- 1Department of Physical Education, College of Education, Seoul National University, Seoul, Republic of Korea
- 2Institute of Aging, Seoul National University, Seoul, Republic of Korea
- 3School of Biological Sciences, Seoul National University, Seoul, Republic of Korea
Introduction: Anxiety and depression are psychiatric disorders that have a deleterious effect on human mental health. Meanwhile, various forms of exercise have been demonstrated to have beneficial effects on the reduction of symptoms of anxiety and depression. However, the disparate effects of each exercise on mood symptoms remain to be elucidated. This research examines the different effects of each type of exercise on mood symptoms and electroencephalography (EEG) activity.
Methods: Accordingly, subjects engaged in six weeks of aerobic and resistance exercises with a 3-week washout period between each intervention. The Score of Hospital Anxiety and Depression Scale (HADS) was employed to assess the severity of both anxiety and depression. For the purposes of EEG analysis, we calculated the values of theta/beta ratio (TBR), Higuchi Fractal Dimension (HFD), and frontal alpha asymmetry (FAA).
Results: Both types of exercise resulted in the alleviation of both anxiety and depression. Notably, aerobic exercise significantly decreased the score of HADS-A (anxiety), while resistance exercise significantly improved HADS-D (depression). In the context of EEG analysis, a significant decrease of TBR in the left frontal region was observed after aerobic exercise.
Discussion: Therefore, these findings emphasize the importance of personalized exercise strategies for those suffering from anxiety and depression. Furthermore, further investigation into the impact of exercise on brain wave activities associated with anxiety and depression are needed.
1 Introduction
Anxiety and depression are mental illnesses that exhibit both similarities and differences (Demyttenaere and Heirman, 2020; Kalin, 2020). Symptoms such as abnormal excitement, dizziness, emotional arousal, and instability are associated with anxiety, whereas anhedonia, lack of energy, and low emotional response are linked to depression (Clark and Watson, 1991; Burns and Eidelson, 1998; American Psychiatric Association, 2013). A notably high prevalence of anxiety and depression among young adults has been recently observed (Barker et al., 2019; Goodwin, 2020). The elevated prevalence of anxiety and depression is a primary contributor to the observed increase in suicidal behaviors (Kalin, 2021). Therefore, it is imperative to develop and implement diverse strategies for the management of mental health concerns.
Recent studies have sought to find neurophysiological markers by measuring the brain activity in patients with anxiety or depression (Knyazev et al., 2005b; Lin et al., 2021; Liang et al., 2021). Among the various neuroimaging and neurophysiological techniques, electroencephalography (EEG) has been employed as a means of analyzing brain activity associated with anxiety and depression (Britton et al., 2016). EEG is conducted by attaching electrodes to the scalp, which allows for high accessibility and high temporal resolution (Song, 2016). EEG is particularly useful for identifying the electrical activity of the brain of various mental disorders (Yasin et al., 2021; Jaiswal et al., 2023). Recent studies have used EEG to compare the brain waves of patients with anxiety or depression with those of healthy controls to determine whether each group has a different level of activity in specific wave patterns (Knyazev et al., 2005a; Blackhart et al., 2006; Trambaiolli and Biazoli, 2020; Minkowski et al., 2021). One study suggested that a higher power spectrum value of alpha waves was observed in patients suffering from depression (Liang et al., 2021).
While various treatment methods are provided for the improvement of anxiety and depression, drug treatments, such as anxiolytic or antidepressant, are the most popular prescription for both diseases (Bandelow et al., 2015; Amare et al., 2017; Köhler et al., 2018). Despite anxiety and depression having different mechanisms (Boyer, 2000), antidepressants are dominantly prescribed for both anxiety and depression. However, these common treatments can be problematic. While anxiety requires immediate symptom relief through anxiolytics, antidepressants require minimum 2 weeks to show their effects (Zoberi and Alec Pollard, 2010). Moreover, antidepressants may carry a risk of excessive physiological reactions and induce additional anxiety as side effects (Ballew, 2021; Fayez and Gupta, 2023). The current situation that antidepressants are prescribed for anxiety treatment despite danger of side effects reveals the lack of accurately established disorder-specific strategies.
Considering that drug treatment has critical side effects (Ramic et al., 2020), a demand for treatment with fewer side effects is increasing. Exercise has been proposed as an effective therapeutic tool for improving mental illness and neurodegenerative conditions (Hearing et al., 2016; Quan et al., 2020). A substantial body of evidence from animal and human studies has demonstrated that exercise effectively increases neurotransmitters, growth factors, blood flow formation, and neurogenesis, ultimately leading to improvements in brain function (Voss et al., 2013; Ryan and Kelly, 2016; Moon et al., 2019). In conclusion, a multifaceted approach may be needed to address each mood.
Long-term exercise has been demonstrated to have beneficial effects on both mood and the symptom relief, as well as on EEG changes (Liang et al., 2021). A number of reviews have examined whether the EEG pattern of patients are altered through exercise interventions (Gramkow et al., 2020; Hosang et al., 2022). However, although previous studies have primarily focused on comparing the effects of aerobic and resistance exercise on anxiety and depression, either separately or in relation to each other, no research has simultaneously addressed all four aspects; comparing which types of exercise are more beneficial for each mood. Furthermore, there is a paucity of studies investigating these effects alongside with changes in EEG patterns. In a previous pilot study, we posited that 6 weeks of aerobic or resistance exercise may result in different patterns of anxiety and depression alleviation and distinct EEG alterations (Yuk et al., 2024). Specifically, 6 weeks of resistance exercise significantly reduced depression-related symptoms, whereas aerobic exercise reduced anxiety-related symptoms. Thus, we would like to propose the possibility that different chronic exercise modalities may have different effects; determining which exercise is more effective for mood-related EEG changes.
In light of these findings, further research is required to gain a deeper understanding of the relationship between changes in brain wave levels and the manifestation of anxiety and depression symptoms. Specifically, this was a secondary study based on the pilot study, which examined the effects of two types of exercise on anxiety and depression symptoms (Yuk et al., 2024). The aim of this study is comparing the effects of resistance and aerobic exercise for the alleviation on mood-related symptoms. We hypothesized that each exercise would play a different role in improving anxiety and depression: aerobic exercise would alleviate anxiety and resistance exercise would reduce depression.
2 Materials and methods
2.1 Ethic declarations
All experimental procedures were approved by the Institutional Review Board of Seoul National University in accordance with the standards of the Declaration of Helsinki of the World Medical Association (IRB No. 2309/004-010). Prior to the experiment, written consent and questionnaire scores (online) were obtained from all participants. All subjects were informed about the procedures and purpose of the study, as well as the potential risks of exercise protocols in both oral and written forms, and they confirmed their willingness to participate.
2.2 Participants
Healthy adults aged 19–39 years were recruited from Seoul National University in response to online/offline advertisements. Participants taking anxiolytics/antidepressants or participating in regular exercise were excluded. Participants were randomly divided into three groups with one control (non-exercise) group (CTL) and two experimental groups.
