- 1Department of Trauma Center, First Affiliated Hospital, China Medical University, Shenyang, China
- 2Department of Cardiac Surgery, First Affiliated Hospital, China Medical University, Shenyang, China
- 3Department of Emergency, The First Hospital of China Medical University, Shenyang, China
Trauma is the fourth leading cause of death globally and the primary cause of mortality in the 15–45 age group, with traumatic brain injury (TBI) at the core of trauma care. Annually, over 50 million TBI patients are reported worldwide. The complex and heterogeneous pathophysiology of TBI presents substantial diagnostic and therapeutic challenges. In recent years, multimodal monitoring has emerged as a crucial tool to guide clinical management. The integration of multimodal monitoring with machine learning offers novel opportunities for TBI assessment and management, given the rapid development and widespread application of machine learning approaches. Therapeutic hypothermia has shown potential neuroprotective benefits in experimental and clinical contexts, though evidence remains mixed and its implementation in practice faces significant challenges. This review summarizes recent advancements in multimodal monitoring and explores how machine learning can optimize the application of therapeutic hypothermia in conjunction with multimodal data. For example, predictive models trained on multimodal signals (e.g., EEG, ICP, cerebral blood flow, and oxygenation) can help identify patient subgroups most likely to benefit from targeted temperature management. By enabling such stratification and adaptive treatment strategies, machine learning may support the development of more personalized and effective therapeutic approaches for TBI.
1 Introduction
Traumatic brain injury (TBI) is a severe public health issue, affecting 10’s of millions of patients globally each year (Dewan et al., 2019; Maas et al., 2022). TBI is typically caused by external force impacting the head or penetrating injuries, potentially leading to a series of complex neurological pathological changes, including primary and secondary injuries (Wu et al., 2022). Primary injuries refer to brain tissue damage caused directly by mechanical forces, such as contusions and hemorrhages, while secondary injuries involve a series of biochemical and physiological cascades, such as brain edema, blood-brain barrier damage, inflammatory responses, oxidative stress, and apoptosis (Minta et al., 2020; Shao et al., 2019; Unadkat et al., 2025). These pathological processes often interact, leading to more extensive neuronal damage and functional impairment (Zhao et al., 2019). Clinical manifestations of TBI are diverse, including consciousness disturbances, motor dysfunction, cognitive deficits, and changes in mood and behavior (Yen et al., 2018; Zhang and Ioachimescu, 2025). Due to the complexity of TBI pathophysiological mechanisms, traditional treatment strategies often face multiple challenges. For example, single drug treatments struggle to address multiple concurrent pathological mechanisms (Lins et al., 2023), and while surgical interventions can quickly address intracranial hematomas and skull fractures, they cannot resolve microscopic neuronal damage. Moreover, individual differences result in varying effects of the same treatment plan among different patients, further increasing the difficulty of TBI treatment (Tenovuo et al., 2021; Zheng et al., 2020).
In recent years, hypothermic neuroprotection has gained widespread attention as a treatment strategy in TBI management. Hypothermic therapy reduces brain metabolic demands by lowering body temperature, thereby alleviating inflammatory responses and oxidative stress, and protecting brain tissue to some extent (Liang et al., 2023; Yan et al., 2022). However, implementing hypothermic therapy requires precise control, including cooling extent, duration, and initiation timing, to achieve optimal therapeutic effects and minimize potential side effects (Kendall et al., 2023). Despite the positive effects shown in clinical and experimental studies, standardization and personalized implementation of hypothermic neuroprotection still require further exploration (Wang et al., 2024; Wu et al., 2021).
Meanwhile, with continuous advancements in medical technology, machine learning techniques have increasingly been applied in the medical field (Greener et al., 2022). Machine learning analyzes and integrates large amounts of complex physiological data, extracting valuable feature information to support clinical decision-making (Cobianchi et al., 2023; Zhang et al., 2021). In TBI management, machine learning can improve diagnostic accuracy and treatment effectiveness by analyzing multimodal monitoring data to identify key physiological and pathological features (Bischof and Cross, 2023; Schroder et al., 2021). Thus, machine learning can monitor and analyze patients’ physiological states in real-time, providing personalized treatment recommendations and optimizing therapeutic strategies like hypothermic neuroprotection.
