- 1Department of Psychiatry and Behavioral Sciences, University of California, San Francisco, San Francisco, CA, United States
- 2Weill Institute for Neurosciences, University of California, San Francisco, San Francisco, CA, United States
- 3Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA, United States
Deep brain stimulation (DBS) is well suited to target disorders with network dysregulation, as is the case in many neuropsychiatric diseases. While DBS is a well-established therapy for Parkinson’s disease, essential tremor, dystonia, and medically refractory epilepsy, it is actively being studied in clinical trials for neuropsychiatric disorders including treatment-refractory major depressive disorder (MDD). Due to the nature of symptomology and participant characteristics, special care must be taken in the design and implementation of clinical trials testing DBS for neuropsychiatric disorders. In particular, these studies typically include multi-year relationships between participants and study staff with frequent interactions, high burden of study activities on participants, and disclosure by participants of sensitive information related to symptoms and disease state. Through our experience with six participants across more than 5 years of the Presidio clinical trial assessing personalized closed-loop DBS for treatment-refractory MDD, we have gathered experience and evidence to inform best practices for conducting these interaction-intensive clinical studies in a vulnerable population. Here, we present these Key Practices along with discussion, informed by multiple fundamental principles: The Belmont Report; emotional and physical safety for study participants and staff; and integrity and validity of scientific outcomes.
Introduction
To ensure the protection and dignity of participants, staff of clinical trials involving human subjects must be guided by the foundational principles of Respect for Persons, Beneficence, and Justice presented in the Belmont Report (U.S. Department of Health and Human Services, 1979). These principles recognize the autonomy of individuals who have the right to make informed decisions about their participation in research, require researchers to maximize benefits and minimize harm to participants, and advocate for fairness in the distribution of the benefits and burdens of research. Specific to deep brain stimulation (DBS), there have been important publications about additional ethical considerations, including outlining the need for risk/benefit analyses, carefully considered inclusion and exclusion criteria, respect for participant autonomy and quality of life, and concerns with recording neural activity (Baker et al., 2023; Bell et al., 2009; Bell et al., 2016; Bell et al., 2011; Bell and Racine, 2013; Fins et al., 2011; Muñoz et al., 2020; Nuttin et al., 2014; Rabins et al., 2009; Acevedo et al., 2022; Synofzik and Schlaepfer, 2011; Park et al., 2017). Here, we provide an additional resource to support researchers involved in clinical trials testing DBS by presenting key considerations to foster productive professional relationships between participants and study staff, maximize benefit and minimize harm to DBS participants, and protect the integrity of trial results by conducting the trial using a scientifically rigorous, explicit protocol. We start by providing background on the current landscape of clinical trials testing DBS for neuropsychiatric indications, particularly the types of activities involved in many of these trials and the composition of study teams required for safely conducting these trials. We then discuss five Key Practices we believe are critical for the success of DBS trials in neuropsychiatric indications: (1) Setting expectations with study participants; (2) delineating scope of study staff responsibilities; (3) establishing and maintaining appropriate boundaries; (4) being mindful of dual-roles; and (5) involving the participant, their family, and caregivers.
Background on clinical trials of DBS for neuropsychiatric indications
DBS involves the surgical implantation of electrodes in the brain and an implantable pulse generator to deliver therapeutic stimulation to targeted brain regions (Miocinovic et al., 2013; Lozano et al., 2019; Herrington et al., 2016). DBS is FDA-approved for Parkinson’s disease (PD) (Hacker et al., 2020; Fang and Tolleson, 2017) and has an FDA humanitarian device exemption (HDE) for the treatment of dystonia and Obsessive Compulsive Disorder (OCD) (U.S. Food and Drug Administration, 2009; U.S. Food and Drug Administration, 2003). It is being actively studied in clinical trials for multiple neuropsychiatric indications (Table 1). Individuals eligible for DBS trials have severe presentations of their disorder with high degrees of impairment and have typically received little to no benefit from standard-of-care treatments. Some may have developed distrust of the medical system or hopelessness for potential symptom relief.