2.3 Study design and procedures
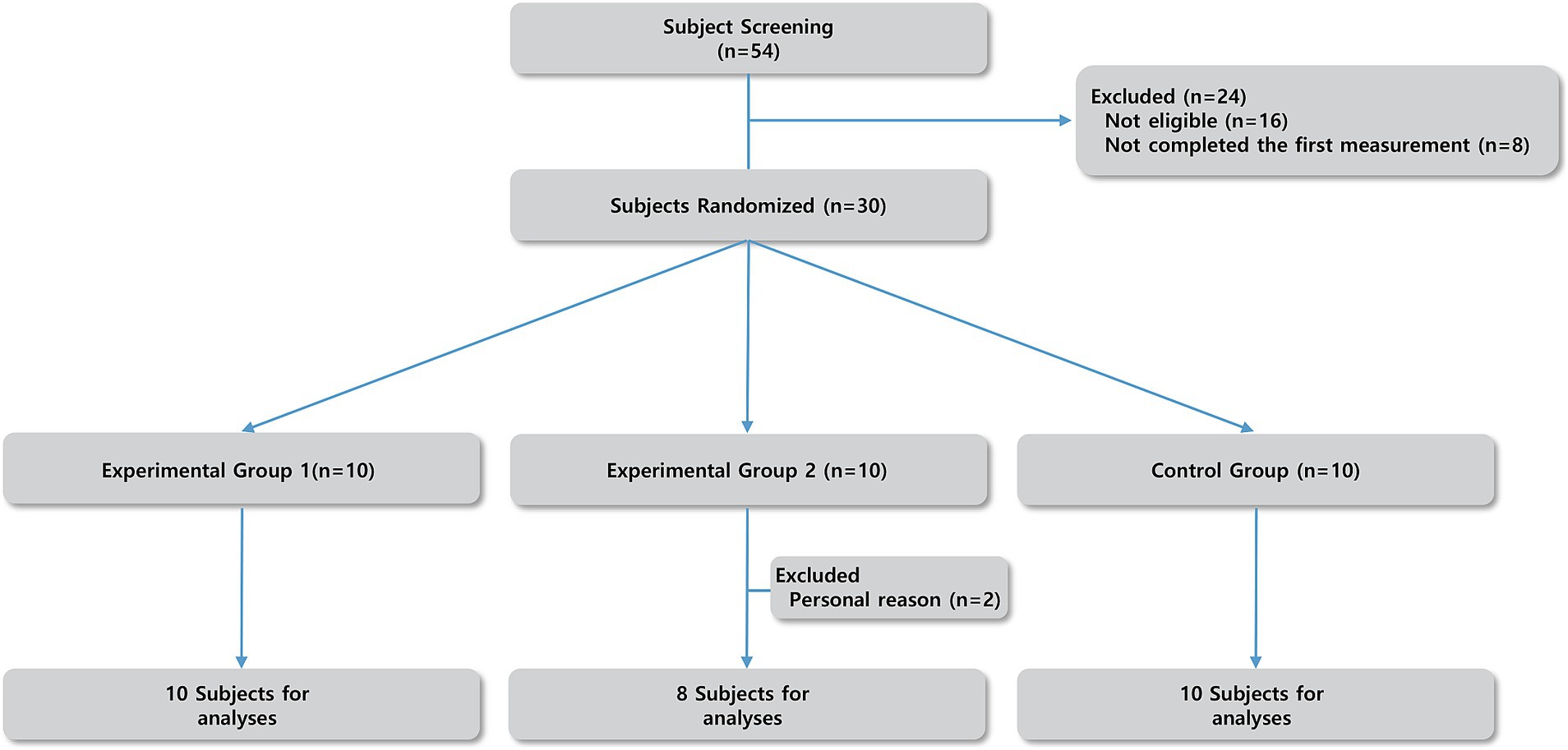
The overall procedure of this study started in September 2023 and was completed in April 2024. Initially, 30 healthy participants (age: 26.32 ± 4.58) volunteered.
The flow diagram of this study is illustrated in Figure 1. Exercise groups performed two 6-week exercise sessions twice a week: a total twelve-week with a 3-week washout (O’Donnell et al., 2011; Sanghuachang et al., 2023) session after one exercise session (Supplementary Figure S1). All interventions were taken by the same researcher and same environment (indoor lab).
Participants in experimental groups attended a total of 24 sessions according to the experiment schedule. The experiment consisted of the following phases: Exercise 1 (Week 1–6) / Wash-out (Week 7–9) / Exercise 2 (Week 10–15) (Supplementary Figure S1).
At the first visit (screening), the Physical Activity Readiness Questionnaire (PAR-Q), International Physical Activity Questionnaire (IPAQ), and the score of Hospital Anxiety and Depression Scale (HADS) were measured. Assessment was done before the first exercise session in Week 1 and 10 and after the last exercise session in Week 6 and 15 (Supplementary Figure S1). The assessment measures include body composition, resting heart rate, HADS, and 10-channel EEG data.
2.4 Exercise procedures
2.4.1 Aerobic exercise program
The aerobic exercise program was conducted based on ACSM guidelines (Pescatello, 2014). Exercise was done on a cycle ergometer (Monark 829E, Sweden). The individual VO₂max value was predicted through a 6-min Åstrand cycle ergometer test (Åstrand and Ryhming, 1954). Each session consisted of a 3-min warm-up and cool-down of light intensity activity (without resistance load). After warm-up, the cycling exercise was done at a moderate intensity [i.e., 60%~70% of individual heart rate reserve (HRR)] lasting 30 min. The exercise intensity was gradually increased based on individual’s heart rate increase. The individual’s heart rate was tracked throughout each session using Apple Watch SE (Apple, United States).
2.4.2 Resistance exercise program
The resistance exercise program was conducted based on ACSM guidelines and previous literature (Pescatello, 2014; LeBouthillier and Asmundson, 2017; O'Sullivan et al., 2023). The specific contents are summarized in Supplementary Table S1. Adaptation session was provided for participants for 30 min prior to exercise sessions (resistance). Participants performed 3 sets of 10–12 repetitions for each movement, with 60–90 s of time intervals between each set (Willardson, 2006). The intervention weight was recorded and progressively increased based on the participants’ adaptation. All human-human interactions were controlled.
2.5 Physical and mood symptom measurements
Weight, body fat percentage, and skeletal muscle mass of participants were measured by InBody 770 (InBody, Korea). During the measurement, the participants were instructed to remove accessories or metallic substances, including socks, earrings, and necklaces. Resting heart rate was measured using Apple Watch SE (Apple, United States) (Wallen et al., 2016; Falter et al., 2019). Blood pressure was measured using BPB10750 (InBody, Korea).
The HADS is a questionnaire composed of total of 42 points, divided into 21 points (anxiety/depression) and maximum 3 points per question (7 questions each), which is used to evaluate the severity of anxiety and depressive symptoms. The Korean version of the HADS was used (Min et al., 1999). The severity of each mood according to HADS scores are as represented: 0–7 normal, 8–10 borderline abnormal, 11–21 abnormal. HADS has a high validity and sensitivity on both anxiety (ɑ = 0.89) and depression (ɑ = 0.86) (Oh et al., 1999).
2.6 EEG analysis
EEG measurements were conducted in a dark, quiet condition to make subjects maintain resting state. After a short adaptation period, subjects were instructed to close their eyes and minimize all movements including eye movements. We provided the subjects a pair of earplugs to minimize external noises. EEG was recorded for 3 min using QEEG-64FX (Laxtha Inc., Korea) (Mahato et al., 2020; Lim et al., 2018). The whole recording was done in accordance with the 10–20 system using 10 channels (Klem et al., 1999). Electrodes were attached to Fp1, Fp2 (prefrontal), F3, F4 (frontal), T7, T8 (temporal), P3, P4 (parietal), O1, and O2 (occipital). The right and left earlobe each served as the ground (GND) and reference load (A2). The sampling rate was 250 Hz/channel, with a 12-bit A/D converter.
After raw data was extracted, it was filtered with a 0.5 Hz low-pass filter, a 50 Hz high-pass filter, and a notch filter set at 60 Hz to eliminate additional movements or electrical noises (Mahato et al., 2020), and was processed with Savitzky–Golay filter (window length: 51) to remove noise artifacts (Al-Qazzaz et al., 2019) using Telescan program (Ver. 3.29., Laxtha Inc. Korea). EEG signals were assessed by three individuals who were blinded to the experiment. Visual inspection was done to exclude signals contaminated by external noise or other artifacts.
Each set of EEG data was analyzed according to previous studies that suggested the relationship between each biomarker and state of anxiety or depression. We set the frequency of each wave: beta (4–7.99 Hz), alpha (8–12.99 Hz), and theta (13–29.99 Hz). As anxiety and depression biomarkers, we calculated the value of the theta/beta ratio (TBR) (Wei et al., 2020; Chang and Choi, 2023), Higuchi Fractal Dimension (HFD) values of each brain region (Bachmann et al., 2013), and frontal alpha asymmetry (FAA) (Seok et al., 2007). HFD values were calculated using Python v3.12.
2.7 Statistical analysis
Statistical analysis was performed using Graph Pad Prism V.10.2.2 (Graph Pad Software Inc., CA, United States) and R (version 4.4.2, R Foundation for Statistical Computing, Vienna, Austria). All diagrams and data are presented as mean ± standard error of the mean (SEM). Data analyses were performed based on three groups according to exercise type [CTL, Aerobic (AE), Resistance (RE)].