Multimodal monitoring technologies play a crucial role in the diagnosis and management of TBI (Roldán et al., 2020). These technologies include electroencephalography (EEG), cerebral blood flow monitoring, intracranial pressure (ICP) monitoring, imaging (such as CT and MRI), and brain oxygen saturation monitoring. Integrating various physiological parameters provides a comprehensive assessment of the brain’s condition (Rohaut et al., 2024). However, the large and complex data volumes make it challenging to efficiently extract key information using traditional methods. The introduction of machine learning offers new tools for analyzing and integrating multimodal monitoring data, enhancing the precision and personalization of TBI diagnosis and treatment (Acosta et al., 2022).
This review adopts a narrative synthesis approach rather than a systematic review method, focusing on the application of multimodal monitoring and machine learning in the management of traumatic brain injury (TBI), with particular emphasis on the integration of therapeutic hypothermia (TTM) and machine learning for personalized treatment strategies. The search strategy involved a comprehensive literature review conducted across PubMed, Scopus, and IEEE Xplore in July 2024, with studies published from January 2000 to June 2025. Key search terms included “Traumatic Brain Injury (TBI),” “Multimodal monitoring,” “Machine learning,” “Hypothermic neuroprotection,” “Therapeutic Hypothermia (TTM),” “Electroencephalography (EEG),” “Cerebral Blood Flow (CBF),” “Intracranial Pressure (ICP),” “Brain Oxygen Saturation (PbtO2)”, and “Clinical Decision Support.” Studies were included if they discussed the application of multimodal monitoring or machine learning in TBI diagnosis, treatment, or management, and those focused on therapeutic hypothermia or the integration of multiple data modalities with machine learning for personalized care. Exclusion criteria comprised articles not addressing TBI or multimodal monitoring, studies limited to animal models, and publications outside the defined time range (before 2000 or after June 2025). The studies were grouped based on monitoring modality (e.g., EEG, CBF, ICP, PbtO2, CT/MRI imaging), task (e.g., diagnosis, prognosis, prediction, therapeutic intervention), and outcome (e.g., effectiveness of monitoring methods, clinical outcomes, machine learning model performance). This review does not perform a formal systematic quality assessment, instead providing a qualitative synthesis of key themes and trends. While areas of conflicting or insufficient evidence were noted, especially concerning the integration of machine learning with therapeutic hypothermia, the methodology remains transparent and reproducible, ensuring that the review’s findings are grounded in the existing literature. By clearly defining inclusion and exclusion criteria, the review enables a comprehensive analysis of multimodal monitoring and machine learning in TBI, offering valuable insights into current practices and highlighting areas for further research and innovation.
This article will delve into the application of hypothermic neuroprotection in TBI management and analyze the role of machine learning in optimizing hypothermic therapy. Additionally, it will review the importance of multimodal monitoring technologies in TBI assessment and how machine learning improves the effectiveness and personalization of TBI treatment through data analysis. Future research will explore advancing personalized and precise TBI management based on these emerging technologies. This review is intended for neurocritical care clinicians, biomedical engineers, and researchers focused on traumatic brain injury (TBI) management and treatment innovation. Its primary aim is to provide decision-support insights for healthcare professionals involved in the acute management of TBI, particularly in the application of multimodal monitoring and machine learning for personalized treatment strategies.
2 Multimodal monitoring technologies
Multimodal monitoring technologies provide clinicians with rich information sources to more comprehensively and accurately assess patients’ neurological states (Appavu et al., 2021; Hwang et al., 2025; Tas et al., 2022). As shown in Figure 1A, the key components of multimodal monitoring are EEG, Cerebral Blood Flow Monitoring, ICP Monitoring, Imaging, Brain Oxygen Saturation Monitoring, etc., Figure 1B shows the typical workflow of a typical machine learning algorithm. The following Table 1 summarizes the key technical parameters and applications of various monitoring techniques used during different phases of Traumatic Brain Injury (TBI). It highlights the core technical features of each technology, such as sampling frequency, spatial resolution, and monitoring depth. In the acute phase, these technologies focus on quick diagnosis and timely intervention, with an emphasis on monitoring brain function, detecting ischemic events, and identifying high intracranial pressure (ICP). In the subacute phase, the technologies are used to assess treatment efficacy and monitor brain recovery. Finally, in the recovery phase, these tools help evaluate long-term outcomes and support decisions regarding rehabilitation strategies. This table provides a comprehensive overview of how each technology is applied in TBI management and recovery, offering insights into their role and effectiveness across different stages of the injury.
