor cortices, with a tendency toward right hemisphere dominance. Higher task difficulty also strengthened FC, particularly in motor-related regions, indicating greater neural coordination. Behaviorally, participants exhibited slower gait parameters and reduced cognitive performance as task complexity increased, highlighting the impact of dual-task interference.
1 Introduction
Most daily activities involve managing motor–cognitive tasks while processing external information, such as crossing a street while observing traffic or carrying a cup of tea while thinking about a shopping list. These motor–cognitive interactions are referred to as motor–cognitive dual task performance (Wollesen and Voelcker-Rehage, 2014). Motor and cognitive dual task training can be categorized into sequential and simultaneous approaches. Sequential motor-cognitive training involves motor and cognitive training occurring at separate times, either before or after physical exercises or on different days (Herold et al., 2018; Tait et al., 2017). Simultaneous motor-cognitive training, on the other hand, involves training both motor and cognitive tasks simultaneously (Lauenroth et al., 2016). A recent review found that simultaneous training significantly improves cognitive performance across various populations, whereas evidence regarding the effectiveness of sequential training remains inconclusive (Tait et al., 2017). Furthermore, the optimal interval between motor and cognitive exercises in sequential training is yet to be determined (Herold et al., 2018; Tait et al., 2017). These findings suggest that simultaneous motor-cognitive training is a more promising and time-efficient approach to enhancing cognitive functions compared to sequential training regimens.
Interactive motor-cognitive dual task is a type of simultaneous motor-cognitive training, in which cognitive task is “incorporated” into the motor task. The cognitive task is a relevant prerequisite to successfully solve the motor-cognitive task (e.g., walking to certain cones in a predefined order or dancing) (Schott, 2015). Several studies have shown that interactive motor-cognitive dual task has many advantages (Herold et al., 2018; Laguë-Beauvais et al., 2015; Plummer et al., 2015; Shams and Seitz, 2008; Yogev-Seligmann et al., 2012). Firstly, interactive motor-cognitive dual task is closer to daily life situations (Herold et al., 2018). For example, it is unlikely that an older person habitually solves an arithmetic task during walking, but it is likely that he/she walks through the supermarket while remembering what goods to buy and where to find those. Secondly, if the cognitive task is incorporated into the motor task, no prioritization effects would occur (Plummer et al., 2015). Such prioritization effects (giving priority either to the cognitive or the motor task) are known to influence motor and cognitive performance (Laguë-Beauvais et al., 2015; Plummer et al., 2015; Yogev-Seligmann et al., 2012). A further advantage of interactive motor-cognitive dual task could be that multiple sensory systems are stimulated due to the execution and control of the cognitive task and the motor task at the same point of time (Shams and Seitz, 2008).
Functional near-infrared spectroscopy (fNIRS), a non-invasive neuroimaging technique, has emerged as a promising tool for investigating brain activation and functional connectivity (FC) (Leff et al., 2011). By measuring fluctuations in oxygenated hemoglobin (HbO) and deoxygenated hemoglobin (HbR) concentrations in response to neuronal activity, fNIRS offers unique insights into the functional dynamics of the brain. FC refers to the interactions between different regions of the cerebral cortex during neurophysiological processes, highlighting important connections among distinct cortical areas (Chen et al., 2022; Liu et al., 2021). Previous fNIRS studies have successfully applied dual task paradigms in both healthy individuals (Yao et al., 2024) and patients, including hypertension (Yao et al., 2024), cognitive impairment (Cao et al., 2022), stroke (Hermand et al., 2019), Parkinson’s disease (Ranchet et al., 2020), and schizophrenic (Ji et al., 2020; Xia et al., 2022; Yang et al., 2020).
Despite the potential of interactive motor-cognitive dual tasking, gaps remain in the current literature. Many of these studies have primarily focused on activation in the prefrontal cortex (PFC), a region known for its involvement in executive functions and cognitive control (Lapanan et al., 2023; Miura et al., 2024). However, research targeting other brain regions associated with motor and cognitive processes, such as the motor cortex, remains scarce. Furthermore, the impact of varying task difficulty on brain activation and FC across these regions is not yet fully understood. Although some studies have reported that increased task difficulty leads to heightened PFC activity (Chen et al., 2022), it is still unclear whether similar patterns are observed in other cortical areas, especially during tasks requiring both motor and cognitive engagement. Thus, further investigation is necessary to elucidate how task complexity influences brain activation beyond the PFC and whether this extends to changes in FC across multiple brain regions.
Given the advantages of interactive motor-cognitive dual task, this study aimed to investigate the neural mechanisms underlying this approach. Specifically, we used fNIRS to assess how varying levels of task difficulty affect brain activation, FC, and both motor (walking) and cognitive performance in healthy adults. By analyzing changes in brain activity and connectivity across different task conditions, we aimed to understand how the brain adapts to increased cognitive and motor demands. Our hypothesis posits that (1) as task difficulty increases, there will be a corresponding increase in brain activation across multiple cortical regions, accompanied by heightened FC between task-relevant regions; (2) behavioral performance will show differential adaptation based on task difficulty.
2 Materials and methods
2.1 Participants
We recruited 28 healthy subjects (age: 22.53 ± 3.46 years, height: 165.71 ± 6.31 cm, weight: 61.97 ± 9.48 kg, education: 14 ± 2.35 years) from Ningbo Rehabilitation Hospital participated in the study, including 14 men and 14 women. Inclusion criteria: participants must have no cognitive impairment, as indicated by a Mini-Mental State Examination (MMSE) score of ≥ 24 (Tombaugh and McIntyre, 1992); voluntary consent to participate in the experiment; absence of cardiovascular, respiratory, musculoskeletal, or neurological diseases; the ability to walk independently; and right-handedness. Right-handed participants were specifically chosen to minimize variability in brain activation patterns, as handedness has been shown to influence functional lateralization and cortical activation in motor and cognitive tasks (Shirzadi et al., 2020). Additionally, while some of the participants were below the legal age of adulthood in certain jurisdictions, they were all university students or young adults with fully developed cognitive and motor capabilities. This age group was selected to ensure homogeneity in cognitive function and neuroplasticity, reducing potential confounding effects that might arise from age-related cognitive decline or neurodevelopmental differences. Exclusion criteria: visual impairment and use of medications affecting the nervous system within the last six months.
The required sample size was calculated using G*power software (version 3.1, University of Kiel, Kiel, Germany) (Kang, 2021). The statistical test used in this study was a one-way repeated measures ANOVA. The input parameters were set as follows: effect size = 0.4, α error probability = 0.05, power (1—ß error probability) = 0.85, and number of groups = 3. Based on these calculations and considering an anticipated rate of approximately 15%, 28 participants were recruited for this study.
Before the fNIRS experiment, the purpose, procedures, fNIRS system, and potential risks of the study were informed in detail with the subjects and their family members to ensure participants could make an informed decision regarding their involvement in the experiment. All participants provided written informed consent before data collection. The experimental protocol was approved by the Institutional Review Board of Ningbo Rehabilitation Hospital (2022-03-G2). All experimental procedures were performed in accordance with the latest guidelines and regulations of the Declaration of Helsinki.
2.2 Experimental protocol
In this study, participants performed an interactive motor-cognitive dual task, which involved walking while completing a variant of the color-word Stroop task. Stroop task is one of the most widely used tasks in cognitive function (Qi et al., 2024). In most traditional versions of the Stroop task, words are displayed on a screen, and participants are instructed to quickly and accurately identify the font color of the words (Qi et al., 2024; Riedel et al., 2024; Wollesen et al., 2016). To increase ecological validity and better approximate real-life situations that require simultaneous motor and cognitive engagement, we adapted the Stroop task into a dynamic, walking-based format.
A 4 × 4 matrix of color words (red, blue, green, and yellow) was placed on the ground in a 6 × 6 meter grid, with each row and column spaced 2 meters apart (Figure 1 and Supplementary Video). Each word was printed in a specific ink color, which could either match or differ from the written word (e.g., the word “red” might be printed in green ink). This created a Stroop-like cognitive interference task, requiring participants to focus on both the written word and its ink color. Participants were instructed to walk at a comfortable pace in an initial point through the grid, guided by the researcher’s verbal instructions. For example, if the researcher instructed participants to find the word “red” displayed in green font, they needed to locate the corresponding card within the matrix (i.e., the card showing the word “red”). After identifying the target, the participant would move to its location and await the next instruction. Subsequent instructions required participants to continue from their current position, searching for the next target without returning to the initial point. During the ET and MT tasks, participants were provided with four possible word options at each instruction, and they were required to select a different word each time, ensuring no repetitions. To reduce the potential influence of learning and adaptation, the three tasks were presented in a randomized order. Each trial lasted one minute, with the card layout reshuffled for every test to further minimize any learning effects. To ensure participants remained engaged and to maintain measurement accuracy, a one-minute rest period was provided between tasks to prevent fatigue.
