- 1Physical Education and Sports School, Soochow University, Suzhou, Jiangsu, China
- 2Rehabilitation Engineering Lab, Department of Kinesiology and Community Health, University of Illinois at Urbana-Champaign, Champaign, IL, United States
Objective: This study investigates the effects of auditory rhythmic adaptation on lower limb joint mechanics in individuals with Functional Ankle Instability (FAI) during drop landings, aiming to explore potential rehabilitation strategies.
Methods: Twenty male FAI individuals performed single-leg drop landings under four rhythmic conditions (no rhythm, 60, 120, 180 bpm) after auditory rhythmic adaptation. Joint mechanics data were collected, and analyzed using two-way repeated measures ANOVA to examine the main effects and interaction effects of rhythm and limb condition. Rhythmic adaptation was assessed using time interval reproduction paradigm.
Results: The ground reaction force (GRF), joint torque and joint stiffness were significantly influenced by side (p< 0.05). Hip and knee joint range of motion (RoM), lower limb and joint stiffness, joint torque were significantly affected by conditions (p< 0.05). Significant interaction effects were observed in joint stiffness and joint torque (p < 0.05).
Conclusion: Rhythmic auditory adaptation modulates motor control strategies in individuals with FAI by influencing joint mechanics during drop landing. In particular, rhythmic adaptation at 120 bpm facilitates a proximal-dominant torque-redistribution strategy, characterized by higher hip and knee extension torques and increased ankle plantarflexion torque on the stable side, and increased hip extension torques on the stable side. These changes suggest the potential of 120 bpm to improve motor control and reduce injury risk.
1 Introduction
Drop landing is a common movement in both daily activities and sports, and the associated ground reaction forces (GRF) can reach several times body weight (Puddle and Maulder, 2013; Saunders et al., 2014), posing a high risk of ankle injuries. Due to its unique anatomical structure, the ankle is particularly prone to plantar flexion and inversion during drop landing, increasing the likelihood of lateral ligaments damage (Fong et al., 2009). Fransz et al. (2018) identified the late dynamic component of GRF during single-leg drop landing as a significant predictor of non-contact lateral ankle sprains, underscoring the close link between altered landing mechanics and injury risk. Therefore, optimizing joint mechanics seems to be critical for reducing the risk of ankle injuries during drop landings.
However, during actual athletic performance, ankle injuries are often underestimated or inadequately managed. Research has shown that more than half of athletes (56.8%) who suffer ankle injuries do not seek professional treatment (McKay et al., 2001), opting instead for self-rehabilitation. Additionally, unresolved post-injury inflammation and inadequate rehabilitation following ankle sprains can disrupt neuromuscular control, leading to movement pattern alterations (Hertel, 2002). These maladaptive changes often involve altered joint kinematics and delayed muscle activation, which persist even after acute symptoms resolve (Marín Fermín et al., 2023). As a consequence of an initial ankle sprain, approximately 40% of individuals go on to develop chronic ankle instability (CAI) (Hertel, 2002). CAI serves as an overarching term that encompasses a range of post-sprain dysfunction and can be categorized into mechanical ankle instability (MAI), which involves structural laxity, and functional ankle instability (FAI), which occurs in the absence of such mechanical deficits but is associated with impaired neuromuscular control(Freeman, 1965). While aging (Konrad et al., 1999) and fatigue (Edwards, 1981) independently contribute to movement dysfunction, the presence of unresolved ankle injuries further amplifies these risks. Over time, FAI individuals may develop compensatory movement patterns as a protective mechanism, which can result in diminished motor control, delayed muscle response time, neuromuscular dysfunction, and lower limb asymmetry (Monteleone et al., 2014). These issues, in turn, may impair the ability of central nervous system (CNS) to integrate and process sensory information (Cyr et al., 2019; Henry and Baudry, 2019).
Proprioceptive dysfunction is considered a hallmark feature of FAI that may contribute to postural instability and inefficient motor strategies (Proske and Gandevia, 2012). Since effective motor control depends on the integration of multiple sensory inputs, including proprioceptive, visual, and auditory information, the disruption of proprioception may lead individuals with FAI to rely more heavily on alternative sensory modalities to maintain postural control and functional performance (Meng et al., 2022). Among these modalities, auditory input has gained increasing attention for its role in enhancing movement coordination. Rhythmic auditory stimulation (RAS), which provides external timing cues through rhythmic sounds, has been shown to facilitate motor control by promoting auditory-motor synchronization in both healthy individuals and clinical populations (Braun Janzen et al., 2021). RAS has been demonstrated to modulate spatiotemporal gait parameters, improve joint kinematics, and enhance motor cortical excitability (Thaut et al., 2019) by engaging cerebellar and subcortical structures involved in timing and coordination (Shin et al., 2015). Based on the neural entrainment framework, RAS provides a temporally structured cueing system that supports anticipatory postural adjustments and improves movement efficiency. This is particularly relevant for individuals with FAI, who often exhibit an earlier peak in vertical GRF and a delay in the activation of musculoskeletal components during drop landing(Jeon et al., 2021), indicating deficits in motor timing and preparatory control. RAS may therefore serve as an effective compensatory mechanism, reinforcing temporal structure in motor execution and mitigating neuromuscular delays associated with proprioceptive deficits.
Preliminary research has revealed the potential of auditory rhythm in improving balance in individuals with ankle instability (Coker et al., 2023). Therefore, auditory rhythmic cues may hold considerable promise in the rehabilitation of individuals with FAI, warranting further exploration to clarify their underlying mechanisms and therapeutic value. We hypothesized that auditory rhythmic cues would modulate lower limb joint range of motion and torques, and that different rhythms would elicit distinct bilateral limb responses during drop landing. Accordingly, our goal was to explore the status of the lower limb joint mechanics among FAI individuals, to identify which outcomes displayed deficits and which outcomes were sensitive to detect these different rhythmic conditions.
2 Materials and methods
2.1 Participants
The sample size for this study was initially estimated using G*Power 3.1 software (Dusseldorf, Germany), with effect size (ES), α, and power (1-β error probability) set to 0.25, 0.05, and 0.95, respectively. The selected ES of 0.25 corresponds to a medium effect, as defined by Cohen (1988), and was based on previous biomechanical studies in individuals with FAI, which reported large ES in joint angles and GRF during landing tasks (Wang et al., 2024). Based on this estimation, the total sample size required was calculated to be 18. In this study, 20 male unilateral functional ankle instability individuals were selected, and both lower limbs underwent the drop landing task [Age (23.50 ± 0.76) years, Height (175.50 ± 5.37) cm, Weight (71.90 ± 7.93) kg; CAIT (Cumberland Ankle Instability Tool) score: unstable side (19.20 ± 2.38), stable side (28.50 ± 0.51), p< 0.001]. The study followed the Declaration of Helsinki and was approved by the Ethics Committee of Soochow University (SUDA20221005H03). All participants were fully informed about the purpose of the study and provided written informed consent. They were diagnosed with FAI based on established screening criteria and had a minimum of 3 years of regular sports training experience.
The inclusion and exclusion criteria for participants were as follows:
Inclusion Criteria:
I. CAIT score less than 24 on the unstable side (Donahue et al., 2011).
II. A CAIT score of 24 or higher for the stable side (Hiller et al., 2007).
III. No history of severe lower limb injuries, such as fractures or serious orthopedic injuries (Wu et al., 2022).
IV. At least one ankle sprain on the unstable side in the past year.
Exclusion criteria:
I. Bilateral ankle sprains (Wang et al., 2022).
II. Acute pathological symptoms in the lower limbs, such as acute fractures, cellulitis, and acute muscle strains, and so on.
III. History of lower limb fractures, surgeries (Kweon et al., 2022), hypertension, heart disease, or other conditions that may interfere with testing.
IV. CNS, vestibular disorders, or visual impairments that could affect balance (Wu et al., 2022).
V. Congenital deformities of the foot, ankle, knee, pelvis, or spine.
VI. Positive results on the anterior drawer test or talar tilt test (Kaminski et al., 1999).
2.2 Data collection
A Vicon motion capture system (Vicon Nexus v.1.5.1) equipped with eight infrared cameras (MX13, United Kingdom) and 28 reflective markers (14 mm in diameter) were used to collect the kinematic data at a sampling frequency of 100 Hz. The placement of markers on the lower limbs is shown in Figure 1. GRFs were recorded synchronously using a force plate (9287B, Kistler, Inc., Switzerland), operating at a sampling frequency of 1,000 Hz and connected to the motion capture system via an analog-to-digital converter.