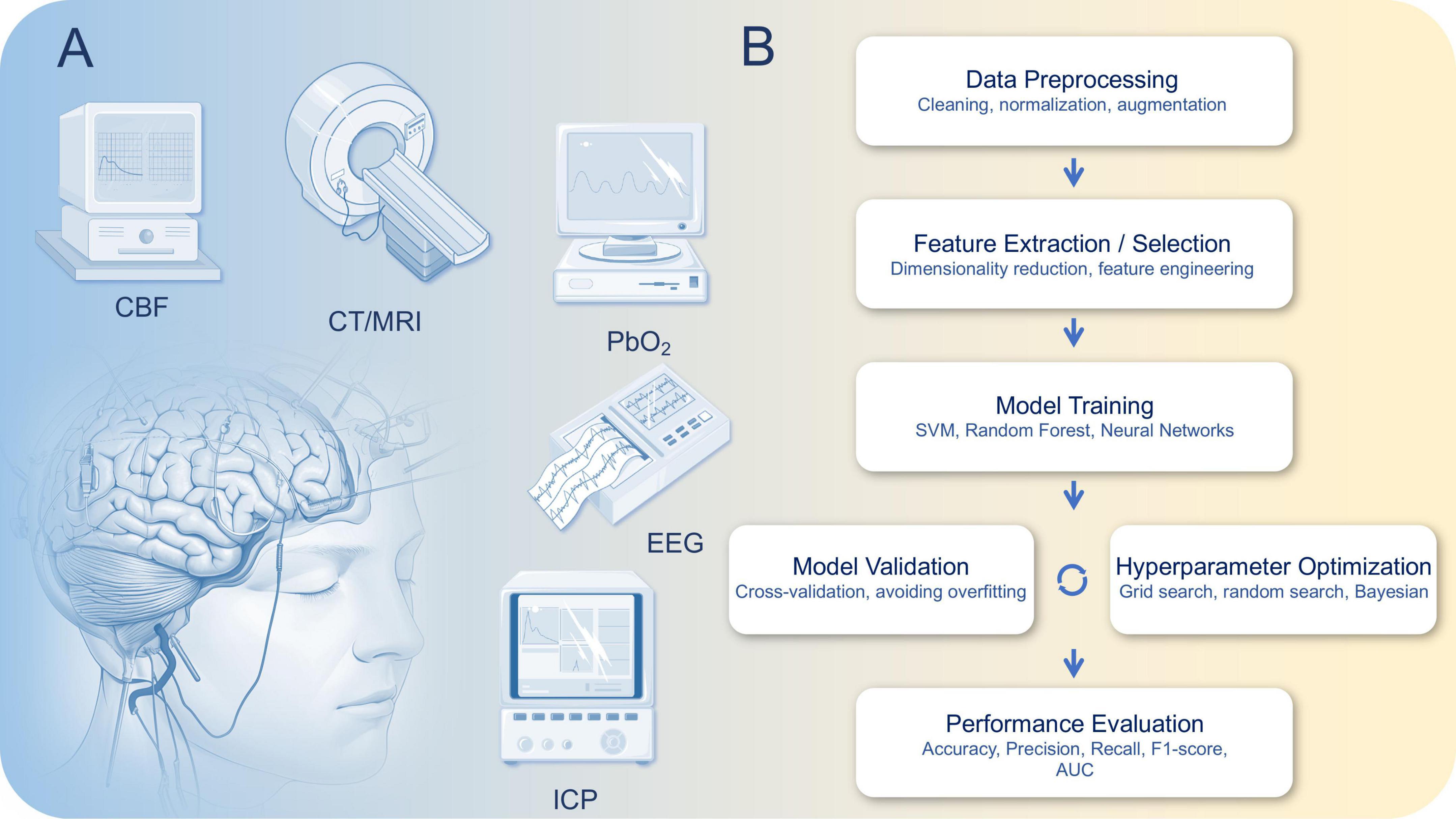
Figure 1. (A) Multimodal monitoring technology for traumatic brain injury (TBI) and (B) typical workflow of a machine learning algorithm, illustrating the main steps including data preprocessing, feature extraction/selection, model training, model validation, hyperparameter optimization, and performance evaluation. This pipeline ensures robust and generalizable model performance for biomedical data analysis.
