- 1School of Sports Medicine and Rehabilitation, Beijing Sport University, Beijing, China
- 2Key Laboratory of Exercise Rehabilitation Science of the Ministry of Education, Beijing Sport University, Beijing, China
Background: The parietal lobe is an important cerebral cortex area for sensory information processing to maintain postural control. High-definition transcranial direct current stimulation (HD-tDCS) can improve the excitability of the target brain region. The purpose of this study was to investigate whether HD-tDCS applied to either unilateral or bilateral parietal lobes would improve postural control.
Method: A single-blind randomized crossover experimental design was used. 18 healthy right-handed adults were recruited for unilateral and bilateral HD-tDCS, as well as sham stimulation. All participants completed the sensory organization test (SOT) and motor control test (MCT) under eyes open and eyes closed conditions before and immediately after each intervention. The equilibrium score (ES), composite score (CS), and sensory score (VIS, SOM, VEST, PREF) from SOT, along with latency and response strength from the MCT, were calculated. Two-way repeated measures analyses of variance (ANOVAs) were used for the dependent variables. Bonferroni’s post hoc tests were used in case of significant ANOVA results.
Results: The composite latency increased significantly after right (p = 0.025) and bilateral (p = 0.004) stimulation under eyes open condition. When the balance plate moved large forward, the latency increased significantly after left (p = 0.003) and bilateral (p = 0.04) stimulation under eyes closed condition. For response strength, when the balance plate moved forward at different magnitude under eyes closed condition, they all decreased significantly after bilateral stimulation (p < 0.05).
Conclusion: The parietal lobe participates in the modulation of automatic postural response. The primary function of the right parietal lobe in postural response is to process visual information, while the left is responsible for processing somatosensory information.
1 Introduction
Sensory information processing are one of the important factors to maintain stability. The integration of sensory information from the somatosensory, visual, and vestibular systems serves to elucidate the complex sensory environment. The dependence of visual, somatosensory, and vestibular sensory cues is dynamically adjusted based on the particular environment, organism, or task conditions (Legrand et al., 2024). When equilibrium is disrupted, the central nervous system (CNS) reprocesses sensory information, selects and initiates appropriate postural responses, thus formulating an effective motor strategy to preserve stability (Slijper and Latash, 2004).
Postural control involves multiple regions of CNS, with the parietal lobe being a significant brain region for processing sensory information (Kamada and Takeuchi, 2023). Research have confirmed that the right parietal lobe area can be activated by visual stimuli (Naito et al., 2003), while the left can be stimulated by somatosensory and vestibular inputs (Ishigaki et al., 2016b). The right parietal lobe area is responsible for maintaining static stability through processing visual information, while the left processes proprioceptive information for the same purpose (Oka et al., 2022). Sensory information processing and integration in the posterior parietal cortex have been shown to be dominated by the right hemisphere (Arshad et al., 2014; Pisella et al., 2011; Convento et al., 2018). Therefore, it suggests that there are functional differences in sensory information processing between the left and right hemispheres of the parietal lobe.
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that delivers weak electrical currents to specific brain regions through the scalp. By inducing depolarization or hyperpolarization of neural tissues, it modulates cortical excitability and promotes changes in neural plasticity. Specifically, anodal stimulation increases neuronal excitability in targeted areas, while cathodal stimulation exerts an inhibitory effect, reducing cortical activity (Geers et al., 2024; Kesikburun, 2022). High-definition transcranial direct current stimulation (HD-tDCS) is capable of simultaneously modulating multiple regions within a distributed brain network (Müller et al., 2022). Research has demonstrated that HD-tDCS achieves focal stimulation by utilizing smaller electrodes arranged in a ring configuration, which effectively confines current flow to the targeted area (Kim et al., 2024). Therefore, an increasing number of studies have applied HD-tDCS to relevant brain regions to explore its impact on postural control, but few have approached it from the perspective of sensory information processing.
The weighting of reliance on sensory information (somatosensory, vision, and vestibular input) during postural control varies. An over-reliance on a specific sensory modality may result from either diminished quality of alternative sensory inputs or compromised neural processing of other sensory information within the brain. Research indicates that certain populations, such as individuals with chronic low back pain (Zafar et al., 2024), adolescents diagnosed with idiopathic scoliosis (Lau et al., 2023), patients recovering from ACL injuries (Ciceklidag et al., 2024), elderly individuals and those at high risk of falls (Unver and Bek, 2022), demonstrate compromised ankle joint proprioceptive integration capabilities. Patients with stroke (Moore et al., 2024) and Parkinson’s disease (Lee et al., 2025) exhibit an over-reliance on visual information for postural control. This compensatory strategy results in a predominant dependence on visual cues, which may include inappropriate or misleading inputs, while underutilizing vestibular and somatosensory feedback mechanisms. If unilateral or bilateral parietal HD-tDCS can optimize sensory information processing—with right-sided stimulation enhancing the visual system and left-sided stimulation enhancing the somatosensory system to maintain stability—it may offer a promising approach to modulate the aforementioned conditions and support the development of more targeted rehabilitation strategies.
Therefore, the primary purpose of this study was to investigate whether HD-tDCS applied to the left, right or bilateral parietal lobes enhances posture control and response, as well as the capacity of visual, somatosensory and vestibular system to maintain stability. The hypothesis of this study was that HD-tDCS of the unilateral and bilateral parietal lobes could improve postural control. The anodal tDCS applied on the right lobe could promote the ability of visual system, and applied on the left lobe could promote the ability of somatosensory system to maintain stability.
2 Materials and methods
This experiment employed a single-blind randomized crossover experimental method and was approved by Sports Science Experiment Ethics Committee of Beijing Sport University (No. 2023179H). Additionally, it obtained the Chinese clinical trial registration number: ChiCTR2300075357.1 All participants signed informed consent in accordance with the Declaration of Helsinki.
2.1 Participants
A total of 18 right-handed healthy young adults aged 18–35 years participated in this study [male, n = 8; female, n = 10; age, 21.88 (1.96); height, 1.71 (0.07); weight, 64.97 (9.83)]. According to G-power (effect size, 0.25; 1-β, 0.9; α, 0.05), 16 subjects needed to be recruited. To mitigate subject attrition, a total of 18 participants were recruited, all of whom completed the experimental procedures. Health status was assessed using Physical Activity Readiness Questionnaire (PAR-Q), while right-handedness was determined using the Edinburgh Handedness Inventory. Subjects with a history of neurological, orthopedic or other medical diseases were excluded.
