- 1Department of Neurology, Osaka Neurological Institute, Osaka, Japan
- 2Department of Pharmacology, School of Pharmacy and Pharmaceutical Sciences, Mukogawa Women’s University, Nishinomiya, Japan
- 3Center for Drug Discovery and Development Sciences, Research Organization of Science and Technology, Ritsumeikan University, Kyoto, Japan
- 4Department of Clinical Neuroscience, Institute of Biomedical Sciences, Tokushima University, Tokushima, Japan
Emerging evidence suggests that striatal striosomes play a key role in the dopaminergic regulation of motor and mental action selection processes, with impairments leading to repetitive stereotyped movements (dystonias), thoughts (obsessions), and behaviors (compulsions). To explore this hypothesis therapeutically, we investigated how idiopathic dystonia and obsessive-compulsive disorder (OCD) respond to a novel dopaminergic treatment using low-dose L-DOPA combined with chlorpromazine (CPZ), which can primarily enhance striosomal D1 dopamine receptor (D1R) signaling in humans. The therapeutic effects of L-DOPA/CPZ were assessed over 1 year in 26 idiopathic dystonia patients (mean age, 55.9 years; 23.1% male) with OCD. The daily doses of L-DOPA/carbidopa and CPZ-phenolphthalinate were increased stepwise to 50 mg and 5 mg, respectively, three times daily over an 8-weeks period, and then maintained for a year. The severity of dystonia and OCD was evaluated using the Burke-Fahn-Marsden Dystonia Movement Scale (BFMDMS) and Yale-Brown Obsessive-Compulsive Scale (Y-BOCS). At a 1-year follow-up, the BFMDMS and Y-BOCS scores improved by approximately 80% (mean difference, −13.8; 95% CI, −16.9 to −10.6; P < 0.0001) and 75% (mean difference, −16.0; 95% CI, −16.1 to −15.8; P < 0.0001), respectively, with no specific adverse effects. Thus, low-dose L-DOPA/CPZ provided striking and lasting benefits to patients with idiopathic dystonia and OCD. Our findings indicate that dystonia and OCD may share a common striatal dysfunction due to altered D1R signaling in the striosomes. Pharmacologic interventions aimed at modulating striosomal D1R signaling could enhance our understanding of the striatal mechanisms involved in the pathophysiology of both dystonia and OCD.
1 Introduction
Dystonia is a clinical syndrome characterized by involuntary, sustained, or repetitive contractions of antagonistic muscles, causing patterned movements and postures (Albanese et al., 2013). This neurological condition is among the most disabling movement disorders, though its pathophysiology is still not fully understood (Sciamanna et al., 2022). Of interest is that dystonia often occurs alongside several neuropsychiatric symptoms (Cavallaro et al., 2002; Voon et al., 2010; Barahona-Corrêa et al., 2011; Timmers et al., 2019). Most notably, idiopathic dystonia (Cavallaro et al., 2002; Voon et al., 2010; Barahona-Corrêa et al., 2011) and SGCE myoclonus-dystonia (Timmers et al., 2019) coexist with obsessive-compulsive disorder (OCD), although no association with DYT1 dystonia has been observed (Heiman et al., 2007). OCD is a highly prevalent neuropsychiatric condition characterized by repetitive and stereotyped thoughts, urges, or images (known as obsessions), along with repetitive, stereotyped behaviors and mental acts (known as compulsions) (American Psychiatric Association [APA], 2013). Prevailing evidence indicates that both dystonia and OCD occur due to disrupted interactions between the frontal cortex and basal ganglia (Hollunder et al., 2024). However, it remains ambiguous as to which anatomical substrates within the cortico-basal ganglia circuits contribute to the overlapping symptomatic characteristics associated with deficits in movement and behavioral inhibition in conditions such as dystonia and OCD. Multimodal therapeutic strategies have been employed in the treatment of these often debilitating conditions, yielding a diverse range of outcomes.
Accruing evidence suggests that imbalances in the activity between D1 and D2-type dopamine receptors (D1Rs and D2Rs) within the striatal striosome-matrix system contribute to the development of various motor and cognitive disorders of corticobasal ganglia origin, including dystonia and OCD (Amemori et al., 2011; Crittenden and Graybiel, 2011; Graybiel and Matsushima, 2023; Goto, 2025). A long-standing hypothesis highlights the critical role of striatal striosomes in the dopaminergic regulation of the mechanisms that facilitates the selection of specific motor and mental actions (for a comprehensive review, see Ref. Graybiel and Matsushima, 2023). This implies that altered dopamine processing in the striosomes may lead to repetitive stereotyped movements (dystonias), thoughts (obsessions), and behaviors (compulsions) (Amemori et al., 2011; Graybiel and Matsushima, 2023). To support this hypothesis within a therapeutic framework, we here present evidence of a significant and durable response of idiopathic dystonia and OCD to a novel dopaminergic treatment using L-DOPA (a dopamine precursor) combined with chlorpromazine (CPZ, a D2 antagonist), which can enhance D1R signaling primarily in the striatal striosomes in humans (Matsumoto et al., 2022, 2024; Matsumoto and Goto, 2024; Goto, 2025).
2 Subjects and methods
2.1 Ethics statement
This study received approval from the Institutional Ethics Committee at the Osaka Neurological Institute (reference number: OR04-3), and written informed consent was obtained from all participants involved in the research. Furthermore, it is registered with the UMIN Clinical Trials Registry (UMIN000027430), which is recognized by the International Committee of Medical Journal Editors.
2.2 Patient sorting, clinical evaluations, and drug administration
The study design is illustrated in Figure 1. This study enrolled patients diagnosed with both idiopathic dystonia and OCD. The diagnosis of idiopathic dystonia was based on the criteria established by Albanese et al. (2013). The severity of dystonia was evaluated using the Burke-Fahn-Marsden Dystonia Movement Scale (BFMDMS) (Burke et al., 1985). The participants exhibited dystonia symptoms affecting various parts of their bodies (Supplementary Tables 1, 2). Genetic tests and brain MRIs were conducted to exclude hereditary and secondary dystonias. All participants tested negative for disease-causing variations in “currently” known dystonia genes identified by whole-exome sequencing (OMIM Phenotypic Series PS128100).1 The patients identified as having idiopathic dystonia were also assessed using the clinician-rated Yale-Brown Obsessive-Compulsive Scale (Y-BOCS), (Goodman et al., 1989) with 10-item scale where each item is rated from 0 to 40. A score of 0–7 is considered subclinical, 8–15 is mild, 16–23 is moderate, 24–31 is severe, and 32–40 is extreme (Goodman et al., 1989). The subtypes of OC symptoms, as categorized by the Y-BOCS Symptom Checklist, are presented in Supplementary Tables 3, 4. Throughout the studies described below, the participants’ concurrent medications, except for L-DOPA and CPZ, remained unchanged (Supplementary Tables 1, 2). Clinical evaluations and video recordings were conducted 1 day before (baseline), at 4 and 8 weeks, and 1 year after initiating the treatment with L-DOPA and/or CPZ.