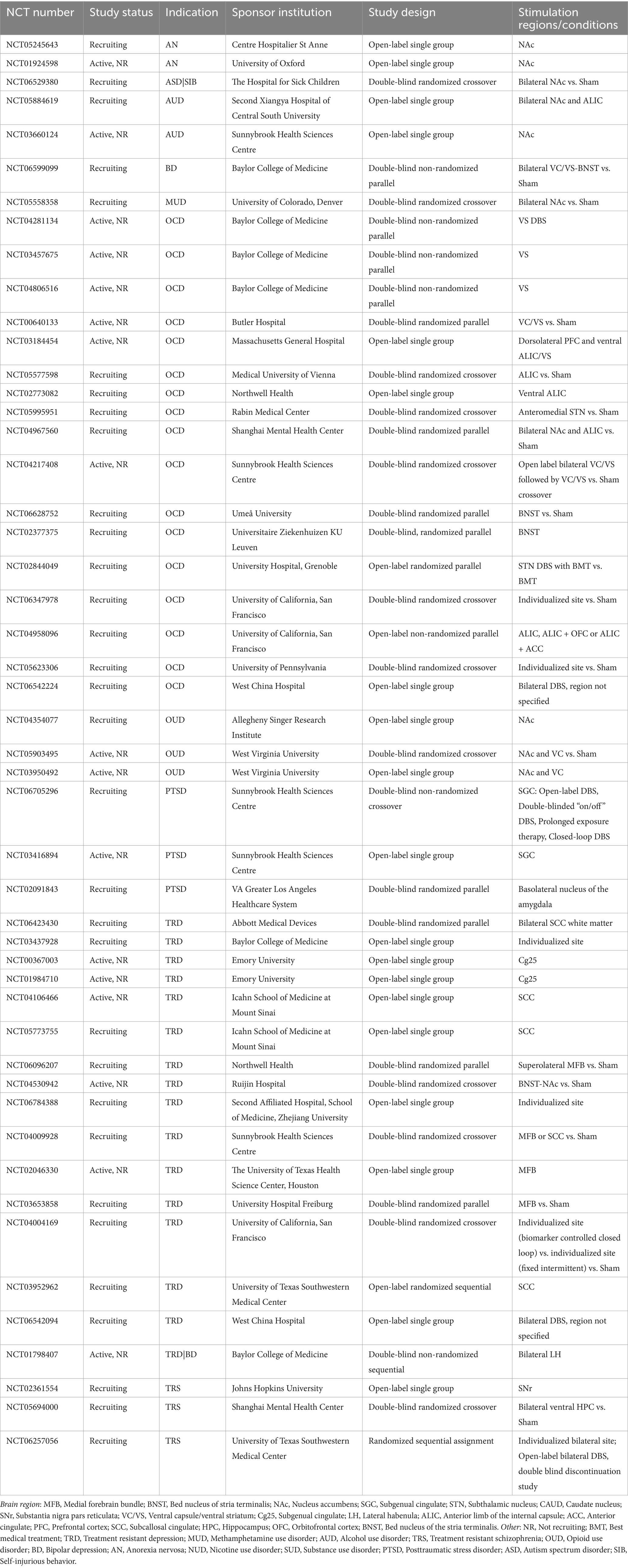
Table 1. Clinical trials testing DBS for neuropsychiatric indications that are currently active (recruiting or not recruiting), based on search of clinicaltrials.gov in February 2025.
There are ethical and practical constraints to performing sham surgeries as a control condition, so randomized controlled trials of DBS often use a within-participant crossover design (Rabins et al., 2009), with each participant receiving both active and sham stimulation (AB/BA design). Stimulation parameters are sometimes optimized before the crossover begins, which introduces a substantial challenge for participants as they have experienced therapeutic stimulation but know that stimulation will be withheld during the crossover. Participants may experience heightened anxiety with upcoming start or switches of crossover arms and need to be reminded that stimulation will only be off temporarily. In addition, protocols should have explicit criteria for prematurely exiting a sham condition due to decompensation. The use of alternate study designs in which only one arm crosses from sham to active stimulation (AA/BA design) (Synofzik and Schlaepfer, 2011) has been proposed to avoid these concerns.
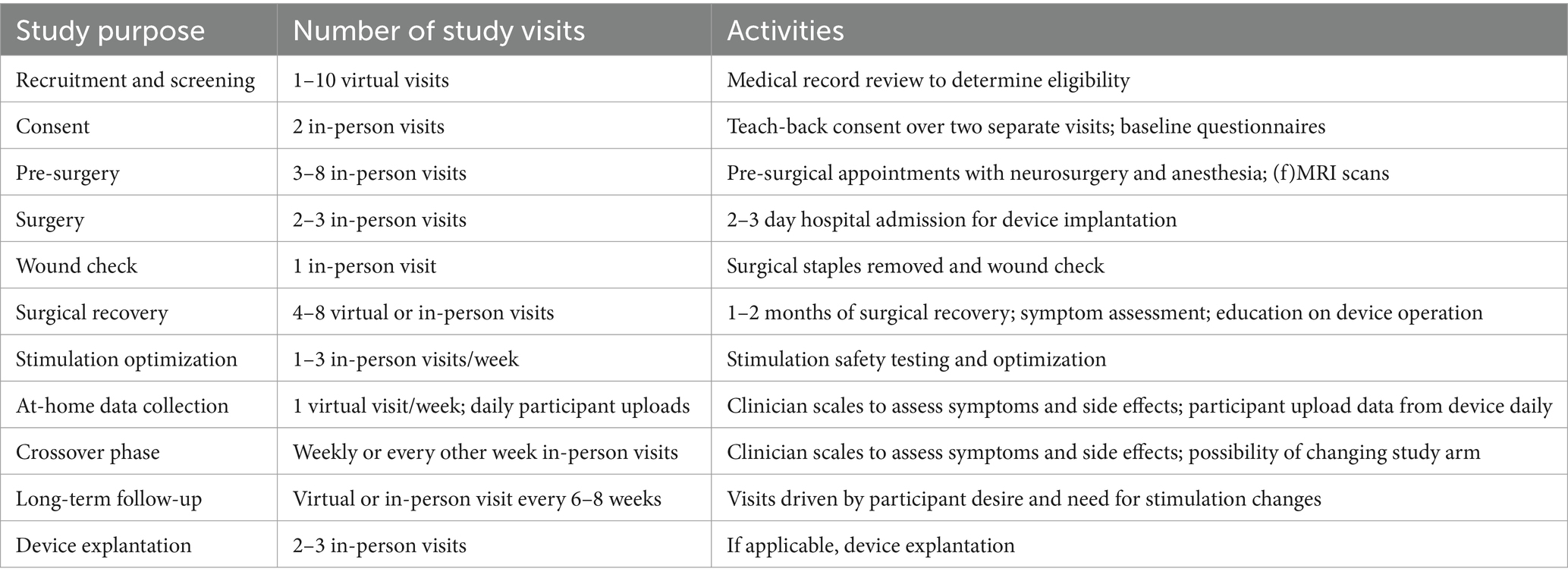
DBS requires ongoing monitoring and parameter adjustments to maximize benefits and ensure safety of participants. There are generally activities undertaken by the participant at home as well as study visits to monitor and change settings. For established indications such as PD, programming adjustments typically occur 3 to 11 times within the first six months following surgery (Ondo and Bronte-Stewart, 2005). Clinical trials assessing DBS for novel indications may require more frequent study visits and symptom reports to fully characterize therapeutic benefit or side-effects of therapy. Table 2 describes typical study activities and their frequency.
Study staff for clinical trials of DBS for neuropsychiatric indications
International psychiatric and neurosurgical societies have reached consensus that experienced multidisciplinary teams are mandatory for the ethical conduct of research on neuropsychiatric DBS or for therapeutic DBS offered through an HDE (Nuttin et al., 2014). Guidelines mandate teams with expertise from the following disciplines: stereotactic and functional neurosurgery, psychiatry, neurology, neuropsychology, and neuroethics. Based on our experience, the study team should also include well-trained clinical research coordinators (CRCs) (Buchanan et al., 2021) clinical psychologists, and if closed-loop stimulation is being employed (which involves analyzing neural activity to identify a symptom-correlated biomarker), individuals experienced in neurophysiology, neural signal processing, and decoding analyses. The study team must also have support for regulatory submissions to the FDA and IRB.
In our experience, CRCs play a critical role in DBS trials. CRCs are “on the front lines” and have the most day-to-day contact and communication with study participants (Davis et al., 2002). Therefore, CRCs are often the first to be aware of participant concerns. It is not a CRC’s job to de-escalate a participant in a crisis; rather, it is their job to recognize situations where participants need assistance and notify the proper study staff clinician(s) (Schatten et al., 2020) based on an established decision tree, as discussed below.