2.2 HD-tDCS
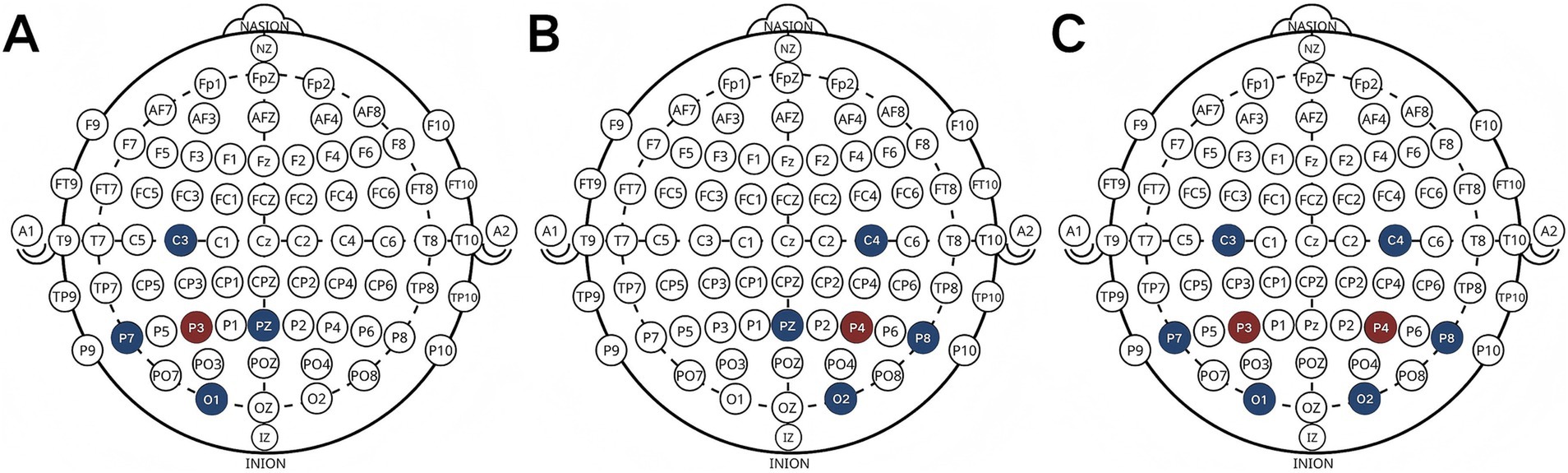
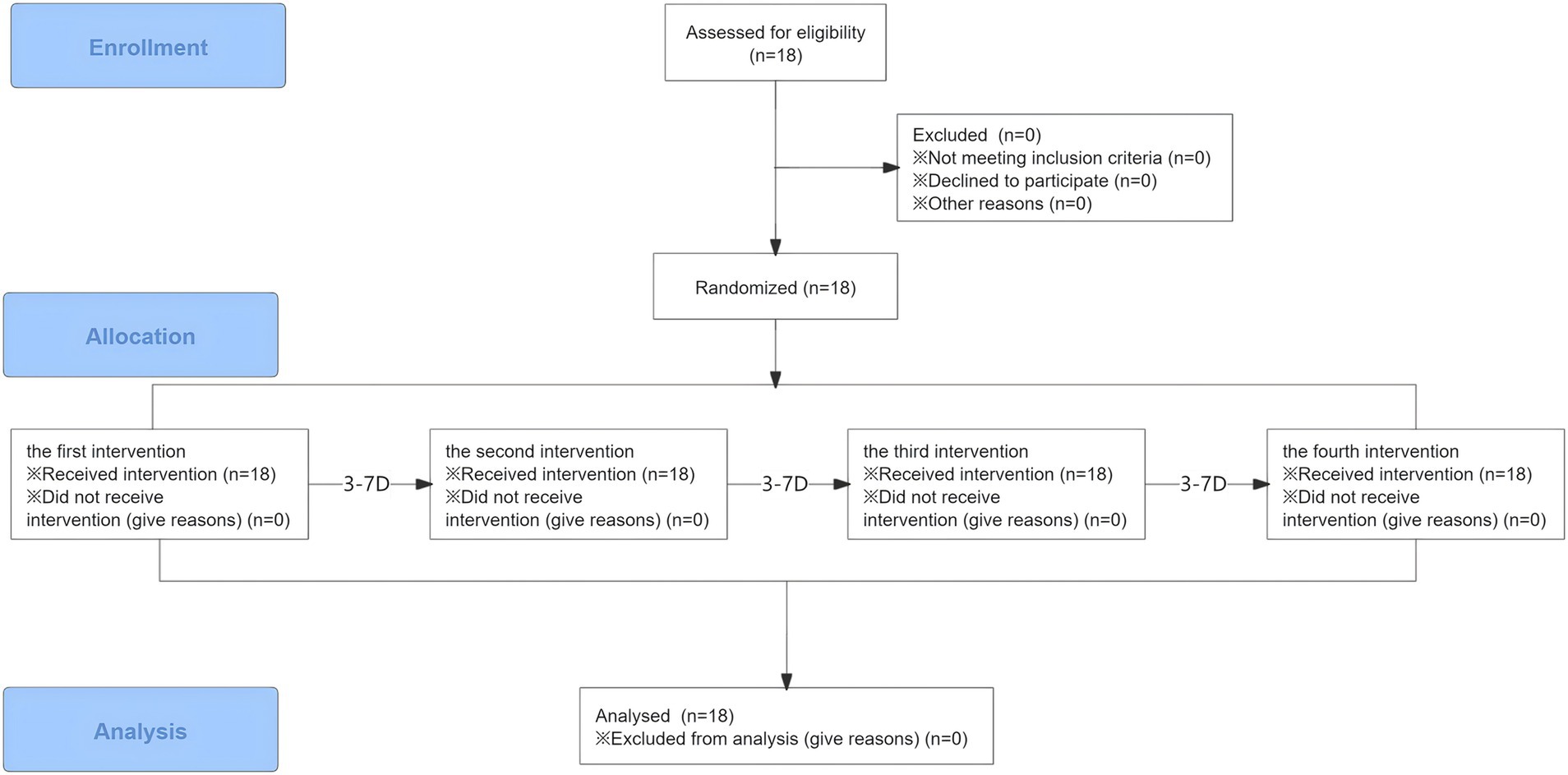
HD-tDCS and sham stimulation were delivered using a battery-driven current stimulator (NE, Starstim 32, Spain). A multifocal HD-tDCS montage was arranged in a 4×1 arrangement across unilateral hemispheres, utilizing five round Ag/AgCl electrodes measuring 3.14cm2. The central electrodes, using the anode, were positioned over the right (P4) or left (P3) parietal areas, with the cathodes serving as surrounding return electrodes (C4, P8, PZ, O2 on the right and C3, P7, PZ, O1on the left). When stimulating both hemispheres bilaterally, eight AgCl electrodes (3.14cm2 each) were used, with the central electrode positioned to stimulate the right (P4) and left (P3) parietal lobes simultaneously. To minimize errors arising from electrode variations, the return electrodes were positioned at P7, P8, C3, C4, O1, and O2. Each anode receives a current intensity of 2 mA, resulting in individual return electrodes receiving 0.50 mA for unilateral stimulation and 0.67 mA for bilateral stimulation. This study primarily investigates the functional disparities between the left and right parietal lobes, necessitating the assurance that each hemisphere receives equivalent intensity during each intervention. Stimulation lasted 20 min with 30s ramp up and ramp down periods. In the sham stimulation group, current was only passed during the ramp-up and ramp-down periods (Figure 1).
2.3 Measures
All subjects completed sensory organization test (SOT) and motor control test (MCT) on the Bertec Balance Advantage System (Columbus, U.S.A). The participants stood barefoot on the platform with their hands naturally hanging at their sides, and were instructed to gaze straight ahead and maintain standing stability to the best of their ability. Throughout the entire test, participants wore a harness, which did not impede their movements but served to ensure safety in the event of balance loss. In the event of the participants experiencing a loss of balance, the re-measurement was conducted.
SOT quantifies the ability of the sensory system (including visual, somatosensory and vestibular system) to maintain balance by inputting various sensory information conditions. This test consists of six conditions. Within each SOT, participants underwent three successive trials for each of the six conditions. Each trial lasted for 20 s, with a 10-s break between trials.
MCT assesses the latency and response strength of automatic postural responses when the platform moves forward or backward at three different magnitudes (small, medium, and large). The participants were tested under conditions of eyes open and eyes closed, respectively. The interval between consecutive tests is randomized and controlled by the computer. Consequently, participants cannot predict the timing, direction, or magnitude of the next platform movement.
2.4 Experimental procedure
The study employed a single-blind, randomized crossover design, in which session order was random and sessions were separated by 3–7 days. The number “1,” “2,” “3,” and “4” represents the right, left, bilateral and sham stimuli, respectively. The test order of each subject was randomly sorted by a professional statistical expert. The order of stimulation sessions was counterbalanced across participants. All electrodes were worn (P3, P4, PZ, P7, P8, C3, C4, O1, O2) during each stimulation, ensuring that participants remained blinded to the details of each stimulation type. Each session consisted of SOT and MCT under eyes open and eyes closed conditions, all before and immediately after either real or sham stimulation. Subjects were instructed to refrain from consuming alcohol or coffee within 24 h prior to the start of the experiment. Throughout the experiment, the laboratory maintained a quiet environment, ensuring participants remained vigilant, refrained from engaging in conversations with others, and abstained from using their phones. If the participants experienced any discomfort, the experiment was terminated. The duration of each session tasks was approximately 1.5 h.
2.5 Data analysis methods
Equilibrium score (ES), composite score (CS) and sensory score (VIS, SOM, VEST and PRE) serves as the primary outcome of the SOT. An equilibrium score is generated under each testing condition, while CS is calculated based on scores from all conditions. The higher the score, the better the balance performance. The CS is the predominant indicator utilized in the SOT (Zhou et al., 2025). Sensory scores are calculated based on the ES, following the formula provided, reflecting the capability of sensory system to maintain balance. PREF represents the capacity to counteract visual interference for the maintenance of postural stability.
The latency and response strength serves as the primary outcome of the MCT. The latency records the time from the onset of perturbation to the reaction of each leg. The latency under different directions and magnitude of platform movement is obtained by averaging the reaction time of both legs. The composite latency is obtained by averaging the reaction times in forward and backward translations at three different magnitude. The response strength quantifies the force applied to the platform, with larger numbers indicating a greater force being applied.
