- 1Department of Radiology, University of Arkansas for Medical Sciences, Little Rock, AR, United States
- 2Department of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, AR, United States
- 3Arkansas Children’s Research Institute, Little Rock, AR, United States
- 4Arkansas Children’s Nutrition Center, Little Rock, AR, United States
- 5University of Arkansas, Fayetteville, AR, United States
Background: Maternal mental health during pregnancy can influence fetal brain development, yet its long-term effects remain unclear. This study investigates the association between prenatal maternal depression and anxiety symptoms and white matter microstructure in the limbic system of 8-year-old children.
Methods: Fifty-one healthy pregnant women and typically developing 8-year-old children dyads were included in this prospective and longitudinal study. Maternal depression and anxiety symptoms were assessed at 12, 24, and 36 weeks of gestation using the Beck Depression Inventory-II (BDI-II) and State–Trait Anxiety Inventory (STAI). Their children underwent a brain MRI examination at age 8 years with multi-shell diffusion imaging analyzed using diffusion tensor imaging (DTI), diffusional kurtosis imaging (DKI), and neurite orientation dispersion and density imaging (NODDI) models for a multi-aspect evaluation of microstructural development. Key diffusion metrics (FA: fractional anisotropy; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity; MK: mean kurtosis; AK: axial kurtosis; RK: radial kurtosis; NDI: neurite density index; ODI: orientation dispersion index; FWF: free water fraction) were extracted from the limbic system white matter structures including cingulum, fornix, and uncinate fasciculus, which are closely associated with emotional and motivational processes.
Results: Higher maternal depression symptom scores were associated with lower FA (R = –0.3126, p = 0.0305, in CGH.R; R = –0.3025, p = 0.0366, in FXC.R) and MK (R = –0.3284, p = 0.0227, in CGG.R) and higher MD (R = 0.2879, p = 0.0472, in CGH.R) and RD (R = 0.3451, p = 0.0163, in CGH.R; R = 0.3456, p = 0.0161, in FXC.R) in predominately right-hemisphere limbic tracts. Higher maternal anxiety symptom scores were associated with increased MD (R = 0.2897, p = 0.0458, in FXC.L; R = 0.2859, p = 0.0488, in UF.L) and RD (R = 0.3168, p = 0.0283, in FXC.L), decreased NDI (R = –0.3787, p = 0.0079, in FXC.L; R = –0.3422, p = 0.0173, in UF.R), and increased AK (R = 0.3154, p = 0.029, in UF.L) in predominately left-hemisphere limbic tracts.
Conclusion: Our findings suggest that maternal depression and anxiety during pregnancy may have long-lasting impacts on offspring white matter microstructure maturation in the limbic system. This highlights the need for prenatal mental health screening and potential interventions to promote brain development and support optimal neurodevelopmental outcomes in children.
1 Introduction
Depression and anxiety are highly prevalent among pregnant women. An analysis of data from the 2010–2019 National Health Interview Survey shows depression prevalence rates of 27.2 to 40.6% for different racial groups among pregnant women aged 18–44 (Sulley et al., 2023). In parallel, gestational anxiety disorders are estimated to occur in approximately 15–20% of pregnancies (Fawcett et al., 2019). Emerging research suggests that prenatal maternal mental health may have long-lasting effects on offspring neurodevelopment, particularly in white matter microstructures (Lebel et al., 2016; Wu et al., 2024). Our previous study demonstrated significant associations between maternal anxiety and depressive symptoms in the third trimester of pregnancy and reduced neonatal brain white matter integrity, as evidenced by decreased fractional anisotropy (FA) values, particularly affecting white matter regions in the frontal lobe, middle frontal gyrus, and limbic system (Graham et al., 2020). Other studies also showed that higher maternal anxiety and depression levels were associated with reduced white matter integrity in the right frontal lobe regions (Dean et al., 2018), lower FA and AD in the right amygdala (Rifkin-Graboi et al., 2013), as well as variations of FA across limbic and prefrontal regions (Rifkin-Graboi et al., 2015). Ross et al. found that by 2–3 years of age, children exposed to prenatal depression showed changes in white matter integrity, including increased FA and decreased mean diffusivity (MD) and radial diffusivity (RD), particularly in commissural and projection fiber regions (Roos et al., 2022). EI Marroun et al. reported that maternal depressive symptoms during pregnancy were associated with white matter microstructural alterations in children aged 6–9 years, specifically increased MD in the uncinate fasciculus and decreased FA and increased MD in the cingulum bundle (El Marroun et al., 2018). Wu et al. reviewed multiple MRI-based studies showing that when mothers experience elevated psychological stress during pregnancy, the fetus or newborn show volumetric or microstructural abnormalities in key brain regions such as the hippocampus, amygdala, and cingulate gyrus (Wu et al., 2024). These findings showed that prenatal maternal mental health may impact child neurodevelopment, particularly in white matter structures involved in motivation, emotion, learning, and memory within the limbic system (Wu et al., 2024; El Marroun et al., 2018; Gray, 1983).
Studies have emphasized white matter microstructural integrity as an important biomarker for mental health issues and proposed white matter trajectory models to evaluate neurodevelopment and neurodegeneration (Kochunov and Hong, 2014; Peters and Karlsgodt, 2015). Diffusion MRI is sensitive in evaluating white matter microstructural development, and different advanced diffusion model (DTI, DKI, and NODDI) can provide valuable information on microstructural integrity from a different perspective. DTI is widely used and sensitive to white matter, but assumes Gaussian diffusion, limiting its capacity to capture complex tissue architectures (Paydar et al., 2014). DKI extends DTI by incorporating non-Gaussian diffusion, thereby enhancing sensitivity to subtle microstructural heterogeneity (Paydar et al., 2014; Shi et al., 2019). NODDI, an advanced multi-compartment dMRI model, refines brain microstructure characterization by modeling neurite density, fiber orientation dispersion, and free water content (Huang et al., 2022; Zhang et al., 2012; Lynch et al., 2020). Combining these three techniques will provide a comprehensive, more nuanced, and biologically meaningful understanding of white matter microstructural development.
Mounting evidence shows that the limbic system—including key tracts such as the cingulum, fornix, and uncinate fasciculus—plays a pivotal role in emotional regulation, motivation, learning, and memory, rendering it particularly vulnerable to anxiety and depression (Gray, 1983; Torrico and Abdijadid, 2023; Van Velzen et al., 2020; Santos et al., 2018; Schmaal et al., 2016). Moreover, maternal mental health issues during pregnancy can significantly shape the development of these limbic pathways in offspring, potentially heightening their risk for future mental health challenges (Lebel et al., 2016; Wu et al., 2024; Graham et al., 2020; Dean et al., 2018; Rifkin-Graboi et al., 2013; Rifkin-Graboi et al., 2015; Roos et al., 2022; El Marroun et al., 2018). Given these considerations, understanding how prenatal maternal anxiety and depression may predispose children to alterations in limbic white matter microstructure is critical. Therefore, in this study, we focus on examining the relationships between maternal mental health during pregnancy and the white matter microstructural integrity of the vital limbic system in 8-year-old children. We hypothesized that higher maternal anxiety and depression symptoms during pregnancy will be associated with lower white matter microstructural development in these regions.
2 Materials and methods
2.1 Study design and participants
This is part of a prospective and longitudinal study (the Glowing Follow Up study, NCT03108001), which is a follow up of a previous prospective, observational study (the Glowing study, clinicaltrials.gov NCT01131117). All study procedures were approved by the institutional review board of the University of Arkansas for Medical Sciences, and appropriate consents/assents were obtained from study participants. In the Glowing study, pregnant women were enrolled and their newborns were initially followed up until age 2 years. Inclusion criteria for the pregnant women in the Glowing study were: pre-pregnancy body mass index (BMI) of 18.5–35, second parity, singleton pregnancy, ≥ 21 years old, conceived without assisted fertility treatments. Exclusion criteria for the pregnant women were preexisting medical conditions, medical complications during pregnancy, use of medications during pregnancy known to influence fetal growth, and tobacco and/or alcohol use. For their offspring, inclusion criteria were healthy and full-term at birth, while exclusion criteria were medications and medical conditions known to influence child growth and development. Participants who were enrolled in the original Glowing study and agreed to be contacted for future studies were contacted to participate in the Glowing Follow Up study. In this follow up study, children underwent an MRI examination of their brain and neurodevelopment assessments at age 8 years. A total of 67 mother/child dyads had both valid mental health assessments during pregnancy and a successful brain diffusion MRI scan at age ~8 years; 16 children were scanned after a scanner system upgrade which resulted in significant data harmonization issues for the diffusion MRI scans which have not been resolved and therefore excluded from this report; 51 mother/child dyads are included in this study report. Table 1 shows the demographic information of the study participants.