The normality of data was assessed through Shapiro–Wilk test. An unpaired t-test or Wilcoxon rank sum test was performed to compare baseline values between groups. A paired t-test was performed to compare the exercise effect after each intervention. A one-way ANOVA was conducted to compare the average score change deviation within each group of participants. A two-way ANOVA with repeated measurements was used to compare the effect of exercise after intervention. A Bonferroni post-hoc test was applied to verify differences within the group. Values of p < 0.05 were considered statistically significant. Effect size (ES) was calculated based on the statistical analysis method. The ES of t-test was measured as Cohen’s d, while the ES of two-way with repeated measurements was measured as partial eta squared ( ).
3 Results
3.1 Participants
In total, 18 participants (female 83.3%) composed the experimental group and 10 participants (female 70%) belonged to the control group. During the experiment, two female participants from experimental group 2 dropped out during the washout session; however, their data from the resistance exercise session was included in the analysis as both participants had completed the entire resistance exercise session.
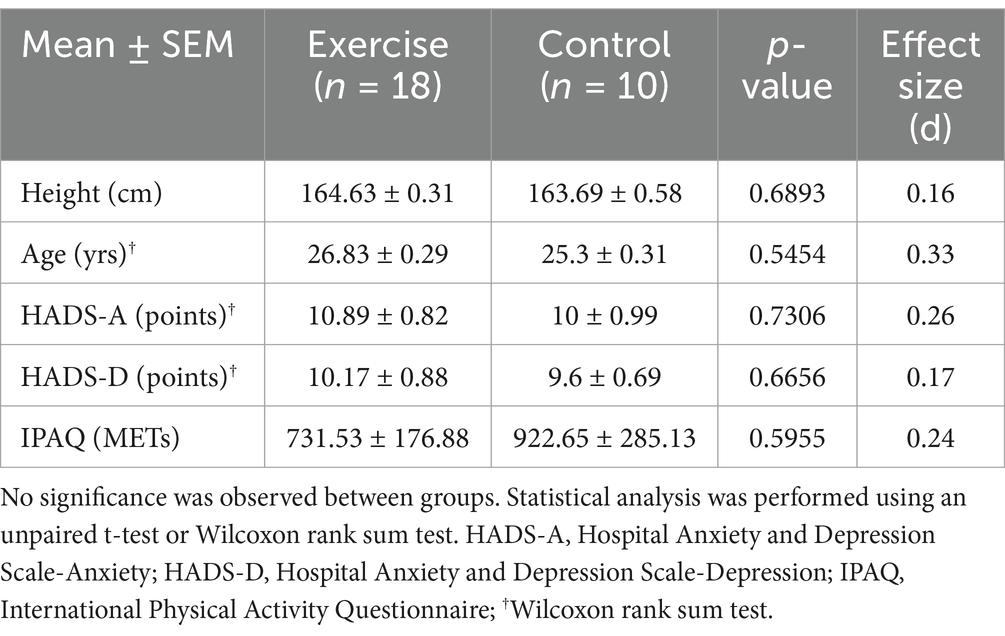
No significant differences were observed in the baseline values of questionnaire scores of HADS-A (anxiety), HADS-D (depression), and the average Metabolic Equivalent Task (MET) value according to IPAQ between the experimental and control groups (Table 1).
3.2 Physical measurements
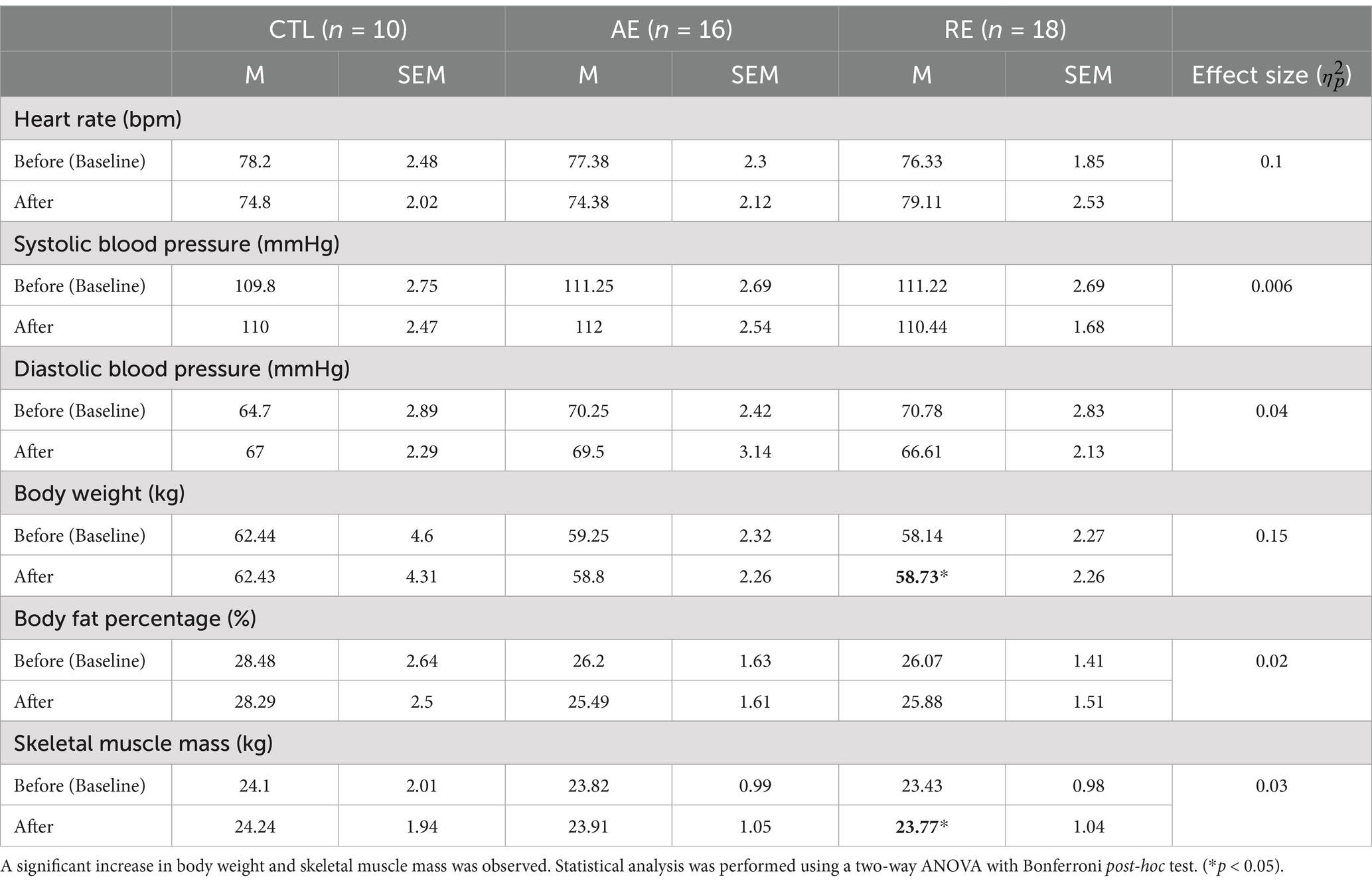
Table 2 presents the changes in physical characteristics observed following each intervention. No significant changes were noted within groups throughout the intervention period in resting heart rate, blood pressure, and body fat percentage following aerobic exercise. However, only resistance exercise resulted in a significant increase in body weight (p = 0.033, ES = 0.15) and skeletal muscle mass (p = 0.039, ES = 0.02) compared to the baseline measurement.
3.3 Mood symptom measurements
We measured the score of HADS to evaluate the changes in anxiety and depression symptoms. A two-way ANOVA on HADS-A scores revealed a significant main effect from group by time interaction F(2,41) = 4.113, p = 0.024, ES = 0.17. A post-hoc analysis revealed a significant decrease in HADS-A score following both AE (p = 0.0003) and RE (p = 0.003) (Figure 2A). There was no significance in within the CTL group (p = 0.647).