All data were analyzed by SPSS 25.0 and presented as Mean ± SD. Using Shapiro–Wilk tests to evaluate the normal distribution of the data. Two-way repeated measures analysis of variance (ANOVA) was used to analyze the dependent variables. Bonferroni’s post hoc tests were used in case of significant ANOVA results. The significance threshold was set at α = 0.05 (Figure 2).
3 Results
No adverse reactions or events occurred during the entire experiment, such as skin redness, headache, dizziness or other events.
3.1 ES CS and sensory score
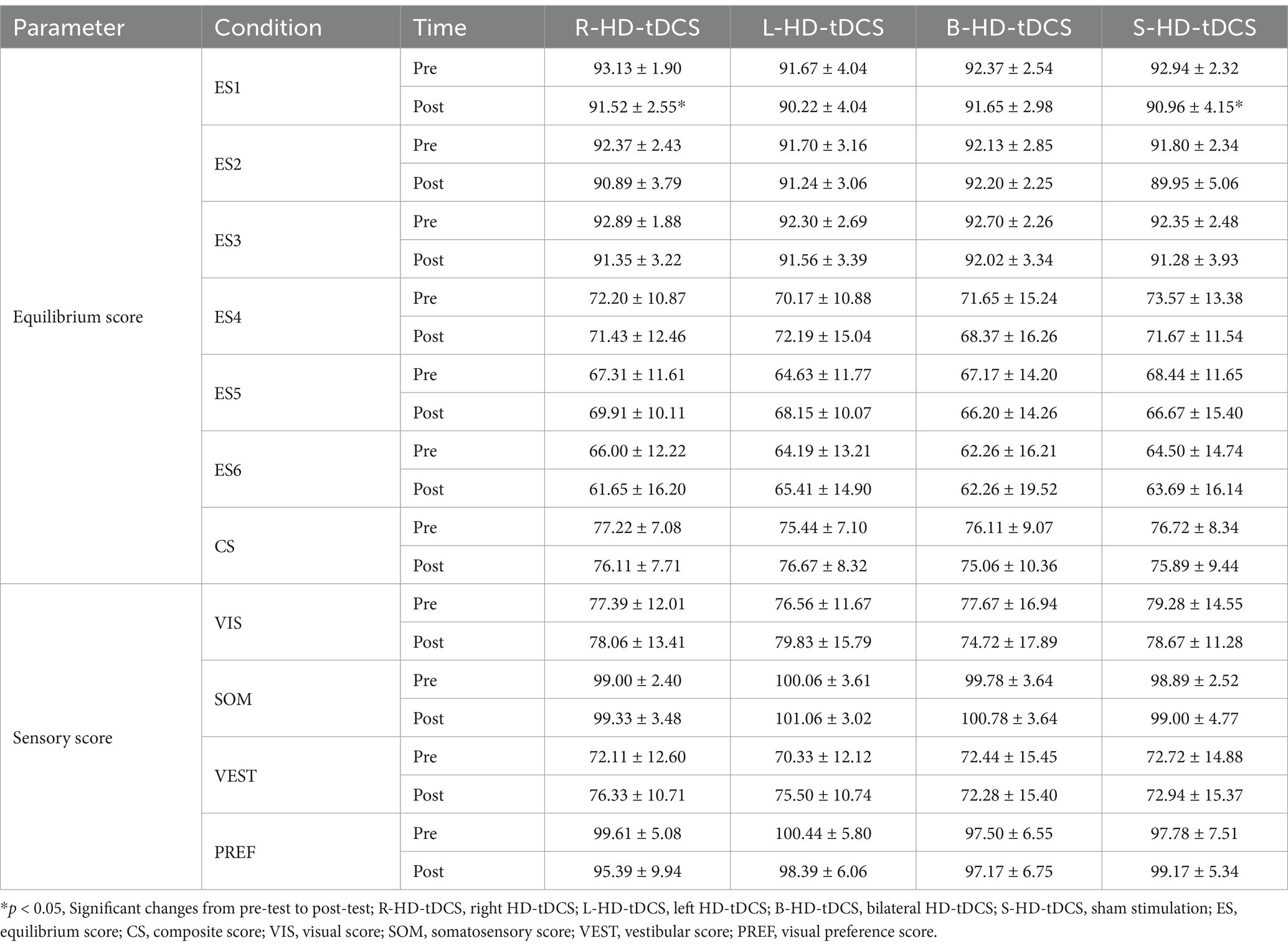
The four experimental sessions showed no significant differences in ES, CS, SOM, VIS, VEST, and PREF at baseline (all values of p > 0.05). The main effect of time is significant different for ES1 [F(1, 17) = 11.328, p = 0.004, ηp2 = 0.4], post-hoc comparisons revealed that ES1 decreased significantly only after right (p = 0.011) and sham (p = 0.035) stimulation. For SOT2-6, there are no significant main effects of time, group, or interaction effects. The results of two-way repeated measures ANOVAs of SOT are presented in Table 1. The means (SD) of SOT are presented in Table 2.
3.2 Latency, response strength
The four experimental sessions showed no significant differences in latency and response strength under eyes open and eyes closed conditions at baseline (all values of p > 0.05).
3.2.1 Latency
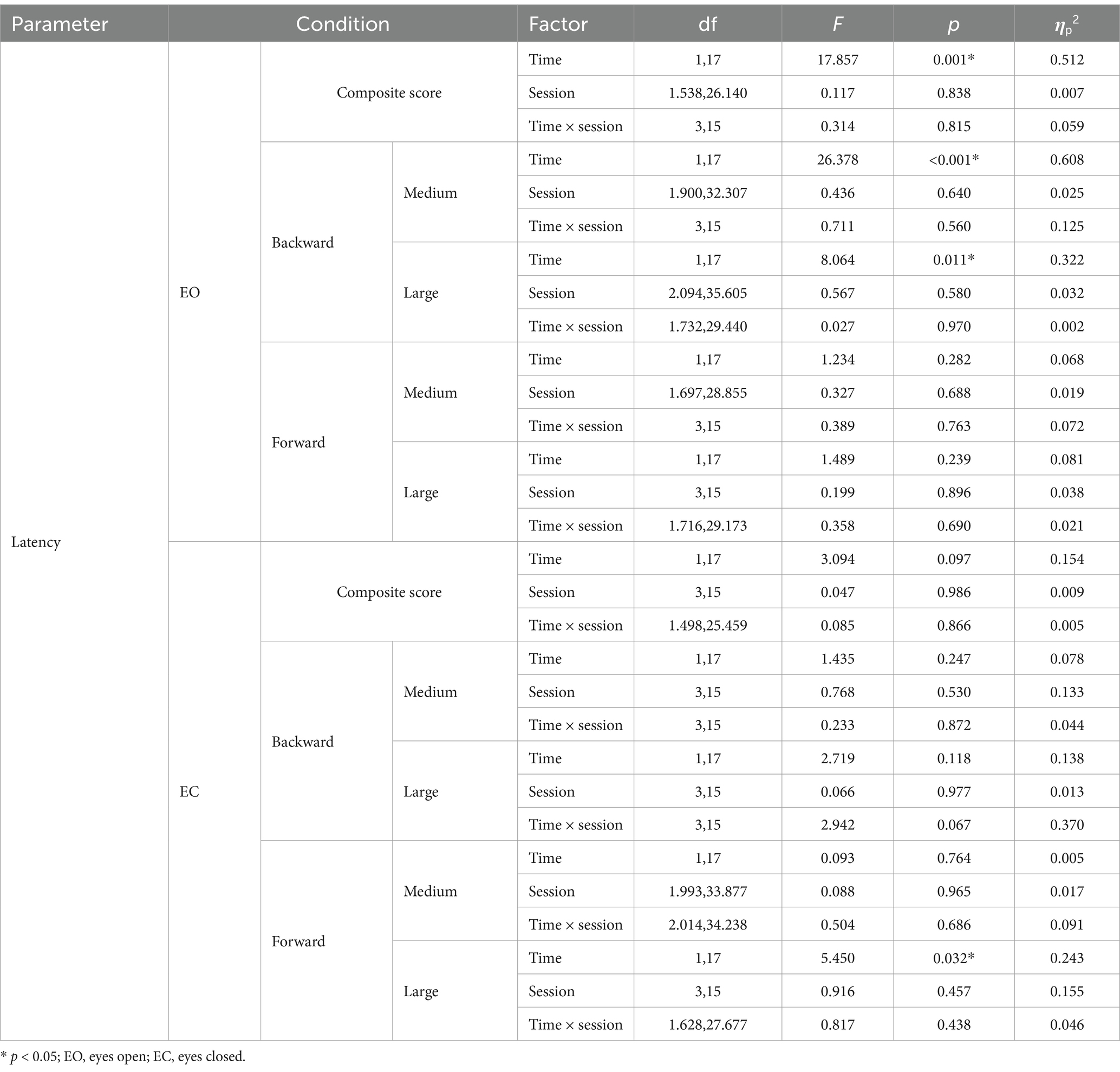
For eyes open, the main effect of time is significant for composite latency [F(1, 17) = 17.857, p = 0.001, ηp2 = 0.512], post-hoc comparisons revealed that composite latency increased significantly after right (p = 0.025) and bilateral (p = 0.004) stimulation. The main effect of time is significant in medium and large backward translation [medium backward: F(1, 17) = 26.378, p < 0.001, ηp2 = 0.608; large backward: F(1, 17) = 8.064, p < 0.001, ηp2 = 0.322], post-hoc comparisons revealed that there is no significant change after true and sham stimulation (p > 0.05).