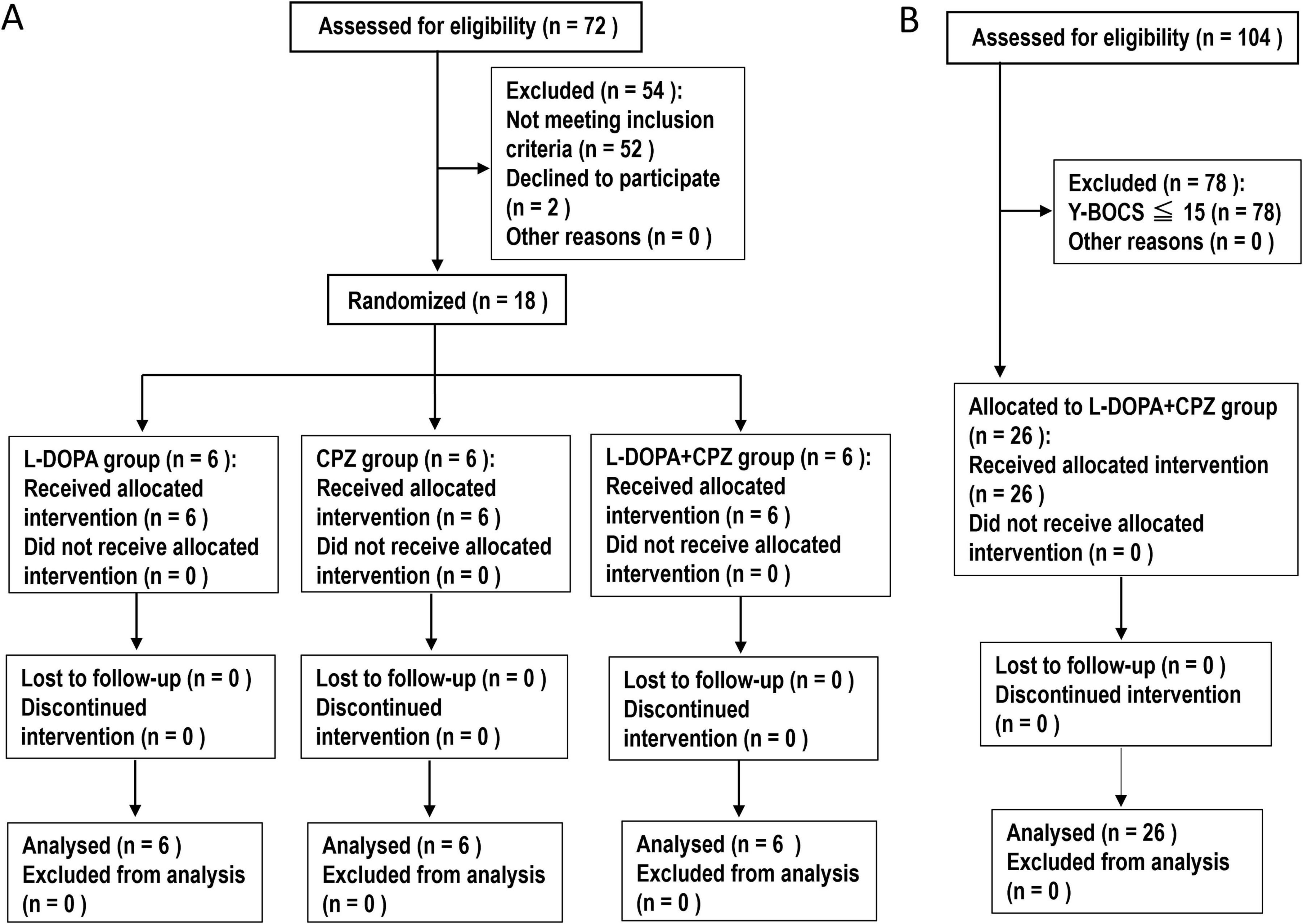
Figure 1. Study Design. (A) An 8-weeks follow-up study. This study involved the enrollment of patients with idiopathic dystonia and OCD. A total of 18 participants were randomly assigned by S. M. and categorized into three distinct groups: L-DOPA (n = 6), chlorpromazine (CPZ) (n = 6), and a combined L-DOPA + CPZ group (n = 6). Over an 8-weeks period, the daily doses of L-DOPA/carbidopa and/or CPZ-phenolphthalinate were systematically increased, reaching a maximum of 50 mg administered three times daily and/or 5 mg administered three times daily, respectively. The study groups consisted of participants who received L-DOPA/carbidopa alone, CPZ-phenolphthalinate alone, and a combination treatment of L-DOPA/carbidopa alongside CPZ-phenolphthalinate. Clinical evaluations and video recordings were conducted at baseline (1 day before treatment initiation) and subsequently at 4 weeks and 8 weeks post-treatment commencement. (B) A 1-year follow-up study. This study involved a cohort of 26 patients with idiopathic dystonia and obsessive-compulsive disorder (OCD), as indicated by a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score of 16 or higher. The administration of L-DOPA/carbidopa in conjunction with CPZ-phenolphthalinate was initiated at low dosages and subsequently adjusted in a methodical manner over an 8-weeks period, ultimately achieving doses of 50 mg administered three times daily and 5 mg administered three times daily, respectively. Following this titration phase, the medication dosages were maintained at a constant level until the study’s conclusion, which included a 1-year follow-up period. Clinical assessments were conducted at baseline, as well as at 4 weeks, 8 weeks, and 1 year following the initiation of treatment with L-DOPA/CPZ.
2.2.1 An 8-weeks follow-up study
In the order of visits, 18 participants were randomly assigned (S. M.) and divided into three distinct groups: L-DOPA, CPZ, and L-DOPA + CPZ groups, as illustrated in Figure 1 and detailed in Supplementary Table 1. In a single-blind study design, participants were not informed of their group assignments. The administration of the drugs was conducted in accordance with the methods that we previously reported (Matsumoto et al., 2022, 2024; Matsumoto and Goto, 2024). Dopacol tablets L50™, containing L-DOPA (50 mg) and carbidopa (5 mg) (Nichi-Iko Pharmaceutical Co., Toyama, Japan), as well as Wintermin™ fine granules (10%; 180 mg of CPZ-phenolphthalinate per gram) (Shionogi Co., Osaka, Japan), were used.
