- 1Additive Manufacturing Laboratory, Department of Biomechanics, University of Nebraska at Omaha, Omaha, NE, United States
- 2Division of Plastic and Reconstructive Surgery, Department of Surgery, University of Nebraska Medical Center, Omaha, NE, United States
- 3Department of Occupational Therapy Education, University of Kansas Medical Center, Kansas City, KS, United States
Phantom limb pain (PLP) after amputation is a multifaceted condition. Targeted muscle reinnervation (TMR) surgery coapts amputated nerves to motor nerves of regional muscles, closing the neuromuscular loop, enabling improved myoelectric prosthesis control and reducing PLP. Long-term effects of TMR and residual limb use have been observed; however, the short-term neural changes and their timeline are not understood. The purpose of this study was to examine the cortical changes shortly after TMR without a prosthesis, specifically the functional connectivity and hemispheric dominance during a motor task involving the affected limb. The case participant is a male 52 years old, with a left traumatic transradial amputation sustained 4 years earlier, scheduled for TMR surgery. Data was collected before and 2 months after TMR. Brain activity was recorded using functional near-infrared spectroscopy (fNIRS) while the participant performed a gross manual dexterity task (box and block test) using their phantom hand. Pain levels were assessed using a 10-point visual analog scale (VAS). Following TMR, the participant reported a VAS score of 0 and increased use of the amputated limb in daily activities. fNIRS analysis during the affected limb task showed a reduction in interhemispheric functional connectivity, prominently in the primary sensory cortex, where the average z-value decreased from 0.29 to 0.12 after TMR. In contrast, connectivity between the premotor and supplementary motor areas increased slightly, from 0.08 to 0.12. Overall, intrahemispheric correlations decreased, with opposite patterns observed across hemispheres. The largest changes occurred ipsilaterally: connectivity between the primary motor and sensory areas increased from 0.23 to 0.27, while contralaterally it decreased from 0.22 to 0.16. Conversely, connectivity between the primary motor and premotor areas increased contralaterally but decreased ipsilaterally. Hemispheric dominance calculated through the Laterality index (LI) shifted from bilateral (LI = 0.079) to ipsilateral (LI = 0.59), primarily driven by reduced activation in the contralateral primary motor cortex. These findings suggest that TMR alone can elicit measurable cortical changes in the early post-surgical period, alongside improvements in pain and functional limb use. They also support fNIRS as a non-invasive method for monitoring neural adaptation after TMR and enhance understanding of PLP mechanisms and recovery timelines.
1 Introduction
In the United States, 31,450 upper limb amputations occur annually, resulting in permanent functional limitation (Dillingham et al., 2002, 1998; Owings and Kozak, 1998). People with amputation commonly (87%) experience phantom limb pain (PLP) (Stankevicius et al., 2021). PLP is a localized pain with enigmatic origins and variable manifestations, tending to be more intense and constant in upper limb amputation, affecting all aspects of life, including mental health, life quality, and employment (Cole et al., 2009; Hill, 1999; Jang et al., 2011; Nikolajsen and Jensen, 2001; Padovani et al., 2015).
Targeted muscle reinnervation (TMR), a promising surgical intervention for PLP, was originally developed to improve myoelectric prosthesis control. TMR reroutes residual limb nerves to alternative muscles, preventing painful neuroma formation and closing the sensorimotor feedback loop. Surface electromyography (EMG) detects neuromuscular signals for myoelectric prosthesis control (Dumanian et al., 2019; Kuiken et al., 2009). Beyond functional benefits, TMR reduces PLP (Serino et al., 2017), and has been associated with altered functional connectivity and hemispheric dominance.
Functional connectivity, defined as the covariation of separate brain regions, provides a framework to study cortical pattern changes (Friston, 1994). Interhemispheric functional connectivity supports coordination of movement and sensory integration across hemispheres and is disrupted in stroke and amputation (Takeuchi et al., 2012). After amputation, interhemispheric sensorimotor connectivity is generally reduced compared to healthy controls (Bramati et al., 2019; Hahamy et al., 2015; Makin et al., 2013a; Zhang et al., 2018). Early evidence suggests TMR further modifies these patterns: one study reported reduced interhemispheric connectivity after TMR combined with therapy (Borrell et al., 2023b), another observed increased intrahemispheric connectivity between motor and sensory cortices, resembling healthy controls (Serino et al., 2017).
Hemispheric dominance offers an additional perspective. Functional magnetic resonance imaging (fMRI) studies use the Laterality Index (LI) to quantify asymmetry in cortical activation, a descriptive measure assessing overall hemispheric dominance (Galaburda et al., 1990; Güntürkün et al., 2020; Hutsler and Galuske, 2003; Nirkko et al., 2001; Seghier, 2008). In unimanual tasks, the sensorimotor cortex predominantly controls the contralateral body side, but amputation disrupts this balance. Reduced inhibition in the affected hemisphere leads to diffuse ipsilateral activation (Chen et al., 2013; Philip and Frey, 2014), a pattern observed in children with congenital limb deficiencies (Zuniga et al., 2021). Following TMR, cortical activity shifts back toward contralateral dominance. One study reported contralateral focused activation, resembling controls (Yao et al., 2015), while another combining TMR with individualized phantom limb therapy observed balanced activation changes, with increased activity in the primary contralateral motor areas (Borrell et al., 2023b). Following hand transplantation, cortical activity may shift back toward contralateral dominance in higher motor planning areas (Piza-Katzer et al., 2007).
Despite these advances, short-term cortical responses to TMR remain poorly defined. Early changes in hemispheric dominance and connectivity, occurring before long-term prosthesis-driven plasticity, are not well characterized (Yao et al., 2015). We address that gap by analyzing connectivity and dominance patterns in the early postoperative period. We hypothesize that TMR induces short-term cortical changes associated with pain reduction, enabling increased limb use before long-term prosthesis use-dependent plasticity and neuromuscular loop closure. Specifically, we hypothesize changes in interhemispheric functional connectivity after TMR, increases in intrahemispheric connectivity between the primary motor (M1) and primary sensory (S1) cortices in the contralateral hemisphere during task performance. Additionally, pre-TMR dominance will be biased away from the contralateral side, shifting post-TMR toward increased contralateral activation, with higher motor planning regions showing stronger activity first.
2 Materials and methods
2.1 Patient recruitment
The participant was referred by the performing surgeon. Inclusion criteria included the ability to follow task instructions, move the phantom limb, and complete both pre- and post-surgical data collection. Due to limited contraindications against fNIRS and the rarity of local TMR cases, there were no further contraindications beyond major upper limb motion limitation. The participant was a 52-year-old right-handed man, self-reported ambidextrous, a retired painter, with hypertension and diabetes, 6′2″ tall, and weighed 275 lbs. On July 4, 2020, he sustained a traumatic left transradial amputation, followed by recurrent neuroma formation and chronic pain requiring multiple excision surgeries. In March 2024, he underwent TMR to treat neuromas and enable myoelectric prosthesis use. The procedure involved excising median and ulnar nerve neuromas and transferring the nerves to the motor branches of multiple muscles at the elbow level. The median nerve was transferred to the motor branches of the pronator quadratus and the flexor digitorum superficialis muscles, and the ulnar nerve to the flexor carpi ulnaris muscle.