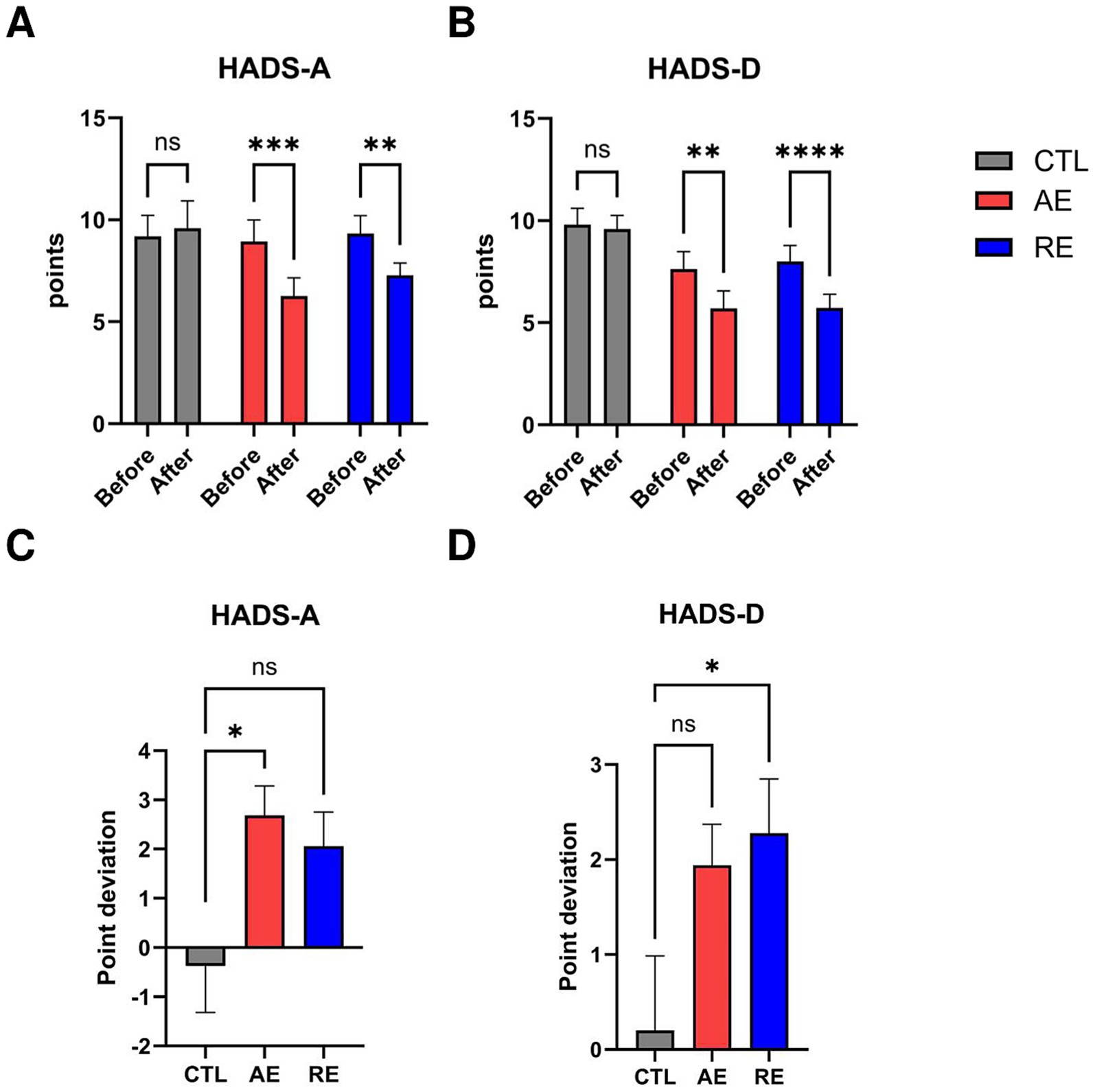
Figure 2. Changes in questionnaire scores after exercise. (A) HADS-A score, (B) HADS-D score, (C) Point deviation of HADS-A, (D) Point deviation of HADS-D. CTL (n = 10), AE (n = 16), and RE (n = 18). Statistical analysis was performed using two-way ANOVA with Bonferroni post-hoc test. Data was presented as mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = not significant). HADS-A, Hospital Anxiety and Depression Scale-Anxiety; HADS-D, Hospital Anxiety and Depression Scale-Depression; CTL, Control; AE, Aerobic exercise; RE, Resistance exercise.
There was a tendency toward a group by time interaction F(2,41) = 3.011, p = 0.06, ES = 0.13 was observed on HADS-D scores. A significant reduction in HADS-D scores was observed following both AE (p = 0.011) and RE (p < 0.0001). There was no significance in the CTL group (p = 0.776) (Figure 2B). The results demonstrated that both 6 weeks of aerobic and resistance exercise caused a significant decrease in anxiety-related scores and depression-related scores.
To ascertain which exercise exerts a more pronounced effect on the reduction of anxiety and depressive symptoms, we conducted a comparative analysis of the mean score change deviation within each participant group. The results showed that aerobic exercise resulted in a statistically significant reduction in anxiety scores compared to the CTL group (p = 0.025) (Figure 2C). Conversely, resistance exercise led to a significant decrease in depression scores compared to the CTL group (p = 0.044) (Figure 2D). In conclusion, both exercises exhibited a notable impact on mood symptoms, with aerobic exercise particularly contributing to anxiety improvements and resistance exercise to depression relief.
3.4 EEG analysis
Prior research indicates that the severity of anxiety may be indicated by TBR of the frontal region and FAA (Seok et al., 2007; Al-Ezzi et al., 2020). Conversely, TBR of the temporal region, HFD, and FAA may be indicative of depression-related EEG activity, as previously observed in studies (Seok et al., 2007; Bachmann et al., 2013; Ren et al., 2020).
While no significant differences were observed in the TBR of frontal region between the RE and CTL groups, a notable decrease of TBR in the left frontal region (ch3) following exercise was verified (p = 0.04, ES = 0.1) (Figure 3). No significant alterations in HFD (ES = 0.004), TBR of the temporal region (ES = 0.07), and FAA were discerned (ES = 0.05) (Figures 4A–C). These finding imply that aerobic exercise may influence EEG patterns, particularly the ratio of theta and beta waves in the frontal region. However, no notable changes were observed following resistance exercise.
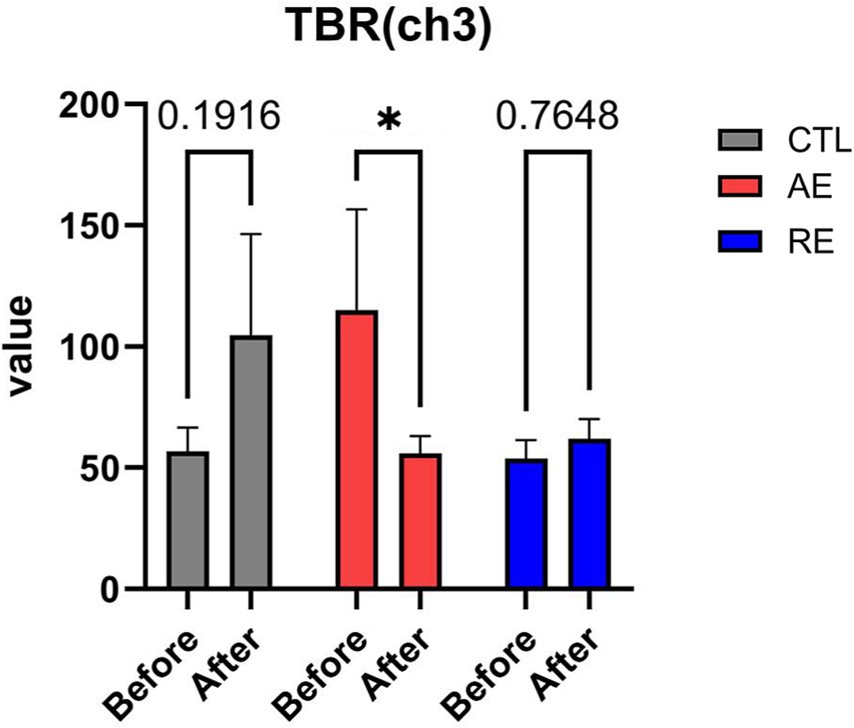
Figure 3. Changes of anxiety-related EEG patterns after exercise. TBR (ch3). CTL (n = 10), AE (n = 16), and RE (n = 18). Statistical analysis was performed using two-way ANOVA with Bonferroni post-hoc test. Data was presented as mean ± SEM (*p < 0.05, ns = not significant). TBR, Theta/beta ratio; CTL, Control; AE, Aerobic exercise; RE, Resistance exercise.
