- 1Shaoxing People's Hospital (Shaoxing Hospital, Zhejiang University School of Medicine), Shaoxing, China
- 2The State Key Laboratory for Brain-Computer Intelligence, and the Key Laboratory for Biomedical Engineering of Ministry of Education of China, Department of Biomedical Engineering, Zhejiang University, Hangzhou, China
- 3Department of Neurosurgery, Shaoxing People's Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 4College of Mathematical Medicine, Zhejiang Normal University, Jinhua, China
- 5The Key Laboratory for Biomedical Engineering of Ministry of Education of China, Department of Biomedical Engineering and Instrument Science, Zhejiang University, Hangzhou, China
- 6The School of Electrical and Information Engineering, Zhengzhou University, Zhengzhou, China
- 7Henan Key Laboratory of Brain Science and Brain Computer Interface Technology, Zhengzhou, China
- 8The Department of Neurosurgery, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
Introduction: Mental fatigue, resulting from prolonged cognitive tasks or sleep deprivation, significantly impacts safety and performance, particularly in high-risk environments. However, effective intervention methods are limited, highlighting the urgent need for new approaches to alleviate mental fatigue. This study explores the effectiveness of Autonomous Sensory Meridian Response (ASMR) as a novel intervention for alleviating mental fatigue.
Methods: A within-subject design was employed in this work, where 28 healthy young subjects (M/F = 17/11, age = 21.82 ± 0.37 years) were requested to perform a continuous 30 min sustained attention task (named No-Break session) and a 30 min task with a 4-min mid-task ASMR break (named ASMR-Break session) at a counterbalanced order. The immediate effect and general effect of ASMR were quantitatively assessed on behavioral performance and EEG characteristics.
Results: Behaviorally, only significant immediate effect was revealed as showing in reduced reaction time. Further interrogation of brain dynamics showed complex patterns of spatio-spectrum alterations and an interaction in small-world metric in theta band. Specifically, the ASMR intervention prevented an increase in small-worldness, and the correlation between changes in small-worldness and reaction times diminished after the intervention.
Discussion: In sum, this preliminary investigation provides insight into ASMR's neural mechanisms and suggests it may help attenuate fatigue. Further research in larger, more diverse samples will be necessary to confirm its utility for mental fatigue management in real-world settings.
1 Introduction
Heuristically, mental fatigue is characterized by subjective mental exhaustion, often caused by prolonged work or insufficient sleep. This condition can lead to decreased alertness and reduced attention to tasks (Zhao et al., 2012), potentially resulting in severe consequences such as fatigue driving (Zhao et al., 2016) and low productivity in working environment (Uehli et al., 2014). In fact, 21% of global annual traffic accidents were found to be attributed to driving fatigue (Vivoli et al., 2006). In Canada, more than 50% of drivers were driving under fatigue and 20% were driving even in semi-sleep (Vingilis et al., 2005); while this ratio was 40% in China according to the China Expressway Network. To reduce these undesirable consequences, understanding the neural mechanisms underlying mental fatigue is crucial for developing effective detection and management strategies, improving work efficiency, and reducing potential hazards in high-stakes environments.
Despite extensive research on mental fatigue, studies on interventions to alleviate it remain relatively scarce. Common methods include rest (Gui et al., 2015), listening to music (Taya et al., 2018), and exercising (Xu et al., 2017). However, these methods have certain limitations. For example, although many studies have reported the effectiveness of rest (Chen et al., 2010; Blasche et al., 2018), mental fatigue does not necessarily recover after rest (Jacquet et al., 2021) and is dependent on the characteristics of the rest (i.e., duration of the rest, when to administer the rest, etc.; Tait et al., 2024; Lim and Kwok, 2016). Listening to music has long been recognized to be associated with positive emotions (McCraty et al., 1998), which may in return alleviate mental fatigue through modulating the motivation. Nevertheless, the impact of music on mental fatigue can vary based on personal preferences and the specific characteristics of the music (McCraty et al., 1998). Similarly, the effects of exercise on mental fatigue can vary widely depending on the type, intensity, and duration of both the mental and physical activities involved (Holgado et al., 2020). In fact, we previously reported that a 15-min cycling exercise failed to exhibit better recovery in comparison with rest (Gao et al., 2022). Therefore, it is particularly important to find a more effective and universally applicable intervention for mental fatigue recovery.
Autonomous sensory meridian response (ASMR) is a pleasurable, head-orientated tingling sensation that is typically in response to specific audiovisual stimuli (i.e., whispers, soft sounds, tapping, etc.; Barratt and Davis, 2015). Despite its non-scientific origins, substantial interests and apparent prevalence were gained on ASMR-related applications. Of note, the somatosensory “tingles” are associated with a reduction in heart rate and an increase in skin conductance responses (Poerio et al., 2018). More importantly, recent studies have revealed positive effect during ASMR, including a feeling of relaxation (Barratt and Davis, 2015) and alleviation of anxiety (Eid et al., 2022), indicating the potential role of ASMR as a novel intervention for mental fatigue.
Taking all the above into consideration, the effects of ASMR intervention on mental fatigue recovery were comprehensively assessed in the current work. Specifically, the work includes a within-subject design comparison of two sessions, that is, No-Break: comprising a continuous 30-min sustained attention task [i.e., Psychomotor Vigilance Task (PVT)], and ASMR-Break: consisting of two 15-min PVT tasks with a 4-min ASMR break in between. The behavior performance in terms of reaction time as well the brain dynamics [as measured in terms of power spectral density (PSD) and brain network metrics] were then quantitatively examined to provide a comprehensive assessment of the effects on fatigue recovery. To the best of our knowledge, this is the first study to explore the effect of ASMR on fatigue recovery and more importantly the underlying neural mechanisms. Based upon previous studies on ASMR (Barratt and Davis, 2015), we hypothesized that ASMR would lead to beneficial effect on mental fatigue. Moreover, based upon prior neuroimaging studies of brain activities during ASMR (Swart et al., 2022; Smith et al., 2017; Fredborg et al., 2021), we further hypothesized that ASMR would lead to complex spatio-spectral patterns as seen in both PSD and functional brain network reorganization.
2 Materials and methods
2.1 Subjects
In this study, 28 healthy university students (males/females = 17/11, age = 21.82 ± 0.37 years) were recruited from Zhejiang University. The same self-administered questionnaire was performed and the ASMR audio was played in a quiet room prior the experiment to ensure participants met the inclusion criteria: (1) no discomfort from the ASMR audio; (2) absence of chronic physical or psychosocial illnesses; (3) no history of significant somatic diseases; (4) have slept for at least 7 h the night prior to the experiment; (5) no intake of alcohol and caffeine on the day of the experiment. After being informed of the experimental protocol, subjects completed a consent form based on the Helsinki Declaration. Ethical approval for this study was obtained from the Institutional Review Board of Zhejiang University (IRB no. [2022]-47).
2.2 Experimental protocol
This study employed a within-subject design to investigate the impact of ASMR on mental fatigue recovery. Specifically, each participant completed two sessions (named No-Break and ASMR-Break session respectively) in a counterbalanced manner with a minimum interval of 5 days between the two sessions to ensure sufficient recovery from fatigue (Figure 1). A 30-min PVT was used to induce a substantial fatigue effect, as described in our previous research (Gao et al., 2020; Sun et al., 2014). In brief, participants were instructed to monitor a screen and promptly press a button upon the appearance of a red dot. The inter-trial interval varied randomly between 2 and 10 seconds (mean = 6 s). In the No-Break session, subjects were required to perform the 30-min PVT continuously without interruption. While in the ASMR-Break session, subjects underwent a 4-min ASMR intervention following the initial 15-min PVT, before proceeding to another 15-min PVT. The total duration of PVT was kept the same between two sessions. Electroencephalography (EEG) was recorded throughout each session.
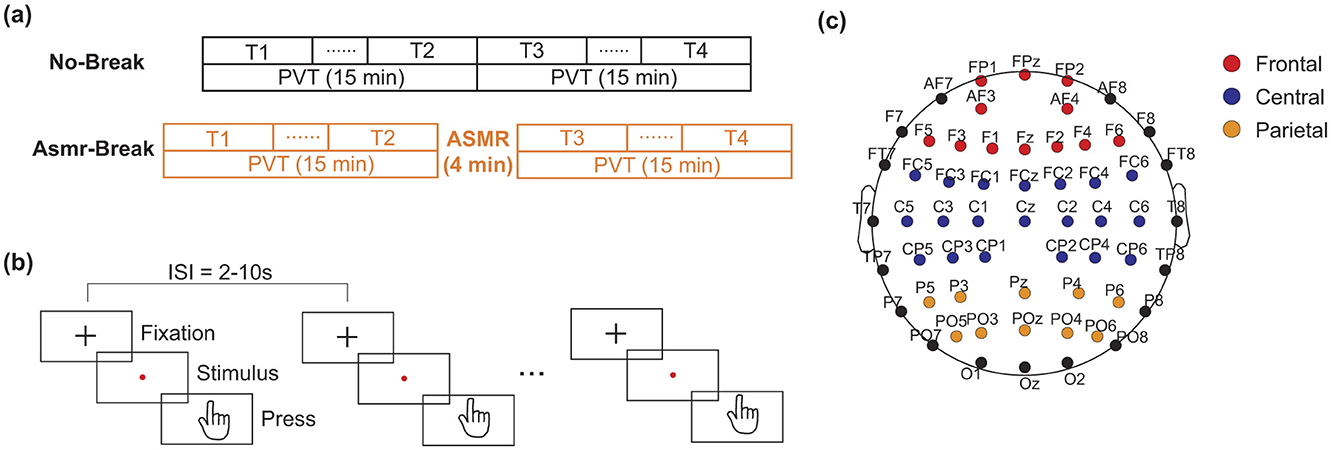
Figure 1. Schematic of the experimental paradigm. (a) The No-Break session consists of a continuous 30-min PVT), whereas the ASMR-Break session includes a 4-min Autonomous Sensory Meridian Response (ASMR) intervention inserted into the PVT. Time points T1 and T4 represent the first and last 6-min of task engagement, respectively. Time points T2 and T3 indicate the 6-min segments immediate before and after the ASMR intervention period. (b) Protocol of the PVT. (c) The assignment of electrodes in frontal, central, and parietal cortex.