2.2 Experimental setup
Data was collected across two visits and supplemented with the surgeon’s notes (Figure 1A). The first visit occurred in February 2024, followed by the TMR procedure 21 days later in March. The second visit was in May 2024, 61 days after TMR; no prosthesis was used during that time. Each visit lasted 2 h; cortical activity was recorded while the participant performed a motor task. The protocol began and concluded with a 3-min rest, with tasks with the non-affected and then affected limb in between. Tasks involved 30 s of rest and 1 min of activity, repeated three times; analysis hereafter will be focused on the affected limb only. Post-TMR, Oxycodone was prescribed for 3–5 days for pain. The participant provided written informed consent, and the study was approved by the University of Nebraska Institutional Review Board.
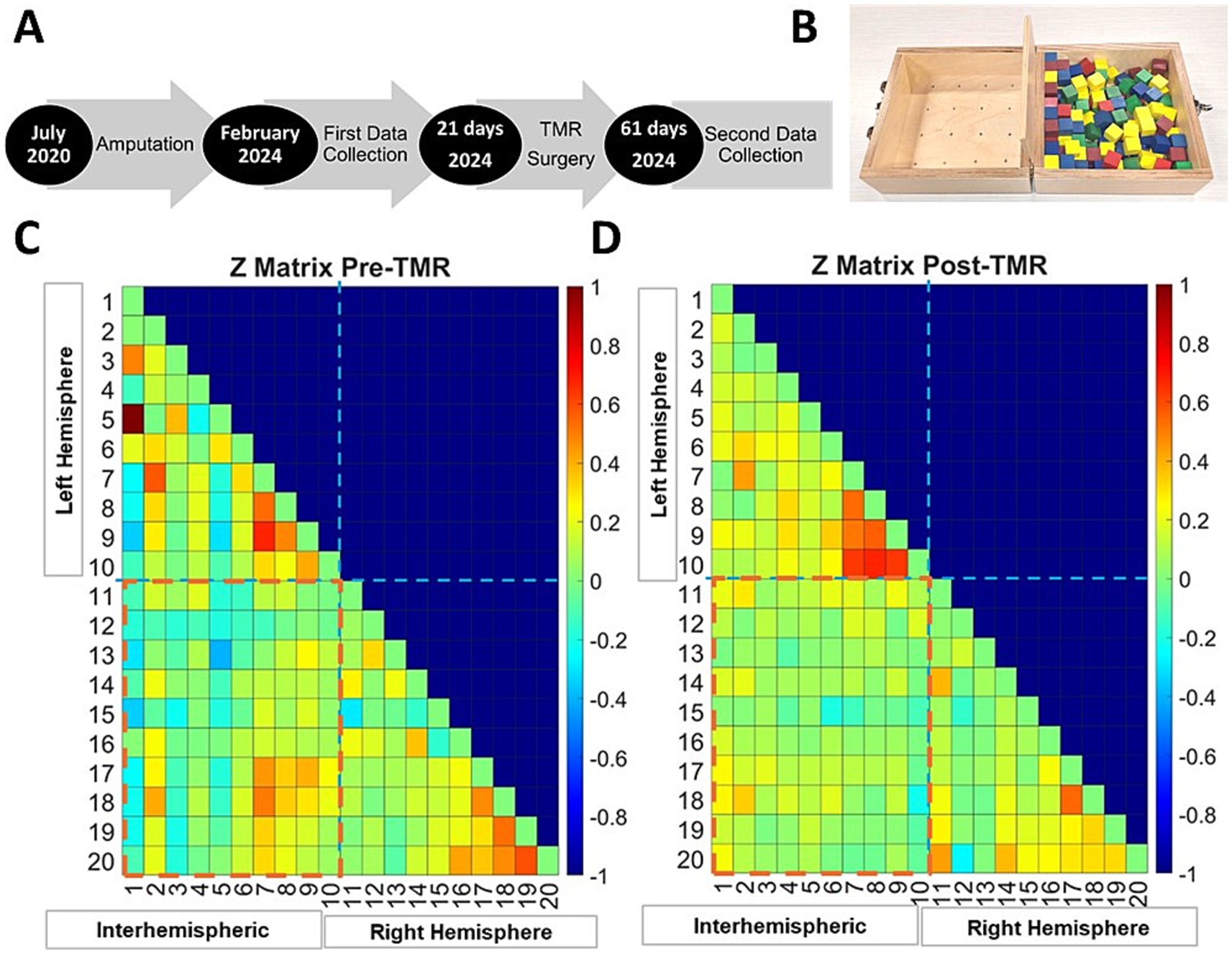
Figure 1. Timeline of surgeries and data collection (A). Box and blocks test, the task during which the fNIRS data was collected (B). Fisher-transformed correlation matrices showing functional connectivity strength in both hemispheres during the movement task. Intrahemispheric connections are displayed for the left and right hemispheres, and interhemispheric connections are indicated across the red dashed line for before TMR (C) and after (D).
2.3 Experimental protocol
2.3.1 Gross manual dexterity task motor imagery
The box and block test (Figure 1B), a measure of gross manual dexterity (Mathiowetz et al., 1985), was used as the motor task performed during cortical activity recording, which involves moving as many 1-inch blocks as possible from one side of the box to the other, across a divider, in 1 min. For the phantom limb despite lacking a physical hand, he attempted to perform each step to move the blocks, picking up the block, transferring it across the divider, and releasing it, without a prosthesis (Raffin et al., 2016; Scaliti et al., 2020). The unaffected limb was tested first for task familiarization.
2.3.2 Pain level
Pain and medication use were documented via interviews on data collection days and medical records. Pain was self-reported on a 10-point visual analog scale (1 = no pain, 10 = very painful) (Price et al., 1983).
2.3.3 Functional Near-Infrared Spectroscopy (fNIRS)
Functional Near-Infrared Spectroscopy (fNIRS) is a non-invasive method for measuring cortical activity a few centimeters below the surface (Pinti et al., 2020), through emitting near-infrared light at two wavelengths and detecting reflected signals to measure hemodynamic responses via changes in oxygenated (HbO2) and deoxygenated (HbR) hemoglobin (Scholkmann et al., 2014). In contrast to the gold standard fMRI, fNIRS is portable, movement-tolerant, and safe, making it suitable for active tasks and clinical populations contraindicated to fMRI (Scholkmann et al., 2014). Recent work demonstrated its utility for monitoring cortical activity, functional connectivity, and hemispheric activation in this group (Bai et al., 2020; Borrell et al., 2024, 2023a, 2023b; Karumattu Manattu et al., 2023; Li et al., 2023; Shen et al., 2025).
fNIRS data were collected using the NIRSport 2 system (NIRx Medical Technologies, LLC, Berlin, Germany), sampled at 8 Hz with wavelengths of 760 and 850 nm. The cap included 15 detectors, 16 sources, and 8 short-separation channels to filter superficial noise (Zhang et al., 2021). It was positioned over the sensorimotor cortex according to the international 10–20 system, covering the C3 and C4 landmarks to target upper limb motor activity (Nishiyori et al., 2016; The International Federation of Clinical Neurophysiology, n.d.).