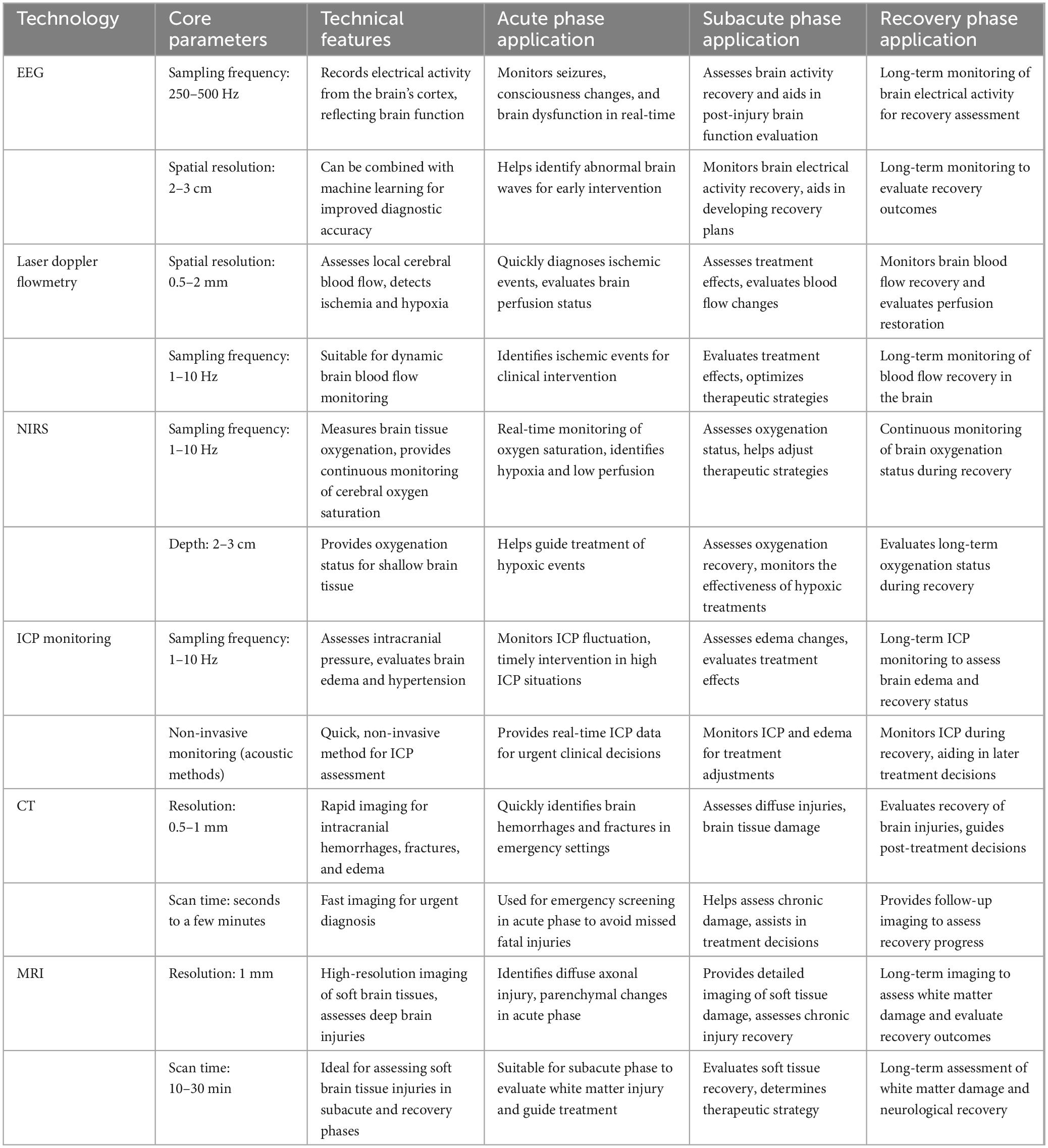
Table 1. Technical parameters and phase-specific applications of monitoring technologies in traumatic brain injury (TBI).