In order to induce the ASMR effect, the audio content was carefully selected, which includes simulated ear-picking sounds (Sakurai et al., 2023), tapping or knocking on wood blocks (Higueras et al., 2023), and whispering by a female voice (Ohta and Inagaki, 2021). These widely-used sounds were chosen based on their effectiveness in eliciting ASMR responses, as documented in prior research (Liu and Zhou, 2019). In the current work, two ASMR audio clips (see Supplementary material for reference), each containing these similar elements, were utilized to ensure consistency and to minimize the impact of any single element on the participants. This approach was aimed at providing a robust ASMR experience while controlling for variability between clips. Both clips were considered effective in triggering ASMR responses in subjects, as validated by previous studies (Liu and Zhou, 2019). After ASMR stimulation, participants were also asked to provide a self-rating of the perceived ASMR intensity. The Short Stress State Questionnaire (SSSQ) was used to assess the mental states before and after each session. The SSSQ consists of 24 items categorized into three subjective experiences: engagement, distress, and worry.
2.3 Data acquisition and preprocessing
EEG data were collected using a 64-channel wireless EEG device (Model: NeuSen W64, Neuracle, China) with the placement according to the international 10–20 system. The reference electrodes were positioned between CPZ and CZ, while the sampling rate was set at 1,000 Hz. During data collection, the impedance of each electrode was maintained below 10 kΩ and a 50 Hz notch filter was employed to avoid main interference. During the data collection, participants were instructed to maintain comfortable sitting position to mitigate potential muscular noise in EEG.
Then a previously-validated standard EEG signal preprocessing pipeline was employed (Dimitrakopoulos et al., 2018). First, the raw data were down-sampled to 500 Hz to reduce data volume and computational load. Then, a 1–40 Hz band-pass filter was applied to remove low-frequency drift (e.g., baseline shifts from environmental or physiological factors) and high-frequency noise such as muscle artifacts. Channels with extremely poor signal-to-noise ratio—often due to loose or detached electrodes—were excluded and interpolated using spherical spline interpolation. All signals were then re-referenced to the average of all electrodes to reduce reference bias. Independent component analysis (ICA) was subsequently performed to identify and remove common artifacts, including eye blinks, muscle activity, and cardiac signals. Specifically, ocular artifacts were characterized by large-amplitude components with broad spectral range; muscle artifacts were identified as sharp, high frequency activity (typically > 25 Hz) originating from scalp muscles; and electrocardiographic artifacts were recognized by their stereotyped, high-frequency waveforms resembling ECG morphology.
The EEG data were then segmented into 6-min bins with 50% overlap, leading to each segment containing about 60 trials. Similar to our previous work (Sun et al., 2014), trials with reaction times (RTs) exceeding 500 ms were considered errors, while trials with RTs below 100 ms were considered false alarms. Thus, only trials with reaction times falling within the range of 100–500 ms were included in subsequent analysis. Furthermore, data from a time window of 500 ms following the occurrence of each stimulus were selected for power analysis and brain network construction. Data preprocessing was performed using in-house scripts and the EEGLAB toolbox in Matlab 2021a (MathWorks Inc., USA; Delorme and Makeig, 2004).
In our previous studies (Gao et al., 2020; Sun et al., 2017), we found mixed findings of the recovery effect of in-between task break. Specifically, a mid-task rest break would lead to beneficial effect toward the end of the task (Sun et al., 2017) while transit effect was revealed immediately after a mid-task exercise break (Gao et al., 2020). Therefore, we set out to comprehensively examine the recovery effect of ASMR from both transit and long-term perspectives. Specifically, data from the segments at the beginning of the task (i.e., defined as T1 hereafter) and the end of the task (defined as T4 hereafter) that were corresponding the most vigilant and fatigued state were utilized to investigate the long-term recovery effect of ASMR. Then, the segments immediately before and after the ASMR break were defined as T2 and T3 respectively to reveal the transit recovery effect. In Figure 1, we showed the schematic diagram of the experiment and the corresponding assignment of the EEG electrodes.
2.4 Power spectral density
Within each 500 ms epoch, the preprocessed EEG data was segmented using a 50% Hamming window of 250 ms and the Welch method was employed to estimate the power spectral density (PSD) in the conventional canonical frequency bands: delta (1–4 Hz), theta (4–7 Hz), alpha (8–13 Hz), and beta (13–30 Hz). Relative power within each channel was computed as the ratio of the power in a specific band to the total PSD estimated across the range of 1–30 Hz. The averaged relative power for each 6-min segment was obtained from all included epochs within T1, T2, T3, and T4. Previous research had indicated that spectral EEG activity in frontal, central, and posterior cortical regions correlate with mental fatigue (Tran et al., 2020). Therefore, in this study, all recorded electrodes were categorized into these three regions to better investigate the recovery effects of ASMR on mental fatigue. The specific electrode assignment was showed in Figure 1c.
2.5 Functional connectivity
In this work, functional connectivity was estimated using the weighted phase lag index (wPLI; Vinck et al., 2011). wPLI is an extension of the PLI (Stam et al., 2007), designed to address certain limitations (i.e., volume conduction and common sources of noise) in capturing true synchronization between brain regions. Heuristically, wPLI calculates the consistency of phase differences between EEG signals recorded from different brain regions and can be estimated as follows:
Let xj(t) represents the time series of the jth channel, then the analytic signal Xj(t) is computed as:
where denotes the Hilbert transform of xj(t) and i is the imaginary unit. Let Xk(t) denotes the analytical signal of the kth channel, the complex-valued cross-spectrum between two channels can be denoted as:
where denotes the complex conjugate of Xk(t). Then wPLI is calculated as:
where ℑ(z) denotes the imaginary component of Z, |·| and 〈·〉 represent the absolute and mean value operations respectively, and sign is the signum function. wPLI values vary between 0 and 1, i.e., a value of 0 indicates absence of phase synchronization, while a value of 1 denotes complete synchronization between two channels.
To obtain frequency-specific brain network characteristics, we band-pass filtered EEG data into four canonical frequency bands. Subsequently, for each frequency band, we computed the wPLI for all electrode pairs in each epoch ([0, 500] ms). This approach allowed us to derive an adjacency matrix of size 59 × 59 for each epoch. These adjacency matrices were then averaged across epoch for each segment (i.e., T1, T2, T3, and T4). Therefore, for each participant, there were four 59 × 59 adjacency matrices per segment.
2.6 Graph theoretical analysis
To quantitatively assess the changes induced by ASMR on the brain network reorganization, we employed graph theoretical analysis to compare the brain network properties at both global and nodal levels. Specifically, characteristic path length (L) and global efficiency (Eglob) were utilized to quantify network integration, while clustering coefficient (C) and local efficiency (Eloc) were employed to measure network segregation. Additionally, the small-worldness index (σ) was used to characterize the balance between network segregation and integration. Of note, σ is the ratio of the clustering coefficient to the path length after both metrics (C and L) have been standardized through normalizing their values by those of equivalent random networks (Humphries et al., 2006): σ = (C/Crand)/(L/Lrand). Crand and Lrand represent the mean clustering coefficient and characteristic path length of 100 matched random networks according to Maslov and Sneppen (2002). At the nodal level, nodal strength (Str) was estimated at the weighted network to reveal the nodal characteristics. Here, graph theoretical analysis was performed using the Brain Connectivity Toolbox (Rubinov and Sporns, 2010). For further details pertaining to the formulations and descriptions of these network metrics could be found in our previous work (Sun et al., 2017) or review in the field (Rubinov and Sporns, 2010).
It is noteworthy mentioning that a widely-used sparsity (i.e., the ratio between the number of existing edges to the number of maximum possible edges) approach was initially applied to remove the spurious connections. In this work, a wide sparsity range of [0.1, 0.4] with a step of 0.01 was adopted. The abovementioned global and nodal network metrics were estimated at each sparsity value. To avoid multiple comparisons at the individual sparsity threshold value and to reduce the dependency of statistical results on the arbitrary choice of a single threshold, a previously-validated integration metric was further estimated that was equivalent to the area under the curve of the network metrics (Achard and Bullmore, 2007). The integrated values were set as input for the following statistical analysis.
