- 1Department of Neurology, Hebei Medical University Third Hospital, Shijiazhuang, Hebei, China
- 2Department of Neurology, Peking University People’s Hospital, Beijing, China
- 3Hebei Medical University Third Hospital, Shijiazhuang, Hebei, China
Background: This study aimed to investigate the mediating effect of enlarged perivascular space (EPVS) in basal ganglia (BG) on the relationship between glycated hemoglobin (HbA1c) levels and mild cognitive impairment (MCI) in patients with cerebral small vessel disease (CSVD).
Methods: Data on HbA1c levels and MOCA scores and CSVD imaging markers, including EPVS volume and distribution patterns were collected. Logistic regression was performed to identify independent risk factors for MCI. A mediation effect analysis was further conducted to determine whether BG-EPVS mediate the impact of HbA1c on cognitive impairment.
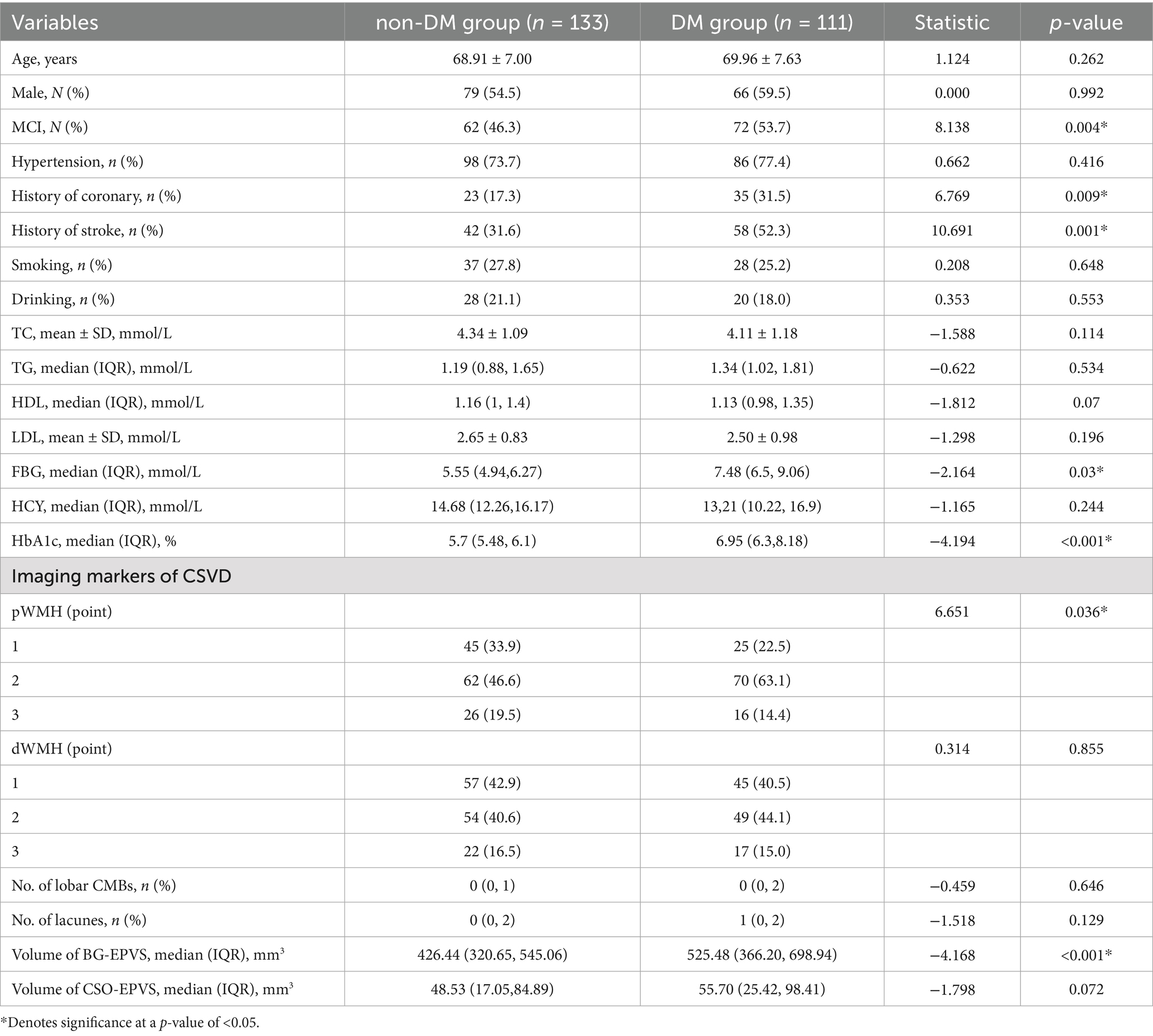
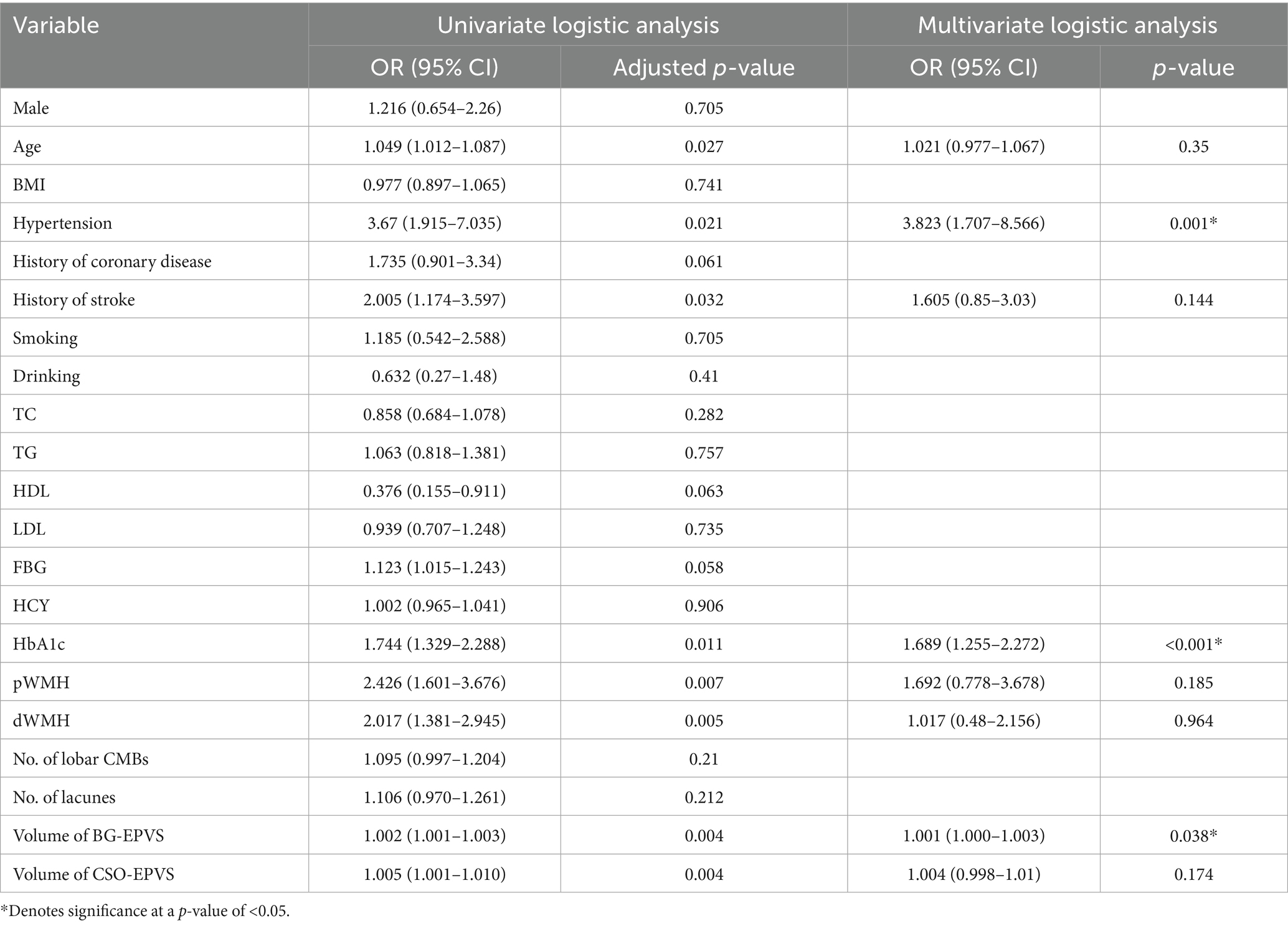
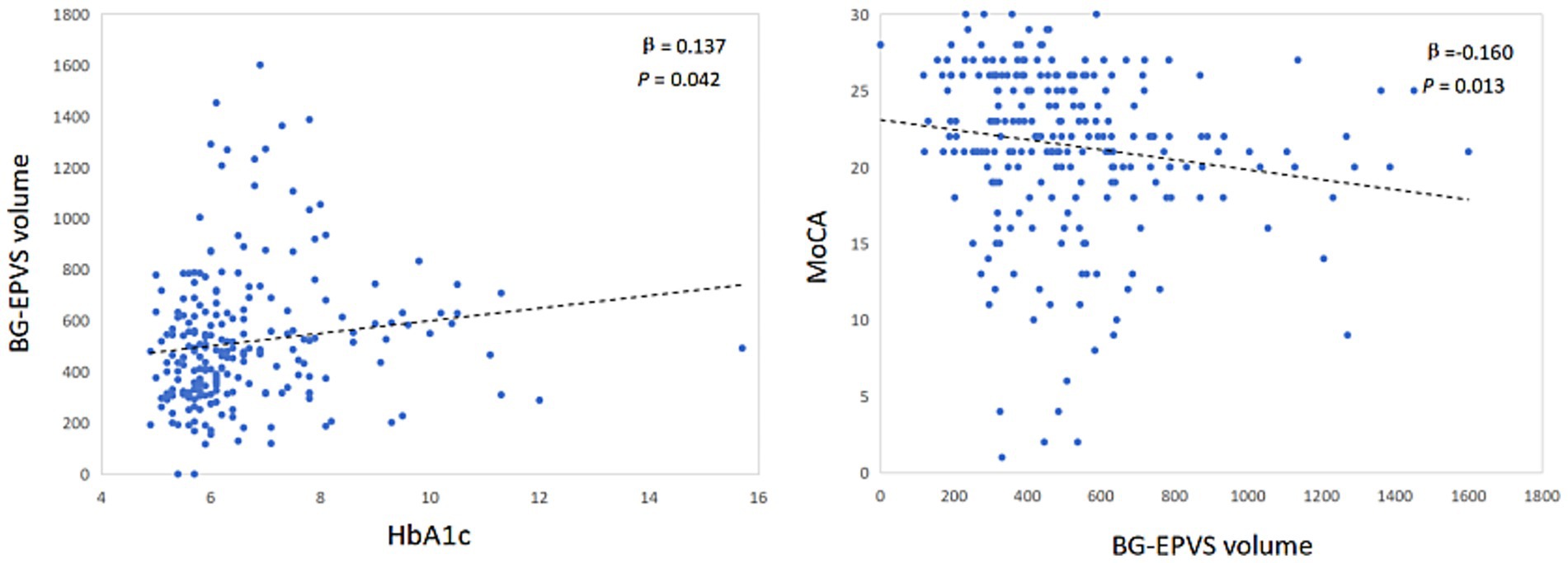
Results: A total of 244 CSVD patients were enrolled in this study. Compared with non-DM patients, DM patients had a significantly greater BG-EPVS volume (p < 0.001) and more severe periventricular white matter hyperintensities (p-WMH) (p = 0.036). Multivariate logistic regression analysis revealed that hypertension [odds ratio (OR) = 3.823; 95% confidence interval (CI):1.707–8.566; p = 0.001], the HbA1c level (OR = 1.689; 95%CI:1.255–2.272; p<0.001) and BG-EPVS volume (OR = 1.001; 95% CI:1.000–1.003; p = 0.038) were independent risk factors for MCI. After adjusting for sex and age, partial correlation analysis revealed a significant positive correlation between BG-EPVS volume and HbA1c (β = 0.137; p = 0.042) and a significant negative correlation with MOCA scores (β = −0.160; p = 0.013). The effect of HbA1c on MCI in patients with CSVD was indirectly mediated by BG-EPVS volume (indirect effect = −0.074; 95% CI: −0.187 to −0.012; the mediating effect ratio was 11.3%).