The L-DOPA group comprised 6 participants who received L-DOPA/carbidopa (50 mg/day) for the first 2 weeks, followed by L-DOPA/carbidopa (50 mg twice daily) for the subsequent 2 weeks, and L-DOPA/carbidopa (50 mg three times daily) for the final 4 weeks. The CPZ group consisted of 6 participants, who ingested CPZ-phenolphthalinate (5 mg/day) for the first 2 weeks, followed by CPZ-phenolphthalinate (5 mg twice daily) for the next 2 weeks, and ultimately, CPZ-phenolphthalinate (5 mg three times daily) for the final 4 weeks. The L-DOPA + CPZ group included 6 participants who received L-DOPA/carbidopa (50 mg/day) in conjunction with CPZ-phenolphthalinate (5 mg/day) for the initial 2 weeks, L-DOPA/carbidopa (50 mg twice daily) alongside CPZ-phenolphthalinate (5 mg twice daily) for the following 2 weeks, and L-DOPA/carbidopa (50 mg three times daily) combined with CPZ-phenolphthalinate (5 mg three times daily) over the final 4 weeks.
2.2.2 A one-year follow-up study
This study enrolled a total of 26 patients with idiopathic dystonia and OCD (Y-BOCS ≥ 16) (see Figure 1 and Supplementary Table 2). As outlined in the protocol for the 8-weeks follow-up study, the daily doses of L-DOPA/carbidopa and CPZ-phenolphthalinate were incrementally increased to 150 mg and 15 mg, respectively, over the 8-weeks period. Following this, the medication dosages remained constant throughout the study, which included a 1-year follow-up period.
2.3 Statistical analyses
All values are presented as means ± standard deviation (SD). Non-parametric statistical analyses were conducted using the Friedman test, followed by Dunn’s multiple comparison test for further evaluation. A significance level of P < 0.05 was set for this study. The analyses were performed using GraphPad Prism version 7.0, developed by GraphPad Software, located in San Diego, California, USA.
3 Results
3.1 Low-dose L-DOPA and CPZ exert therapeutic effects synergistically
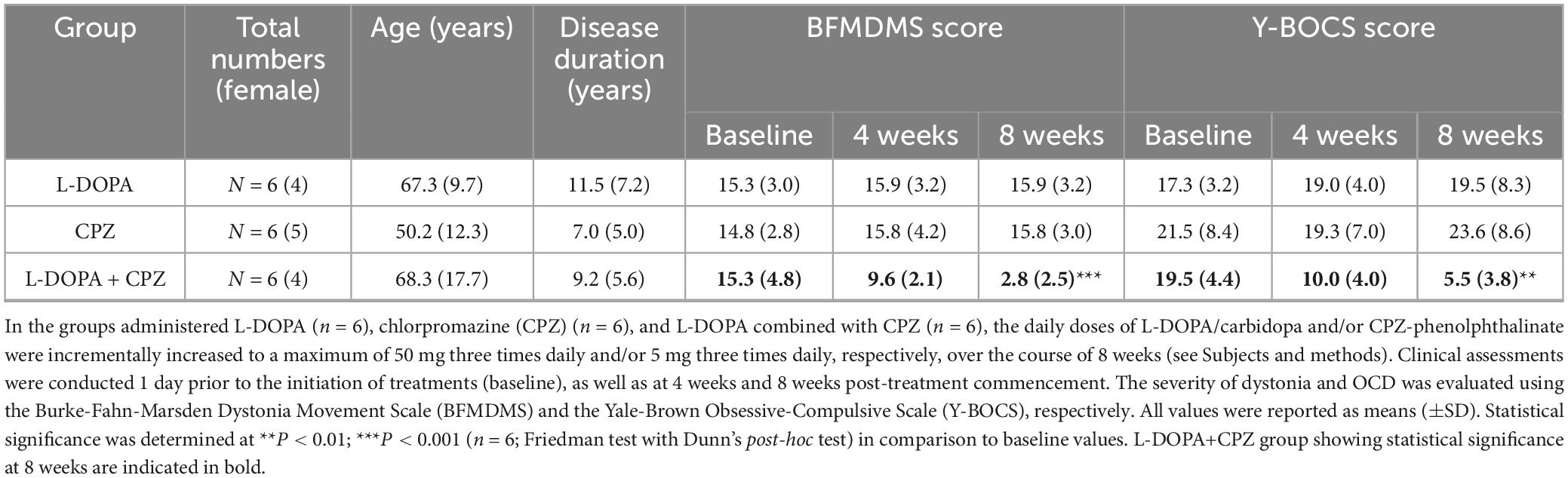
The therapeutic effects of L-DOPA + CPZ, L-DOPA alone, or CPZ alone were assessed over 8 weeks in 18 patients with idiopathic dystonia and OCD, with a mean age of 61.9 years (SD 15.4) and a mean disease duration of 9.2 years (SD 6.0) (see Table 1).
At an 8-weeks follow-up, the combined administration of L-DOPA/carbidopa (50 mg three times daily) with CPZ-phenolphthalinate (5 mg three times daily) resulted in significant improvements in both the BFMDMS and Y-BOCS scores, with reductions of approximately 80% (n = 6; mean difference, −12.5; 95% CI, −15.0 to −10.1; P = 0.0016) and 70% (n = 6; mean difference, −14.0; 95% CI, −14.5 to −13.4; P = 0.0016), respectively, when compared to baseline levels. In contrast, the administration of L-DOPA/carbidopa (50 mg three times daily) alone or CPZ-phenolphthalinate (5 mg three times daily) alone did not yield any significant changes in the BFMDMS or Y-BOCS scores from baseline in either the L-DOPA group (n = 6) or the CPZ group (n = 6).
Thus, medication with low doses of L-DOPA and CPZ is superior to either one alone, suggesting that low-dose L-DOPA and CPZ synergistically act to produce effective therapeutic results in treating idiopathic dystonia and OCD. This notion corroborates our previous reports on patients with idiopathic blepharospasm (Matsumoto et al., 2022) and cervical dystonia (Matsumoto et al., 2024).
3.2 Low-dose L-DOPA/CPZ exerts long-lasting therapeutic effects
The therapeutic effects of L-DOPA/CPZ were assessed over 1 year in 26 patients (23.1% male) with idiopathic dystonia and OCD (Y-BOCS ≥ 16), with a mean age of 55.9 years (SD 16.1) and a mean disease duration of 8.6 years (SD 6.9) (see Table 2).