2.4 Analysis
Analysis focused on the affected limb task. Trials with motion artifacts were discarded and repeated. Short separation channels were used to filter superficial physiological noise. This single-case study employed descriptive analysis to characterize observed patterns.
2.4.1 Pain
Pain was recorded on the visual analog scale, requiring no further analysis.
2.4.2 Task-based connectivity
For functional connectivity, the raw fNIRS data were processed with the NIRS Brain AnalyzIR toolbox (Santosa et al., 2018). The data were down-sampled to 4 Hz, optical density was computed, and the modified Beer–Lambert Law was applied to obtain Oxygenated hemoglobin (HbO) concentrations. Pearson correlation coefficients (r) were calculated using the toolbox’s ‘connectivity’ module, which uses an autoregressive robust correlation function to reduce confounding effects (Huppert, 2016; Santosa et al., 2017). Each correlation coefficient (r-value) represents the relationship between the hemodynamic signals of two channels and serves as a surrogate for functional connectivity. To normalize variance, connectivity values were transformed into Z-scores using Fisher’s transformation (Fisher, 1915).
2.4.3 Hemispheric dominance
Hemispheric dominance was computed using the laterality index (LI) formula (Equation 1). This formula incorporated all the oxygenated hemoglobin values from regions of interest, including the primary motor (M1) and primary sensory (S1) cortices independently, while the premotor cortex (PMC) and supplementary motor area (SMA) were combined for each hemisphere (Borrell et al., 2023a; Seghier, 2008). HbOL and HbOR represent the average oxygenated hemoglobin response over the three one-minute activity tasks, in the left and right hemispheres, respectively. The LI ranges from −1 to +1, where negative values indicate right hemispheric dominance, positive values indicate left hemispheric dominance, and LI values between −0.2 and +0.2 reflect bilateral dominance.
3 Results
3.1 Pain level
At the pre-TMR interview, the participant reported a pain level of 4 out of 10. Postoperatively, the reported pain level was zero, indicating complete relief.
3.2 Movement task functional connectivity
The Fisher transformed matrices (Figures 1C,D) illustrate fNIRS channels correlation strength, reflecting interhemispheric (red dashed box) and intrahemispheric connectivity for each hemisphere before (Figure 1C) and shortly after TMR (Figure 1D). Connectivity was analyzed across the primary motor cortex (M1), premotor/supplementary motor area (PMC/SMA), and primary sensory cortex (S1).
Interhemispheric functional connectivity changed following TMR (Figure 2). The first row illustrates the interhemispheric connectivity strength and the absolute corresponding Pearson correlation coefficients for motor-related channels, M1, PMC/SMA, and S1. Before the intervention (Figure 2A), most interhemispheric channel pairs showed weak correlations (r < 0.3), with moderate correlations (r = 0.4–0.55) in a few S1 channels. Following TMR (Figure 2B), the number of channels between the S1 regions decreased (Baseline = 5, Follow-up = 1), while those between the PMC/SMA increased (Baseline = 0, Follow-up = 4). The second row shows the same interhemispheric channels averaged per region, and overall hemispheric average. Pre-TMR (Figure 2C), the largest average value was between S1areas (z = 0.29), while smaller in M1 (z = 0.12) and PMC/SMA (z = 0.08). After TMR (Figure 2D), there was a decrease in both S1 (z = 0.12) and M1 (z = 0.07), with a slight increase between PMC/SMA (z = 0.12).
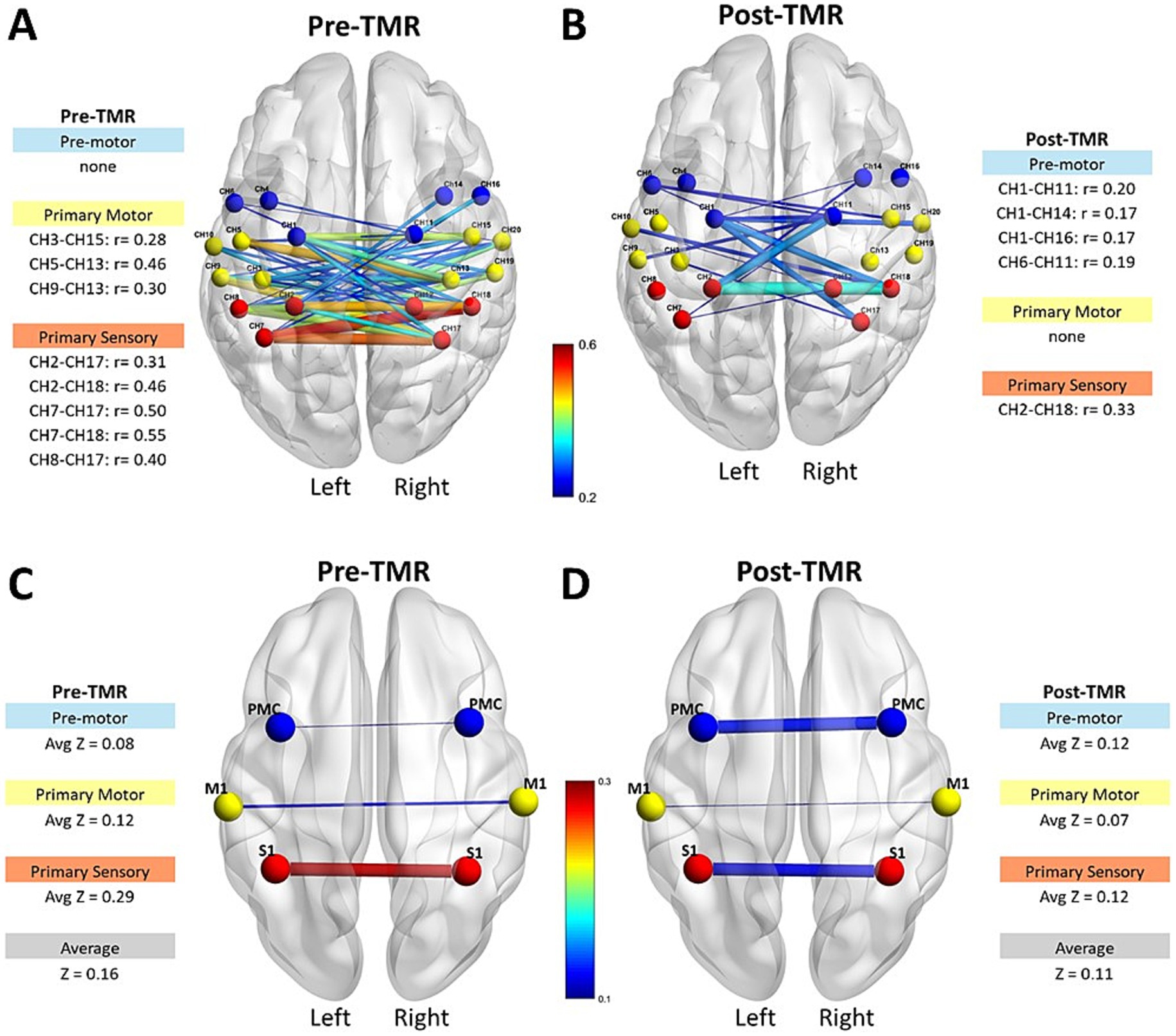
Figure 2. Interhemispheric connectivity strength and Pearson correlation coefficients in motor-related areas pre- and post-TMR intervention. The first row shows connectivity strength and correlation coefficients for M1, PMC/SMA, and S1 regions before (A) and after (B) the TMR surgery. The second row displays average z values per region and overall hemisphere connectivity before (C) and after (D) the TMR surgery.