Conclusion: HbA1c is an independent risk factor for MCI. Increased BG-EPVS volume mediates the partial effect of HbA1c on CSVD-related cognitive dysfunction.
1 Introduction
Glycated hemoglobin (HbA1c), acts as a key marker for evaluating glucose intolerance, is widely used in assessment of the risk of diabetes-related complications. Recent research has shown that elevated HbA1c (≥8%) is an important risk factor for cognitive dysfunction in patients with diabetes mellitus (DM) (Xiao et al., 2025). However, the impact of HbA1c on cognition does not seem to be limited to patients with diabetes mellitus. Systematic review revealed that HbA1c was significantly associated with structural damage to the brain, including volume decline, cortical thickness and impaired functional connectivity (Soleymani et al., 2024). Consequently we hypothesize that HbA1c induced MCI correlates with changes in brain structure.
DM is an independent determinant of CSVD. Chronic hyperglycemia potentiates brain injury through three mechanisms: blood–brain barrier disruption, deposition of advanced glycation end products, and subsequent impairment of glymphatic clearance (Chen et al., 2021; Zebarth et al., 2023). Enlarged perivascular spaces (EPVS), as imaging markers of CSVD, reflect glymphatic clearance dysfunction (Hayden, 2024; Hannawi, 2024). Previous studies have demonstrated that the mechanism underlying EPVS may involve regional heterogeneity across different brain regions. Basal ganglia (BG)-EPVS are primarily distributed around precapillary arterioles, and their formation mechanisms are potentially associated with hemodynamic disruption. In contrast, centrum semiovale (CSO)-EPVS are predominantly distributed around postcapillary venules, and their underlying mechanisms are related primarily to the accumulation of metabolic products, including β-amyloid and tau proteins (Na et al., 2023; Oltmer et al., 2024; Rowsthorn et al., 2023). However, the distribution patterns and pathological mechanisms of EPVS in diabetic mellitus (DM) patients remain undefined. Recent studies have confirmed that an increased EPVS burden is associated with the progression of cognitive impairment (Paradise et al., 2021). However, the effect of EPVS distribution on cognition remains controversial. Clinical studies in diabetic populations have revealed that compared with cognitively normal controls, patients with MCI have a greater BG-EPV burden (Teng et al., 2022). Other studies have shown that CSO-EPVS and hippocampal EPVS may be specifically linked to memory impairment in DM patients (Zhao et al., 2022; Pan et al., 2025). Based on this, we hypothesize that whether the distribution of EPVS mediates the impact of HbA1c on cognition.
Therefore, this study aimed to analyze the mediating effect of EPVS on the relationship between HbA1c and MCI in patients with CSVD to elucidate the mechanism of the metabolic-vascular-glycemic-like network in CSVD-related cognitive impairment and provide a theoretical basis for the development of precise intervention strategies targeting MCI in patients with DM.
2 Methods
2.1 Study design and population
This retrospective study consecutively enrolled patients diagnosed with CSVD in the Department of Neurology at the Hebei Medical University Third Hospital between July 2023 and February 2025. The inclusion criteria were as follows: (1) the diagnosis of CSVD conformed to the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) criteria (Wardlaw et al., 2013); and (2) age was ≥60 years. The exclusion criteria were as follows: (1) secondary cognitive impairment due to alcohol consumption, substance abuse, vitamin B12 deficiency, or hypothyroidism; (2) secondary cognitive impairment from neurodegenerative diseases, acute cerebral infarction, or cerebral amyloid angiopathy; and (3) inability to undergo magnetic resonance imaging (MRI) or the MoCA assessment. The study was approved by the Ethics Committee of the Hebei Medical University Third Hospital (Approval No. W2024-065-1). All patients signed the informed consent form.
2.2 Baseline data collection
Demographic data, including sex, age, weight, and height, and medical history, including hypertension, coronary heart disease, DM, stroke, and smoking/alcohol consumption history, were collected from medical records. Blood analyses were performed for high-density lipoprotein (HDL), total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), homocysteine (Hcy), glycated hemoglobin (HbA1c), and fasting blood glucose (FBG) levels. The diagnosis of diabetes was based on the diagnostic criteria of the World Health Organization (Alberti and Zimmet, 1998).
2.3 Neuropsychological assessment
MCI assessments were performed by two standardized trained clinicians using the Beijing Version of MoCA (Hong et al., 2022). MCI was identified as a MoCA score <26 (an additional 1 point for education <12 years) (Lu et al., 2011).
2.4 Imaging
Cerebral imaging was performed utilizing a 3.0 Tesla MRI system (Philips, Netherlands). The scanning protocols included three-dimensional (3D) magnetization-prepared rapid gradient-echo (MPRAGE) sequences for T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), 3D fluid-attenuated inversion recovery (T2-FLAIR) sequences, diffusion-weighted imaging (DWI), and susceptibility-weighted imaging (SWI). Parameters for the 3D T1WI sequence were as follows: (repetition time/echo time = 500 ms/20 ms, flip angle = 8°, image resolution = 1 mm × 1.2 mm × 1 mm). 3D T2WI was performed with the following parameter settings: TR = 3,000 ms, TE = 90 ms, a flip angle of 90°, an image resolution maintained at 1 repetition time (TR) set to 500 ms, an echo time (TE) of 20 ms, a flip angle of 8°, and an image size of 1 mm × 1 mm × 1 mm. The parameters for 3D T2-FLAIR imaging were as follows: TR = 7,000 ms, TE = 120 ms, a flip angle of 90°, and an image resolution consistent with that of T2WI (1 mm × 1 mm × 1 mm). The DWI scanning parameters were as follows: TR = 5,000 ms, TE was set to the shortest, b0 and b1000 were used, and the resolution was 1.4 mm × 2.0 mm × 6 mm. SWI was conducted as follows: TR = 31 ms, TE = 7.2 ms, field of view of 230 mm, and V oxide size of 0.6 mm × 0.6 mm × 2.0 mm.