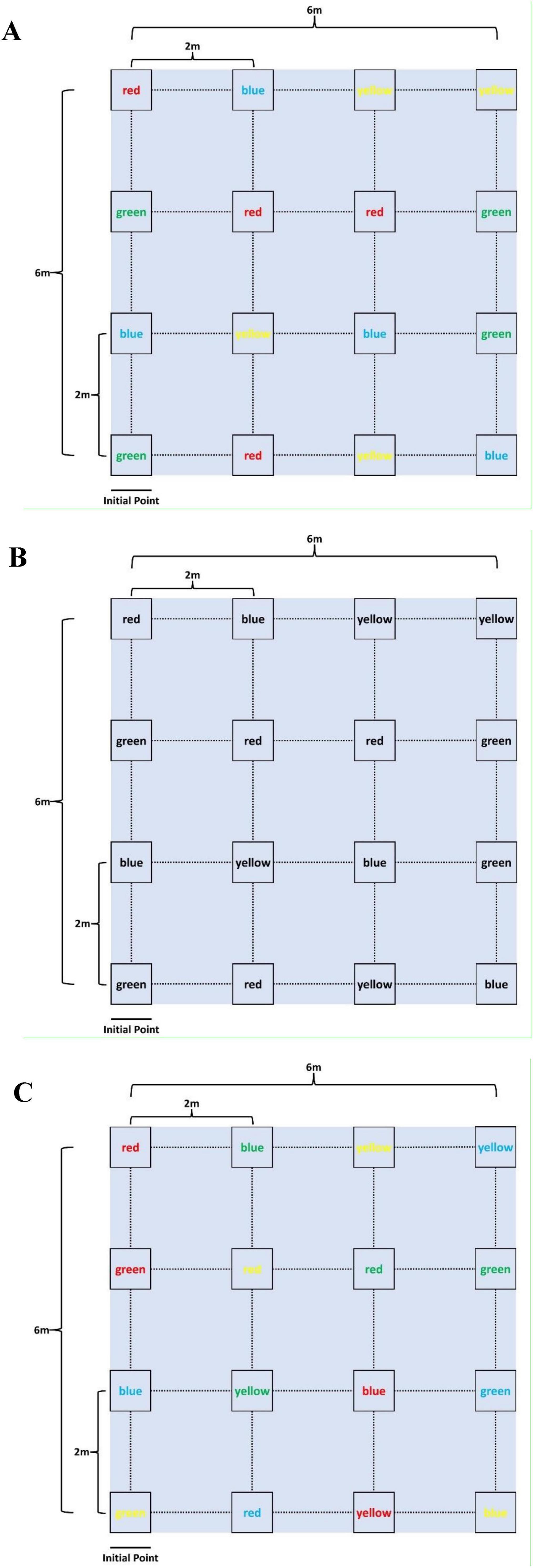
Figure 1. Interactive motor-cognitive dual task diagram. (A) Easy task (ET), (B) medium task (MT), (C) difficult task (DT).
Three levels of difficulty were introduced to the interactive motor-cognitive dual task:
1. Easy task (ET): The font color and the word itself were congruent, presenting no conflict (e.g., the word “red” was printed in red ink, as shown in Figure 1A). This task required minimal cognitive effort, focusing mainly on motor activity.
2. Medium task (MT): The words were printed in black ink, removing the color-coding element. Participants had to rely solely on reading and interpreting the text for the cognitive challenge (Figure 1B), increasing the cognitive demand.
3. Difficult task (DT): The font color and the word were incongruent (e.g., the word “red” was printed in green ink, as shown in Figure 1C), creating a Stroop-like interference. This task required participants to suppress the automatic tendency to respond to the font color, significantly increasing the cognitive load.
2.3 Data acquisition
2.3.1 fNIRS data acquisition
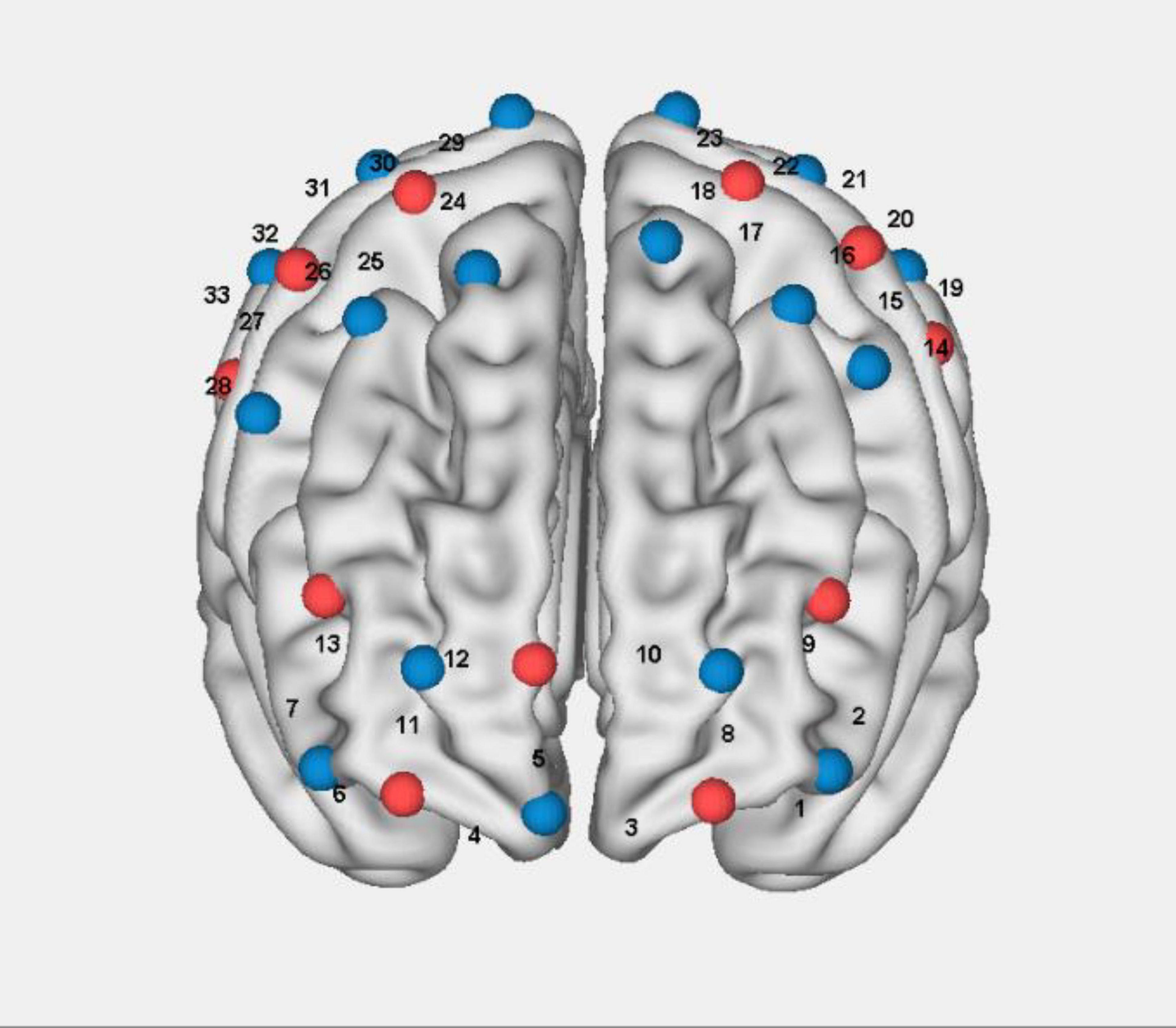
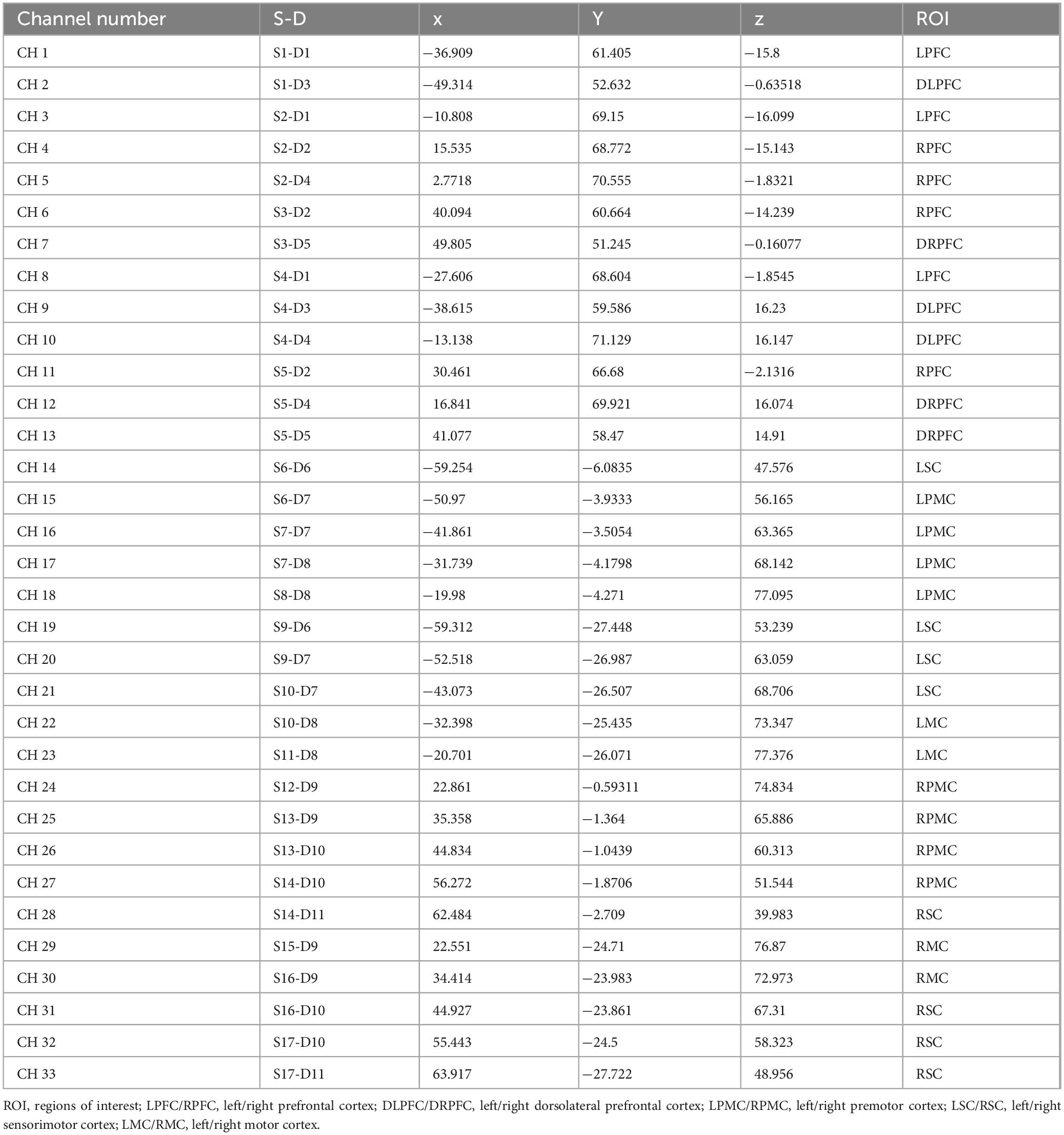
A continuous-wave fNIRS system (Nirsmart, Danyang Huichuang Medical Equipment Co., Ltd., China) was employed in this study to measure cortical hemodynamic responses associated with interactive motor-cognitive dual tasks. This system has been widely utilized in cognitive and motor neuroscience research, demonstrating its reliability in detecting functional brain activity during movement-based tasks (Chen et al., 2022; Li et al., 2020; Li et al., 2023; Miura et al., 2024; Ou et al., 2024). The system operates at two wavelengths (760 and 850 nm) and collects data at a sampling rate of 11 Hz. The setup consisted of 33 channels, formed by 17 source optodes and 11 detector optodes, which were symmetrically placed over both hemispheres following the 10/20 international electrode placement system (Figure 2). These channels covered multiple brain regions of interest (ROIs), including the left and right prefrontal cortex (LPFC/RPFC), dorsolateral prefrontal cortex (DLPFC/DRPFC), premotor cortex (LPMC/RPMC), sensorimotor cortex (LSC/RSC), and motor cortex (LMC/RMC) (Table 1). The selection of these ROIs was based on Brodmann areas (BA) and anatomical references relevant to motor and cognitive processes. To ensure standardized spatial localization, the acquired optode coordinates were transformed into Montreal Neurological Institute (MNI) coordinates and mapped onto the MNI standard brain template using the NirSpace software (Danyang Huichuang Medical Equipment Co., Ltd., China) (Li et al., 2023). The emitter-detector distance was 3 cm. Prior to each recording, a NIR gain quality check was performed to ensure moderate data acquisition, avoiding both under-gained and over-gained conditions.
