- 1Department of Neurology, Dalian Friendship Hospital, Dalian, China
- 2Department of Critical Care Medicine, The First Hospital of Qiqihaer, Qiqihar, China
1 Introduction
Immunoglobulin G4 (IgG4)-related disease (IgG4-RD) is a systemic, chronic, inflammatory, and fibrotic condition related to the immune system that can affect various parts of the body (Al-Mujaini et al., 2018). However, neurological involvement is rare, particularly in reported cases involving the dura mater (Okazaki et al., 2011). Currently, both domestically and internationally, research on IgG4-related spinal pachymeningitis (IgG4-RSP) is mostly limited to case reports. There is a lack of research on the clinical features of this disease. This study retrospectively analyzed individual case reports of IgG4-RSP published in the PubMed, Web of Science, and Embase databases from 2009 to the present. The study then summarized and analyzed the clinical features of IgG4-RSP. The specific content is reported below.
2 Methods
2.1 Data sources
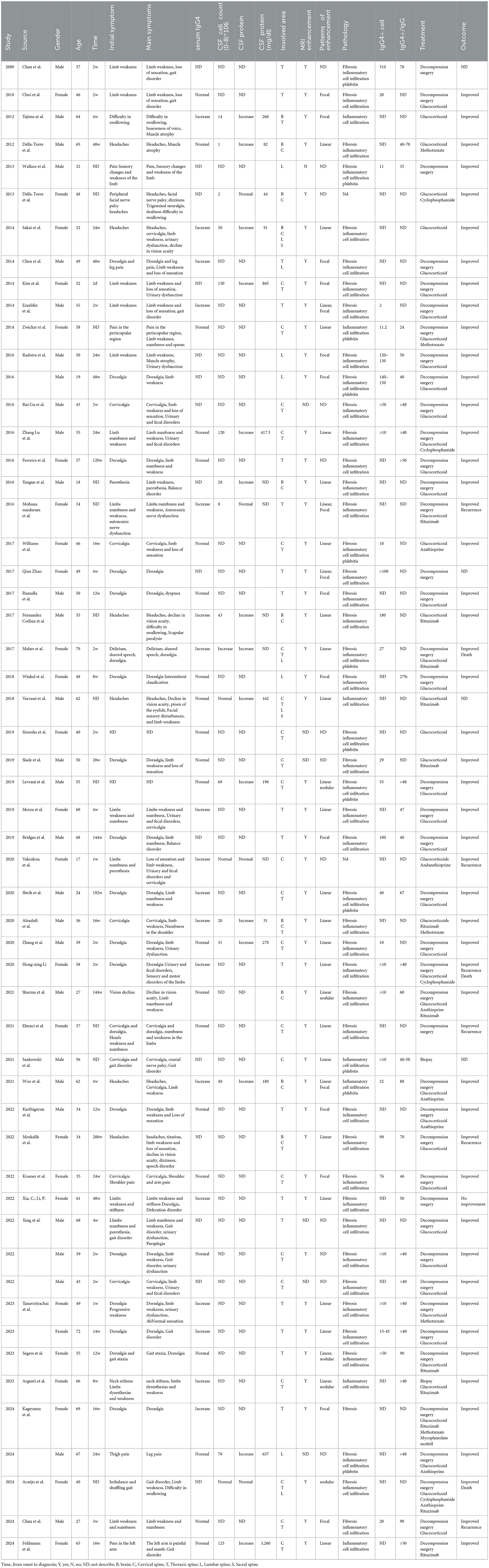
Using the search terms “immunoglobulin G4,” “hypertrophic spinal pachymeningitis,” “IgG4-related disease,” and “IgG4-related spinal pachymeningitis,” case reports and single-center studies published in the PubMed, Web of Science, and Embase databases were retrieved from domestic and international sources up to September 2024. A total of 362 articles were retrieved: 162 from PubMed, 98 from the Web of Science, and 102 from the Embase database. Of these, 140 were duplicates. After reading the full texts, irrelevant medical records and incomplete reports were excluded, resulting in 50 eligible articles comprising data on 55 patients. All patients were diagnosed with IgG4-RSP by a specialist clinician, and they had relatively complete clinical records. The articles were read in detail, and the following information was extracted: patient gender, age of onset, initial symptoms, clinical manifestations, laboratory tests, sites of lesions, pathological results, treatment methods, prognosis, and situations of misdiagnosis (Table 1).