Assessment of CSVD imaging markers: Two radiologists reassessed neuroimaging markers, including white matter hyperintensities (WMH), lacunes, cerebral microbleeds (CMBs), and enlarged perivascular spaces (EPVS), in the original imaging data. When they disagreed, a consensus was reached through discussion. EPVS were defined as spaces with a diameter >2 mm and a signal intensity consistent with that of cerebrospinal fluid, which showed hyperintense signals on T2-weighted images and hypointense signals on T2-FLAIR images. EPVS were segmented and measured with a deep learning-based segmentation approach—the EPVS Automatic Segmentation System (VB-Net) (Zhang et al., 2024). The revised Fazekas score was used for white matter hyperintensity (WMH) assessment (Zheng et al., 2023). To ensure assessment consistency, two radiologists were blinded to the diagnosis of the patients. When they disagreed, a consensus was reached through discussion.
2.5 Statistical analysis
First, participants were grouped by the presence of diabetes mellitus to analyze baseline characteristics and EPVS distribution patterns among diabetic patients. Second, participants were stratified by the presence of MCI to compare baseline and imaging parameters between groups. In the univariate regression analysis for MCI risk factors, the Benjamini–Hochberg method was applied for FDR correction to control false positive risk, and variables with an adjusted p-value < 0.05 were included in the multivariate logistic regression model. Partial correlation analysis, controlling for age and sex, was performed in the MCI group to explore the relationships between HbA1c levels and BG-EPVS severity, as well as between BG-EPVS severity and the MoCA score. For the mediation analysis, the PROCESS toolbox was applied to investigate the mediating role of BG-EPVS in the association between HbA1c and MCI in patients with CSVD. A multivariate linear regression model was employed with model number 4 selected; 5,000 bootstrap samples were included, and bootstrap inference was selected for the model coefficients. A p-value ≤0.05 was considered to indicate statistical significance.
3 Results
3.1 Participant characteristics between patients with DM and those without DM
This retrospective study included 244 eligible CSVD patients, there were 134 patients with MCI, 110 patients without MCI. All enrolled patients were divided into non-DM group (n = 133) and a DM group (n = 111). Table 1 shows the differences between the two groups. Patients with DM had a greater incidence of coronary heart disease and stroke (p < 0.05, Table 1). The DM group also had significantly higher levels of FBG and HbA1c than the non-DM group did (p < 0.05, Table 1). In terms of CSVD imaging markers, DM patients presented more severe periventricular WMH and a larger volume of BG-EPVS, with statistically significant differences (p < 0.05, Table 1).
3.2 Logistic regression analysis of risk factors for MCI in patients with CSVD
Univariate and multivariate logistic regressions were performed to identify risk factors for MCI in CSVD patients, and the results are shown in Table 2. Univariate logistic analysis revealed that age, hypertension, History of HbA1c, pWMH, dWMH, BG-EPVS volume, and centrum semiovale EPVS volume were significantly associated with MCI (adjusted p < 0.05, Table 2). After adjusting for confounding factors, multivariate logistic regression analysis revealed that hypertension [odds ratio (OR): 3.823, 95% confidence interval (CI): 1.707–8.566, p = 0.001], HbA1c levels (OR: 1.689, 95% CI: 1.255–2.272, p<0.001), and BG-EPVS volume (OR: 1.001, 95% CI: 1.000–1.003, p = 0.038) were independently associated with MCI (Table 2).
All patients were divided into two subgroups for univariate and multivariate regression analyses to identify the risk factors for MCI based on the presence or absence of comorbid DM. The results showed that hypertension, HbA1c levels and BG-EPVS volume were independent predictor of MCI only in DM group (Supplementary materials 1, 2).
3.3 Partial correlation analysis of BG-EPVS volume with HbA1c levels and the MoCA score
After controlling for sex, age, hypertension and history of stroke, the BG-EPVS volume was significantly correlated with HbA1c levels (β = 0.137, p = 0.042) and significantly negatively correlated with the MoCA score (β = −0.160, p = 0.013) (Figure 1).
3.4 Indirect effects of HbA1c levels on MCI mediated by BG-EPVS volume
Mediation analysis revealed significant natural indirect (β = −0.074, 95%CI: −0.187 to −0.012), direct (β = −0.583, p = 0.016), or total effects (β = −0.657, p = 0.006) of HbA1c levels on cognitive impairment that were mediated by BG-EPVS volume in patients with CSVD, with a 11.3% mediating effect (Figure 2). However, the aforementioned mediation effect does not hold in the DM group (Supplementary material 3).