Intrahemispheric functional connectivity also changed following TMR (Figure 3). The first row illustrates the intrahemispheric connectivity strength and corresponding absolute Pearson correlation coefficients within motor-related cortical channels, M1, PMC/SMA, and S1 within each hemisphere. Before TMR (Figure 3A), moderately strong correlations (r = 0.4–0.55) were observed in several channels within M1 and S1 in both hemispheres, with the sensory area showing the highest correlation (r = 0.6) on the ipsilateral (left) side. After TMR (Figure 3B), correlations decreased across all sensory channels, with the highest correlation dropping to r = 0.49 in both hemispheres. In the left PMC/SMA, the number of correlated channels increased from zero to two after TMR, though correlations remained weak. (r < 0.3). In the right PMC/SMA, three premotor channels were initially correlated; after TMR, two weakened, while one channel (CH11–CH14) strengthened from r = 0.256 to 0.365. The second row shows average z values for intrahemispheric channels between regions. Prior to TMR (Figure 3C), the left hemisphere (ipsilateral side) showed the highest z value between the PMC/SMA-M1 (z = 0.27), while the z value between the primary sensory and premotor areas was 0.23. On the right hemisphere, the pattern was reversed: the highest z value was between the S1-M1 (z = 0.22), and between PMC/SMA-M1 was 0.15, giving an overall average of z = 0.12, considerably lower than the left hemisphere (Z = 0.20). After TMR (Figure 3D), this pattern shifted. On the left hemisphere, the z value between PMC/SMA-M1 decreased to 0.20, while the z value between M1-S1 increased to 0.27. On the right hemisphere, the values remained lower and showed the reversed pattern.
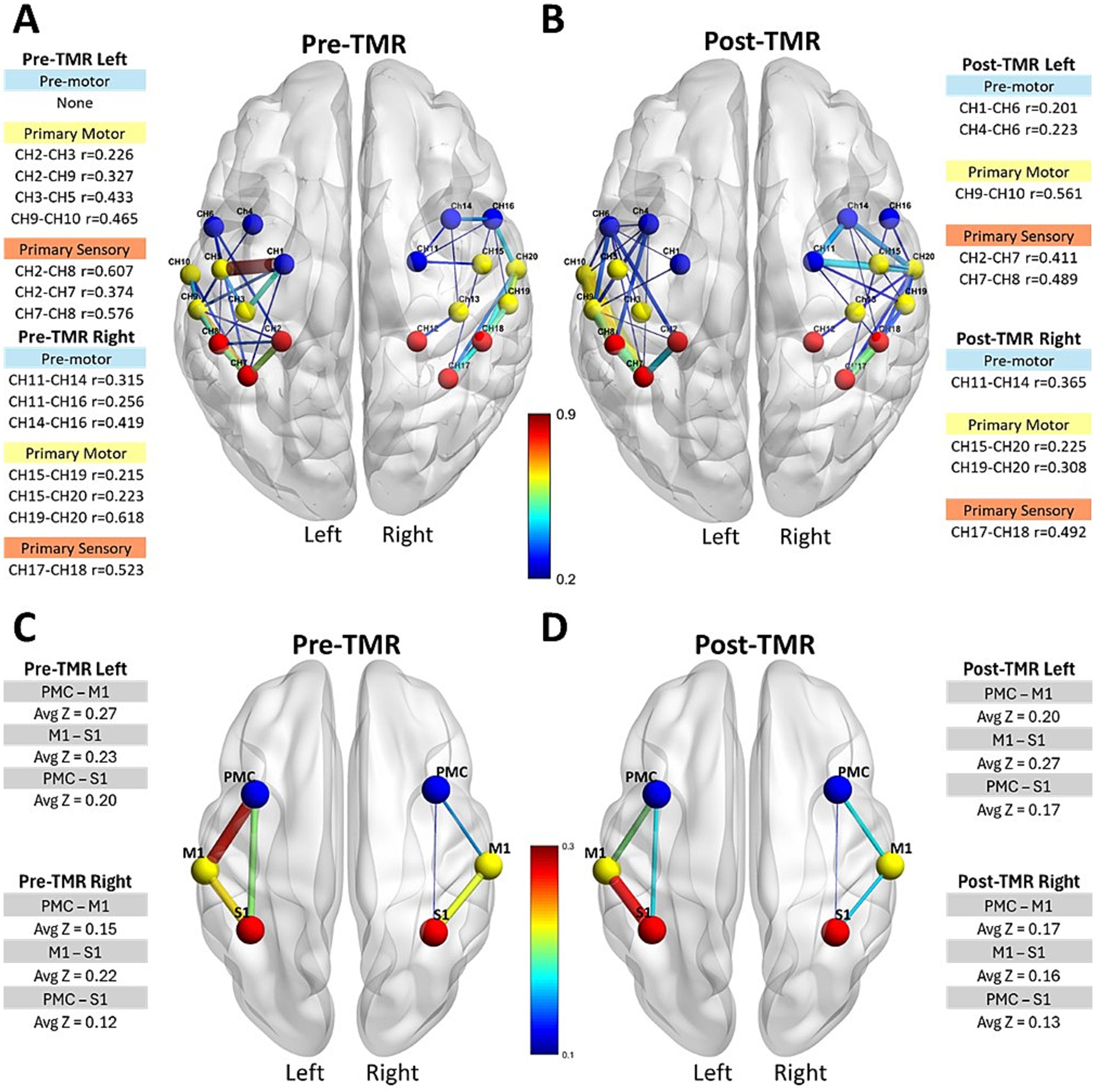
Figure 3. Intrahemispheric connectivity strength and Pearson correlation coefficients in motor-related cortical areas. The first row shows connectivity strength and correlation coefficients for M1, PMC/SMA, and S1 regions within each hemisphere pre-TMR (A) and post-TMR Intervention (B). The second row displays average z values per region before (C) and after (D) the TMR surgery.