3 Statistical analysis
The data were analyzed using SPSS 26.0 statistical software. The measurement data approximately conforming to a normal distribution and a skewed distribution were expressed as mean ± standard deviation (x ± s) and median (the first quartile, the third quartile) [M(Q1, Q3)], respectively. A p-value of < 0.05 was considered statistically significant.
4 Results
4.1 General information
Of the 55 patients, 29 were men and 26 were women, resulting in a male-to-female ratio of approximately 1.1:1. The mean age at onset was 48.29 years, ranging from 17 to 79 years. For 44 patients, the time from symptom onset to the diagnosis of IgG4-RSP upon admission was 7.93 (range 0.50–6.00) months.
4.2 Initial and principal symptoms
The initial symptoms of 53 patients were recorded, and the incidence rates, from highest to lowest, were as follows: pain (71.7%, 38/53), sensory abnormalities or weakness (32.1%, 17/53), gait disorders (7.5%, 4/53), and other symptoms such as difficulty swallowing and visual impairment. Among the patients whose initial symptom was pain, 47% (18/38) experienced dorsalgia, 21.1% (8/38) experienced cervicalgia, and the remaining patients experienced headache, limb pain, and scapular region pain, in that order. Among the 53 patients, the most common symptoms were sensory abnormalities or weakness (75.5%, 40/53) and pain (75.5%, 40/53). The other symptoms included urinary and fecal disorders (28.3%, 15/53), gait disorders, and cranial nerve involvement. Furthermore, 17% (9/53) of the patients experienced involvement of other organ systems.
4.3 Imaging manifestations
All 55 patients underwent at least one spinal magnetic resonance imaging (MRI) scan. The images revealed focal or diffuse thickening of the dura mater. A total of 49 patients were evaluated for enhanced MRI information, and of them, 98.0% (48/49) exhibited lesion enhancement on the enhanced MRI. Enhanced lesions were observed in 43 patients, including 29 cases of diffuse linear enhancement, 14 cases of focal or nodular enhancement, and 8 cases exhibiting both diffuse linear and focal or nodular enhancements. All spinal cord segments could be involved; the highest incidence was in the thoracic spine (n = 41, 74.5%), followed by the cervical spine (n = 31, 56.4%), lumbar spine (n = 10, 18.2%), and sacral spine (n = 2, 3.6%). Combined involvement of the cervical and thoracic spines was relatively common (n = 22, 40%). In addition, dura mater involvement in the brain was observed in 10 patients.
4.4 Laboratory tests
Among the 55 patients, serum IgG4 levels were measured in 39 patients, of whom 41.0% (16/39) had elevated serum IgG4 levels. Of the 17 patients who underwent routine cerebrospinal fluid examinations, cell count and protein levels in the cerebrospinal fluid increased in 70.6% (12/17) and 82.4% (14/17), respectively. The cerebrospinal fluid cell count ranged from 1 × 106 to 150 × 106, with a median of 31 × 106. The cerebrospinal fluid protein level ranged from 44 mg/dL to 865 mg/dL, with a median of 188 mg/dL.
4.5 Pathological examination
Of the 55 patients, 53 underwent pathological biopsy. The pathological changes were primarily characterized by lymphocyte and plasma cell infiltration. In addition, 86.8% (46/53) of the patients exhibited mat fibrosis, while 22.6% (12/53) exhibited obliterated phlebitis. Furthermore, 94.7% (36/38) had an IgG4-to-IgG cell ratio of more than 40% or more than 10 IgG4/high-power field (HPF) in pathological tissue.
4.6 Treatment and prognosis
A total of 98.2% (54/55) of the patients received at least one treatment protocol. Among them, 77.8% (42/54) underwent decompression surgery, 88.9% (48/54) received glucocorticoids, 25.9% (14/54) received traditional immunosuppressants (including azathioprine, methotrexate, and cyclophosphamide), and 22.2% (12/54) received rituximab. Furthermore, 40% (22/54) of the patients received a treatment regimen combining glucocorticoids with immunosuppressants and/or rituximab. Of the 55 patients, four were lost to follow-up and three died, two of whom died from secondary infections following glucocorticoid and immunosuppressant treatment. Six cases had symptom recurrence, and 98.0% (50/51) of the patients experienced varying degrees of symptom relief.