For eyes closed, the main effect of time is significant in large forward translation [F(1, 17) = 4.450, p = 0.032, ηp2 = 0.243], post-hoc comparisons revealed that latency increased significantly after left (p = 0.003) and bilateral (p = 0.04) stimulation. The results of two-way repeated-measures ANOVAs of latency are presented in Table 3. The means (SD) of latency are presented in Table 4.
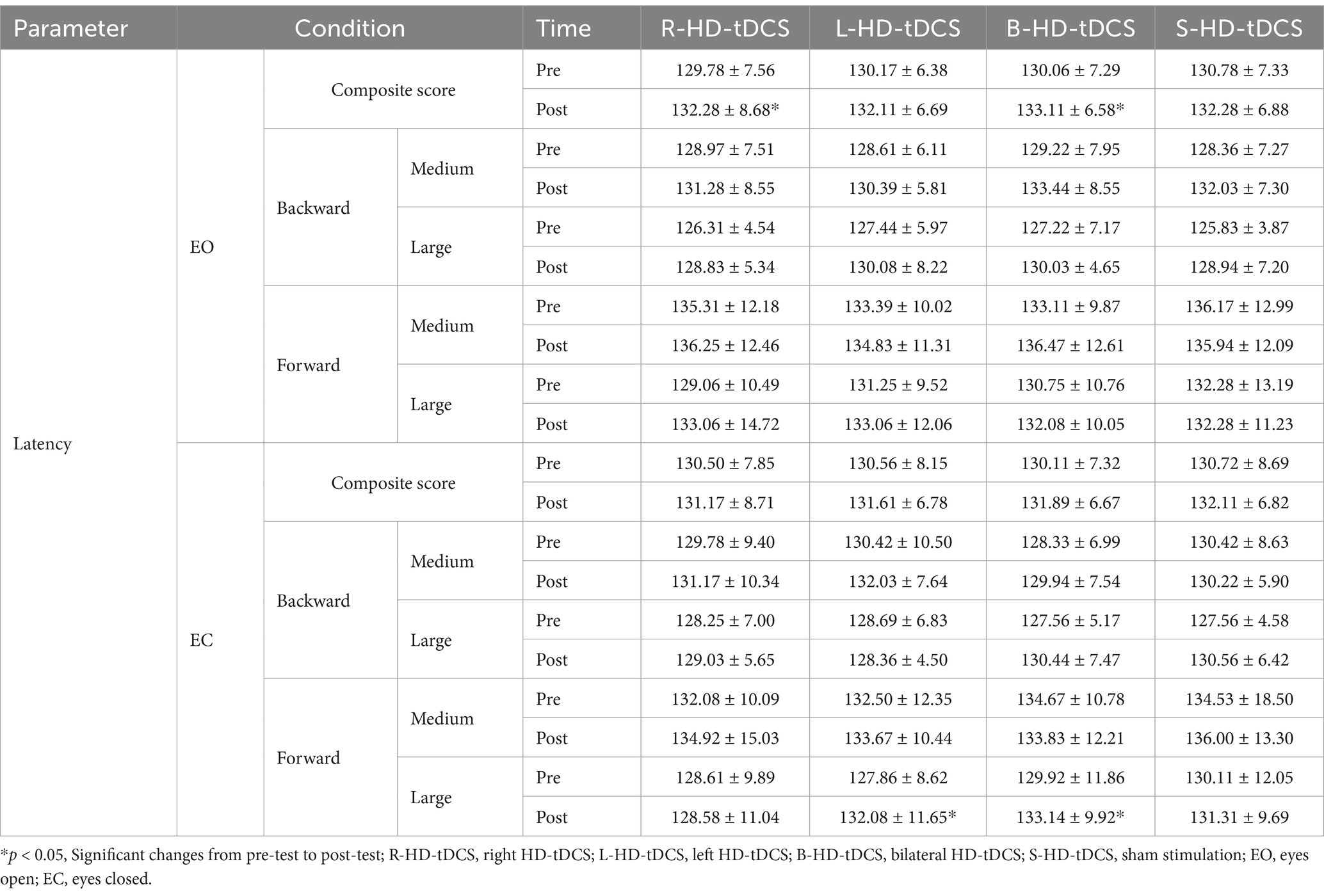
Table 4. Latency under eyes open and eyes closed before and after HD-tDCS or sham intervention (Mean±SD).
3.2.2 Response strength
For eyes open, in forward and backward translations at three different magnitudes, the main effect of time is significant. However there are no significant main effect of session and the interaction effect.
For eyes closed, in forward and backward translations at three different magnitudes, the main effect of time is significant. Post-hoc comparisons revealed that for forward translation at three different magnitudes, response strength decreased significantly after only bilateral stimulation (p < 0.05). The results of two-way repeated-measures ANOVAs of response strength are presented in Table 5. The means (SD) of response strength are presented in Table 6.
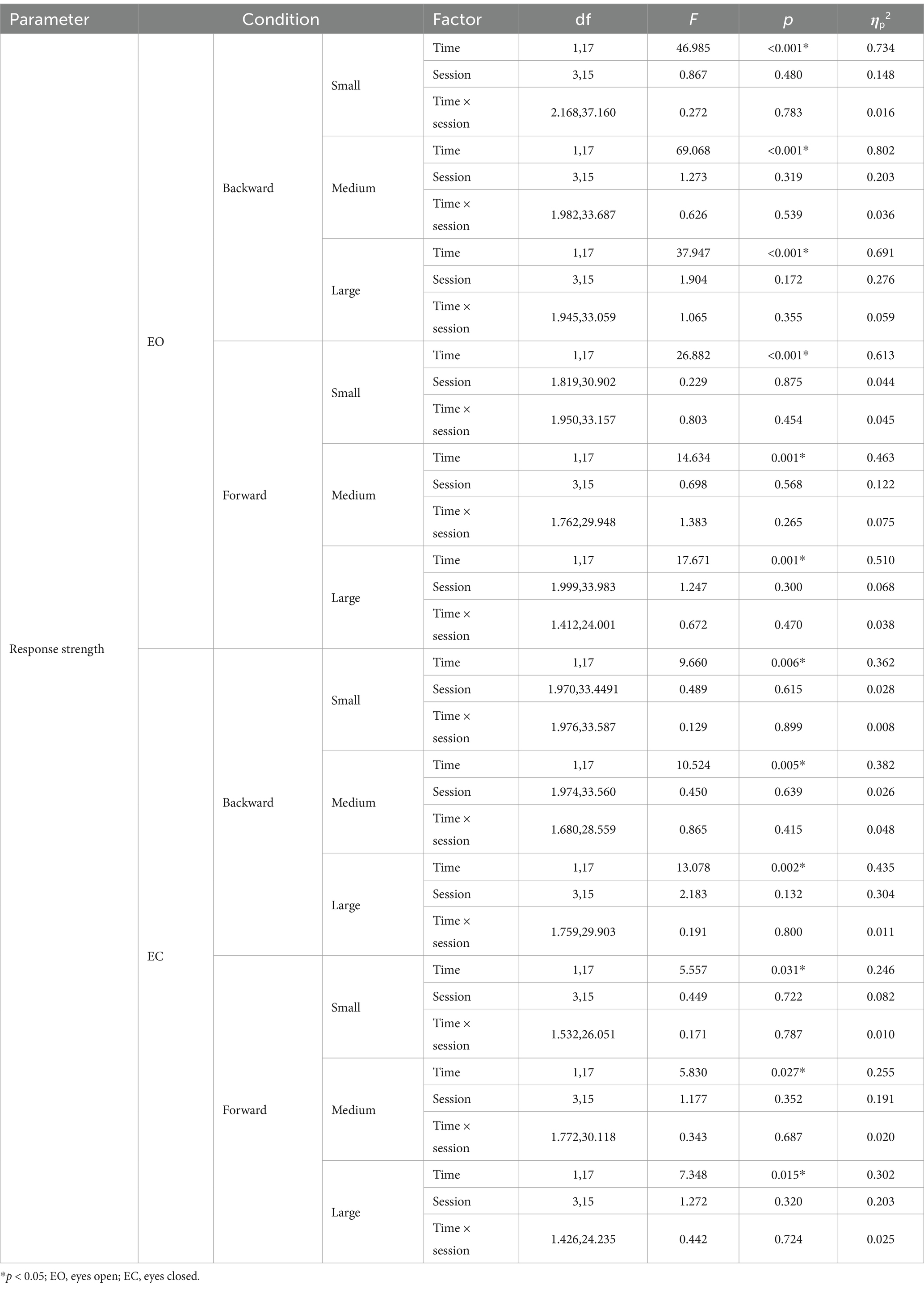
Table 5. Results of two-way repeated-measures ANOVAs of response strength under eyes open and eyes closed.