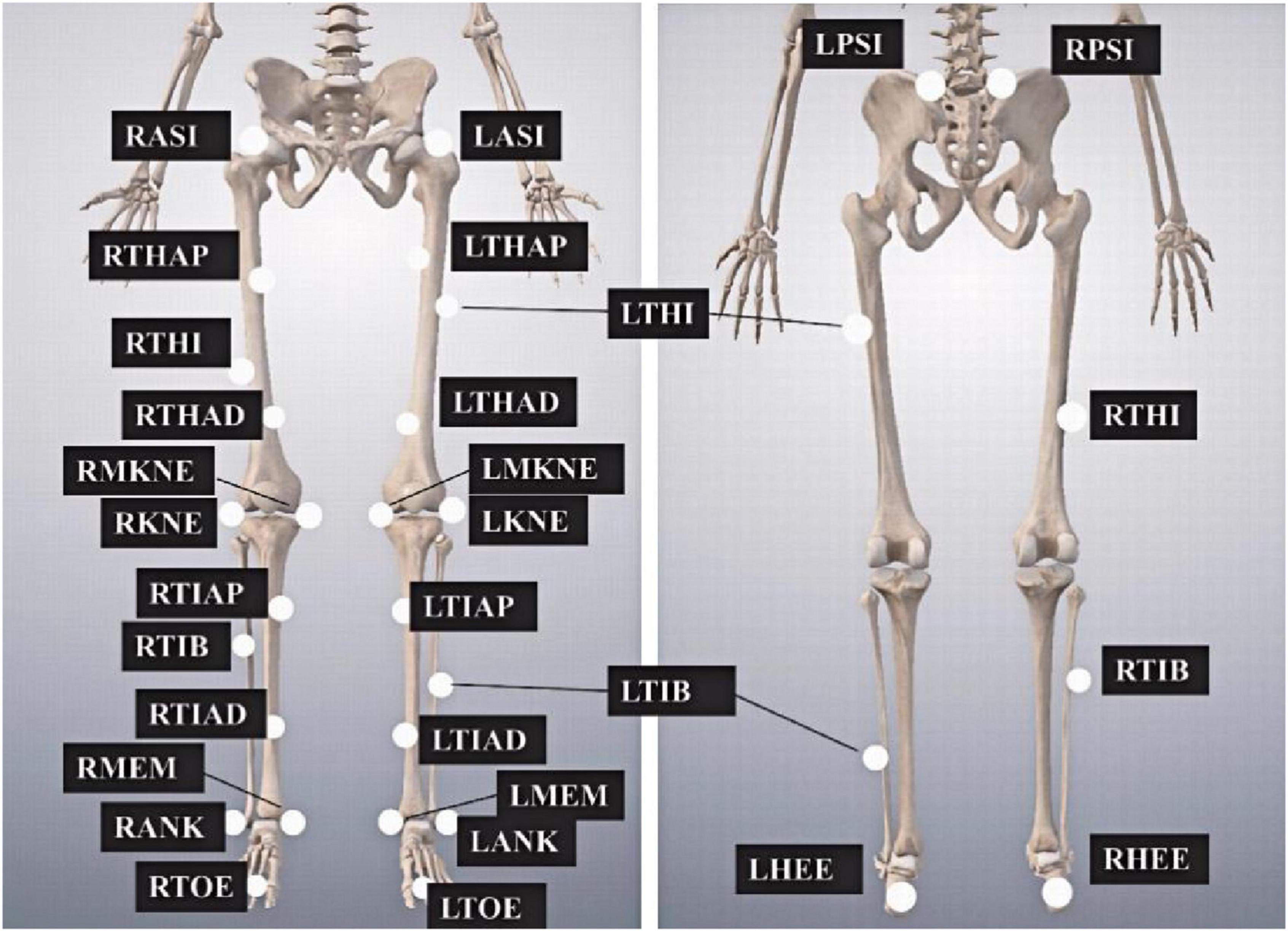
Figure 1. Markers placement on the lower limbs. The abbreviations are as follows, LASI and RASI, left and right anterior superior iliac spines, respectively; LTHAP and RTHAP, left and right thigh alignment upwards; LTHI and RTHI, left and right thigh; LTHAD and RTHAD, left and right thigh alignment downwards; LMKNE and RMKNE, left and right medial knee; LKNE and RKNE, left and right knee; LTIAP and RTIAP, left and right tibia alignment upwards; LTIB and RTIB, left and right tibia; LTIAD and RTIAD, left and right tibia alignment downwards; LMEM and RMEM, left and right medial malleoli; LANK and RANK, left and right ankle; LTOE and RTOE, left and right toe; LPSI and RPSI, left and right posterior superior iliac spines; LHEE and RHEE, left and right heel.
2.3 Experimental procedure
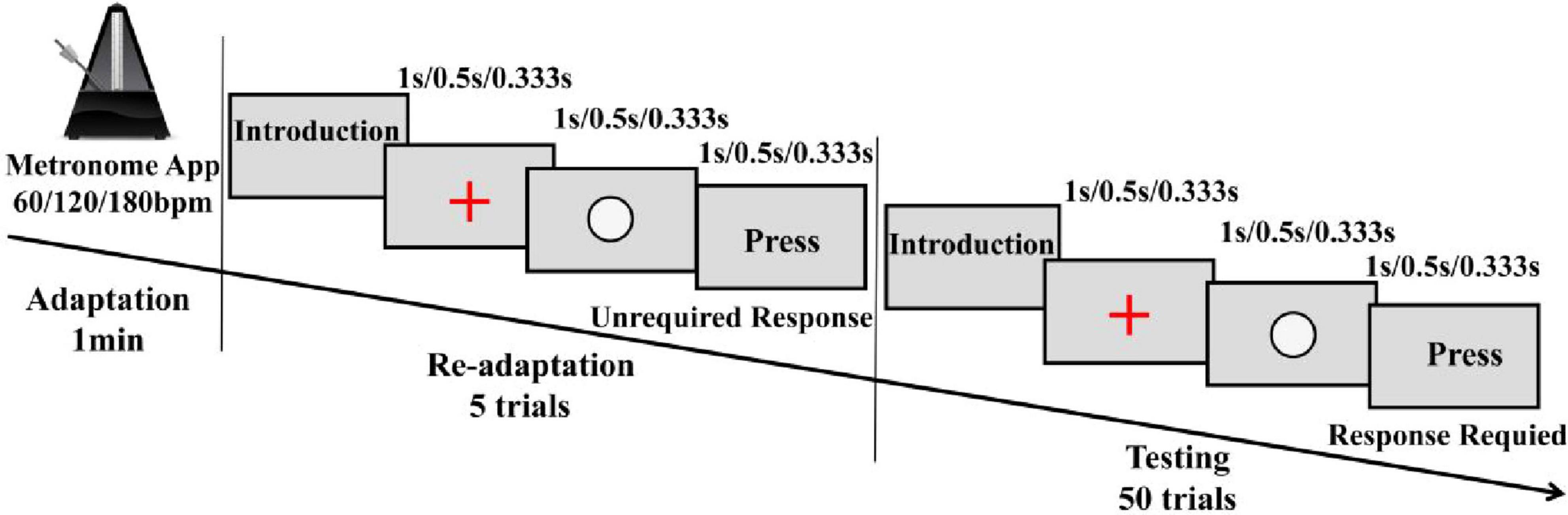
By integrating slow, moderate, and fast tempo conditions (Kim et al., 2023; Kirkland et al., 2018; Rivera-Tello et al., 2023), this experimental design included four rhythm conditions (no rhythm, 60, 120, and 180 bpm) to investigate the effects of auditory rhythm on lower limb joint mechanics during single-leg drop landing in individuals with FAI. Before the experiment, participants changed into standardized athletic shoes (Feiyue, China), cotton socks, and experimental attire, and the laboratory temperature was maintained at a constant 23°C. Participants refrained from strenuous activity for 24 h before the experiment to prevent muscle fatigue. After completing a 5-min warm-up on a treadmill at 2.2 m/s, reflective markers were promptly placed by the same experienced experimenter within 3 min to preserve the effects of the warm-up. Before performing the drop landing task under rhythm conditions, a structured rhythm adaptation paradigm was implemented to enhance rhythmic adaptation effects. Specifically, the single-leg drop landing task began with the control condition (CC), which did not involve rhythmic stimuli and therefore did not require prior rhythm adaptation. The remaining three conditions (60, 120, and 180 bpm) were completed in a randomized order. During the period of rhythm adaptation, participants were seated at a distance of 55 cm from the computer screen (Lenovo Legion R7000, 15.6-inch, 144 Hz) and underwent a rhythm adaptation phase for 1-min firstly, using a metronome software app (Metronome, v15.2, Japan), which generated auditory beats at predefined tempos. To ensure that the rhythm variations reflected differences in tempo rather than beat strength, all rhythms were represented in terms of 1/4-note lengths. After that, the three rhythm conditions (60, 120, and 180 bpm) were converted into time inter-onset interval (IOI, ms) of 1,000 500, and 333.33 ms, respectively, using the formula (1).
In this formula, 60,000 represents the total number of milliseconds in 1 min, IOI refers to the inter-onset interval in milliseconds, which is the time between two consecutive beats.
These calculated IOIs were applied within the readaptation and test phases of rhythm adaptation paradigm, which designed using Python-based Psychopy software (v 2023.2) to objectively assess participants’ rhythm adaptation based on their response time errors. In the readaptation phase, participants were presented five times with three elements on the screen (a “+” symbol, a white circle, and a “Press” text)—which were displayed sequentially according to the rhythm (Figure 2), the IOI between them was identical to the target interval (Breska and Ivry, 2018). During this phase, participants did not need to respond but only familiarized themselves with the rhythmic structure. However, they were instructed to always pay close attention to the auditory rhythm cues. In the test phase, the elements displayed were the same, participants were instructed to respond by pressing the spacebar when the “Press” text appeared at rhythmically defined time points. This phase included 50 trials, and the timing of the keypress was recorded. The accuracy of the response was assessed based on the error between the participant’s keypress and the expected rhythm. A correct response was defined as a keypress occurring within ± 50 ms of the expected rhythm (Darabi and Svensson, 2021). Rhythm adaptation was considered successful if accuracy was at least 80% (Fuller and Fienup, 2018). If this threshold was not met, participants had to undergo re-adaptation, with a 120–s rest period provided between two adaptation attempts.
After successfully completing the rhythm adaptation task, participants moved on to the single-leg drop landing task. They stood on a 30 cm high platform with their hands on their hips (Figure 3A) to avoid interfering with the motion capture markers and to minimize the influence of arm movement. Participants in the rhythm conditions listened to the beats and independently determined when to step off the platform and land on their test leg, which was randomized between the left and right leg for each trial. For all conditions, the experimenter provided a verbal instruction (“Prepare, left/right foot, trial 1/2/3”) before each trial. The left/right foot cue was included because the experimenter was unaware of the unstable side, and the trial number helped track the three required valid trials for each condition. A trial was considered valid if the participant landed with the forefoot transitioning to the heel and maintained a stable single-leg stance for 3 s (Ardakani et al., 2019). Data collection began when the experimenter issued the command and ended once the participant stabilized the landing and exited the cushioning phase. Each participant collected three valid drop landings per condition, with a 1-min rest period between trials to minimize fatigue(Wernli et al., 2016). To enhance data reliability and reduce inter-trial variability, mean values from three valid drop landings per condition were calculated and used for subsequent analysis(Troester et al., 2018). No additional rest period was provided between different rhythm conditions, as participants naturally rested on their bodies during the rhythm adaptation paradigm task. All single-leg drop landing tests (2 sides: stable and unstable × 4 conditions: CC, 60, 120, 180 bpm) were completed within a single experimental day.