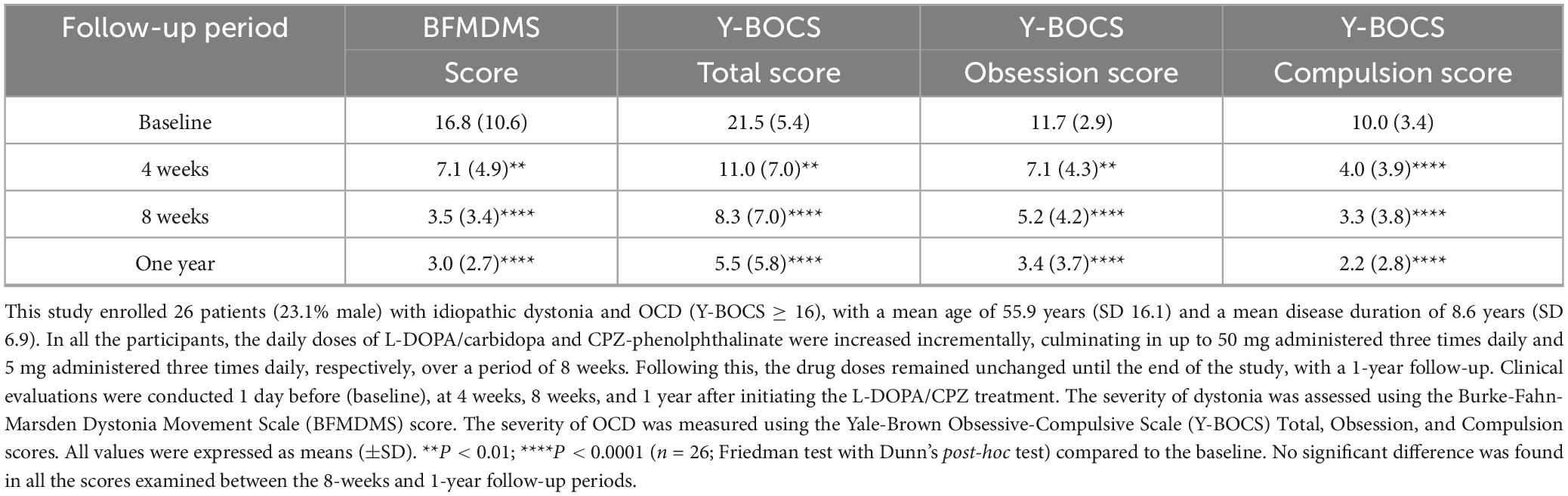
Table 2. A 1-year follow-up study on the therapeutic effects of low-dose L-DOPA combined with chlorpromazine (CPZ) in patients with idiopathic dystonia and OCD.
The administration of L-DOPA/CPZ resulted in a significant enhancement of the BFMDMS and Y-BOCS scores among all 26 participants involved in the study. At the 1-year follow-up, utilizing daily doses of L-DOPA/carbidopa (50 mg three times daily) and CPZ-phenolphthalein (5 mg three times daily), the BFMDMS score showed an improvement of approximately 80% (mean difference, −13.8; 95% CI, −16.9 to −10.6; P < 0.0001) in comparison to baseline measurements. Concurrently, the Y-BOCS total, obsession, and compulsion scores, improved by approximately 75% (mean difference, −16.0; 95% CI, −15.8 to −16.1; P < 0.0001), 70% (mean difference, −8.5; 95% CI, −8.7 to −8.1; P < 0.0001), and 80% (mean difference, −7.8; 95% CI, −8.0 to −7.6; P < 0.0001), respectively. Importantly, no significant differences were observed in the scores evaluated between the 8-weeks and 1-year follow-up periods. It is plausible that the symptoms of dystonia and OCD were primarily mitigated during the initial 8-weeks treatment phase.
Thus, low-dose L-DOPA/CPZ could exert a highly effective and lasting therapeutic impact on idiopathic dystonia (for example, see Supplementary Videos 1–5) and OCD. During this study, none of the participants reported any specific adverse problems, including gastrointestinal issues.
4 Discussion
There is a consensus that alterations in the network activity of the cortico-striato-thalamo-cortical (CSTC) loops play a significant role in the pathogenesis of both dystonia and OCD (Alexander et al., 1986; Hollunder et al., 2024). This study seeks to elucidate the mechanisms underlying the overlapping repetitive and stereotyped symptoms associated with the motor and mental impairments inherent to dystonia and OCD, respectively. We present evidence indicating that low-dose L-DOPA/CPZ can provide significant and enduring therapeutic benefits for individuals with idiopathic dystonia and OCD. Since L-DOPA acts as a D1/D2 agonist and CPZ functions as a D2 antagonist, our findings suggest that targeting D1R signaling within the CSTC circuits may offer a promising approach for enhancing the understanding of the pathophysiology of both conditions.
4.1 The role of striatal (striosomal) dopamine signaling in the CSTC circuits
Striatal dopamine processing, mediated by D1Rs and D2Rs, is closely linked to a wide range of motor and mental processes, particularly in the context of reinforcement learning (RL) and decision-making (Graybiel and Matsushima, 2023). D1R activation promotes goal-directed behaviors and rewards salience, while D2R stimulation inhibits specific responses and regulates stimulus incentive value (Graybiel and Matsushima, 2023). A balanced interaction between D1Rs and D2Rs is essential for optimizing motor control and cognitive flexibility, facilitating motor and behavioral adaptation informed by prior experiences and anticipated outcomes (Graybiel and Matsushima, 2023). It has significant implications for maladaptive movement and behaviors observed in dystonia and OCD. Interestingly, a recent study in humans has shown that a low dose of L-DOPA or haloperidol (a D2 antagonist) can regulate decision thresholds in RL, thereby contributing to the mechanisms underlying the selection of specific mental actions (Chakroun et al., 2023).
Mesostriatal dopaminergic inputs are principally integrated by medium spiny neurons (MSNs) expressing D1Rs and D2Rs (D1-MSNs and D2-MSNs), which constitute the vast majority of striatal neurons and form the major striatal efferent system integral to the CSTC loops (Crittenden and Graybiel, 2011; Graybiel and Matsushima, 2023). Within the parallel interacting CSTC loops, the putamen participates in the sensorimotor circuit, while the caudate nucleus involves the cognitive circuit (Alexander et al., 1986). Hence, dystonia and OCD may occur due to alterations in dopamine processing in the putamen and caudate nucleus, respectively (see Figure 2A). The striosomes may function as a key regulator of dopamine processing in both the dystonia-associated and OCD-associated CSTC circuits at the striatal level in humans.