Having provided an overview of structure and design of clinical trials assessing DBS for neuropsychiatric indications, we next discuss the five Key Practices we believe are critical for successfully conducting these trials.
Key practices
Key practice 1: setting expectations with study participants
Clear and reiterated expectations are critical for successfully conducting DBS clinical trials. This includes (a) managing expectations the participant has about DBS and the clinical trial, and (b) establishing expectations the study team has of the participant.
DBS should not be thought of as a “cure-all” for the neuropsychiatric condition being investigated (Thomson and Carter, 2020). Participants who are eligible for neuropsychiatric DBS trials may have reached a point of desperation due to prior failed treatments and feel that DBS is their last and only hope for improvement (Thomson et al., 2021). However, DBS is only one aspect of a comprehensive treatment program (similar to traditional neuropsychiatric treatments), and participants may require extensive psychosocial rehabilitation (Nuttin et al., 2014; Rabins et al., 2009). Participants often contend with altered identity once stimulation alleviates disease symptoms which have been a core aspect of their lives for many years. Participants often have the desire to re-engage with education, employment, or social activities but are unable to make tangible steps toward these goals. Occupational or relational therapy and connecting with community services may be highly beneficial in these cases. We suggest creating a pamphlet that lists local, state, and federal agencies and support systems that can be distributed to both interested and enrolled participants.
There is always the possibility that DBS may be unsuccessful or only partly successful in mitigating disease symptoms for an individual. We have noticed that some participants drift in their mindset and think the study team has a ‘good’ setting for them and is ‘choosing’ to withhold that setting. While this may be true in the limited context of the sham condition during crossover periods, the study team may have been unsuccessful in identifying therapeutically beneficial stimulation or stimulation may have become less effective over time. During informed consent and following, direct conversations with the participant regarding all potential outcomes should be used to reorient and correct any therapeutic or trial misconceptions. Appropriate expectations may need to be reiterated several times, especially during a change in study condition or moving between symptomatic and asymptomatic states. A mnemonic has been deployed for DBS recipients for PD with success to aid in adjusting participants’ expectations to be more realistic (Okun and Foote, 2004). Lastly, participants must have accurate expectations that their contact with study staff will decrease and potentially cease as formal clinical trial activities are concluded.