2.2 Prenatal maternal depression and anxiety symptoms
Mental health of the pregnant women, specifically depression and anxiety symptoms during pregnancy, was assessed at three time points throughout the pregnancy─12 weeks (T1), 24 weeks (T2), and 36 weeks (T3) of gestation by trained psychological examiners. Depression symptoms were measured using the Beck Depression Inventory-II (BDI-II) (Beck et al., 1996), a validated and widely used 21-item self-report questionnaire, where each item is scored 0–3, resulting in a total score ranging from 0 to 63, with higher scores showing greater depression symptoms. State anxiety symptoms were assessed using the State–Trait Anxiety Inventory (STAI) (Speilberger et al., 1983), a 20-item self-report tool with each item scored 1–4, and total scores ranging from 20 to 80, where higher scores showing greater state anxiety symptoms. The measured BDI and STAI scores for the study cohort are presented in Figure 1.
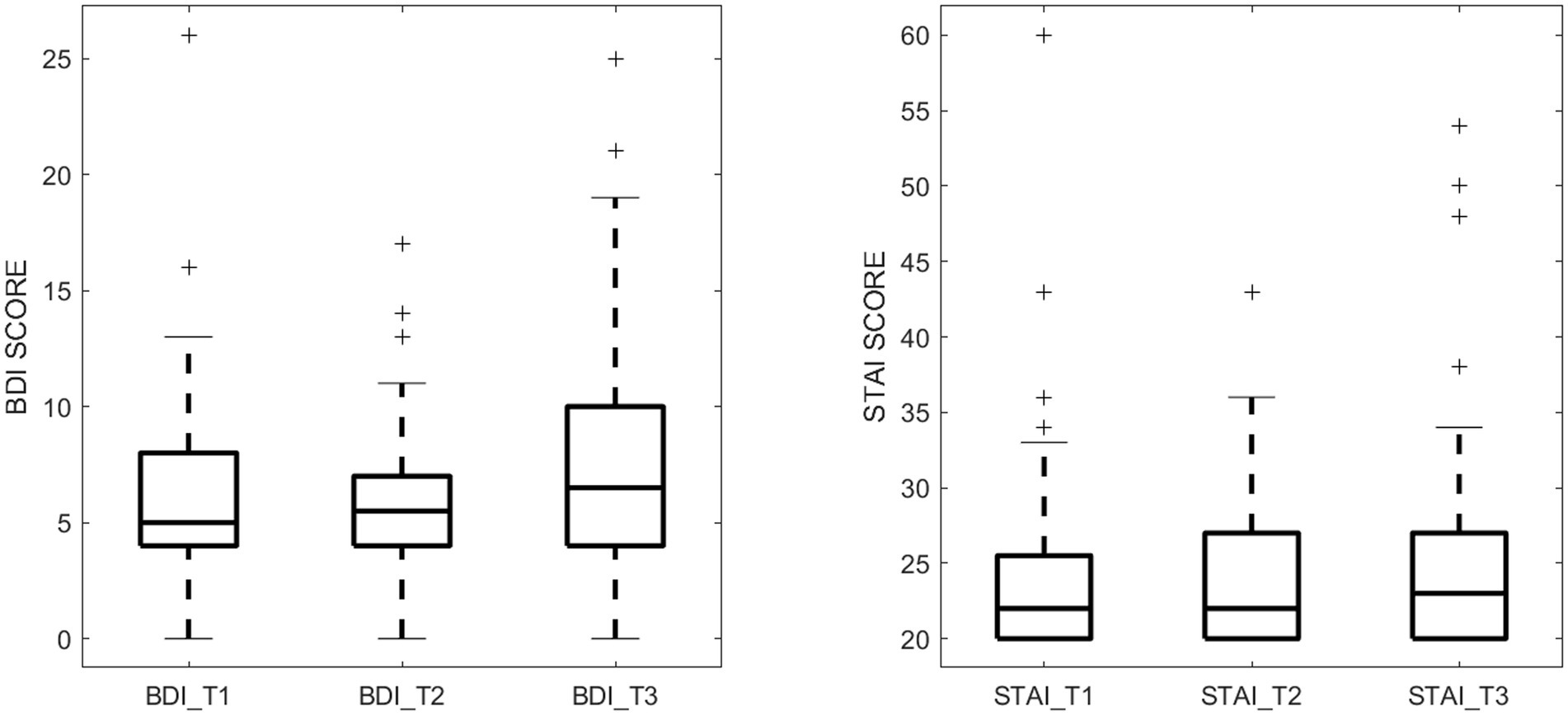
Figure 1. Maternal depression symptom scores (measured by BDI) and anxiety symptom scores (measured by STAI) during the 3 pregnancy trimesters (T1, T2, and T3).
2.3 Brain MRI data acquisition
At ~8 years of age, each child underwent an MRI scan of the brain without sedation using a PRISMA 3 T MRI scanner (Siemens Healthneers, USA) and a 20-channel head coil. The imaging protocol included a sagittal MPRAGE 3D T1-weighted sequence with TR 2,400 ms, TE 2.22 ms, 8 degree flip angle, turbo factor of 256, 192 sagittal slices, and a resolution of 0.8 mm x 0.8 mm x 0.8 mm, and an axial Multiband EPI pulse sequence for diffusion MRI data acquisition with TR 3,230 ms, TE 89.2 ms, 92 slices, resolution 1.5 mm x 1.5 mm x 1.5 mm, multiband factor 4. A multi-shell diffusion scheme was used with b-values of 0, 1,500, and 3,000 s/mm2, sampled using 7, 47, and 46 diffusion directions, respectively. Diffusion-weighted images at all b-values were acquired in both anterior–posterior (AP) and posterior–anterior (PA) phase-encoding directions to enable susceptibility distortion correction using the TOPUP approach.
Although some advanced diffusion protocols (such as early DKI protocols) often limited maximum b-values to 2000 s/mm2, recent studies support the inclusion of higher b-values—up to 3,000 s/mm2—for improved sensitivity to microstructural complexity in brain tissue without violating model assumptions (Chou et al., 2017; Olson et al., 2018; Bano et al., 2024). This b-value selection reflects a balance between increased diffusion contrast and reduced signal-to-noise ratio (SNR), which can be mitigated by modern acquisition techniques (Yan et al., 2013; Chuhutin et al., 2017; Kuo et al., 2018; Ezequiel Farrher and Shah, 2012).
2.4 Imaging data preprocessing
The diffusion imaging data were preprocessed using MRtrix3, FSL, and ANTs (Tournier et al., 2019; Jenkinson et al., 2012; Avants et al., 2009). All diffusion-weighted datasets underwent a standardized preprocessing workflow as follows. First, the raw diffusion images were denoised using a Marchenko-Pastur principal components analysis (MP-PCA) approach to minimize thermal noise. Next, Gibbs ringing artifacts were removed prior to correcting for geometric and motion-related distortions. Specifically, head motion, eddy current, and susceptibility-induced distortions were addressed using FSL’s Topup (for dual AP-PA acquisitions) followed by Eddy to correct for motion and remaining eddy currents. Subsequently, bias field correction was applied to reduce low-frequency intensity inhomogeneities across the image volume. Finally, global intensity normalization was performed by normalizing the input diffusion-weighted MRI images to have the same B0 white matter median value to remove intensity variations due to T2-weighting and RF inhomogeneity. This preprocessing pipeline ensures consistent image quality and facilitates robust downstream diffusion modeling and parameter mapping. To extract diffusion parameters, we utilized three diffusion models of white matter microstructure. DTI metrics were computed using the MRtrix3, yielding FA, axial diffusivity (AD), RD, and MD (Tournier et al., 2019). DKI model fitting was conducted with DIPY toolbox,1 providing mean kurtosis (MK), axial kurtosis (AK), and radial kurtosis (RK) (Henriques et al., 2021). NODDI parameters were derived using the open-source tool AMICO,2 generating neurite density index (NDI), orientation dispersion index (ODI), and free water fraction (FWF) (Daducci et al., 2015). Together, these models enabled a detailed and multi-faceted assessment of brain diffusion properties, offering a refined characterization of white matter microstructure.
2.5 Region of interest (ROI) extraction
The limbic system white matter regions of interest (ROIs) were extracted using the Johns Hopkins University (JHU) white matter atlas (Mori et al., 2008). Specifically, the white matter ROIs encompassed the cingulum gyrus (CGG) and the cingulum hippocampus (CGH), fornix (FNX), and uncinate fasciculus (UF) were reconstructed and average diffusion parameters within each ROI were calculated for each subject. A visual representation of the limbic system white matter regions studied is provided in Figure 2.