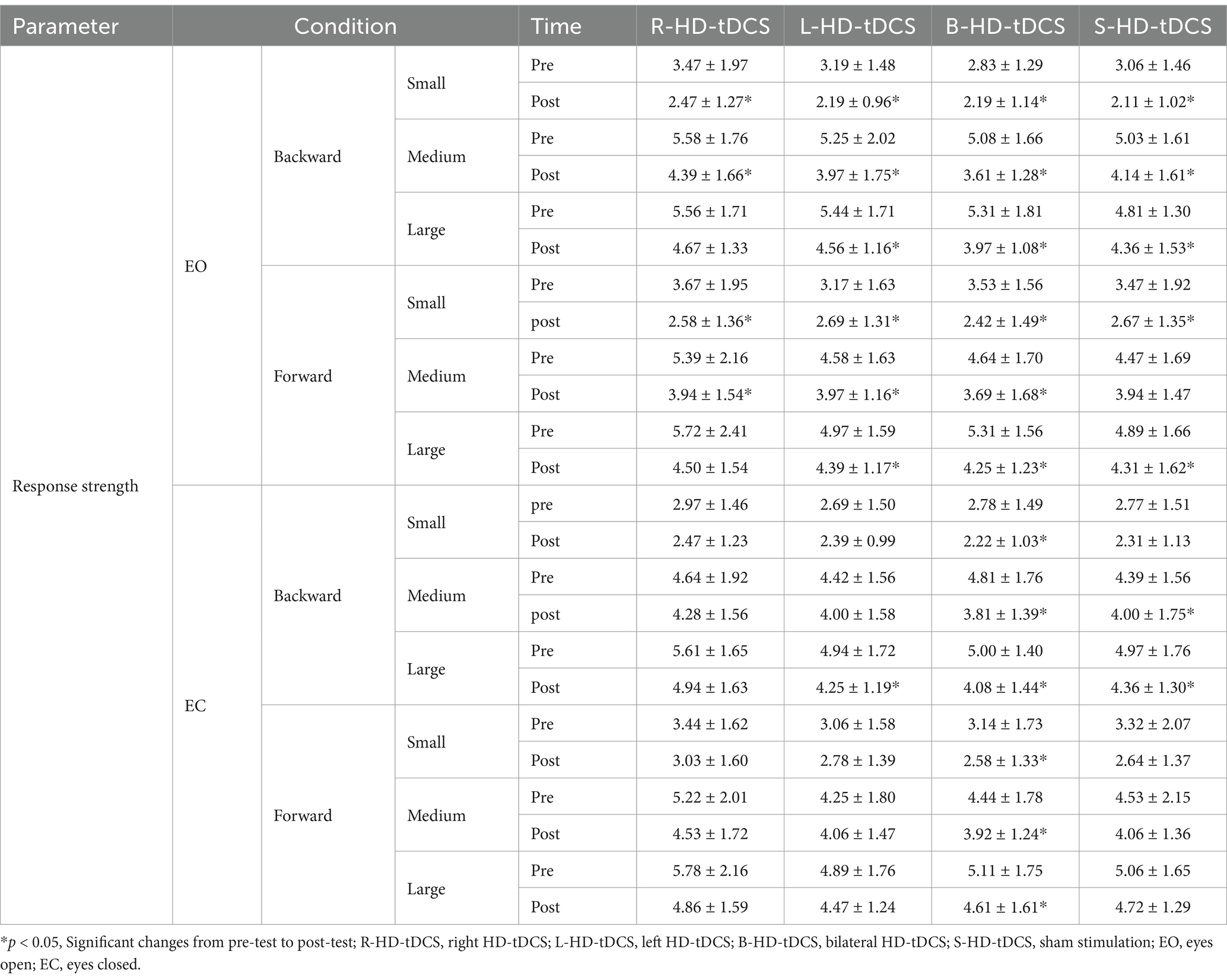
Table 6. Response strength under eyes open and eyes closed before and after HD-tDCS or sham intervention (Mean±SD).
4 Discussion
The current study observed that enhancing the excitability of the right parietal lobes under eyes open conditions and the left parietal lobes under eyes closed conditions using HD-tDCS led to a prolongation of the latency of automatic postural responses. These findings demonstrate that the right parietal lobe is involved in modulating automatic responses when visual information is input normally, while the left parietal lobe is involved when visual information is shielded. When the platform was translated forward at three different magnitudes under eyes closed conditions, response strength decreased only after bilateral stimulation. This suggests that bilateral parietal HD-tDCS is beneficial for enhancing the ability of postural responses. The study did not find that unilateral or bilateral parietal lobes HD-tDCS could improve the ability of vision, somatosensory and vestibular system to maintain postural control in young healthy adults.
Rapid response to external interference is necessary in daily life to maintain stability. For example, it is important to take corresponding balance strategies in time to prevent falls while walking on a wet or icy road. Postural responses can be categorized into two phases: an early phase, from the onset of perturbation to the initial rapid reaction, known as the automatic postural response; and a later phase, involving the establishment of a new steady-state posture (Honeine et al., 2015). Early studies have suggested that postural response is mainly regulated at subcortical level (Sherrington, 1910). Currently, an increasing number of studies have shown that the cerebral cortex also participates in modulating postural responses (Bolton, 2015; Barollo et al., 2022; Jacobs and Horak, 2007). It is primarily involved in the later stage, optimizing responses to adapt to the surrounding environment and meet individual goals (Jacobs and Horak, 2007; Maki and McIlroy, 2007). Meanwhile, the early stage, which is highly automated, is controlled by the subcortical level (Honeine et al., 2015).
J. V. Jacobs had studied the early and late phase of latency. The authors believe that brainstem circuits initiate an automatic response, which is subsequently optimized by cortical circuits during later phases. The cerebral cortex directly regulates posture responses through the corticospinal loop, leading to a longer latency period. Alternatively, it can indirectly shorten the latency by initiating coordination through the brainstem (Jacobs and Horak, 2007). The increased excitability of parietal lobe directly correlates with its heightened involvement in the postural response.
Studies suggest that the cerebellar-cortical loop is accountable for adapting postural responses based on prior experience (Thach and Bastian, 2004), while the basal ganglia-cortical loop is accountable for pre-selecting and optimizing postural responses based on current context (Takakusaki et al., 2004; Grillner et al., 2005). Increased cortical involvement in the early stage may facilitate interaction between the cortex and subcortical structures, potentially benefiting later-stage adjustments. This hypothesis is consistent with previous research findings. Motor cortex (C3/C4) tDCS activates postural synergies involving brainstem networks via descending projections (Nonnekes et al., 2014). Meanwhile, brainstem networks project to the cortical cortex, expediting cortical processing (Sibley et al., 2014; Jones, 2008). These result suggest that increasing cortical excitability can promote its interaction with subcortical structures.
While this study interprets prolonged latency as indicative of heightened cortical engagement, the previous studies suggest that such electrophysiological delays may reflect compromised neural processing efficiency or subclinical impairment, potentially associated with postural instability and increased fall risk in clinical populations (Lee et al., 2020). Comprehensive investigation of postural reactions necessitates moving beyond exclusive focus on latency to quantify the total stabilization period, defined as the temporal interval from perturbation onset to complete balance recovery. Biomechanical investigations have revealed that athletes with concussion histories exhibit shorter step latency compared to controls, yet paradoxically demonstrate prolonged time-to-stabilization (Monoli et al., 2025). A limitation of this study is reliance on latency to assess postural responses, therefore future research should integrate evaluation of total response time. The observed prolongation of automatic postural responses in this study suggests that enhanced cortical engagement facilitates cortico-subcortical network interactions to optimize posture response. Moreover, the outcome of response strength in the present study further support the hypothesis.
