- 1Department of Neurology, The 940th Hospital of Joint Logistics Support Force of the Chinese People’s Liberation Army, Lanzhou, China
- 2Department of Endocrinology, The 940th Hospital of Joint Logistics Support Force of the Chinese People’s Liberation Army, Lanzhou, China
Introduction: Dyke-Davidoff-Masson syndrome (DDMS) is a rare neurological disorder characterized by unilateral hemiparesis, facial asymmetry, severe epilepsy, and intellectual disability. While congenital DDMS is predominantly attributed to anterior circulation anomalies [e.g., internal carotid artery (ICA) or middle cerebral artery (MCA) hypoplasia], posterior circulation involvement remains unreported. Here, we present the first documented case of congenital DDMS resulting from a rare combination of hypoplastic left posterior cerebral artery (PCA) and ipsilateral fetal-type posterior communicating artery (FTP).
Case presentation: A 19-year-old male exhibited atypical DDMS manifestations: absence seizures, preserved motor function, and occipitotemporal cognitive deficits (MoCA: 20/30). Neuroimaging revealed classic DDMS features. Angiography confirmed left PCA hypoplasia with FTP persistence, while CT perfusion demonstrated chronic left PCA hypoperfusion. Lamotrigine (100 mg/day) and regular cognition rehabilitative training resulted in good symptom control.
Conclusion: This case identifies PCA hypoplasia with FTP as a novel DDMS etiology, challenging the MCA/ICA-centric paradigm. The “posterior phenotype” (absence seizures, preserved motor function, occipitotemporal cognitive deficits) expands DDMS heterogeneity. Multimodal imaging (angiography/perfusion) is diagnostic gold-standard, while personalized therapy optimizes outcomes.
Introduction
Dyke-Davidoff-Mason syndrome (DDMS) is a rare neurological disorder characterized predominantly by unilateral hemiparesis, facial asymmetry, severe epilepsy, and intellectual disability (Alam et al., 2018). Its typical neuroimaging features include atrophy of the unilateral hemisphere, enlargement of the ipsilateral lateral ventricle, and thickening of the ipsilateral cranial vault (Rondao et al., 2023; Zamora and Kontzialis, 2015). Etiologically, DDMS can be classified into congenital and acquired subtypes (Aguiar et al., 1998; Rondao et al., 2023). Previous studies have demonstrated that vascular anomalies leading to decreased cerebral blood flow supply are the main causes of congenital DDMS (Ayas et al., 2017; Gökçe et al., 2017; Rondao et al., 2023). However, to our best known, available congenital DDMS cases associated with definitive vascular anomalies occurring in anterior cerebral circulation [e.g., internal carotid artery (ICA) or middle cerebral artery (MCA)], whereas no case reports have been reported for unilateral posterior cerebral artery (PCA) (Afifi, 1987; Aggarwal et al., 2017; AlHatmi et al., 2023; Bagazgoitia et al., 2010; Bekci et al., 2016; Gökçe et al., 2017; Gul et al., 2024; Liao et al., 2018; Pinto et al., 2013; Ruggieri et al., 2012; Ruggieri et al., 2016; Sarikaya and Sarikaya, 2007; Sener and Jinkins, 1992; Stred et al., 1986; TEAL et al., 1973; Ünal et al., 2004; Yadav et al., 2009) (Supplementary Table 1).
Here, we present the first documented case of congenital DDMS in a man resulting from a rare combination of hypoplastic left PCA and ipsilateral fetal-type posterior communicating artery (FTP), with atypical manifestations (absence seizures, preserved motor function, occipitotemporal cognitive deficits). This finding provides novel evidence that contributes to the expansion of the spectrum of vascular etiology and the heterogeneity of clinical manifestations in DDMS.
Case presentation
A 19-year-old, right-handed male was admitted to our department due to the occurrence of recurrent absence seizures over a period of 6 months. Initially, he had episodes of absence seizures that attacked two to three times per day and lasted for approximately 1 min. Gradually, his seizures became more severe, with approximately 6–7 attacks per day, each lasting 2–3 min. He was delivered via an uneventful caesarean section at a local hospital after a gestation of 39 weeks. There were no reported complications during pregnancy or the perinatal period, and no accidents have been noted in his medical history. The family history is unremarkable. Notably, his mother revealed that he displayed weaker learning abilities compared to peers starting at the age of 6. The notable co-occurrence of both learning disabilities and recurrent seizure in our patient prompted medical evaluation.