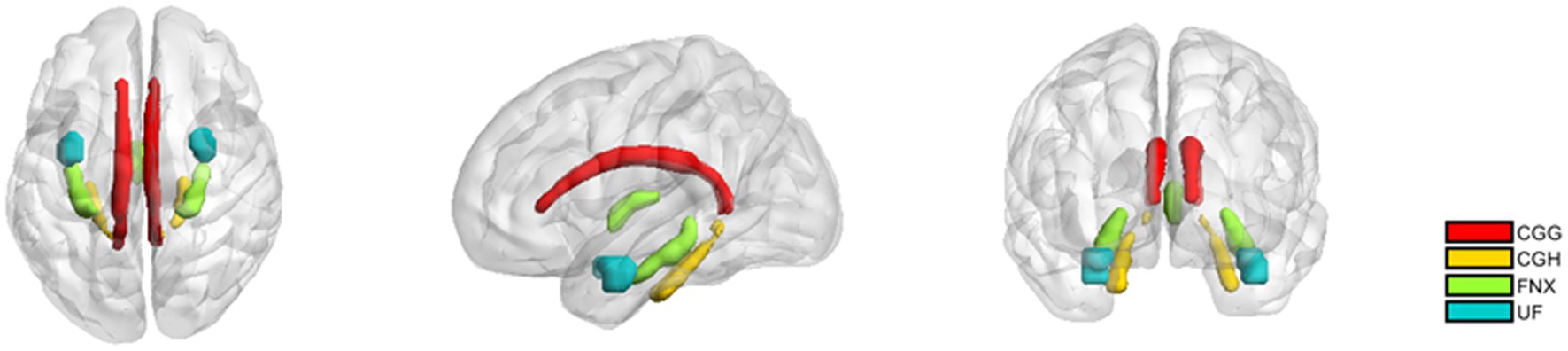
Figure 2. The white matter regions of interest in the limbic system. CGG: Cingulum (cingulate gyrus); CGH: Cingulum (hippocampus); fornix (FNX); uncinate fasciculus (UF). The definitions of these regions come from the JHU atlas.
2.6 Statistical analysis
To examine the relationships between prenatal maternal mental health and child white matter microstructure at 8 years of age, we conducted Spearman correlation analyses between maternal BDI and STAI scores (at T1, T2, and T3) and the diffusion metrics (FA, MD, AD, RD, MK, AK, RK, NDI, ODI, and FWF) extracted from the limbic system white matter ROIs. Child age and sex were included as covariates to control for potential confounding effects in the analysis. Spearman rank correlation coefficients (ρ) were used to assess associations between maternal symptom scores and diffusion MRI metrics. This non-parametric method was chosen due to the non-normal distribution of several imaging variables and to account for potential non-linear but monotonic relationships between psychological measures and neuroimaging indices.
3 Results
3.1 Relationships between BDI-II and diffusion metrics
3.1.1 Time points
At each of the three trimesters during pregnancy (T1, T2, T3), significant correlations between maternal depression symptoms and child white matter microstructure were observed (4 for T1; 5 for T2 and 7 for T3), as shown in Table 2.
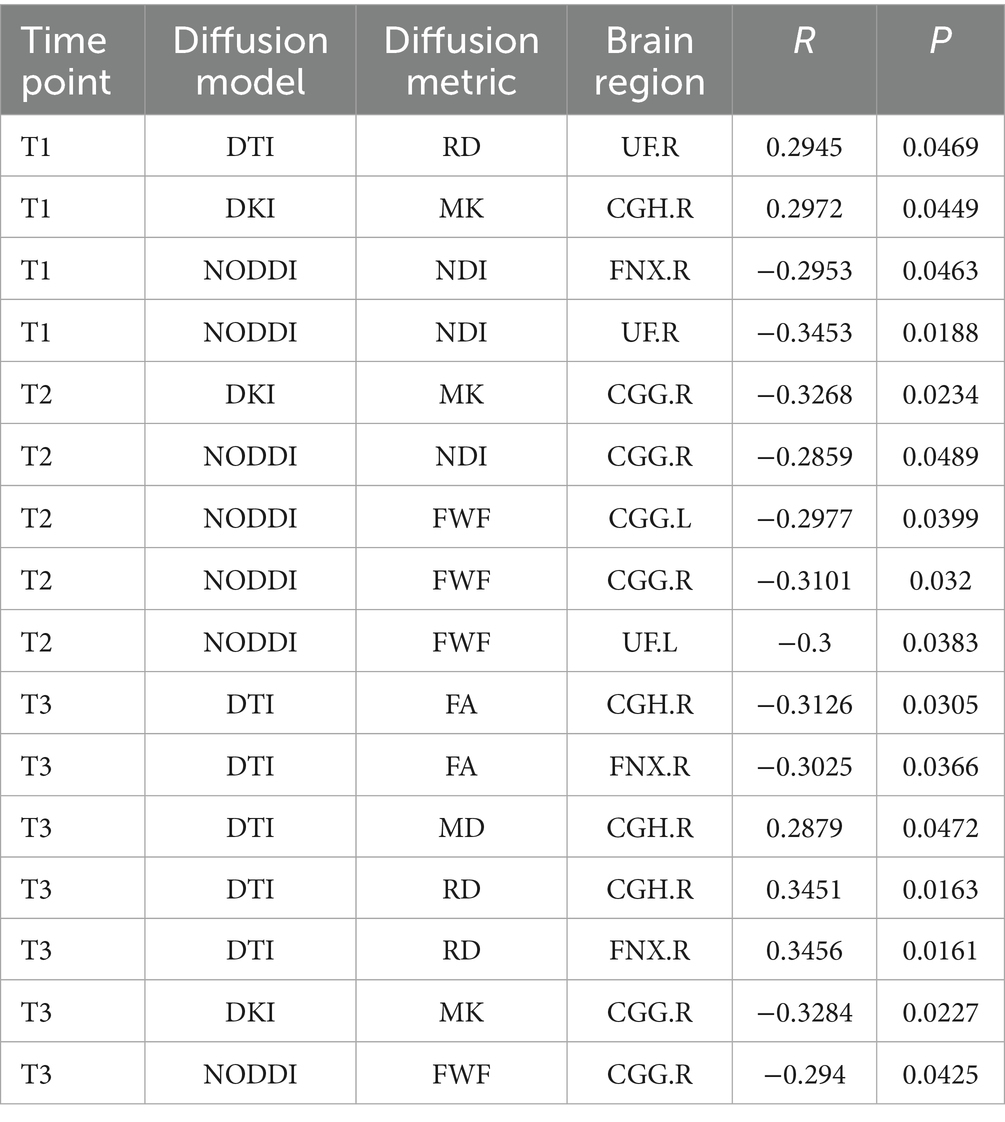
Table 2. Significant correlation between maternal depression symptom scores (measured by BDI) during the 3 pregnancy trimesters (T1, T2, and T3) and children brain diffusion imaging parameters at age 8 years.
3.1.2 Diffusion modality
Fractional anisotropy showed a significant negative correlation with BDI scores, indicating reduced white matter fiber integrity in individuals with maternal depression. MD and RD showed significant positive correlations with BDI scores, suggesting increased diffusivity in white matter. A scatter plot depicting these relationships is presented in Figure 3. Additionally, MK and FWF showed significant negative correlations with BDI scores. Scatter plots illustrating these relationships are presented in Figures 4, 5.
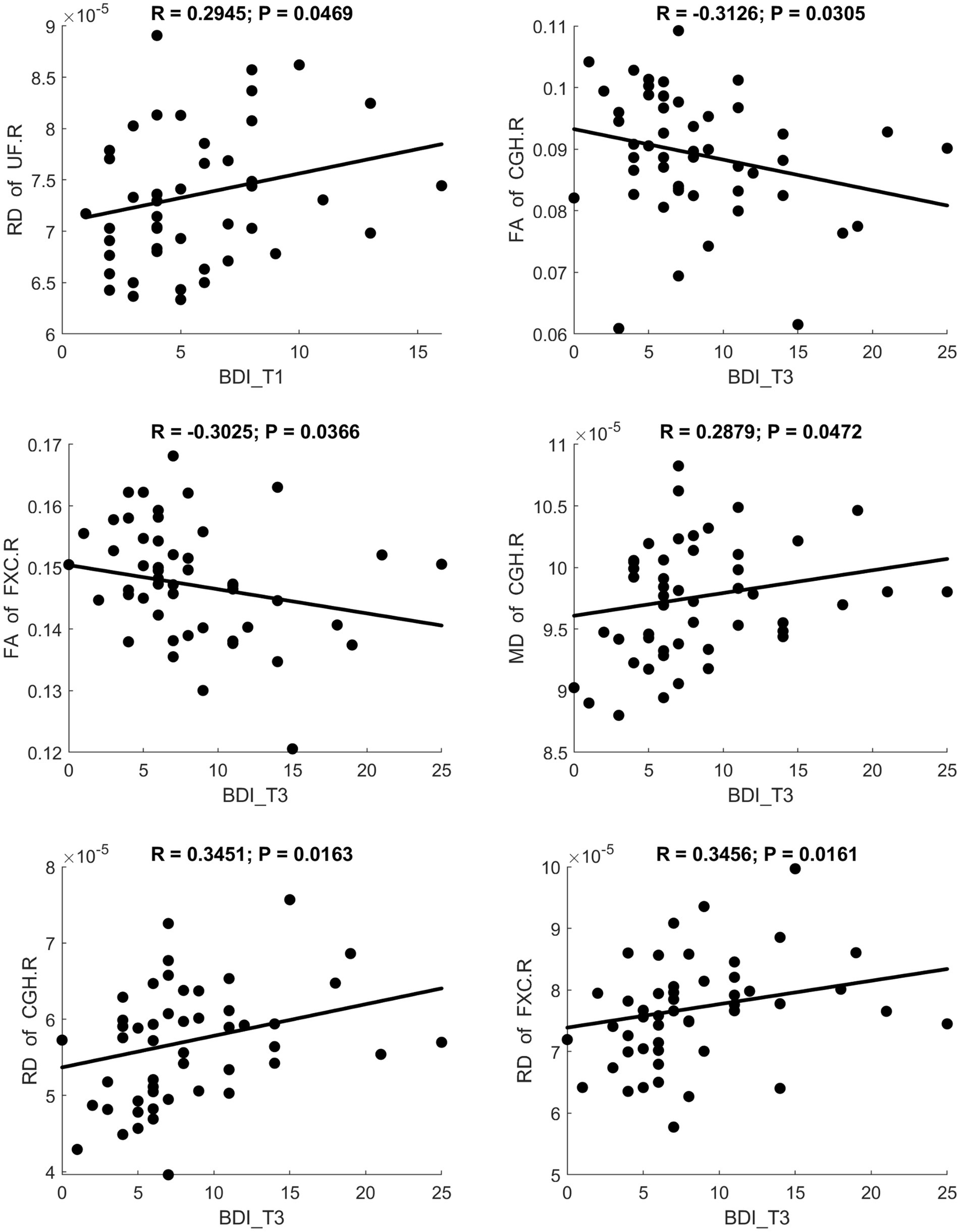
Figure 3. Significant correlation between maternal depression symptom scores (measured by BDI) during the 3 pregnancy trimesters (T1, T2, and T3) and children brain diffusion imaging parameters (measured by DTI) at age 8 years.