2.7 Statistical analysis
Prior to the statistical analysis, normality was assessed using the Shapiro–Wilk test for all variables. When normality was satisfied (p > 0.05), parametric paired-sample t-tests were conducted. If normality was not satisfied (p < 0.05), non-parametric Wilcoxon matched-pairs signed rank tests were used instead. For within-subject 2 × 2 comparisons, two-way repeated-measures ANOVAs were applied. Specifically, subjective feelings as assessed by the SSSQ scores were quantitatively compared using two-way repeated measures ANOVA with factor #1 time (i.e., before and after PVT) and factor #2 session (i.e., No-break v.s. ASMR-break). Of note, separate repeated measures ANOVA was performed for three SSSQ categories (i.e., engagement, distress, and worry). Then, power spectral intensity and brain network metrics were analyzed using two-way repeated measures ANOVA with different factors (i.e., factor #1: time, T1 vs. T4 in the general effect whereas T2 vs. T3 in the immediate effect; factor #2: session, No-break vs. ASMR-break). In addition, four paired-samples t-tests (No-break T1 vs. No-break T2; No-break T1 vs. ASMR-break T1; ASMR-break T1 vs. ASMR-break T2; No-break T2 vs. ASMR-break T2) were conducted with 95% confidence intervals. To correct for multiple comparisons in the t-test results, the false discovery rate was controlled using the Benjamini–Hochberg procedure, with a threshold of q = 0.05 applied to the resulting p-values. This correction was also applied to all node-level and connectivity-based p-values. Analyses focused on within band changes; consequently, graph metrics were not directly compared across different bands. Spearman's rank correlation coefficient was employed to evaluate the relationship between the changes of behavioral reaction times (ΔRTs) and network metrics (Δnetwork metrics). In order to limit the number of relationship assessments, only global network metrics with significant main effect was included for the association analysis. Statistical analysis for this study was conducted using SPSS software version 26 (IBM, New York), with statistical significance set at p < 0.05.
3 Results
3.1 Behavioral performance
We first examined changes in subjective feelings before and after the PVT. Significant main time effect (F1,27 = 5.250, p = 0.030, η2 = 0.163) was revealed in the Engagement. Further post-hoc analysis showed the main time effect was attributed to a significant decrease of Engagement in the No-break session (t1,27 = 2.536, pF DR = 0.030, Cohen′s d = 0.479), indicating the PVT paradigm led to successful subjective experience of mental fatigue. We then quantitatively compared the behavioral metrics (Figure 2). As for the immediate effect (T2 vs. T3), a significant main time-by-session interaction effect was revealed (F1, 27 = 6.225, p = 0.020, η2 = 0.187). In terms of the general effect (T1 vs. T4), significant main time effect (F1,27 = 52.047, p < 0.001, η2 = 0.658) and session effect (F1,27 = 4.445, p = 0.044, η2 = 0.141) were revealed. Specifically, in comparison with a monotonically increase pattern of RT in the No-break session, the ASMR related recovery revealed at T3 appeared to maintain toward the end of experiment, leading to a marginally significant decrease of RT at T4 (t1,27 = 2.172, pFDR = 0.072, Cohen′s d = 0.410).
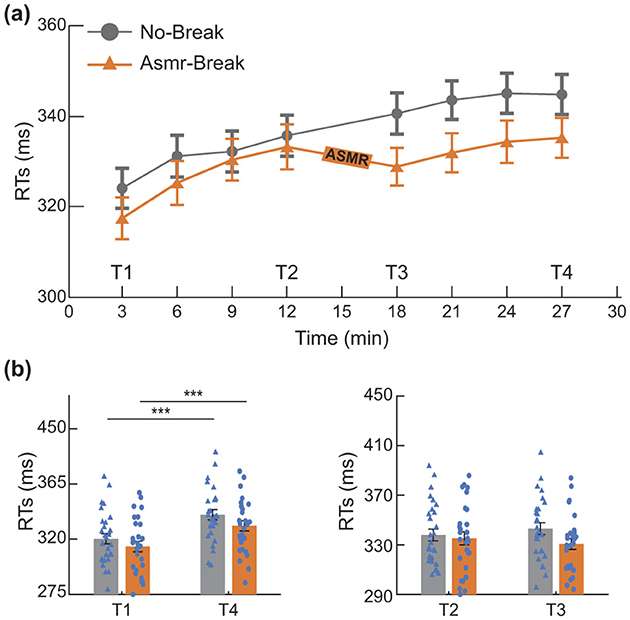
Figure 2. Behavioral performance. (a) Reaction times (RTs) during the PVT, binned into sliding 6-min segments (50% overlap) to assess performance fluctuations over time. (b) Evaluation of the general and immediate effects of the ASMR intervention. *p < 0.05; ***p < 0.001. Error bars represent the mean ± SEM.
To assess whether individual differences in ASMR sensitivity or sex influenced the behavioral outcomes, repeated-measures ANCOVAs were conducted with both self-reported ASMR intensity and sex included as covariates. For the general effect, neither the main effect of ASMR sensitivity (F1,27 = 0.081, p = 0.778, η2 = 0.003) nor sex (F1,27 = 0.107, p = 0.747, η2 = 0.004) was significant. No significant interactions involving sensitivity or sex were observed (all p > 0.1). Similarly, for the immediate effect, the main effects of ASMR sensitivity (F1,27 = 0.003, p = 0.955, η2 = 0.000) and sex (F1,27 = 0.746, p = 0.396, η2 = 0.028) were both non-significant, and all associated interactions were also non-significant (all p > 0.2). These results suggest that neither ASMR sensitivity nor sex had a measurable impact on the primary behavioral findings.
3.2 ASMR-related alterations in PSD
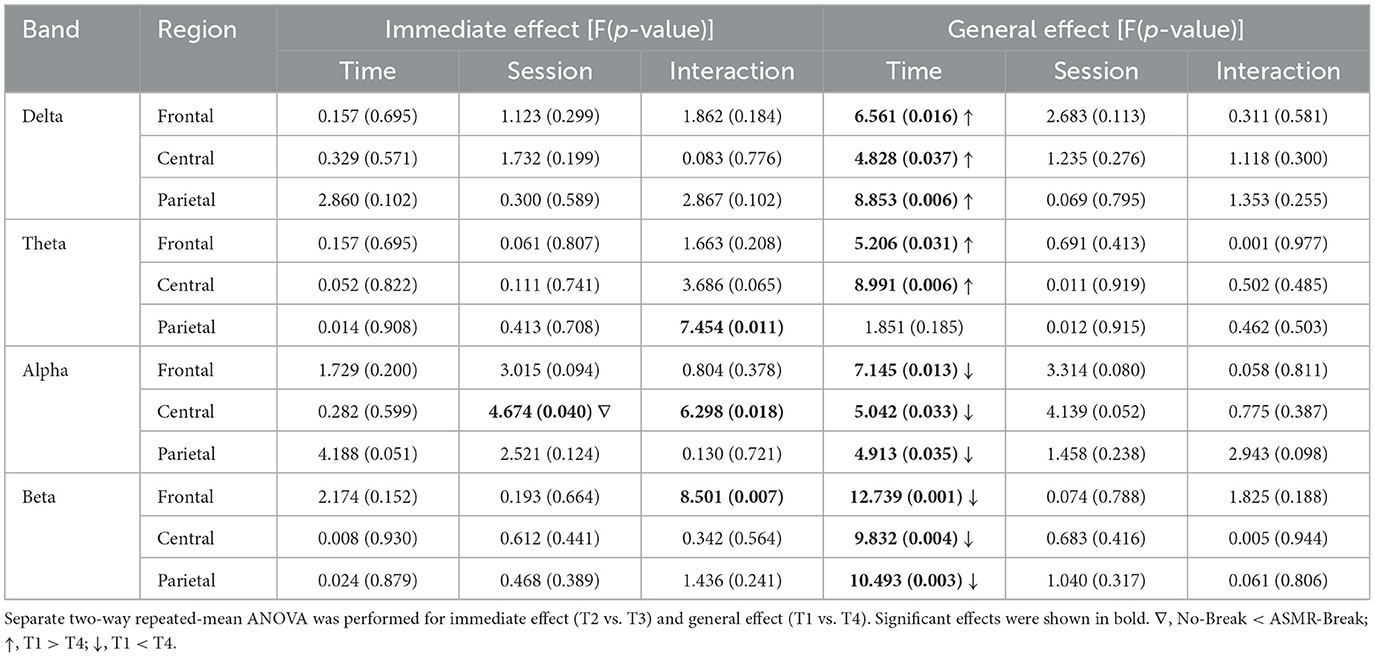
In Figure 3, we showed the ASMR-related alterations in PSD. Distinct spatio-spectral pattern was revealed for the immediate and general effect. Specifically, we found significant time-by-session interaction in one delta-band EEG channel (Figure 3a) for the immediate effect. As for the general effect, we found the EEG channels with significant interaction was in delta band (n = 3) with a left parietal region predilection (Figure 3b). We then interrogated the ASMR-related alterations in different brain regions (Table 1). In terms of the immediate effect, we found significant interaction effect in the parietal region of theta band (F1,27 = 7.454, p = 0.011, η2 = 0.216), central region of alpha band (F1,27 = 6.298, p = 0.018, η2 = 0.189), and frontal region of beta band (F1,27 = 8.501, p = 0.007, η2 = 0.239); whereas significant main session effect was only revealed in the central region of alpha band (F1,27 = 4.674, p = 0.040, η2 = 0.148). Main time effect failed to pass the significance threshold. As for the general effect, significant time effect (p < 0.05) was revealed in most of the regions across four frequency bands except the parietal regions in theta band (F1,27 = 1.851, p = 0.185, η2 = 0.064). Specifically, with the time-on-task increase, a decrease in delta and theta with an increase in alpha and beta power was observed. Comprehensive details of the post-hoc statistical analyses are provided in the Supplementary material.
