- 1Academy of Music, Southwest University, Chongqing, China
- 2Mental Health Research Institute of Chinese Music, Southwest University, Chongqing, China
- 3Department of Radiology, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI, United States
- 4Research Center for Cross-Straits Cultural Development, Fujian Normal University, Fuzhou, Fujian, China
Objective: Although music has been shown to affect brain function, the structural characteristics of the brain in musicians compared to non-musicians are often overlooked. This limited attention restricts the practical use of music’s emotional, cognitive, and motor functions. The current study aimed to investigate structural differences in the brains of musicians compared to non-musicians in order to better understand the neuroanatomical basis of musical training.
Methods: Sixteen musicians and seventeen age-matched non-musicians underwent a brain structural neuroimaging scan. Group differences in structural morphometry were assessed.
Results: Significant differences were found in cortical thickness, fractal dimensionality, gyrification, and sulcal depth measures. Compared to non-musicians, musicians showed greater cortical thickness in the left superior frontal gyrus and right central parietal region, and showed structural advantages in fractal dimensionality and sulcal depth in the left fusiform gyrus and right central region. In contrast, non-musicians showed greater gyrification in the bilateral insula, right superior parietal lobule, and right supramarginal gyrus. Notably, significant interactive effects were observed between gender and cortical thickness, fractal dimensionality, gyrification, and sulcal depth in regions of the limbic system, including the hippocampus, cingulate gyrus, insula, fusiform gyrus, and precuneus.
Conclusion: Structural differences in the frontal cortex, limbic system, and sensorimotor areas between musicians and non-musicians highlight the changes in brain structure associated with musical training. These findings provide insight into the underlying mechanisms of music-related brain function and may provide guidance for future applications of music to improve mental health and neuroplasticity.
1 Introduction
The brain is not a static organ; rather, it changes dynamically across an individual’s lifespan. Throughout human development, the brain adapts in response to various experiences. Music, as one of the most important experiences of emotion regulation, not only enhances emotional experience, cognitive processing, and social interaction, but also affects the morphological and functional properties of internal cranial nerves. Long term exposure to music—whether it be through listening, active training, or passive exposure—can induce stable morphological changes in the brain, continuing to shape one’s cognitive processing, emotional expression, and behavioral responses. These music-induced changes in plasticity, often referred to as music-based interventions (Sihvonen et al., 2017), have become a growing focus in neurological rehabilitation in recent years.
Traditional studies on music and the brain have mainly focused on how musical training affects functional neural activity. One key finding from these studies is that the dopamine reward system—comprised of the hippocampus, hypothalamus, ventral tegmental area, and nucleus accumbens—is activated during music-evoked emotion (Koelsch, 2010; Sachs et al., 2015). This reward system also interacts with functional structures involved in auditory perception (as a predictive process) to enhance an individual’s hedonic experience (Belfi et al., 2019), and contributes to emotion regulation, empathic feelings, and prosocial behavior (Ferreri et al., 2019). Even in extremely preterm infants, music exposure has been shown to enhance the structural maturation of emotion-related neural pathways—such as in the external capsule, claustrum, extreme capsule, and uncinate fasciculus—and to increase amygdala volume when compared to infants receiving the standard-of-care (Sa de Almeida et al., 2020).
Musicians also demonstrate superior integration of motor patterns and sensory processing across somatosensory and auditory domains. Structural and functional advantages have been observed in areas such as the posterior-superior cerebellar hemisphere, the dominant primary sensorimotor cortex, the left Heschl’s gyrus (Gebel et al., 2013), and fractional anisotropy (Halwani et al., 2011). When comparing brain network activity during cello playing and singing, overlapping activation was observed in the intraparietal sulcus (IPS) and supramarginal gyrus (SMG) when participants were asked to either compensate for or ignore introduced pitch perturbations, and in the posterior superior temporal gyrus (pSTG) and dorsal pre-motor cortex (dPMC) during the same task. Differences between singing and playing were most prominent in the primary motor cortex (M1), centered on the relevant motor effectors (e.g., hand, larynx) (Segado et al., 2021).
However, the human brain also functions during resting states, not just during active tasks. A well-regulated resting state supports more effective brain function during task performance, and music is an important stimulus that can influence brain structure even at rest. Just as mindfulness meditation influences brain cortical structure, musical training can also lead to non-task related structural changes—such as alterations in cortical thickness, fractal dimensionality, gyrification, and sulcal depth—which may enhance cognitive processing and regulate neural activity during resting or non-task states.
Most research up to this point has focused on differences in cortical thickness and surface area in sensorimotor regions when comparing brain surface morphometry in musicians and non-musicians. One study identified 17 regions—9 cerebellar and 8 sensorimotor—that differed between musicians and non-musicians, as well as between early-learning and late-learning musicians (Shenker et al., 2023). Moreover, musicianship was found to be correlated with greater cortical thickness and gray matter volumes in the frontal and temporal regions, and musicians also showed more localized structural whole-brain covariance compared to non-musicians (Bermudez et al., 2009). These findings support the commonly accepted view that musical activity requires sophisticated dynamic interplay between multisensory and motor behaviors, sub-served by the auditory, visual, tactile, and motor systems of the brain in particular (Moller et al., 2021).
Cortical thickness examines the distance between the top of the brain and the white matter boundary in the neocortex, while also examining gray matter morphology (Hirakawa et al., 2016; Seiger et al., 2018). However, this measure is limited to cortical areas, and does not capture information from non-cortical areas of the brain (Bermudez et al., 2009). Therefore, additional measures—such as fractal dimensionality, gyrification, and sulcal depth—can complement cortical thickness to make up for its limitations. Fractal dimensionality examines structural complexity beyond the capabilities of cortical thickness (Di Ieva et al., 2014, 2015; Madan and Kensinger, 2017; Yotter et al., 2011b), and has been shown to be more sensitive to structural variability (Chen et al., 2020; Madan and Kensinger, 2017; Zhang et al., 2008). Gyrification reflects the amount of local cortical folding, thus serving as an indicator for the integrality between cortical and subcortical circuits (Li et al., 2021). Sulcal depth, measured as the Euclidean distance between the cortex and outer surface (Li et al., 2021; Yun et al., 2013), captures changes in gray and white matter (Im et al., 2008; Jin et al., 2018; Kim et al., 2008; Kochunov et al., 2008). Because sulcal depth is not limited to gray matter alone, it is sensitive to the complex folding patters of the cortical surface, and serves as a relatively new way to observe the cerebral cortex (Jin et al., 2018).