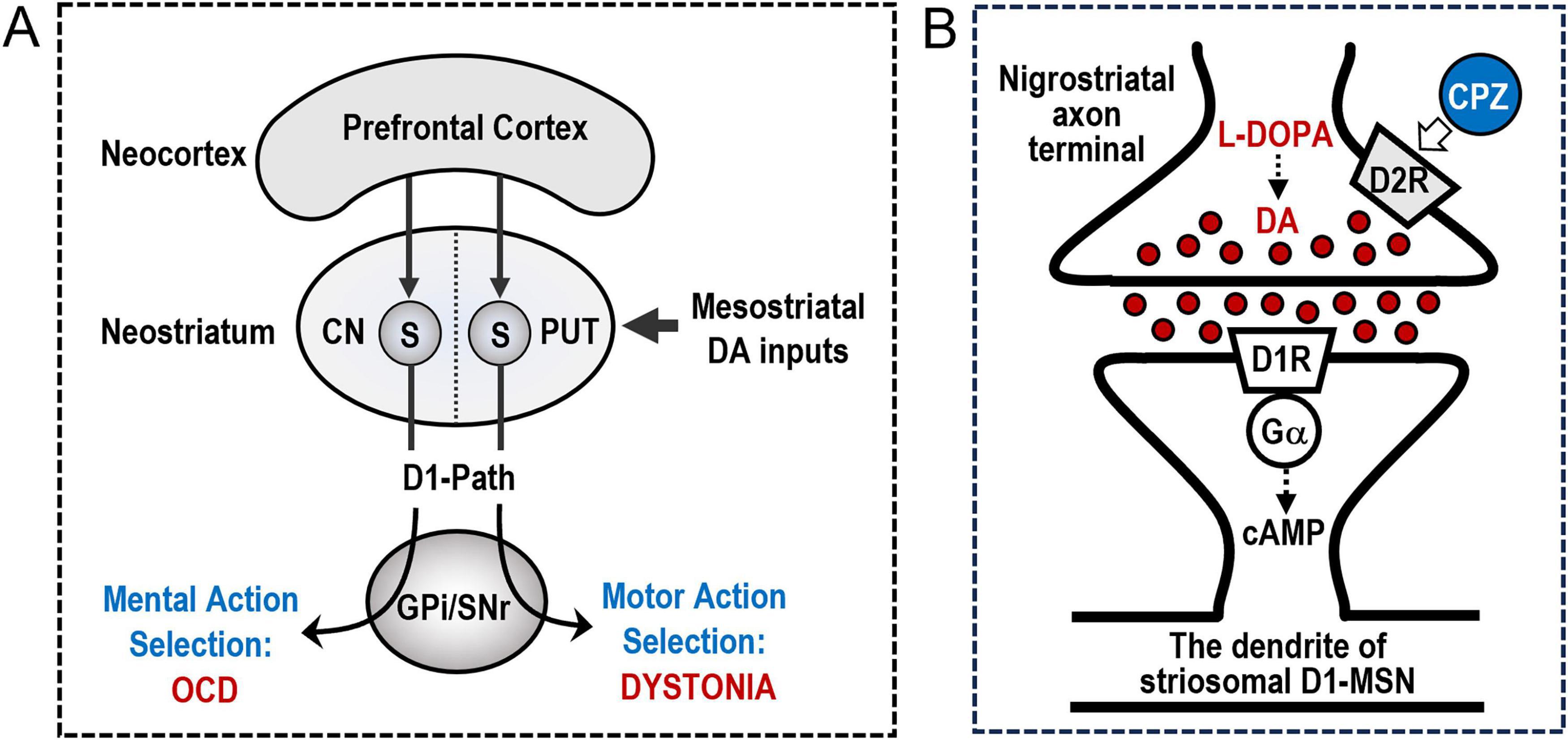
Figure 2. Striosomal D1 Dopamine Signaling in Dystonia and Obsessive-Compulsive Disorder (OCD). (A) A hypothetical role of the prefronto-striosomal network in dystonia and OCD. The presented diagram provides a simplified representation of the mechanisms by which the cortico-striatal circuit governs the selection of mental and motor actions. Medium spiny neurons that express D1-type dopamine receptors (D1-MSNs) in the striatal striosomes (S) receive cortico-striatal inputs predominantly from the limbic-associated prefrontal cortex. These D1-MSNs constitute the striosomal D1 output pathway (D1-Path) to the basal ganglia output nuclei, specifically the globus pallidus internus (GPi) and substantia nigra pars reticulata (SNr). Additionally, striosomal D1-MSNs receive dopaminergic signals via the mesostriatal pathway. Within the parallel and interacting cortico-striatal-thalamo-cortical loops, the putamen (PUT) is involved in the sensorimotor circuit, whereas the caudate nucleus (CN) is associated with cognitive functions. Consequently, alterations in the activity of striosomal D1-MSNS within the PUT may disrupt specific mechanisms related to “motor” action selection, potentially leading to the development of dystonia. Conversely, changes in the activity of striosomal D1-MSNs within the CN may hinder specific mechanisms associated with “mental” action selection, potentially contributing to the manifestation of OCD. (B) The therapeutic mechanisms for idiopathic dystonia and OCD. The diagram provided delineates a theoretical model that elucidates the mechanisms through which low doses of L-DOPA and chlorpromazine (CPZ) exert therapeutic effects in treating idiopathic dystonia and OCD. D1-type dopamine receptors (D1Rs) are heavily enriched in the striosome, but not the matrix, compartment of the human neostriatum. Consequently, the administration of low doses of L-DOPA may selectively affect D1R-expressing medium spiny neurons (D1-MSNs) situated within the striosomes due to its D1 agonistic properties. The activation of D1Rs induces an increase in cyclic adenosine monophosphate (cAMP) production via the olfactory-type G-protein α subunit (Gα), thereby enhancing the activity of the striosomal D1-MSNs. Moreover, the concurrent use of low doses of CPZ may synergistically enhance the D1-agonistic effects of L-DOPA on the activity of striosomal D1-MSNs. This phenomenon occurs because low-dose D2 antagonists, such as CPZ, primarily target presynaptic D2-type dopamine receptors (D2Rs), also known as D2R autoreceptors, located on the mesostriatal axon terminals. This targeting mechanism facilitates increased dopamine release in the striatum, thereby enhancing overall striatal dopaminergic activity. Therefore, the combined use of low doses of L-DOPA and CPZ represents a novel pharmacological strategy for the treatment of idiopathic dystonia and OCD by augmenting the activity of striosomal D1-MSNs.