It may be useful to create a ‘Research Engagement Agreement’, an IRB-approved document separate from the consent form. This agreement should be presented and discussed with the participant and their signature obtained; it can be referenced if corrective actions need to be taken. Topics which should be considered for inclusion in such an agreement include (a) Respectful engagement and non-discrimination; (b) Appropriate use of contact information; (c) Communication about study visit rescheduling; (d) Notification of any abrupt changes in symptoms or side effects believed to be related to stimulation; (e) Notification about changes in medical status or events which may impact the stimulation device (e.g., head impact). Finally, participants should know to contact 911 for any medical emergencies.
Key practice 2: delineating scope of study staff responsibilities
Two key areas of study staff responsibility include managing suicide risk and long-term care considerations (Higgins et al., 2024; Dasgupta et al., 2024). Many neuropsychiatric clinical trials inquire about suicidality; the Columbia Suicide Severity Rating Scale is a commonly used instrument (Posner et al., 2011). A robust risk mitigation plan must be in place to respond to disclosure of suicidality from participants (Schatten et al., 2020). Assessing for suicide does not have a prospective iatrogenic effect (DeCou and Schumann, 2018), and should be conducted at every study interaction in clinical populations with a high propensity for suicide. If study visits occur remotely via telephone or video, participants should be asked where they are located in case it becomes necessary to send emergency services.
Each study should have an explicit decision tree to guide study staff following participant disclosure of suicidality. Participants determined to be at high-risk should be referred to study clinicians for immediate further evaluation. If the participant is in imminent danger of attempting suicide, ask the participant to call 911 or admit themselves to a hospital emergency department. Having the participant take this action themselves helps to preserve participant autonomy; however, if the participant is unwilling or unable to do so, study staff should contact emergency services directly to ensure participant safety. Clinicians have additional responsibilities based on the ethical principle of non-abandonment, which obligates them to follow care for the participant longitudinally or until transfer of care to a qualified clinician occurs (Nuttin et al., 2014).
Implanted DBS devices can remain functional for a decade or longer (Sette et al., 2019; Van Riesen et al., 2016). While the formal study activities for clinical trials typically do not last this long, a plan for long-term management of implanted DBS devices should be included in the trial design (Sankary et al., 2020). Although follow-up phases of 10 years or longer are being advised to better understand the long-term effects of DBS, it is unclear how entities can reliably financially support ongoing treatment maintenance over such a long period (Rabins et al., 2009). In some cases, participant care can be transferred to established clinics. However, due to either the types of devices implanted or the indication, some study participants may need to be followed indefinitely by the study team if therapy remains on. If such long-term monitoring is not feasible, devices may need to be explanted or deactivated to ensure long-term safety for participants.
Key practice 3: establishing and maintaining appropriate boundaries
Many clinical trials testing DBS for neuropsychiatric diseases involve multi-year relationships between participants and study staff. Building rapport and trust with study participants is important but must be balanced with maintaining appropriate boundaries. The relationships between study staff and participants are fundamentally unbalanced, similar to the imbalances in provider/patient relationships (Afolabi, 2015; Baca, 2011). Study staff are in a position of power given their ability to ‘gate-keep’ access to therapy. Study staff may ask participants numerous questions about their prior life traumas, symptoms, activities of daily living, relationships, and other personal topics as a way to track disease symptoms, treatment efficacy, and side effects. However, disclosure of this type of information from study staff about themselves to participants is largely inappropriate.
We recommend having a conversation early on with participants about boundaries between study staff and participants. This helps to establish that study staff will ask many questions of the participant, but should not be asked reciprocal questions, and will decline to provide comparable information about themselves. To further support maintaining appropriate boundaries, all phone and text communication between CRCs and study participants should be done using a dedicated study phone number or device. Participants can be provided with contact information for the Principal Investigator and study Clinical Psychologist along with instructions regarding who to contact in case of emergency or other needs.
Study visits should ideally be conducted with two or more staff members present with the study participant in a designated professional space, which facilitates appropriate boundaries and the safety of visits. Dependent upon inclusion criteria, participants may have the potential for aggressive language or behavior, and staff safety should be prioritized. DBS side effects may also include agitation and hyperactivity (Seritan et al., 2021). Conducting a visit with two staff members also allows one person to remain with a participant in crisis while the other contacts emergency assistance, if required. If study visits must be conducted independently, ensure that someone else on the study team knows the time and location of the visit and have the in-person staff member check in with this colleague during and following the study visit.
Key practice 4: being mindful of dual-roles
There are two forms of dual-roles involved in clinical trials for DBS. Individuals receiving treatment are both participants and patients, and the study staff includes those who are researchers and clinicians. The duality of these roles must be carefully monitored and care must be taken not to exceed the scope that is appropriate within a clinical trial.