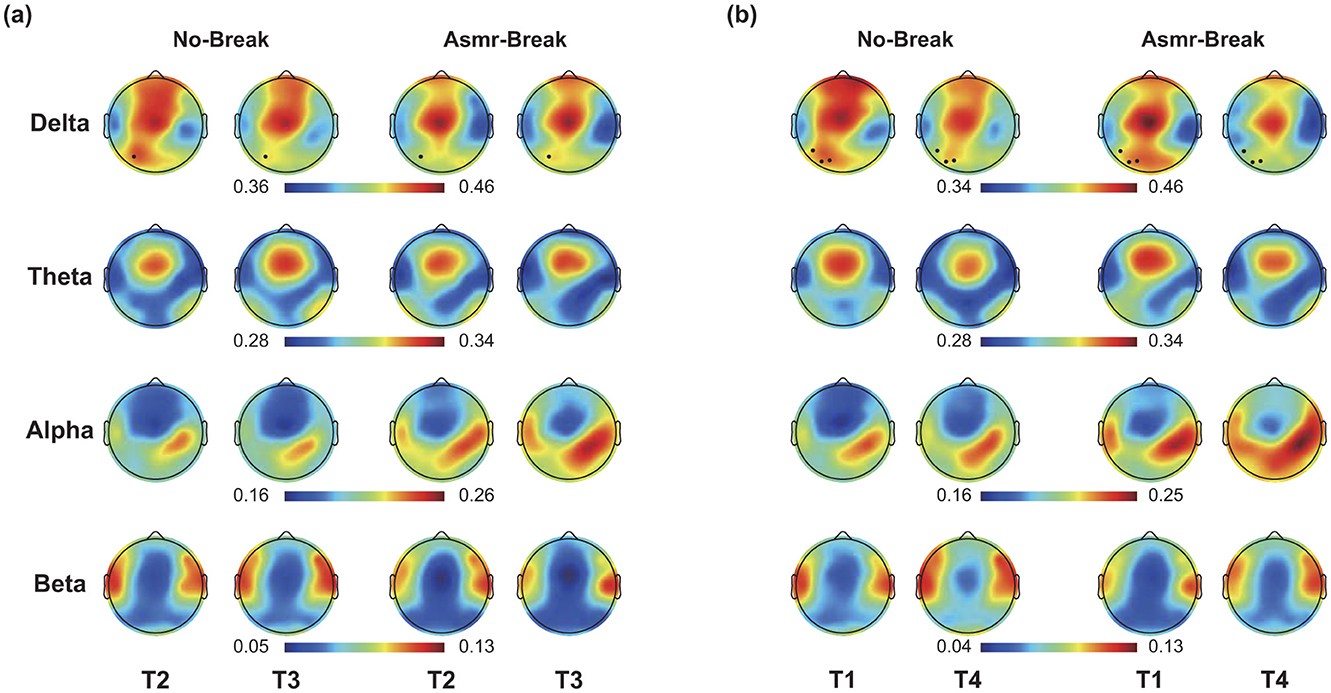
Figure 3. (a) Immediate effect (T2 vs. T3) and (b) General effect (T1 vs. T4) of ASMR on EEG PSD in four frequency bands. Black dots indicate EEG channel with a significant time-by-session interaction effect.
3.3 ASMR effect on network reorganization
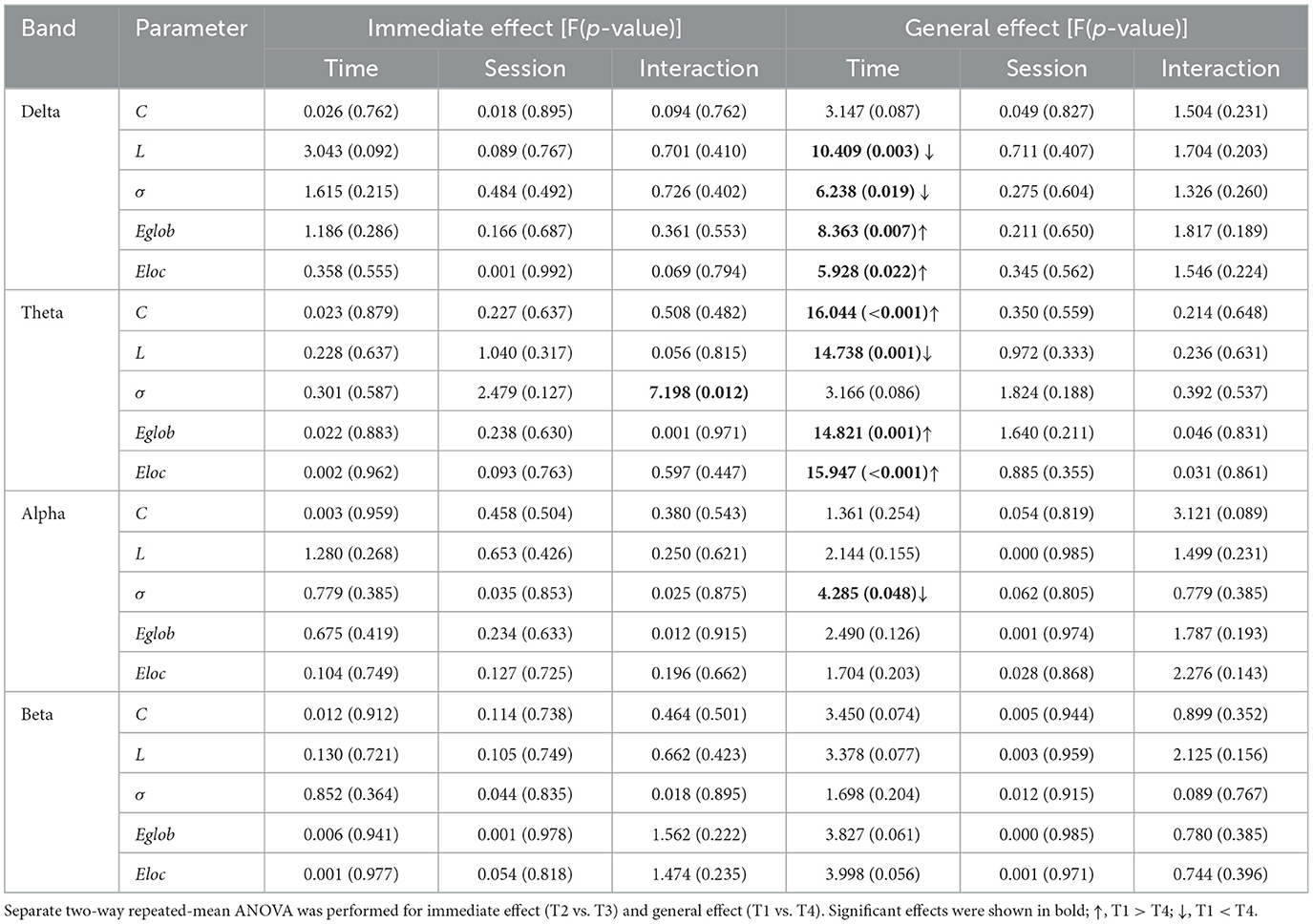
We then looked into the ASMR effect on brain network reorganization. In Table 2, we showed the statistical results of network metrics. In terms of immediate effect, only σ in theta band exhibited a significant time-by-session interaction effect (F1,27 = 7.189, p = 0.012, η2 = 0.210). Following post-hoc analysis suggested that the significant interaction effect was attributed to a significant increase of σ from T2 to T3 in the No-break session compared to a non-significant decrease in the ASMR-break session (t1,27 = 2.473, pFDR = 0.040, Cohen′s d = 0.467). Main time and session effect failed to pass the significance threshold. As for the general effect, significant time effect was observed in network metrics in delta, theta and alpha band (Table 2). Further inspection of the main time effect, we found a less optimal network topology as shown in reduced local segregation as well as global integration toward the end of task. Detailed post-hoc analyses of the statistical results could be found in Supplementary material. In Figure 4, we showed the nodal characteristics. However, no EEG channel exhibited a significant time-by-session interaction for either the immediate or general effect.