3.3 Hemispheric dominance
The overall Laterality Index (LI) (Figure 4) before TMR was 0.079, indicating bilateral hemispheric dominance slightly biased ipsilaterally. After TMR, LI increased to 0.59, indicating ipsilateral dominance. Descriptive, region-specific analysis, not illustrated here, suggested that the shift was primarily driven by reduced activation in the contralateral M1 cortex, while other regions remained relatively stable.
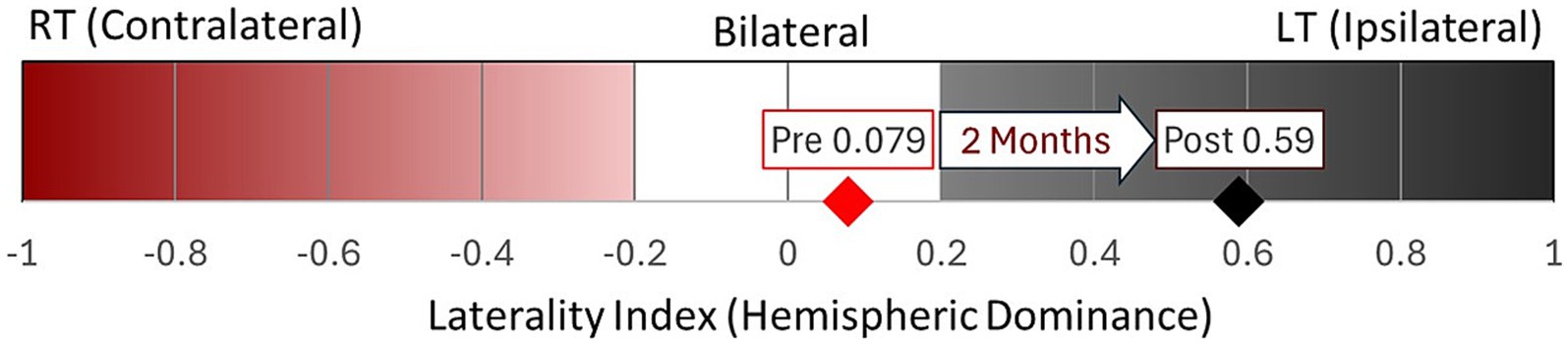
Figure 4. Overall laterality Index within motor-related regions pre- and post-TMR. Post-TMR, activation increased in the primary sensory areas bilaterally, with decreased activation in the contralateral primary motor and premotor regions.
4 Discussion
4.1 Pain
The etiology of PLP remains enigmatic. Traditionally, centrally, it was attributed to somatosensory cortex maladaptive reorganization, with early studies suggesting that increased use of the affected limb could reverse maladaptive plasticity and reduce pain (Flor et al., 1995; Lotze et al., 1999; Nikolajsen and Jensen, 2001). However, recent investigations critically challenge this view (Makin et al., 2013b; Makin and Bensmaia, 2017; Tucciarelli et al., 2024), concluding that cortical changes do not fulfil the criteria for “true reorganization,” which demands the emergence of novel input, novel computation, and distinct connectional fingerprint producing a new functional role. Cortical limb representation remains remarkably stable after sensory loss, and apparent remapping is better explained by the potentiation of pre-existing neural architecture. Given this and the short-term nature of our investigation, interpretations are limited to functional changes, as major structural reorganization, if present, is unlikely within this timeframe. In our participant, the pain was resolved, likely due to neuroma excisions.
4.2 Task based connectivity
4.2.1 Interhemispheric functional connectivity
Consistent with previous research in traumatic upper limb loss, interhemispheric connectivity was weak (Hahamy et al., 2015; Makin et al., 2013b). We hypothesized that connectivity would increase after TMR, following pain reduction and subsequent increased affected limb use without a prosthesis, since in people with limb loss, frequent engagement in bimanual tasks strengthens interhemispheric connectivity and pain modulates connectivity patterns (Hahamy et al., 2015; Makin et al., 2013a). Contrary to this, overall interhemispheric connectivity decreased. Reductions were most pronounced in M1 and S1, while areas involved in motor planning (PMC/SMA) (Roland et al., 1980) showed a slight increase.
The corpus callosum inhibits the contralateral hemisphere during unilateral tasks, silencing unused areas. Amputation disrupts this balance, reducing interhemispheric connectivity, especially among individuals reporting higher pain (Sparling et al., 2024). Microstructural alterations, including reduced white matter integrity as indicated by lower fractional anisotropy values in fibers connecting PMC/SMA, are associated with decreased interhemispheric communication in people with limb loss (Bramati et al., 2019; Li et al., 2017) while others show no significant differences compared to controls (Tucciarelli et al., 2024). After 2 months, the small, localized increase in PMC/SMA connectivity may reflect early functional adaptation and possible corpus callosum involvement, although this cannot be determined without structural data. Still, these regions are critical for motor planning and complex motor tasks, and controlling a missing hand without sensory feedback may heavily load these networks, potentially driving the observed connectivity increase (Li et al., 2024; Roland et al., 1980). This is particularly relevant as the participant reported increased limb use and no pain following surgery.
During motor imagery tasks, interhemispheric connectivity in the S1 cortex decreased to zero, consistent with a hand transplantation case study (Piza-Katzer et al., 2007). This may reflect pain relief following neuroma excision and, marginally, transient postoperative medication. In M1, connectivity also decreased, likely reflecting absent functional motor output as newly coapted motor nerves had not yet reinnervated target muscles. Reduced connectivity between M1 and S1 may indicate functional decoupling of the missing limb’s representation from corresponding contralateral areas (Borrell et al., 2023b; Makin et al., 2015). Together with localized PMC/SMA increases, this suggests early-stage functional adaptation after TMR.
4.2.2 Intrahemispheric functional connectivity
No consistent global pattern emerged across intrahemispheric connectivity analyses, with major changes occurring in the ipsilateral (left) rather than the contralateral (right) hemisphere. Before TMR, contralateral hemisphere connectivity was lower than ipsilateral, and both hemispheres showed further reductions after TMR. Across hemispheres, PMC/SMA–M1 connectivity was inversely related to M1–S1 connectivity. Regionally, ipsilateral PMC/SMA–M1 connectivity decreased post-TMR, while M1–S1 connectivity increased; contralateral connectivity remained low, with slight increases in PMC/SMA–M1 and decreases in M1–S1.