2.3.2 Behavioral data acquisition
The Inertial Measurement Unit (IMU) sensor was positioned approximately 5 cm above the subject’s medial ankle to collect walking performance data during the task. The walking performance index included step speed, step length, and stride width. Researchers recorded the number of words each subject found during each task as an indicator of cognitive performance.
2.4 Data processing and analysis
2.4.1 Preprocessing
We preprocessed the data collected using fNIRS in the preprocessing module of the NirSpark (Danyang Huichuang Medical Equipment Co., Ltd., China) software, which has been used in previous experiments (Li et al., 2020; Li et al., 2023). The preprocessing steps were as follows:
The standard deviation threshold was set to 6.0, and the amplitude threshold was set to 0.5. Motion artifacts were removed using standard deviation combined with cubic spline interpolation. Interference signals caused by heart rate, breathing rate, and Mayer waves were removed using a 0.01 to 0.2 Hz band-pass filter. Differential path-length factors were set to 6.0. Optical density was converted to blood oxygen concentration using the modified Beer-Lambert law.
2.4.2 Brain activation
In this study, we selected the concentration of HbO as the target indicator of brain activation, as the HbO signals have a higher signal-to-noise ratio (Zhang et al., 2023). The steps for analyzing the HbO time-series data were:
The initial time of the hemodynamic response function (HRF) was set to −2 s and the end time to 60 s. The baseline state was retained from −2 s to 0 s, while the single block paradigm lasted from 0 s to 60 s. The generalized linear model (GLM) was used to generate an ideal HRF for each task. Experimental HRF values were compared with the ideal HRF values to determine the corresponding range. The beta value, indicating the extent of brain activation, served as an indicator for estimating the HRF prediction of the HbO signal. The mean HbO for each ROI in the task states was obtained by dividing the concentration of HbO in all channels in each ROI by the number of channels in each ROI.
2.4.3 Functional connectivity
The FC matrix was computed in NirSpark using Pearson’s correlation analysis between the time series for each pair of channels. Fisher’s r-to-z transformation was conducted to improve normality. For each participant, a 33 × 33 correlation matrix was generated. The ROI-to-ROI correlation coefficients for each participant were calculated. The time series of all channel pairs were averaged for each participant, contributing to the general FC value for each participant. To ensure statistical robustness, we applied a false discovery rate (FDR) correction at q < 0.05 to control for multiple comparisons when analyzing connectivity data.
2.4.4 Laterality index
The lateralization index (LI) evaluates interhemispheric regional activation asymmetry during interactive motor-cognitive dual task. The calculation of the LI was performed as follows:
HbO_L represents the average oxygenated hemodynamic response of channels in the left hemisphere, HbO_R represents the average oxygenated hemodynamic response of channels in the right hemisphere. The value of the LI ranges between −1 and +1. An LI value of “−1” indicates complete left hemisphere dominance, while an LI value of “+1” indicates complete right hemisphere dominance (Borrell et al., 2023; Seghier, 2008).
2.5 Statistical analysis
Statistical analysis was performed using SPSS 27.0 software. The Shapiro–Wilk test was applied to assess data normality, and Levene’s test was used to evaluate the homogeneity of variances. Given that this study involved the same group of participants performing multiple tasks under different conditions (ET, MT, and DT), a one-way repeated measures ANOVA was chosen to analyze differences in: walking performance (speed, step length, and stride width), cognitive performance (number of words found), fNIRS data (ROI activation, channel activation, channel FC, ROI-to-ROI FC, and LI). Post-hoc comparisons were corrected using the Bonferroni method. FDR correction was applied to the fNIRS data. All P-values reported are based on two-sided tests, with values less than 0.05 considered statistically significant.
3 Results
3.1 fNIRS data
3.1.1 Brain activation
The results from Figures 3A, B, which show the activation in the left and right ROIs, indicate the following: the activation of LPMC in MT (0.032 ± 0.016) was higher than in ET (0.014 ± 0.016), the activation of LMC in both DT (0.029 ± 0.026) and ET (0.028 ± 0.018) was higher than in MT (0.026 ± 0.026), the activation of DRPFC in ET (0.056 ± 0.029) was higher than in MT (0.039 ± 0.039), the activation of RPMC in both MT (0.041 ± 0.037) and DT (0.049 ± 0.032) was higher than in ET (0.029 ± 0.015), and the activation of RSC and RMC in DT (0.053 ± 0.035, 0.050 ± 0.026) were higher than in ET (0.041 ± 0.029, 0.038 ± 0.034).
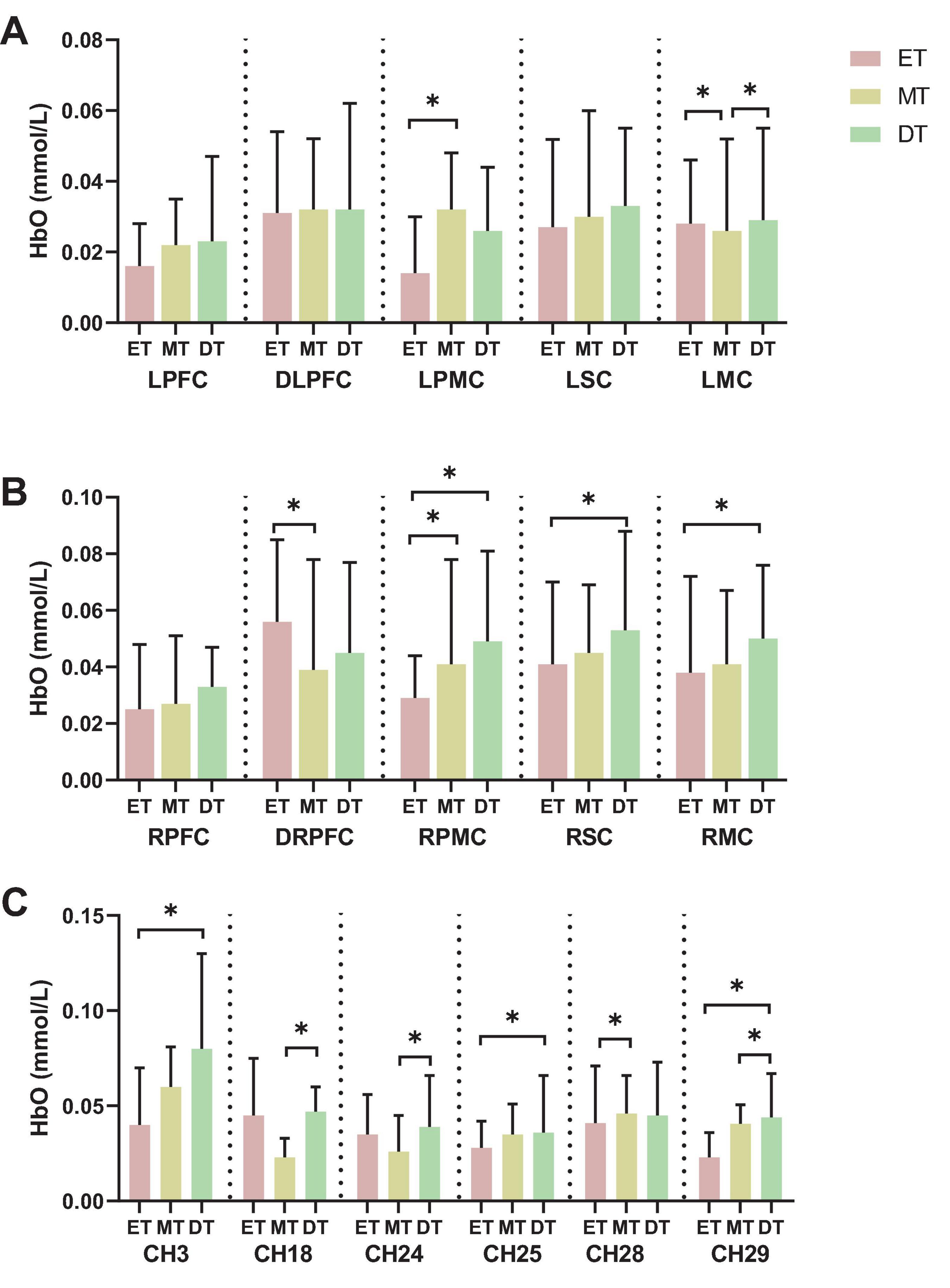
Figure 3. Results of brain activation. (A) left hemisphere activation, (B) right hemisphere activation, (C) channel activation. *P < 0.05. ET, easy task; MT, medium task; DT, difficult task; LPFC/RPFC, left/right prefrontal cortex; DLPFC/DRPFC, left/right dorsolateral prefrontal cortex; LPMC/RPMC, left/right premotor cortex; LSC/RSC, left/right sensorimotor cortex; LMC/RMC, left/right motor cortex.