People receiving DBS in clinical trials are by definition research participants. They give informed consent to research activities which follow an approved protocol. As such, they have the right to decline to perform study activities and can withdraw from the study at any time without penalty. If participants do decide to withdraw, special considerations may be needed to ensure long-term safety. This may involve explantation of the device system, turning off active stimulation, or establishing the individual with a clinic which can manage ongoing DBS therapy.
People receiving DBS in clinical trials are treated as patients in the context of device implantation (appointments with neurosurgery and anesthesia, hospital admission, pain management during the postoperative period, follow-up wound check). These participants receive medical care in the context of the clinical trial, but they should not receive other medical care from the study team. As such, we encourage verbiage such as ‘study visit’ rather than ‘clinic visit’ and ‘participant call’ vs. ‘telehealth appointment’ when conducting study activities. Of note, ensuring safety and directing participants to resources in the context of suicidality is within the scope of clinical trial activities and is mandated by the Belmont Report.
Researchers are responsible for contributing to the clinical trial protocol design, collecting and analyzing the data, and reporting results to clinical and research communities. These individuals may have other scientific interests which dovetail with DBS clinical trials. Some of these other research endeavors may be covered under separate IRB protocols which trial participants can decide if they want to participate in Thomson et al. (2021) and Morain et al. (2021). It should be made clear to trial participants which activities are related to the clinical trial (and therefore may directly benefit them if therapy is successful) versus other activities which do not have the potential for personal benefit. Declining to participate in ancillary activities should in no way negatively affect their participation in the clinical trial. Researchers must also ensure that research activities do not interfere with required clinical activities (e.g., those associated with DBS implantation).
Clinicians often switch roles between being a researcher and a clinician in DBS clinical trials (Mergenthaler et al., 2021). Clinicians provide medical care and assessments in the context of study activities, but they should not provide other medical care to study participants. Particular care must be taken if medications are prescribed in the context of the clinical trial. The trial-related purpose of these medications must be made abundantly clear, and any requests for renewal of other medications must be redirected to a non-study-related physician. Ongoing communication with a participant’s non-study-related medical providers may be required as other diagnoses or treatments may affect study activities, ongoing participant eligibility, or data interpretability.
Key practice 5: involving the participant, their family, and caregivers
Clinical research is ideally a collaborative endeavor between study participants, their family, caregivers, and study staff. Inclusion in research development, trial processes, and social support not only helps participants comply with study activities, it also improves the safety profile of participation (Numans et al., 2019; Bird et al., 2020; Grady, 2022; Fins et al., 2017). We recommend that each study participant have a consented ‘study partner’ for the duration of the study. The study team can contact the study partner for additional information related to the participant’s symptoms or side effects and study partners serve as an important resource for support following surgery and in case of emergency (Thomson and Segrave, 2017; Thomson et al., 2023).
Because participants are central to DBS research (Acevedo et al., 2023), we have found it helpful to periodically offer continuing education and updates to participants about the utility of the data they provide, especially as repeated symptom reports over months can be burdensome and lead to participation fatigue. The principle of respect for persons requires informing research participants of study results if they are interested (Rabins et al., 2009). Participants often demonstrate interest in study procedures and are curious about how their experience maps onto the data and decisions of the research team (De Haan et al., 2015; Klein et al., 2016). Quarterly updates and end-of-year reviews that contain information about the quantity of data participants have provided, the team’s scientific output, and next steps can serve as an opportunity to thank participants for their hard work and share publicly available results with participants. However, the specifics of how much information is shared and when this occurs are study specific, and some study results should only be shared after full study completion. For example, unblinding an individual study participant’s crossover condition order may inadvertently unblind the conditions of other participants given small sample sizes and typically counterbalanced conditions across the sample within a study. Unblinding may also bias study staff and how they interact with future participants. With regard to blinding, many participants experience high anxiety leading up to the start of the crossover periods, and we have found that a blinded onset to a potential setting change can be beneficial to mitigate this anxiety and further aid in blinding staff involved in symptom ratings and clinician scales. Also note that while it is imperative for study staff to protect the privacy of research participants, participants are free to publicly disclose their participation in a clinical trial, such as via social media or in DBS support groups. Therefore, if premature release of information may be damaging to the integrity of the study results or the experience of other participants in the study, sharing results with participants may need to be delayed.