Physical examination revealed no remarkable abnormalities in our patient. The Montreal Cognitive Assessment (MoCA) yielded a score of 20 in our patient, suggesting mild cognitive impairment with predominant deficits in the language and delayed recall domains. The electroencephalogram showed interhemispheric asymmetry, increased activity of sharp slow complex waves in the left occipital and left middle-posterior temporal regions. Cranial computed tomography (CT) and magnetic resonance imaging (MRI) revealed left hemisphere atrophy (Figures 1A–F), thickening of the ipsilateral cranial vault (Figures 1A,B), dilatation of the ipsilateral frontal sinus (Figure 1B), and enlargement of the left ipsilateral ventricle (Figures 1C–F). Furthermore, CT angiography (CTA) showed hypoplasia of the right vertebral artery (VA), and revealed that the left PCA originated from the ipsilateral ICA with a hypoplastic P1 segment, a slender subsequent artery stem, and sparse branches (Figures 2A,B). Further CT perfusion imaging (CTP) analysis confirmed the areas supplied by the left PCA exhibited hypoperfusion (Figures 2C–F). Other items consisting of psychological assessment, magnetic resonance venography, ultrasonography of the heart and other internal organs, chest CT scan, blood routine test, blood biochemistry index, blood coagulation analysis, urinalysis, and autoimmune disease parameters, were within normal limits.
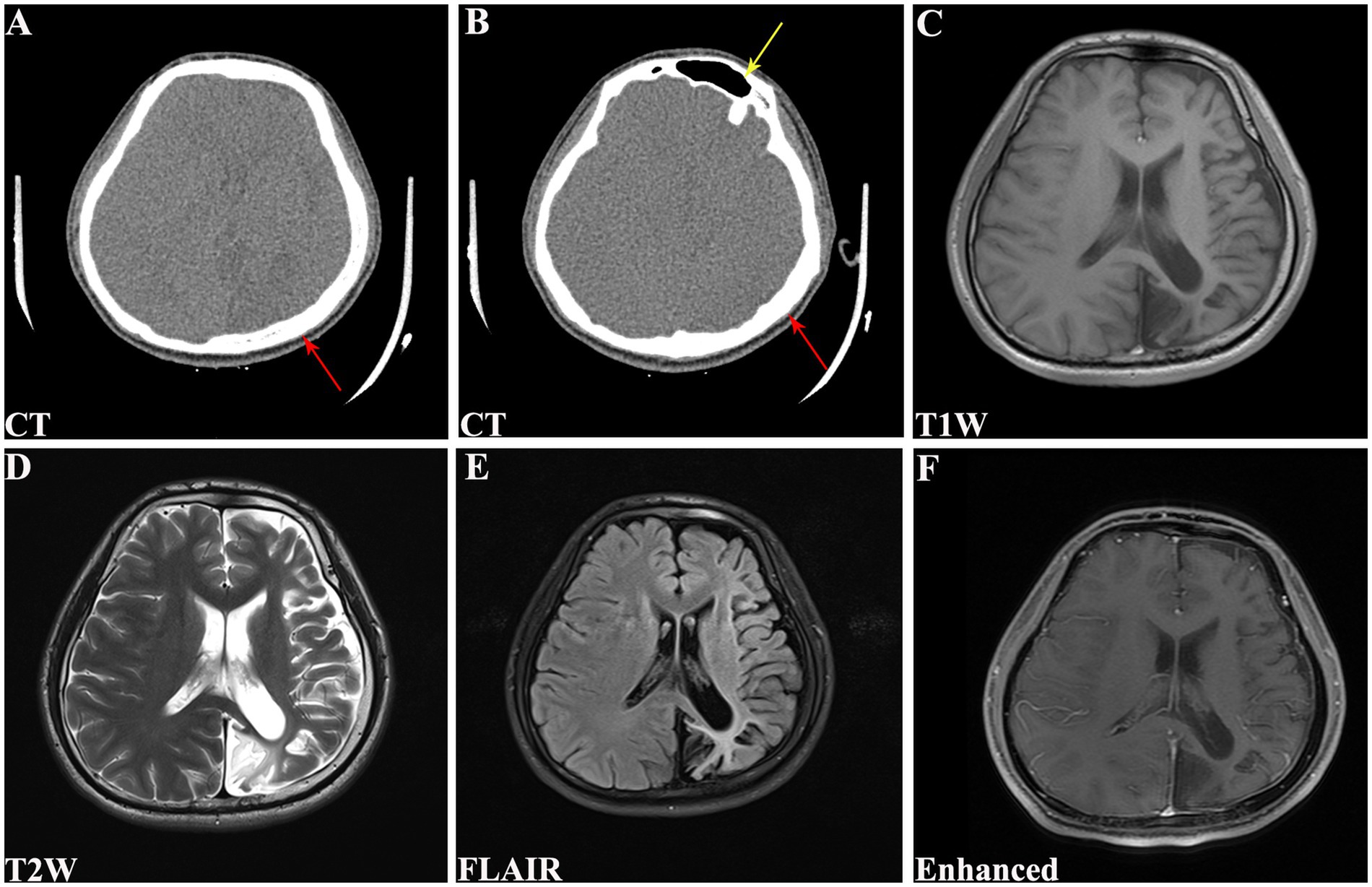
Figure 1. Cranial CT and MRI scans of the patient. (A) CT revealing left hemisphere atrophy with Compensated cranial thickening (red arrow). (B) CT demonstrating hyperpneumatization of the left frontal sinus (yellow arrow) with compensated cranial thickening (red