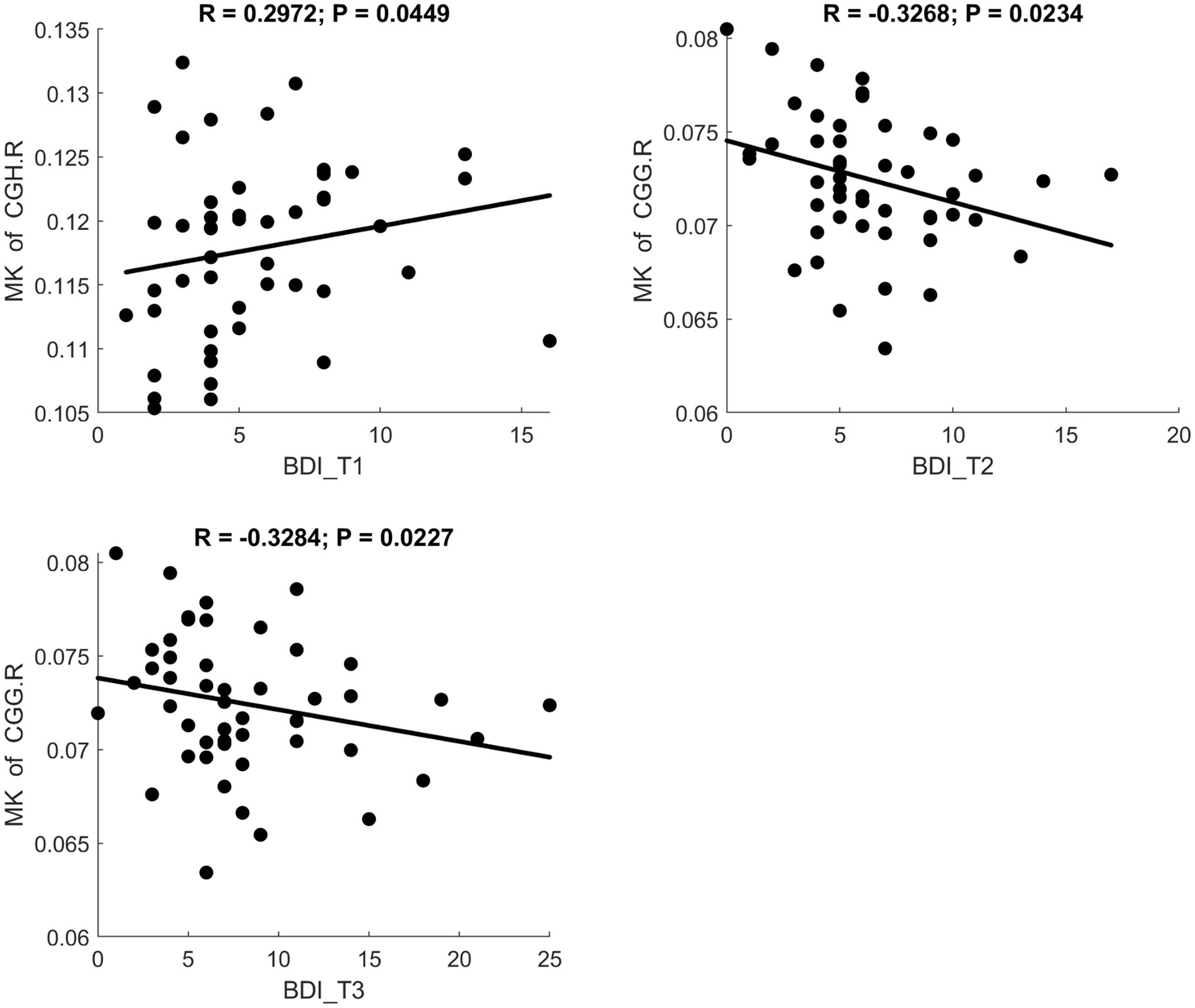
Figure 4. Significant correlation between maternal depression symptom scores (measured by BDI) during the 3 pregnancy trimesters (T1, T2, and T3) and children brain diffusion imaging parameters (measured by DKI) at age 8 years.
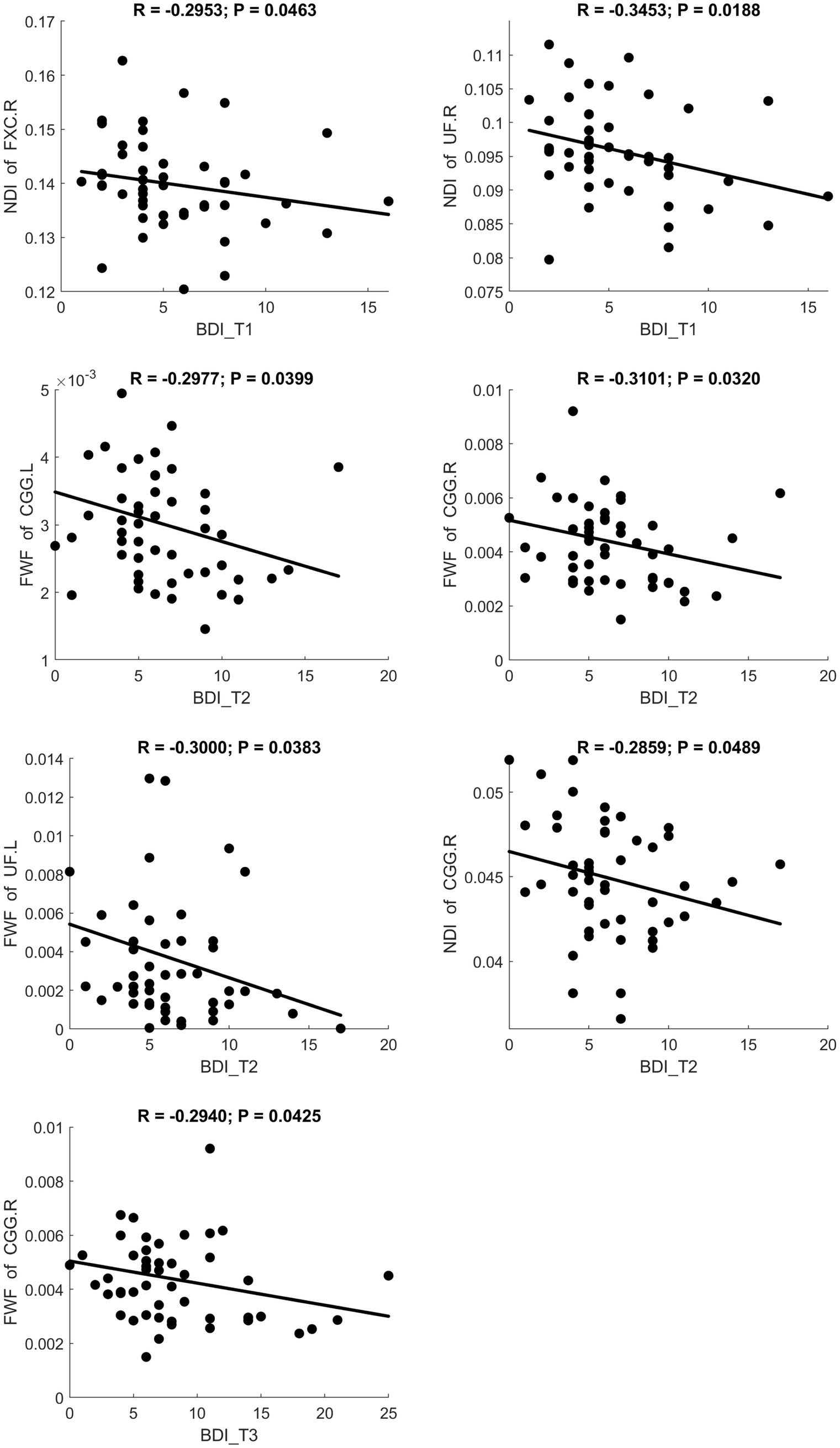
Figure 5. Significant correlation between maternal depression symptom scores (measured by BDI) during the 3 pregnancy trimesters (T1, T2, and T3) and children brain diffusion imaging parameters (measured by NODDI) at age 8 years.