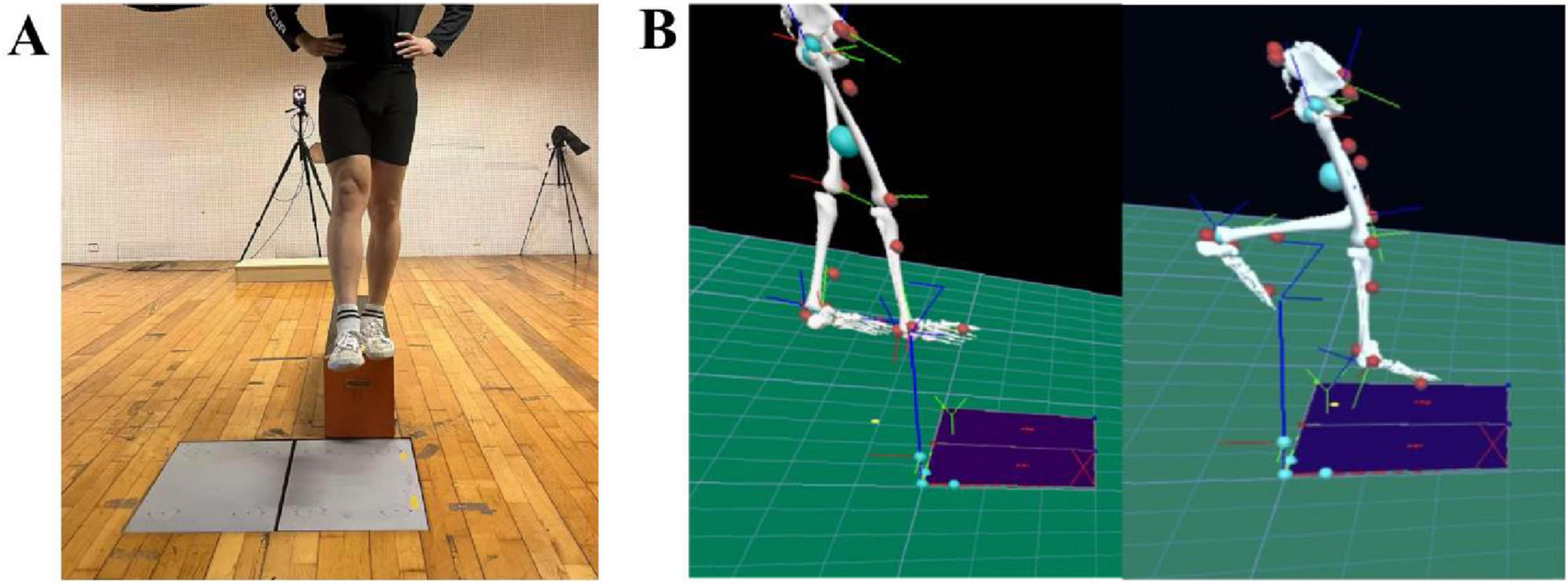
Figure 3. Preparation for the drop landing test (A) and lower limb model (B). (A) represents the step-off motion during the drop landing movement, while (B) illustrates the pyCGM2-lowerLimb_CGM23 lower-limb model in Visual3D.
2.4 Experimental indicators
In this study, various GRF and joint kinetic variables were selected for analysis under different rhythm conditions during drop landing task. The GRF parameters included vertical ground reaction force (vGRF, N) at initial ground contact (IC) and peak vertical ground reaction force (PvGRF) were normalized to body weight (BW), and the time to peak vertical ground reaction force (T_PvGRF, ms) was also recorded. IC was defined as the moment when the vGRF first exceeded 10N (Christoforidou et al., 2017). The range of motion (RoM, °) for the hip, knee, and ankle joints was also measured in degrees. The RoM was calculated as the difference between the maximum and minimum joint angles recorded during the entire drop landing phase. The entire landing phase was defined from IC until the vGRF returned to 1 BW, indicating the end of the drop landing buffering process(Nauwelaerts and Aerts, 2006).
The pyCGM2-lowerLimb CGM23lower-limb model in Visual3D (C-Motion, United States, Figure 3B) was used to calculate joint kinetics. The joint torques (Nm/kg) at the PvGRF moment for the hip, knee, and ankle joints at sagittal plane were analyzed. Additionally, stiffness parameters were assessed, including lower limb stiffness (Khip, BW/m) and joint stiffness (Kjoint, dNm/kg/°).
To improve the numerical representation of joint stiffness, we expressed the stiffness values in deci-Newton meters per kilogram per degree (dNm/kg/°), where 1 dNm = 0.1 Nm. This unit adjustment allows for greater precision and more decimal places in the presentation of small stiffness values, without changing the underlying physical measurement. The symbol “+” denotes hip extension, knee extension, and ankle plantarflexion, while “-” denotes hip flexion, knee flexion, and ankle dorsiflexion. The lower limb stiffness was calculated using Eq. (2), while joint stiffness was derived from Eq. (3):
where PvGRF represents the peak vertical GRF, which refers to the maximum vGRF. ΔL represents the vertical displacement of the hip joint center. ΔM represents the change in joint torques, and RoM represents the change in joint angle. The Kjoint model was based on the ratio of net muscle moment change to joint angular displacement in the sagittal plane during the ground-contact phase. This approach assumes that the joint behaves approximately as a torsional spring when the time difference between peak torque and peak angular displacement is minimal (typically less than 10% of the movement cycle). Under such conditions, the joint stiffness metric provides a reasonable mechanical approximation of dynamic joint behavior.
2.5 Statistical analysis
Data analysis was performed using SPSS 26.0 and Excel 2021 software, with a significance level set at α = 0.05. All data were presented as mean ± standard deviation (M ± SD). First, the Shapiro-Wilk test was used to assess whether the data followed a normal distribution. If the data were normally distributed, two-way repeated measures analysis of variance (rmANOVA) was used to analyze the main effects, interaction effects, and simple effects of side (stable side vs. unstable side) and rhythm conditions (no rhythm, 60, 120, and 180 bpm) on the variables during the drop landing task. For variables that were not normally distributed, non-parametric tests such as the Friedman test were used to examine overall differences, followed by post hoc comparisons using the Wilcoxon signed-rank test with Bonferroni correction to control for multiple comparisons.
3 Results
3.1 GRF
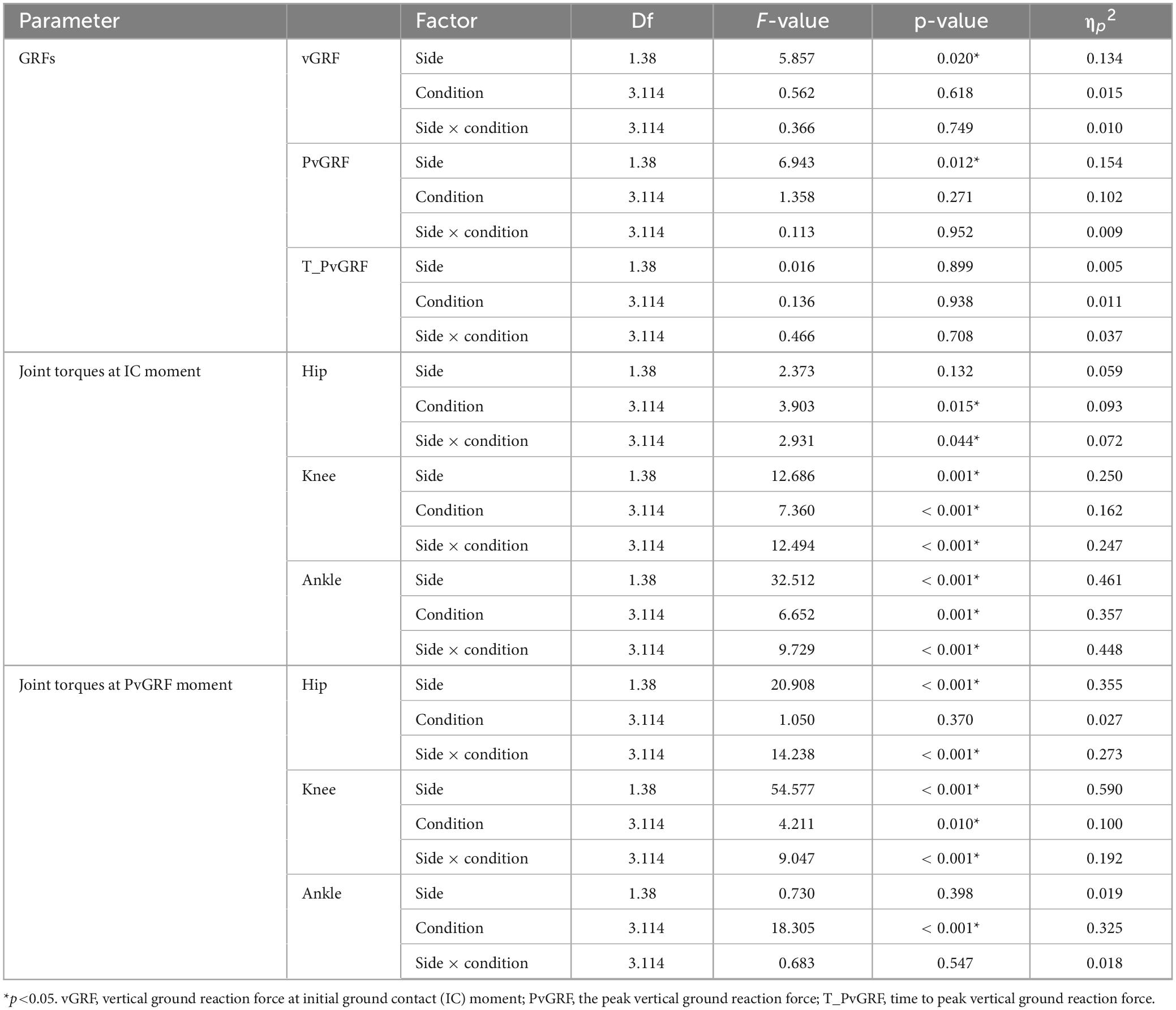
The diagram of vGRF during the entire drop landing phase is shown in Figure 4. Significant main effect of side was observed for both the vGRF at IC moment and PvGRF (p< 0.05). Specifically, the vGRF at IC moment was higher on the unstable side than on the stable side (F1,38 = 5.857, p = 0.020, ηp2 = 0.134), and the PvGRF was also higher on the unstable side (F1,38 = 6.943, p = 0.012, ηp2 = 0.154). No significant differences were found for T_PvGRF (p> 0.05), as detailed in Table 1.