arrow). (C) T1W, (D) T2W, (E) FLAIR, and (F) enhanced MRI showing left cerebral hemiatrophy along with enlargement of ipsilateral lateral ventricle. CT, computed tomography; MRI, magnetic resonance imaging; T1W, T1-weighted; T2W, T2-weighted; FLAIR, fluid attenuated inversion recovery.
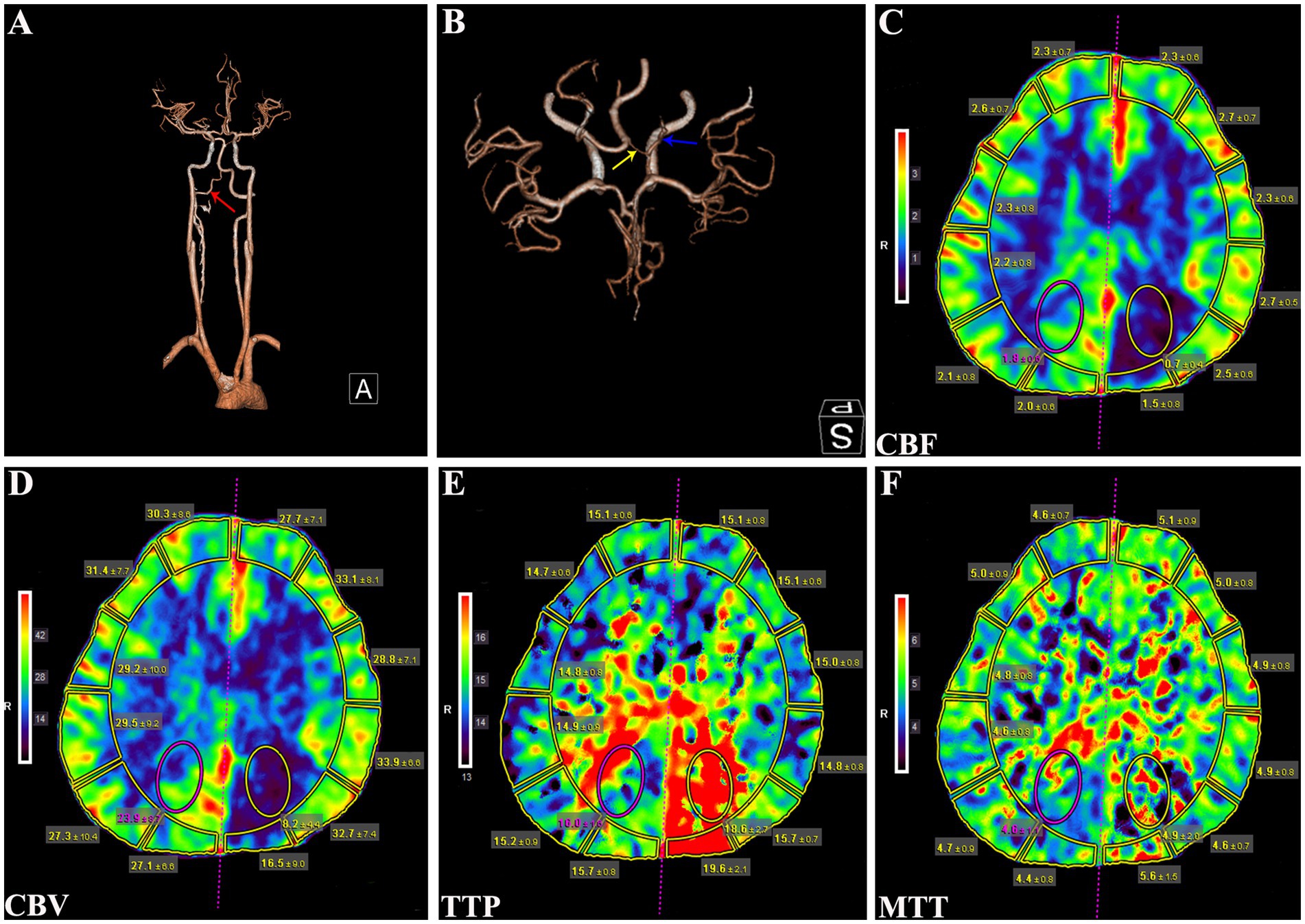
Figure 2. CTA and CTP analysis of the patient. (A) CTA showing a hypoplastic right VA (red arrow). (B) CTA revealing the left PCA originating from ipsilateral ICA with a hypoplastic P1 segment (yellow arrow) and a slenderly subsequent artery stem (blue arrow). (C) CBF, (D) CBV, (E) TTP, and (F) MTT parameters of CTP analysis demonstrating hypoperfusion in the left PCA supplying regions. CTA, computed tomography angiography; VA, vertebral artery; PCA, posterior cerebral artery; ICA, internal carotid artery; CBF, cerebral blood flow; CBV, cerebral blood volume; TTP, time to peak; MTT, mean transit time.
Based on the classical neuroimaging features, our patient was diagnosed with DDMS and received treatment with oral lamotrigine 50 mg twice daily, as well as regular cognition rehabilitative training. Since then, he has been regularly followed up by our department. During the 12-month follow-up period, the patient maintained complete seizure freedom (0 events/month) on lamotrigine monotherapy at a maintenance dose of 100 mg/day. Cognitive function demonstrated mild improvement (Supplementary Table 2).
Discussion
This case is the first report of congenital DDMS caused by hypoplasia of left PCA combined with ipsilateral FTP, offering novel insights into the etiology and clinical phenotypes of this rare disorder. Traditional perspectives posit that congenital DDMS is mainly