3.1.3 Brain regions
Significant results were predominantly observed in the right hemisphere white matter fiber tracts, specifically, 14 out of 16 significant relationships involved the limbic system in the right hemisphere, as shown in Table 2.
3.2 Relationships between STAI and diffusion metrics
3.2.1 Time points
At the three trimesters during pregnancy (T1, T2, T3), significant correlations between maternal anxiety symptoms and child white matter microstructures were also observed (3 for T1, 3 for T2 and 6 for T3), as shown in Table 3.
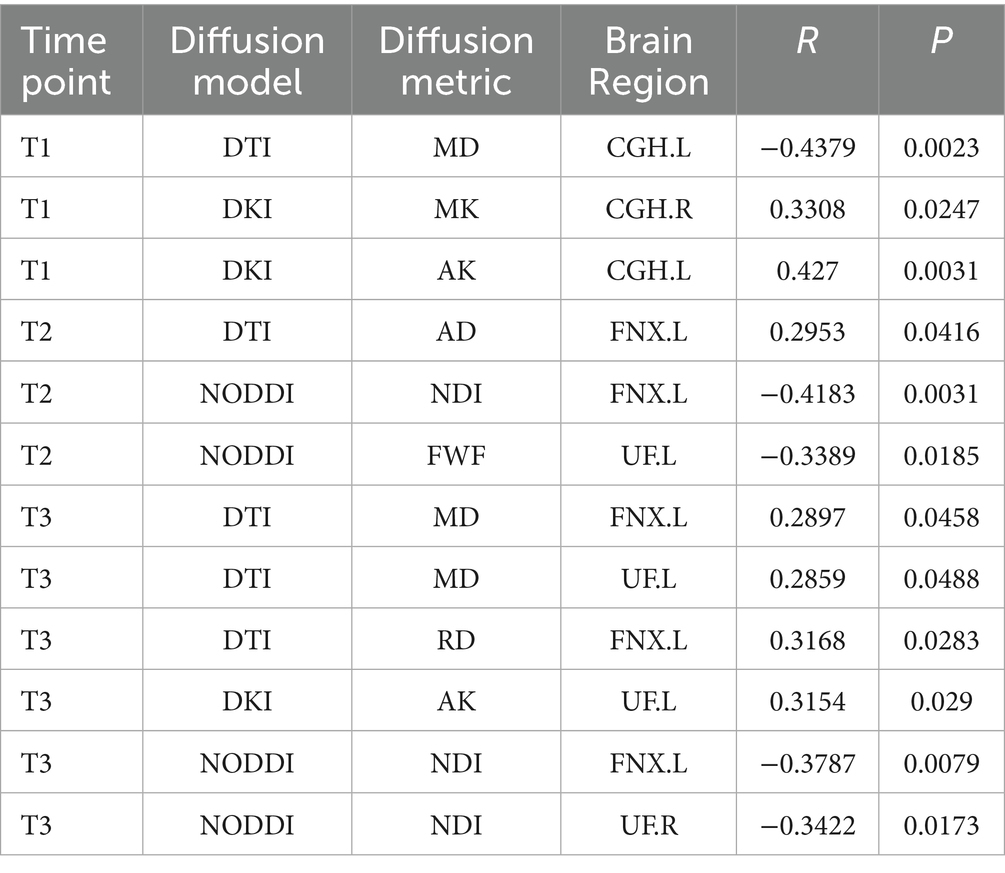
Table 3. Significant correlation between maternal anxiety symptom scores (measured by STAI) during the 3 pregnancy trimesters (T1, T2, and T3) and children brain diffusion imaging parameters at age 8 years.
3.2.2 Diffusion modality
MD and RD were significantly positively correlated with STAI scores. AK showed a significant positive correlation with STAI. NDI showed a significant negative correlation with STAI. Scatter plots illustrating these relationships are presented in Figures 6–8, respectively.
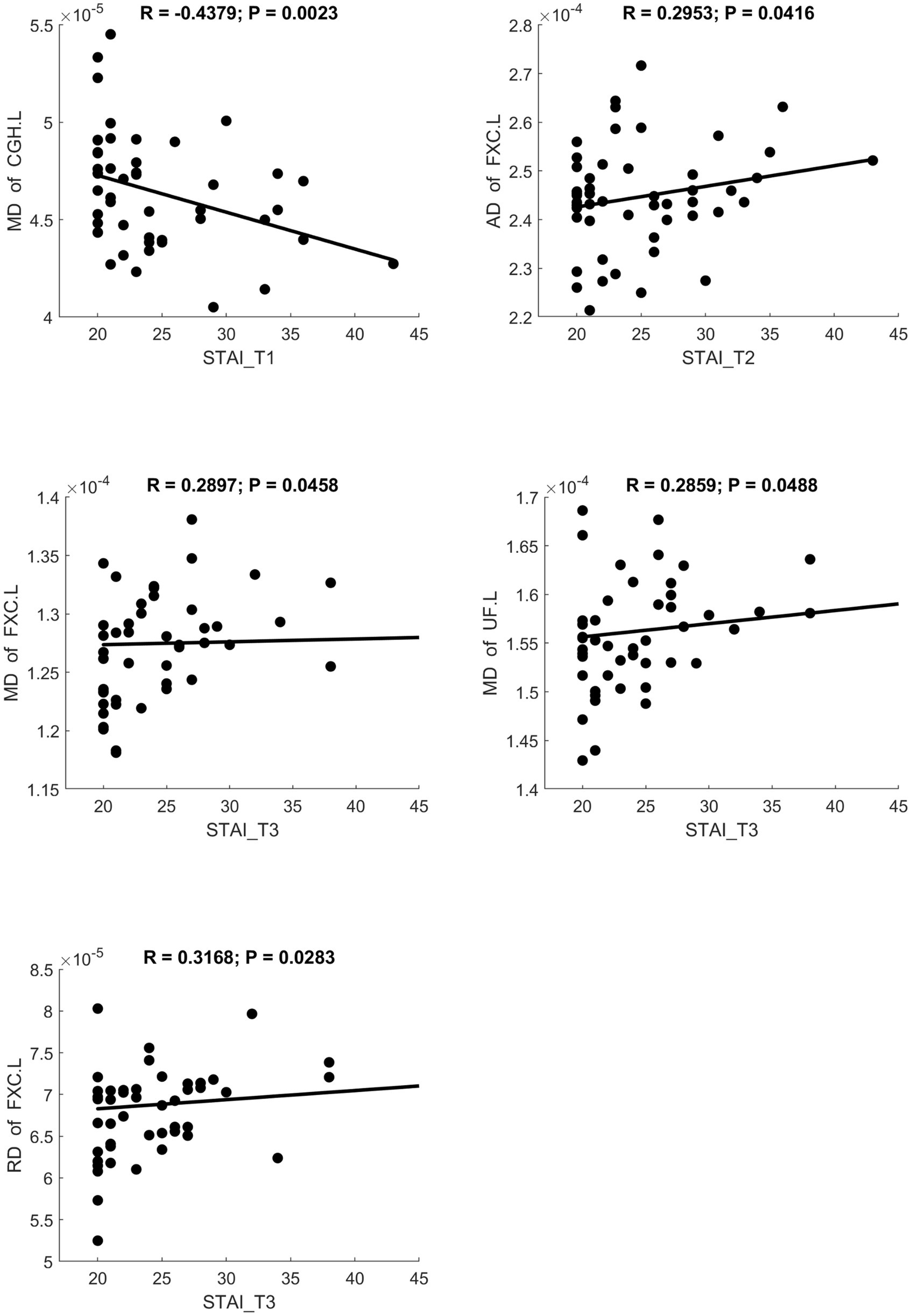
Figure 6. Significant correlation between maternal anxiety symptom scores (measured by STAI) during the 3 pregnancy trimesters (T1, T2, and T3) and children brain diffusion imaging parameters (measured by DTI) at age 8 years.