Discussion
Clinical trials for DBS span early feasibility studies to pivotal trials. These trials require careful consideration due to the vulnerable population, implanted devices, sensitive nature of disclosures, and longitudinal nature of the treatment. Here, we provide five Key Practices for the successful completion of DBS trials in neuropsychiatric conditions. These Key Practices were developed from over half a decade’s worth of experience with participants in an intensive DBS clinical trial testing closed-loop DBS for MDD. The Key Practices were created with the goals of ethically conducting and completing clinical trials, but they do not ensure a positive outcome in terms of beneficial therapy. These Practices are not an exhaustive list of the ethical and practical considerations for successful DBS clinical trials, but we hope they support current work and foster continued discussion. As DBS technology and therapy continue to evolve, there is an ongoing need to critically evaluate how to conduct clinical trials responsibly to maximize benefit, minimize risk, and maintain scientific rigor.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by University of California, San Francisco Human Research Protection Program. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AT-M: Investigation, Writing – original draft, Writing – review & editing. EH: Investigation, Writing – original draft, Writing – review & editing. NB: Investigation, Writing – review & editing. DA: Investigation, Writing – review & editing. AK: Funding acquisition, Investigation, Writing – review & editing. KS: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. KS received a UCSF Community Wellbeing Grant to host seminars to discuss topics ultimately included in this publication. The clinical trial was supported by the Ray and Dagmar Dolby Family Fund through the Department of Psychiatry at UCSF and by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number UH3NS123310. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acevedo, N. J., Castle, D., Bosanac, P., Groves, C., and Rossell, S. L. (2023). Patient feedback and psychosocial outcomes of deep brain stimulation in people with obsessive–compulsive disorder. J. Clin. Neurosci. 112, 80–85. doi: 10.1016/j.jocn.2023.04.012
Acevedo, N., Castle, D., Groves, C., Bosanac, P., Mosley, P. E., and Rossell, S. (2022). Clinical recommendations for the care of people with treatment-refractory obsessive-compulsive disorder when undergoing deep brain stimulation. Aust. N. Z. J. Psychiatry 56, 1219–1225. doi: 10.1177/00048674221100947
Afolabi, O. E. (2015). Dual relationships and boundary crossing: a critical issues in clinical psychology practice. Int. J. Psychol. Counsel. 7, 29–39. doi: 10.9857/IJCP2014.0287
Baca, M. (2011). Professional boundaries and dual relationships in clinical practice. J. Nurse Pract. 7, 195–200. doi: 10.1016/j.nurpra.2010.10.003
Baker, S., Fenstermacher, E., Davis, R. A., Kern, D. S., Thompson, J. A., Felsen, G., et al. (2023). Ethical considerations in closed loop deep brain stimulation. Deep Brain Stimulat. 3, 8–15. doi: 10.1016/j.jdbs.2023.11.001
Bell, E., Leger, P., Sankar, T., and Racine, E. (2016). Deep brain stimulation as clinical innovation: an ethical and organizational framework to sustain deliberations about psychiatric deep brain stimulation. Neurosurgery 79, 3–10. doi: 10.1227/NEU.0000000000001207
Bell, E., Mathieu, G., and Racine, E. (2009). Preparing the ethical future of deep brain stimulation. Surg. Neurol. 72, 577–586. doi: 10.1016/j.surneu.2009.03.029
Bell, E., Maxwell, B., McAndrews, M. P., Sadikot, A., and Racine, E. (2011). Deep brain stimulation and ethics: perspectives from a multisite qualitative study of Canadian neurosurgical centers. World Neurosurg. 76, 537–547. doi: 10.1016/j.wneu.2011.05.033
Bell, E., and Racine, E. (2013). “Ethics guidance for neurological and psychiatric deep brain stimulation” in Handbook of clinical neurology, vol. 116 (Amsterdam: Elsevier), 313–325.
Bird, M., Ouellette, C., Whitmore, C., Li, L., Nair, K., McGillion, M. H., et al. (2020). Preparing for patient partnership: a scoping review of patient partner engagement and evaluation in research. Health Expect. 23, 523–539. doi: 10.1111/hex.13040
Buchanan, D. A., Goldstein, J., Pfalzer, A. C., Lin, Y. C., Kang, H., and Claassen, D. O. (2021). Empowering the clinical research coordinator in academic medical centers. Mayo Clin. Proc. 5, 265–273. doi: 10.1016/j.mayocpiqo.2020.09.014
Dasgupta, I., Klein, E., Cabrera, L. Y., Chiong, W., Feinsinger, A., Fins, J. J., et al. (2024). What happens after a neural implant study? neuroethics expert workshop on post-trial obligations. Neuroethics 17:22. doi: 10.1007/s12152-024-09549-2