In order to investigate the effects of musical training on brain structure, the current study examined cortical thickness, fractal dimensionality, gyrification, and sulcal depth in a group of age-matched musicians and non-musicians. Additionally, we also examined the gender effect on brain surface morphometry between musicians and non-musicians, as it is known that gender can influence neural processing during music perception. For example, an event-related potentials (ERP) study found that females display early right anterior negativity (ERAN) and mismatch negativity (MMN) in both hemispheres during music syntactic processing, whereas males exhibit right hemispheric dominance (Koelsch et al., 2003). A neuroimaging study also found that, compared to females, males generally rely on the left lateralized hemisphere (e.g., the anterior and posterior perisylvian areas and cerebellum) during music pitch processing (Gaab et al., 2003). Therefore, the goal of this study was to identify key structural differences between musicians and non-musicians, and to examine how gender may influence these differences. By doing so, we hope to contribute to the growing body of work on music-induced neuroplasticity and its implications for cognitive and emotional functioning.
2 Methods
2.1 Data source
Structural T1-weighted images (NIFTI format) were obtained from a public dataset via OpenNeuro with accession number ds003146.1 There were sixteen musicians (age range: 20–42 years old, mean age: 28 ± 7 years old; 8 males and 8 females) who had at least 3 years of formal musical training or studies experiences (either singing or playing instruments) who were also currently involved in daily musical activities. Fourteen musicians practiced multiple instruments (such as piano, keyboard, guitar, saxophone, bass etc.), although there was one or two main instruments practiced. Among them, four singing musicians also had instrumental practice, although they mainly had singing practice (classified as singing musicians). In addition, two musicians only practiced the piano. Their mean practice years were 19 ± 12.264 years, the age of music practicing onset was 11.188 ± 6.473 years, and weekly practice time was 12.688 ± 8.404 hours. The participants’ detailed information can be seen in the S1 Table of https://doi.org/10.1371/journal.pone.0222796. Seventeen age-matched non-musicians (age range: 20–45 years old, mean age: 27 ± 6 years old; 8 males and 9 females) did not receive extra-curricular music instruction beyond a mandatory school music course. All participants were Spanish-speaking, right-handed, and had normal hearing. They gave their written informed consent before the MRI scanning session. The research protocol was approved by the Ethics Committee of the Institute of Neurobiology at the Universidad Nacional Autónoma de México and was conducted in accordance with the international standards of the Declaration of Helsinki of 1964 (Angulo-Perkins and Concha, 2019).
2.2 MRI data acquisition
Images were acquired on a 3 T Discovery MR750 scanner (General Electric, Waukesha, Wisconsin) with a 32-channel coil. The parameters were: TR = 2,300 ms, TE = 3 ms, feld of view = 256 × 256 mm2, voxel size = 1 × 1 × 1 mm3.
2.3 Cortical surface preprocessing
Image preprocessing was performed with the Computational Anatomy Toolbox (CAT12, http://www.neuro.uni-jena.de/cat/), a software plugin for Statistical Parametric Mapping (SPM12, https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) within MATLAB. CAT12 is not only more precise and accurate compared to earlier voxel-based morphometry (VBM) plug-ins (Farokhian et al., 2017; Yuksel et al., 2018), but is also fully automated for surface-based analysis (Zhuang et al., 2017). The preprocessing steps in CAT12 consisted of bias-field correction, skull-stripping, alignment to the Montreal Neurological Institute (MNI) structural template (to classify gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF), and spatial normalization [with the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) registration 1.5 mm)] (Kurth et al., 2015; Yuksel et al., 2018; Zhuang et al., 2017). After these initial preprocessing steps, a spherical harmonic approach (Yotter et al., 2011a) was used to reparametrize the brain surface mesh in order to reduce brain area distortions (Yotter et al., 2011c) and repair topological defects (Chen et al., 2020; Yotter et al., 2011a,c).
Cortical thickness was preprocessed based on the established CAT12 workflow (Dahnke et al., 2013). This algorithm uses tissue segmentation to evaluate WM distance and projects the local maxima to the GM voxels. Values at the outer GM boundary within the WM distance map are then projected back to the inner GM boundary to generate GM thickness (Li et al., 2021). A central surface was then generated between the GM thickness and WM distance (Li et al., 2021). Spatial normalization was applied with the DARTEL registration (Li et al., 2021), and spatial smoothing was performed using a 15 mm full-width at half maximum (FWHM) Gaussian kernel.
Fractal dimensionality, which examines cortical complexity, was derived from spherical harmonic reconstructions (Li et al., 2021; Yotter et al., 2011a). This technique calculates the slope between a logarithmic plot of surface area and the maximum value, representing the bandwidth of frequencies used to reconstruct the cortical surface shape (Li et al., 2021; Yotter et al., 2011b). Spatial smoothing for the fractal dimensionality was performed using a 20 mm FWHM Gaussian kernel.
Gyrification, considered an indicator of cortical folding, was calculated using absolute mean curvature, an extrinsic surface measure that captures changes in the normal direction of the brain surface (Li et al., 2021; Luders et al., 2006). Spatial smoothing was once again performed with a 20 mm FWHM Gaussian kernel.
Sulcal depth was calculated as the Euclidean distance between the central surface and its convex hull, and the resulting values were then transformed using the sqrt function (Li et al., 2021). Spatial smoothing for sulcal depth was also performed using a 20 mm FWHM Gaussian kernel.
2.4 Statistical analysis
The current study used a two-factor between-subjects design with music group (musicians vs. non-musicians) and gender (males vs. females) as independent variables. Two-way (music group × gender) ANCOVAs were conducted to examine differences between the groups on the main study variables, with age and intracranial volumes considered as covariates. All morphometric analyses were performed using CAT12 and were analyzed via a non-parametric permutation technique. Threshold-Free Cluster Enhancement (TFCE) was used in permutation testing with 5,000 permutations (Smith and Nichols, 2009). TFCE p < 0.05 was used for multiple comparison correction. The brain regions with cluster size of at least 100 vertices (cluster size × percentage covered in the specific region produced by CAT12) were reported. The Desikan–Killiany atlas (DK40) (Desikan et al., 2006) was used to label the cortical regions and the results were visualized using CAT12.
3 Results
3.1 Age
As expected, the two-way ANOVA revealed no significant age differences among the four subgroups (group: F (1,33) = 1.123, η2 = 0.037, p = 0.298; gender: F (1,33) = 0.230, η2 = 0.008, p = 0.635; group x gender: F (1,33) = 1.034, η2 = 0.034, p = 0.318), indicating that participants were successfully matched.