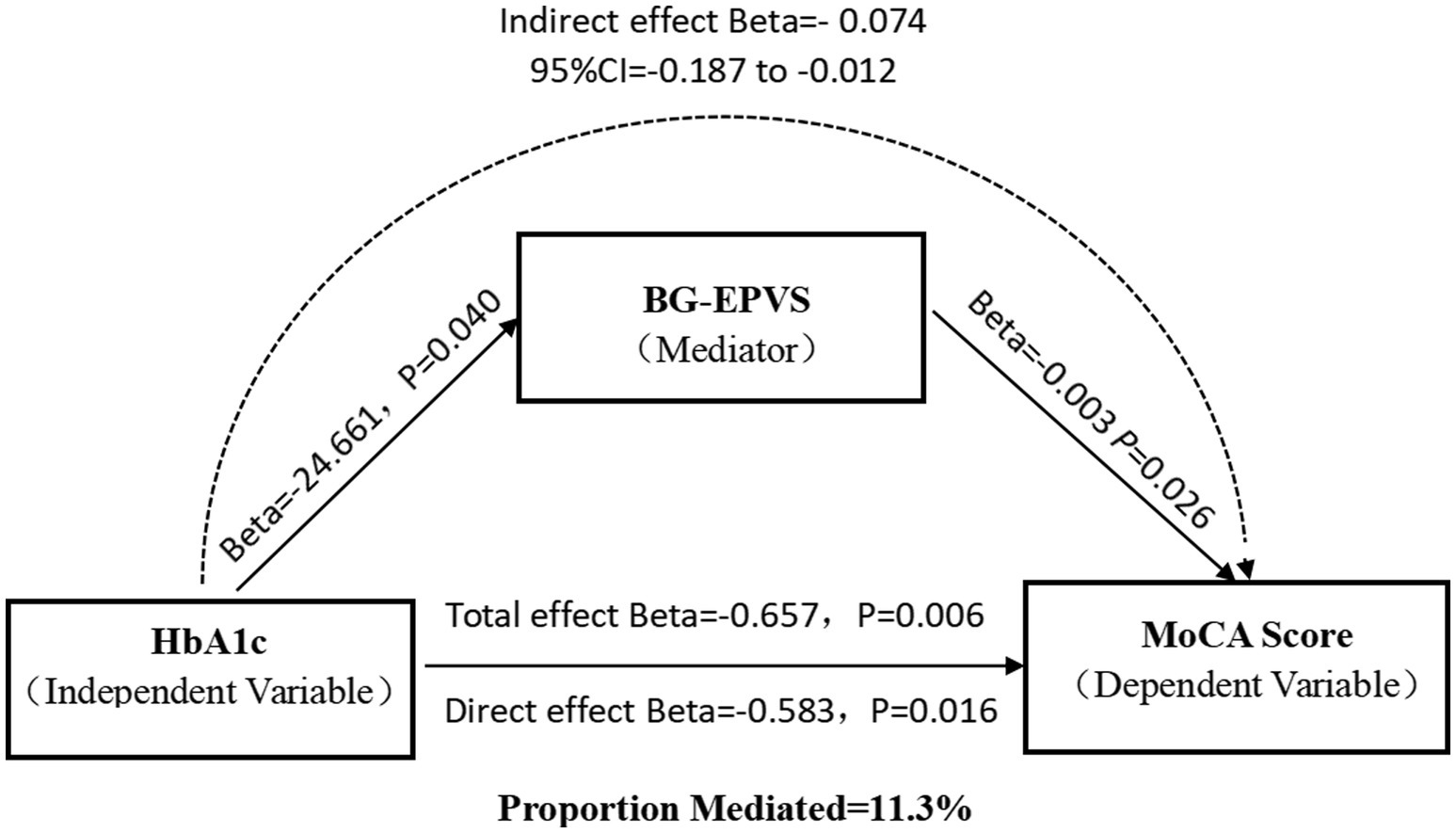
Figure 2. Mediation effect diagram. Association of HbA1c with basal ganglia EPVS volume. Association of basal ganglia EPVS volume with MoCA score. Total effect of HbA1c on MoCA score. Indirect effect of HbA1c on MoCA score mediated by BG-EPVS volume. Direct effect of HbA1c on MoCA score.
4 Discussion
MCI is a crucial clinical manifestation of CSVD and is a significant risk factor for subsequent decreases in activities of daily living and an increased fall risk (Zhou et al., 2024). T2DM is a significant risk factor for both CSVD and MCI. Recent studies have indicated that the prevalence of MCI in individuals with T2DM ranges from 30.7% to 44.1% (Yu and Yu, 2025; Zhao et al., 2025). Our study investigated the role of CSVD imaging markers in the pathway through which glycemic metabolic indicators influence cognitive function. The findings revealed that the HbA1c level and BG-EPVS volume were independent risk factors for cognitive impairment in patients with CSVD, but the association was not significant in patients without DM. Moreover, the BG-EPVS volume was positively correlated with the HbA1c level (β = 0.137; p = 0.042) and significantly negatively correlated with the MoCA score (β = −0.160; p = 0.013). In all patients, mediation analysis indicated that the BG-EPVS volume partially mediated the negative effect of the HbA1c level on the MoCA score, supporting the role of CSVD as a pathway through which metabolic dysfunction affects brain health. However, when stratifying patients by presence or absence of DM, this mediation effect disappeared.
This study demonstrated that the HbA1c level serves as an independent predictor of MCI in patients with CSVD, especially among those with DM. Specifically, every 1% increase in HbA1c was associated with a 77.7% increase in the risk of MCI development, which aligns with previous findings indicating that every 1% increase in HbA1c was associated with a 0.021-point decrease in cognitive screening assessment scores (Cukierman-Yaffe et al., 2021). Importantly, these findings demonstrate population-level generalizability and are independent of ethnicity. Epidemiological research among Asian Americans in the United States has demonstrated that elevated HbA1c levels are linked to declines in executive cognitive function and an increased incidence of MCI (OR = 1.20; 95% CI: 1.11–1.29) (Gonzalez et al., 2024). Studies of Latino communities have also increasingly recognized an association between the HbA1c level and cognitive impairment (Yu and Siang Ng, 2023). The quantitative threshold of the effect of HbA1c on cognition is generally consistent across studies. A meta-analysis of Chinese DM patients indicated that an HbA1c level ≥ 8.5% is an independent predictor of cognitive dysfunction (Peng et al., 2023). Another study revealed a U-shaped association of mean HbA1c with cognitive dysfunction, where HbA1c levels ≥8% conferred a greater risk than stable ranges did (Xiao et al., 2025). Notably, elevated HbA1c levels remain significantly associated with cognitive impairment even in studies focusing on coronary heart disease (Zheng et al., 2025).