associated with vascular anomalies in anterior circulation anomalies (e.g., ICA or MCA), leading to unilateral cerebral hypoperfusion and secondary cerebral atrophy (Atalar et al., 2007; Rondao et al., 2023). Notably, isolated PCA anomalies have not been previously documented in this context. In the present case, neuroimaging revealed an anomalous left PCA origin from the ipsilateral ICA with P1 segment hypoplasia, resulting in territorial hypoperfusion. This vascular configuration suggests that chronic hemodynamic impairment during critical neurodevelopmental stages precipitated prolonged hemispheric hypoperfusion, ultimately inducing characteristic anatomical features of congenital DDMS—including ipsilateral cerebral atrophy, progressive ventriculomegaly, and compensatory calvaria thickening (Bhol et al., 2021; Zilkha, 1980).
The coexistence of FTP further underscores the role of Will’s circle variations in the pathogenesis of congenital DDMS. FTP, a common anatomical variant that occurs in 20–30% of the general population, has historically been considered benign (Davidoiu et al., 2023). However, recent studies indicate that FTP is strongly associated with increased risk of cerebrovascular injury, such as white matter hyperintensities, lacunar infarcts and posterior circulation infarctions (Feng et al., 2023; Hsu et al., 2021; Mann et al., 2021). In this case, FTP likely aggravated hypoperfusion in the left PCA-supplied area by impairing the posterior–anterior collateral circulation (Srichawla and Garcia-Dominguez, 2024). This finding not only expands the spectrum of vascular etiologies in congenital DDMS, but also proposes a unique pathogenic pattern combining PCA hypoplasia with ipsilateral FTP.