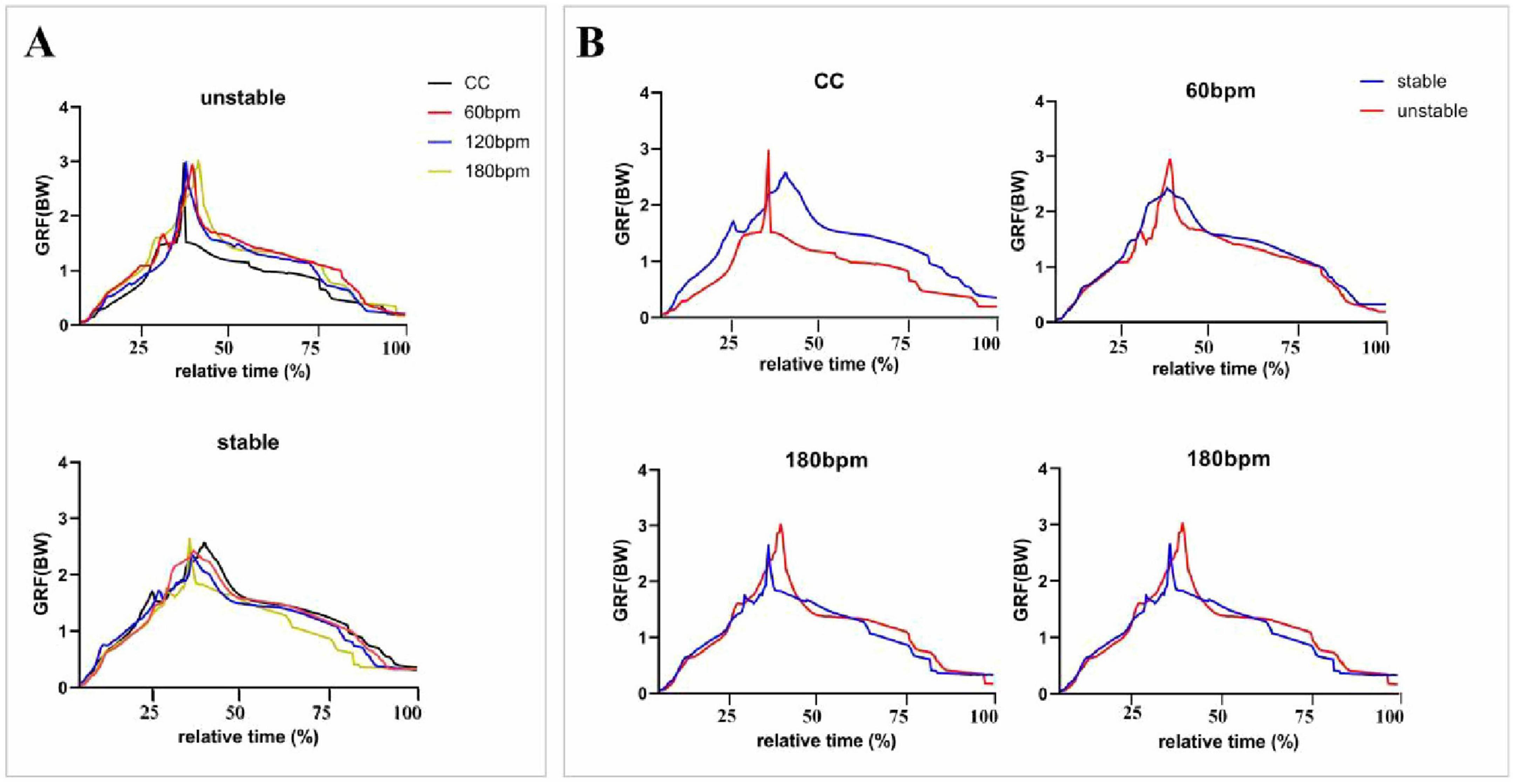
Figure 4. Vertical ground reaction forces (vGRFs) during the entire drop landing phase. The vGRF (N) data were normalized by dividing by body weight (BW), and the results were expressed as multiples of BW. CC, the condition of no rhythm (control condition). The x-axis represents relative time (%), indicating the progression of vGRF from the IC moment to the PvGRF moment and the end of the cushioning phase. (A) Presents an overall comparison of vGRF trends across all rhythmic conditions for both the stable and unstable sides, while (B) depicts the vGRF trends separately for the stable and unstable sides under each rhythmic condition.
3.2 Lower limb joint torques
3.2.1 Joint torques at IC moment
As shown in Table 1, at the IC moment, the hip joint torque was significantly influenced by the interaction effects of rhythm condition and side, and by the main effect of rhythm condition (p< 0.05). Simple effects analysis revealed that the hip extension torque on the unstable side under CC was greater than under 180 bpm (p = 0.010). Under 120 bpm condition, the hip extension torque on the stable side was greater than on the unstable side (F1,38 = 6.242, p = 0.017, ηp2 = 0.141), and the 180 bpm condition also showed a greater hip extension torque on the stable side (F1,38 = 4.239, p = 0.046, ηp2 = 0.100).
As for the knee joint torque, there was a significant interaction between the effects of rhythm condition and side (p< 0.001), as well as the main effects of rhythm condition (p< 0.001) and side (p = 0.001). Simple effects analysis revealed that, the knee extension torque on the stable side, under CC was greater than under both 60 and 180 bpm (p< 0.001). On the stable side, the knee extension torque under 120 bpm was greater than under both 60 bpm (p = 0.005) and 180 bpm (p< 0.001). On the unstable side, the knee extension torque at 60 bpm was greater than under CC (p = 0.023), 120 bpm (p = 0.008), and 180 bpm (p< 0.001). Under CC and 120 bpm, the knee extension torque on the stable side was greater than on the unstable side (F1,38 = 17.314, p< 0.001, ηp2 = 0.313; F1,38 = 14.863, p<0.001, ηp2 = 0.281, respectively), whereas at 60 bpm, the knee extension torque on the unstable side was greater than on the stable side (F1,38 = 8.220, p = 0.007, ηp2 = 0.178).
Ankle joint torque was significantly influenced by the interaction effects of rhythm condition and side (p<0.001), as well as the main effects of rhythm condition (p = 0.001) and side (p<0.001; Table 1). Specifically, the ankle plantarflexion torque on the stable side under 180 bpm was smaller than under CC (p = 0.001) and 60 bpm (p = 0.007). On the unstable side, the ankle plantarflexion torque under CC was greater than under both 60 (p = 0.001) and 120 bpm (p = 0.08).,The ankle plantarflexion torque on the stable side under 60 bpm was greater than the unstable side (F1,38 = 55.231, p< 0.001, ηp2 = 0.592), and the ankle plantarflexion torque on the stable side under 120 bpm was greater than on the unstable side (F1,38 = 7.365, p = 0.010, ηp2 = 0.162) (Figure 5).
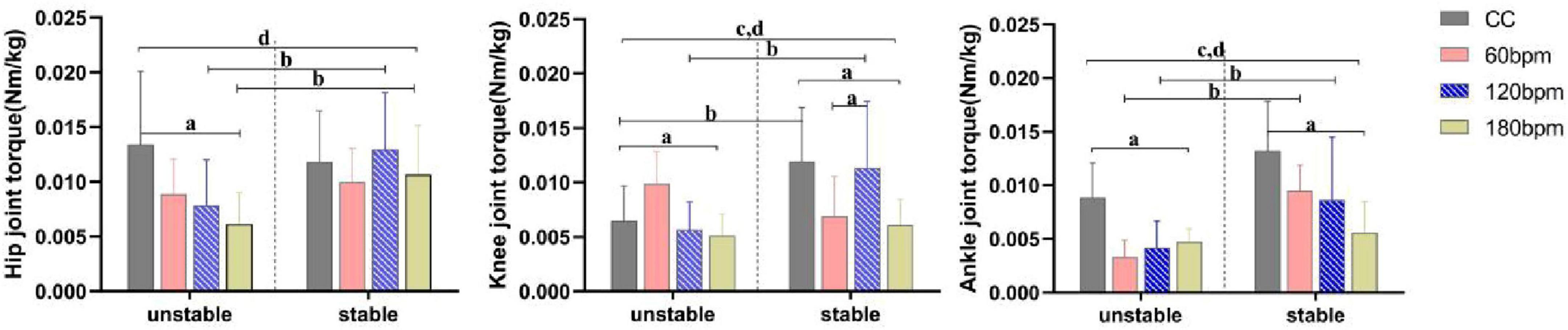
Figure 5. Joint torques at initial ground contact (IC) moment. a,b Indicate a statistically significant interaction effect between side and rhythm condition (p <0.05). a Indicates a significant simple effect of rhythm; b Indicates a significant simple effect of side. c Indicates a statistically significant main effect of side (p < 0.05). d Indicates a statistically significant main effect of rhythm condition (p < 0.05).
3.2.2 Joint torques at PvGRF moment
At the PvGRF moment, hip joint torque was significantly influenced by the interaction effects of rhythm condition and side, as well as by the main effect of side (p< 0.001, Table 1). On the stable side, the hip extension torque under CC was greater than under the 180 bpm condition (p = 0.037). On the unstable side, the hip extension torque under CC was smaller than under both the 120 (p = 0.025) and 180 bpm (p = 0.003). Additionally, the hip extension torque on the unstable side at 60 bpm was smaller than at 120 (p = 0.001) and 180 bpm (p< 0.001). On the other hand, the hip extension torque on the unstable side was greater than the stable side under both 120 and 180 bpm (F1,38 = 20.175, p< 0.001,ηp2 = = 0.347; F1,38 = 44.232, p< 0.001,ηp2 = = 0.538, respectively).
Knee joint torque was significantly influenced by the interaction effects of rhythm condition and side (p<0.001), as well as by the main effects of condition (p = 0.010) and side (p< 0.001, Table 1). Specifically, the knee extension torque on the stable side under 180 bpm was smaller than under CC (p = 0.026), 60 bpm (p = 0.016) and 120 bpm conditions (p< 0.001). The knee extension torque on the unstable side under CC was smaller than under the 60 (p = 0.013) and 180 bpm (p = 0.035) conditions. Regardless of the rhythm condition, the knee extension torque on the stable side was always greater than on the unstable side (CC, F1,38 = 32.487, p<0.001, ηp2 = 0.461; 60 bpm, F1,38 = 11.932, p = 0.001,ηp2 = 0.239; 120 bpm, F1,38 = 44.965, p<0.001,ηp2 = 0.542; 180 bpm, F1,38 = 4.365, p = 0.043, ηp2 = 0.103).