EPVS represent imaging manifestations of impaired intracranial lymphatic circulation and are most commonly observed in the BG and CSO regions. Our research revealed that compared with non-T2DM individuals, T2DM patients presented larger BG-EPVS volume, whereas no significant difference in CSO-EPVS volume was detected between the two groups. Partial correlation analysis in CSVD patients confirmed a dose-dependent association between HbA1c levels and BG-EPVS severity, reinforcing the link between glycemic control and BG-EPVS formation. Studies by Zou et al. (2022) and Bown et al. (2023) have indicated that cerebrovascular risk factors, including hypertension and diabetes, significantly contribute to the severity of Choi et al. (2023) BG-EPVS. Choi et al. (2023) demonstrated that type 2 diabetes mellitus (T2DM) patients exhibit progressive basal ganglia volume reduction, which is correlated with gray matter atrophy in cortical-striatal-limbic circuits during disease progression. Notably, studies in nondiabetic older adults have demonstrated an association between BG-EPVS formation and insulin resistance (Wu et al., 2020), whereas no such correlation was observed for CSO-EPVS. These findings collectively demonstrate that the basal ganglia are a selectively vulnerable target of dysglycemia-induced neurotoxicity and that dysglycemia may selectively modulate EPVS in the BG region.
This study also revealed that increased BG-EPVS volume was significantly associated with MCI and served as an independent risk factor for MCI in CSVD patients. However, no significant correlation was detected between CSO-EPVS volume and MCI. Teng et al. (2022) demonstrated that cognitive decline in T2DM patients was associated with BG-EPVS formation (OR = 3.84; 95% CI: 1.81–8.13; p < 0.001), and as the severity of the BG-EPVS increased, the MoCA score significantly decreased. Another study similarly suggested that BG-EPVS severity might serve as a potential imaging marker for cognitive impairment in T2DM patients, outperforming CSO-EPVS (Choi et al., 2021), which aligns with our findings. However, Choe et al. (2022) reported that BG-EPVS were associated with only executive dysfunction, and this correlation weakened after adjusting for lacunar infarcts and WMH, indicating that the influence of BG-EPVS on executive function may be modulated by other CSVD imaging markers.
Our study further indicated that BG-EPVS partially mediated the effect of HbA1c levels on MCI in patients with CSVD. Elevated HbA1c levels promote the progression of CSVD, and this pathway contributes to the exacerbation of cognitive decline. In animal models of DM, reduced glymphatic transport and impaired cerebrospinal fluid–interstitial fluid exchange have been observed, suggesting that DM leads to dysfunction of neurovascular–glial complexes, which contributes to EPVS formation (Benveniste and Nedergaard, 2022). HbA1c levels specifically regulate EPVS distribution, with BG-EPVS contributing to HbA1c-induced MCI through the following mechanisms: (1) BG-EPVS primarily localize around precapillary microarteries (Na et al., 2023), and their formation is linked to atherosclerotic/hemodynamic injury and drives vascular cognitive impairment (Zhong et al., 2023; Yamasaki et al., 2022). The indirect effect of hyperglycemia on MCI via BG-EPVS suggests a mechanism involving cerebral hemodynamic injury, thereby illuminating the role of the “metabolism–vascular–glymphatic” interactive network in CSVD-related MCI. In contrast, CSO-EPVS predominantly localize around postcapillary venules (Na et al., 2023). Their pathogenesis is associated primarily with β-amyloid and tau protein deposition, which is a critical factor in Alzheimer’s disease-related dementia (Perosa et al., 2022). However, notably, the effect of HbA1c on the MoCA score in DM patients is not mediated by BG-EPVS volume. This negative result stems from the absence of a significant correlation between HbA1c and BG-EPVS, suggesting that the development of BG-EPVS in patients with DM is not dependent on HbA1c levels. Instead, the development of BG-EPVS in patients with DM may be influenced by other cerebrovascular risk factors. This speculation is supported by our finding that compared with the non-DM group, the DM group was more likely to have comorbid cerebrovascular disease risk factors.
The main strength of our study is that we analyzed the impact of HbA1c levels on both BG-EPVS and cognitive function and further investigated the role of increased BG-EPVS volume in the association between elevated HbA1c levels and cognitive impairment. This approach helps elucidate the potential pathogenic mechanisms underlying cognitive dysfunction in diabetic patients. However, several limitations should be acknowledged. First, as this was a single-center study with a relatively small sample size, selection bias may have affected the results. Second, the potential confounding effects of glucose-lowering medications on the outcomes were not considered. Additionally, the MoCA score is significantly influenced by educational level, resulting in a lack of a unified cutoff value for diagnosing MCI using the MoCA. In this study, only two stratifications were made on the basis of years of education during the MCI identification process, which may introduce bias into MCI diagnosis on the basis of MoCA scores. In the future, stratified studies of the educational background of the population and multicenter large-scale studies are warranted to address these limitations and validate our findings.
5 Conclusion
In summary, our findings demonstrate that elevated HbA1c levels are associated with increased BG-EPVS volumes and MCI. Furthermore, the partial effect of HbA1c elevation on cognitive impairment may be mediated by the BG-EPVS volume, which provides novel insights into the pathogenic mechanisms underlying cognitive impairment in diabetic patients. These findings highlight BG-EPVS as a potential neuroimaging marker for assessing the risk of MCI in patients with SCVD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethical Review Committee of The Third Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft. WaL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Visualization, Writing – original draft. YY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. YX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. WeL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft. JW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. HR: Conceptualization, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing – review & editing. JL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Medical Science Research Project of Hebei (grant number 20242292).