Davis, A. M., Hull, S. C., Grady, C., Wilfond, B. S., and Henderson, G. E. (2002). The invisible hand in clinical research: the study Coordinator’s critical role in human subjects protection. J. Law Med. Ethics 30, 411–419. doi: 10.1111/j.1748-720x.2002.tb00410.x
De Haan, S., Rietveld, E., Stokhof, M., and Denys, D. (2015). Effects of deep brain stimulation on the lived experience of obsessive-compulsive disorder patients: in-depth interviews with 18 patients. PLoS One 10:e0135524. doi: 10.1371/journal.pone.0135524
DeCou, C. R., and Schumann, M. E. (2018). On the iatrogenic risk of assessing suicidality: a meta-analysis. Suicide Life Threat Behav. 48, 531–543. doi: 10.1111/sltb.12368
Fang, J. Y., and Tolleson, C. (2017). The role of deep brain stimulation in Parkinson’s disease: an overview and update on new developments. NDT 13, 723–732. doi: 10.2147/NDT.S113998
Fins, J. J., Kubu, C. S., Mayberg, H. S., Merkel, R., Nuttin, B., and Schlaepfer, T. E. (2017). Being open minded about neuromodulation trials: finding success in our “failures”. Brain Stimul. 10, 181–186. doi: 10.1016/j.brs.2016.12.012
Fins, J. J., Mayberg, H. S., Nuttin, B., Kubu, C. S., Galert, T., Sturm, V., et al. (2011). Misuse of the FDA’s humanitarian device exemption in deep brain stimulation for obsessive-compulsive disorder. Health Aff. 30, 302–311. doi: 10.1377/hlthaff.2010.0157
Grady, C. (2022). The evolution of research participant as partner: the seminal contributions of bob Veatch. Theor. Med. Bioeth. 43, 267–276. doi: 10.1007/s11017-022-09579-y
Hacker, M. L., Turchan, M., Heusinkveld, L. E., Currie, A. D., Millan, S. H., Molinari, A. L., et al. (2020). Deep brain stimulation in early-stage Parkinson disease: five-year outcomes. Neurology 95, e393–e401. doi: 10.1212/WNL.0000000000009946
Herrington, T. M., Cheng, J. J., and Eskandar, E. N. (2016). Mechanisms of deep brain stimulation. J. Neurophysiol. 115, 19–38. doi: 10.1152/jn.00281.2015
Higgins, N., Gardner, J., Wexler, A., Kellmeyer, P., O'Brien, K., and Carter, A. (2024). Post-trial access to implantable neural devices: an exploratory international survey. BMJ Surg. Interv. Health Technol. 6:e000262. doi: 10.1136/bmjsit-2024-000262
Klein, E., Goering, S., Gagne, J., Shea, C. V., Franklin, R., Zorowitz, S., et al. (2016). Brain-computer interface-based control of closed-loop brain stimulation: attitudes and ethical considerations. Brain-Comput. Interfaces 3, 140–148. doi: 10.1080/2326263X.2016.1207497
Lozano, A. M., Lipsman, N., Bergman, H., Brown, P., Chabardes, S., Chang, J. W., et al. (2019). Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol. 15, 148–160. doi: 10.1038/s41582-018-0128-2
Mergenthaler, J. V., Chiong, W., Dohan, D., Feler, J., Lechner, C. R., Starr, P. A., et al. (2021). A qualitative analysis of ethical perspectives on recruitment and consent for human intracranial electrophysiology studies. AJOB Neurosci. 12, 57–67. doi: 10.1080/21507740.2020.1866098
Miocinovic, S., Somayajula, S., Chitnis, S., and Vitek, J. L. (2013). History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 70:163. doi: 10.1001/2013.jamaneurol.45
Morain, S. R., Largent, E. A., and Wexler, A. (2021). Getting into their heads: when the investigator is also the treating physician. AJOB Neurosci. 12, 68–70. doi: 10.1080/21507740.2020.1866103
Muñoz, K. A., Kostick, K., Sanchez, C., Kalwani, L., Torgerson, L., Hsu, R., et al. (2020). Researcher perspectives on ethical considerations in adaptive deep brain stimulation trials. Front. Hum. Neurosci. 14:578695. doi: 10.3389/fnhum.2020.578695
Numans, W., Van Regenmortel, T., and Schalk, R. (2019). Partnership research: a pathway to realize multistakeholder participation. Int. J. Qual. Methods 18:1609406919884149. doi: 10.1177/1609406919884149
Nuttin, B., Wu, H., Mayberg, H., Hariz, M., Gabriëls, L., Galert, T., et al. (2014). Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J. Neurol. Neurosurg. Psychiatry 85, 1003–1008. doi: 10.1136/jnnp-2013-306580
Okun, M. S., and Foote, K. D. (2004). A mnemonic for Parkinson disease patients considering DBS: a tool to improve perceived outcome of surgery. Neurologist 10:290. doi: 10.1097/01.nrl.0000138737.97544.7c
Ondo, W. G., and Bronte-Stewart, H. (2005). The North American survey of placement and adjustment strategies for deep brain stimulation. Stereotact. Funct. Neurosurg. 83, 142–147. doi: 10.1159/000088654
Park, R. J., Singh, I., Pike, A. C., and Tan, J. O. A. (2017). Deep brain stimulation in anorexia nervosa: Hope for the hopeless or exploitation of the vulnerable? The Oxford Neuroethics gold standard framework. Front. Psych. 8:44. doi: 10.3389/fpsyt.2017.00044