Additionally, when comparing the activation of channels across the three groups, the results show significant differences in channels 3, 18, 24, 25, 28, and 29 (Figure 3C). Specifically: the activation of channel 3 and 25 in DT (0.080 ± 0.050, 0.036 ± 0.030) was higher than in ET (0.040 ± 0.030, 0.028 ± 0.014), the activation of channel 18 and 24 in DT (0.047 ± 0.013, 0.039 ± 0.027) was higher than in MT (0.023 ± 0.010, 0.026 ± 0.019), the activation of channel 28 in MT (0.046 ± 0.020) was higher than in ET (0.041 ± 0.030), and the activation of channel 29 in DT (0.044 ± 0.023) were higher than in ET (0.023 ± 0.013) and MT (0.041 ± 0.010).
3.1.2 Functional connectivity
The connectivity strength between channels for each group is shown in Figure 4. The mean channel-to-channel connectivity strength was 0.46 ± 0.21 for the ET (Figure 4A), 0.58 ± 0.22 for the MT (Figure 4B), and 0.61 ± 0.21 for the DT (Figure 4C). There was a significant difference in FC values among the three groups (F = 3.24, P = 0.04). The group-averaged FC in the DT was significantly higher than that in the ET (P = 0.023; Figure 4D). However, there were no significant differences between the ET and MT (P = 0.15) and between the MT and DT (P = 0.26).
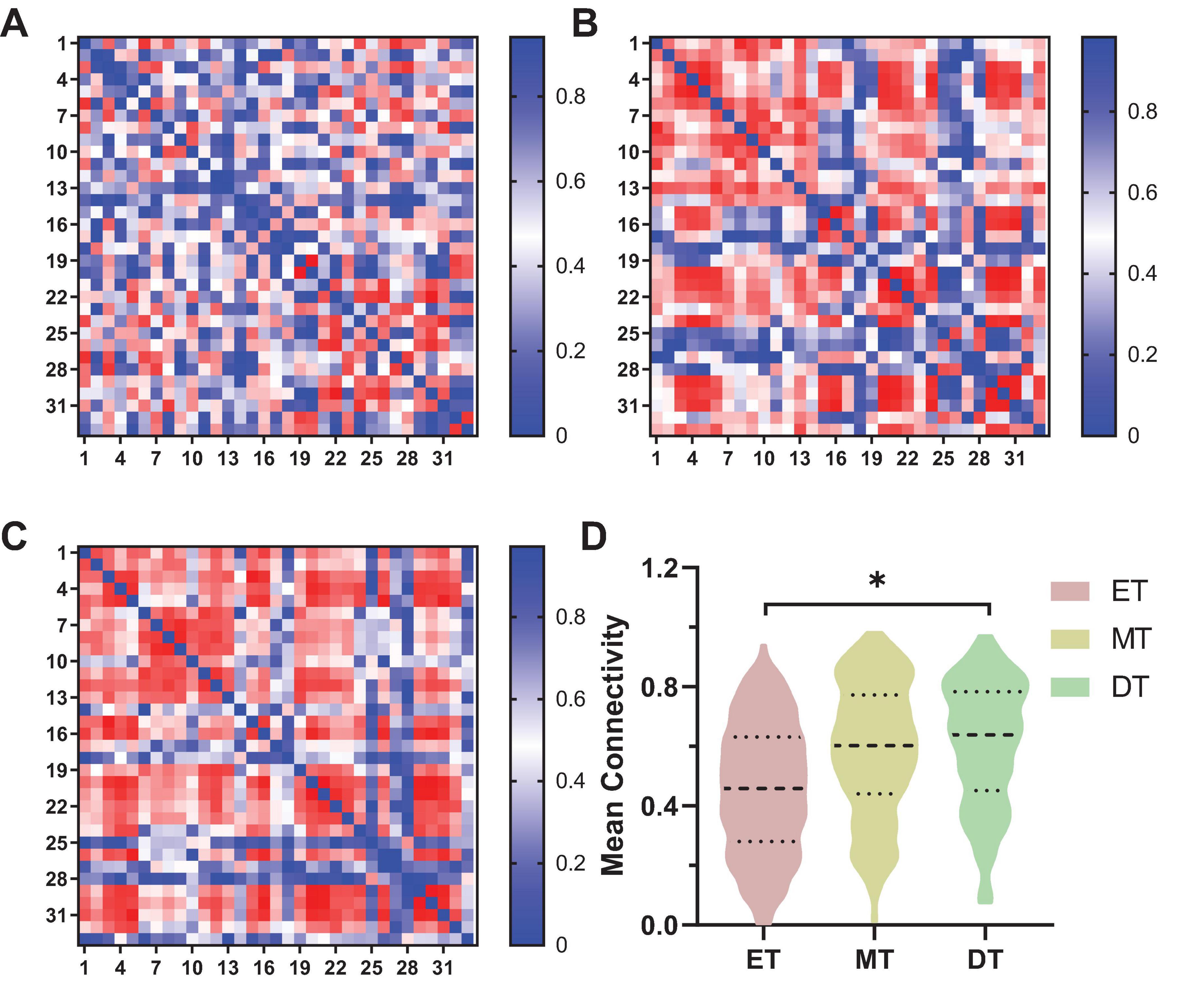
Figure 4. Results of FC of channel-to-channel. *P < 0.05. (A) ET, (B) MT, (C) DT. (D) Mean connectivity strength of 33 channels among three groups. ET, easy task; MT, medium task; DT, difficult task.
Figure 5 shows the FC of ROI-to-ROI among the three groups (Figure 5A). Specifically, the FC results showed that the connectivity strength of ET (0.18 ± 0.16) between LSC and RPMC was significantly lower than DT (0.44 ± 0.03, P = 0.033; Figure 5B). Additionally, the connectivity strength of DT between RPMC and RSC (0.38 ± 0.15; Figure 5C) and between RSC and RMC (0.52 ± 0.17; Figure 5D) was significantly higher than both ET (P = 0.014) and MT (P = 0.034).
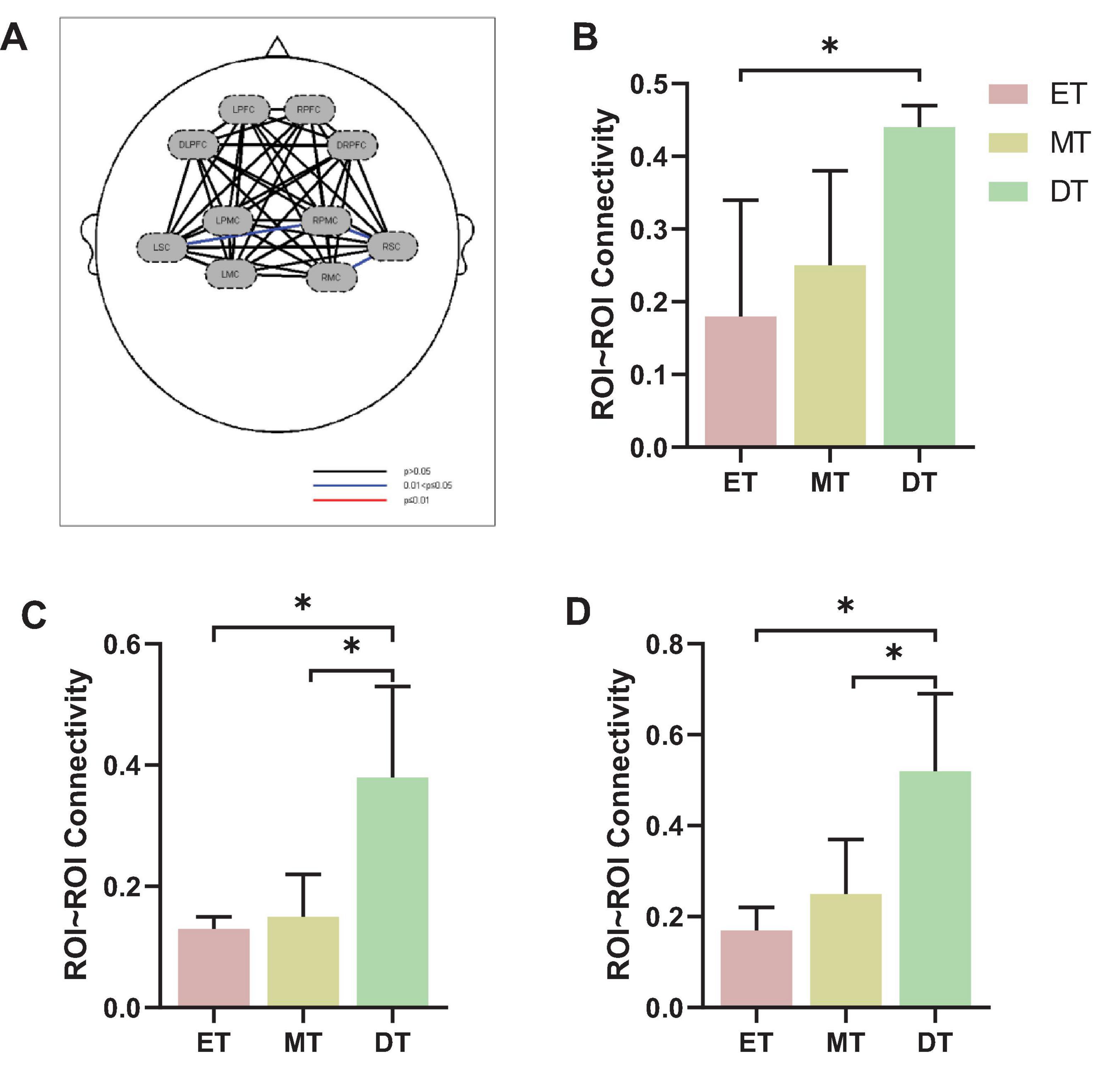
Figure 5. Results of FC of ROI-to-ROI. (A) FC of ROI-to-ROI, (B) LSC∼RPMC, (C) RPMC∼RSC, (D) RSC∼RMC. *P < 0.05. ET, easy task; MT, medium task; DT, difficult task; RPMC, right premotor cortex; LSC/RSC, left/right sensorimotor cortex; RMC, right motor cortex.