Notably, this case demonstrates a distinct cognitive profile: whereas profound intellectual disability typically manifests in childhood among classic DDMS patients (Ayas et al., 2017; Rondao et al., 2023), our patient exhibited relatively preserved overall cognition, with deficits selectively localized to language and delayed recall domains. This distinct cognitive phenotype may be attributed to the following pathophysiological mechanisms: (I) vascular sparing of anterior circulation networks preserved prefrontal-mediated executive functions (working memory/attention), contrasting with impaired posterior vascular territories (Park et al., 2011; Popplau and Hanganu-Opatz, 2024); (II) left PCA territory ischemia directly disrupting occipito-temporal hubs critical for language processing (lingual/fusiform gyri) (Cook et al., 2014; Papagno et al., 2023), consistent with verbal fluency deficits observed here and in left PCA infarct patients (90.1% verbal memory impairment in left PCA vs. 71% in right PCA) (Benke et al., 2022); (III) chronic hippocampal hypoperfusion via compromised PCA-temporal branches, inducing subclinical neuronal loss and selective recall deficits without global amnesia—mirroring extrahippocampal lesion effects in PCA stroke (Benke et al., 2022; Watanabe et al., 2019); (IV) neuroimaging did not reveal any cerebral penetrating malformations, suggesting that the hypoperfusion event may have occurred in the late gestational or perinatal period, when neuroplasticity allowed synaptic reorganization and network remodeling to partially compensate for the ischemic damage (Rondao et al., 2023). Collectively, this case delineates a vascular-territory-specific cognitive phenotype in congenital DDMS, where left PCA hypoperfusion targets: (a) language networks, (b) hippocampal-thalamic pathways, while sparing (c) frontal-executive domains.
Moreover, a recent systematic review consisting 188 DDMS cases revealed that motor deficits (hemiparesis in 97.6% and facial asymmetry in 100% of congenital DDMS patients) and generalized tonic–clonic seizures represent hallmark clinical features (Rondao et al., 2023). However, this case demonstrated marked phenotypic divergence, exhibiting neither hemiparesis nor facial asymmetry. The predominant epileptic manifestation was characterized by atypical absence seizures. This discrepancy can be attributed to: (I) the ischemic damage was strictly limited to the areas supplied by the PCA (occipital and temporal lobes), without involving the motor system (Xalxo et al., 2025); and (II) the focal injury to the left temporal-occipital lobe may have triggered absence seizures through abnormal synchronized discharges of the temporal-occipital and thalamocortical networks (Danielson et al., 2011; Groulx-Boivin et al., 2024; Kumar et al., 2023). Interestingly, according to the literature search, only one previous case of DDMS was reported to have absence seizures (Parker et al., 1972). The present case represents a notable exception, as it is the second documented instance of DDMS associated with such an atypical epilepsy phenotype.
This case also highlights three important implications for clinical practice: first, attention should be paid to atypical DDMS manifestations even in the absence of hemiparesis and facial asymmetry, and the possibility of DDMS should be considered when the patient presents with mild cognitive impairment and atypical epilepsy (e.g., absence seizures) to avoid underdiagnosis. Second, characteristic neuroimaging findings remain the gold-standard criteria for DDMS (Durcan et al., 2018; Zamora and Kontzialis, 2015). High-resolution MRA or CTA, combined with perfusion studies, is essential to delineate cerebrovascular abnormalities and guide prognosis. In addition, combining multidisciplinary disciplines such as neuroimaging, epilepsy specialties, and neurorehabilitation to develop personalized therapeutic regimens may improve symptom control and functional outcomes.
This report is limited by the absence of definitive prenatal imaging to confirm the congenital etiology of DDMS, which introduces diagnostic uncertainty. While the developmental trajectory and radiological characteristics strongly support a congenital origin, future studies documenting early-stage imaging would be required to establish causal relationships. Consequently, this case represents presumed congenital DDMS rather than a definitively proven congenital anomaly.
In conclusion, this report identifies for the first time that unilateral PCA hypoplasia with ipsilateral FTP as a novel etiology of congenital DDMS, challenging the traditional cognitive framework that considers MCA/ICA developmental abnormalities as the core etiology of congenital DDMS. The unique clinical triad (absence seizures, the absence of motor deficits, and mild cognitive impairment) expands the phenotypic spectrum of DDMS. Neuroimaging in combination with angiography is key to a definitive diagnosis, while personalized antiepileptic therapy and cognitive rehabilitation can effectively improve patients’ quality of life.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the 940th Hospital of Joint Logistics Support Force of the Chinese People’s Liberation Army. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HH: Funding acquisition, Visualization, Writing – original draft, Data curation, Investigation. CW: Writing – original draft, Visualization. HJH: Data curation, Investigation, Writing – review & editing. YZ: Data curation, Investigation, Project administration, Writing – review & editing. BZ: Supervision, Writing – review & editing, Conceptualization. DW: Supervision, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported in part by funding from the Young Talent Reserve Initiative under the High-Level Talent Cultivation Program of the 940th Hospital, Joint Logistics Support Force, Chinese PLA (Grant No. 2024-G3-6), and Postdoctoral Fellow Matching Fund of the 940th Hospital, Joint Logistics Support Force, Chinese PLA (Grant No. 2024YYZZBSH-2).