Ankle joint torque was significantly influenced by the main effect of rhythm condition (p<0.001, Table 1). The ankle plantarflexion torque under CC was greater than under all three rhythm conditions, and the ankle plantarflexion torque at 60 bpm was the lowest (p< 0.001). No significant main effect of side (F1,38 = 0.730, p = 0.398,ηp2 = 0.019) or interaction effect (F3,114 = 0.683, p = 0.547,ηp2 = = 0.018) was observed for ankle joint torque (Figure 6).
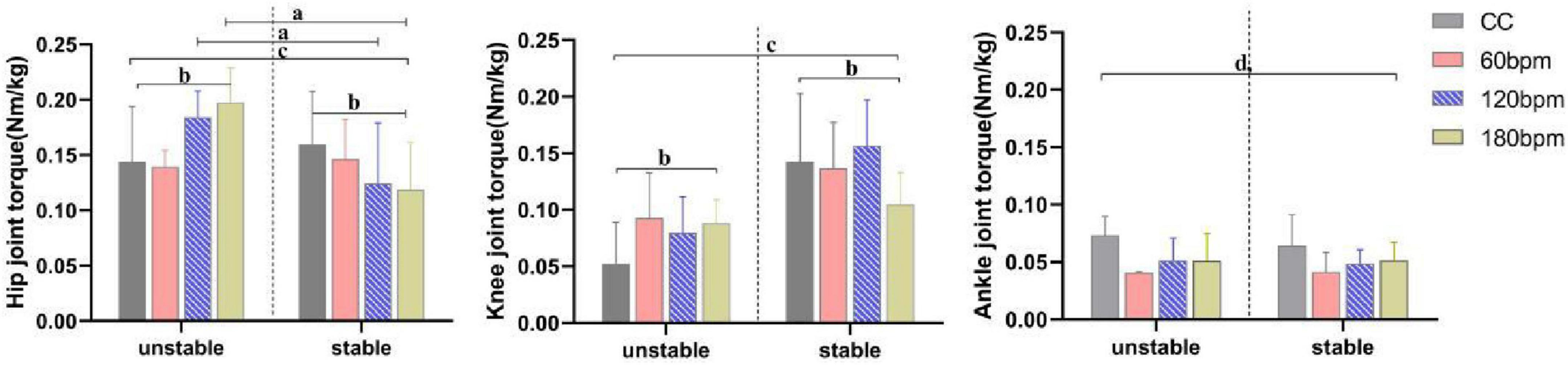
Figure 6. Joint torques at PvGRF moment. a,b Indicate a statistically significant interaction effect between side and rhythm condition (p < 0.05). a Indicates a significant simple effect of rhythm (p < 0.05). b Indicates a significant simple effect of side (p < 0.05). c Indicates a statistically significant main effect of side (p < 0.05). d Indicates a statistically significant main effect of rhythm condition (p < 0.05).
3.3 RoM
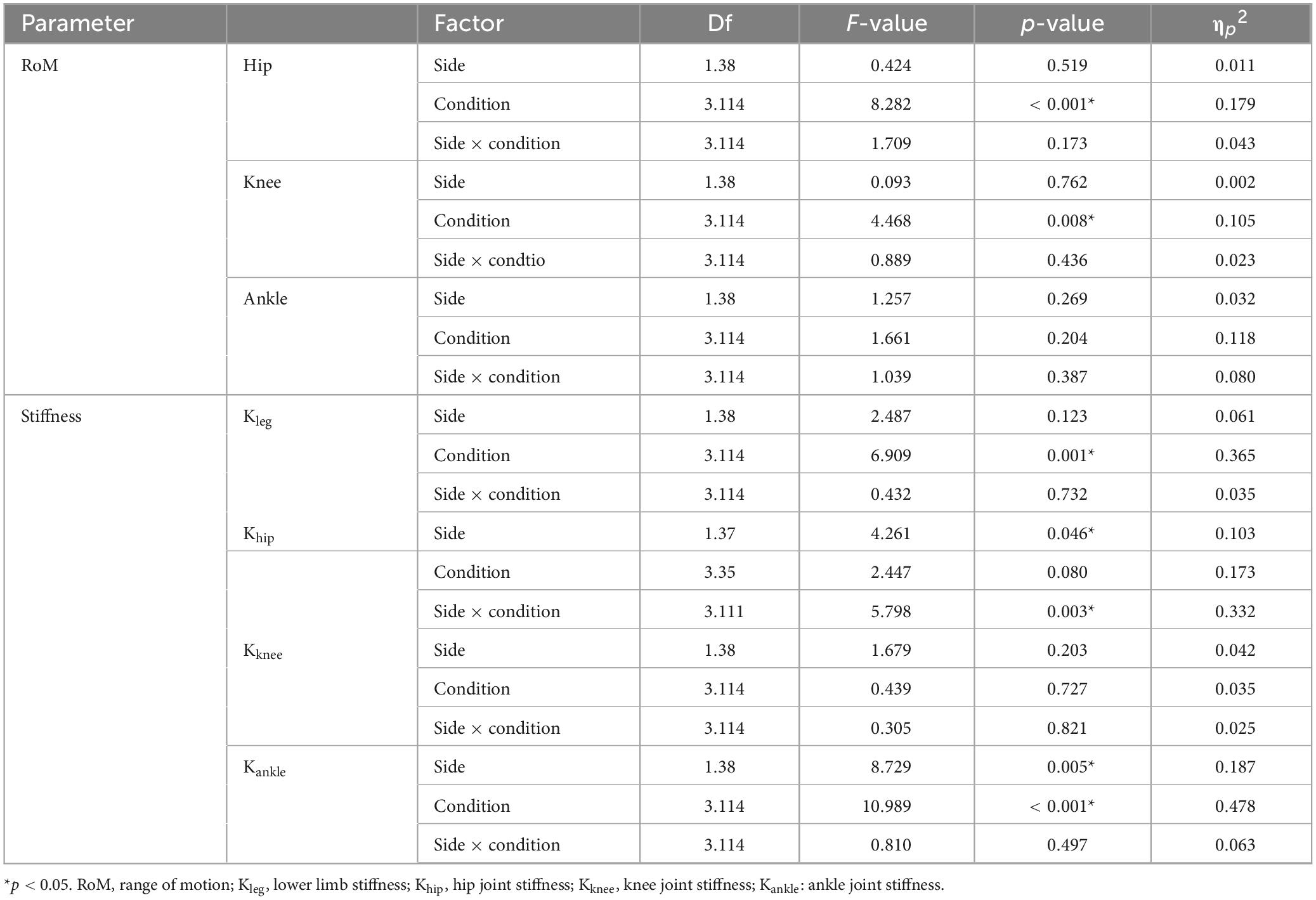
Table 2 presents the descriptive statistics of joint RoM under different rhythmic conditions for both limbs, and the results of the ANOVA are summarized in Table 3. The RoM in the hip joint and knee joint was significantly affected by rhythm condition (F3,114 = 8.282, p<0.001, ηp2 = 0.179; F3,114 = 4.468, p = 0.008, ηp2 = 0.105, respectively). The hip RoM under CC was smaller than under the rhythmic conditions, and the hip RoM under 180 bpm was higher than other conditions. The knee RoM under CC was the highest. No significant differences were observed for ankle RoM (p > 0.05).
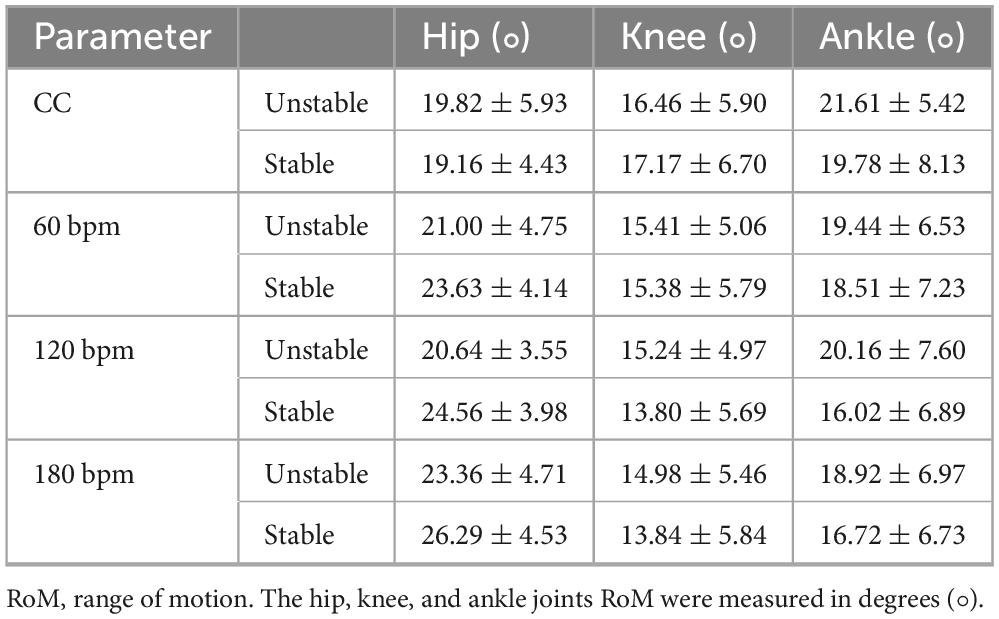
Table 2. The Hip, knee and ankle RoM across four auditory rhythm conditions for both limbs (M ± SD).
3.4 Stiffness
Khip was significantly influenced by rhythm condition (F3,36 = 6.909, p = 0.001, ηp2 = 0.365). The stiffness at 60 bpm was significantly lower than under CC, 120 and 180 bpm conditions. Khip was significantly influenced by the interaction of rhythm condition and side (F3,111 = 5.798, p = 0.003, ηp2 = 0.332), as well as by the main effect of side (F1,37 = 4.261, p = 0.046,ηp2 = 0.103). Specifically, the Khip on the stable side under 60 bpm was greater than under CC (p = 0.003), 120 (p = 0.04) and 180 bpm (p = 0.006). At 60 bpm, the Khip on the stable side was greater than on the unstable side (F1,37 = 16.586, p< 0.001, ηp2 = 0.310). No significant differences were observed for Kknee (p> 0.05). Kankle was significantly influenced by the main effects of rhythm condition and side (F3,36 = 10.986, p<0.001,ηp2 = 0.478; F1,38 = 8.729, p = 0.005, ηp2 = 0.187, respectively). The stiffness on the unstable side was greater than on the stable side, with the Kankle at 180 bpm being the highest and the stiffness at 60 bpm being the lowest, as the ANOVA results detailed in Table 3 and the descriptive statistics detailed in Table 4.