4.2 The prefronto-striatal (striosomal) network in dystonia and OCD
Repetitive stereotyped motor and cognitive symptoms are key clinical features of both dystonia (Amemori et al., 2011; Crittenden and Graybiel, 2011; Albanese et al., 2013; Matsumoto et al., 2022, 2024; Matsumoto and Goto, 2024; Goto, 2025) and OCD (Amemori et al., 2011; Crittenden and Graybiel, 2011; American Psychiatric Association [APA], 2013; Graybiel and Matsushima, 2023). It has long been suggested that these symptoms may occur due to altered function of a neural circuit that connects the prefrontal cortex to the striosomes, which regulate the dopaminergic control of limbic cortical-striatal circuit processing (Graybiel and Matsushima, 2023). This hypothesis highlights the crucial roles of striosomal D1-MSNs in the dopaminergic regulation of RL and decision-making (Graybiel and Matsushima, 2023). Consequently, they contribute to specific motor and mental action selection mechanisms, of which impairments can lead to repetitive stereotyped symptoms in both dystonia and OCD (Figure 2A; Amemori et al., 2011; Crittenden and Graybiel, 2011; Graybiel and Matsushima, 2023). It is therefore plausible that striosomal D1-MSNs may serve as an anatomical substrate responsible for the development of dystonia and OCD.
Notably, the network activity of the prefronto-striosomal pathway is responsive to modulation by incoming signals through mono-synaptic and/or multi-synaptic pathways that originate from diverse brain regions, including the thalamus, cerebellum, and brainstem. This indicates the potential for symptoms associated with dystonia and OCD to arise from functional impairments in any of the brain regions through the striatal striosome mechanism. Moreover, the output activities of striosomal D1-MSNs can be profoundly affected by their intricate interactions with a range of neurotransmitters, including gamma-aminobutyric acid (GABA), acetylcholine, and glutamate (Graybiel and Matsushima, 2023; Goto, 2025). Within the intricate striatal microcircuits, these neurotransmitter interactions foster a dynamic interplay that shapes the overall functionality of striosomal D1-MSNs (Graybiel and Matsushima, 2023; Goto, 2025). Consequently, considering dystonia and OCD as network disorders underscores the importance of gaining a comprehensive understanding of these dynamics, which may provide valuable insights into the pathophysiology of both conditions.
4.3 Therapeutic role of striosomal D1R signaling in dystonia OCD in humans
In the human striatum, D1Rs are heavily enriched in the striosomes, in contrast to their limited presence in the matrix compartment (Goto, 2023; Goto, 2025). This novel finding indicates that in humans, D1R-mediated signals are primarily processed through striosome-based circuits, and the processes of motor and mental action selection are primarily reliant on the activity of striosomal D1-MSNs (Goto, 2025). This specific compartmental distinction in D1R density enables the investigation of dopaminergic modulation targeting striosomal D1-MSNs (Matsumoto et al., 2022, 2024; Matsumoto and Goto, 2024). When exposed to low-dose L-DOPA, its D1-agonistic effects primarily act upon striosomal D1-MSNs, activating D1Rs to promote cAMP production, which in turn enhances striosomal D1-MSN activity (Figure 2B). The strategic concomitant use of low-dose CPZ may amplify the D1-agonistic effects of L-DOPA on striosomal D1-MSNs in a synergistic manner, (Matsumoto et al., 2022, 2024) as shown in Table 1. This is because low-dose D2 antagonists (e.g., CPZ) predominantly affect presynaptic D2Rs, known as D2 autoreceptors (D2ARs), located on the mesostriatal axon terminals, thereby increasing striatal dopamine release and activity (Figure 2B; Chen et al., 2020; Chakroun et al., 2023; Goto, 2025). The efficacy of this D2AR-mediated feedback mechanism depends on a balanced occupancy of both presynaptic and postsynaptic D2Rs by D2 antagonists within the striatum (Chen et al., 2020; Chakroun et al., 2023). Consequently, it is plausible that a combination of low-dose L-DOPA and CPZ could enhance striosomal D1R signaling, thereby restoring the proper functionality of the CTSC loops at the striatal level, which could lead to improvements in idiopathic dystonia and OCD. This implies that reduced striatal dopamine signaling may contribute to both dystonia and OC symptoms. This notion bears relevance to clinical observations indicating that dystonia can coexist with OCD in hypodopaminergic conditions, such as Parkinson’s disease (Mallet et al., 2002) and dopa-responsive dystonia (Antelmi et al., 2015). Also, a significant proportion of schizophrenia patients receiving high-dose antipsychotics (D2 antagonists) subsequently develop dystonia (Goto, 2025) and/or OCD (Biria et al., 2019) as a side effect. Notably, a recent study in rodents reveals that the effectiveness of high-dose antipsychotics is primarily linked to the modulation of D1-MSNs, rather than D2-MSNs, in the striatum (Yun et al., 2023).
4.4 Dystonia and OCD as syndromes associated with multiple etiologies
Dystonia and OCD are multifaceted conditions that occur due to a variety of etiological factors (Balint et al., 2018; Singh et al., 2023). An understanding of their complexities is essential for developing effective therapeutic interventions. It is important to note that repetitive stereotyped motor and cognitive symptoms can arise from either a paucity or an excess of striosomal D1-MSN activity (Amemori et al., 2011; Goto, 2025). Specific subgroups of patients may exhibit dystonia (Goto, 2025) and/or OCD (Crittenden et al., 2021) associated with heightened striosomal D1R signaling. In such instances, the antagonism of D1Rs, which can be achieved through the use of D1 antagonists (Goto, 2025) or high-dose D2 antagonists (Yun et al., 2023; Goto, 2025), may prove beneficial.
In the context of OCD, there is a concept that excessive dopamine signaling contributes to the condition, primarily due to the “phasic” release of dopamine within the CSTC (Wong et al., 2008; Sesia et al., 2013; Stein et al., 2019; Yin et al., 2023). According to this excessive dopamine hypothesis of OCD, dopamine receptor antagonists have been employed in treating OCD; however, their clinical efficacy remains limited, even at high doses, and their use carries a potential risk of serious side effects (Pittenger, 2021; van Roessel et al., 2023). In hypodopaminergic conditions, such as those induced by high-dose antipsychotic medications, a significant decrease in dopamine signaling can lead to an increased sensitivity of dopamine receptors to episodic dopamine surges. This phenomenon, known as dopamine (receptor) supersensitivity, is particularly attributed to the heightened activity of D2Rs coupling with adenosine A2A receptors (Kostrzewa et al., 2018). These insights may elucidate the beneficial yet limited efficacy of dopamine antagonists in the treatment of OCD. For individuals presenting hypodopaminergic states, it is crucial to prioritize the enhancement of dopamine signaling as a core strategy for effectively addressing OCD. In these instances, the use of high-dose D2 or D1 antagonists may disrupt the logical order of treatment strategy and could lead to serious adverse effects over time, such as tardive syndrome (Goto, 2025). Based on the current findings, these considerations may also be relevant to the treatment of idiopathic dystonia.