Acknowledgments
The authors would like to thank the patients for letting us publish his case and all the contributors for their input and work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2025.1629156/full#supplementary-material
References
Afifi, A. K. (1987). Cerebral hemiatrophy, hypoplasia of internal carotid artery, and intracranial aneurysm. Arch. Neurol. 44:232. doi: 10.1001/archneur.1987.00520140090024
Aggarwal, A., Aggarwal, A. K., Kapoor, A., Kapoor, R., and Bansal, A. (2017). Hemiatrophy of brain: antenatal ultrasonography and MRI/postnatal MRI diagnosis with the introduction of “shifted falx sign”. J. Med. Ultrason. 44, 147–151. doi: 10.1007/s10396-016-0744-7
Aguiar, P. H., Liu, C. W., Leitao, H., Issa, F., Lepski, G., Figueiredo, E. G., et al. (1998). MR and CT imaging in the Dyke-Davidoff-Masson syndrome. Report of three cases and contribution to pathogenesis and differential diagnosis. Arq. Neuropsiquiatr. 56, 803–807. doi: 10.1590/s0004-282x1998000500016
Alam, M., Ayaz Ul Haq, M., Ali, F., Mehwish, H., and Nawab, K. (2018). Dyke-Davidoff-Masson syndrome: an unusual cause of status epilepticus and refractory seizures. J. Coll. Physicians Surg. Pak. 28, S99–S101. doi: 10.29271/jcpsp.2018.06.S99
AlHatmi, A., Almashaikhi, T., and Ajmi, E. A. (2023). Imaging features of Dyke-Davidoff-Masson syndrome. Sultan Qaboos Univ. Med. J. 23, 122–124. doi: 10.18295/squmj.9.2022.055
Atalar, M. H., Icagasioglu, D., and Tas, F. (2007). Cerebral hemiatrophy (Dyke–Davidoff–Masson syndrome) in childhood: clinicoradiological analysis of 19 cases. Pediatr. Int. 49, 70–75. doi: 10.1111/j.1442-200X.2007.02299.x
Ayas, Z. O., Asil, K., and Ocal, R. (2017). The clinico-radiological spectrum of Dyke-Davidoff-Masson syndrome in adults. Neurol. Sci. 38, 1823–1828. doi: 10.1007/s10072-017-3074-7
Bagazgoitia, L., Garcia-Penas, J. J., Duat-Rodriguez, A., Hernandez-Martin, A., and Torrelo, A. (2010). Facial capillary malformation and Dyke-Davidoff-Masson syndrome. Pediatr. Neurol. 43, 202–204. doi: 10.1016/j.pediatrneurol.2010.04.011
Bekci, T., Bilgici, M. C., Turgut, E., and Aslan, K. (2016). A rare combination: Sturge–Weber syndrome and accompanying Dyke–Davidoff–Masson syndrome. Acta Neurol. Belg. 116, 79–81. doi: 10.1007/s13760-015-0511-3
Benke, T., Bodner, T., Wiesen, D., and Karnath, H. O. (2022). The amnestic syndrome of posterior cerebral artery infarction. Eur. J. Neurol. 29, 2987–2995. doi: 10.1111/ene.15449
Bhol, D., Chandrasekar, S., John, J., and Satapathy, A. K. (2021). Dyke-Davidoff-Masson syndrome: a rare cause of acquired cerebral hemiatrophy. Asian J. Neurosurg. 16, 579–581. doi: 10.4103/ajns.AJNS_499_20
Cook, P. A., McMillan, C. T., Avants, B. B., Peelle, J. E., Gee, J. C., and Grossman, M. (2014). Relating brain anatomy and cognitive ability using a multivariate multimodal framework. NeuroImage 99, 477–486. doi: 10.1016/j.neuroimage.2014.05.008
Danielson, N. B., Guo, J. N., and Blumenfeld, H. (2011). The default mode network and altered consciousness in epilepsy. Behav. Neurol. 24, 55–65. doi: 10.3233/BEN-2011-0310
Davidoiu, A. M., Minca, D. I., Rusu, M. C., Hostiuc, S., and Toader, C. (2023). The fetal type of posterior cerebral artery. Medicina (Kaunas) 59:231. doi: 10.3390/medicina59020231
Durcan, R., Smyth, S., and Bolster, F. (2018). Teaching neuroimages: Dyke-Davidoff-Masson syndrome. Neurology 90, e2097–e2098. doi: 10.1212/WNL.0000000000005640