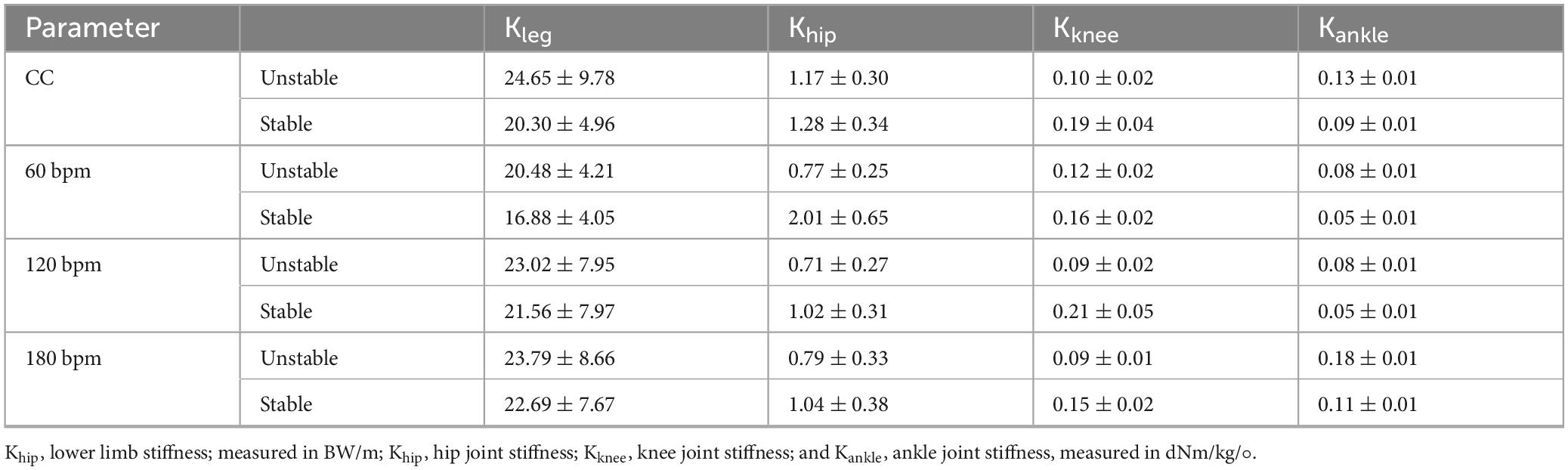
Table 4. The lower limb stiffness and joints stiffness across four auditory rhythm conditions for both limbs (M ± SD).
4 Discussion
This study investigates the effects of auditory rhythm adaptation on lower limb joint mechanics in FAI individuals. The results confirmed our hypotheses, rhythmic auditory cues modulated range of motion and torques, and different rhythm conditions elicited distinct bilateral mechanical responses during single-leg drop landing. Compared to the control condition, plantarflexion torque at the PvGRF moment was significantly reduced under any rhythmic conditions, particularly at 60 bpm, while the 120 bpm elicited increased hip and knee extension torques. Meanwhile, the stable side showed greater RoM. In contrast, the unstable side consistently exhibited reduced hip and knee torques and restricted hip RoM across all conditions, reflecting impaired neuromuscular control.
4.1 The effect of different auditory rhythms adaptation
At the PvGRF moment, the plantarflexion torque under CC was higher than any rhythmic conditions. A higher plantarflexion moment may reflect greater mechanical demands on the ankle joint. This increased torque may indicate compensatory strategies or inefficient load absorption mechanisms, both of which have been associated with elevated susceptibility to reinjury in unstable ankles (Delahunt et al., 2006; Gribble et al., 2013). Therefore, compared to conditions with rhythmic stimulation, the absence of rhythm may lead to suboptimal joint loading patterns and compromised joint protection. Auditory rhythms can influence motor output by entraining motor-related neural oscillations, thereby aligning cortical activity with rhythmic timing and facilitating more temporally coordinated movement (Clements-Cortes and Bartel, 2018). This synchronization often referred to as entrainment (Braunlich et al., 2019). In the context of motor control, such entrainment may serve as a mechanism through which external auditory cues modulate joint-level movement patterns. Interestingly, plantarflexion torque at 60 bpm was the lowest on both the stable and unstable side. One possible explanation is that at 60 bpm, the ankle joint may not have had sufficient time to generate an effective shock absorption response, leading to reduced torque output. Although slower rhythm theoretically offers a longer preparatory window, the reduced Khip and torque outputs suggest that the entrainment effect was suboptimal. This finding aligns with the idea that suboptimal rhythmic entrainment may interfere with anticipatory postural adjustments (APAs)—the preemptive neuromuscular activations critical for preparing the body for landing. Ineffective APAs at this slower rhythm could result in delayed or inefficient muscle coordination, contributing to altered joint mechanics during complex motor tasks. Instead, the system may have redistributed neuromuscular control to proximal joints, minimizing direct ankle load but requiring longer postural adjustment times.
In contrast, under 120 bpm, the hip, knee, and ankle torques on the stable side were higher than those on the unstable side at IC moment, suggesting a torque redistribution strategy in which the stable limb assumes a greater mechanical role in impact attenuation at IC moment. The effectiveness of 120 bpm may be attributed to its alignment with natural human physiological rhythm (Iwanaga, 1995), enhancing sensory-motor synchronization and the coupling of perception and action. Studies have found that rhythms in the range of 76-122 bpm induce stronger neural activity in the auditory cortex, posterior cingulate cortex, and inferior parietal lobule (Liu et al., 2018). Styns et al. (2007) also found that rhythms between 106 and 130 bpm elicited a favorable synchronization with human body movements. These findings further substantiate the significant role of a 120 bpm rhythm in enhancing athletic performance and motor control. This entrainment effect at 120 bpm may offer unique benefits by enhancing temporal predictability and facilitating rhythm-driven motor timing. Meanwhile, at the PvGRF moment, the hip extension torque on the unstable side exceeded that of the stable side, may reflect a neuromuscular adaptation in response to impaired ankle function, whereby joint loading is shifted proximally to reduce mechanical stress on the compromised distal segments. Such a hip-dominant control strategy likely contributes to postural stabilization and reflects an internal protective mechanism to maintain functional integrity during high-impact tasks. These findings highlight the role of auditory rhythm adaptation in modulating joint-specific motor strategies. The observed improvements in joint torque profiles and load redistribution suggest that rhythm-based interventions may serve as a promising non-invasive approach to enhance joint coordination and motor control in individuals with FAI.
However, under 180 bpm, although the stable side demonstrated the greatest hip RoM, it simultaneously exhibited reduced RoM in both the knee and ankle joints, along with the lowest ankle plantarflexion torque. These results suggest that faster rhythms may increase the motor execution demands. This suggests that while rhythm adaptation can enhance control, excessively rapid tempos may impose constraints on inter-joint coordination and functional stability.
4.2 Differential joint mechanics in the bilateral lower limbs of FAI individuals
At the IC moment, the vGRF and the PvGRF were higher on the unstable side, suggesting that the unstable side may exhibit deficits in neuromuscular control, thus increasing the risk of injury during drop landing.
The study also revealed that, under both the 120 and 180 bpm, the stable side exhibited higher hip and knee extension torques at IC moment, along with a greater hip RoM, compared to the unstable side. These rhythms fall within the range of human-preferred movement rhythms and are commonly used in sports and rehabilitation settings to guide coordination and movement timing. The observed increase in joint extension at IC moment may serve as a preparatory strategy to absorb impact forces, with the extended posture facilitating elastic deformation and energy dissipation during drop landing (Zhang et al., 2000).
On the other hand, the unstable side under CC exhibited lower hip and knee extension torques at PvGRF moment and smaller hip RoM compared to rhythmic conditions. However, under 120 bpm, the hip extension torque on the unstable side increased, higher than the stable side, indicating an enhanced proximal joint contribution. This change may reflect the modulatory role of auditory rhythm adaptation in enhancing neuromuscular coordination, particularly through the temporal synchronization of sensory input and motor output (Thaut et al., 1999; Todd and Lee, 2015). Prior studies have shown that auditory-motor synchronization improves movement accuracy, enhances preparatory motor activity, and supports postural regulation during dynamic tasks (Repp, 2005; Schaefer, 2014). Thus, the mechanism of drop landing is not merely mechanical, involving shock absorption and energy conversion, but also includes neurophysiological modulation of motor timing and postural strategies. This integrated response may be especially beneficial for individuals with sensorimotor deficits, such as those with FAI.
According to Fernández-Sotos et al. (2016), rhythmic units consist of one or more pulses in a sustained pattern, serving as temporal cues that can guide movement. These rhythmic pulses may influence motor control by enhancing sensorimotor integration. Damm et al. (2020) demonstrated that auditory rhythm can couple with motor instructions, enhancing the connection between perceptual and motor regions. Consistent with these findings, our results suggest that auditory rhythm adaptation may enhance neuromuscular control on the unstable side of FAI individuals, potentially improving motor performance, and the hip joint played a key role in stability and control. Notably, the increased engagement of proximal joints observed under the 120 bpm condition may represent a general motor adaptation strategy that supports efficient torque redistribution, optimized load management.