Previous studies have concluded that the MCT has no learning effect (Hill et al., 2018; Hale et al., 2009), which generates the two sub-strategies of postural control: ankle strategy and hip-knee strategy (Pollock et al., 2000). Repeated exposure to MCT allows CNS to select a more effective motor strategy, transitioning from a hip-knee to an ankle strategy (Hill et al., 2018). This change may result in a decrease in response strength. Under moderate disturbance, the default strategy is the ankle strategy. As the disturbance difficulty increases, there is a shift from the ankle strategy to the hip strategy. The participants in the previous study reported that maintaining balance is more difficult when the platform moves forward compared to moving backward (Hill et al., 2018). The activation levels of lower extremity muscles increase with the perturbation magnitude during the MCT (Hill et al., 2018). Meanwhile, in the case of closed eyes, maintaining posture becomes more challenging, resulting in increased activation of muscles. When the platform moves forward under eyes closed conditions, bilateral parietal stimulation may increase the involvement of the cerebral cortex, promoting coordination between the parietal lobe and subcortical structures. These resulted in the CNS selecting a more effective balance strategy and reducing response strength. Rapid adaptation of the neuromuscular system’s reactive to novel perturbations decreases the possibility of improper motor response (Hill et al., 2018). Bilateral tDCS on PPC enhances postural adaptation following tilt in healthy young adults (Young et al., 2020). Parietal tDCS may function by enhancing the rapid adaptation to facilitate optimized postural responses, thereby reducing response strength.
Postural control is predominantly dependent on the integration of sensory information derived from visual, somatosensory, and vestibular inputs. The right hemisphere-driven processing of multisensory information in the posterior parietal cortex (Arshad et al., 2014; Pisella et al., 2011; Convento et al., 2018) may account for the prolonged latency observed with right stimulation during eyes open condition, while the left stimulation’s lack of effect suggests a different mechanism at play. In daily life and during physical activities, individuals are exposed to constantly changing visual environments, requiring them to direct their attention to external control points and maintain neuromuscular to ensure postural stability (Tasci et al., 2024). The visual system provides a fundamental mechanism for coordinating, regulating, and controlling movement, while also managing interactions within the environment (Unell et al., 2021). Spatial reference frames of the body within the environment affects posture reactions. Self-centered spatial maps must be constantly updated when individuals are disturbed, enabling compensatory movements to be rapidly to executed to maintain balance (Maki and McIlroy, 2007). Spatial remapping, updating information across eyes movements, is an important mechanism for trans-saccadic perception, which is the process of integrating the content of individual fixations over space and time into a stable, internal representation of the environment (Prime et al., 2011). The right parietal lobe primarily governs spatial navigation and trans-saccadic perception (Pisella et al., 2011; Ten Brink et al., 2019). The latency remained unchanged during right stimulation under eyes closed conditions, further suggesting that the right parietal lobe primarily processes visual information during postural reactions.
Proprioception (the sense of body position and movement) includes signals from mechanoreceptors (proprioceptors) located in muscles, tendons, and joint capsules. Proprioception plays a critical role in constantly updating information on body segment positions, detecting body sway and monitoring changes in muscle length, particularly surrounding the ankle joint, to maintain steady upright standing (Henry and Baudry, 2019). The vestibular organs, located within the inner ear, consist of bilateral otoliths and three semicircular canals, which detect linear and angular accelerations of the head. Vestibular signals provide real-time environmental information in both egocentric and allocentric reference frames to ensure postural stability (Xie et al., 2024). The left posterior parietal cortex can be activated by somatosensory input, such as light touch from an external stable reference frame (Ishigaki et al., 2016b), as well as by vestibular inputs, like caloric stimulation or galvanic vestibular stimulation (Bense et al., 2001). In a previous study employing repetitive transcranial magnetic stimulation (rTMS) to attenuate the function of the left posterior parietal cortex, the inhibition in cortical function was only observed under the eyes closed condition. Specifically, when participants had their eyes closed, those in the stimulation group exhibited decreased stability and diminished proprioception in both the ankle and knee joints. Moreover, they displayed symptoms akin to sensory ataxia (Bertuccelli et al., 2024). Research indicates that the left parietal lobe primarily processes proprioception and vestibular sensation during posture control (Ishigaki et al., 2016b; Oka et al., 2022). Blocking of visual information leads to reliance on proprioception and vestibular sensation for posture control. Participants exhibit a preference for proprioception over vestibular sensation during the horizontal translation of the support surface (Akçay et al., 2021). MCT is assessed through forward and backward movements on a platform. These findings can explain why left stimulation prolonged latency just under the eyes closed condition. In summary, the parietal lobe shows lateralized roles in postural control: the right side processes visual information, while the left side handles somatosensory input, aligning with prior research.
Perhaps because the subjects in the present study did not have any sensory information processing issues, no significant differences were observed after intervention in the ability of each sensory system to maintain balance.
The results of previous study are consistent with the present. Using a sway-referencing paradigm to assess sensory reweighing processes, the previous study explored the effect of M1 tDCS on postural control in healthy older and young adults (Craig and Doumas, 2017). Many prior investigations into the influence of tDCS on postural control have yielded significant outcomes, focusing on individuals with clinical ailments (Benninger et al., 2010; Kaski et al., 2014; Kaski et al., 2013). A meta-analysis investigating the effects of non-invasive brain stimulation on sensory function in post-stroke patients revealed that tDCS significantly enhances sensory recovery compared to control groups, particularly when targeting the primary motor cortex (M1), primary somatosensory cortex (S1), or combined M1-S1 stimulation (Chen et al., 2023). Combined intervention of bilateral S1 tDCS with sensorimotor training significantly enhanced light touch perception, stereognosis, and proprioception in post-stroke patients (Li et al., 2023). Consequently, the subjects recruited may have influenced outcomes. In future studies, participants with sensory integration deficits could be selected to investigate whether HD-tDCS of the parietal lobe improves the stability-maintaining abilities of the visual, somatosensory, and vestibular systems.
Currently, the effects of parietal tDCS on postural control remain inconclusive. Cathodal tDCS applied to the PPC reduces the influence of light touch on postural control, but does not affect static standing balance (Ishigaki et al., 2016a). However, unilateral parietal cathodal tDCS can impair static standing balance in healthy young individuals (Oka et al., 2022). Previous studies have largely confirmed the role of the parietal lobe in postural control by using cathodal stimulation to attenuate its function. However, whether anodal stimulation of the parietal lobe can enhance static or dynamic postural control remains to be further investigated. Bilateral tDCS on PPC enhances postural adaptation following tilt in healthy young adults. Notably, this improvement is independent of stimulation polarity, as both configurations—anodal stimulation over the left PPC with cathodal placement on the right, and vice versa—demonstrate comparable efficacy in augmenting postural adaptability (Young et al., 2020). Postural responses are regulated through the interaction between cortical and subcortical structures. The prolongation of latency induced by parietal tDCS suggests that tDCS may modulate sensorimotor functions via corticospinal and subcortical pathway. The previous study suggested that increased corticospinal excitability following anodal tDCS may result from activation of voltage-sensitive Ca2+ channels, increased influx of Ca2+, which subsequently elevates NMDA receptor sensitivity, thereby leading to increased motor evoked potentials (MEPs). Although afferent feedback may regulate pacing during self-paced exercise, enhanced corticospinal excitability did not improve motor performance (Kristiansen et al., 2021). Emerging evidence indicated that corticospinal excitability was suppressed during anodal tDCS, with no persistent modulation observed after stimulation (Takano et al., 2023). Additionally, whether stimulation of different hemispheres would produce different outcomes is also a key focus for future research.
5 Study limitations
This study mainly discusses the impact of parietal HD-tDCS on postural control from the perspective of sensory information processing. The indicators involve equilibrium score, composite score, sensory score, latency and response strength under different test conditions, which have certain limitations for the expression of results. The postural response solely evaluated the latency in automatic posture response without assessing the time course of reaction. The analysis of motor strategy primarily relies on reaction strength, overlooking the muscle activation of different strategies. It is important to record electromyography (EMG) of trunk and lower extremity to enhance the precision of conclusions. Matching the difficulty of the test task with the capabilities of the subjects may lead to better-presented test results. The participants included in such experiment should be carefully considered. In future studies, the utilization of functional near-infrared brain imaging and EEG analysis can provide an intuitive understanding of cortical activity during posture control.