3.2 Structural morphometry differences
3.2.1 Cortical thickness
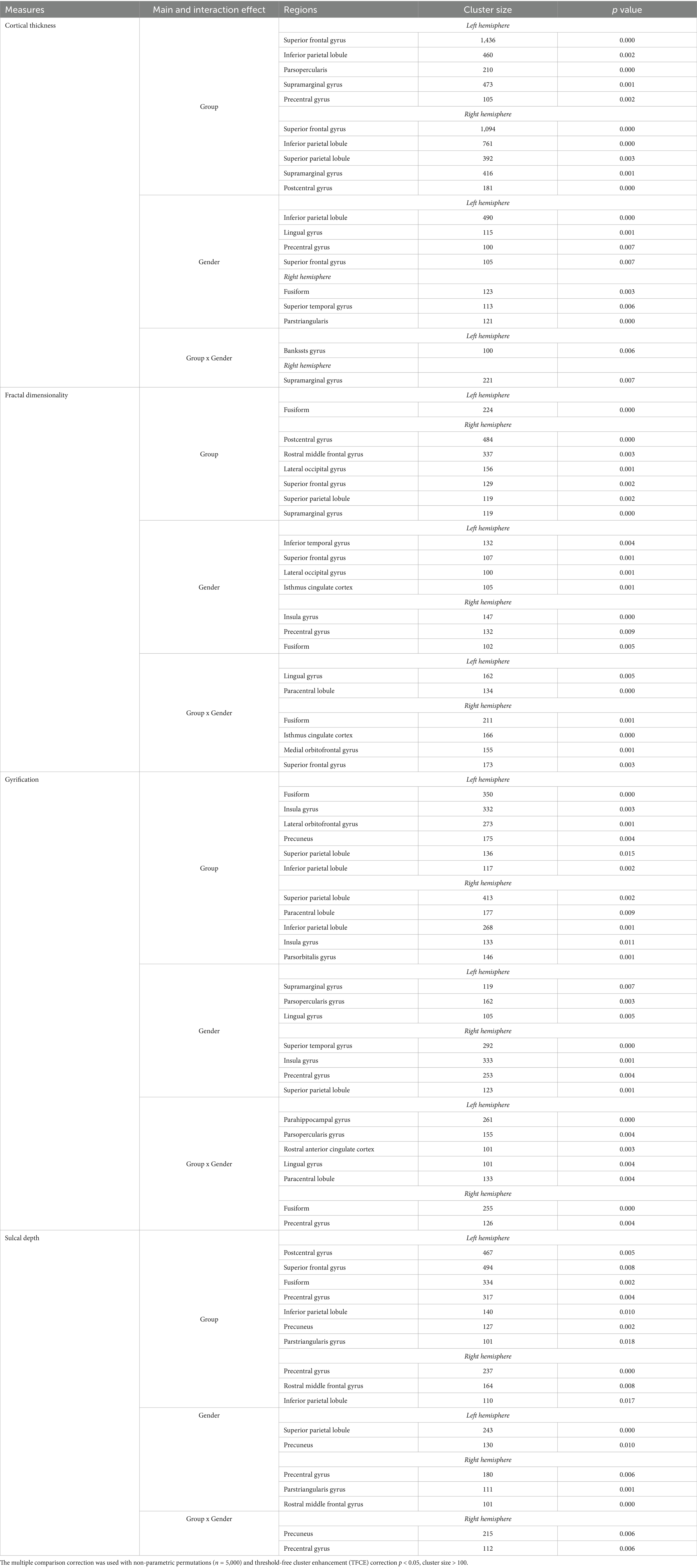
(1) The main effect of musical training was observed in the left pars opercularis and precentral gyri, postcentral gyrus, superior parietal lobule, and in the superior frontal, supramarginal gyri, and inferior parietal lobule in both hemispheres. (2) Gender had a main effect on the left lingual, precentral, superior frontal gyri, and inferior parietal lobule as well as the right fusiform, superior temporal, and pars triangularis gyri. (3) Musical training and gender had a significant interaction effect in the left banks of the superior temporal sulcus (bankssts) and right supramarginal gyrus (see Table 1 and Figure 1A).
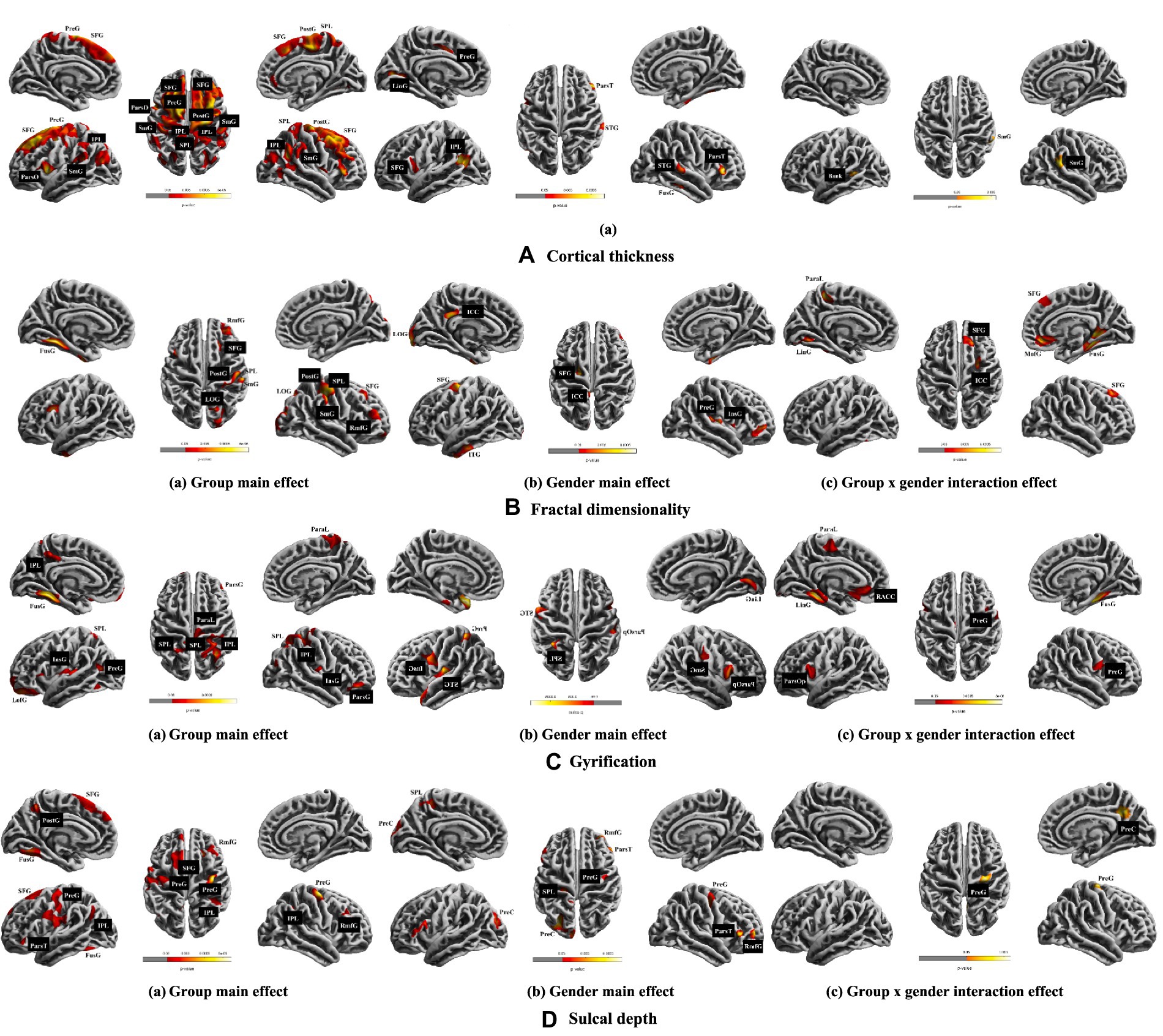
Figure 1. Results of ANCOVAs (with age and cranium volume as covariates) for all main variables. The multiple comparison correction was used with non-parametric permutations (n = 5,000) and threshold-free cluster enhancement (TFCE) correction p < 0.05, cluster size > 100. The brain left/right hemisphere in the figure is also the actual brain left/right hemisphere. A, B, C, and D shows the cortical thickness, fractal dimensionality, gyrification, and sulcal depth, respectively. SFG, superior frontal gyrus; SPL, superior parietal lobule; IPL, inferior parietal lobule; ParaL, paracentral lobule; ParsO, parsopercularis; ParsT, Parstriangularis; ParsG, parsopercularis gyrus; SmG, supramarginal gyrus; PreG, precentral gyrus; PostG, postcentral gyrus; LinG, lingual gyrus; STG, superior temporal gyrus; ITG, inferior temporal gyrus; Bank, bankssts gyrus; RmfG, rostral middle frontal gyrus; LOG, lateral occipital gyrus; ICC, isthmus cingulate cortex; InsG, insula gyrus; FusG, fusiform gyrus; LofG, lateral orbitofrontal gyrus; PreC, precuneus; RACC, rostral anterior cingulate cortex.