3.1.3 Laterality index
Figure 6 shows the cortical activation symmetry. The LI ranged from 0.10 to 0.35, indicating that subjects mainly realized right brain lateralization while performing interactive motor-cognitive dual task. There was a significant difference in the LSC/RSC between ET (0.21 ± 0.35) and DT (0.24 ± 0.31). Additionally, the LI value of LMC/RMC was smaller during ET (0.15 ± 0.36) than during MT (0.26 ± 0.26) and DT (0.28 ± 0.33). No significant differences in LI values were detected between the other groups (P > 0.05).
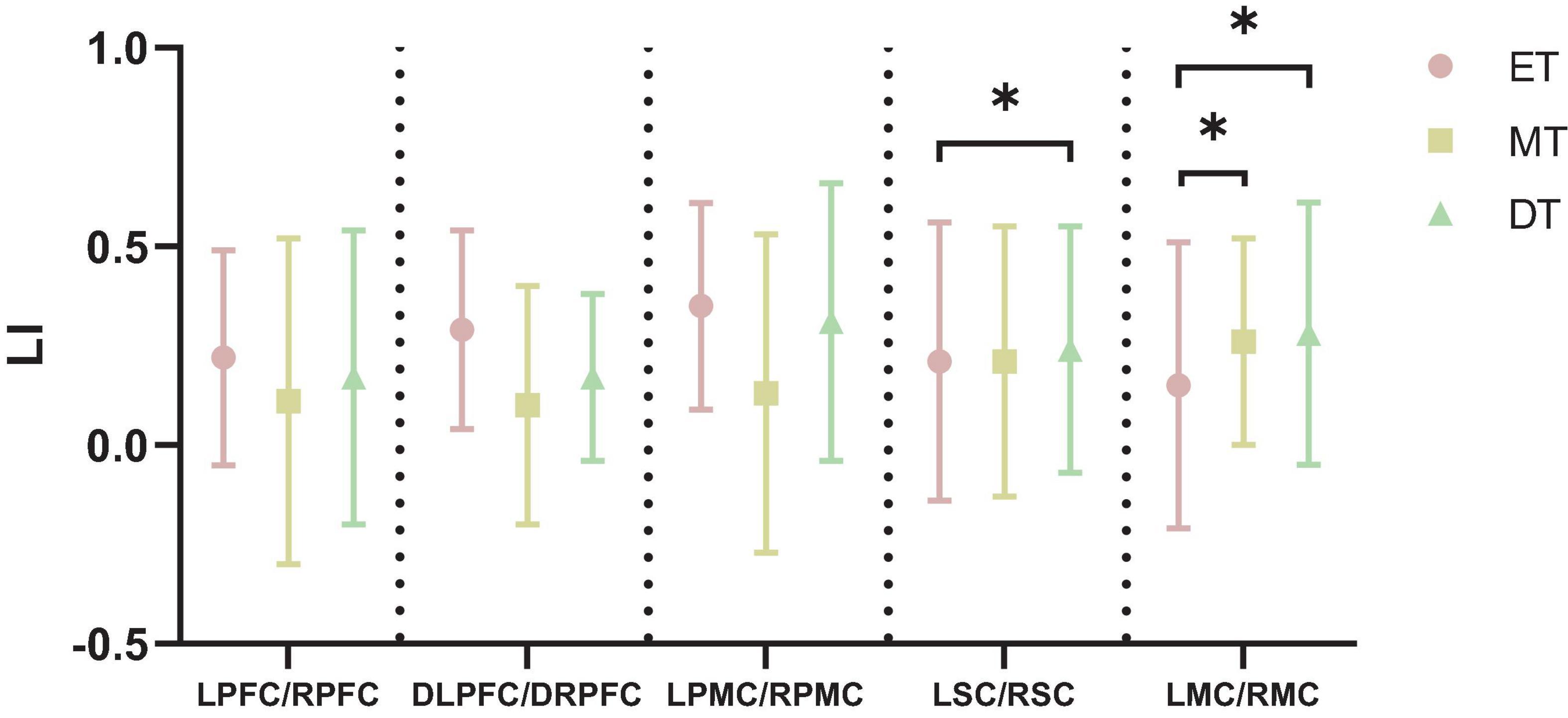
Figure 6. Results of LI. *P < 0.05. ET, easy task; MT, medium task; DT, difficult task; LI, laterality index; LPFC/RPFC, left/right prefrontal cortex; DLPFC/DRPFC, left/right dorsolateral prefrontal cortex; LPMC/RPMC, left/right premotor cortex; LSC/RSC, left/right sensorimotor cortex; LMC/RMC, left/right motor cortex.
3.2 Behavioral data
3.2.1 Walking performance
The speed (F = 8.69, P = 0.031; Figure 7A) and stride length (F = 2.53, P = 0.047; Figure 7B) during DT were significantly smaller than during MT. There was no significant difference in stride width between the three tasks (F = 1.73, P = 0.062; Figure 7C).
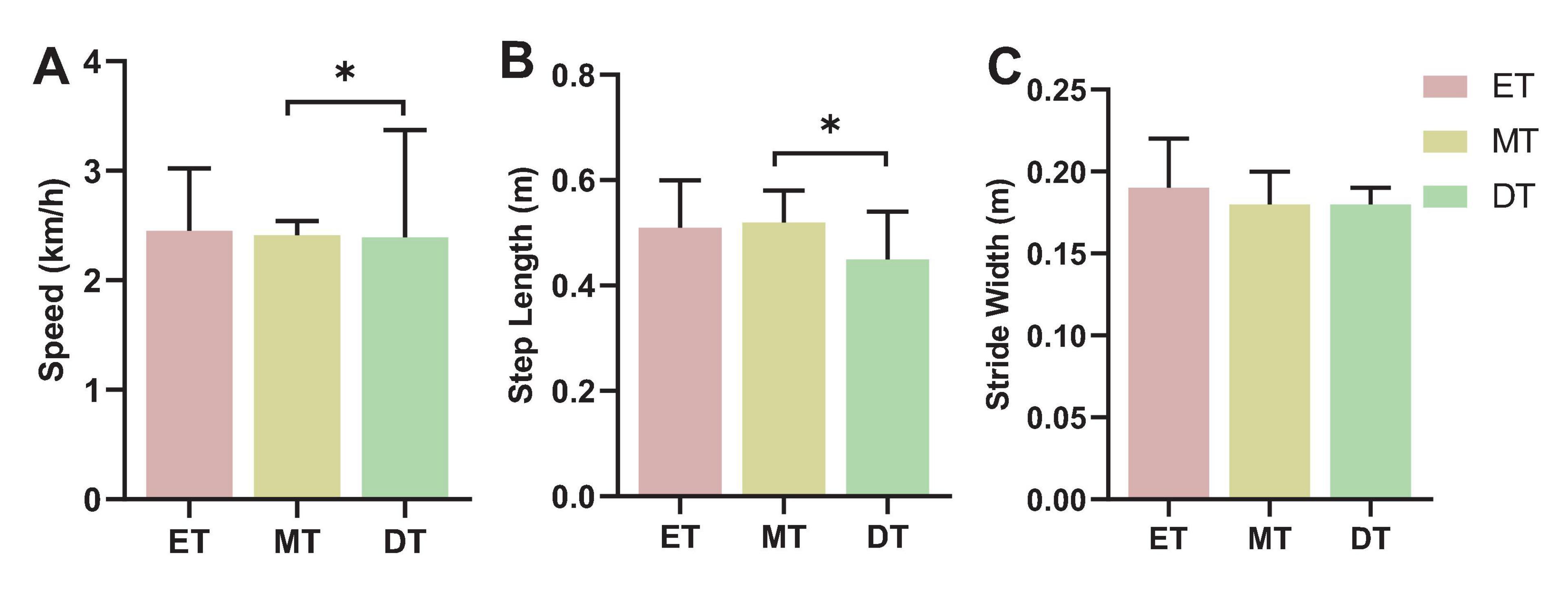
Figure 7. Results of walking performance. *P < 0.05. (A) Speed, (B) step length, (C) stride width. ET, easy task; MT, medium task; DT, difficult task.