6 Conclusion
The parietal lobe participates in modulating automatic postural responses. The primary role of the right parietal lobe in postural response is to process visual information, while the left parietal lobe processes somatosensory information.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by ethics committee of Beijing Sport University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FY: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. YL: Conceptualization, Investigation, Writing – review & editing. XL: Conceptualization, Investigation, Writing – review & editing. YH: Investigation, Writing – review & editing. JG: Investigation, Writing – review & editing. PZ: Investigation, Writing – review & editing. QL: Investigation, Writing – review & editing. ZP: Investigation, Writing – review & editing. JQ: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by grants from the Fundamental Research Funds for the Central Universities (Exercise Rehabilitation Science Laboratory, No. 2023KFZX003) and the Beijing High-Grade, Precision, and Advanced Disciplines Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CNS, central nervous system; CS, composite score; ES, equilibrium score; HD-tDCS, high-definition transcranial direct current stimulation; MCT, motor control test; M1, primary motor cortex; PAR-Q, Physical Activity Readiness Questionnaire; PREF, visual preference score; SOM, somatosensory score; SOT, sensory organization test; S1, primary somatosensory cortex; VEST, Vestibular score; VIS, visual score.
Footnotes
References
Akçay, M. E., Lippi, V., and Mergner, T. (2021). Visual modulation of human responses to support surface translation. Front. Hum. Neurosci. 15:615200. doi: 10.3389/fnhum.2021.615200
Arshad, Q., Nigmatullina, Y., Roberts, R. E., Bhrugubanda, V., Asavarut, P., and Bronstein, A. M. (2014). Left cathodal trans-cranial direct current stimulation of the parietal cortex leads to an asymmetrical modulation of the vestibular-ocular reflex. Brain Stimul. 7, 85–91. doi: 10.1016/j.brs.2013.07.002
Barollo, F., Hassan, M., Petersen, H., Rigoni, I., Ramon, C., Gargiulo, P., et al. (2022). Cortical pathways during postural control: new insights from functional EEG source connectivity. IEEE Trans. Neural Syst. Rehabil. Eng. 30, 72–84. doi: 10.1109/TNSRE.2022.3140888
Benninger, D. H., Lomarev, M., Lopez, G., Wassermann, E. M., Li, X., Considine, E., et al. (2010). Transcranial direct current stimulation for the treatment of parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 81, 1105–1111. doi: 10.1136/jnnp.2009.202556
Bense, S., Stephan, T., Yousry, T. A., Brandt, T., and Dieterich, M. (2001). Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J. Neurophysiol. 85, 886–899. doi: 10.1152/jn.2001.85.2.886
Bertuccelli, M., Bisiacchi, P., and Del Felice, A. (2024). Disentangling cerebellar and parietal contributions to gait and body Schema: a repetitive transcranial magnetic stimulation study. Cerebellum 23, 1848–1858. doi: 10.1007/s12311-024-01678-x
Bolton, D. A. E. (2015). The role of the cerebral cortex in postural responses to externally induced perturbations. Neurosci. Biobehav. Rev. 57, 142–155. doi: 10.1016/j.neubiorev.2015.08.014
Chen, G., Wu, M., Chen, J., Cai, G., Liu, Q., Zhao, Y., et al. (2023). Non-invasive brain stimulation effectively improves post-stroke sensory impairment: a systematic review and meta-analysis. J. Neural Transm. (Vienna) 130, 1219–1230. doi: 10.1007/s00702-023-02674-x
Ciceklidag, M., Kaya, I., Ayanoglu, T., Ayas, I. H., Ozer, M., Ataoglu, M. B., et al. (2024). Proprioception after primary repair of the anterior cruciate ligament. Am. J. Sports Med. 52, 1199–1208. doi: 10.1177/03635465241228839
Convento, S., Romano, D., Maravita, A., and Bolognini, N. (2018). Roles of the right temporo-parietal and premotor cortices in self-location and body ownership. Eur. J. Neurosci. 47, 1289–1302. doi: 10.1111/ejn.13937
Craig, C. E., and Doumas, M. (2017). Anodal transcranial direct current stimulation shows minimal, measure-specific effects on dynamic postural control in young and older adults: a double blind, sham-controlled study. PLoS One 12:e0170331. doi: 10.1371/journal.pone.0170331
Geers, L., Dormal, V., Bonato, M., Vandermeeren, Y., Masson, N., and Andres, M. (2024). Modulation of initial leftward bias in visual search by parietal tDCS. PLoS One 19:e0315715. doi: 10.1371/journal.pone.0315715
Grillner, S., Hellgren, J., Ménard, A., Saitoh, K., and Wikström, M. A. (2005). Mechanisms for selection of basic motor programs – roles for the striatum and pallidum. Trends Neurosci. 28, 364–370. doi: 10.1016/j.tins.2005.05.004
Hale, L., Miller, R., Barach, A., Skinner, M., and Gray, A. (2009). Motor control test responses to balance perturbations in adults with an intellectual disability. J. Intellect. Develop. Disabil. 34, 81–86. doi: 10.1080/13668250802683810
Henry, M., and Baudry, S. (2019). Age-related changes in leg proprioception: implications for postural control. J. Neurophysiol. 122, 525–538. doi: 10.1152/jn.00067.2019
Hill, C. M., Wilson, S., Mouser, J. G., Donahue, P. T., and Chander, H. (2018). Motor adaption during repeated motor control testing: attenuated muscle activation without changes in response latencies. J. Electromyogr. Kinesiol. 41, 96–102. doi: 10.1016/j.jelekin.2018.05.007
Honeine, J. L., Crisafulli, O., Sozzi, S., and Schieppati, M. (2015). Processing time of addition or withdrawal of single or combined balance-stabilizing haptic and visual information. J. Neurophysiol. 114, 3097–3110. doi: 10.1152/jn.00618.2015
Ishigaki, T., Imai, R., and Morioka, S. (2016a). Cathodal transcranial direct current stimulation of the posterior parietal cortex reduces steady-state postural stability during the effect of light touch. Neuroreport 27, 1050–1055. doi: 10.1097/WNR.0000000000000654
Ishigaki, T., Ueta, K., Imai, R., and Morioka, S. (2016b). EEG frequency analysis of cortical brain activities induced by effect of light touch. Exp. Brain Res. 234, 1429–1440. doi: 10.1007/s00221-015-4545-9
Jacobs, J. V., and Horak, F. B. (2007). Cortical control of postural responses. J. Neural Transm. (Vienna) 114, 1339–1348. doi: 10.1007/s00702-007-0657-0
Jones, B. E. (2008). Modulation of cortical activation and behavioral arousal by cholinergic and orexinergic systems. Ann. N. Y. Acad. Sci. 1129, 26–34. doi: 10.1196/annals.1417.026
Kamada, H., and Takeuchi, N. (2023). Transcranial direct current stimulation over the Temporoparietal junction modulates posture control in unfamiliar environments. Brain Sci. 13:1514. doi: 10.3390/brainsci13111514
Kaski, D., Dominguez, R. O., Allum, J. H., and Bronstein, A. M. (2013). Improving gait and balance in patients with leukoaraiosis using transcranial direct current stimulation and physical training: an exploratory study. Neurorehabil. Neural Repair 27, 864–871. doi: 10.1177/1545968313496328
Kaski, D., Dominguez, R. O., Allum, J. H., Islam, A. F., and Bronstein, A. M. (2014). Combining physical training with transcranial direct current stimulation to improve gait in parkinson’s disease: a pilot randomized controlled study. Clin. Rehabil. 28, 1115–1124. doi: 10.1177/0269215514534277
Kesikburun, S. (2022). Non-invasive brain stimulation in rehabilitation. Turk. J. Phys. Med. Rehabil. 68, 1–8. doi: 10.5606/tftrd.2022.10608
Kim, E., Lee, G., Lee, J., and Kim, Y. H. (2024). Simultaneous high-definition transcranial direct current stimulation and robot-assisted gait training in stroke patients. Sci. Rep. 14:4483. doi: 10.1038/s41598-024-53482-6