2.1 EEG
Electroencephalography records electrical activity from the brain’s cortex, providing a non-invasive and real-time method for assessing brain function. In TBI patients, EEG can monitor phenomena such as seizures, consciousness changes, and brain dysfunction. Common EEG technical parameters include: Sampling Frequency: Typically, between 250 and 500 Hz, used to capture high-frequency cortical activity. Spatial Resolution: Generally, 2–3 cm, used to localize electrical activity in the cerebral cortex.
Patients with TBI often face the risk of seizures; EEG helps in the timely identification and monitoring of seizure activities for early intervention (Pyrzowski et al., 2024; Sconzo et al., 2025). Additionally, EEG is used to evaluate consciousness levels and the extent of brain dysfunction, aiding clinical decision-making (Bai et al., 2021). However, traditional EEG data interpretation relies on experienced neurologists and is susceptible to noise. Combining machine learning technology allows for automated analysis of EEG data, identifying specific brain wave patterns and improving analysis efficiency and accuracy (Parsa et al., 2023). For example, machine learning algorithms can quickly detect abnormal brain activities and assist clinicians in making more accurate diagnoses, using deep learning to automatically detect epileptiform abnormalities in EEG from TBI. They demonstrated that a recurrent neural network (RNN) trained with continuous electroencephalogram (EEG) data can effectively identify epileptiform activity (EA), achieving an accuracy of up to 80.78%. This lays the foundation for robust and automated detection of epileptiform activity in traumatic brain injury (TBI) patients (Faghihpirayesh et al., 2021).
In the acute phase of TBI, EEG is primarily used to monitor epileptic activity for early detection and intervention. During the subacute phase, EEG can assess the recovery of brain function. In the recovery phase, EEG is used to evaluate long-term neurological recovery. Machine learning techniques can analyze abnormal waveforms in EEG signals, automatically detect seizures, and assist clinical diagnosis, thus enhancing the efficiency of analysis.
2.2 Cerebral blood flow monitoring
Cerebral blood flow monitoring assesses the brain’s blood perfusion status, providing crucial information about ischemia and hypoxia. The technical parameters are as follows: Laser Doppler Flowmetry: Spatial Resolution: Approximately 0.5–2 mm, used to monitor dynamic changes in local cerebral blood flow. Sampling Frequency: Typically 1–10 Hz, used for real-time blood flow fluctuation assessment. NIRS: Oxygen Saturation Detection Range: Usually between 60% and 100%. Depth: NIRS is generally used for monitoring oxygenation status of superficial brain tissue, with a depth of 2–3 cm.
The stability of cerebral blood flow is vital for maintaining normal brain function (Vu et al., 2024) In TBI, cerebral blood flow monitoring can be achieved through techniques like laser Doppler flowmetry, thermal diffusion, and near-infrared spectroscopy. Laser Doppler flowmetry evaluates local brain blood flow changes by measuring laser reflection (Ayasse et al., 2025), while thermal diffusion uses thermal probes to measure temperature changes in local tissues, indirectly reflecting blood flow (Hartings et al., 2020). Near-infrared spectroscopy measures cerebral oxygen saturation, providing continuous and non-invasive information on central nervous system hemoglobin oxygen saturation (Barud et al., 2021). These technologies play a crucial role in identifying ischemic events and assessing treatment effects. Cerebral blood flow data are often complex and dynamic, and machine learning can more accurately interpret these data through pattern recognition and trend analysis, providing decision support. By analyzing real-time cerebral blood flow data, machine learning can identify potential ischemic risks and offer recommendations for clinical intervention.
In the acute phase of TBI, laser Doppler flowmetry helps identify cerebral ischemic events. During the subacute phase, NIRS can continuously monitor the oxygenation status of brain tissue. In the recovery phase, these techniques assist in evaluating cerebral blood flow recovery.
2.3 ICP monitoring
Intracranial pressure monitoring is a key tool for evaluating intracranial hypertension and brain edema (Shang et al., 2024). Elevated ICP is a common complication in TBI, potentially leading to insufficient brain perfusion and neuronal damage. Common monitoring methods include external sensors and non-invasive techniques. Key parameters include: Sampling Frequency: Typically, 1–10 Hz, used for real-time ICP fluctuation monitoring. Non-invasive ICP Monitoring: ICP changes are estimated using cranial acoustic techniques, suitable for initial assessments in acute phase patients.