3.2.2 Cognitive performance
The number of words found by the subjects in the DT was lower than in the ET and MT (F = 4.55, P = 0.042; Figure 8).
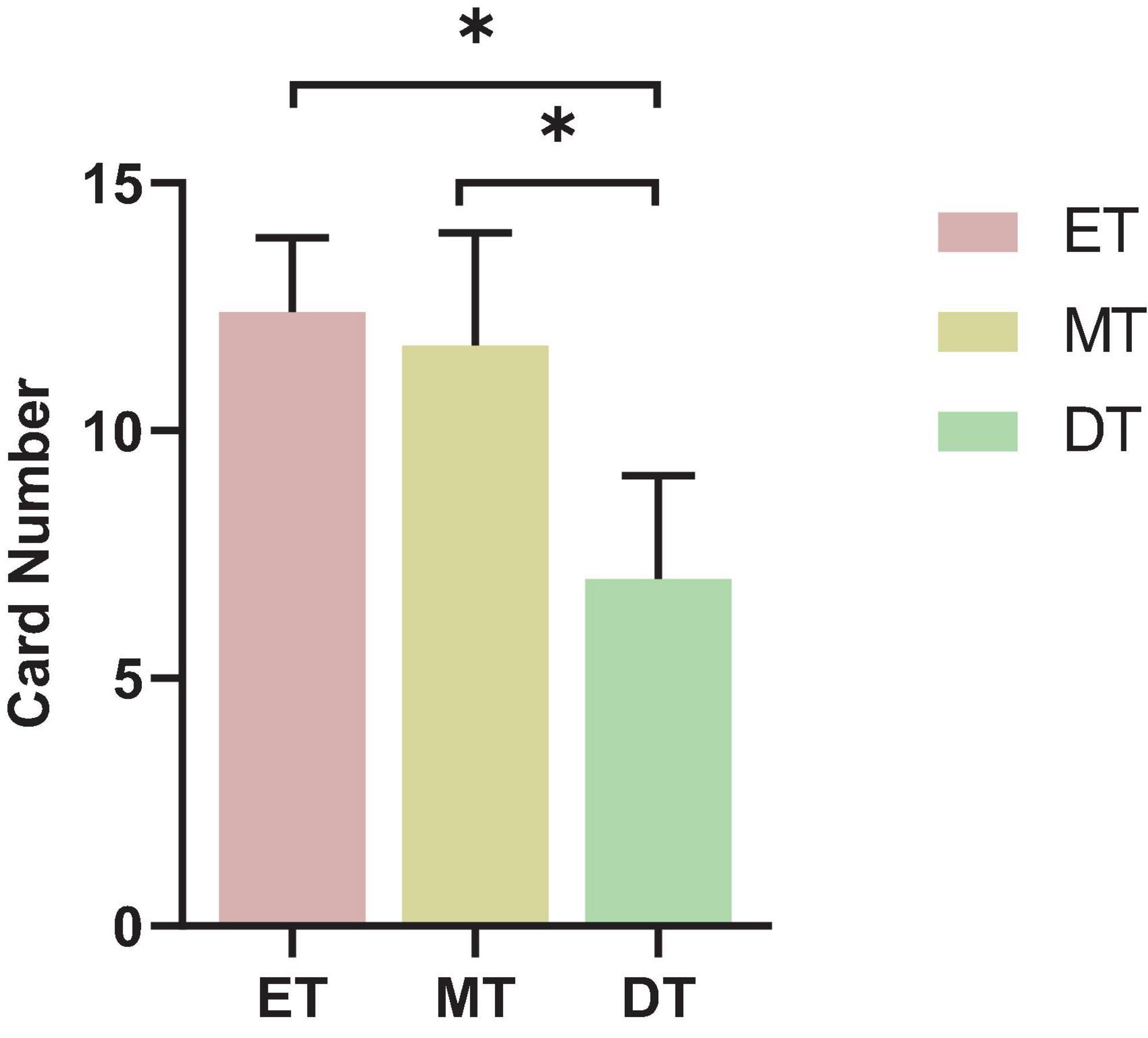
Figure 8. Results of cognitive performance. *P < 0.05. ET, easy task; MT, medium task; DT, difficult task.
4 Discussion
This study aimed to explore the impact of varying difficulty levels of interactive cognitive-motor dual task on brain activation, FC, and behavioral performance in healthy adults using fNIRS. By examining the hemodynamic responses across the PFC and motor cortex, significant HbO differences were observed in the DRPFC, LPMC, RPMC, RSC, LMC, and RMC across the ET, MT, and DT conditions. Additionally, FC analysis showed distinct connectivity strengths across these regions as task difficulty increased, particularly between regions such as LSC∼RPMC, RPMC∼RSC, and RSC∼RMC. These results support our initial hypothesis that as task difficulty increased, both brain activation and FC across multiple cortical regions also increased. Our findings reveal notable adaptations in brain activation patterns and FC strength in response to increasing task difficulty, offering valuable insights into the neural mechanisms underlying motor-cognitive integration.
In this study, significant ROI-level activation differences were primarily observed in the DRPFC, LPMC, RPMC, RSC, LMC, and RMC regions. Elevated metabolic activity in the PFC has proven that it is strongly associated with increased planning and attention in motor and cognitive tasks (Hamacher et al., 2015; Wang et al., 2023). The motor cortex plays a critical role in the planning, coordination, and execution of motor functions, which are essential for gait control during dual-task walking (Ou et al., 2024; Rubenstein and Rakic, 2020). Previous studies on dual-task walking have predominantly focused on PFC activation, with findings showing that PFC activation generally increases during dual-task walking compared to single-task walking (Al-Yahya et al., 2019; Chen et al., 2022; Stuart et al., 2018). However, in our study, DLPFC activation during MT was lower than during ET. This discrepancy may be attributed to the specific nature of the motor-cognitive interaction, where the demands on motor coordination might outweigh those on cognitive engagement, supporting the “posture-first” strategy (Bloem et al., 2006). This strategy, in which individuals prioritize the walking task over other concurrent tasks, is typically used by younger individuals but less frequently by older adults (Bloem et al., 2006). In this scenario, motor cortex, rather than the PFC, may take on a more prominent role as the cognitive component is deprioritized. These findings imply that the allocation of cognitive and motor resources could shift depending on task complexity, with some motor-cognitive tasks diverting processing demands from the PFC to motor regions. Furthermore, as task difficulty increased in this study, activation levels in the LPMC/RPMC and LMC/RMC regions also rose, reinforcing this explanation.
Further examination of channel-specific activation revealed significant differences in channels 3, 18, 24, 25, 28, and 29, located in the LPFC, LPMC, RPMC, RSC, and RMC. These findings closely correspond to the activation patterns observed in the ROIs, indicating that task complexity influences not only larger cortical areas but also distinct neural pathways essential for motor-cognitive integration. Channel 3 in the LPFC appears to play a crucial role in higher-order cognitive processing, while the increased activation in channels 18, 24, 25, 28, and 29 likely reflects a more nuanced response within motor cortex regions, facilitating precise motor control under higher task demands. These findings have implications for designing personalized rehabilitation protocols based on channel- and ROI-specific activation patterns. For patients in need of cognitive rehabilitation, exercises can be designed to intensively engage the LPFC, enhancing executive function and cognitive control during physical tasks. Conversely, for patients who require improved motor coordination, activities engaging the LPMC, RPMC, RSC, and RMC can be prioritized to bolster motor control and sensory processing. This targeted approach to rehabilitation may optimize both cognitive and motor function recovery based on individual activation patterns.
Our analysis of FC showed that the average channel-to-channel connectivity strength was lower during the ET compared to the DT, consistent with previous studies indicating that FC strength typically increases with greater task difficulty and cognitive load (Chen et al., 2022; Ding et al., 2024). This suggests a neural recruitment mechanism, where higher task complexity enhances coordination between brain regions to optimize the synchronization of cognitive and motor resources (Chen et al., 2022; Chirles et al., 2017). Interestingly, despite the increase in cognitive task difficulty, we observed stronger FC in motor regions, particularly between LSC∼RPMC, RPMC∼RSC, and RSC∼RMC. This aligns with our activation results, which showed significant engagement of the LPMC, RPMC, RSC, and RMC, suggesting that motor regions play a more prominent role under higher cognitive load. One possible explanation is that greater cognitive demand increases the need for motor planning and coordination, requiring greater motor cortex involvement to maintain gait stability and movement control (Rubenstein and Rakic, 2020). This supports previous research showing that higher task complexity recruits additional motor resources to prevent performance deterioration (Chen et al., 2022; Chirles et al., 2017; Obrig et al., 2000). Additionally, the strengthened FC in motor regions further supports the “posture-first” strategy, where individuals prioritize stable motor execution over cognitive engagement under high cognitive demand. These findings suggest that even when task difficulty is primarily cognitive, the motor system adapts to maintain performance, highlighting the strong interaction between cognitive and motor networks in dual-task conditions.
The LI analysis in this study revealed a dominant role of the right hemisphere during the interactive motor-cognitive dual tasks. Traditionally, the right hemisphere is specialized in functions such as spatial cognition, visual-spatial processing, and motor control (Borrell et al., 2023; Güntürkün et al., 2020). In our dual-task setup, which combined walking with a Stroop task, participants were required to identify both the color and spatial position of words, tasks that heavily rely on spatial processing. This explains the stronger involvement of the right hemisphere, as it aligns with the cognitive demands of the task. Our findings are consistent with recent neuroimaging studies, which have shown right-lateralized activation in the DLPFC during dual-task paradigms involving spatial navigation and walking (Möller et al., 2024; Rahman et al., 2021). This suggests that the right hemisphere plays a crucial role in integrating cognitive and motor functions, particularly when tasks require simultaneous spatial processing and movement control. Additionally, previous studies have suggested that handedness is one of the factors affecting the lateralization pattern (Shirzadi et al., 2020). All participants in this study were right-handed, which is representative of the general population. Research suggests that both right- and left-handed individuals tend to show varying degrees of right-hemisphere dominance for spatial processing (Powell et al., 2012). Some studies further indicate that while right-handed individuals typically rely on the right hemisphere for spatial tasks, left-handed individuals may display less pronounced hemispheric specialization (Shirzadi et al., 2020; Vogel et al., 2003). These findings highlight the complexity of hemispheric dominance and its role in spatial-motor integration.