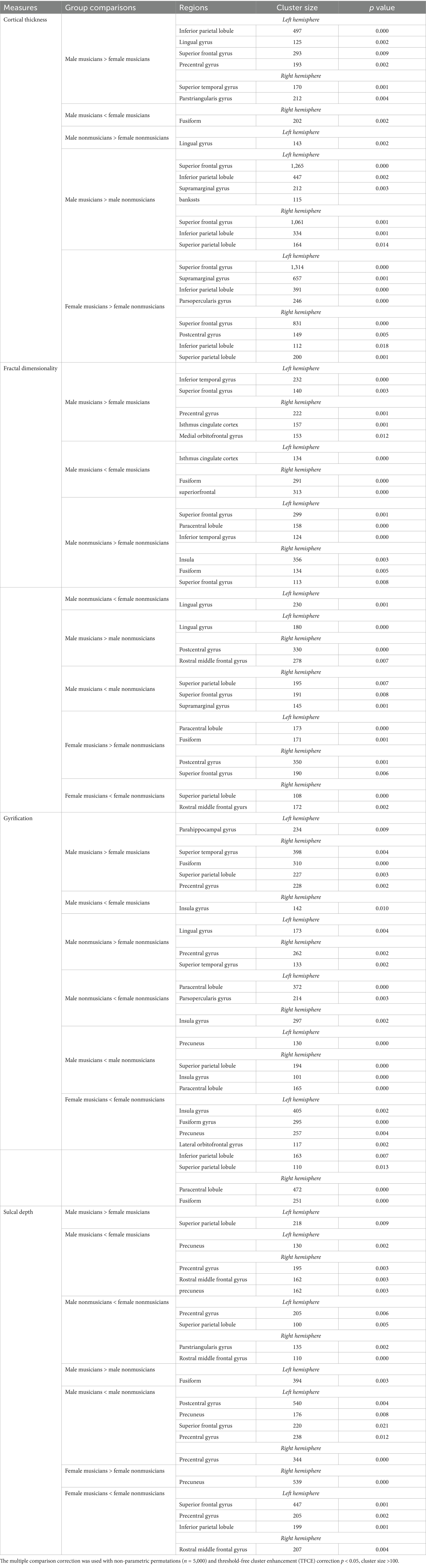
Post hoc analysis indicated the following: (1) Male musicians exhibited greater cortical thickness in the left superior frontal, lingual, precentral, and inferior parietal lobule, as well as in the right superior temporal and pars triangularis gyri, but reduced cortical thickness in the right fusiform gyrus compared to female musicians. (2) Male non-musicians showed greater cortical thickness in the left lingual gyrus compared to female non-musicians. (3) Male musicians had greater cortical thickness compared to male non-musicians in the left superior frontal, supramarginal, bankssts gyri, and inferior parietal lobule, as well as in the right superior frontal, superior and inferior parietal lobules (4) Female musicians exhibited increased cortical thickness compared to female non-musicians in the left superior frontal, supramarginal, pars opercularis gyri, inferior parietal lobule, and in the right superior frontal, postcentral gyri, and superior and inferior parietal lobules (see Table 2).
3.2.2 Fractal dimensionality
(1) Musical training had a main effect on the left fusiform gyrus and the right postcentral, rostral middle frontal, superior frontal, lateral occipital, supramarginal gyri, and superior parietal lobule. (2) Gender had a main effect on the left inferior temporal, superior frontal, lateral occipital and isthmus cingulate gyri, as well as on the right insula, precentral, and fusiform gyri. (3) Interaction effects between musical training and gender were seen in the left lingual gyrus and paracentral lobule, and in the right fusiform, isthmus cingulate, medial orbitofrontal, and superior frontal gyri (see Table 1 and Figure 1B).
Post hoc analysis showed that: (1) Male musicians had greater fractal dimensionality in the left superior frontal and inferior temporal gyri, and in the right precentral, medial orbitofrontal gyri, and isthmus cingulate cortex, but reduced fractal dimensionality in the left isthmus cingulate cortex and right superior frontal and fusiform gyri, compared to female musicians. (2) Male non-musicians had higher fractal dimensionality in the left superior frontal and inferior temporal gyri and paracentral lobule, as well as in the right insula, fusiform, and superior frontal gyri, but lower fractal dimensionality in the left lingual gyrus, than female nonmusicians. (3) Male musicians had increased fractal dimensionality compared to male non-musicians in the left lingual gyrus and right postcentral and rostral middle frontal gyri, but had reduced values in the right superior frontal, supramarginal gyri, and superior parietal lobule. (4) Female musicians had increased fractal dimensionality compared to female non-musicians in the left fusiform gyrus and paracentral lobule, and in the right postcentral and superior frontal gyri, but reduced values in the right rostral middle frontal gyrus and superior parietal lobule (see Table 2).
3.2.3 Gyrification
(1) Musical training had a main effect on the left fusiform, lateral orbitofrontal, and precuneus gyri, and on the right pars orbitalis gyrus and paracentral lobule, as well as on the insula gyrus and superior and inferior parietal lobules in both hemispheres. (2) Gender had a main effect on the left supramarginal, pars opercularis and lingual gyri, and on the right superior temporal, insula, precentral gyri and superior parietal lobule. (3) Interaction effects between musical training and gender were observed in the left parahippocampus, pars opercularis, rostral anterior cingulate, lingual gyri, and paracentral lobule, and in the right fusiform and precentral gyri (see Table 1 and Figure 1C).