The hip, knee, and ankle joints together form an integrated kinetic chain in the lower limb, and these joints work in concert to perform movement tasks. When dysfunction occurs in one joint, the body adapts by modifying its movement strategy, relying on adjacent or distal joints to compensate for the impaired joint (Lee et al., 2018). In this study, the unstable side exhibited a greater hip extension moment under CC and at the PvGRF moment. This suggests that, in the presence of ankle instability, individuals subconsciously rely more on the hip joint to buffer the impact forces during drop landing. However, this reliance on the hip joint may represent a passive compensatory movement pattern. Proprioception is crucial for neuromuscular control. Ankle proprioceptors relay joint position, movement status, and muscle force information to the brain, which then processes this feedback to precisely regulate neuromuscular activity (Fernandes et al., 2000). On the unstable side, impaired proprioceptive input may cause “neurological input blockage” (Pereira et al., 2022), impairing the CNS ability to respond effectively (Linford et al., 2006). As a result, individuals may become passively dependent on the hip joint, reducing the ankle’s ability to absorb shock during drop landing (Hubbard and Wikstrom, 2010). Following an ankle injury, the body may adapt by developing movement patterns that limit load on both the injured and uninjured ankle.
Kjoint refers to the joint’s resistance to deformation under external force, indicating the joint’s stability and flexibility. While a stiffer joint inherently requires greater force to achieve the same deformation, this does not equate to improved stability or reduced flexibility in isolation. Instead, stiffness represents a trade-off between energy absorption and structural rigidity during dynamic tasks like drop landing. During impact absorption, higher Kjoint enhances force dissipation capacity, thereby reducing peak stress on articular surfaces and soft tissues. Our findings revealed that Khip at 60 bpm was highest on the stable limb, aligning with its role in load-bearing stability. Conversely, the unstable limb exhibited paradoxically higher Kankle at 180 bpm, despite the ankle’s functional requirement for greater flexibility. This counterintuitive result suggests a compensatory mechanism that reduced ankle mobility, evidenced by RoM decrements at 180 bpm, may force the joint into a stiffer mechanical state to maintain positional control, potentially increasing injury risk through altered load distribution.
4.3 Limitations and future directions
It is important to acknowledge that our estimation of Kjoint is based on a simplified biomechanical model that considers only external joint torque and joint angular displacement, and does not incorporate muscle properties such as viscosity, activation timing, or viscoelastic behavior. Consequently, the model may omit critical neuromuscular factors that influence joint stiffness under real-life dynamic conditions. Future studies should consider integrating electromyography (EMG) and muscle ultrasound data to capture the active muscular contributions to joint stabilization and to more comprehensively assess joint protection strategies during high-impact tasks. Second, the drop landing task may not fully capture delayed motor responses that could occur under 60 bpm. Future research could explore the biomechanical adaptations of FAI individuals when performing rhythmic entrainment tasks under slow-tempo constraints using gait or stepping tasks.
Finally, given that rhythmic cues can support motor coordination and sensory function (Hsu et al., 2021), incorporating rhythmic elements into conventional training, such as balance exercises, strength training, and proprioceptive neuromuscular facilitation (PNF) techniques, may potentially enhance timing control, movement consistency, and the efficiency of motor learning. Such combinations yield additive or synergistic benefits could also be systematically investigated.
5 Conclusion
Rhythmic auditory adaptation influences lower limb joint mechanics and modulates motor control strategies in individuals with FAI during single-leg drop landing. Specifically, auditory stimulation at 120 bpm elicited a proximal-dominant torque distribution pattern on the stable limb at IC moment, characterized by increased hip and knee extension torques. Meanwhile, torque redistribution and increased hip joint involvement on the unstable side at PvGRF moment reflects compensatory adaptations aimed at mitigating stress on the unstable ankle. These findings highlight the potential of rhythm-based interventions, particularly 120 bpm, to optimize anticipatory motor strategies and reduce reinjury risk in FAI individuals through non–invasive modulation of sensorimotor coordination.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Soochow University (SUDA20221005H03). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – original draft, Writing – review & editing. ZW: Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. YL: Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation. YT: Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software. YZ: Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Methodology. XW: Data curation, Software, Writing – review & editing, Conceptualization. XM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. QZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Jiangsu Provincial Department of Education under the Jiangsu Key Project of Philosophy and Social Sciences Research in Colleges and Universities (2023SJZD143) and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX25_3518).
Acknowledgments
We are grateful for the support of the Sports Biomechanics Laboratory at the Physical Education and Sports School of Soochow University, as well as all the subjects and staff.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ardakani, M. K., Wikstrom, E. A., Minoonejad, H., Rajabi, R., and Sharifnezhad, A. (2019). Hop-Stabilization training and landing biomechanics in athletes with chronic ankle instability: A randomized controlled trial. J. Athl. Train. 54, 1296–1303. doi: 10.4085/1062-6050-550-17
Braun Janzen, T., Koshimori, Y., Richard, N. M., and Thaut, M. H. (2021). Rhythm and music-based interventions in motor rehabilitation: Current evidence and future perspectives. Front. Hum. Neurosci. 15:789467. doi: 10.3389/fnhum.2021.789467
Braunlich, K., Seger, C. A., Jentink, K. G., Buard, I., Kluger, B. M., and Thaut, M. H. (2019). Rhythmic auditory cues shape neural network recruitment in Parkinson’s disease during repetitive motor behavior. Eur. J. Neurosci. 49, 849–858. doi: 10.1111/ejn.14227
Breska, A., and Ivry, R. B. (2018). Double dissociation of single-interval and rhythmic temporal prediction in cerebellar degeneration and Parkinson’s disease. Proc. Natl. Acad. Sci. U. S. A. 115, 12283–12288. doi: 10.1073/pnas.1810596115
Christoforidou, A, Patikas, D. A., Bassa, E., Paraschos, I., Lazaridis, S., Christoforidis, C., et al. (2017). Landing from different heights: Biomechanical and neuromuscular strategies in trained gymnasts and untrained prepubescent girls. J. Electromyogr. Kinesiol. 32, 1–8. doi: 10.1016/j.jelekin.2016.11.003
Clements-Cortes, A., and Bartel, L. (2018). Are we doing more than we know? possible mechanisms of response to music therapy. Front. Med. (Lausanne) 5:255. doi: 10.3389/fmed.2018.00255
Cohen, J. (1988). Statistical power analysis for the behavioral sciences, Second Edn. Mahwah, NJ: Lawrence Eribaum Associated, Psychology Press.
Coker, E., Harel, D., Roginska, A., and Lubetzky, A. V. (2023). Weighting of visual and auditory inputs in dancers with and without previous ankle injury. Hum. Mov. Sci. 92:103155. doi: 10.1016/j.humov.2023.103155
Cyr, J. P., Anctil, N., and Simoneau, M. (2019). Balance control mechanisms do not benefit from successive stimulation of different sensory systems. PLoS One 14:e0226216. doi: 10.1371/journal.pone.0226216
Damm, L., Varoqui, D., De Cock, V. C., Dalla Bella, S., and Bardy, B. (2020). Why do we move to the beat? A multi-scale approach, from physical principles to brain dynamics. Neurosci. Biobehav. Rev. 112, 553–584. doi: 10.1016/j.neubiorev.2019.12.024
Darabi, N., and Svensson, U. P. (2021). Dynamic systems approach in sensorimotor synchronization: Adaptation to tempo step-change. Front. Physiol. 12:667859. doi: 10.3389/fphys.2021.667859
Delahunt, E., Monaghan, K., and Caulfield, B. (2006). Altered neuromuscular control and ankle joint kinematics during walking in subjects with functional instability of the ankle joint. Am. J. Sports Med. 34, 1970–1976. doi: 10.1177/0363546506290989
Donahue, M., Simon, J., and Docherty, C. L. (2011). Critical review of self-reported functional ankle instability measures. Foot Ankle Int. 32, 1140–1146. doi: 10.3113/fai.2011.1140
Edwards, R. H. (1981). Human muscle function and fatigue. Ciba Found Symp. 82, 1–18. doi: 10.1002/9780470715420.ch1
Farley, C. T., Houdijk, H. H., Van Strien, C., and Louie, M. (1998). Mechanism of leg stiffness adjustment for hopping on surfaces of different stiffnesses. J. Appl. Physiol. 85, 1044–1055. doi: 10.1152/jappl.1998.85.3.1044
Fernandes, N., Allison, G. T., and Hopper, D. (2000). Peroneal latency in normal and injured ankles at varying angles of perturbation. Clin. Orthop. Relat. Res. 193–201. doi: 10.1097/00003086-200006000-00023
Fernández-Sotos, A., Fernández-Caballero, A., and Latorre, J. M. (2016). Influence of tempo and rhythmic unit in musical emotion regulation. Front. Comput. Neurosci. 10:80. doi: 10.3389/fncom.2016.00080
Fong, D. T., Chan, Y. Y., Mok, K. M., Yung, P. S., and Chan, K. M. (2009). Understanding acute ankle ligamentous sprain injury in sports. Sports Med. Arthrosc. Rehabil. Ther. Technol. 1:14. doi: 10.1186/1758-2555-1-14
Fransz, D. P., Huurnink, A., Kingma, I., de Boode, V. A., Heyligers, I. C., and van Dieën, J. H. (2018). Performance on a single-legged drop-jump landing test is related to increased risk of lateral ankle sprains among male elite soccer players: A 3-year prospective cohort study. Am. J. Sports Med. 46, 3454–3462. doi: 10.1177/0363546518808027
Freeman, M. A. (1965). Instability of the foot after injuries to the lateral ligament of the ankle. J. Bone Joint Surg. Br. 47, 669–677.
Fuller, J. L., and Fienup, D. M. (2018). A preliminary analysis of mastery criterion level: Effects on response maintenance. Behav. Anal. Pract. 11, 1–8. doi: 10.1007/s40617-017-0201-0
Gribble, P. A., Delahunt, E., Bleakley, C., Caulfield, B., Docherty, C. L., Fourchet, F., et al. (2013). Selection criteria for patients with chronic ankle instability in controlled research: A position statement of the International Ankle Consortium. J. Orthop Sports Phys. Ther. 43, 585–591. doi: 10.2519/jospt.2013.0303
Henry, M., and Baudry, S. (2019). Age-related changes in leg proprioception: Implications for postural control. J. Neurophysiol. 122, 525–538. doi: 10.1152/jn.00067.2019
Hertel, J. (2002). Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J. Athl. Train. 37, 364–375.