In people with lower-limb amputation and phantom sensation but no pain, increased intra-hemispheric connectivity occurs in the deafferented hemisphere during residual and intact limb stimulation, including primary and secondary sensory cortices (S1–S2) and primary motor and premotor areas (Bramati et al., 2019). In our upper-limb TMR patient, only slight increases in contralateral M1–PMC/SMA connectivity were observed post-TMR, with no clear S1–S2 changes. This likely reflects methodological and physiological factors, as no sensory stimulation was used, somatosensory regions were analyzed globally, and measurements occurred early post-TMR, before stabilization. These findings suggest early TMR plasticity emerges in motor circuits, with somatosensory changes appearing later or requiring task-based activation. If replicated, these findings may serve as a neuromarker, guiding early targeted upper motor rehabilitation post-TMR.
4.3 Interhemispheric dominance
Before TMR, hemispheric dominance was bilateral, consistent with reports that amputation disrupts interhemispheric connectivity and causes functional decoupling (Borrell et al., 2023b; Makin et al., 2015, 2013b). After TMR, dominance shifted ipsilateral, driven by reduced contralateral M1 activation rather than increased ipsilateral activity. This contrasts with contralateral dominance, typically reported in long-term studies involving repetitive motor practice, reinforcing sensorimotor networks (Serino et al., 2017; Yao et al., 2015). The shift likely reflects passive contralateral suppression due to recently severed efferent pathways, leaving contralateral M1 temporarily disconnected. Comparable reductions in excitability occur in stroke patients (Traversa et al., 1997), and in healthy individuals after short-term immobilization (Ngomo et al., 2012). We interpret this ipsilateral dominance as transient; over time, axonal regrowth and reinnervation should shift towards contralateral dominance. However, axonal regrowth occurs at approximately 1 mm/day (Gordon, 2020) with reliable EMG activity detected at 6 months (Al-Ajam et al., 2022).
5 Conclusion
Our short-term post-TMR investigation provides insights into neural plasticity, highlighting changes in both interhemispheric and intrahemispheric connectivity within the sensorimotor cortex and premotor regions. An increase in interhemispheric premotor connectivity appears to be one of the earliest observable signs of cortical adaptation during task-based connectivity assessments. Notably, hemispheric dominance shifts toward the ipsilateral side early in the process, likely due to reduced activation in the primary motor area of the contralateral hemisphere, before newly connected muscles begin to produce EMG signals. This case study demonstrates the potential of functional neuroimaging to assess cortical connectivity changes following TMR, offering a valuable tool for understanding and optimizing motor rehabilitation strategies.
6 Limitations
This single-case study has several limitations. Without controls, it is unclear whether changes reflect normalization or TMR-specific adaptations. The participant’s ambidexterity and profession as a painter limit the generalizability of observations to typical TMR patients. Effects of pain reduction, loop closure, and use-dependent plasticity could not be isolated. Additional confounders may have influenced the results, including psychological and mental health factors that affect cortical plasticity.
Methodologically, fNIRS spatial resolution limits analysis to cortical surface activity, and the single motor task may not represent broader changes. Without a longer follow-up, neural activity cannot be linked to functional improvements.
Finally, field gaps complicate interpretation. Limited literature document clear patterns of cortical change following upper limb amputation with clear timelines, inconsistent methodologies, and outcome measures. This hinders the prediction of specific neural adaptation patterns.
Together, these limitations highlight the need for controlled longitudinal studies with larger samples, standardized protocols, and multiple outcome measures.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The University of Nebraska at Omaha (UNMC) Institutional Review Board (IRB). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TMA: Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. KFW: Data curation, Software, Supervision, Writing – review & editing. KY: Data curation, Investigation, Writing – review & editing. JB: Supervision, Validation, Writing – review & editing. JZ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their gratitude to the University of Nebraska at Omaha and the University of Nebraska Medical Center for their invaluable support and resources. The authors would additionally like to thank the research participants for volunteering for the study and the clinical staff associated with the TMR procedure.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Ajam, Y., Woollard, A., and Kang, N. (2022). Advances in upper limb loss rehabilitation: the role of targeted muscle reinnervation and regenerative peripheral nerve interfaces. Plast. Aesthet. Res. 9:63. doi: 10.20517/2347-9264.2022.24
Bai, Z., Fong, K. N. K., Zhang, J., and Hu, Z. (2020). Cortical mapping of mirror visual feedback training for unilateral upper extremity: a functional near-infrared spectroscopy study. Brain Behav. 10:e01489. doi: 10.1002/BRB3.1489
Borrell, J. A., Fraser, K., Manattu, A. K., and Zuniga, J. M. (2023a). Laterality index calculations in a control study of functional near infrared spectroscopy. Brain Topogr. 36, 210–222. doi: 10.1007/S10548-023-00942-3
Borrell, J. A., Karumattu Manattu, A., Copeland, C., Fraser, K., D’Ovidio, A., Granatowicz, Z., et al. (2024). Prosthetic home intervention induces cortical plasticity in paediatrics with congenital limb reduction. Brain Commun. 6:fcae044. doi: 10.1093/BRAINCOMMS/FCAE044
Borrell, J. A., Manattu, A. K., Copeland, C., Fraser, K., D’Ovidio, A., Granatowicz, Z., et al. (2023b). Phantom limb therapy improves cortical efficiency of the sensorimotor network in a targeted muscle reinnervation amputee: a case report. Front. Neurosci. 17:50. doi: 10.3389/FNINS.2023.1130050
Bramati, I. E., Rodrigues, E. C., Simões, E. L., Melo, B., Höfle, S., Moll, J., et al. (2019). Lower limb amputees undergo long-distance plasticity in sensorimotor functional connectivity. Sci. Rep. 9:2518. doi: 10.1038/S41598-019-39696-Z
Chen, A., Yao, J., Kuiken, T., and Dewald, J. P. A. (2013). Cortical motor activity and reorganization following upper-limb amputation and subsequent targeted reinnervation. Neuroimage Clin. 3, 498–506. doi: 10.1016/J.NICL.2013.10.001