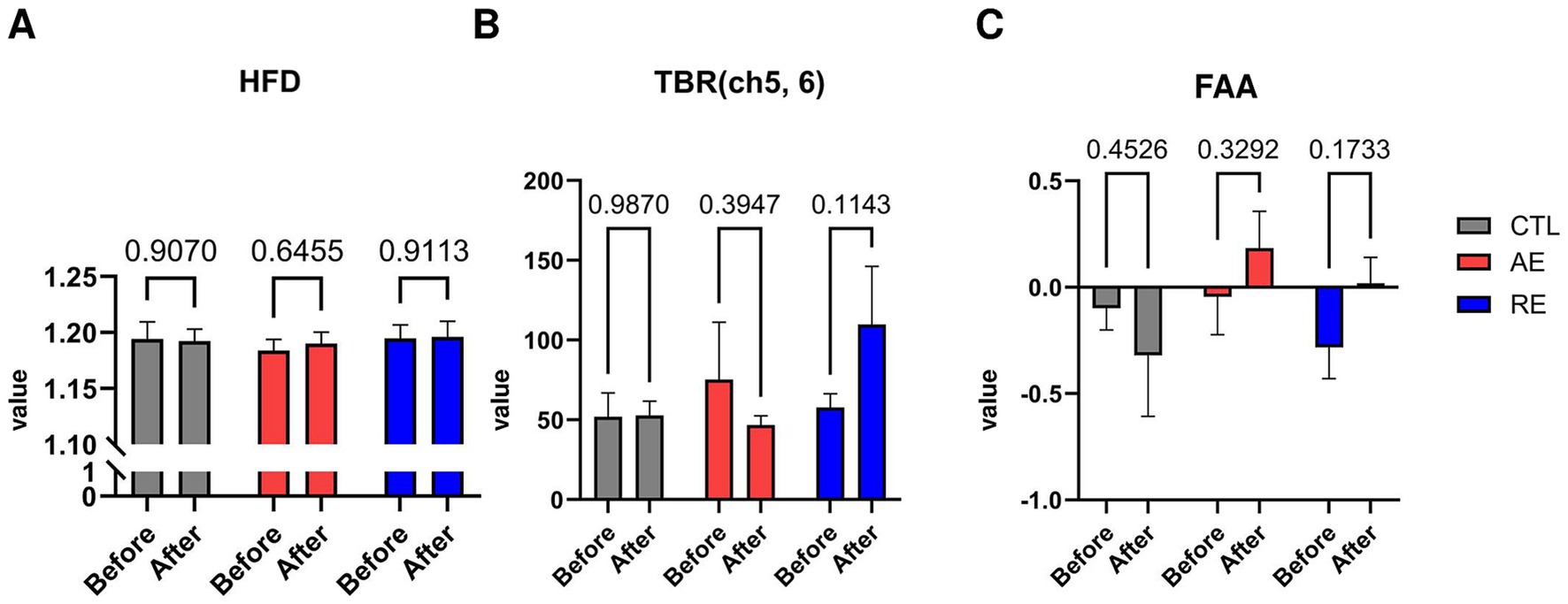
Figure 4. Changes of depression-related EEG patterns after exercise. (A) HFD, (B) TBR (ch5, 6), (C) FAA. CTL (n = 10), AE (n = 16), and RE (n = 18). Statistical analysis was performed using two-way ANOVA with Bonferroni post-hoc test. Data was presented as mean ± SEM (*p < 0.05, ns = not significant). HFD, Higuchi Fractal Dimension; TBR, Theta/beta ratio; FAA, Frontal alpha asymmetry; CTL, Control; AE, Aerobic exercise; RE, Resistance exercise.
4 Discussion
A substantial body of evidence from both non-clinical and clinical studies indicates the potential to differentiate between anxiety and depression at the mechanistic level (Renner et al., 2018) and to apply different therapeutic strategies for each mood state (Yuk et al., 2023). With regard to the findings of our pilot trial, the objective was to find the disparate effects of aerobic and resistance exercise on the reduction of anxiety/depression-related symptoms and the alteration of brain wave patterns. We observed a reduction in anxiety symptoms after aerobic exercise, while a reduction in depressive symptoms was observed after resistance exercise. We were also able to confirm EEG pattern changes associated with anxiety symptoms. These findings provide valuable insights for promoting exercise for the enhancement of mental health, especially applying individualized exercise strategies according to one’s mental status.
Firstly, with regard to the body composition results, 6 weeks of resistance exercise resulted in a significant increase in skeletal muscle mass and body weight. Nevertheless, no significant differences were observed after aerobic exercise. Given that our aerobic exercise regimen consisted of 2 days of cycling at moderate intensity, it is plausible that a higher frequency or intensity may be required to induce significant alterations in body composition (Mendes et al., 2013; Bryner et al., 1997). Notwithstanding, the Åstrand test results demonstrated a significant increase in VO₂max among all participants following aerobic exercise (Supplementary Figure S2A), which corroborates the findings of a previous study (Mendes et al., 2013). In addition, the relative load calculation results exhibited a notable increase in load throughout the resistance session (Supplementary Figure S2B). This suggests that 6 weeks of resistance exercise may effectively enhance skeletal muscle mass, consistent with the previous finding that a 6–12 repetition maximum (RM) may facilitate muscle hypertrophy (Kraemer and Ratamess, 2004). Our findings indicate that 6 weeks of aerobic exercise is effective for elevating cardiorespiratory fitness ability, while resistance exercise may promote muscle mass gain.
Secondly, changes in the questionnaire scores were observed in order to investigate the changes in anxiety and depressive symptoms. Consequently, both types of exercise resulted in a reduction in the severity of both anxiety and depression, as indicated by HADS criteria, from mild to normal levels. In accordance with our findings, previous studies have highlighted that exercise has a beneficial impact on human mental health (Fetzner and Asmundson, 2015; LeBouthillier and Asmundson, 2017; O'Sullivan et al., 2023). It is noteworthy that the comparison of questionnaire score deviations for each exercise revealed that each exercise can specifically target the alleviation of different mood symptoms. While several studies support that aerobic exercise effectively reduces anxiety symptoms (Fetzner and Asmundson, 2015; Hill et al., 2019) and resistance exercise contributes to a decrease in depression symptoms (Zhao et al., 2020), our results suggest that the effects of different types of exercise may be differentiated. Specifically, 6 weeks of aerobic and resistance exercises (once per week) have been shown to, respectively, reduce BAI and BDI scores (Yuk et al., 2024). Thus, participating in 6 weeks of moderate aerobic and resistance exercise has the potential to offer comprehensive benefits with regards to mental health enhancement. However, participating in different types of exercise based on one’s current mood symptoms, aerobic exercise for anxiety-like symptoms and resistance exercise for depression-like symptoms can be a more efficacious approach.
Next, we investigated the EEG pattern changes according to previous literature, which confirmed the difference between anxiety or depression patients and healthy controls. In light of the notable decline in frontal TBR, recent studies have indicated a positive correlation between TBR and anxiety level scores (Putman et al., 2014; Al-Ezzi et al., 2020). Therefore, when considering the alterations in questionnaire scores and outcomes of TBR, it can be postulated that aerobic exercise may serve to mitigate anxiety symptoms and EEG patterns. Moreover, given that our subjects were not clinically diagnosed as patients, our findings may imply that frontal TBR functions as an anxiety-specific biomarker for individuals without a clinical diagnosis of anxiety disorders. Notably, aerobic exercise may significantly alter.