5 Discussion
IgG4-related disease (IgG4-RD) is an inflammatory and fibrotic disorder that affects multiple organs. It is mainly characterized by elevated serum IgG4 levels and the enlargement of affected organs and tissues. The most frequently affected glands include the pancreas, submandibular gland, and lacrimal gland. Involvement of the nervous system is rare (Wallace et al., 2019). In the nervous system, the dura mater and pituitary gland are the most frequently affected sites. Previous studies have statistically shown that the incidence of IgG4-related pachymeningitis is 1.9% (Wallace et al., 2015), while the incidence of hypophysitis is between 1.7% and 2.3% (Zhang et al., 2017; Lin et al., 2015). However, IgG4-RSP is primarily reported as individual cases. This disease primarily affects middle-aged individuals, with a male-to-female ratio of approximately 1.1:1. The clinical manifestations are complex and diverse, posing significant challenges to diagnosis. This study summarizes the clinical and pathological characteristics of patients with IgG4-RSP to enhance neurologists' awareness of this disease and enable early diagnosis and treatment.
The clinical manifestations of IgG4-RSP are non-specific and associated with the affected spinal cord regions. They mainly manifest as radicular pain and symptoms of spinal cord compression. Among the 53 cases described in this article, 38 patients reported pain as their initial symptom. Of those patients, 47% experienced dorsalgia and approximately 21% experienced cervicalgia. The most prevalent symptoms were weakness and/or sensory abnormalities in the trunk or limbs (75.5%), pain in the trunk or limbs (75.5%), and urination and bowel disorders (28.3%).
The symptoms of IgG4-RSP are attributed to compression of the spinal nerve roots and spinal cord by thickened and hyperplastic dura mater. Spinal cord imaging examinations are essential for diagnosis. Magnetic resonance imaging (MRI) of the spinal cord is the preferred diagnostic method for IgG4-RSP because it can accurately display the lesion's location, range, and degree of spinal cord compression, as well as its progression during follow-up. The condition is characterized by uneven thickening of the affected dura mater, which appears as a low signal on T1WI images and a low or isointense signal on T2WI images. Cross-sectional images show that the thickened dura mater compresses the adjacent spinal cord, causing it to become thinner. An enhanced scan reveals dura mater enhancement (Alsulaiman, 2020). In the patients with IgG4-RSP summarized in this article, spinal cord involvement most commonly occurred in the thoracic spine, followed by the cervical spine. This finding is consistent with previous studies (Yang et al., 2022). If a patient exhibits symptoms of spinal cord compression and MRI of the spinal cord shows the aforementioned manifestations, the possibility of IgG4-RSP should be considered. Additional examinations should be performed to aid in the diagnosis.
There are no separate diagnostic criteria for IgG4-RSP. Diagnosis primarily depends on the diagnosis of IgG4-related disease and involvement of the spinal dura mater. The Comprehensive Diagnostic Criteria for IgG4-Related Diseases (2011) (Umehara et al., 2012), established in Japan in 2011, were the earliest comprehensive diagnostic guidelines for IgG4-RD. They encompass three aspects: clinical manifestations, serum IgG4 levels, and pathological features. The criteria were updated and discussed in 2020 (Umehara et al., 2021). Simple lymph node enlargement does not meet the requirements for clinical and imaging features. Typical tissue fibrosis, especially mat fibrosis, and obliterative phlebitis have been added to the pathological diagnostic features. Serum IgG4 levels are elevated in up to 90% of patients with IgG4-RD, but this estimate varies substantially depending on the type of patients included in studies (Katz and Stone, 2022). A 2016 Japanese survey revealed that 84.5% of patients with type 1 autoimmune pancreatitis (AIP) had high serum IgG4 levels (Masamune et al., 2020). Alessia Buglioni's research demonstrated that serum IgG4 levels were increased in 81% of patients with IgG4-related kidney disease (IgG4-RKD) (Buglioni et al., 2024). Xia et al. (2023) recruited a cohort of 40 patients diagnosed with IgG4-related sialadenitis (IgG4-RS), all exhibited elevated serum IgG4 levels. While elevated serum IgG4 levels are important for diagnosing IgG4-RD, some patients with IgG4-RSP do not exhibit elevated levels. The case results summarized in this article showed that approximately two-thirds of the patients had normal serum IgG4 levels, which is consistent with previous findings (Lu et al., 2016).