Intracranial pressure monitoring is typically performed using external sensors and non-invasive techniques. External sensors measure ICP directly through intracranial pressure sensors, while non-invasive monitoring estimates ICP changes using cranial acoustic techniques. Real-time monitoring of ICP changes provides a basis for managing intracranial hypertension and aids in determining whether surgical intervention or treatment adjustments are necessary (Fernando et al., 2019). However, due to the dynamic nature of ICP data and individual variations, personalized solutions are required (Zeiler et al., 2022). Machine learning can help identify potential crisis moments through big data analysis and provide personalized management plans. Machine learning models can also predict ICP trends, helping doctors take preventive measures in advance.
In the acute phase, ICP monitoring is used to identify elevated intracranial pressure early and guide treatment. During the subacute phase, ICP monitoring helps assess changes in brain edema, and in the recovery phase, it is used to evaluate ICP recovery and guide long-term management.
2.4 Imaging
Imaging is a fundamental method for TBI assessment, providing structural and functional information (Hu et al., 2022). Common imaging techniques include computed tomography (CT) (Mader et al., 2021), magnetic resonance imaging (MRI) (Pinggera et al., 2020), and proton magnetic resonance spectroscopy (Bartnik-Olson et al., 2021). Technical parameters include: CT: Resolution: Typically, 0.5–1 mm, used for rapid identification of intracranial hemorrhage and fractures. Scan Time: The rapidity of CT scanning makes it the method of choice during the acute phase. MRI: Resolution: Typically, 1 mm, used for assessing soft brain tissue damage, diffuse axonal injury, and white matter lesions.
Computed tomography scans quickly identify intracranial hemorrhages, fractures, and edema, making it the preferred imaging method in emergency situations, while MRI offers higher-resolution imaging of brain soft tissues, detecting diffuse axonal injury and parenchymal changes. Imaging provides precise localization of lesions, assesses the extent and nature of damage, and forms the basis for personalized treatment plans. However, interpreting imaging data requires expertise, and machine learning can enhance analysis efficiency through automated image recognition and segmentation. Machine learning algorithms can automatically identify lesion areas in CT or MRI scans, assisting doctors in more accurate evaluations (Ling et al., 2025).
In the acute phase, CT is used for rapid screening of hemorrhage and fractures. In the subacute phase, MRI helps assess diffuse injuries and white matter changes, with advantages in evaluating soft brain tissues. During the recovery phase, MRI is used to detect neural repair and functional brain recovery.
2.5 Brain oxygen saturation monitoring
Brain oxygen saturation monitoring assesses the oxygenation status of brain tissue, providing critical information for identifying hypoxia and low perfusion. Technical parameters include: Oxygen Saturation Detection Range: Typically, between 60% and 100%, reflecting the oxidative status of brain tissue in real time. Monitoring Depth: NIRS is typically used for monitoring superficial brain tissue with a depth of 2–3 cm.
Near-infrared spectroscopy (NIRS) is a commonly used technique for monitoring brain oxygen saturation (Gomez et al., 2023a). This technology evaluates oxygenation status by measuring changes in tissue light absorption, enabling real-time monitoring of brain oxygen saturation, identifying potential hypoxic events, and assessing the impact of treatment on brain oxygenation. An article from the Canadian High-Resolution Traumatic Brain Injury (CAHR-TBI) cohort study explored the predictive value of regional oxygen saturation (rSO2) and cerebrovascular reactivity index (CVRi) measured by near-infrared spectroscopy in acute traumatic brain injury patients’ prognosis. The study found that these two indicators were significantly correlated with patients’ clinical outcomes, with low rSO2 and CVRi associated with poor prognosis, suggesting that NIRS may become an effective tool for assessing acute TBI prognosis and guiding clinical treatment decisions (Gomez et al., 2024). Brain oxygen saturation data are easily influenced by external environment and physiological factors. Machine learning can improve data accuracy through data cleaning and feature extraction, help identify potential oxygenation issues, and detect key features related to prognosis, thus guiding personalized treatment (Gomez et al., 2023b).