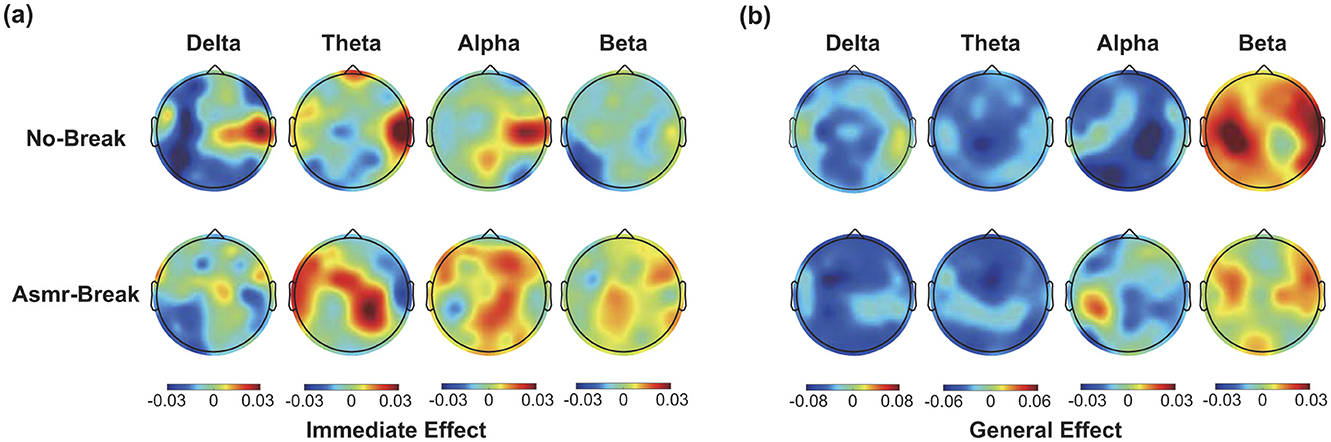
Figure 4. The spatial distribution of ΔStr (absolute differences) across four frequency bands in this figure (a) immediate effect (ΔStr = StrT3 – Str T2) and (b) general effect ΔStr = Str T4 – Str T1. Black dots indicate nodes with significant time-by-session interaction effect.
3.4 Correlation
For those global network metrics that exhibited significant main effect, only small-worldness in theta band of No–break session was significantly correlated with behavioral performance for the general effect (Figure 5). That is, Δσθ was significantly correlated with ΔRT in the No–break session (R = 0.397, p = 0.036), indicating brain network tends to become more small-world toward the end of task to compensate the behavioral deterioration. The significant correlation was absent in the ASMR – break session (R = 0.161, p = 0.410).
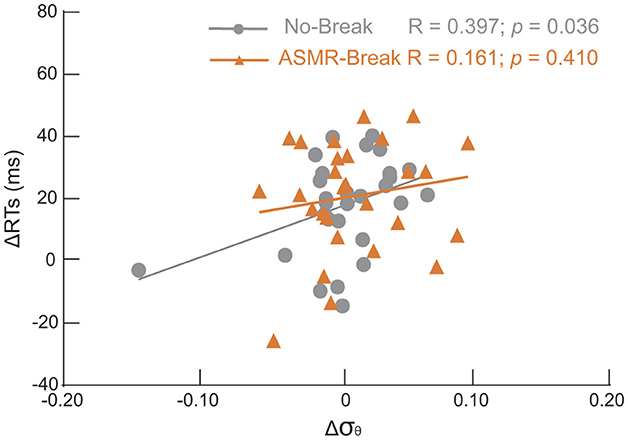
Figure 5. Scatter plots of changes of small-worldness (Δσ) in theta band between T4 and T1 and the corresponding changes of reaction time (ΔRT). Significantly positive correlation was revealed only in the No-break session (gray circles); that is the larger RT increment the higher Δσθ. Such significant association was absent in the ASMR-rest session.
4 Discussion
In this study, we examined the restorative effects of ASMR on mental fatigue and delved into the underlying neural mechanisms facilitating this recovery. Our key findings are as follows: first, ASMR demonstrated an immediate restorative effect on behavioral performance, though general beneficial effects were not observed. Second, significant EEG power alterations were noted following ASMR exposure, notably on Pθ_parietal, Pα_central, and Pβ_frontal. Particularly, a significant decrease in Pβ_frontal was observed during the ASMR-Break session. Third, a significant interaction effect was observed in the small-worldness within theta band, suggesting an immediate restorative effect of ASMR. Fourth, during the No-Break session, changes of RT were significantly positively correlated with the σ in the theta band, but this correlation was absent in the ASMR-Break session. These findings are discussed in greater detail below.
4.1 Behavioral effect of ASMR
According to the resource model theory (Boksem and Tops, 2008), prolonged tasks deplete cognitive resources, leading to the time-on-task effect, which manifests as a performance decline (Liu and Zhang, 2023). Consistent with previous fatigue studies, behavioral performance significantly deteriorated after performing continuous PVT (Dai et al., 2022). Introducing ASMR during tasks revealed significant interactions in the immediate effect analysis. As noted by Barratt and Davis (2015), ASMR induces a distinct, flow-like mental state that enhances relaxation and enriches the subjective feeling of pleasure and calmness (Inagaki and Ohta, 2022). This mental state may help reduce mental workloads. Moreover, ASMR has been reported to be associated with reduced heart rate, which is indicative of relaxation (Poerio et al., 2018). This effect is supported by other research (Lohaus et al., 2023) showing that ASMR induces a higher state of relaxation in comparison with pure rest. Consistently, we observed that the significant decrease in engagement and increase in distress typically caused by continuous PVT were mitigated following the ASMR intervention. However, we did not observe a significant interaction effect concerning the general effect. At the first blush, this may seem counterintuitive especially when significant beneficial effect was observed for the immediate effect. Of note, there have been several instances where the recuperative effect of a mid-task break on task performance has not been observed (Lim and Kwok, 2016; Sun et al., 2017). In fact, in our previous work where a 5-min mid-task rest was administered to provide recovery effect of mental fatigue (Sun et al., 2017). Similar to the current work, we failed to reveal the recuperative effect on behavioral performance. In line with these findings, Lim and colleagues showed that taking a longer break leads to higher immediate rebound in performance yet greater subsequent TOT decline, pointing an important moderator of break duration on the fatigue recovery (Lim and Kwok, 2016). The duration–effect principle also applies to ASMR: Most ASMR sensitive individuals report tingles and relaxation within the first 2–4 min of a video (Valtakari et al., 2019). Another study showed that around 3 min of ASMR viewing led to decreases in heart rate and increases in skin conductance, indicating an ASMR state. Tingle intensity and subjective relaxation ratings increased with 5–10 min of exposure compared to shorter viewing times (Engelbregt et al., 2022; Terashima et al., 2024). Additionally, longer ASMR durations significantly shorten sleep latency (Wu et al., 2024). Another key moderator is the type of break. We previously reported that a mid-task 15-min cycling exercise would lead to both immediate and general recuperative effect (Gao et al., 2020). In sum, recovery of cognitive performance following a mid-task break is not a given and is heavily relied on the characteristics of the break (including duration, types, and administration time), highlighting the need for caution in interpreting these results and more importantly in practical applications for fatigue recovery.