Hiller, C. E., Refshauge, K. M., Herbert, R. D., and Kilbreath, S. L. (2007). Balance and recovery from a perturbation are impaired in people with functional ankle instability. Clin. J. Sport Med. 17, 269–275. doi: 10.1097/JSM.0b013e3180f60b12
Hsu, H. Y., Lin, C. W., Lin, Y. C., Wu, P. T., Kato, H., Su, F. C., et al. (2021). Effects of vibrotactile-enhanced music-based intervention on sensorimotor control capacity in the hand of an aging brain: A pilot feasibility randomized crossover trial. BMC Geriatr. 21:660. doi: 10.1186/s12877-021-02604-0
Hubbard, T. J., and Wikstrom, E. A. (2010). Ankle sprain: Pathophysiology, predisposing factors, and management strategies. Open Access J. Sports Med. 1, 115–122. doi: 10.2147/oajsm.s9060
Iwanaga, M. (1995). Relationship between heart rate and preference for tempo of music. Percept. Mot. Skills 81, 435–440. doi: 10.1177/003151259508100215
Jeon, H. G., Lee, S. Y., Park, S. E., and Ha, S. (2021). Ankle instability patients exhibit altered muscle activation of lower extremity and ground reaction force during landing: A systematic review and meta-analysis. J. Sports Sci. Med. 20, 373–390. doi: 10.52082/jssm.2021.373
Kaminski, T. W., Perrin, D. H., and Gansneder, B. M. (1999). Eversion strength analysis of uninjured and functionally unstable ankles. J. Athl. Train. 34, 239–245.
Kim, H. W., Lee, K. M., and Lee, Y. S. (2023). Sensorimotor and working memory systems jointly support development of perceptual rhythm processing. Dev. Sci. 26:e13261. doi: 10.1111/desc.13261
Kirkland, M. C., Chen, A., Downer, M. B., Holloway, B. J., Wallack, E. M., Lockyer, E. J., et al. (2018). Bipedal hopping timed to a metronome to detect impairments in anticipatory motor control in people with mild multiple sclerosis. Clin. Biomech. (Bristol) 55, 45–52. doi: 10.1016/j.clinbiomech.2018.04.009
Konrad, H. R., Girardi, M., and Helfert, R. (1999). Balance and aging. Laryngoscope 109, 1454–1460. doi: 10.1097/00005537-199909000-00019
Kweon, S. J., Harrison, K., Williams, D. S. B. III, and Kwon, Y. U. (2022). Foot and shank coordination during walking in copers compared with patients with chronic ankle instability and controls. Orthop. J. Sports Med. 10:23259671221139482. doi: 10.1177/23259671221139482
Lee, J., Song, Y., and Shin, C. S. (2018). Effect of the sagittal ankle angle at initial contact on energy dissipation in the lower extremity joints during a single-leg landing. Gait Posture 62, 99–104. doi: 10.1016/j.gaitpost.2018.03.019
Linford, C. W., Hopkins, J. T., Schulthies, S. S., Freland, B., Draper, D. O., and Hunter, I. (2006). Effects of neuromuscular training on the reaction time and electromechanical delay of the peroneus longus muscle. Arch. Phys. Med. Rehabil. 87, 395–401. doi: 10.1016/j.apmr.2005.10.027
Liu, Y., Liu, G., Wei, D., Li, Q., Yuan, G., Wu, S., et al. (2018). Effects of musical tempo on musicians’ and non-musicians’ emotional experience when listening to music. Front. Psychol. 9:2118. doi: 10.3389/fpsyg.2018.02118
Marín Fermín, T., Al-Dolaymi, A. A., and D’Hooghe, P. (2023). Acute ankle sprain in elite athletes: How to get them back to the game? Foot Ankle Clin. 28, 309–320. doi: 10.1016/j.fcl.2022.12.007
McKay, G. D., Goldie, P. A., Payne, W. R., and Oakes, B. W. (2001). Ankle injuries in basketball: Injury rate and risk factors. Br. J. Sports Med. 35, 103–108. doi: 10.1136/bjsm.35.2.103
Meng, L., Kong, L., Kong, L., Zhang, Q., Shen, J., and Hao, Y. (2022). Effects of visual deprivation on the injury of lower extremities among functional ankle instability patients during drop landing: A kinetics perspective. Front. Physiol. 13:1074554. doi: 10.3389/fphys.2022.1074554
Monteleone, B. J., Ronsky, J. L., Meeuwisse, W. H., and Zernicke, R. F. (2014). Ankle kinematics and muscle activity in functional ankle instability. Clin. J. Sport Med. 24, 62–68. doi: 10.1097/01.jsm.0000432858.86929.80
Nauwelaerts, S., and Aerts, P. (2006). Take-off and landing forces in jumping frogs. J. Exp. Biol. 209(Pt 1), 66–77. doi: 10.1242/jeb.01969
Pereira, M., Swash, M., and de Carvalho, M. (2022). Exercise following immobility increases lower motor neuron excitability: F-wave and H-reflex studies. Neurophysiol. Clin. 52, 147–156. doi: 10.1016/j.neucli.2021.12.004
Proske, U., and Gandevia, S. C. (2012). The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 92, 1651–1697. doi: 10.1152/physrev.00048.2011
Puddle, D. L., and Maulder, P. S. (2013). Ground reaction forces and loading rates associated with parkour and traditional drop landing techniques. J. Sports Sci. Med. 12, 122–129.
Repp, B. H. (2005). Sensorimotor synchronization: A review of the tapping literature. Psychon. Bull. Rev. 12, 969–992. doi: 10.3758/bf03206433
Rivera-Tello, S., Romo-Vázquez, R., González-Garrido, A. A., and Ramos-Loyo, J. (2023). Musical tempo affects EEG spectral dynamics during subsequent time estimation. Biol. Psychol. 178:108517. doi: 10.1016/j.biopsycho.2023.108517
Saunders, N. W., Hanson, N., Koutakis, P., Chaudhari, A. M., and Devor, S. T. (2014). Landing ground reaction forces in figure skaters and non-skaters. J. Sports Sci. 32, 1042–1049. doi: 10.1080/02640414.2013.877593
Schaefer, R. S. (2014). Auditory rhythmic cueing in movement rehabilitation: Findings and possible mechanisms. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 369:20130402. doi: 10.1098/rstb.2013.0402
Shin, Y. K., Chong, H. J., Kim, S. J., and Cho, S. R. (2015). Effect of rhythmic auditory stimulation on hemiplegic gait patterns. Yonsei Med. J. 56, 1703–1713. doi: 10.3349/ymj.2015.56.6.1703
Styns, F., van Noorden, L., Moelants, D., and Leman, M. (2007). Walking on music. Hum. Mov. Sci. 26, 769–785. doi: 10.1016/j.humov.2007.07.007
Thaut, M. H., Kenyon, G. P., Schauer, M. L., and McIntosh, G. C. (1999). The connection between rhythmicity and brain function. IEEE Eng. Med. Biol. Mag. 18, 101–108. doi: 10.1109/51.752991
Thaut, M. H., Rice, R. R., Braun Janzen, T., Hurt-Thaut, C. P., and McIntosh, G. C. (2019). Rhythmic auditory stimulation for reduction of falls in Parkinson’s disease: A randomized controlled study. Clin. Rehabil. 33, 34–43. doi: 10.1177/0269215518788615
Todd, N. P., and Lee, C. S. (2015). The sensory-motor theory of rhythm and beat induction 20 years on: A new synthesis and future perspectives. Front. Hum. Neurosci. 9:444. doi: 10.3389/fnhum.2015.00444
Troester, J. C., Jasmin, J. G., and Duffield, R. (2018). Reliability of single-leg balance and landing tests in rugby union; prospect of using postural control to monitor fatigue. J. Sports Sci. Med. 17, 174–180.
Wang, B., Zhang, X., Zhu, F., Zhu, W., Wang, X., Jia, F., et al. (2022). A randomized controlled trial comparing rehabilitation with isokinetic exercises and Thera-Band strength training in patients with functional ankle instability. PLoS One 17:e0278284. doi: 10.1371/journal.pone.0278284
Wang, Z., Lu, M., Kong, L., Meng, L., Xue, J., Zheng, Y., et al. (2024). Impact of cognitive tasks on biomechanical adjustments during single-leg drop landings in individuals with functional ankle instability. Appl. Sci. 14:10297. doi: 10.3390/app142210297
Wernli, K., Ng, L., Phan, X., Davey, P., and Grisbrook, T. (2016). The relationship between landing sound, vertical ground reaction force, and kinematics of the lower limb during drop landings in healthy men. J. Orthop. Sports Phys. Ther. 46, 194–199. doi: 10.2519/jospt.2016.6041
Wu, H. W., Chang, Y. S., Arefin, M. S., You, Y. L., Su, F. C., and Lin, C. F. (2022). Six-Week remodeled bike pedal training improves dynamic control of lateral shuffling in athletes with functional ankle instability. Sports Health 14, 348–357. doi: 10.1177/19417381211035781
Yin, L., Hu, X., Lai, Z., Liu, K., and Wang, L. (2020). Leg stiffness and vertical stiffness of habitual forefoot and rearfoot strikers during running. Appl. Bion. Biomech. 2020:8866340. doi: 10.1155/2020/8866340
Keywords: functional ankle instability, drop landing, auditory, rhythmic adaptation, joint mechanics
Citation: Meng L, Wang Y, Wang Z, Liu Y, Tan Y, Zhang Y, Wei X, Mao X and Zhang Q (2025) Effects of auditory rhythmic adaptation on lower limb joint mechanics during single-leg drop landings in individuals with functional ankle instability. Front. Hum. Neurosci. 19:1579260. doi: 10.3389/fnhum.2025.1579260
Received: 20 February 2025; Accepted: 10 June 2025;
Published: 02 July 2025.
Edited by:
Takao Yamasaki, Minkodo Minohara Hospital, JapanReviewed by:
Ringo Tang-Long Zhu, Hong Kong Polytechnic University, Hong Kong SAR, ChinaMichał Pawłowski, Jerzy Kukuczka Academy of Physical Education in Katowice, Poland
Huimeng Chen, Beijing Sport University, China
Copyright © 2025 Meng, Wang, Wang, Liu, Tan, Zhang, Wei, Mao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuxia Zhang, cXh6aGFuZ0BzdWRhLmVkdS5jbg==; Xiaokun Mao, eGttYW9Ac3VkYS5lZHUuY24=
 Lingyue Meng
Lingyue Meng Yubo Wang
Yubo Wang Zilong Wang
Zilong Wang Yongan Liu1
Yongan Liu1 Qiuxia Zhang
Qiuxia Zhang