Post hoc analysis showed that: (1) Male musicians had increased gyrification in the left parahippocampus and in the right superior temporal, fusiform, and precentral gyri, and superior parietal lobule, but had reduced gyrification in the right insula compared to female musicians. (2) Male non-musicians exhibited greater gyrification in the left lingual gyrus and right precentral and superior temporal gyri, but had reduced gyrification in the left pars opercularis, paracentral lobule, and right insula compared to female non-musicians. (3) Male musicians had reduced gyrification compared to male nonmusicians in the left precuneus and in the right insula, superior parietal, and paracentral lobules. (4) Female musicians showed reduced gyrification compared to female non-musicians in the left insula, fusiform, precuneus, lateral orbitofrontal gyri, and superior and inferior parietal lobules, as well as in the right fusiform gyrus and paracentral lobule (see Table 2).
3.2.4 Sulcal depth
(1) Musical training had a main effect on the left postcentral, superior frontal, fusiform, precuneus, and pars triangularis gyri, on the right rostral middle frontal gyrus, and on the precentral gyrus and inferior parietal lobule in both hemispheres. (2) Gender had a main effect on the left precuneus and superior parietal lobule, and on the right precentral, pars triangularis, and rostral middle frontal gyri. (3) Musical training and gender had interaction effects in the right precuneus and precentral gyri (see Table 1 and Figure 1D).
Post hoc analysis showed that: (1) Male musicians had greater sulcal depth in the left superior parietal lobule, but had reduced sulcal depth in the left precuneus and in the right precentral, rostral middle frontal, and precuneus compared to female musicians. (2) Male non-musicians exhibited reduced sulcal depth compared female non-musicians in the left precentral gyrus and superior parietal lobule, as well as in the right pars triangularis and rostral middle frontal gyri. (3) Male musicians had greater sulcal depth compared to male non-musicians in the left fusiform gyrus, but reduced sulcal depth in the left postcentral, precentral, precuneus, and superior frontal gyri, as well as in the right precentral gyrus. (4) Female musicians showed increased sulcal depth in the right precuneus, but decreased sulcal depth in the left superior frontal and precentral gyri, inferior parietal lobule, and in the right rostral middle frontal gyrus compared to female non-musicians (see Table 2).
4 Discussion
In the current study, we investigated differences in cortical thickness, fractal dimensionality, gyrification, and sulcal depth between musicians and non-musicians. In addition to comparing the effects of musical training on brain structural development, we also analyzed how these structural indicators vary across specific brain regions in conjunction with gender characteristics.
4.1 Cortical thickness—musicians versus non-musicians
Cortical thickness in the frontal regions of the brain serves as important evidence for the structural impact of musical training. In the current study, musicians demonstrated significantly greater cortical thickness in the bilateral superior frontal gyrus and the right middle frontal gyrus compared to non-musicians. These findings are consistent with prior research suggesting that increased cortical thickness in the superior frontal gyrus may reflect the substantial cognitive demands placed on networks involved in mnemonic retention, monitoring, and retrieval during many years of musical training (Bermudez et al., 2009; Petrides and Pandya, 2002). Similar patterns were also observed in child musicians (ages 9–11) and elderly musicians (ages 50–80), where the posterior segment of the superior frontal gyrus was thicker than in non-musicians (Ghosh et al., 2024; Habibi et al., 2020), suggesting that frontal regions play an important role in lifetime musical development.
Additionally, musicians showed greater cortical thickness in the caudal and rostral middle frontal gyrus, consistent with previous studies (Bermudez et al., 2009). This region has been shown to activate during both passive listening and explicit sound identity comparison tasks, suggesting its involvement in developing a stable representation of the environment in the face of variable auditory information – an essential function for musicians (Giordano et al., 2014). Furthermore, the precentral gyrus, a core sensorimotor area, was also thicker in musicians, as consistent with prior research (Bermudez et al., 2009). Long-term training with drums and wind instruments has been associated with plasticity in the region, and is linked to motor execution (Bruchhage et al., 2020; Choi et al., 2018). In addition, clinical and behavioral studies have shown that this cluster is further associated with motor processes requiring perceptive feedback and strong attentional control (Bruchhage et al., 2020), suggesting that musical training may enhance some aspects of attention, even in an extra-musical context (Roman-Caballero et al., 2021).
Altogether, these findings provide structural evidence for musicians’ cognitive advantages in frontal regions of the brain and perceptual processing strengths in the precentral gyrus. Notably, both regions are also key components of the functional brain networks that are activated when listening to music (Liu et al., 2021, 2022). Future studies may benefit from incorporating these regions into structural network analyses in order to further explore the influence of music on brain plasticity.
4.2 Cortical thickness—interactions between group and gender
When examining the interactions between musical training and gender, the left bankssts was found to be thicker in male musicians compared to male non-musicians. Bankssts thickness is an important brain index for gender differences in brain development and executive function, including working memory, reading comprehension, and fluency (Wierenga et al., 2019). In Wierenga’s two-year longitudinal study, it was found that the left bankssts in boys had a steeper decline in surface area compared to the left bankssts in girls, suggesting a distinct developmental trajectory. Given the prominent executive functions of musicians, the thicker bankssts in male musicians may reflect a developmental advantage.
Interestingly, however, the left bankssts was found to be the area of the brain with the highest β-amyloid deposition in cognitively normal elderly adults (Guo et al., 2020), making it a potential early biomarker for Alzheimer’s disease (AD) (Wee et al., 2013). The thicker bankssts in male musicians compared with male-nonmusicians suggests that musical training and cortical thickness may serve as analytical evidence for cognitive processing and may even be used to predict cognitive decline, ultimately enhancing clinical diagnostic strategies.
4.3 Fractal dimensionality and sulcal depth—musicians versus nonmusicians
Our analysis revealed that musicians exhibited higher fractal dimensionality and sulcal depth in the left fusiform and right postcentral gyri compared to non-musicians. Fractal dimensionality is a useful measure of cortical complexity, ranging from molecular architecture to whole-brain morphometry (Di Ieva et al., 2014, 2015; Meregalli et al., 2022). Musical training has been associated with increased activation in the left fusiform gyrus (Schmithorst and Holland, 2003), which has also been implicated in the perception of sound richness in a PET study (Satoh et al., 2015). Furthermore, in a systematic review of the literature on clinical and nonclinical samples, the fractal dimensionality of the left fusiform was also found to be higher in healthy participants compared to patients with bipolar disorder (Meregalli et al., 2022), suggesting that this structural advantage may reflect enhanced emotional regulation and cognitive development in musicians.
Sulcal depth has been studied as an important neuroimaging biomarker for brain diseases and has been widely used to study the morphological characteristics of cerebral folding (Im et al., 2008; Shin et al., 2022). The increased sulcal depth in the fusiform gyrus seen in musicians may reflect domain-specific perceptual expertise. This region’s close relationship with the postcentral gyrus further supports its role in cognitive development (Yao et al., 2023). While fractal dimensionality and sulcal depth have not been discussed in conjunction with existing research, the integration of these two metrics may lead to improved computational analyses of brain structure.