Overall, it is imperative to recognize that both dystonia and OCD can manifest in conditions with either deficient or excessive dopamine signaling in the striatum. This understanding is crucial for determining the most suitable dopaminergic treatment for individual patients with dystonia or OCD. Furthermore, it may elucidate the reasons behind the often conflicting or unsatisfactory outcomes associated with conventional dopaminergic medications in the treatment of these challenging disorders.
5 Conclusion
This study presents compelling evidence that low-dose L-DOPA combined with CPZ can produce significant improvements in idiopathic dystonia and OCD by enhancing striatal (striosomal) D1R signaling. Our findings suggest a shared pathophysiology between these conditions, linked to striatal dysfunction due to dysregulated D1R activity in the striosomes. This insight contributes to the understanding of their pathophysiology and suggests that dopaminergic treatments aimed at restoring proper striosomal dopamine activity could serve as a practical approach for treating dystonia and OCD. However, the present study is limited by its relatively small participant pool, underscoring the need for further validation of the findings through research that utilizes a larger sample size and employs double-blinded, multi-center analyses, as well as comprehensive documentation of all adverse events that occur during the research process. Finally, we advocate for the advancement of clinical research focused on pharmacologically modulating striosomal D1R signaling to elucidate the intricate striatal mechanisms that govern the selection and execution of specific motor and mental actions, in both normative and pathological contexts. In pursuit of this objective, we express the strong expectation that advancements in high-resolution, cutting-edge in vivo brain imaging techniques, such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), will provide researchers with dynamic insights into the functioning of the striatal striosome-matrix dopamine system in humans. These advanced imaging capabilities are anticipated to enhance our understanding of the intricate interplay between motor functions and cognitive processes, potentially leading to the development of innovative treatment strategies for debilitating basal ganglia disorders, including dystonia and OCD.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study received approval from the Institutional Ethics Committee at the Osaka Neurological Institute (reference number: OR04-3), and written informed consent was obtained from all participants involved in the research. Furthermore, it is registered with the UMIN Clinical Trials Registry (UMIN000027430), which is recognized by the International Committee of Medical Journal Editors.
Author contributions
SM: Conceptualization, Data curation, Investigation, Writing – original draft. HS: Conceptualization, Writing – original draft. SG: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank Dr. Kenichi Amemori of Kyoto University for his helpful comments and discussion about the pathomechanism of dystonia and OCD based on a modular computational model of the basal ganglia circuits.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2025.1621054/full#supplementary-material
Supplementary Video 1 | The case with Meige syndrome.
Supplementary Video 2 | The case with cervical dystonia (horizontal type).
Supplementary Video 3 | The case with cervical dystonia (antecollis).
Supplementary Video 4 | The case with truncal dystonia.
Supplementary Video 5 | The case with focal hand dystonia.
Footnotes
References
Albanese, A., Bhatia, K., Bressman, S., Delong, M., Fahn, S., Fung, V., et al. (2013). Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 28, 863–873. doi: 10.1002/mds.25475
Alexander, G., DeLong, M., and Strick, P. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381. doi: 10.1146/annurev.ne.09.030186.002041
Amemori, K., Gibb, L., and Graybiel, A. (2011). Shifting responsibly: The importance of striatal modularity to reinforcement learning in uncertain environments. Front. Hum. Neurosci. 5:47. doi: 10.3389/fnhum.2011.00047
American Psychiatric Association [APA] (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association.
Antelmi, E., Stamelou, M., Liguori, R., and Bhatia, K. (2015). Nonmotor symptoms in dopa-responsive dystonia. Mov. Disord. Clin. Pract. 2, 347–356. doi: 10.1002/mdc3.12211
Balint, B., Mencacci, N., Valente, E., Pisani, A., Rothwell, J., Jankovic, J., et al. (2018). Dystonia. Nat. Rev. Dis. Primers 4:25. doi: 10.1038/s41572-018-0023-6
Barahona-Corrêa, B., Bugalho, P., Guimarães, J., and Xavier, M. (2011). Obsessive-compulsive symptoms in primary focal dystonia: A controlled study. Mov. Disord. 26, 2274–2278. doi: 10.1002/mds.23906
Biria, M., Huang, F., Worbe, Y., Fineberg, N., Robbins, T., and Fernandez-Egea, E. (2019). A cross sectional study of impact and clinical risk factors of antipsychotic-induced OCD. Eur. Neuropsychopharmacol. 29, 905–913. doi: 10.1016/j.euroneuro.2019.06.006
Burke, R., Fahn, S., Marsden, C., Bressman, S., Moskowitz, C., and Friedman, J. (1985). Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 35, 73–77. doi: 10.1212/wnl.35.1.73
Cavallaro, R., Galardi, G., Cavallini, M., Henin, M., Amodio, S., Bellodi, L., et al. (2002). Obsessive compulsive disorder among idiopathic focal dystonia patients: An epidemiological and family study. Biol. Psychiatry 52, 356–361. doi: 10.1016/s0006-3223(02)01332-x
Chakroun, K., Wiehler, A., Wagner, B., Mathar, D., Ganzer, F., van Eimeren, T., et al. (2023). Dopamine regulates decision thresholds in human reinforcement learning in males. Nat. Commun. 14:5369. doi: 10.1038/s41467-023-41130-y
Chen, R., Ferris, M., and Wang, S. (2020). Dopamine D2 autoreceptor interactome: Targeting the receptor complex as a strategy for treatment of substance use disorder. Pharmacol. Ther. 213:107583. doi: 10.1016/j.pharmthera.2020.107583