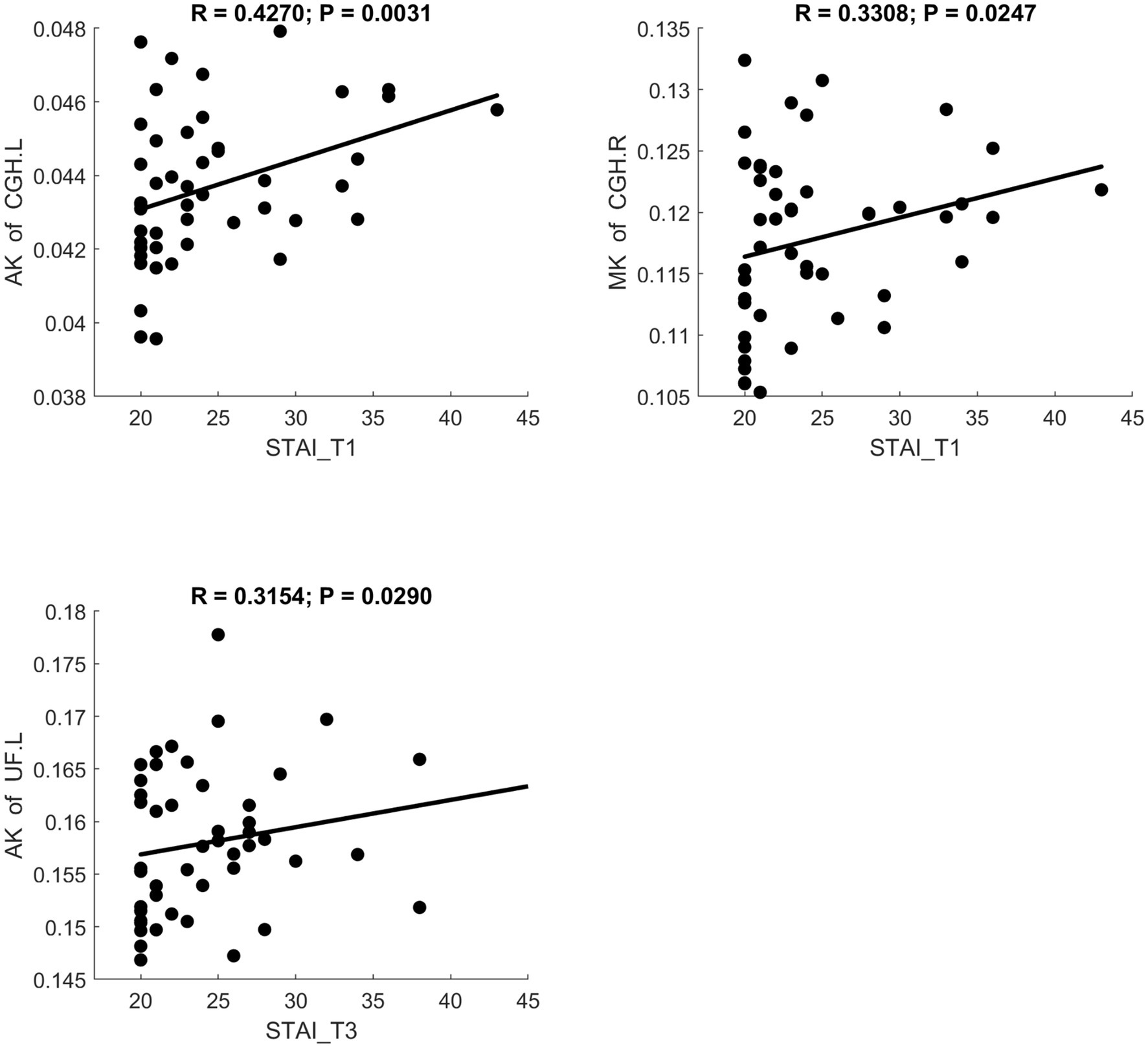
Figure 7. Significant correlation between maternal anxiety symptom scores (measured by STAI) during the 3 pregnancy trimesters (T1, T2, and T3) and children brain diffusion imaging parameters (measured by DKI) at age 8 years.
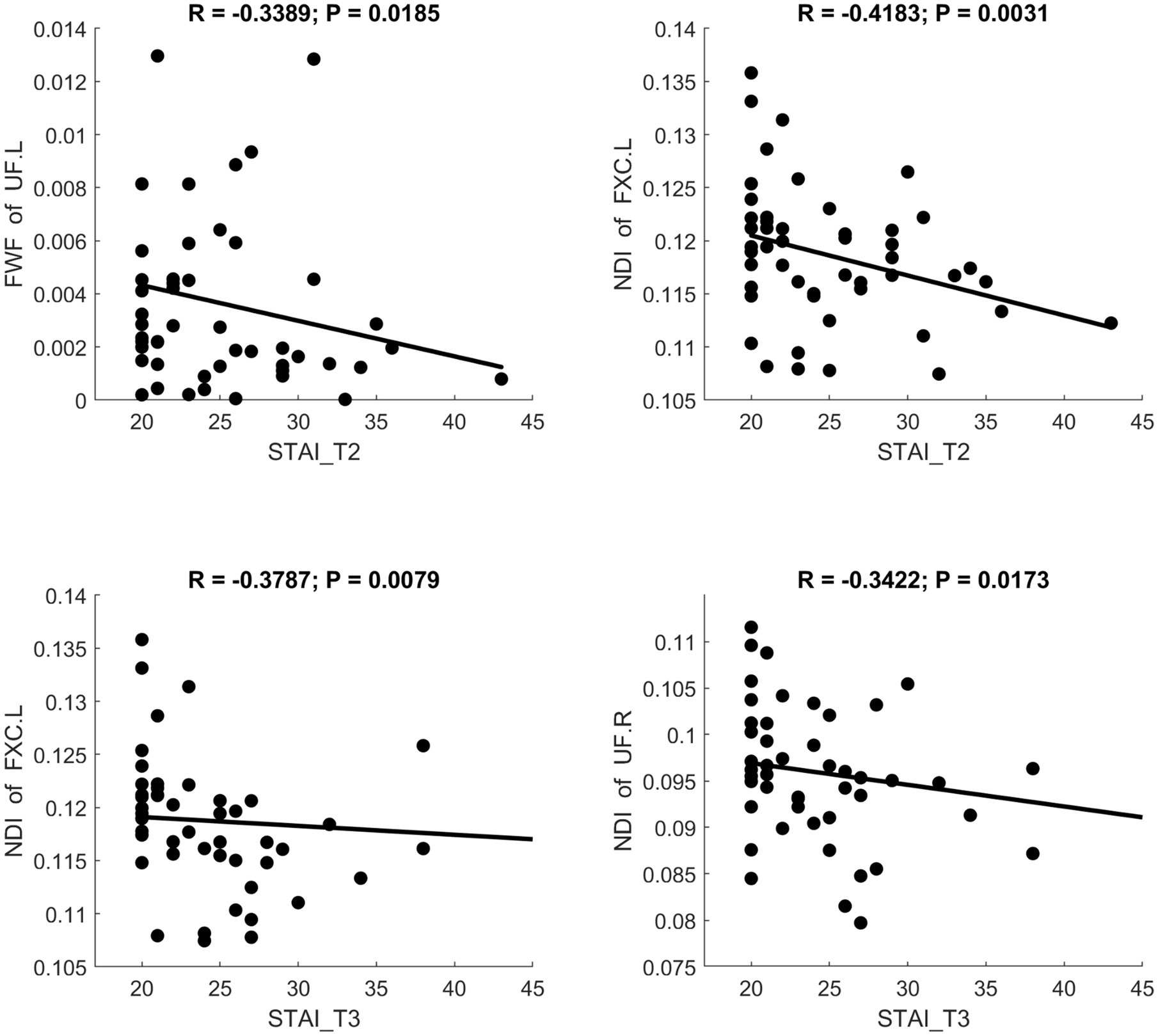
Figure 8. Significant correlation between maternal anxiety symptom scores (measured by STAI) during the 3 pregnancy trimesters (T1, T2, and T3) and children brain diffusion imaging parameters (measured by NODDI) at age 8 years.
3.2.3 Brain regions
Significant results were predominately observed in the left hemisphere white matter fiber tracts, specifically, 10 out of 12 significant relationships involved the limbic system in the left hemisphere, as shown in Table 3.
4 Discussion
This study aimed to investigate relationships between maternal mental health during pregnancy and long-term brain development of offspring. Specifically, we tracked the white matter microstructural development of 51 eight-year-old children and identified significant correlations between child brain white matter microstructural properties and their mothers’ depression (BDI) and anxiety (STAI) symptoms assessed at different gestational stage (12 weeks, 24 weeks, and 36 weeks of pregnancy, respectively). By utilizing advanced diffusion imaging and applying DTI, DKI, and NODDI models, we extracted FA, MD, AD, RD, MK, AK, RK, NDI, ODI, and FWF parameters, with a particular focus on limbic white matter regions—namely, the cingulum (both cingulate gyrus and hippocampus), fornix, and uncinate fasciculus. Overall, our results showed that maternal depression symptoms correlated predominantly with white matter microstructural integrity in the right brain, whereas maternal anxiety symptoms correlated predominantly with white matter microstructural integrity in the left brain. Our findings suggest that prenatal maternal mental health may be associated with changes in the development of fetal limbic system white matter, which may persist to later ages in childhood.