Therefore, elevated serum IgG4 levels are not specific to the diagnosis of IgG4-RD. However, clinical studies have shown (Wallace et al., 2015; Lin et al., 2015) that serum IgG4 levels can reflect disease activity to some extent and are positively correlated with the number of affected organs and the degree of organ fibrosis. In 2019, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) jointly introduced the first international classification standard for IgG4-RD (Wallace et al., 2020), emphasizing typical clinical manifestations of affected organs and changes observed in laboratory and imaging studies. This standard assigns weighted scores to each assessment indicator. This addresses the issue of diagnosing IgG4-RD when serum IgG4 levels are normal and pathological results are lacking. It is also more applicable to patients with IgG4-RD involving multiple organs. A diagnosis of IgG4-RSP still depends on pathological alterations. The characteristic histological features of IgG4-RD are as follows: (1) massive lymphoplasmacytic infiltration, (2) mat fibrosis, and (3) obliterative phlebitis (Deshpande et al., 2012). For immunoglobulin G4-related hypertrophic pachymeningitis (IgG4-RHP), immunohistochemical staining requires more than 10 IgG4-positive plasma cells per high-power field (HPF), and the IgG4-positive/IgG-positive plasma cell ratio must be greater than 40% (Saitakis and Chwalisz, 2021). The pathological diagnosis of IgG4-RSP mainly refers to that of IgG4-RHP.
IgG4-RD is an immune-mediated, chronic, inflammatory disease. Currently, drug treatment is based on glucocorticoids, supplemented by immunosuppressants and even biological agents (Perugino and Stone, 2020). When IgG4-RSP causes progressive neurological symptoms due to spinal cord compression, surgical decompression is effective. Patients with IgG4-RD respond well to glucocorticoids (Seegobin et al., 2021), but there are no clear standards for the dosage, tapering schedule, or duration of maintenance treatment. Clinical experience is the main guide for treatment. While nearly all patients with IgG4-RD respond to glucocorticoids, approximately 40% do not achieve complete remission and relapse within 1 year (Yunyun et al., 2017). When used in combination with glucocorticoids, immunosuppressants such as methotrexate, cyclophosphamide, azathioprine, tacrolimus, and mycophenolate mofetil can improve outcomes, reduce recurrence, accelerate the taper process, and reduce glucocorticoid side effects (de Pretis et al., 2017; Della-Torre et al., 2015; Luo et al., 2020; Buechter et al., 2014). If the aforementioned treatments are ineffective, biological agents can be considered. Rituximab (RTX) is the first and most widely used biological agent for treating IgG4-RD. It can significantly reduce serum IgG4 levels (Khosroshahi et al., 2010). RTX has a significant therapeutic effect on IgG4-RD remission (Carruthers et al., 2015), and regular use can reduce IgG4-RD recurrence (Campochiaro et al., 2019). Of the 55 patients discussed in this article, 48 received glucocorticoids, 14 received immunosuppressants, and 12 received rituximab. While the majority of the patients showed short-term improvement, no long-term improvement was observed, and some experienced recurrence during follow-up.
5.1 Limitations
As the dataset was based on case reports and small-scale studies, there may be inconsistencies in the descriptions of clinical symptoms, laboratory and imaging findings, and treatment plans.