Crittenden, J., Gipson, T., Smith, A., Bowden, H., Yildirim, F., Fischer, K., et al. (2021). Striatal transcriptome changes linked to drug-induced repetitive behaviors. Eur. J. Neurosci. 53, 2450–2468. doi: 10.1111/ejn.15116
Crittenden, J., and Graybiel, A. (2011). Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat. 5:59. doi: 10.3389/fnana.2011.00059
Goodman, W., Price, L., Rasmussen, S., Mazure, C., Fleischmann, R., Hill, C., et al. (1989). The yale-brown obsessive compulsive scale. I. Development, use, and reliability. Arch. Gen. Psychiatry 46, 1006–1011. doi: 10.1001/archpsyc.1989.01810110048007
Goto, S. (2023). Specificity of striatal dopamine D1 system in humans: implications for clinical use of D1 receptor-agonists in Parkinson’s disease. Front. Hum. Neurosci. 17:1178616. doi: 10.3389/fnhum.2023.1178616
Goto, S. (2025). Functional pathology of neuroleptic-induced dystonia based on the striatal striosome-matrix dopamine system in humans. J. Neurol. Neurosurg. Psychiatry 96, 177–183. doi: 10.1136/jnnp-2024-334545
Graybiel, A., and Matsushima, A. (2023). Striosomes and matrisomes: Scaffolds for dynamic coupling of volition and action. Annu. Rev. Neurosci. 46, 359–380. doi: 10.1146/annurev-neuro-121522-025740
Heiman, G., Ottman, R., Saunders-Pullman, R., Ozelius, L., Risch, N., and Bressman, S. (2007). Obsessive-compulsive disorder is not a clinical manifestation of the DYT1 dystonia gene. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144B, 361–364. doi: 10.1002/ajmg.b.30431
Hollunder, B., Ostrem, J., Sahin, I., Rajamani, N., Oxenford, S., Butenko, K., et al. (2024). Mapping dysfunctional circuits in the frontal cortex using deep brain stimulation. Nat. Neurosci. 27, 573–586. doi: 10.1038/s41593-024-01570-1
Kostrzewa, R., Wydra, K., Filip, M., Crawford, C., McDougall, S., Brown, R., et al. (2018). Dopamine D2 receptor supersensitivity as a spectrum of neurotoxicity and status in psychiatric disorders. J. Pharmacol. Exp. Ther. 366, 519–526. doi: 10.1124/jpet.118.247981
Mallet, L., Mesnage, V., Houeto, J., Pelissolo, A., Yelnik, J., Behar, C., et al. (2002). Compulsions, Parkinson’s disease, and stimulation. Lancet 360, 1302–1304. doi: 10.1016/S0140-6736(02)11339-0
Matsumoto, S., and Goto, S. (2024). Meige syndrome as a craniofacial type of dystonia treatable by dual dopaminergic modulation using L-DOPA/Chlorpromazine: A case report. J. Mov. Disord. 17, 233–235. doi: 10.14802/jmd.23265
Matsumoto, S., Koizumi, H., Shimazu, H., and Goto, S. (2024). Therapeutic effects of dual dopaminergic modulation with l-DOPA and chlorpromazine in patients with idiopathic cervical dystonia. Neurol Clin. Pract. 14:e200254. doi: 10.1212/CPJ.0000000000200254
Matsumoto, S., Koizumi, H., Shimazu, H., Kaji, R., and Goto, S. (2022). A dual dopaminergic therapy with L-3,4-dihydroxyphenylalanine and chlorpromazine for the treatment of blepharospasm, a focal dystonia: Possible implications for striosomal D1 signaling. Front. Neurol. 13:25. doi: 10.3389/fneur.2022.922333
Pittenger, C. (2021). Pharmacotherapeutic strategies and new targets in OCD. Curr. Top. Behav. Neurosci. 49, 331–384. doi: 10.1007/7854_2020_204
Sciamanna, G., El Atiallah, I., Montanari, M., and Pisani, A. (2022). Plasticity, genetics and epigenetics in dystonia: An update. Handb. Clin. Neurol. 184, 199–206. doi: 10.1016/B978-0-12-819410-2.00011-4
Sesia, T., Bizup, B., and Grace, A. (2013). Evaluation of animal models of obsessive-compulsive disorder: Correlation with phasic dopamine neuron activity. Int. J. Neuropsychopharmacol. 16, 1295–1307. doi: 10.1017/S146114571200154X
Singh, A., Anjankar, V., and Sapkale, B. (2023). Obsessive-Compulsive Disorder (OCD): A comprehensive review of diagnosis, comorbidities, and treatment approaches. Cureus 15:e48960. doi: 10.7759/cureus.48960
Stein, D., Costa, D., Lochner, C., Miguel, E., Reddy, Y., Shavitt, R., et al. (2019). Obsessive-compulsive disorder. Nat. Rev. Dis. Primers 5:52. doi: 10.1038/s41572-019-0102-3
Timmers, E., Smit, M., Kuiper, A., Bartels, A., van der Veen, S., van der Stouwe, A., et al. (2019). Myoclonus-dystonia: Distinctive motor and non-motor phenotype from other dystonia syndromes. Parkinsonism Relat. Disord. 69, 85–90. doi: 10.1016/j.parkreldis.2019.10.015
van Roessel, P., Grassi, G., Aboujaoude, E., Menchón, J., Van Ameringen, M., and Rodríguez, C. (2023). Treatment-resistant OCD: Pharmacotherapies in adults. Compr. Psychiatry 120:152352. doi: 10.1016/j.comppsych.2022.152352
Voon, V., Butler, T., Ekanayake, V., Gallea, C., Ameli, R., Murphy, D., et al. (2010). Psychiatric symptoms associated with focal hand dystonia. Mov. Disord. 25, 2249–2252. doi: 10.1002/mds.23250
Wong, D., Brasić, J., Singer, H., Schretlen, D., Kuwabara, H., Zhou, Y., et al. (2008). Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: Clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology 33, 1239–1251. doi: 10.1038/sj.npp.1301528
Yin, L., Han, F., Yu, Y., and Wang, Q. (2023). A computational network dynamical modeling for abnormal oscillation and deep brain stimulation control of obsessive-compulsive disorder. Cogn. Neurodyn. 17, 1167–1184.
Keywords: dystonia, obsessive-compulsive disorder, striosome, dopamine D1 receptors, D2 antagonist, dopaminergic treatment
Citation: Matsumoto S, Shimazu H and Goto S (2025) Therapeutic effects of striatal dopaminergic modulation on idiopathic dystonia and OCD in humans: insights from the striosome hypothesis. Front. Hum. Neurosci. 19:1621054. doi: 10.3389/fnhum.2025.1621054
Received: 30 April 2025; Accepted: 28 July 2025;
Published: 20 August 2025.
Edited by:
Daniel Kent Leventhal, University of Michigan, United StatesReviewed by:
Ken-ichi Amemori, Kyoto University, JapanHuaibin Cai, National Institute on Aging (NIH), United States
Copyright © 2025 Matsumoto, Shimazu and Goto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoshi Goto, c2dvdG8wMzI2QG91dGxvb2suanA=
†ORCID: Shinichi Matsumoto, orcid.org/0000-0002-6755-8549; Hideki Shimazu, orcid.org/0000-0002-8507-5938; Satoshi Goto, orcid.org/0000-0002-8238-5762
 Shinichi Matsumoto
Shinichi Matsumoto Hideki Shimazu2†
Hideki Shimazu2† Satoshi Goto
Satoshi Goto