4.2 EEG power changes induced by ASMR
In terms of the underlying neural mechanisms, we first interrogated the spatio-spectral alterations. Immediately following the ASMR intervention, there was a clear modulation in the power spectral densities across several frequency bands. Specifically, notable EEG changes immediately following ASMR exposure included a decrease in beta power in frontal regions, along with alterations in theta and alpha bands across various cortical areas. These alterations suggest a complex interplay between ASMR stimuli and brain electrophysiological responses, impacting cognitive states and fatigue levels. Previous studies have indicated that ASMR may reduce beta activity (Inagaki and Ohta, 2022). Increased beta activity is often reported in state of mental fatigue (Craig et al., 2012), which is associated with heightened alertness (Kamiński et al., 2012) and focused attention (Palacios-García et al., 2021). And beta-gamma coupling in the frontal was observed during driving fatigue (Liu et al., 2023). Therefore, ASMR stimuli may promote a shift from states of heightened alertness to a more balanced neural oscillatory profile, thereby supporting recovery of cognitive resources. Alpha waves, typically associated with wakeful relaxation, may reflect a calm but alert state (Lombardi et al., 2023). Previous research indicates that alpha power increases in states of mental fatigue (Magnuson et al., 2021), aiding in attention maintenance by suppressing distracting inputs (Zhao et al., 2023). Recent EEG-based neurometrics have demonstrated that the alpha-based Mental Drowsiness index can reliably detect fatigue during driving in real time (Ronca et al., 2022; Giorgi et al., 2023). ASMR has been shown to trigger increased alpha wave activity (Fredborg et al., 2021), which is linked to the relaxation. Therefore, the enhancement of alpha activity due to ASMR in our study is more likely indicative of relaxation, rather than a direct response to mental fatigue or functional inhibition (Fredborg et al., 2021). Theta activity has consistently been reported as a reliable indicator of mental fatigue (Cao et al., 2014; Wascher et al., 2014; Brietzke et al., 2021), with its enhancement associated with poorer performance in tasks requiring sustained attention (Kao et al., 2013). Contrary to the traditional view that links theta activity to increased task engagement (Onton et al., 2005; Smit et al., 2005). The enhancement of theta activity contradicts the core idea that it is associated with an increasing reluctance to further engage in tasks during the process of mental fatigue (Grandjean, 1979; Tops and Boksem, 2010). Moreover, studies utilizing event-related potential (ERP) analysis methods consistently show that as task duration increases, the amplitude of theta-related ERPs declines (Guo et al., 2016; Hopstaken et al., 2015; Möckel et al., 2015). Our findings also show a significant reduction in theta as mental fatigue progresses, possibly because the time window we captured is the stimulus window. Evidence suggests that while theta activity may increase during inter-trial intervals in states of fatigue, task-related theta activity diminishes (Arnau et al., 2021). Another study indicated that theta activity in the parietal region was linked to attention (Yang et al., 2017). Similar to the alpha band, the observed reduction in theta due to ASMR may stem from its relaxing effects. As for the general effect, a systematic change is evident, typically characterized by a decrease in delta and theta power alongside an increase in alpha and beta power. These trends are consistent with prior observations that mental fatigue is accompanied by shifts in spectral power (Craig et al., 2012; Magnuson et al., 2021; Möckel et al., 2015; Yirikogullari et al., 2025). Although the behavioral analysis indicates that ASMR did not completely eliminate the accumulation of fatigue, the fatigue-related spectral changes were partially mitigated in the ASMR session. For example, the significant increase in Pβ frontal from T1 to T4 was observed only in the No-Break session, suggesting that ASMR intervention may help curb the up-regulation of beta activity that is typically associated with heightened alertness or compensatory efforts under fatigue. Overall, the EEG power dynamics alterations observed in response to ASMR interventions underscore its potential role in modulating brain function to enhance relaxation and cognitive efficiency. By increasing alpha power, ASMR seems to promote a relaxed state that aids in maintaining focus amidst mental fatigue, while decreases in beta and theta power might lessen the overall cognitive load and reduce active engagement in demanding tasks. These insights expand our understanding of ASMR's benefits beyond mere relaxation, suggesting its utility in fostering cognitive and psychological resilience in high-demand scenarios.
4.3 Network alterations induced by ASMR
In comparison with the widely-investigated spectrum alterations of mental fatigue and fatigue recovery, effects of fatigue recovery on brain network reorganization are less understood (Qi et al., 2019). Here, we reported the topological reorganizations of brain network due to ASMR modulation. In terms of immediate effects, our results indicate that ASMR significantly counteracts the increase in σ within the theta band. This immediate modulation appears to improve cognitive resilience and improve performance in tasks that require sustained attention. By stabilizing small-worldness, ASMR may help preserve the balance between local specialization and global integration within brain networks, which is essential for optimal cognitive functioning (Pisarchik et al., 2023). This finding suggests that ASMR could play a crucial role in maintaining efficient brain network dynamics under fatigue-inducing conditions. When considering the general effect, mental fatigue is marked by the significant time effect, that is, network reorganization within the delta and theta band. This reorganization is characterized by notable decreases in both global and local efficiency, alongside a significant increase in characteristic path length, together suggesting a comprehensive decline in network connectivity efficiency. Previous research has reported similar increase trend in the characteristic path length (Peng et al., 2022) as well as reduced global efficiency and increased local clustering (Giannakopoulou et al., 2023) under fatigue. Notably, although ASMR did not completely prevent these general network changes, it did disrupt the correlation between the σ and RTs. The disruption of the σ-RTs correlation further supports the potential of ASMR to mitigate the effects of fatigue, enabling individuals to sustain attention and performance levels over extended periods. These insights provide a promising approach for enhancing cognitive resilience and performance in high-demand environments.
4.4 Methodological considerations
Several issues need to be acknowledged when interpreting our findings. First, a within-subject design was employed in this work to explore the effect of ASMR for mental fatigue. Although this could be an advantage to account for the well-known inter-individual differences (Sun et al., 2014), the sample size was relatively small and primarily composed of healthy college students, which limits the generalizability of our conclusions. As our work is one of the first exploratory studies to investigate the restorative effect of ASMR on fatigue recovery, future research with larger, more diverse cohorts—including a broader age range—is needed to validate and extend our findings. Second, the regulation of ASMR exhibits significant individual differences. Although we carefully screened and selected the audio stimuli based on preliminary evaluations (Sakurai et al., 2023), individual variability in sensitivity and response to ASMR may still influence the outcomes (Poerio et al., 2022) and complicated the interpretation of our results. Investigating how neuro-diversity or other psychological factors modulate ASMR responsiveness will be a crucial step in understanding its full potential. Finally, the ASMR intervention in this study was set at a duration of 4 min, while previous research suggests that the duration of an intervention can significantly affect fatigue recovery. However, Lim and colleagues found that taking a longer break leads to greater immediate redound in performance yet greater subsequent TOT decline (Lim and Kwok, 2016) that might be attributed to motivation switch. Moreover, we have previously investigated the effect of mid-task break on fatigue recovery with various durations [i.e., 5.4 min rest break (Sun et al., 2017), 15 min exercise break (Gao et al., 2020)]. Divergent findings were revealed, highlighting the equivalent importance of both the content and length of the break on fatigue recovery. Future studies should explore various types and durations of ASMR stimulation to determine the optimal parameters for fatigue alleviation in practical applications.
5 Conclusion
In conclusion, this study explored ASMR's neural mechanisms in alleviating mental fatigue through behavioral and electrophysiological assessments. Our findings confirm that ASMR provides immediate relief from mental fatigue by interacting with the brain's networks, not just by countering fatigue directly. This research underscores ASMR's potential as a therapeutic tool in high-demand settings and sets the stage for further exploration of non-pharmacological interventions to enhance mental resilience and performance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was obtained from the Institutional Review Board of Zhejiang University, affiliated with the Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, China (IRB No. [2022]-47). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YSi: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. YSu: Conceptualization, Resources, Project administration, Funding acquisition, Writing – original draft, Writing – review & editing. KW: Investigation, Data curation, Writing – review & editing. LG: Investigation, Data curation, Writing – review & editing. SW: Investigation, Data curation, Writing – review & editing. MX: Investigation, Data curation, Writing – review & editing. XQ: Supervision, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by the Zhejiang Provincial Natural Science Foundation of China (Grant no. LTGY23H180015), in part by the Zhejiang Provincial Natural Science Foundation of China (Grant no. LR23F010003), in part by the National Natural Science Foundation of China (Grant no. 82172056), in part by the National Key Research and Development Program of China (Grant no. 2022ZD0117902), in part by the Key Research and Developmental Program of Zhejiang Province (Grant no. 2024C03042), and in part by Science and Technology Special Project of the Institute of Wenzhou, Zhejiang University (Grant no. XMGL-KJZX-202203, XMGL-CX-202401).