6 Conclusion
IgG4-related spinal pachymeningitis (IgG4-RSP) is relatively uncommon in clinical settings. Its clinical symptoms and imaging results are not specific and depend on the affected area. Symptoms include local pain and spinal cord compression, which can result in progressive neurological impairment and paralysis. In patients with normal serum IgG4 levels and no other systemic involvement, the possibility of IgG4-RSP should be considered, and a dural biopsy should be performed promptly. Once the diagnosis is confirmed, early treatment is necessary; the prognosis for most treated patients is relatively good. The gold standard for diagnosing the disease is a spinal cord biopsy, but obtaining the sample is difficult and highly invasive. Some scholars have proposed using the quantitative concentration of IgG4 and the IgG4 index in cerebrospinal fluid as an alternative to biopsy. However, there are only a few case reports on IgG4 concentrations in cerebrospinal fluid, so its clinical application is limited. This may serve as a potential direction for future studies.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: no.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
BZ: Data curation, Writing – original draft, Writing – review & editing. LK: Software, Writing – original draft. XL: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Mujaini, A., Al-Khabori, M., Shenoy, K., and Wali, U. (2018). Immunoglobulin G4-related disease: an update. Oman Med. J. 33, 97–103. doi: 10.5001/omj.2018.20
Alsulaiman, A. (2020). Idiopathic hypertrophic spinal pachymeningitis: a diagnostic challenge: a case report and review of the literature. J. Neurosci. Rural Pract. 11, 175–177. doi: 10.1055/s-0039-1698008
Buechter, M., Klein, C. G., Kloeters, C., Schlaak, J. F., Canbay, A., Gerken, G., et al. (2014). Tacrolimus as a reasonable alternative in a patient with steroid-dependent and thiopurine-refractory autoimmune pancreatitis with IgG4-associated cholangitis. Z. Gastroenterol. 52, 564–568. doi: 10.1055/s-0034-1366331
Buglioni, A., Jenkins, S. M., Nasr, S. H., Zhang, P., Gibson, I. W., Alexander, M. P., et al. (2024). Clinicopathologic features of IgG4-related kidney disease. Kidney Int. Rep. 9, 2462–73. doi: 10.1016/j.ekir.2024.05.011
Campochiaro, C., Della-Torre, E., Lanzillotta, M., Bozzolo, E., Baldissera, E., Milani, R., et al. (2019). Long-term efficacy of maintenance therapy with Rituximab for IgG4-related disease. Eur. J. Intern. Med. 74:92–8. doi: 10.1016/j.ejim.2019.12.029
Carruthers, M. N., Topazian, M. D., Khosroshahi, A., Witzig, T. E., Wallace, Z. S., Hart, P. A., et al. (2015). Rituximab for IgG4-related disease: a prospective, open-label trial. Ann. Rheum. Dis. 74, 1171–1177. doi: 10.1136/annrheumdis-2014-206605
de Pretis, N., Amodio, A., Bernardoni, L., Campagnola, P., Capuano, F., Chari, S. T., et al. (2017). Azathioprine maintenance therapy to prevent relapses in autoimmune pancreatitis. Clin. Transl. Gastroenterol. 8:e90. doi: 10.1038/ctg.2017.17
Della-Torre, E., Campochiaro, C., Bozzolo, E. P., Dagna, L., Scotti, R., Nicoletti, R., et al. (2015). Methotrexate for maintenance of remission in IgG4-related disease. Rheumatology 54, 1934–1936. doi: 10.1093/rheumatology/kev244
Deshpande, V., Zen, Y., Chan, J. K., Yi, E. E., Sato, Y., Yoshino, T., et al. (2012). Consensus statement on the pathology of IgG4-related disease. Mod. Pathol. 25, 1181–1192. doi: 10.1038/modpathol.2012.72
Katz, G., and Stone, J. H. (2022). Clinical perspectives on IgG4-related disease and its classification. Annu. Rev. Med. 73, 545–562. doi: 10.1146/annurev-med-050219-034449
Khosroshahi, A., Bloch, D. B., Deshpande, V., and Stone, J. H. (2010). Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthr. Rheum. 62, 1755–1762. doi: 10.1002/art.27435
Lin, W., Lu, S., Chen, H., Wu, Q., Fei, Y., Li, M., et al. (2015). Clinical characteristics of immunoglobulin G4–related disease: a prospective study of 118 Chinese patients. Rheumatology 54, 1982–1990. doi: 10.1093/rheumatology/kev203