4.4 Fractal dimensionality—interactions between group and gender
When considering the interaction between musical training and gender, female musicians showed greater fractal dimensionality in the left fusiform gyrus compared to female non-musicians, while male musicians showed greater fractal dimensionality in the right postcentral gyrus compared to male non-musicians. These gender-specific patterns may suggest that female musicians are more attenuated to emotional perception via fusiform activation, while male musicians may engage sensorimotor processes via the postcentral gyrus (Shah et al., 2021).
4.5 Gyrification—musicians versus non-musicians
In terms of gyrification, non-musicians exhibited greater cortical folding in several regions, including the bilateral insula, right inferior and superior parietal lobules, posterior cingulate cortex, and superior temporal gyrus compared to musicians. Gyrification is often quantified using the ‘gyrification index’ (Zilles et al., 1988), and higher values indicate a higher degree of cortical folding, a developmental marker for brain maturation (White et al., 2010). In contrast to cortical thickness, fractal dimensionality, and sulcal depth—gyrification was more prominent in non-musicians. These differences were widely distributed across multiple brain regions, including the insula, parietal lobules, central gyrus, and temporal gyrus. Cortical folding is the result of complex cellular and mechanical processes that involve neural stem progenitor cells and their lineages, the migration and differentiation of neurons, and the genetic programs that regulate and fine-tune these processes (Fernandez and Borrell, 2023). Increased gyrification has been seen in the auditory cortices of both young and elderly musicians (Benner et al., 2017; Rus-Oswald et al., 2022). The occurrence of nonmusicians’ increased gyrification here may be due to a false outcome caused by insufficient sample size. In the future, an increased sample size will allow for the investigation of differences in gyrification between musicians and nonmusicians.
4.6 Limitations
The current study had several limitations. First, the relatively small sample size in musicians and non-musicians necessitates future follow-up studies with larger samples to validate these findings. Second, although singing musicians and instrumental musicians have similar or overlapping neural functions or structures, there are also differences between them (Ghosh et al., 2024); for example, singers may exhibit enhanced activation in language-dominant brain regions more than instrumentalists; this difference stems from the unique nature of vocal motor training, which involves the physical production of sound through speech-like movements, contrasting with playing an instrument using one’s hands (Christiner and Reiterer, 2015). Therefore, it is necessary for future research to further group instrumental musicians and singing musicians for more detailed investigation. Third, no behavioral measure was utilized in the current study, which may not adequately capture the multifaceted aspects of music acquisition. Future studies should therefore incorporate behavioral measures and explore their associations with brain structural brain morphometry changes to achieve a more integrative understanding.
5 Conclusion
As one of the few studies to systematically analyze the effects of musical training on structural brain development, the current study identified key differences in brain morphology between musicians and non-musicians across four measures: cortical thickness, fractal dimensionality, gyrification, and sulcal depth. Our findings suggest that increased cortical thickness in the frontal lobe, along with greater fractal dimensionality and sulcal depth in the left fusiform and right postcentral gyri, provide important structural evidence for enhanced brain development in musicians. Conversely, stronger gyrifications in multiple brain regions in non-musicians offers new insight into the development of brain plasticity.
Despite these findings, our current understanding of the brain’s structural adaptations to music is still limited. Rather than relying on single morphological indicators, future research should aim to develop an analytical method that integrates multiple morphological indicators in order to provide a more comprehensive understanding of how the brain processes music and how musical training affects neuroplasticity.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The data used in current manuscript is a secondary data analysis of published data. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YL: Conceptualization, Funding acquisition, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. QH: Investigation, Methodology, Visualization, Writing – review & editing. TH: Investigation, Methodology, Supervision, Visualization, Writing – review & editing. JH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Fujian Normal University Research Start-Up Funding (Y0720304K05), Natural Science Foundation of China (32200822), Basic Research Project of Central University of China (SWU2209508), and Chongqing Social Science Planning Project (2018 and 2019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Angulo-Perkins, A., and Concha, L. (2019). Discerning the functional networks behind processing of music and speech through human vocalizations. PLoS One 14:e0222796. doi: 10.1371/journal.pone.0222796
Belfi, A. M., Kasdan, A., and Tranel, D. (2019). Anomia for musical entities. Aphasiology 33, 382–404. doi: 10.1080/02687038.2017.1409871
Benner, J., Wengenroth, M., Reinhardt, J., Stippich, C., Schneider, P., and Blatow, M. (2017). Prevalence and function of Heschl's gyrus morphotypes in musicians. Brain Struct. Funct. 222, 3587–3603. doi: 10.1007/s00429-017-1419-x
Bermudez, P., Lerch, J. P., Evans, A. C., and Zatorre, R. J. (2009). Neuroanatomical correlates of musicianship as revealed by cortical thickness and voxel-based morphometry. Cereb. Cortex 19, 1583–1596. doi: 10.1093/cercor/bhn196
Bruchhage, M. M. K., Ngo, G. C., Schneider, N., D'Sa, V., and Deoni, S. C. L. (2020). Functional connectivity correlates of infant and early childhood cognitive development. Brain Struct. Funct. 225, 669–681. doi: 10.1007/s00429-020-02027-4
Chen, J. H., Huang, N. X., Zou, T. X., and Chen, H. J. (2020). Brain cortical complexity alteration in amyotrophic lateral sclerosis: a preliminary fractal dimensionality study. Biomed. Res. Int. 2020:1521679. doi: 10.1155/2020/1521679