Cole, J., Crowle, S., Austwick, G., and Henderson Slater, D. (2009). Exploratory findings with virtual reality for phantom limb pain; from stump motion to agency and analgesia. Disabil. Rehabil. 31, 846–854. doi: 10.1080/09638280802355197
Dillingham, T. R., Pezzin, L. E., and MacKenzie, E. J. (1998). Incidence, acute care length of stay, and discharge to rehabilitation of traumatic amputee patients: an epidemiologic study. Arch. Phys. Med. Rehabil. 79, 279–287. doi: 10.1016/S0003-9993(98)90007-7
Dillingham, T. R., Pezzin, L. E., and MacKenzie, E. J. (2002). Limb amputation and limb deficiency: epidemiology and recent trends in the United States. South. Med. J. 95, 875–883. doi: 10.1097/00007611-200208000-00018
Dumanian, G. A., Potter, B. K., Mioton, L. M., Ko, J. H., Cheesborough, J. E., Souza, J. M., et al. (2019). Targeted muscle Reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann. Surg. 270, 238–246. doi: 10.1097/SLA.0000000000003088
Fisher, R. A. (1915). Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika 10:507. doi: 10.2307/2331838
Flor, H., Elbert, T., Knecht, S., Wienbruch, C., Pantev, C., Birbaumers, N., et al. (1995). Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375, 482–484. doi: 10.1038/375482A0
Friston, K. J. (1994). Functional and effective connectivity in neuroimaging: a synthesis. Hum. Brain Mapp. 2, 56–78. doi: 10.1002/HBM.460020107
Galaburda, A. M., Rosen, G. D., and Sherman, G. F. (1990). Individual variability in cortical organization: its relationship to brain laterality and implications to function. Neuropsychologia 28, 529–546. doi: 10.1016/0028-3932(90)90032-J
Gordon, T. (2020). Peripheral nerve regeneration and muscle Reinnervation. Int. J. Mol. Sci. 21:8652. doi: 10.3390/IJMS21228652
Güntürkün, O., Ströckens, F., and Ocklenburg, S. (2020). Brain lateralization: a comparative perspective. Physiol. Rev. 100, 1019–1063. doi: 10.1152/PHYSREV.00006.2019
Hahamy, A., Sotiropoulos, S. N., Slater, D. H., Malach, R., Johansen-Berg, H., and Makin, T. R. (2015). Normalisation of brain connectivity through compensatory behaviour, despite congenital hand absence. eLife 2015:e04605. doi: 10.7554/ELIFE.04605.001
Hill, A. (1999). Phantom limb pain: a review of the literature on attributes and potential mechanisms. J. Pain Symptom Manag. 17, 125–142. doi: 10.1016/S0885-3924(98)00136-5
Huppert, T. J. (2016). Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy. Neurophotonics 3:010401. doi: 10.1117/1.NPH.3.1.010401
Hutsler, J., and Galuske, R. A. W. (2003). Hemispheric asymmetries in cerebral cortical networks. Trends Neurosci. 26, 429–435. doi: 10.1016/S0166-2236(03)00198-X
Jang, C. H., Yang, H. S., Yang, H. E., Lee, S. Y., Kwon, J. W., Yun, B. D., et al. (2011). A survey on activities of daily living and occupations of upper extremity amputees. Ann. Rehabil. Med. 35, 907–921. doi: 10.5535/ARM.2011.35.6.907
Karumattu Manattu, A., Borrell, J. A., Copeland, C., Fraser, K., and Zuniga, J. M. (2023). Motor cortical functional connectivity changes due to short-term immobilization of upper limb: an fNIRS case report. Front. Rehabil. Sci. 4:1156940. doi: 10.3389/FRESC.2023.1156940
Kuiken, T. A., Li, G., Lock, B. A., Lipschutz, R. D., Miller, L. A., Stubblefield, K. A., et al. (2009). Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA 301, 619–628. doi: 10.1001/JAMA.2009.116
Li, H., Fu, X., Lu, L., Guo, H., Yang, W., Guo, K., et al. (2023). Upper limb intelligent feedback robot training significantly activates the cerebral cortex and promotes the functional connectivity of the cerebral cortex in patients with stroke: a functional near-infrared spectroscopy study. Front. Neurol. 14:1042254. doi: 10.3389/FNEUR.2023.1042254
Li, Z., Li, C., Fan, L., Jiang, G., Wu, J., Jiang, T., et al. (2017). Altered microstructure rather than morphology in the corpus callosum after lower limb amputation. Sci. Rep. 7, 1–10. doi: 10.1038/SREP44780
Li, W., Zhu, G., Jiang, Y., Miao, C., Zhang, G., and Xu, D. (2024). Cortical response characteristics of passive, active, and resistance movements: a multi-channel fNRIS study. Front. Hum. Neurosci. 18:1419140. doi: 10.3389/FNHUM.2024.1419140
Lotze, M., Grodd, W., Birbaumer, N., Erb, M., Huse, E., and Flor, H. (1999). Does use of a myoelectric prosthesis prevent cortical reorganization and phantom limb pain? Nat. Neurosci. 2, 501–502. doi: 10.1038/9145
Makin, T. R., and Bensmaia, S. J. (2017). Stability of sensory topographies in adult cortex. Trends Cogn. Sci. 21, 195–204. doi: 10.1016/J.TICS.2017.01.002
Makin, T. R., Cramer, A. O., Scholz, J., Hahamy, A., Slater, D. H., Tracey, I., et al. (2013a). Deprivation-related and use-dependent plasticity go hand in hand. eLife 2, 1–15. doi: 10.7554/ELIFE.01273
Makin, T. R., Filippini, N., Duff, E. P., Henderson Slater, D., Tracey, I., and Johansen-Berg, H. (2015). Network-level reorganisation of functional connectivity following arm amputation. NeuroImage 114, 217–225. doi: 10.1016/J.NEUROIMAGE.2015.02.067
Makin, T. R., Scholz, J., Filippini, N., Henderson Slater, D., Tracey, I., and Johansen-Berg, H. (2013b). Phantom pain is associated with preserved structure and function in the former hand area. Nat. Commun. 4:1570. doi: 10.1038/NCOMMS2571
Mathiowetz, V., Volland, G., Kashman, N., and Weber, K. (1985). Adult norms for the box and block test of manual dexterity. Am. J. Occup. Ther. 39, 386–391. doi: 10.5014/AJOT.39.6.386
Ngomo, S., Leonard, G., and Mercier, C. (2012). Influence of the amount of use on hand motor cortex representation: effects of immobilization and motor training. Neuroscience 220, 208–214. doi: 10.1016/j.neuroscience.2012.06.018
Nikolajsen, L., and Jensen, T. S. (2001). Phantom limb pain. Br. J. Anaesth. 87, 107–116. doi: 10.1093/BJA/87.1.107
Nirkko, A. C., Ozdoba, C., Redmond, S. M., Bürki, M., Schroth, G., Hess, C. W., et al. (2001). Different ipsilateral representations for distal and proximal movements in the sensorimotor cortex: activation and deactivation patterns. NeuroImage 13, 825–835. doi: 10.1006/NIMG.2000.0739
Nishiyori, R., Bisconti, S., and Ulrich, B. (2016). Motor cortex activity during functional motor skills: an fNIRS study. Brain Topogr. 29, 42–55. doi: 10.1007/S10548-015-0443-5
Owings, M. F., and Kozak, L. J., (1998). Ambulatory and inpatient procedures in the United States, 1996. Vital and health statistics, series 13: data on health resources utilization 13. Hyattsville, Maryland: U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention National Center for Health Statistics.