Lu, Z., Tongxi, L., Jie, L., Yujuan, J., Wei, J., Xia, L., et al. (2016). IgG4-related spinal pachymeningitis. Clin. Rheumatol. 35, 1549–1553. doi: 10.1007/s10067-015-3104-x
Luo, X., Peng, Y., Zhang, P., Li, J., Liu, Z., Lu, H., et al. (2020). Comparison of the effects of cyclophosphamide and mycophenolate mofetil treatment against immunoglobulin G4-related disease: a retrospective cohort study. Front. Med. 7:253. doi: 10.3389/fmed.2020.00253
Masamune, A., Kikuta, K., Hamada, S., Tsuji, I., Takeyama, Y., Shimosegawa, T., et al. (2020). Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2016. J. Gastroenterol. 55, 462–470. doi: 10.1007/s00535-019-01658-7
Okazaki, K., Uchida, K., Koyabu, M., Miyoshi, H., and Takaoka, M. (2011). Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. J. Gastroenterol. 46, 277–288. doi: 10.1007/s00535-011-0386-x
Perugino, C. A., and Stone, J. H. (2020). IgG4-related disease: an update on pathophysiology and implications for clinical care. Nat. Rev. Rheumatol. 16, 702–714. doi: 10.1038/s41584-020-0500-7
Saitakis, G., and Chwalisz, B. K. (2021). The neurology of IGG4-related disease. J. Neurol. Sci. 424:117420. doi: 10.1016/j.jns.2021.117420
Seegobin, K., Moustafa, M. A., Gannon, N., Keller, K., Hastings, J., Gupta, V., et al. (2021). Successful treatment of IgG4-related hypertrophic pachymeningitis with induction rituximab and dexamethasone followed by maintenance rituximab. Clin. Case Rep. 9, 1610–1614. doi: 10.1002/ccr3.3855
Umehara, H., Okazaki, K., Kawa, S., Takahashi, H., Goto, H., Matsui, S., et al. (2021). The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod. Rheumatol. 31, 529–533. doi: 10.1080/14397595.2020.1859710
Umehara, H., Okazaki, K., Masaki, Y., Kawano, M., Yamamoto, M., Saeki, T., et al. (2012). Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod. Rheumatol. 22, 21–30. doi: 10.3109/s10165-011-0571-z
Wallace, Z. S., Deshpande, V., Mattoo, H., Mahajan, V. S., Kulikova, M., Pillai, S., et al. (2015). IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 67, 2466–2475. doi: 10.1002/art.39205
Wallace, Z. S., Naden, R. P., Chari, S., Choi, H., Della-Torre, E., Dicaire, J. F., et al. (2020). The 2019 American College of Rheumatology/European league against rheumatism classification criteria for IgG4-related disease. Arthritis Rheumatol. 72, 7–19. doi: 10.1002/art.41120
Wallace, Z. S., Zhang, Y., Perugino, C. A., Naden, R., Choi, H. K., Stone, J. H., et al. (2019). Clinical phenotypes of IgG4-related disease: an analysis of two international cross-sectional cohorts. Ann. Rheum. Dis. 78, 406–412. doi: 10.1136/annrheumdis-2018-214603
Xia, R. H., Hu, Y. H., Qian, J. J., Wang, M., Zhang, Y., Liu, Y., et al. (2023). Analysis of clinicopathological characteristics of IgG4-related sialadenitis. Zhonghua Kou Qiang Yi Xue Za Zhi. 58, 815–820. doi: 10.3760/cma.j.cn112144-20230506-00186
Yang, F., Liu, Z., Zhang, Y., Li, P., Zhu, Y., Zhu, Q., et al. (2022). Case report: Clinical highlights and radiological classification of IgG4-related spinal pachymeningitis: a rare case series and updated review of the literature. Front. Oncol. 12:1035056. doi: 10.3389/fonc.2022.1035056
Yunyun, F., Yu, C., Panpan, Z., Hua, C., Di, W., Lidan, Z., et al. (2017). Efficacy of cyclophosphamide treatment for immunoglobulin G4-related disease with addition of glucocorticoids. Sci. Rep. 7:6195. doi: 10.1038/s41598-017-06520-5
Keywords: IgG4-related disease, IgG4-related disease (IgG4-RD), spinal pachymeningitis, clinical features, spinal, spinal cord injury
Citation: Zhang B, Kang L and Li X (2025) Clinical features of immunoglobulin G4-related spinal pachymeningitis. Front. Hum. Neurosci. 19:1541096. doi: 10.3389/fnhum.2025.1541096
Received: 07 December 2024; Accepted: 24 June 2025;
Published: 17 July 2025.
Edited by:
Nico Melzer, Universitätsklinikum Düsseldorf, GermanyCopyright © 2025 Zhang, Kang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohong Li, eGhsaWhoQDEyNi5jb20=
 Bingxue Zhang
Bingxue Zhang Lenan Kang
Lenan Kang Xiaohong Li
Xiaohong Li