As task difficulty increased, participants exhibited enhanced FC, yet their behavioral performance declined, with slower gait parameters and reduced word identification. This suggests that although the brain recruits additional neural resources to compensate for increased cognitive load, this adaptation is insufficient to fully counteract dual-task interference. When cognitive demands exceed the brain’s processing capacity, performance declines become inevitable (Chen et al., 2022; Hairu et al., 2021). Several theories explain this phenomenon (Pashler et al., 2001; Wickens, 2002; Wollesen and Voelcker-Rehage, 2014). The central bottleneck theory suggests that a bottleneck in information processing allows only one task to be processed at a time; thus, processing of a second task cannot begin until the first task is complete, often resulting in delayed responses in a dual task setting (Pashler et al., 2001). The four-dimensional multiple resource model posits that interference between tasks increases when they share stages, sensory modalities, processing codes, or visual information channels (Wickens, 2002). Additionally, the attentional resource theory explains declines in motor–cognitive functioning under dual task conditions as stemming from competing demands for attentional resources, leading to interference and reduced performance in one or both tasks (Wollesen and Voelcker-Rehage, 2014). Beyond experimental findings, these deficits have real-world implications, such as an increased risk of falls in older adults or individuals with neurological conditions. While enhanced FC may indicate a neural compensation mechanism, it does not fully offset the behavioral impairments caused by higher cognitive demands. This suggests that under high task loads, the brain’s ability to allocate resources remains constrained. Future research should investigate whether dual task training can improve cognitive-motor integration, potentially reducing interference effects and enhancing both movement stability and cognitive performance in daily life.
This study provides a critical foundation for developing intervention strategies in populations with cognitive impairments. The observed brain activation patterns suggest that personalized dual-task training (e.g., walking combined with cognitive challenges) can be designed to enhance specific brain region functions: LPFC-focused interventions may improve executive function, while premotor and motor cortex training could optimize motor coordination. Additionally, the progressive strengthening of FC with increasing task difficulty supports the implementation of gradual difficulty progression training, which promotes efficient neural resource integration and reduces cognitive-motor interference in daily life. The dominance of the right hemisphere in spatial processing and motor control, highlights its clinical significance for patients with right-hemisphere impairments (e.g., Alzheimer’s disease or right-hemisphere stroke). Future interventions could prioritize spatial-oriented dual tasks that activate the right hemisphere, tailored to individual lateralization patterns, to maximize rehabilitation outcomes.
Despite the valuable insights offered by this study, several limitations should be acknowledged. First, the sample size was relatively small, which may limit the statistical power and generalizability of the results; future studies with larger cohorts are needed to confirm these findings. Second, the study exclusively included healthy young adults, which restricts the applicability of the results to clinical populations. Replicating the study in individuals with neurological impairments would provide greater insight into the potential translational value of the findings for rehabilitation. Finally, although the interactive Stroop-walking paradigm was designed to mimic real-world dual-task situations, it may not fully capture the complexity and unpredictability of everyday environments. Therefore, the ecological validity of the task remains limited and should be further examined in future research.
5 Conclusion
We found that increased task difficulty heightened activation in the premotor and motor cortices, with a tendency toward right hemisphere dominance. Higher task difficulty also strengthened FC, particularly in motor-related regions, indicating greater neural coordination. Behaviorally, participants exhibited slower gait parameters and reduced cognitive performance as task complexity increased, highlighting the impact of dual-task interference.
These findings provide valuable insights into the neural mechanisms of motor-cognitive integration. The right hemisphere’s crucial role in spatial processing and motor control highlights the potential benefits of spatially oriented dual-task training for patients with right-hemisphere impairments, such as stroke or Alzheimer’s disease. Furthermore, the observed increase in FC under high cognitive load supports the use of progressive dual-task training to improve cognitive-motor coordination. For individuals with cognitive deficits, interventions targeting the LPFC may enhance executive function, while motor cortex-focused training could improve movement stability. Future studies should investigate these effects in populations with neurological conditions and explore the long-term impact of dual-task training on brain plasticity and functional recovery.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Ningbo Rehabilitation Hospital (2022-03-G2). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XL: Data curation, Writing – original draft. LT: Data curation, Writing – original draft. YZ: Data curation, Writing – original draft. LY: Data curation, Writing – original draft. LZ: Writing – review and editing. MT: Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This research was funded by the research project of Ningbo Rehabilitation Hospital (Project Numbers: 2024KY01, 2022KY05), the 2023 Annual Pathophysiology Technology Research Key Laboratory Open Fund of Zhejiang Province (Project Number: 202307), Medicine and Healthcare in Zhejiang Province Science and Technology Plan Projects of Traditional Chinese Medicine (Project Number: 2024ZL954), the Collaborative Innovation Center for Health and Elderly Care Application Technology and Standards (Project Number: JKYL2022010), and Ningbo Key Research and Development Plan Project (Project Number: 2023Z173).
Acknowledgments
We thank all the participants for participating in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2025.1464617/full#supplementary-material
References
Al-Yahya, E., Mahmoud, W., Meester, D., Esser, P., and Dawes, H. (2019). Neural substrates of cognitive motor interference during walking; Peripheral and central mechanisms. Front. Hum. Neurosci. 12:536. doi: 10.3389/fnhum.2018.00536
Bloem, B., Grimbergen, Y., van Dijk, J., and Munneke, M. (2006). The posture second; strategy: A review of wrong priorities in Parkinson’s disease. J. Neurol. Sci. 248, 196–204. doi: 10.1016/j.jns.2006.05.010
Borrell, J., Fraser, K., Manattu, A., and Zuniga, J. (2023). Laterality index calculations in a control study of functional near infrared spectroscopy. Brain Topogr. 36, 210–222. doi: 10.1007/s10548-023-00942-3
Cao, X., Zhao, Z., Kang, Y., Tian, Y., Song, Y., Wang, L., et al. (2022). The burden of cardiovascular disease attributable to high systolic blood pressure across China, 2005-18: A population-based study. Lancet Public Health 7, e1027–e1040. doi: 10.1016/S2468-2667(22)00232-8
Chen, Y., Cao, Z., Mao, M., Sun, W., Song, Q., and Mao, D. (2022). Increased cortical activation and enhanced functional connectivity in the prefrontal cortex ensure dynamic postural balance during dual-task obstacle negotiation in the older adults: A fNIRS study. Brain Cogn. 163:105904. doi: 10.1016/j.bandc.2022.105904
Chirles, T., Reiter, K., Weiss, L., Alfini, A., Nielson, K., and Smith, J. (2017). Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J. Alzheimers Dis. 57, 845–856. doi: 10.3233/JAD-161151
Ding, Q., Ou, Z., Yao, S., Wu, C., Chen, J., Shen, J., et al. (2024). Cortical activation and brain network efficiency during dual tasks: An fNIRS study. Neuroimage 289:120545. doi: 10.1016/j.neuroimage.2024.120545
Güntürkün, O., Ströckens, F., and Ocklenburg, S. (2020). Brain lateralization: A comparative perspective. Physiol. Rev. 100, 1019–1063. doi: 10.1152/physrev.00006.2019
Hairu, R., Close, J., Lord, S., Delbaere, K., Wen, W., Jiang, J., et al. (2021). The association between white matter hyperintensity volume and gait performance under single and dual task conditions in older people with dementia: A cross-sectional study. Arch. Gerontol. Geriatr. 95:104427. doi: 10.1016/j.archger.2021.104427
Hamacher, D., Herold, F., Wiegel, P., Hamacher, D., and Schega, L. (2015). Brain activity during walking: A systematic review. Neurosci. Biobehav. Rev. 57, 310–327. doi: 10.1016/j.neubiorev.2015.08.002
Hermand, E., Tapie, B., Dupuy, O., Fraser, S., Compagnat, M., Salle, J., et al. (2019). Prefrontal cortex activation during dual task with increasing cognitive load in subacute stroke patients: A pilot study. Front. Aging Neurosci. 11:160. doi: 10.3389/fnagi.2019.00160
Herold, F., Hamacher, D., Schega, L., and Müller, N. (2018). Thinking while moving or moving while thinking - Concepts of motor-cognitive training for cognitive performance enhancement. Front. Aging Neurosci. 10:364696. doi: 10.3389/fnagi.2018.00228