Feng, L., Zhai, F. F., Li, M. L., Zhou, L. X., Ni, J., Yao, M., et al. (2023). Association between anatomical variations of the circle of Willis and covert vascular brain injury in the general population. Cerebrovasc. Dis. 52, 480–486. doi: 10.1159/000527432
Gökçe, E., Beyhan, M., and Sade, R. (2017). Radiological imaging findings of Dyke–Davidoff–Masson syndrome. Acta Neurol. Belg. 117, 885–893. doi: 10.1007/s13760-017-0778-7
Groulx-Boivin, E., Bouchet, T., and Myers, K. A. (2024). Understanding of consciousness in absence seizures: a literature review. Neuropsychiatr. Dis. Treat. 20, 1345–1353. doi: 10.2147/NDT.S391052
Gul, E., Atalar, M. H., and Atik, I. (2024). Evaluation of the contralateral hemisphere with DWI in pediatric patients with Dyke–Davidoff–Masson syndrome. Acta Neurol. Belg. 124, 911–918. doi: 10.1007/s13760-024-02473-5
Hsu, C. F., Chen, K. W., Su, C. H., Shen, C. Y., and Chi, H. Y. (2021). Bilateral vertebral artery hypoplasia and fetal-type variants of the posterior cerebral artery in acute ischemic stroke. Front. Neurol. 12:582149. doi: 10.3389/fneur.2021.582149
Kumar, A., Lyzhko, E., Hamid, L., Srivastav, A., Stephani, U., and Japaridze, N. (2023). Neuronal networks underlying ictal and subclinical discharges in childhood absence epilepsy. J. Neurol. 270, 1402–1415. doi: 10.1007/s00415-022-11462-8
Liao, Y., Wu, C., and Wang, J. (2018). A case report of Dyke-Davidoff-Masson syndrome. Chin. J. Neurol. 51, 60–61. doi: 10.3760/cma.j.issn.1006-7876.2018.01.013
Lu, J., Li, D., Li, F., Zhou, A., Wang, F., Zuo, X., et al. (2011). Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J. Geriatr. Psychiatry Neurol. 24, 184–190. doi: 10.1177/0891988711422528
Mann, L., Preece, R., Haslam, L., Paravastu, S., Bulbulia, R. A., and Kulkarni, S. R. (2021). Posterior cerebral circulation stroke secondary to foetal origin of posterior communicating artery: an indication for carotid endarterectomy. EJVES Vasc. Forum 50, 7–11. doi: 10.1016/j.ejvsvf.2020.12.022
Papagno, C., Pascuzzo, R., Ferrante, C., Casarotti, A., Riva, M., Antelmi, L., et al. (2023). Deficits in naming pictures of objects are associated with glioma infiltration of the inferior longitudinal fasciculus: a study with diffusion MRI tractography, volumetric MRI, and neuropsychology. Hum. Brain Mapp. 44, 4011–4027. doi: 10.1002/hbm.26325
Park, K. C., Yoon, S. S., and Rhee, H. Y. (2011). Executive dysfunction associated with stroke in the posterior cerebral artery territory. J. Clin. Neurosci. 18, 203–208. doi: 10.1016/j.jocn.2010.05.026
Parker, C. E., Harris, N., and Mavalwala, J. (1972). Dyke-Davidoff-Masson syndrome. Five case studies and deductions from dermatoglyphics. Clin. Pediatr. (Phila) 11, 288–292. doi: 10.1177/000992287201100513
Pinto, W. B. V. D., Souza, P. V. S. D., Pedroso, J. L., and Barsottini, O. G. P. (2013). Dyke-Davidoff-Masson syndrome: a combination of clinical and radiological signs not to be missed. Arq. Neuropsiquiatr. 71, –911. doi: 10.1590/0004-282X20130137
Popplau, J. A., and Hanganu-Opatz, I. L. (2024). Development of prefrontal circuits and cognitive abilities. Cold Spring Harb. Perspect. Biol. 16:a041502. doi: 10.1101/cshperspect.a041502
Rondao, M., Hsu, B., Centeno, R. S., and de Aguiar, P. (2023). Dyke-Davidoff-Masson syndrome: main clinical and radiological findings-systematic literature review. Seizure 110, 58–68. doi: 10.1016/j.seizure.2023.04.020
Ruggieri, M., Milone, P., Pavone, P., Falsaperla, R., Polizzi, A., Caltabiano, R., et al. (2012). Nevus vascularis mixtus (cutaneous vascular twin nevi) associated with intracranial vascular malformation of the Dyke–Davidoff–Masson type in two patients. Am. J. Med. Genet. A 158A, 2870–2880. doi: 10.1002/ajmg.a.35221