Choi, S., Park, S. G., and Lee, H. H. (2018). The analgesic effect of music on cold pressor pain responses: the influence of anxiety and attitude toward pain. PLoS One 13:e0201897. doi: 10.1371/journal.pone.0201897
Christiner, M., and Reiterer, S. M. (2015). A Mozart is not a Pavarotti: singers outperform instrumentalists on foreign accent imitation. Front. Hum. Neurosci. 9:482. doi: 10.3389/fnhum.2015.00482
Dahnke, R., Yotter, R. A., and Gaser, C. (2013). Cortical thickness and central surface estimation. NeuroImage 65, 336–348. doi: 10.1016/j.neuroimage.2012.09.050
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Di Ieva, A., Esteban, F. J., Grizzi, F., Klonowski, W., and Martin-Landrove, M. (2015). Fractals in the neurosciences, part II: clinical applications and future perspectives. Neuroscientist 21, 30–43. doi: 10.1177/1073858413513928
Di Ieva, A., Grizzi, F., Jelinek, H., Pellionisz, A. J., and Losa, G. A. (2014). Fractals in the neurosciences, part I: general principles and basic neurosciences. Neuroscientist 20, 403–417. doi: 10.1177/1073858413513927
Farokhian, F., Beheshti, I., Sone, D., and Matsuda, H. (2017). Comparing CAT12 and VBM8 for detecting brain morphological abnormalities in temporal lobe epilepsy. Front. Neurol. 8:428. doi: 10.3389/fneur.2017.00428
Fernandez, V., and Borrell, V. (2023). Developmental mechanisms of gyrification. Curr. Opin. Neurobiol. 80:102711. doi: 10.1016/j.conb.2023.102711
Ferreri, L., Mas-Herrero, E., Zatorre, R. J., Ripolles, P., Gomez-Andres, A., Alicart, H., et al. (2019). Dopamine modulates the reward experiences elicited by music. Proc. Natl. Acad. Sci. USA 116, 3793–3798. doi: 10.1073/pnas.1811878116
Gaab, N., Keenan, J. P., and Schlaug, G. (2003). The effects of gender on the neural substrates of pitch memory. J. Cogn. Neurosci. 15, 810–820. doi: 10.1162/089892903322370735
Gebel, B., Braun, C., Kaza, E., Altenmuller, E., and Lotze, M. (2013). Instrument specific brain activation in sensorimotor and auditory representation in musicians. NeuroImage 74, 37–44. doi: 10.1016/j.neuroimage.2013.02.021
Ghosh, A., Singh, S., S, M., Jagtap, T., and Issac, T. G. (2024). Music and the aging brain - exploring the role of long-term Carnatic music training on cognition and gray matter volumes. J. Neurosci. Rural Pract. 15, 327–333. doi: 10.25259/JNRP_605_2023
Giordano, B. L., Egermann, H., and Bresin, R. (2014). The production and perception of emotionally expressive walking sounds: similarities between musical performance and everyday motor activity. PLoS One 9:e115587. doi: 10.1371/journal.pone.0115587
Guo, T., Landau, S. M., and Jagust, W. J.Alzheimer's Disease Neuroimaging, I (2020). Detecting earlier stages of amyloid deposition using PET in cognitively normal elderly adults. Neurology 94, e1512–e1524. doi: 10.1212/WNL.0000000000009216
Habibi, A., Ilari, B., Heine, K., and Damasio, H. (2020). Changes in auditory cortical thickness following music training in children: converging longitudinal and cross-sectional results. Brain Struct. Funct. 225, 2463–2474. doi: 10.1007/s00429-020-02135-1
Halwani, G. F., Loui, P., Ruber, T., and Schlaug, G. (2011). Effects of practice and experience on the arcuate fasciculus: comparing singers, instrumentalists, and non-musicians. Front. Psychol. 2:156. doi: 10.3389/fpsyg.2011.00156
Hirakawa, H., Akiyoshi, J., Muronaga, M., Tanaka, Y., Ishitobi, Y., Inoue, A., et al. (2016). FKBP5 is associated with amygdala volume in the human brain and mood state: a voxel-based morphometry (VBM) study. Int. J. Psychiatry Clin. Pract. 20, 106–115. doi: 10.3109/13651501.2016.1144772
Im, K., Lee, J. M., Seo, S. W., Hyung Kim, S., Kim, S. I., and Na, D. L. (2008). Sulcal morphology changes and their relationship with cortical thickness and gyral white matter volume in mild cognitive impairment and Alzheimer's disease. NeuroImage 43, 103–113. doi: 10.1016/j.neuroimage.2008.07.016
Jin, K., Zhang, T., Shaw, M., Sachdev, P., and Cherbuin, N. (2018). Relationship between Sulcal characteristics and brain aging. Front. Aging Neurosci. 10:339. doi: 10.3389/fnagi.2018.00339
Kim, J. S., Lee, J. S., Park, M. H., Kang, H., Lee, J. J., Lee, H. J., et al. (2008). Assessment of cerebral glucose metabolism in cat deafness model: strategies for improving the voxel-based statistical analysis for animal PET studies. Mol. Imaging Biol. 10, 154–161. doi: 10.1007/s11307-008-0140-9
Kochunov, P., Thompson, P. M., Coyle, T. R., Lancaster, J. L., Kochunov, V., Royall, D., et al. (2008). Relationship among neuroimaging indices of cerebral health during normal aging. Hum. Brain Mapp. 29, 36–45. doi: 10.1002/hbm.20369
Koelsch, S. (2010). Towards a neural basis of music-evoked emotions. Trends Cogn. Sci. 14, 131–137. doi: 10.1016/j.tics.2010.01.002
Koelsch, S., Maess, B., Grossmann, T., and Friederici, A. D. (2003). Electric brain responses reveal gender differences in music processing. Neuroreport 14, 709–713. doi: 10.1097/00001756-200304150-00010
Kurth, F., Gaser, C., and Luders, E. (2015). A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM). Nat. Protoc. 10, 293–304. doi: 10.1038/nprot.2015.014
Li, Y., Wang, N., Wang, H., Lv, Y., Zou, Q., and Wang, J. (2021). Surface-based single-subject morphological brain networks: effects of morphological index, brain parcellation and similarity measure, sample size-varying stability and test-retest reliability. NeuroImage 235:118018. doi: 10.1016/j.neuroimage.2021.118018
Liu, Y., Lian, W., Zhao, X., Tang, Q., and Liu, G. (2021). Spatial connectivity and temporal dynamic functional network connectivity of musical emotions evoked by dynamically changing tempo. Front. Neurosci. 15:700154. doi: 10.3389/fnins.2021.700154
Liu, Y., Zhao, X., Tang, Q., Li, W., and Liu, G. (2022). Dynamic functional network connectivity associated with musical emotions evoked by different tempi. Brain Connect. 12, 584–597. doi: 10.1089/brain.2021.0069
Luders, E., Thompson, P. M., Narr, K. L., Toga, A. W., Jancke, L., and Gaser, C. (2006). A curvature-based approach to estimate local gyrification on the cortical surface. NeuroImage 29, 1224–1230. doi: 10.1016/j.neuroimage.2005.08.049
Madan, C. R., and Kensinger, E. A. (2017). Age-related differences in the structural complexity of subcortical and ventricular structures. Neurobiol. Aging 50, 87–95. doi: 10.1016/j.neurobiolaging.2016.10.023