There are some similarities between our BDI/STAI and brain correlation analyses findings. Both BDI and STAI showed positive correlations with MD and RD measured by DTI. These results suggest that higher maternal psychological stress in pregnancy may reduce tissue density or levels of myelination, thereby increasing water diffusivity and compromising the organization of white matter. Both BDI and STAI also showed negative correlations with NDI measured by NODDI, indicating that maternal psychological stress may inhibit optimal neuronal growth or myelination in the developing brain and therefore impact neurite density.
There are also some interesting differences between our BDI/STAI and brain correlation analyses findings. In particular, maternal depression symptoms measured by BDI were more prominently related with right-sided limbic white matter (e.g., right cingulum and right fornix), which are crucial for emotional regulation and memory processing, as shown in Table 2. In contrast, maternal anxiety symptoms measured by STAI was more prominently related with left-sided limbic white matter, as shown in Table 3, which are crucial for language, cognitive processing of emotions, and possibly internal worry loops. Our finding that maternal depression significantly correlated with right hemisphere limbic white matter in children is consistent with literature findings reporting abnormal right hemisphere brain function involved in depression. According to Hecht, depression is often characterized by hyperactivation of the right hemisphere and relative hypoactivation of the left hemisphere, manifesting as amplified negative emotional processing, pessimistic thinking, and enhanced self-focus or rumination (Hecht, 2010). Belden et al. found that children with a smaller insula in the right hemisphere of the brain — related either to depression or excessive guilt — were more likely to have recurrent episodes of clinical depression as they got older (Belden et al., 2015). Likewise, Li et al. reported increased regional homogeneity in multiple right-hemisphere regions—such as frontal, temporal, and cingulate regions—in depressed patients compared to healthy controls, indicating a predominance of right-hemisphere involvement in depression (Li et al., 2018). Our findings of maternal anxiety correlation with left-sided limbic white matter in children echoes Blackmon et al. who found that higher subclinical anxiety levels in healthy adults were associated with smaller left amygdala volumes but increased cortical thickness in the left lateral orbitofrontal cortex and temporoparietal junction (Blackmon et al., 2011).
In addition to these lateralization patterns, our study combined DTI, DKI, and NODDI to capture multiple dimensions of white matter microstructural differences associated with maternal depression and anxiety. Numerous studies have reported the sensitivity of DTI parameters to white matter microstructural changes. Previous work by Paydar et al. has also shown that DKI metrics (e.g., MK, AK, RK) may detect subtle developmental changes from childhood into adolescence even beyond what FA reveals using DTI (Paydar et al., 2014). Meanwhile, Zhao et al. observed that NDI is especially sensitive to early changes in myelination and neurite density from birth through adolescence (Zhao et al., 2021). The combination of DTI/DKI/NODDI in the same study provided a comprehensive characterization of white matter microstructural changes in the limbic system associated with maternal depression and anxiety.
Despite the strength of longitudinal and prospective design and comprehensive characterization of maternal depression and anxiety throughout pregnancy and white matter microstructural development at age 8 years, our study has several limitations. First, the sample size is relatively small, limiting statistical power for more strict multiple comparison correction and the generalizability of results; larger cohorts should be considered in future research. Second, we only measured brain imaging data at one time point (age 8 years) after the newborn period, restricting our ability to capture potential developmental trajectories across childhood. Research MRI in younger children without sedation can be completed with high success rate and future study should implement longitudinal scans at multiple ages. Third, there are many other prenatal and postnatal factors which may also impact child white matter microstructural development and they are not included in this study as potential confounders. We cannot rule out the influence of genetic factors or postnatal environmental exposures. Maternal depression and anxiety may reflect broader psychosocial contexts, including parenting quality or ongoing maternal mental health, which were not assessed in this study. Our findings suggest correlation rather than causation, and interpretations should take into account the potential influences of both prenatal and postnatal factors. Future large-scale studies with comprehensive assessments of maternal and child mental and physical health, family environment, and lifestyle factors are warranted. Finally, although we focused on limbic white matter regions due to their known roles in emotion and memory that are impacted by mental health, many other brain networks and/or regions may potentially also show long-lasting changes associated with maternal mental health, and whole-brain analysis will be beneficial.
5 Conclusion
In summary, this study demonstrates that maternal depression and anxiety symptoms during pregnancy are significantly associated with children’s white matter microstructural development in the limbic system at age 8 years. These findings highlight the importance of prenatal mental health screening and potential interventions to promote brain development and support optimal neurodevelopmental outcomes in children.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the University of Arkansas for Medical Science’s Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YH: Conceptualization, Formal analysis, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. TK: Formal analysis, Writing – review & editing. AA: Writing – review & editing. JB: Writing – review & editing. CG: Data curation, Writing – review & editing. AR: Writing – review & editing. XO: Funding acquisition, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Agriculture Research Service of the U.S. Department of Agriculture (6026-10700-001-000D) and was partly supported by the National Institute of Health (R01 HD099099).
Acknowledgments
This work was supported by the Agriculture Research Service of the U.S. Department of Agriculture (6026-10700-001-000D) and was partly supported by the National Institute of Health (R01 HD099099).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Avants, B. B., Tustison, N., and Song, G. (2009). Advanced normalization tools (ANTS). Insight J. 2, 1–35. doi: 10.54294/uvnhin
Bano, W., Pulli, E., Cantonas, L., Sorsa, A., Hämäläinen, J., Karlsson, H., et al. (2024). Implementing ABCD studyⓇ MRI sequences for multi-site cohort studies: practical guide to necessary steps, preprocessing methods, and challenges. MethodsX 12:102789. doi: 10.1016/j.mex.2024.102789
Beck, A. T., Steer, R. A., and Brown, G. (1996). Beck depression inventory–II. United States: Psychological assessment.
Belden, A. C., Barch, D. M., Oakberg, T. J., April, L. M., Harms, M. P., Botteron, K. N., et al. (2015). Anterior insula volume and guilt: neurobehavioral markers of recurrence after early childhood major depressive disorder. JAMA Psychiatry 72, 40–48. doi: 10.1001/jamapsychiatry.2014.1604
Blackmon, K., Barr, W. B., Carlson, C., Devinsky, O., DuBois, J., Pogash, D., et al. (2011). Structural evidence for involvement of a left amygdala-orbitofrontal network in subclinical anxiety. Psychiatry Res. Neuroimaging 194, 296–303. doi: 10.1016/j.pscychresns.2011.05.007
Chou, M.-C., Ko, C.-W., Chiu, Y.-H., Chung, H. W., and Lai, P. H. (2017). Effects of B value on quantification of rapid diffusion kurtosis imaging in normal and acute ischemic brain tissues. J. Comput. Assist. Tomogr. 41, 868–876. doi: 10.1097/RCT.0000000000000621
Chuhutin, A., Hansen, B., and Jespersen, S. N. (2017). Precision and accuracy of diffusion kurtosis estimation and the influence of b-value selection. NMR Biomed. 30:e3777. doi: 10.1002/nbm.3777
Daducci, A., Canales-Rodríguez, E. J., Zhang, H., Dyrby, T. B., Alexander, D. C., and Thiran, J. P. (2015). Accelerated microstructure imaging via convex optimization (AMICO) from diffusion MRI data. NeuroImage 105, 32–44. doi: 10.1016/j.neuroimage.2014.10.026
Dean, D. C., Planalp, E. M., Wooten, W., Kecskemeti, S. R., Adluru, N., Schmidt, C. K., et al. (2018). Association of prenatal maternal depression and anxiety symptoms with infant white matter microstructure. JAMA Pediatr. 172, 973–981. doi: 10.1001/jamapediatrics.2018.2132
El Marroun, H., Zou, R., Muetzel, R. L., Jaddoe, V. W., Verhulst, F. C., White, T., et al. (2018). Prenatal exposure to maternal and paternal depressive symptoms and white matter microstructure in children. Depress. Anxiety 35, 321–329. doi: 10.1002/da.22722
Ezequiel Farrher, F.G., and Shah, N. Jon. Optimal b-value range in diffusion kurtosis imaging. In ISMRM. (2012). Proceedings of the ISMRM 2012 Annual Conference.