Acknowledgments
Authors would like to thank Yawen Wang and Yufeng Liu for their valuable contributions to the experimental design and data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2025.1619424/full#supplementary-material
References
Achard, S., and Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 3:e17. doi: 10.1371/journal.pcbi.0030017
Arnau, S., Brümmer, T., Liegel, N., and Wascher, E. (2021). Inverse effects of time-on-task in task-related and task-unrelated theta activity. Psychophysiology 58:e13805. doi: 10.1111/psyp.13805
Barratt, E. L., and Davis, N. J. (2015). Autonomous sensory meridian response (ASMR): a flow-like mental state. PeerJ 3:e851. doi: 10.7717/peerj.851
Blasche, G., Szabo, B., Wagner-Menghin, M., Ekmekcioglu, C., and Gollner, E. (2018). Comparison of rest-break interventions during a mentally demanding task. Stress Health 34, 629–638. doi: 10.1002/smi.2830
Boksem, M. A., and Tops, M. (2008). Mental fatigue: costs and benefits. Brain Res. Rev. 59, 125–139. doi: 10.1016/j.brainresrev.2008.07.001
Brietzke, C., Vinícius, Í., Franco-Alvarenga, P. E., Canestri, R., Goethel, M. F., Santos, L. E. R., et al. (2021). Proof-of-concept and test-retest reliability study of psychological and physiological variables of the mental fatigue paradigm. Int. J. Environ. Res. Public Health. 18:9532. doi: 10.3390/ijerph18189532
Cao, T., Wan, F., Wong, C. M., da Cruz, J. N., and Hu, Y. (2014). Objective evaluation of fatigue by EEG spectral analysis in steady-state visual evoked potential-based brain-computer interfaces. Biomed. Eng. Online 13, 1–13. doi: 10.1186/1475-925X-13-28
Chen, L., Sugi, T., Shirakawa, S., Zou, J., and Nakamura, M. (2010). Integrated design and evaluation system for the effect of rest breaks in sustained mental work based on neuro-physiological signals. Int. J. Control Autom. Syst. 8, 862–867. doi: 10.1007/s12555-010-0419-x
Craig, A., Tran, Y., Wijesuriya, N., and Nguyen, H. (2012). Regional brain wave activity changes associated with fatigue. Psychophysiology 49, 574–582. doi: 10.1111/j.1469-8986.2011.01329.x
Dai, J., Wang, H., Yang, L., Wang, C., Cheng, S., Zhang, T., et al. (2022). The neuroelectrophysiological and behavioral effects of transcranial direct current stimulation on executive vigilance under a continuous monotonous condition. Front. Neurosci. 16:910457. doi: 10.3389/fnins.2022.910457
Delorme, A., and Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. doi: 10.1016/j.jneumeth.2003.10.009
Dimitrakopoulos, G. N., Kakkos, I., Dai, Z., Wang, H., Sgarbas, K., Thakor, N., et al. (2018). Functional connectivity analysis of mental fatigue reveals different network topological alterations between driving and vigilance tasks. IEEE Trans. Neural Syst. Rehabil. Eng. 26, 740–749. doi: 10.1109/TNSRE.2018.2791936
Eid, C. M., Hamilton, C., and Greer, J. M. (2022). Untangling the tingle: investigating the association between the autonomous sensory meridian response (ASMR), neuroticism, and trait and state anxiety. PLoS ONE 17:e0262668. doi: 10.1371/journal.pone.0262668
Engelbregt, H. J., Brinkman, K., Van Geest, C. C. E., Irrmischer, M., and Deijen, J. B. (2022). The effects of autonomous sensory meridian response (ASMR) on mood, attention, heart rate, skin conductance and EEG in healthy young adults. Exp. Brain Res. 240, 1727–1742. doi: 10.1007/s00221-022-06377-9
Fredborg, B. K., Champagne-Jorgensen, K., Desroches, A. S., and Smith, S. D. (2021). An electroencephalographic examination of the autonomous sensory meridian response (ASMR). Conscious. Cogn. 87:103053. doi: 10.1016/j.concog.2020.103053
Gao, L., Yu, J., Zhu, L., Wang, S., Yuan, J., Li, G., et al. (2022). Dynamic reorganization of functional connectivity during post-break task reengagement. IEEE Trans. Neural Syst. Rehabil. Eng. 30, 157–166. doi: 10.1109/TNSRE.2022.3142855
Gao, L., Zhu, L., Hu, L., Hu, H., Wang, S., Bezerianos, A., et al. (2020). Mid-task physical exercise keeps your mind vigilant: evidences from behavioral performance and EEG functional connectivity. IEEE Trans. Neural Syst. Rehabil. Eng. 29, 31–40. doi: 10.1109/TNSRE.2020.3030106
Giannakopoulou, O., Kakkos, I., Dimitrakopoulos, G. N., Sun, Y., Matsopoulos, G. K., and Koutsouris, D. D. (2023). Time-dependent adaptations of brain networks in driving fatigue. Eng. Proc. 50:6. doi: 10.3390/engproc2023050006
Giorgi, A., Ronca, V., Vozzi, A., Aricò, P., Borghini, G., Capotorto, R., et al. (2023). Neurophysiological mental fatigue assessment for developing user-centered artificial intelligence as a solution for autonomous driving. Front. Neurorobot. 17:1240933. doi: 10.3389/fnbot.2023.1240933
Grandjean, E. (1979). Fatigue in industry. Occup. Environ. Med. 36, 175–186. doi: 10.1136/oem.36.3.175
Gui, D., Xu, S., Zhu, S., Fang, Z., Spaeth, A. M., Xin, Y., et al. (2015). Resting spontaneous activity in the default mode network predicts performance decline during prolonged attention workload. Neuroimage 120, 323–330. doi: 10.1016/j.neuroimage.2015.07.030
Guo, Z., Chen, R., Zhang, K., Pan, Y., and Wu, J. (2016). The impairing effect of mental fatigue on visual sustained attention under monotonous multi-object visual attention task in long durations: an event-related potential based study. PLoS ONE 11:e0163360. doi: 10.1371/journal.pone.0163360
Higueras, T. G., Peinado, A. J., Gajate, B. H., López, M. R., Arjona, L. M., and Ruzafa, M. R. (2023). Effects of autonomous sensory meridian response (ASMR) on mental health. Eur. Psychiatry 66, S963–S963. doi: 10.1192/j.eurpsy.2023.2045
Holgado, D., Sanabria, D., Perales, J. C., and Vadillo, M. A. (2020). Mental fatigue might be not so bad for exercise performance after all: a systematic review and bias-sensitive meta-analysis. J. Cogn. 3:38. doi: 10.5334/joc.126
Hopstaken, J. F., Van Der Linden, D., Bakker, A. B., and Kompier, M. A. (2015). A multifaceted investigation of the link between mental fatigue and task disengagement. Psychophysiology 52, 305–315. doi: 10.1111/psyp.12339
Humphries, M. D., Gurney, K., and Prescott, T. J. (2006). The brainstem reticular formation is a small-world, not scale-free, network. Proc. R. Soc. B Biol. Sci. 273, 503–511. doi: 10.1098/rspb.2005.3354
Inagaki, K., and Ohta, Y. (2022). Capacity of autonomous sensory meridian response on the reduction of mental stress. Int. J. Environ. Res. Public Health 19:14577. doi: 10.3390/ijerph192114577
Jacquet, T., Poulin-Charronnat, B., Bard, P., and Lepers, R. (2021). Persistence of mental fatigue on motor control. Front. Psychol. 11:588253. doi: 10.3389/fpsyg.2020.588253
Kamiński, J., Brzezicka, A., Gola, M., and Wróbel, A. (2012). Beta band oscillations engagement in human alertness process. Int. J. Psychophysiol. 85, 125–128. doi: 10.1016/j.ijpsycho.2011.11.006
Kao, S. C., Huang, C. J., and Hung, T. M. (2013). Frontal midline theta is a specific indicator of optimal attentional engagement during skilled putting performance. J. Sport Exerc. Psychol. 35, 470–478. doi: 10.1123/jsep.35.5.470
Lim, J., and Kwok, K. (2016). The effects of varying break length on attention and time on task. Hum. Factors 58, 472–481. doi: 10.1177/0018720815617395
Liu, M., and Zhou, Q. (2019). A preliminary compilation of a digital video library on triggering autonomous sensory meridian response (ASMR): a trial among 807 Chinese college students. Front. Psychol. 10:2274. doi: 10.3389/fpsyg.2019.02274
Liu, S., Wong, C. M., Liu, X., Wang, H., Bezerianos, A., Sun, Y., et al. (2023). Driving fatigue effects on cross-frequency phase synchrony embedding in multilayer brain network. IEEE Trans. Instrum. Meas. 72, 1–14. doi: 10.1109/TIM.2023.3271740
Liu, S., and Zhang, L. (2023). Theories and research advances related to self-depletion. Int. J. Clin. Exp. Med. 7:15. doi: 10.26855/ijcemr.2023.04.015
Lohaus, T., Yüksekdag, S., Bellingrath, S., and Thoma, P. (2023). The effects of autonomous sensory meridian response (ASMR) videos vs. walking tour videos on ASMR experience, positive affect and state relaxation. PLoS ONE 18:e0277990. doi: 10.1371/journal.pone.0277990
Lombardi, F., Herrmann, H. J., Parrino, L., Plenz, D., Scarpetta, S., Vaudano, A. E., et al. (2023). Beyond pulsed inhibition: alpha oscillations modulate attenuation and amplification of neural activity in the awake resting state. Cell Rep. 42:113162. doi: 10.1016/j.celrep.2023.113162
Magnuson, J. R., Doesburg, S. M., and McNeil, C. J. (2021). Development and recovery time of mental fatigue and its impact on motor function. Biol. Psychol. 161:108076. doi: 10.1016/j.biopsycho.2021.108076
Maslov, S., and Sneppen, K. (2002). Specificity and stability in topology of protein networks. Science. 296, 910–913. doi: 10.1126/science.1065103
McCraty, R., Barrios-Choplin, B., Atkinson, M., and Tomasino, D. (1998). The effects of different types of music on mood, tension, and mental clarity. Altern. Ther. Health Med. 4, 75–84.