Posner, K., Brown, G. K., Stanley, B., Brent, D. A., Yershova, K. V., Oquendo, M. A., et al. (2011). The Columbia–suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. AJP 168, 1266–1277. doi: 10.1176/appi.ajp.2011.10111704
Rabins, P., Appleby, B. S., Brandt, J., DeLong, M. R., Dunn, L. B., Gabriëls, L., et al. (2009). Scientific and ethical issues related to deep brain stimulation for disorders of mood, behavior, and thought. Arch. Gen. Psychiatry 66:931. doi: 10.1001/archgenpsychiatry.2009.113
Sankary, L. R., Nallapan, A. M., Hogue, O., Machado, A. G., and Ford, P. J. (2020). Publication of study exit procedures in clinical trials of deep brain stimulation: a focused literature review. Front. Hum. Neurosci. 14:581090. doi: 10.3389/fnhum.2020.581090
Schatten, H. T., Gaudiano, B. A., Primack, J. M., Arias, S. A., Armey, M. F., Miller, I. W., et al. (2020). Monitoring, assessing, and responding to suicide risk in clinical research. J. Abnorm. Psychol. 129, 64–69. doi: 10.1037/abn0000489
Seritan, A. L., Spiegel, L. L., Weinstein, J. L., Racine, C. A., Brown, E. G., Volz, M., et al. (2021). Elevated mood states in patients with Parkinson’s disease treated with deep brain stimulation: diagnosis and management strategies. JNP 33, 314–320. doi: 10.1176/appi.neuropsych.20080205
Sette, A. L., Seigneuret, E., Reymond, F., Chabardes, S., Castrioto, A., Boussat, B., et al. (2019). Battery longevity of neurostimulators in Parkinson disease: a historic cohort study. Brain Stimul. 12, 851–857. doi: 10.1016/j.brs.2019.02.006
Synofzik, M., and Schlaepfer, T. E. (2011). Electrodes in the brain—ethical criteria for research and treatment with deep brain stimulation for neuropsychiatric disorders. Brain Stimul. 4, 7–16. doi: 10.1016/j.brs.2010.03.002
Thomson, C., and Carter, A. (2020). Ethical issues in experimental treatments for psychiatric disorders: lessons from deep brain stimulation. Transl. Issues Psychol. Sci. 6, 240–246. doi: 10.1037/tps0000267
Thomson, C., and Segrave, R. (2017). “I miss you too”: more voices needed to examine the phenomenological effects of deep brain stimulation. AJOB Neurosci. 8, 122–123. doi: 10.1080/21507740.2017.1320321
Thomson, C. J., Segrave, R. A., and Carter, A. (2021). Informed consent and voluntariness: balancing ethical demands during trial recruitment. AJOB Neurosci. 12, 83–85. doi: 10.1080/21507740.2020.1867667
Thomson, C. J., Segrave, R. A., Fitzgerald, P. B., Richardson, K. E., Racine, E., and Carter, A. (2021). “Nothing to lose, absolutely everything to gain”: patient and caregiver expectations and subjective outcomes of deep brain stimulation for treatment-resistant depression. Front. Hum. Neurosci. 15:755276. doi: 10.3389/fnhum.2021.755276
Thomson, C. J., Segrave, R. A., Fitzgerald, P. B., Richardson, K. E., Racine, E., and Carter, A. (2023). Personal and relational changes following deep brain stimulation for treatment-resistant depression: a prospective qualitative study with patients and caregivers. PLoS One 18:e0284160. doi: 10.1371/journal.pone.0284160
U.S. Department of Health and Human Services (1979). National Research act of 1974 – protection of human subjects of biomedical and behavioral research. The Belmont Report. Washington, DC: U.S. Department of Health and Human Services.
U.S. Food and Drug Administration. (2003). Implanted subcortical electrical stimulator (motor disorders). Neurology Committee: Humanitarian Device Exemption.
U.S. Food and Drug Administration. (2009). Deep brain stimulator for obsessive compulsive disorder (OCD). Neurology Committee: Humanitarian Device Exemption.
Keywords: clinical trials, best practices, deep brain stimulation, neuropsychiatric diseases, major depressive disorder, neuroethics
Citation: Tremblay-McGaw AG, Hamlat EJ, Becker NC, Astudillo Maya DA, Krystal AD and Sellers KK (2025) Best practices for clinical trials of deep brain stimulation for neuropsychiatric indications. Front. Hum. Neurosci. 19:1572972. doi: 10.3389/fnhum.2025.1572972
Edited by:
Caterina Cinel, University of Essex, United KingdomReviewed by:
Cassandra J. Thomson, University of Tasmania, AustraliaCopyright © 2025 Tremblay-McGaw, Hamlat, Becker, Astudillo Maya, Krystal and Sellers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristin K. Sellers, a3Jpc3Rpbi5zZWxsZXJzQHVjc2YuZWR1
 Alexandra G. Tremblay-McGaw
Alexandra G. Tremblay-McGaw Elissa J. Hamlat
Elissa J. Hamlat Natalie C. Becker
Natalie C. Becker Daniela A. Astudillo Maya1,2
Daniela A. Astudillo Maya1,2 Andrew D. Krystal
Andrew D. Krystal Kristin K. Sellers
Kristin K. Sellers