Meregalli, V., Alberti, F., Madan, C. R., Meneguzzo, P., Miola, A., Trevisan, N., et al. (2022). Cortical complexity estimation using fractal dimension: a systematic review of the literature on clinical and nonclinical samples. Eur. J. Neurosci. 55, 1547–1583. doi: 10.1111/ejn.15631
Moller, C., Stupacher, J., Celma-Miralles, A., and Vuust, P. (2021). Beat perception in polyrhythms: time is structured in binary units. PLoS One 16:e0252174. doi: 10.1371/journal.pone.0252174
Petrides, M., and Pandya, D. N. (2002). Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 16, 291–310. doi: 10.1046/j.1460-9568.2001.02090.x
Roman-Caballero, R., Martin-Arevalo, E., and Lupianez, J. (2021). Attentional networks functioning and vigilance in expert musicians and non-musicians. Psychol. Res. 85, 1121–1135. doi: 10.1007/s00426-020-01323-2
Rus-Oswald, O. G., Benner, J., Reinhardt, J., Burki, C., Christiner, M., Hofmann, E., et al. (2022). Musicianship-related structural and functional cortical features are preserved in elderly musicians. Front. Aging Neurosci. 14:807971. doi: 10.3389/fnagi.2022.807971
Sa de Almeida, J., Lordier, L., Zollinger, B., Kunz, N., Bastiani, M., Gui, L., et al. (2020). Music enhances structural maturation of emotional processing neural pathways in very preterm infants. NeuroImage 207:116391. doi: 10.1016/j.neuroimage.2019.116391
Sachs, M. E., Damasio, A., and Habibi, A. (2015). The pleasures of sad music: a systematic review. Front. Hum. Neurosci. 9:404. doi: 10.3389/fnhum.2015.00404
Satoh, M., Yuba, T., Tabei, K., Okubo, Y., Kida, H., Sakuma, H., et al. (2015). Music therapy using singing training improves psychomotor speed in patients with Alzheimer's disease: a neuropsychological and fMRI study. Dement. Geriatr. Cogn. Dis. Extra 5, 296–308. doi: 10.1159/000436960
Schmithorst, V. J., and Holland, S. K. (2003). The effect of musical training on music processing: a functional magnetic resonance imaging study in humans. Neurosci. Lett. 348, 65–68. doi: 10.1016/s0304-3940(03)00714-6
Segado, M., Zatorre, R. J., and Penhune, V. B. (2021). Effector-independent brain network for auditory-motor integration: fMRI evidence from singing and cello playing. NeuroImage 237:118128. doi: 10.1016/j.neuroimage.2021.118128
Seiger, R., Ganger, S., Kranz, G. S., Hahn, A., and Lanzenberger, R. (2018). Cortical thickness estimations of FreeSurfer and the CAT12 toolbox in patients with Alzheimer's disease and healthy controls. J. Neuroimaging 28, 515–523. doi: 10.1111/jon.12521
Shah, M., Kurth, F., and Luders, E. (2021). The impact of aging on the subregions of the fusiform gyrus in healthy older adults. J. Neurosci. Res. 99, 263–270. doi: 10.1002/jnr.24586
Shenker, J. J., Steele, C. J., Zatorre, R. J., and Penhune, V. B. (2023). Using cortico-cerebellar structural patterns to classify early- and late-trained musicians. Hum. Brain Mapp. 44, 4512–4522. doi: 10.1002/hbm.26395
Shin, S. J., Kim, A., Han, K. M., Tae, W. S., and Ham, B. J. (2022). Reduced Sulcal depth in central sulcus of major depressive disorder. Exp. Neurobiol. 31, 353–360. doi: 10.5607/en22031
Sihvonen, A. J., Sarkamo, T., Leo, V., Tervaniemi, M., Altenmuller, E., and Soinila, S. (2017). Music-based interventions in neurological rehabilitation. Lancet Neurol. 16, 648–660. doi: 10.1016/S1474-4422(17)30168-0
Smith, S. M., and Nichols, T. E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44, 83–98. doi: 10.1016/j.neuroimage.2008.03.061
Wee, C. Y., Yap, P. T., and Shen, D.Alzheimer's Disease Neuroimaging, I (2013). Prediction of Alzheimer's disease and mild cognitive impairment using cortical morphological patterns. Hum. Brain Mapp. 34, 3411–3425. doi: 10.1002/hbm.22156
White, T., Su, S., Schmidt, M., Kao, C. Y., and Sapiro, G. (2010). The development of gyrification in childhood and adolescence. Brain Cogn. 72, 36–45. doi: 10.1016/j.bandc.2009.10.009
Wierenga, L. M., Bos, M. G. N., van Rossenberg, F., and Crone, E. A. (2019). Sex effects on development of brain structure and executive functions: greater variance than mean effects. J. Cogn. Neurosci. 31, 730–753. doi: 10.1162/jocn_a_01375
Yao, J. K., Voorhies, W. I., Miller, J. A., Bunge, S. A., and Weiner, K. S. (2023). Sulcal depth in prefrontal cortex: a novel predictor of working memory performance. Cereb. Cortex 33, 1799–1813. doi: 10.1093/cercor/bhac173
Yotter, R. A., Dahnke, R., Thompson, P. M., and Gaser, C. (2011a). Topological correction of brain surface meshes using spherical harmonics. Hum. Brain Mapp. 32, 1109–1124. doi: 10.1002/hbm.21095
Yotter, R. A., Nenadic, I., Ziegler, G., Thompson, P. M., and Gaser, C. (2011b). Local cortical surface complexity maps from spherical harmonic reconstructions. NeuroImage 56, 961–973. doi: 10.1016/j.neuroimage.2011.02.007
Yotter, R. A., Thompson, P. M., and Gaser, C. (2011c). Algorithms to improve the reparameterization of spherical mappings of brain surface meshes. J. Neuroimaging 21, e134–e147. doi: 10.1111/j.1552-6569.2010.00484.x
Yuksel, D., Engelen, J., Schuster, V., Dietsche, B., Konrad, C., Jansen, A., et al. (2018). Longitudinal brain volume changes in major depressive disorder. J. Neural Transm. (Vienna) 125, 1433–1447. doi: 10.1007/s00702-018-1919-8
Yun, H. J., Im, K., Jin-Ju, Y., Yoon, U., and Lee, J. M. (2013). Automated sulcal depth measurement on cortical surface reflecting geometrical properties of sulci. PLoS One 8:e55977. doi: 10.1371/journal.pone.0055977
Zhang, L., Butler, A. J., Sun, C. K., Sahgal, V., Wittenberg, G. F., and Yue, G. H. (2008). Fractal dimension assessment of brain white matter structural complexity post stroke in relation to upper-extremity motor function. Brain Res. 1228, 229–240. doi: 10.1016/j.brainres.2008.06.008
Zhuang, Y., Zeng, X., Wang, B., Huang, M., Gong, H., and Zhou, F. (2017). Cortical surface thickness in the middle-aged brain with White matter Hyperintense lesions. Front. Aging Neurosci. 9:225. doi: 10.3389/fnagi.2017.00225
Keywords: structural brain morphometry, gender, musical training, musicians, non-musicians
Citation: Liu Y, Huang Q, Hosseini T and Hou J (2025) Differences in structural brain morphometry between musicians and non-musicians. Front. Hum. Neurosci. 19:1638813. doi: 10.3389/fnhum.2025.1638813
Edited by:
Shyam Diwakar, Amrita Vishwa Vidyapeetham, IndiaReviewed by:
Edgar R. Eslit, St. Michael's College (Iligan), PhilippinesDalia Elleuch, University of Sfax, Tunisia
Copyright © 2025 Liu, Huang, Hosseini and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiancheng Hou, Ym9uam92aV9ob3VAMTYzLmNvbQ==
 Ying Liu
Ying Liu Qilin Huang1
Qilin Huang1 Thomas Hosseini
Thomas Hosseini Jiancheng Hou
Jiancheng Hou