Acknowledgments
The authors would like to thank the editors and the reviewers for their valuable comments and suggestions to improve the quality of the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2025.1673301/full#supplementary-material
Abbreviations
DM, diabetes mellitus; CSVD, cerebral small vessel disease; HbA1c, hemoglobin A1c; MCI, mild cognitive impairment; EPVS, enlarged perivascular spaces; BG, basal ganglia; MoCA, montreal cognitive assessment; WMH, white matter hyperintensity; CSO, centrum semiovale; HDL, high-density lipoprotein; TC, total cholesterol; TG, triglyceride; LDL, low-density lipoprotein; Hcy, homocysteine; FBG, fasting blood glucose; CMBs, cerebral microbleeds; SD, standard deviation; IQR, interquartile range.
References
Alberti, K. G., and Zimmet, P. Z. (1998). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15, 539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
Benveniste, H., and Nedergaard, M. (2022). Cerebral small vessel disease: a glymphopathy? Curr. Opin. Neurobiol. 72, 15–21. doi: 10.1016/j.conb.2021.07.006
Bown, C. W., Khan, O. A., Liu, D., Remedios, S. W., Pechman, K. R., Terry, J. G., et al. (2023). Enlarged perivascular space burden associations with arterial stiffness and cognition. Neurobiol. Aging 124, 85–97. doi: 10.1016/j.neurobiolaging.2022.10.014
Chen, J., Mooldijk, S. S., Licher, S., Waqas, K., Ikram, M. K., Uitterlinden, A. G., et al. (2021). Assessment of advanced glycation end products and receptors and the risk of dementia. JAMA Netw. Open 4:e2033012. doi: 10.1001/jamanetworkopen.2020.33012
Choe, Y. M., Baek, H., Choi, H. J., Byun, M. S., Yi, D., Sohn, B. K., et al. (2022). Association between enlarged perivascular spaces and cognition in a memory clinic population. Neurology 99, e1414–e1421. doi: 10.1212/WNL.0000000000200910
Choi, K. S., Hwang, I., Moon, J. H., and Kim, M. (2023). Progressive reduction in basal ganglia explains and predicts cerebral structural alteration in type 2 diabetes. J. Cereb. Blood Flow Metab. 43, 2096–2104. doi: 10.1177/0271678X231197273
Choi, E. Y., Park, Y. W., Lee, M., Kim, M., Lee, C. S., Ahn, S. S., et al. (2021). Magnetic resonance imaging-visible perivascular spaces in the basal ganglia are associated with the diabetic retinopathy stage and cognitive decline in patients with type 2 diabetes. Front. Aging Neurosci. 13:666495. doi: 10.3389/fnagi.2021.666495
Cukierman-Yaffe, T., McClure, L. A., Risoli, T., Bosch, J., Sharma, M., Gerstein, H. C., et al. (2021). The relationship between glucose control and cognitive function in people with diabetes after a lacunar stroke. J. Clin. Endocrinol. Metab. 106, e1521–e1528. doi: 10.1210/clinem/dgab022
Gonzalez, H. M., Tarraf, W., Stickel, A. M., Morlett, A., Gonzalez, K. A., Ramos, A. R., et al. (2024). Glycemic control, cognitive aging, and impairment among diverse Hispanic/Latino individuals: study of Latinos- investigation of neurocognitive aging (Hispanic community health study/study of Latinos). Diabetes Care 47, 1152–1161. doi: 10.2337/dc23-2003
Hannawi, Y. (2024). Cerebral small vessel disease: a review of the pathophysiological mechanisms. Transl. Stroke Res. 15, 1050–1069. doi: 10.1007/s12975-023-01195-9
Hayden, M. R. (2024). A closer look at the perivascular unit in the development of enlarged perivascular spaces in obesity, metabolic syndrome, and type 2 diabetes mellitus. Biomedicine 12:96. doi: 10.3390/biomedicines12010096
Hong, Y., Zeng, X., Zhu, C. W., Neugroschl, J., Aloysi, A., Sano, M., et al. (2022). Evaluating the Beijing version of Montreal cognitive assessment for identification of cognitive impairment in monolingual Chinese American older adults. J. Geriatr. Psychiatry Neurol. 35, 586–593. doi: 10.1177/08919887211036182
Lu, J., Li, D., Li, F., Zhou, A., Wang, F., Zuo, X., et al. (2011). Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J. Geriatr. Psychiatry Neurol. 24, 184–190. doi: 10.1177/0891988711422528
Na, H. K., Kim, H. K., Lee, H. S., Park, M., Lee, J. H., Ryu, Y. H., et al. (2023). Role of enlarged perivascular space in the temporal lobe in cerebral amyloidosis. Ann. Neurol. 93, 965–978. doi: 10.1002/ana.26601
Oltmer, J., Mattern, H., Beck, J., Yakupov, R., Greenberg, S. M., Zwanenburg, J. J., et al. (2024). Enlarged perivascular spaces in the basal ganglia are associated with arteries not veins. J. Cereb. Blood Flow Metab. 44, 1362–1377. doi: 10.1177/0271678X241260629
Pan, P., Zhang, D., Li, J., Tang, M., Yan, X., Zhang, X., et al. (2025). The enlarged perivascular spaces in the hippocampus is associated with memory function in patients with type 2 diabetes mellitus. Sci. Rep. 15:3644. doi: 10.1038/s41598-025-87841-8
Paradise, M., Crawford, J. D., Lam, B. C. P., Wen, W., Kochan, N. A., Makkar, S., et al. (2021). Association of dilated perivascular spaces with cognitive decline and incident dementia. Neurology 96, e1501–e1511. doi: 10.1212/WNL.0000000000011537
Peng, J., Ming, L., Wu, J., Li, Y., Yang, S., and Liu, Q. (2023). Prevalence and related factors of cognitive frailty in diabetic patients in China: a systematic review and meta-analysis. Front. Public Health 11:1249422. doi: 10.3389/fpubh.2023.1249422