Ji, X., Quan, W., Yang, L., Chen, J., Wang, J., and Wu, T. (2020). Classification of schizophrenia by seed-based functional connectivity using prefronto-temporal functional near infrared spectroscopy. J. Neurosci. Methods 344:108874. doi: 10.1016/j.jneumeth.2020.108874
Kang, H. (2021). Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 18:17. doi: 10.3352/jeehp.2021.18.17
Laguë-Beauvais, M., Fraser, S., Desjardins-Crépeau, L., Castonguay, N., Desjardins, M., Lesage, F., et al. (2015). Shedding light on the effect of priority instructions during dual-task performance in younger and older adults: A fNIRS study. Brain Cogn. 98, 1–14. doi: 10.1016/j.bandc.2015.05.001
Lapanan, K., Kantha, P., Nantachai, G., Hemrungrojn, S., and Maes, M. (2023). The prefrontal cortex hemodynamic responses to dual-task paradigms in older adults: A systematic review and meta-analysis. Heliyon 9:e17812. doi: 10.1016/j.heliyon.2023.e17812
Lauenroth, A., Ioannidis, A., and Teichmann, B. (2016). Influence of combined physical and cognitive training on cognition: A systematic review. BMC Geriatr. 16:141. doi: 10.1186/s12877-016-0315-1
Leff, D., Orihuela-Espina, F., Elwell, C., Athanasiou, T., Delpy, D., Darzi, A., et al. (2011). Assessment of the cerebral cortex during motor task behaviours in adults: A systematic review of functional near infrared spectroscopy (fNIRS) studies. Neuroimage 54, 2922–2936. doi: 10.1016/j.neuroimage.2010.10.058
Li, H., Fu, X., Lu, L., Guo, H., Yang, W., Guo, K., et al. (2023). Upper limb intelligent feedback robot training significantly activates the cerebral cortex and promotes the functional connectivity of the cerebral cortex in patients with stroke: A functional near-infrared spectroscopy study. Front. Neurol. 14:1042254. doi: 10.3389/fneur.2023.1042254
Li, Q., Feng, J., Guo, J., Wang, Z., Li, P., Liu, H., et al. (2020). Effects of the multisensory rehabilitation product for home-based hand training after stroke on cortical activation by using NIRS methods. Neurosci. Lett. 717:134682. doi: 10.1016/j.neulet.2019.134682
Liu, Y., Huo, C., Lu, K., Liu, Q., Xu, G., Ji, R., et al. (2021). Correlation between gait and near-infrared brain functional connectivity under cognitive tasks in elderly subjects with mild cognitive impairment. Front. Aging Neurosci. 13:482447. doi: 10.3389/fnagi.2021.482447
Miura, H., Ono, Y., Suzuki, T., Ogihara, Y., Imai, Y., Watanabe, A., et al. (2024). Regional brain activity and neural network changes in cognitive-motor dual-task interference: A functional near-infrared spectroscopy study. Neuroimage 297:120714. doi: 10.1016/j.neuroimage.2024.120714
Möller, S., Hagberg, K., and Ramstrand, N. (2024). Cognitive load in individuals with a transfemoral amputation during single- and dual-task walking: A pilot study of brain activity in people using a socket prosthesis or a bone-anchored prosthesis. J. Rehabil. Med. 56:jrm40111. doi: 10.2340/jrm.v56.40111
Obrig, H., Neufang, M., Wenzel, R., Kohl, M., Steinbrink, J., Einhäupl, K., et al. (2000). Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage 12, 623–639. doi: 10.1006/nimg.2000.0657
Ou, Z., Ding, Q., Yao, S., Zhang, L., Li, Y., Lan, Y., et al. (2024). Functional near-infrared spectroscopy evidence of cognitive-motor interference in different dual tasks. Eur. J. Neurosci. 59, 3045–3060. doi: 10.1111/ejn.16333
Pashler, H., Johnston, J., and Ruthruff, E. (2001). Attention and performance. Annu. Rev. Psychol. 52, 629–651. doi: 10.1146/annurev.psych.52.1.629
Plummer, P., Apple, S., Dowd, C., and Keith, E. (2015). Texting and walking: Effect of environmental setting and task prioritization on dual-task interference in healthy young adults. Gait Posture. 41, 46–51. doi: 10.1016/j.gaitpost.2014.08.007
Powell, J., Kemp, G., and García-Finaña, M. (2012). Association between language and spatial laterality and cognitive ability: An fMRI study. Neuroimage 59, 1818–1829. doi: 10.1016/j.neuroimage.2011.08.040
Qi, L., Wang, G., Yang, Y., Yang, S., Liu, L., and Zhang, J. (2024). Positive effects of brisk walking and Tai Chi on cognitive function in older adults: An fNIRS study. Physiol. Behav. 273:114390. doi: 10.1016/j.physbeh.2023.114390
Rahman, T., Polskaia, N., St-Amant, G., Salzman, T., Vallejo, D., Lajoie, Y., et al. (2021). An fNIRS investigation of discrete and continuous cognitive demands during dual-task walking in young adults. Front. Hum. Neurosci. 15:711054. doi: 10.3389/fnhum.2021.711054
Ranchet, M., Hoang, I., Cheminon, M., Derollepot, R., Devos, H., Perrey, S., et al. (2020). Changes in prefrontal cortical activity during walking and cognitive functions among patients with Parkinson’s disease. Front. Neurol. 11:601686. doi: 10.3389/fneur.2020.601686
Riedel, N., Herzog, M., Stein, T., and Deml, B. (2024). Cognitive-motor interference during walking with modified leg mechanics: A dual-task walking study. Front. Psychol. 15:1375029. doi: 10.3389/fpsyg.2024.1375029
Rubenstein, J., and Rakic, P. (2020). Neural Circuit and Cognitive Development: Comprehensive Developmental Neuroscience. Cambridge, MA: Academic Press.
Schott, N. (2015). [Trail walking test for assessment of motor cognitive interference in older adults. Development and evaluation of the psychometric properties of the procedure]. Z. Gerontol. Geriatr. 48, 722–733. doi: 10.1007/s00391-015-0866-3
Seghier, M. (2008). Laterality index in functional MRI: Methodological issues. Magn. Reson. Imaging 26, 594–601. doi: 10.1016/j.mri.2007.10.010
Shams, L., and Seitz, A. (2008). Benefits of multisensory learning. Trends Cogn. Sci. 12, 411–417. doi: 10.1016/j.tics.2008.07.006
Shirzadi, S., Einalou, Z., and Dadgostar, M. (2020). Investigation of functional connectivity during working memory task and hemispheric lateralization in left-and right-handers measured by fNIRS. Optik 221:165347. doi: 10.1016/j.ijleo.2020.165347
Stuart, S., Vitorio, R., Morris, R., Martini, D., Fino, P., and Mancini, M. (2018). Cortical activity during walking and balance tasks in older adults and in people with Parkinson’s disease: A structured review. Maturitas 113, 53–72. doi: 10.1016/j.maturitas.2018.04.011
Tait, J., Duckham, R., Milte, C., Main, L., and Daly, R. (2017). Influence of sequential vs. simultaneous dual-task exercise training on cognitive function in older adults. Front. Aging Neurosci. 9:368. doi: 10.3389/fnagi.2017.00368
Tombaugh, T., and McIntyre, N. (1992). The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 40, 922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x
Vogel, J., Bowers, C., and Vogel, D. (2003). Cerebral lateralization of spatial abilities: A meta-analysis. Brain Cogn. 52, 197–204. doi: 10.1016/s0278-2626(03)00056-3
Wang, Q., Dai, W., Xu, S., Zhu, S., Sui, Y., Kan, C., et al. (2023). Brain activation of the PFC during dual-task walking in stroke patients: A systematic review and meta-analysis of functional near-infrared spectroscopy studies. Front. Neurosci. 17:1111274. doi: 10.3389/fnins.2023.1111274
Wickens, C. (2002). Multiple resources and performance prediction. Theoretical issues in ergonomics science. Thero Issue Ergonomics Sci. 3, 159–177. doi: 10.1080/14639220210123806
Wollesen, B., and Voelcker-Rehage, C. (2014). Training effects on motor–cognitive dual-task performance in older adults: A systematic review. Eur. Rev. Aging Phys. Activity 11, 5–24. doi: 10.1007/s11556-013-0122-z
Wollesen, B., Voelcker-Rehage, C., Regenbrecht, T., and Mattes, K. (2016). Influence of a visual-verbal Stroop test on standing and walking performance of older adults. Neuroscience 318, 166–177. doi: 10.1016/j.neuroscience.2016.01.031
Xia, D., Quan, W., and Wu, T. (2022). Optimizing functional near-infrared spectroscopy (fNIRS) channels for schizophrenic identification during a verbal fluency task using metaheuristic algorithms. Front. Psychiatry 13:939411. doi: 10.3389/fpsyt.2022.939411
Yang, J., Ji, X., Quan, W., Liu, Y., Wei, B., and Wu, T. (2020). Classification of schizophrenia by functional connectivity strength using functional near infrared spectroscopy. Front. Neuroinform 14:40. doi: 10.3389/fninf.2020.00040
Yao, Q., Chen, L., Qu, H., Fan, W., He, L., Li, G., et al. (2024). Comparable cerebral cortex activity and gait performance in elderly hypertensive and healthy individuals during dual-task walking: A fNIRS study. Brain Behav. 14:e3568. doi: 10.1002/brb3.3568
Yogev-Seligmann, G., Hausdorff, J., and Giladi, N. (2012). Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Mov. Disord. 27, 765–770. doi: 10.1002/mds.24963
Keywords: fNIRS, interactive dual task, brain activation, functional connectivity, lateralization
Citation: Li X, Tang L, Zhang Y, Ye L, Zhou L and Tang M (2025) The impact of interactive motor-cognitive dual tasking on brain activation, functional connectivity, and behavioral performance in healthy adults: an fNIRS study. Front. Hum. Neurosci. 19:1464617. doi: 10.3389/fnhum.2025.1464617
Received: 14 July 2024; Accepted: 02 June 2025;
Published: 25 June 2025.
Edited by:
Zhen Yuan, University of Macau, ChinaReviewed by:
Tongning Wu, China Academy of Information and Communications Technology, ChinaRateb Katmah, Khalifa University, United Arab Emirates
Copyright © 2025 Li, Tang, Zhang, Ye, Zhou and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Tang, dGFuZ21pbjg4NzI1OTJAc2luYS5jb20=
†These authors have contributed equally to this work and share senior authorship
 Xiaohan Li
Xiaohan Li Lifeng Tang
Lifeng Tang Yuting Zhang
Yuting Zhang Lin Ye1
Lin Ye1