Möckel, T., Beste, C., and Wascher, E. (2015). The effects of time on task in response selection-an ERP study of mental fatigue. Sci. Rep. 5:10113. doi: 10.1038/srep10113
Ohta, Y., and Inagaki, K. (2021). “Evaluation of the effect of ASMR on reduction of mental stress: EEG study,” in 2021 IEEE 3rd Global Conference on Life Sciences and Technologies (Institute of Electrical and Electronics Engineers), 88−89. doi: 10.1109/LifeTech52111.2021.9391945
Onton, J., Delorme, A., and Makeig, S. (2005). Frontal midline EEG dynamics during working memory. Neuroimage 27, 341–356. doi: 10.1016/j.neuroimage.2005.04.014
Palacios-García, I., Silva, J., Villena-González, M., Campos-Arteaga, G., Artigas-Vergara, C., Luarte, N., et al. (2021). Increase in beta power reflects attentional top-down modulation after psychosocial stress induction. Front. Hum. Neurosci. 15:630813. doi: 10.3389/fnhum.2021.630813
Peng, Y., Li, C., Chen, Q., Zhu, Y., and Sun, L. (2022). Functional connectivity analysis and detection of mental fatigue induced by different tasks using functional near-infrared spectroscopy. Front. Neurosci. 15:771056. doi: 10.3389/fnins.2021.771056
Pisarchik, A. N., Andreev, A. V., Kurkin, S. A., Stoyanov, D., Badarin, A. A., Paunova, R., et al. (2023). Topology switching during window thresholding fMRI-based functional networks of patients with major depressive disorder: consensus network approach. Chaos. 33:93122. doi: 10.1063/5.0166148
Poerio, G. L., Blakey, E., Hostler, T. J., and Veltri, T. (2018). More than a feeling: Autonomous sensory meridian response (ASMR) is characterized by reliable changes in affect and physiology. PLoS ONE 13:e0196645. doi: 10.1371/journal.pone.0196645
Poerio, G. L., Ueda, M., and Kondo, H. M. (2022). Similar but different: high prevalence of synesthesia in autonomous sensory meridian response (ASMR). Front. Psychol. 13:990565. doi: 10.3389/fpsyg.2022.990565
Qi, P., Ru, H., Gao, L., Zhang, X., Zhou, T., Tian, Y., et al. (2019). Neural mechanisms of mental fatigue revisited: new insights from the brain connectome. Engineering 5, 276–286. doi: 10.1016/j.eng.2018.11.025
Ronca, V., Di Flumeri, G., Vozzi, A., Giorgi, A., Aricò, P., Sciaraffa, N., et al. (2022). “Validation of an EEG-based neurometric for online monitoring and detection of mental drowsiness while driving,” in 2022 44th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (Institute of Electrical and Electronics Engineers), 3714–3717. doi: 10.1109/EMBC48229.2022.9871505
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Sakurai, N., Nagasaka, K., Takahashi, S., Kasai, S., Onishi, H., and Kodama, N. (2023). Brain function effects of autonomous sensory meridian response (ASMR) video viewing. Front. Neurosci. 17:1025745. doi: 10.3389/fnins.2023.1025745
Smit, A. S., Eling, P. A., Hopman, M. T., and Coenen, A. M. (2005). Mental and physical effort affect vigilance differently. Int. J. Psychophysiol. 57, 211–217. doi: 10.1016/j.ijpsycho.2005.02.001
Smith, S. D., Katherine Fredborg, B., and Kornelsen, J. (2017). An examination of the default mode network in individuals with autonomous sensory meridian response (ASMR). Soc. Neurosci. 12, 361–365. doi: 10.1080/17470919.2016.1188851
Stam, C. J., Nolte, G., and Daffertshofer, A. (2007). Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 28, 1178–1193. doi: 10.1002/hbm.20346
Sun, Y., Lim, J., Dai, Z., Wong, K., Taya, F., Chen, Y., et al. (2017). The effects of a mid-task break on the brain connectome in healthy participants: a resting-state functional MRI study. Neuroimage 152, 19–30. doi: 10.1016/j.neuroimage.2017.02.084
Sun, Y., Lim, J., Kwok, K., and Bezerianos, A. (2014). Functional cortical connectivity analysis of mental fatigue unmasks hemispheric asymmetry and changes in small-world networks. Brain Cogn. 85, 220–230. doi: 10.1016/j.bandc.2013.12.011
Swart, T. R., Banissy, M. J., Hein, T. P., Bruna, R., Pereda, E., and Bhattacharya, J. (2022). ASMR amplifies low frequency and reduces high frequency oscillations. Cortex 149, 85–100. doi: 10.1016/j.cortex.2022.01.004
Tait, J. L., Aisbett, B., Corrigan, S. L., Drain, J. R., and Main, L. C. (2024). Recovery of cognitive performance following multi-stressor military training. Hum. Factors 66, 389–403. doi: 10.1177/00187208221086686
Taya, F., Dimitriadis, S. I., Dragomir, A., Lim, J., Sun, Y., Wong, K. F., et al. (2018). Fronto-parietal subnetworks flexibility compensates for cognitive decline due to mental fatigue. Hum. Brain Mapp. 39, 3528–3545. doi: 10.1002/hbm.24192
Terashima, H., Tada, K., and Kondo, H. M. (2024). Predicting tingling sensations induced by autonomous sensory meridian response (ASMR) videos based on sound texture statistics: a comparison to pleasant feelings. Philos. Trans. R. Soc. B 379:20230254. doi: 10.1098/rstb.2023.0254
Tops, M., and Boksem, M. A. (2010). Absorbed in the task: personality measures predict engagement during task performance as tracked by error negativity and asymmetrical frontal activity. Cogn. Affect. Behav. Neurosci.10, 441–453. doi: 10.3758/CABN.10.4.441
Tran, Y., Craig, A., Craig, R., Chai, R., and Nguyen, H. (2020). The influence of mental fatigue on brain activity: evidence from a systematic review with meta-analyses. Psychophysiology 57:e13554. doi: 10.1111/psyp.13554
Uehli, K., Mehta, A. J., Miedinger, D., Hug, K., Schindler, C., Holsboer-Trachsler, E., et al. (2014). Sleep problems and work injuries: a systematic review and meta-analysis. Sleep Med. Rev. 18, 61–73. doi: 10.1016/j.smrv.2013.01.004
Valtakari, N. V., Hooge, I. T., Benjamins, J. S., and Keizer, A. (2019). An eye-tracking approach to autonomous sensory meridian response (ASMR): the physiology and nature of tingles in relation to the pupil. PLoS ONE 14:e0226692. doi: 10.1371/journal.pone.0226692
Vinck, M., Oostenveld, R., Van Wingerden, M., Battaglia, F., and Pennartz, C. M. (2011). An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 55, 1548–1565. doi: 10.1016/j.neuroimage.2011.01.055
Vingilis, E., McLeod, A. I., Seeley, J., Mann, R. E., Beirness, D., and Compton, C. P. (2005). Road safety impact of extended drinking hours in Ontario. Accid. Anal. Prev. 37, 549–556. doi: 10.1016/j.aap.2004.05.006
Vivoli, R., Bergomi, M., Rovesti, S., Bussetti, P., and Guaitoli, G. M. (2006). Biological and behavioral factors affecting driving safety. J. Prev. Med. Hyg. 47, 69–73.
Wascher, E., Rasch, B., Sänger, J., Hoffmann, S., Schneider, D., Rinkenauer, G., et al. (2014). Frontal theta activity reflects distinct aspects of mental fatigue. Biol. Psychol. 96, 57–65. doi: 10.1016/j.biopsycho.2013.11.010
Wu, Z., He, C., and Zhao, K. (2024). The effects of autonomous sensory meridian response (ASMR) on sleep quality improvement in adolescents. medRxiv. doi: 10.1101/2024.09.14.24312582
Xu, L., Wang, B., Xu, G., Wang, W., Liu, Z., and Li, Z. (2017). Functional connectivity analysis using fNIRS in healthy subjects during prolonged simulated driving. Neurosci. Lett. 640, 21–28. doi: 10.1016/j.neulet.2017.01.018
Yang, F. C., Jacobson, T. K., and Burwell, R. D. (2017). Single neuron activity and theta modulation in the posterior parietal cortex in a visuospatial attention task. Hippocampus 27, 263–273. doi: 10.1002/hipo.22691
Yirikogullari, H., Dalmizrak, E., and Güntekin, B. (2025). Frontocentral delta and theta oscillatory responses are sensitive to sleep deprivation during a working memory task. Clin. EEG Neurosci. doi: 10.1177/15500594251316914. [Epub ahead of print].
Zhao, C., Kong, Y., Li, D., Huang, J., Kong, L., Li, X., et al. (2023). Suppression of distracting inputs by visual-spatial cues is driven by anticipatory alpha activity. PLoS Biol. 21:e3002014. doi: 10.1371/journal.pbio.3002014
Zhao, C., Zhao, M., Liu, J., and Zheng, C. (2012). Electroencephalogram and electrocardiograph assessment of mental fatigue in a driving simulator. Accid. Anal. Prev. 45, 83–90. doi: 10.1016/j.aap.2011.11.019
Keywords: mental fatigue, autonomous sensory meridian response (ASMR), psychomotor vigilance task (PVT), mental fatigue recovery, functional brain connectivity
Citation: Si Y, Sun Y, Wu K, Gao L, Wang S, Xu M and Qi X (2025) Effects of ASMR on mental fatigue recovery revealed by EEG power and brain network analysis. Front. Hum. Neurosci. 19:1619424. doi: 10.3389/fnhum.2025.1619424
Received: 30 April 2025; Accepted: 23 June 2025;
Published: 16 July 2025.
Edited by:
Marco Bilucaglia, IULM University, ItalyReviewed by:
Ricardo Zavala-Yoé, Monterrey Institute of Technology and Higher Education (ITESM), MexicoVincenzo Ronca, Sapienza University of Rome, Italy
Copyright © 2025 Si, Sun, Wu, Gao, Wang, Xu and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuchen Qi, cWl4dWNoZW5Aemp1LmVkdS5jbg==; Yu Sun, eXVzdW5Aemp1LmVkdS5jbg==
 Yueguang Si
Yueguang Si Yu Sun
Yu Sun Kuijun Wu
Kuijun Wu Lingyun Gao6,7
Lingyun Gao6,7 Sujie Wang
Sujie Wang Xuchen Qi
Xuchen Qi