Perosa, V., Oltmer, J., Munting, L. P., Freeze, W. M., Auger, C. A., Scherlek, A. A., et al. (2022). Perivascular space dilation is associated with vascular amyloid-beta accumulation in the overlying cortex. Acta Neuropathol. 143, 331–348. doi: 10.1007/s00401-021-02393-1
Rowsthorn, E., Pham, W., Nazem-Zadeh, M. R., Law, M., Pase, M. P., and Harding, I. H. (2023). Imaging the neurovascular unit in health and neurodegeneration: a scoping review of interdependencies between MRI measures. Fluids Barriers CNS 20:97. doi: 10.1186/s12987-023-00499-0
Soleymani, Y., Batouli, S. A. H., Ahangar, A. A., and Pourabbasi, A. (2024). Association of glycosylated hemoglobin concentrations with structural and functional brain changes in the normoglycemic population: a systematic review. J. Neuroendocrinol. 36:e13437. doi: 10.1111/jne.13437
Teng, Z., Feng, J., Liu, R., Dong, Y., Chen, H., Xu, J., et al. (2022). Cerebral small vessel disease is associated with mild cognitive impairment in type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 15, 1985–1994. doi: 10.2147/DMSO.S368725
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., et al. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838. doi: 10.1016/S1474-4422(13)70124-8
Wu, D., Yang, X., Zhong, P., Ye, X., Li, C., and Liu, X. (2020). Insulin resistance is independently associated with enlarged perivascular space in the basal ganglia in nondiabetic healthy elderly population. Am. J. Alzheimers Dis. Other Dement. 35:1533317520912126. doi: 10.1177/1533317520912126
Xiao, Y., Hong, X., Neelagar, R., and Mo, H. (2025). Association between glycated hemoglobin A1c levels, control status, and cognitive function in type 2 diabetes: a prospective cohort study. Sci. Rep. 15:5011. doi: 10.1038/s41598-025-89374-6
Yamasaki, T., Ikawa, F., Ichihara, N., Hidaka, T., Matsuda, S., Ozono, I., et al. (2022). Factors associated with the location of perivascular space enlargement in middle-aged individuals undergoing brain screening in Japan. Clin. Neurol. Neurosurg. 223:107497. doi: 10.1016/j.clineuro.2022.107497
Yu, K., and Siang Ng, T. K. (2023). Investigating biological pathways underpinning the longitudinal association between loneliness and cognitive impairment. J. Gerontol. A Biol. Sci. Med. Sci. 78, 1417–1426. doi: 10.1093/gerona/glac213
Yu, Q., and Yu, H. (2025). Development and validation of a risk prediction model for cognitive frailty in elderly patients with type 2 diabetes mellitus. J. Clin. Nurs. 34, 3261–3275. doi: 10.1111/jocn.17508
Zebarth, J., Kamal, R., Perlman, G., Ouk, M., Xiong, L. Y., Yu, D., et al. (2023). Perivascular spaces mediate a relationship between diabetes and other cerebral small vessel disease markers in cerebrovascular and neurodegenerative diseases. J. Stroke Cerebrovasc. Dis. 32:107273. doi: 10.1016/j.jstrokecerebrovasdis.2023.107273
Zhang, Z., Ding, Z., Chen, F., Hua, R., Wu, J., Shen, Z., et al. (2024). Quantitative analysis of multimodal MRI markers and clinical risk factors for cerebral small vessel disease based on deep learning. Int. J. Gen. Med. 17, 739–750. doi: 10.2147/IJGM.S446531
Zhao, H., Wang, F., Luo, G. H., Lei, H., Peng, F., Ren, Q. P., et al. (2022). Assessment of structural brain changes in patients with type 2 diabetes mellitus using the MRI-based brain atrophy and lesion index. Neural Regen. Res. 17, 618–624. doi: 10.4103/1673-5374.320996
Zhao, Y., Wang, H., Tang, G., Wang, L., Tian, X., and Li, R. (2025). Risk factors for mild cognitive impairment in type 2 diabetes: a systematic review and meta-analysis. Front. Endocrinol. 16:1617248. doi: 10.3389/fendo.2025.1617248
Zheng, K., Wang, Z., Chen, X., Chen, J., Fu, Y., and Chen, Q. (2023). Analysis of risk factors for white matter hyperintensity in older adults without stroke. Brain Sci. 13:835. doi: 10.3390/brainsci13050835
Zheng, W., Xin, Q. J., Wang, X. X., Li, S., Wang, X., and Nie, S. P. (2025). Association between glycated hemoglobin and cognitive impairment in older adults with coronary heart disease: a multicenter prospective cohort study. J. Geriatr. Cardiol. 22, 381–388. doi: 10.26599/1671-5411.2025.03.010
Zhong, J., Lin, W., Chen, J., and Gao, Q. (2023). Higher critical closing pressure is independently associated with enlarged basal ganglia perivascular spaces. Front. Neurol. 14:1165469. doi: 10.3389/fneur.2023.1165469
Zhou, X., Yin, W. W., Huang, C. J., Sun, S. L., Li, Z. W., Li, M. X., et al. (2024). Distinctive gait variations and neuroimaging correlates in Alzheimer's disease and cerebral small vessel disease. J. Cachexia. Sarcopenia Muscle 15, 2717–2728. doi: 10.1002/jcsm.13616
Keywords: enlarged perivascular spaces, cerebral small vessel disease, glycated hemoglobin, diabetes mellitus, mild cognitive impairment
Citation: Liu C, Liu W, Yang Y, Xu Y, Li W, Wang J, Ren H and Liu J (2025) Enlarged perivascular spaces in the basal ganglia mediate the negative impact of HbA1c levels on mild cognitive impairment. Front. Hum. Neurosci. 19:1673301. doi: 10.3389/fnhum.2025.1673301
Edited by:
Zhen Yuan, University of Macau, ChinaReviewed by:
Erik J. Behringer, Loma Linda University, United StatesYunus Soleymani, Tehran University of Medical Sciences, Iran
Copyright © 2025 Liu, Liu, Yang, Xu, Li, Wang, Ren and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyan Liu, MzYzMDAzNTFAaGVibXUuZWR1LmNu
 Cuicui Liu
Cuicui Liu Wanhu Liu2
Wanhu Liu2 Huiling Ren
Huiling Ren