Previous studies have shown that individuals diagnosed with major depressive disorder exhibited elevated HFD values (Bachmann et al., 2013; de Aguiar Neto and Rosa, 2019) and alterations in theta oscillatory activity (Ren et al., 2020; Chang and Choi, 2023). Accordingly, we initially assessed the values of HFD and the TBR of the temporal region as potential depression-specific biomarkers. However, the results revealed that the implementation of 6 weeks of aerobic and resistance exercises did not cause a discernible alteration in the values of depression-specific biomarkers across all groups. While some studies question whether HFD can explain the depression severity (Ahmadlou et al., 2012; Lord and Allen, 2023), the majority of previous studies observed significant higher HFD from patients diagnosed with depression compared to that of healthy controls (Akar et al., 2015a; Akar et al., 2015b). As our study participants did not have any clinical diagnosis of mood disorders, we postulated that HFD may serve as a biomarker only when the symptom severity of an individual exceeds a certain level sufficient to be diagnosed as a disease; the severity of the participants was not sufficient to detect HFD differences. Nonetheless, further studies are required to elucidate the relationship between exercise and HFD is needed. With regard to temporal TBR, our findings were consistent with a study that did not find a significant difference between depressed patients and healthy controls (Chang and Choi, 2023). This is the first study to confirm the exercise effect on depression through HFD and temporal TBR. However, the effects of each exercise on these two EEG biomarkers remain unclear.
Another mood-related biomarker we measured was FAA. The FAA serves as a biomarker indicative of disparate activities within the left and right frontal cortex. In particular, increased alpha absolute power in the left hemisphere is indicative of a lack of approach behavior, which is common phenomenon observed in patients with depression (de Aguiar Neto and Rosa, 2019). However, this may be reversed by increasing anxiety severity (Seok et al., 2007). Throughout our trial, we did not observe a significant change in FAA after each intervention. These results were similar to those of our pilot study, which also did not observe any significance after both types of exercise. There are numerous studies examining the changes in FAA together with depression (Bond et al., 2018; Hong et al., 2020); however, the results explaining how exercise regulates the asymmetry patterns are yet contradictory (Reid et al., 1998; Hong et al., 2020). One meta-analysis study questioned the validity of FAA, since patterns of FAA varied according to age or sex, and a single variable that explains the relationship between FAA and depression was not found (Van Der Vinne et al., 2017). Therefore, while some studies utilize FAA as a representative biomarker to explain depression symptoms, further studies are needed to precisely organize the relationship between FAA and mood disorders, and to clarify the effect of exercise on FAA as well.
Our study has some limitations. Firstly, the program G-Power 3.1 (Heinrich-Heine-Universität Düsseldorf, Germany) recommended to recruit total of 30 participants (assumed as ‘ANOVA: Repeated measures, within-between interaction,’ effect size = 0.25, power = 0.8, alpha error probability = 0.05). However, due to dropouts, the final sample size was smaller than the recommended number. Since the G-Power post-hoc power analysis suggests the statistical power of this study as 0.6 (assumed as “ANOVA: Repeated measures, within-between interaction,” effect size = 0.25, total sample size = 28, alpha error probability = 0.05), future research conducted with a larger sample size (N = 42 for power 0.8) could display meaningful results. Second, the absence of objective biomarkers precludes the determination of whether the designated washout period was sufficient to eliminate the effects of exercise on mood. A 3-week wash-out period was implemented, based on the findings of a previous study which suggested that 3-week washout may eliminate exercise effects (Otsuka et al., 2021). Although our study did not reveal any significant discrepancies in the baseline scores of Week 1 and Week 10 (Supplementary Figure S3), the necessity for a precise biomarker to demonstrate the wash-out period remains. Thirdly, we did not control confounding lifestyle variables, such as diet, sleep patterns, etc. Lastly, we had technical limitations regarding EEG analysis: measuring and controlling the impedance was difficult using a dry cap. Moreover, additional artifacts such as signal noise, movement, etc. may have influenced the results. These limitations highlight the need for further studies to address these issues.
In conclusion, both types of exercise have been shown to help reduce symptoms associated with anxiety and depression. Nevertheless, moderate aerobic exercise may be an effective method of reducing anxiety symptoms, whereas moderate resistance exercise has been shown to be more effective in reducing depressive symptoms. The results of the EEG analysis results indicated that aerobic exercise may contribute to a tendency for decrease in TBR (ch3, 4) and a significant reduction in TBR (ch3). However, there were no significant changes in, HFD, and FAA. These findings suggest the possibility that different types of exercise may alleviate different mood symptoms and also lead to changes in mood EEG biomarkers. To the best of our knowledge, this is the first study to directly compare two types of exercise in terms of mood symptoms and EEG pattern changes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Seoul National University IRB No. 2309/004-010. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing, Funding acquisition, Resources. JL: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing. SK: Conceptualization, Formal analysis, Methodology, Project administration, Writing – review & editing. TK: Formal analysis, Methodology, Writing – review & editing. HM: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Research Foundation (NRF) of Korea (NRF-2022R1I1A4053049) and was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1A5A8019303).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2025.1562702/full#supplementary-material
References
Ahmadlou, M., Adeli, H., and Adeli, A. (2012). Fractality analysis of frontal brain in major depressive disorder. Int. J. Psychophysiol. 85, 206–211. doi: 10.1016/j.ijpsycho.2012.05.001
Akar, S. A., Kara, S., Agambayev, S., and Bilgiç, V. (2015a). Nonlinear analysis of EEG in major depression with fractal dimensions. In: 2015 37th annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE, 7410–7413.
Akar, S. A., Kara, S., Agambayev, S., and Bilgiç, V. (2015b). Nonlinear analysis of Eegs of patients with major depression during different emotional states. Comput. Biol. Med. 67, 49–60. doi: 10.1016/j.compbiomed.2015.09.019
Al-Ezzi, A., Kamel, N., Faye, I., and Ebenezer, E. G. M. (2020). EEG frontal theta-beta ratio and frontal midline theta for the assessment of social anxiety disorder. In: 2020 10th IEEE international Conference on Control System, Computing and Engineering (ICCSCE). IEEE, 107–112.
Al-Qazzaz, N. K., Sabir, M. K., Ali, S., Ahmad, S. A., and Grammer, K. (2019). Effective EEG channels for emotion identification over the brain regions using differential evolution algorithm. In: 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE, 4703–4706.
Amare, A. T., Schubert, K. O., and Baune, B. T. (2017). Pharmacogenomics in the treatment of mood disorders: strategies and opportunities for personalized psychiatry. EPMA J. 8, 211–227. doi: 10.1007/s13167-017-0112-8
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders: Dsm-5. Washington, DC: American psychiatric association.
Åstrand, P.-O., and Ryhming, I. (1954). A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during submaximal work. J. Appl. Physiol. 7, 218–221. doi: 10.1152/jappl.1954.7.2.218
Bachmann, M., Lass, J., Suhhova, A., and Hinrikus, H. (2013). Spectral asymmetry and Higuchi’s fractal dimension measures of depression electroencephalogram. Comput. Math. Methods Med. 2013, 1–8. doi: 10.1155/2013/251638
Ballew, K. (2021). Relationship between anti-anxiety and anti-depressant medication on therapy and coping mechanisms. OSU Dissertations. Oklahoma State University. Retrieved from https://openresearch.okstate.edu/entities/publication/9b42cc35-0278-4235-aeab-ce583af13c41/full
Bandelow, B., Reitt, M., Röver, C., Michaelis, S., Görlich, Y., and Wedekind, D. (2015). Efficacy of treatments for anxiety disorders: a meta-analysis. Int. Clin. Psychopharmacol. 30, 183–192. doi: 10.1097/YIC.0000000000000078
Barker, M. M., Beresford, B., Bland, M., and Fraser, L. K. (2019). Prevalence and incidence of anxiety and depression among children, adolescents, and young adults with life-limiting conditions: a systematic review and meta-analysis. JAMA Pediatr. 173, 835–844. doi: 10.1001/jamapediatrics.2019.1712
Blackhart, G. C., Minnix, J. A., and Kline, J. P. (2006). Can Eeg asymmetry patterns predict future development of anxiety and depression? A preliminary study. Biol. Psychol. 72, 46–50. doi: 10.1016/j.biopsycho.2005.06.010
Bond, V., Osby, A., Obisesan, T., Kumar, K., Pemminati, S., Gorantla, V. R., et al. (2018). Effects of aerobic exercise on frontal Eeg asymmetry, coherence and mood: a pilot study. J Clin Diagn Res 12, CC05–CC10. doi: 10.7860/JCDR/2018/32657.11645
Boyer, P. (2000). Do anxiety and depression have a common pathophysiological mechanism? Acta Psychiatr. Scand. 102, 24–29. doi: 10.1111/j.0065-1591.2000.acp29-04.x
Britton, J. W., Frey, L. C., Hopp, J. L., Korb, P., Koubeissi, M. Z., Lievens, W. E., et al. (2016). Electroencephalography (Eeg): An introductory text and atlas of Normal and abnormal findings in adults, children, and infants. Chicago: American Epilepsy Society.