Kristiansen, M., Thomsen, M. J., Nørgaard, J., Aaes, J., Knudsen, D., and Voigt, M. (2021). Anodal transcranial direct current stimulation increases corticospinal excitability, while performance is unchanged. PLoS One 16:e0254888. doi: 10.1371/journal.pone.0254888
Lau, K. K. L., Law, K. K. P., Kwan, K. Y. H., Cheung, J. P. Y., and Cheung, K. M. C. (2023). Proprioception-related gene mutations in relation to the aetiopathogenesis of idiopathic scoliosis: a scoping review. J. Orthop. Res. 41, 2694–2702. doi: 10.1002/jor.25626
Lee, C. Y., Huisinga, J. M., Choi, I. Y., Lynch, S. G., and Lee, P. (2020). Correlation between spinal cord diffusion tensor imaging and postural response latencies in persons with multiple sclerosis: a pilot study. Magn. Reson. Imaging 66, 226–231. doi: 10.1016/j.mri.2019.11.004
Lee, J., Kang, S. H., and Koh, S. B. (2025). Postural instability and gait disturbance are associated with abnormal stereopsis in Parkinson’s disease. PLoS One 20:e0317935. doi: 10.1371/journal.pone.0317935
Legrand, T., Mongold, S. J., Muller, L., Naeije, G., Ghinst, M. V., and Bourguignon, M. (2024). Cortical tracking of postural sways during standing balance. Sci. Rep. 14:30110. doi: 10.1038/s41598-024-81865-2
Li, C., Chen, Y., Tu, S., Lin, J., Lin, Y., Xu, S., et al. (2023). Dual-tDCS combined with sensorimotor training promotes upper limb function in subacute stroke patients: a randomized, double-blinded, sham-controlled study. CNS Neurosci. Ther. 30:e14530. doi: 10.1111/cns.14530
Maki, B. E., and McIlroy, W. E. (2007). Cognitive demands and cortical control of human balance-recovery reactions. J. Neural Transm. (Vienna) 114, 1279–1296. doi: 10.1007/s00702-007-0764-y
Monoli, C., Morris, A. J., Crofts, R., Fino, N. F., Petersell, T. L., Jameson, T., et al. (2025). Acute and longitudinal effects of concussion on reactive balance in collegiate athletes. Neurorehabil. Neural Repair 39, 263–273. doi: 10.1177/15459683241309569
Moore, R. T., Piitz, M. A., Singh, N., Dukelow, S. P., and Cluff, T. (2024). The independence of impairments in proprioception and visuomotor adaptation after stroke. J. Neuroeng. Rehabil. 21:81. doi: 10.1186/s12984-024-01360-7
Müller, D., Habel, U., Brodkin, E. S., and Weidler, C. (2022). High-definition transcranial direct current stimulation (HD-tDCS) for the enhancement of working memory - a systematic review and meta-analysis of healthy adults. Brain Stimul. 15, 1475–1485. doi: 10.1016/j.brs.2022.11.001
Naito, Y., Tateya, I., Hirano, S., Inoue, M., Funabiki, K., Toyoda, H., et al. (2003). Cortical correlates of vestibulo-ocular reflex modulation: a PET study. Brain 126, 1562–1578. doi: 10.1093/brain/awg165
Nonnekes, J., Arrogi, A., Munneke, M. A. M., van Asseldonk, E. H. F., Oude Nijhuis, L. B., Geurts, A. C., et al. (2014). Subcortical structures in humans can be facilitated by transcranial direct current stimulation. PLoS One 9:e107731. doi: 10.1371/journal.pone.0107731
Oka, S., Ikeda, T., Mitsutake, T., Ogata, K., and Goto, Y. (2022). Unilateral cathodal transcranial direct current stimulation over the parietal area modulates postural control depending with eyes open and closed. PLoS One 17:e0269145. doi: 10.1371/journal.pone.0269145
Pisella, L., Alahyane, N., Blangero, A., Thery, F., Blanc, S., and Pelisson, D. (2011). Right-hemispheric dominance for visual remapping in humans. Philos. Trans. R. Soc. B Biol. Sci. 366, 572–585. doi: 10.1098/rstb.2010.0258
Pollock, A. S., Durward, B. R., Rowe, P. J., and Paul, J. P. (2000). What is balance? Clin. Rehabil. 14, 402–406. doi: 10.1191/0269215500cr342oa
Prime, S. L., Vesia, M., and Crawford, J. D. (2011). Cortical mechanisms for trans-saccadic memory and integration of multiple object features. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 366, 540–553. doi: 10.1098/rstb.2010.0184
Sherrington, C. S. (1910). Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J. Physiol. 40, 28–121. doi: 10.1113/jphysiol.1910.sp001362
Sibley, K. M., Mochizuki, G., Lakhani, B., and McIlroy, W. E. (2014). Autonomic contributions in postural control: a review of the evidence. Rev. Neurosci. 25, 687–697. doi: 10.1515/revneuro-2014-0011
Slijper, H., and Latash, M. L. (2004). The effects of muscle vibration on anticipatory postural adjustments. Brain Res. 1015, 57–72. doi: 10.1016/j.brainres.2004.04.054
Takakusaki, K., Saitoh, K., Harada, H., and Kashiwayanagi, M. (2004). Role of basal ganglia-brainstem pathways in the control of motor behaviors. Neurosci. Res. 50, 137–151. doi: 10.1016/j.neures.2004.06.015
Takano, K., Katagiri, N., Sato, T., Jin, M., Koseki, T., Kudo, D., et al. (2023). Changes in corticospinal excitability and motor control during cerebellar transcranial direct current stimulation in healthy individuals. Cerebellum 22, 905–914. doi: 10.1007/s12311-022-01469-2
Tasci, S., Celik, H., and Kirazci, S. (2024). Effects of environmental and task related conditions on postural control under concurrent visual feedback. Hum. Mov. Sci. 94:103186. doi: 10.1016/j.humov.2024.103186
Ten Brink, A. F., Fabius, J. H., Weaver, N. A., Nijboer, T. C. W., and Van der Stigchel, S. (2019). Trans-saccadic memory after right parietal brain damage. Cortex 120, 284–297. doi: 10.1016/j.cortex.2019.06.006
Thach, W. T., and Bastian, A. J. (2004). Role of the cerebellum in the control and adaptation of gait in health and disease. Prog. Brain Res. 143, 353–366. doi: 10.1016/S0079-6123(03)43034-3
Unell, A., Eisenstat, Z. M., Braun, A., Gandhi, A., Gilad-Gutnick, S., Ben-Ami, S., et al. (2021). Influence of visual feedback persistence on visuo-motor skill improvement. Sci. Rep. 11:17347. doi: 10.1038/s41598-021-96876-6
Unver, B., and Bek, N. (2022). Plantar sensation, plantar pressure, and postural stability alterations and effects of visual status in older adults. Somatosens. Mot. Res. 39, 55–61. doi: 10.1080/08990220.2021.1994940
Xie, H., Liang, M., Mo, Y., Schmidt, C., Wang, C., and Chien, J. H. (2024). Comparison between effects of galvanic and vibration-based vestibular stimulation on postural control and gait performance in healthy participants: a systematic review of cross-sectional studies. Ann. Biomed. Eng. 52, 757–793. doi: 10.1007/s10439-023-03425-5
Young, D. R., Parikh, P. J., and Layne, C. S. (2020). Non-invasive brain stimulation of the posterior parietal cortex alters postural adaptation. Front. Hum. Neurosci. 14:248. doi: 10.3389/fnhum.2020.00248
Zafar, T., Zaki, S., Alam, M. F., Sharma, S., Babkair, R. A., Nuhmani, S., et al. (2024). Effect of whole-body vibration exercise on pain, disability, balance, proprioception, functional performance and quality of life in people with non-specific chronic low Back pain: a systematic review and meta-analysis. J. Clin. Med. 13:1639. doi: 10.3390/jcm13061639
Keywords: postural control, postural response, transcranial direct current stimulation, parietal lobe, sensory information processing
Citation: Yang F, Liu Y, Lv X, He Y, Gao J, Zhang P, Li Q, Peng Z and Qian J (2025) Influence of high-definition transcranial direct current stimulation to the parietal cortex on postural control: a single-blind randomized crossover study. Front. Hum. Neurosci. 19:1546631. doi: 10.3389/fnhum.2025.1546631
Edited by:
Daniel Rojas-Valverde, National University of Costa Rica, Costa RicaReviewed by:
Xuetong Zhai, University of Pittsburgh, United StatesRongjuan Zhu, Shaanxi Normal University, China
Copyright © 2025 Yang, Liu, Lv, He, Gao, Zhang, Li, Peng and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinghua Qian, amluZ2h1YXFpYW44OTEyNzNAMTI2LmNvbQ==
 Fan Yang
Fan Yang Yang Liu1,2
Yang Liu1,2 Yaqi He
Yaqi He Qing Li
Qing Li Jinghua Qian
Jinghua Qian