Ruggieri, M., Polizzi, A., Strano, S., Schepis, C., Morano, M., Belfiore, G., et al. (2016). Mixed vascular nevus syndrome: a report of four new cases and a literature review. Quant. Imaging Med. Surg. 6, 515–524. doi: 10.21037/qims.2016.10.09
Sarikaya, B., and Sarikaya, S. (2007). Dyke–Davidoff–Masson syndrome revisited: a didactic case with interesting imaging findings. Australas Radiol. 51, B10–B13. doi: 10.1111/j.1440-1673.2007.01834.x
Sener, R. N., and Jinkins, J. R. (1992). MR of craniocerebral hemiatrophy. Clin. Imaging 16, 93–97. doi: 10.1016/0899-7071(92)90119-T
Srichawla, B. S., and Garcia-Dominguez, M. A. (2024). Regional dynamic cerebral autoregulation across anterior and posterior circulatory territories: a detailed exploration and its clinical implications. World J. Crit. Care Med. 13:97149. doi: 10.5492/wjccm.v13.i4.97149
Stred, S. E., Byrum, C. J., Bove, E. L., and Oliphant, M. (1986). Coarctation of the midaortic arch presenting with monoparesis. Ann. Thorac. Surg. 42, 210–212. doi: 10.1016/S0003-4975(10)60522-X
Teal, J. S., Rumbaugh, C. L., Bergeron, R. T., and Segali, H. D. (1973). Congenital absence of the internal carotid artery associated with cerebral hemiatrophy, absence of the external carotid artery, and persistence of the stapedial artery. Am. J. Roentgenol. 118, 534–545. doi: 10.2214/ajr.118.3.534
Ünal, Ö., Tombul, T., Çırak, B., Anlar, Ö., İncesu, L., and Kayan, M. (2004). Left hemisphere and male sex dominance of cerebral hemiatrophy (Dyke–Davidoff–Masson syndrome). Clin. Imaging 28, 163–165. doi: 10.1016/S0899-7071(03)00158-X
Watanabe, J., Ogata, T., Tsuboi, Y., and Inoue, T. (2019). Impact of cerebral large-artery disease and blood flow in the posterior cerebral artery territory on cognitive function. J. Neurol. Sci. 402, 7–11. doi: 10.1016/j.jns.2019.04.037
Xalxo, N., Ratanpara, L., Patil, K. S., Chauhan, P. R., and Mehra, S. (2025). Morphology and variations of the posterior cerebral artery: a literature review. Cureus 17:e81205. doi: 10.7759/cureus.81205
Yadav, P., Kumar, A., Saili, A., and Datta, V. (2009). Congenital hypoplasia of the internal carotid artery. Indian J. Pediatr. 76, 1061–1062. doi: 10.1007/s12098-009-0203-1
Zamora, C. A., and Kontzialis, M. (2015). Teaching neuroimages: Dyke-Davidoff-Masson in Sturge–Weber syndrome. Neurology 85:e128. doi: 10.1212/WNL.0000000000002043
Keywords: Dyke-Davidoff-Masson syndrome, cerebral hemiatrophy, cerebrovascular disease, vascular malformation, cognitive impairment, epilepsy
Citation: Huang H, Wang C, Hua H, Zhang Y, Zhao B and Wan D (2025) Case Report: Dyke-Davidoff-Masson syndrome resulting from a rare combination of hypoplastic left posterior cerebral artery and ipsilateral fetal-type posterior communicating artery. Front. Hum. Neurosci. 19:1629156. doi: 10.3389/fnhum.2025.1629156
Edited by:
Shihao He, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Geng Guo, First Hospital of Shanxi Medical University, ChinaEnes Gül, Cumhuriyet University, Türkiye
Copyright © 2025 Huang, Wang, Hua, Zhang, Zhao and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Zhao, ZHJ6aGFvYm8xOTc5QDE2My5jb20=; Dongjun Wan, d2FuZG9uZ2p1bjIwMDZAMTYzLmNvbQ==
†These authors have contributed equally to this work
 He Huang
He Huang Chunyu Wang
Chunyu Wang Huijuan Hua
Huijuan Hua Yingju Zhang
Yingju Zhang Bo Zhao
Bo Zhao Dongjun Wan
Dongjun Wan