Bryner, R., Toffle, R., Ullrich, I., and Yeater, R. (1997). The effects of exercise intensity on body composition, weight loss, and dietary composition in women. J. Am. Coll. Nutr. 16, 68–73. doi: 10.1080/07315724.1997.10718651
Burns, D. D., and Eidelson, R. J. (1998). Why are depression and anxiety correlated? A test of the tripartite model. J. Consult. Clin. Psychol. 66, 461–473. doi: 10.1037/0022-006X.66.3.461
Chang, J., and Choi, Y. (2023). Depression diagnosis based on electroencephalography power ratios. Brain Behav 13:e3173. doi: 10.1002/brb3.3173
Clark, L. A., and Watson, D. (1991). Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J. Abnorm. Psychol. 100, 316–336. doi: 10.1037/0021-843X.100.3.316
De Aguiar Neto, F. S., and Rosa, J. L. G. (2019). Depression biomarkers using non-invasive Eeg: a review. Neurosci. Biobehav. Rev. 105, 83–93. doi: 10.1016/j.neubiorev.2019.07.021
Demyttenaere, K., and Heirman, E. (2020). The blurred line between anxiety and depression: hesitations on comorbidity, thresholds and hierarchy. Int. Rev. Psychiatry 32, 455–465. doi: 10.1080/09540261.2020.1764509
Falter, M., Budts, W., Goetschalckx, K., Cornelissen, V., and Buys, R. (2019). Accuracy of apple watch measurements for heart rate and energy expenditure in patients with cardiovascular disease: cross-sectional study. JMIR Mhealth Uhealth 7:e11889. doi: 10.2196/11889
Fetzner, M. G., and Asmundson, G. J. (2015). Aerobic exercise reduces symptoms of posttraumatic stress disorder: a randomized controlled trial. Cogn. Behav. Ther. 44, 301–313. doi: 10.1080/16506073.2014.916745
Goodwin, E. A. (2020). Trends in anxiety among adults in the United States, 2008–2018: rapid increases among young adults. J. Psychiatr. Res. 130, 441–446. doi: 10.1016/j.jpsychires.2020.08.014
Gramkow, M. H., Hasselbalch, S. G., Waldemar, G., and Frederiksen, K. S. (2020). Resting state Eeg in exercise intervention studies: a systematic review of effects and methods. Front. Hum. Neurosci. 14:155. doi: 10.3389/fnhum.2020.00155
Hearing, C., Chang, W., Szuhany, K., Deckersbach, T., Nierenberg, A., and Sylvia, L. G. (2016). Physical exercise for treatment of mood disorders: a critical review. Curr. Behav. Neurosci. Rep. 3, 350–359. doi: 10.1007/s40473-016-0089-y
Hill, M., Gibson, A.-M., Wagerman, S., Flores, E., and Kelly, L. (2019). The effects of aerobic and resistance exercise on state anxiety and cognitive function. Sci Sports 34, 216–221. doi: 10.1016/j.scispo.2018.09.004
Hong, J. S., Kim, S. M., Kang, K. D., Han, D. H., Kim, J. S., Hwang, H., et al. (2020). Effect of physical exercise intervention on mood and frontal alpha asymmetry in internet gaming disorder. Ment. Health Phys. Act. 18:100318. doi: 10.1016/j.mhpa.2020.100318
Hosang, L., Mouchlianitis, E., Guérin, S. M., and Karageorghis, C. I. (2022). Effects of exercise on electroencephalography-recorded neural oscillations: a systematic review. Int. Rev. Sport Exerc. Psychol. 17, 1–54. doi: 10.1080/1750984X.2022.2103841
Jaiswal, S., Huang, S.-L., Juan, C.-H., Huang, N. E., and Liang, W.-K. (2023). Resting state dynamics in people with varying degrees of anxiety and mindfulness: a nonlinear and nonstationary perspective. Neuroscience 519, 177–197. doi: 10.1016/j.neuroscience.2023.03.012
Kalin, N. H. (2020). The critical relationship between anxiety and depression. Am Psychiatric Assoc 177, 365–367. doi: 10.1176/appi.ajp.2020.20030305
Kalin, N. H. (2021). Anxiety, depression, and suicide in youth. Am J Psychiatry 178, 275–279. doi: 10.1176/appi.ajp.2020.21020186
Klem, G. H., Luders, H. O., Jasper, H. H., and Elger, C. (1999). The ten-twenty electrode system of the international federation: The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 52, 3–6.
Knyazev, G. G., Savostyanov, A. N., and Levin, E. A. (2005a). Anxiety and synchrony of alpha oscillations. Int. J. Psychophysiol. 57, 175–180. doi: 10.1016/j.ijpsycho.2005.01.004
Knyazev, G. G., Savostyanov, A. N., and Levin, E. A. (2005b). Uncertainty, anxiety, and brain oscillations. Neurosci. Lett. 387, 121–125. doi: 10.1016/j.neulet.2005.06.016
Köhler, C. A., Freitas, T. H., Stubbs, B., Maes, M., Solmi, M., Veronese, N., et al. (2018). Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol. Neurobiol. 55, 4195–4206. doi: 10.1007/s12035-017-0632-1
Kraemer, W. J., and Ratamess, N. A. (2004). Fundamentals of resistance training: progression and exercise prescription. Med. Sci. Sports Exerc. 36, 674–688. doi: 10.1249/01.MSS.0000121945.36635.61
Lebouthillier, D. M., and Asmundson, G. J. G. (2017). The efficacy of aerobic exercise and resistance training as transdiagnostic interventions for anxiety-related disorders and constructs: a randomized controlled trial. J. Anxiety Disord. 52, 43–52. doi: 10.1016/j.janxdis.2017.09.005
Liang, A., Zhao, S., Song, J., Zhang, Y., Zhang, Y., Niu, X., et al. (2021). Treatment effect of exercise intervention for female college students with depression: analysis of electroencephalogram microstates and power spectrum. Sustain. For. 13:6822. doi: 10.3390/su13126822
Lim, J.-H., Kim, H., Jeon, C., and Cho, S. (2018). The effects on mental fatigue and the cognitive function of mechanical massage and binaural beats (brain massage) provided by massage chairs. Complement. Ther. Clin. Pract. 32, 32–38. doi: 10.1016/j.ctcp.2018.04.008
Lin, I. M., Chen, T.-C., Lin, H.-Y., Wang, S.-Y., Sung, J.-L., and Yen, C.-W. (2021). Electroencephalogram patterns in patients comorbid with major depressive disorder and anxiety symptoms: proposing a hypothesis based on hypercortical arousal and not frontal or parietal alpha asymmetry. J. Affect. Disord. 282, 945–952. doi: 10.1016/j.jad.2021.01.001
Lord, B., and Allen, J. J. (2023). Evaluating Eeg complexity metrics as biomarkers for depression. Psychophysiology 60:e14274. doi: 10.1111/psyp.14274
Mahato, S., Goyal, N., Ram, D., and Paul, S. (2020). Detection of depression and scaling of severity using six channel Eeg data. J. Med. Syst. 44:118. doi: 10.1007/s10916-020-01573-y
Mendes, T. T., Fonseca, T. R., Ramos, G. P., Wilke, C. F., Cabido, C. E. T., De Barros, C. L. M., et al. (2013). Six weeks of aerobic training improves Vo 2max and Mlss but does not improve the time to fatigue at the Mlss. Eur. J. Appl. Physiol. 113, 965–973. doi: 10.1007/s00421-012-2501-y
Min, K.-J., Oh, S. M., and Park, D.-B. (1999). A study on the standardization of the hospital anxiety and depression scale for Koreans. J. Korean Neuropsychiatr. Assoc. 38, 289–296.