Padovani, M. T., Martins, M. R. I., Venâncio, A., and Forni, J. E. N. (2015). Anxiety, depression and quality of life in individuals with phantom limb pain. Acta Ortop Bras 23, 107–110. doi: 10.1590/1413-78522015230200990
Philip, B. A., and Frey, S. H. (2014). Compensatory changes accompanying chronic forced use of the nondominant hand by unilateral amputees. J. Neurosci. 34, 3622–3631. doi: 10.1523/JNEUROSCI.3770-13.2014
Pinti, P., Tachtsidis, I., Hamilton, A., Hirsch, J., Aichelburg, C., Gilbert, S., et al. (2020). The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 1464, 5–29. doi: 10.1111/NYAS.13948
Piza-Katzer, H., Brenneis, C., Löscher, W. N., Benke, T., Schocke, M., Gabl, M. F., et al. (2007). Cortical motor activation patterns following hand transplantation and replantation. Acta Neurochir. Suppl. 100, 113–115. doi: 10.1007/978-3-211-72958-8_24
Price, D. D., McGrath, P. A., Rafii, A., and Buckingham, B. (1983). The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 17, 45–56. doi: 10.1016/0304-3959(83)90126-4
Raffin, E., Richard, N., Giraux, P., and Reilly, K. T. (2016). Primary motor cortex changes after amputation correlate with phantom limb pain and the ability to move the phantom limb. NeuroImage 130, 134–144. doi: 10.1016/j.neuroimage.2016.01.063
Roland, P. E., Larsen, B., Lassen, N. A., and Skinhoj, E. (1980). Supplementary motor area and other cortical areas in organization of voluntary movements in man. J. Neurophysiol. 43, 118–136. doi: 10.1152/JN.1980.43.1.118
Santosa, H., Aarabi, A., Perlman, S. B., and Huppert, T. J. (2017). Characterization and correction of the false-discovery rates in resting state connectivity using functional near-infrared spectroscopy. J. Biomed. Opt. 22:055002. doi: 10.1117/1.JBO.22.5.055002
Santosa, H., Zhai, X., Fishburn, F., and Huppert, T. (2018). The NIRS brain AnalyzIR toolbox. Algorithms 11:73. doi: 10.3390/A11050073
Scaliti, E., Gruppioni, E., and Becchio, C. (2020). And yet it moves: what we currently know about phantom arm movements. Neuroscientist 26, 328–342. doi: 10.1177/1073858420904326
Scholkmann, F., Kleiser, S., Metz, A. J., Zimmermann, R., Mata Pavia, J., Wolf, U., et al. (2014). A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. NeuroImage 85, 6–27. doi: 10.1016/J.NEUROIMAGE.2013.05.004
Seghier, M. L. (2008). Laterality index in functional MRI: methodological issues. Magn. Reson. Imaging 26, 594–601. doi: 10.1016/J.MRI.2007.10.010
Serino, A., Akselrod, M., Salomon, R., Martuzzi, R., Blefari, M. L., Canzoneri, E., et al. (2017). Upper limb cortical maps in amputees with targeted muscle and sensory reinnervation. Brain 140, 2993–3011. doi: 10.1093/BRAIN/AWX242
Shen, D., Yang, B., Li, J., and Gao, S. (2025). Effect of acupuncture treatment for upper limb on cortical activation and functional connectivity: a fNIRS study. Biomed. Signal Process. Control 99:106915. doi: 10.1016/J.BSPC.2024.106915
Sparling, T., Iyer, L., Pasquina, P., and Petrus, E. (2024). Cortical reorganization after limb loss: bridging the gap between basic science and clinical recovery. J. Neurosci. 44:e1051232024. doi: 10.1523/JNEUROSCI.1051-23.2023
Stankevicius, A., Wallwork, S. B., Summers, S. J., Hordacre, B., and Stanton, T. R. (2021). Prevalence and incidence of phantom limb pain, phantom limb sensations and telescoping in amputees: a systematic rapid review. Eur. J. Pain 25, 23–38. doi: 10.1002/EJP.1657
Takeuchi, N., Oouchida, Y., and Izumi, S. I. (2012). Motor control and neural plasticity through interhemispheric interactions. Neural Plast. 2012:823285. doi: 10.1155/2012/823285
The International Federation of Clinical Neurophysiology The ten-twenty electrode system of the International Federation, (n.d.) Available online at: https://pubmed.ncbi.nlm.nih.gov/10590970/ (Accessed January 11, 2025).
Traversa, R., Cicinelli, P., Bassi, A., Rossini, P. M., and Bernardi, G. (1997). Mapping of motor cortical reorganization after stroke. Stroke 28, 110–117. doi: 10.1161/01.STR.28.1.110
Tucciarelli, R., Ejaz, N., Wesselink, D. B., Kolli, V., Hodgetts, C. J., Diedrichsen, J., et al. (2024). Does ipsilateral remapping following hand loss impact motor control of the intact hand? J. Neurosci. 44:e0948232023. doi: 10.1523/JNEUROSCI.0948-23.2023
Yao, J., Chen, A., Kuiken, T., Carmona, C., and Dewald, J. (2015). Sensory cortical re-mapping following upper-limb amputation and subsequent targeted reinnervation: a case report. Neuroimage Clin 8, 329–336. doi: 10.1016/J.NICL.2015.01.010
Zhang, F., Cheong, D., Khan, A. F., Chen, Y., Ding, L., and Yuan, H. (2021). Correcting physiological noise in whole-head functional near-infrared spectroscopy. J. Neurosci. Methods 360:109262. doi: 10.1016/J.JNEUMETH.2021.109262
Zhang, J., Zhang, Y., Wang, L., Sang, L., Li, L., Li, P., et al. (2018). Brain functional connectivity plasticity within and beyond the sensorimotor network in lower-limb amputees. Front. Hum. Neurosci. 12:401731. doi: 10.3389/FNHUM.2018.00403/BIBTEX
Keywords: functional near infrared spectroscopy, targeted muscle reinnervation (TMR), phantom limb pain (PLP), amputation, hemodynamic response, neural plasticity, functional connectivity, hemispheric dominance
Citation: Mootaz AboElnour T, Fraser Wilsey K, Yang K, Borrell JA and Zuniga J (2025) Changes in hemispheric dominance following targeted muscle reinnervation: a case study. Front. Hum. Neurosci. 19:1665931. doi: 10.3389/fnhum.2025.1665931
Edited by:
Zhen Yuan, University of Macau, ChinaReviewed by:
Emily Petrus, National Institutes of Health (NIH), United StatesAndrea Demofonti, Campus Bio-Medico University, Italy
Copyright © 2025 Mootaz AboElnour, Fraser Wilsey, Yang, Borrell and Zuniga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jorge Zuniga, am16dW5pZ2FAdW5vbWFoYS5lZHU=
 Toka Mootaz AboElnour
Toka Mootaz AboElnour Kaitlin Fraser Wilsey
Kaitlin Fraser Wilsey Kai Yang2
Kai Yang2 Jordan Alexander Borrell
Jordan Alexander Borrell Jorge Zuniga
Jorge Zuniga