Fawcett, E. J., Fairbrother, N., Cox, M. L., Whte, I. R., and Fawcett, J. M. (2019). The prevalence of anxiety disorders during pregnancy and the postpartum period: a multivariate Bayesian meta-analysis. J. Clin. Psychiatry 80:1181. doi: 10.4088/JCP.18r12527
Graham, R., Jiang, L., McCorkle, G., Graham, R. M., Bellando, B. J., Sorensen, S. T., et al. (2020). Maternal anxiety and depression during late pregnancy and newborn brain white matter development. Am. J. Neuroradiol. 41, 1908–1915. doi: 10.3174/ajnr.A6759
Hecht, D. (2010). Depression and the hyperactive right-hemisphere. Neurosci. Res. 68, 77–87. doi: 10.1016/j.neures.2010.06.013
Henriques, R. N., Correia, M. M., Marrale, M., Huber, E., Kruper, J., Koudoro, S., et al. (2021). Diffusional kurtosis imaging in the diffusion imaging in python project. Front. Hum. Neurosci. 15:675433. doi: 10.3389/fnhum.2021.675433
Huang, S., Huang, C., Li, M., Zhang, H., and Liu, J. (2022). White matter abnormalities and cognitive deficit after mild traumatic brain injury: comparing DTI, DKI, and NODDI. Front. Neurol. 13:803066. doi: 10.3389/fneur.2022.803066
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Behrens, T. E. J., Woolrich, M. W., Smith, S. M., et al. (2012). FSL. NeuroImage 62, 782–790. doi: 10.1016/j.neuroimage.2011.09.015
Kochunov, P., and Hong, L. E. (2014). Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr. Bull. 40, 721–728. doi: 10.1093/schbul/sbu070
Kuo, Y. S., Yang, S. C., Chung, H. W., and Wu, W. C. (2018). Toward quantitative fast diffusion kurtosis imaging with b-values chosen in consideration of signal-to-noise ratio and model fidelity. Med. Phys. 45, 605–612. doi: 10.1002/mp.12711
Lebel, C., Walton, M., Letourneau, N., Giesbrecht, G. F., Kaplan, B. J., and Dewey, D. (2016). Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biol. Psychiatry 80, 859–868. doi: 10.1016/j.biopsych.2015.12.004
Li, M., Xu, H., and Lu, S. (2018). Neural basis of depression related to a dominant right hemisphere: a resting-state fMRI study. Behav. Neurol. 2018, 1–10. doi: 10.1155/2018/5024520
Lynch, K. M., Cabeen, R. P., Toga, A. W., and Clark, K. A. (2020). Magnitude and timing of major white matter tract maturation from infancy through adolescence with NODDI. NeuroImage 212:116672. doi: 10.1016/j.neuroimage.2020.116672
Mori, S., Oishi, K., Jiang, H., Jiang, L., Li, X., Akhter, K., et al. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 40, 570–582. doi: 10.1016/j.neuroimage.2007.12.035
Olson, D. V., Arpinar, V. E., and Muftuler, L. T. (2018). Assessing diffusion kurtosis tensor estimation methods using a digital brain phantom derived from human connectome project data. Magn. Reson. Imaging 48, 122–128. doi: 10.1016/j.mri.2017.12.026
Paydar, A., Fieremans, E., Nwankwo, J., Nwankwo, J. I., Lazar, M., Sheth, H. D., et al. (2014). Diffusional kurtosis imaging of the developing brain. AJNR Am. J. Neuroradiol. 35, 808–814. doi: 10.3174/ajnr.A3764
Peters, B. D., and Karlsgodt, K. H. (2015). White matter development in the early stages of psychosis. Schizophr. Res. 161, 61–69. doi: 10.1016/j.schres.2014.05.021
Rifkin-Graboi, A., Bai, J., Chen, H., Hameed, W. B.’., Sim, L. W., Tint, M. T., et al. (2013). Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol. Psychiatry 74, 837–844. doi: 10.1016/j.biopsych.2013.06.019
Rifkin-Graboi, A., Meaney, M. J., Chen, H., Bai, J., Hameed, W. B., Tint, M. T., et al. (2015). Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. J. Am. Acad. Child Adolesc. Psychiatry 54:e2, 313–321. doi: 10.1016/j.jaac.2015.01.013
Roos, A., Wedderburn, C. J., Fouche, J.-P., Joshi, S. H., Narr, K. L., Woods, R. P., et al. (2022). Prenatal depression exposure alters white matter integrity and neurodevelopment in early childhood. Brain Imaging Behav. 16, 1324–1336. doi: 10.1007/s11682-021-00616-3
Santos, M. A. O., Bezerra, L. S., Carvalho, A. R. M. R., and Brainer-Lima, A. M. (2018). Global hippocampal atrophy in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Trends Psychiatry Psychother. 40, 369–378. doi: 10.1590/2237-6089-2017-0130
Schmaal, L., Veltman, D. J., van Erp, T. G., Sämann, P. G., Frodl, T., Jahanshad, N., et al. (2016). Subcortical brain alterations in major depressive disorder: findings from the ENIGMA major depressive disorder working group. Mol. Psychiatry 21, 806–812. doi: 10.1038/mp.2015.69
Shi, J., Yang, S., Wang, J., Huang, S., Yao, Y., Zhang, S., et al. (2019). Detecting normal pediatric brain development with diffusional kurtosis imaging. Eur. J. Radiol. 120:108690. doi: 10.1016/j.ejrad.2019.108690
Speilberger, C. D., Gorsuch, R., Lushene, R., Vagg, P. R., and Jacobs, P. A. (1983). Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists.
Sulley, S., Adzrago, D., Mamudu, L., Odame, E. A., Atandoh, P. H., Tagoe, I., et al. (2023). Assessment of prenatal depression among US pregnant women without access to paid sick leave and regular place of care: national health interview survey of US-born and non-US-born. Prev. Med. Rep. 35:102322. doi: 10.1016/j.pmedr.2023.102322
Torrico, T. J., and Abdijadid, S. (2023). Neuroanatomy, limbic system, in StatPearls. United States: StatPearls Publishing.
Tournier, J.-D., Smith, R., Raffelt, D., Tabbara, R., Dhollander, T., Pietsch, M., et al. (2019). MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 202:116137. doi: 10.1016/j.neuroimage.2019.116137
Van Velzen, L. S., Kelly, S., Isaev, D., Aleman, A., Aftanas, L., Bauer, J., et al. (2020). White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol. Psychiatry 25, 1511–1525. doi: 10.1038/s41380-019-0477-2
Wu, Y., De Asis-Cruz, J., and Limperopoulos, C. (2024). Brain structural and functional outcomes in the offspring of women experiencing psychological distress during pregnancy. Mol. Psychiatry 29, 2223–2240. doi: 10.1038/s41380-024-02449-0
Yan, X., Zhou, M., Ying, L., Yin, D., Fan, M., Yang, G., et al. (2013). Evaluation of optimized b-value sampling schemas for diffusion kurtosis imaging with an application to stroke patient data. Comput. Med. Imaging Graph. 37, 272–280. doi: 10.1016/j.compmedimag.2013.04.007
Zhang, H., Schneider, T., Wheeler-Kingshott, C. A., and Alexander, D. C. (2012). NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61, 1000–1016. doi: 10.1016/j.neuroimage.2012.03.072
Zhao, X., Shi, J., Dai, F., Wei, L., Zhang, B., Yu, X., et al. (2021). Brain development from newborn to adolescence: evaluation by neurite orientation dispersion and density imaging. Front. Hum. Neurosci. 15:616132. doi: 10.3389/fnhum.2021.616132
Glossary
BDI-II - Beck Depression Inventory-II
STAI - State–Trait Anxiety Inventory
MRI - magnetic resonance imaging
DTI - diffusion tensor imaging
DKI - diffusion kurtosis imaging
NODDI - neurite orientation dispersion and density imaging
FA - fractional anisotropy
MD - mean diffusivity
AD - axial diffusivity
RD - radial diffusivity
MK - mean kurtosis
AK - axial kurtosis
RK - radial kurtosis
NDI - neurite density index
ODI - orientation dispersion index
FWF - free water fraction
ROI - region of interest
BMI - body mass index
MP-PCA - Marchenko-Pastur principal component analysis
EPI - echo planar imaging
TR - repetition time
TE - echo time
AMICO - accelerated microstructure imaging via convex optimization
DIPY - diffusion imaging in python
CGG - cingulum–cingulate gyrus
CGH - cingulum– hippocampus
FXC - Fornix Cres
UF - uncinate fasciculus
Keywords: prenatal maternal mental health, children’s neurodevelopment, limbic system white matter, diffusion tensor imaging (DTI), diffusion kurtosis imaging (DKI), neurite orientation dispersion and density imaging (NODDI)
Citation: Huang Y, Koscik TR, Andres A, Bellando J, Glasier CM, Ram A and Ou X (2025) Brain white matter development in 8-year-old children is associated with maternal mental health during pregnancy. Front. Hum. Neurosci. 19:1603022. doi: 10.3389/fnhum.2025.1603022
Edited by:
Song Qiao, Zhejiang Hospital, ChinaReviewed by:
Nora Vanegas-Arroyave, Baylor College of Medicine, United StatesJin Gao, Emory University, United States
Copyright © 2025 Huang, Koscik, Andres, Bellando, Glasier, Ram and Ou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiawei Ou, b3V4aWF3ZWlAdWFtcy5lZHU=
 Yali Huang
Yali Huang Timothy R. Koscik
Timothy R. Koscik Aline Andres
Aline Andres Jayne Bellando2,4
Jayne Bellando2,4 Xiawei Ou
Xiawei Ou