Minkowski, L., Mai, K. V., and Gurve, D. (2021). Feature extraction to identify depression and anxiety based on EEG. Annu Int Conf Ieee Eng Med Biol Soc 2021, 6322–6325. doi: 10.1109/EMBC46164.2021.9630821
Moon, H. Y., Javadi, S., Stremlau, M., Yoon, K. J., Becker, B., Kang, S.-U., et al. (2019). Conditioned media from Aicar-treated skeletal muscle cells increases neuronal differentiation of adult neural progenitor cells. Neuropharmacology 145, 123–130. doi: 10.1016/j.neuropharm.2018.10.041
O’donnell, D. E., Casaburi, R., Vincken, W., Puente-Maestu, L., Swales, J., Lawrence, D., et al. (2011). Effect of indacaterol on exercise endurance and lung hyperinflation in Copd. Respir. Med. 105, 1030–1036. doi: 10.1016/j.rmed.2011.03.014
Oh, S.-M., Min, K.-J., and Park, D.-B. (1999). A study on the standardization of the hospital anxiety and depression scale for Koreans: a comparison of normal, depressed and anxious groups. J. Korean Neuropsychiatr. Assoc. 38, 289–296.
O'sullivan, D., Gordon, B. R., Lyons, M., Meyer, J. D., and Herring, M. P. (2023). Effects of resistance exercise training on depressive symptoms among young adults: a randomized controlled trial. Psychiatry Res. 326, –115322. doi: 10.1016/j.psychres.2023.115322
Otsuka, S., Sakakima, H., Tani, A., Nakanishi, K., Takada, S., Norimatsu, K., et al. (2021). Effects of detraining on preconditioning exercise-induced neuroprotective potential after ischemic stroke in rats. Brain Struct. Funct. 226, 2169–2180. doi: 10.1007/s00429-021-02317-5
Pescatello, B. (2014). ACSM’s guidelines for exercise testing and prescription. 9th ed. Wolters Kluwer/Lippincott Williams & Wilkins Health.
Putman, P., Verkuil, B., Arias-Garcia, E., Pantazi, I., and Van Schie, C. (2014). Eeg theta/beta ratio as a potential biomarker for attentional control and resilience against deleterious effects of stress on attention. Cogn. Affect. Behav. Neurosci. 14, 782–791. doi: 10.3758/s13415-013-0238-7
Quan, H., Koltai, E., Suzuki, K., Aguiar, A. S., Pinho, R., Boldogh, I., et al. (2020). Exercise, redox system and neurodegenerative diseases. Biochim Biophys Acta 1866:165778. doi: 10.1016/j.bbadis.2020.165778
Ramic, E., Prasko, S., Gavran, L., and Spahic, E. (2020). Assessment of the antidepressant side effects occurrence in patients treated in primary care. Mater Soc Med 32:131. doi: 10.5455/msm.2020.32.131-134
Reid, S. A., Duke, L. M., and Allen, J. J. (1998). Resting frontal electroencephalographic asymmetry in depression: inconsistencies suggest the need to identify mediating factors. Psychophysiology 35, 389–404. doi: 10.1111/1469-8986.3540389
Ren, Y., Pan, L., Du, X., Li, X., Hou, Y., Bao, J., et al. (2020). Theta oscillation and functional connectivity alterations related to executive control in temporal lobe epilepsy with comorbid depression. Clin. Neurophysiol. 131, 1599–1609. doi: 10.1016/j.clinph.2020.03.038
Renner, K.-H., Hock, M., Bergner-Köther, R., and Laux, L. (2018). Differentiating anxiety and depression: the state-trait anxiety-depression inventory. Cognit. Emot. 32, 1409–1423. doi: 10.1080/02699931.2016.1266306
Ryan, S. M., and Kelly, Á. M. (2016). Exercise as a pro-cognitive, pro-neurogenic and anti-inflammatory intervention in transgenic mouse models of Alzheimer’s disease. Ageing Res. Rev. 27, 77–92. doi: 10.1016/j.arr.2016.03.007
Sanghuachang, W., Hengudomsub, P., Chaimongkol, N., and Kotchabhakdi, N. (2023). Effectiveness of neurobic exercise program on memory performance in community-dwelling older adults with mild cognitive impairment: a randomized controlled crossover trial. Belitung Nurs J 9, 100–109. doi: 10.33546/bnj.2476
Seok, L. J., Hwan, Y. B., Oh, D.-Y., and Kim, K. (2007). Eeg A1, A2, and percent asymmetry indices in major depressive disorder; the importance of symptom severity of depression and anxiety. Singyŏng chŏngsin ŭihak 46, 179–184. Available at: https://www.kci.go.kr/kciportal/landing/article.kci?arti_id=ART001052542&utm_source=chatgpt.com
Trambaiolli, L. R., and Biazoli, C. E. (2020). Resting-state global Eeg connectivity predicts depression and anxiety severity. Annu Int Conf IEEE Eng Med Biol Soc 2020, 3707–3710. doi: 10.1109/EMBC44109.2020.9176161
Van Der Vinne, N., Vollebregt, M. A., Van Putten, M. J., and Arns, M. (2017). Frontal alpha asymmetry as a diagnostic marker in depression: fact or fiction? A meta-analysis. Neuroimage 16, 79–87. doi: 10.1016/j.nicl.2017.07.006
Voss, M. W., Vivar, C., Kramer, A. F., and Van Praag, H. (2013). Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 17, 525–544. doi: 10.1016/j.tics.2013.08.001
Wallen, M. P., Gomersall, S. R., Keating, S. E., Wisløff, U., and Coombes, J. S. (2016). Accuracy of heart rate watches: implications for weight management. PLoS One 11:e0154420. doi: 10.1371/journal.pone.0154420
Wei, H., Chang, L., Huang, Q., and Zhou, R. (2020). Relation between spontaneous electroencephalographic theta/beta power ratio and test anxiety. Neurosci. Lett. 737:135323. doi: 10.1016/j.neulet.2020.135323
Willardson, J. M. (2006). A brief review: factors affecting the length of the rest interval between resistance exercise sets. J. Strength Cond. Res. 20, 978–984. doi: 10.1519/R-17995.1
Yasin, S., Hussain, S. A., Aslan, S., Raza, I., Muzammel, M., and Othmani, A. (2021). Eeg based major depressive disorder and bipolar disorder detection using neural networks: a review. Comput. Methods Prog. Biomed. 202:106007. doi: 10.1016/j.cmpb.2021.106007
Yuk, K. H., Lee, S. M., Bae, W. R., Park, J. Y., Woo, S. W., Song, P., et al. (2023). Distinct effect of exercise modes on mood-related behavior in mice. Biochem. Biophys. Res. Commun. 646, 36–43. doi: 10.1016/j.bbrc.2023.01.047
Yuk, K., Lim, J., and Moon, H. Y. (2024). Comparing the effects of resistance and aerobic exercise on mood-related symptoms and Eeg activity in young healthy adults: a non-randomized pilot study. Ment. Health Phys. Act. 27:100626. doi: 10.1016/j.mhpa.2024.100626
Zhao, J. L., Jiang, W. T., Wang, X., Cai, Z. D., Liu, Z. H., and Liu, G. R. (2020). Exercise, brain plasticity, and depression. CNS Neurosci. Ther. 26, 885–895. doi: 10.1111/cns.13385
Zoberi, K., and Alec Pollard, C. (2010). Treating anxiety without Ssris. J. Fam. Pract. 59, 148–154. Available at: https://www.mdedge.com/jfponline/article/59413/mental-health/treating-anxiety-without-ssris
Keywords: aerobic exercise, resistance exercise, anxiety, depression, EEG
Citation: Yuk K, Lim J, Kim S, Kim TY and Moon HY (2025) Exploring the effects of aerobic and resistance exercise on mood-related symptoms and EEG activity. Front. Hum. Neurosci. 19:1562702. doi: 10.3389/fnhum.2025.1562702
Edited by:
Inbaraj Ganagarajan, National Institute of Mental Health and Neurosciences (NIMHANS), IndiaReviewed by:
Satish Jaiswal, University of California, San Diego, United StatesAlessandro Quaglieri, Sapienza University of Rome, Italy
Copyright © 2025 Yuk, Lim, Kim, Kim and Moon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyo Youl Moon, c2t5bWFuMTlAc251LmFjLmty
 Kihoon Yuk
Kihoon Yuk Jawon Lim
Jawon Lim Sangyun Kim
Sangyun Kim Tae Yeon Kim
Tae Yeon Kim Hyo Youl Moon
Hyo Youl Moon