In the acute phase of TBI, NIRS is used for continuous monitoring of brain tissue oxygenation. During the subacute phase, combined with other multimodal data, NIRS helps evaluate brain hypoxia and guide treatment. In the recovery phase, this technology assists in evaluating the oxygenation status during the process of neurological recovery.
In addition to non-invasive modalities such as NIRS, invasive brain tissue oxygenation techniques [e.g., LICOX (Früh et al., 2024), Raumedic (Rot et al., 2020)] remain the standard in many centers, and recent trials such as BOOST and BONANZA are exploring their role in TBI management.
3 Application of machine learning in TBI
The application of machine learning in TBI management is increasingly widespread, demonstrating significant potential. The success of machine learning models in diagnosing and assessing TBI relies heavily on efficient feature extraction. To achieve this, both traditional statistical methods, such as Principal Component Analysis (PCA) and Independent Component Analysis (ICA), and deep learning approaches, such as Convolutional Neural Networks (CNN), are employed to extract key information from multimodal data, including EEG, CT, and MRI. In the training and validation process, the dataset is typically split into training and validation sets at ratios of 70%–30% or 80%–20%, with cross-validation occasionally used to further assess the model’s generalization capability. During model training, hyperparameters are optimized through techniques like grid search or random search to enhance model accuracy and stability. For model validation and performance evaluation, a test set is used, and performance is assessed through tools such as AUC-ROC curves and confusion matrices. In addition to standard metrics like accuracy, precision, and recall, supplementary indicators such as F1-score and Mean Squared Error (MSE) are used for a more comprehensive evaluation. Common algorithms used in this context include CNNs, which automatically extract spatial features from EEG data to aid in TBI diagnosis, and Support Vector Machines (SVM), which are effective for classifying small, high-dimensional datasets, often used to distinguish between mild and severe TBI. Through automated analysis and data-driven insights, machine learning is transforming TBI diagnosis and treatment, especially in the integration of multimodal monitoring data, development of personalized treatment plans, and optimization of treatment strategies (as shown in Figure 2).
3.1 Data integration and feature extraction
Traumatic brain injury patients often undergo multiple monitoring techniques, generating large amounts of complex physiological data. Machine learning can integrate these multimodal monitoring data to extract key features that help identify important information related to TBI. For example, extracting brain activity patterns from EEG, detecting lesion areas from imaging data, and identifying ischemic states from cerebral blood flow and oxygen saturation data. This feature extraction capability not only improves diagnostic accuracy but also provides strong support for developing personalized treatment plans.
EEG data analysis: Machine learning can automate the analysis of EEG signals to identify specific brain activity patterns, such as warning signals for seizures. This helps in early intervention before the patient shows obvious symptoms. For instance, Vishwanath et al. (2021), explored the possibility of detecting mild traumatic brain injury (mTBI) through machine learning and deep learning techniques applied to human sleep EEG data. The study found that these algorithms effectively identified mTBI patients from EEG data, showing high accuracy and sensitivity.
Imaging data processing: In CT and MRI imaging data, deep learning algorithms can automatically identify and segment lesion areas, reducing the burden on doctors and increasing diagnostic efficiency. For example, Muller et al. (2023) successfully developed a model for classifying chronic TBI using machine learning techniques on mixed diffusion imaging data. This study revealed the potential of machine learning to enhance the accuracy of chronic TBI diagnosis, especially in identifying damage patterns and differentiating stages of injury. This work provides clinicians with a data-driven tool to improve the diagnosis and treatment planning for chronic TBI patients (Muller et al., 2023).
Multisource data integration: By integrating data from different monitoring technologies, machine learning can generate a comprehensive patient status model, enabling more accurate disease assessment and prognosis prediction.
The following Table 2 provides a comparison of various monitoring modalities used in TBI management, detailing their respective data sources, prediction or diagnosis tasks, model types, validation methods, performance metrics, and limitations. It summarizes the application of different machine learning models across diverse modalities such as EEG, ICP, CBF, PbtO2, and imaging (CT/MRI), highlighting key aspects like the model class, primary metrics (e.g., AUC, accuracy, sensitivity), and potential limitations. By examining these parameters, the table offers insights into the strengths and challenges associated with each monitoring modality, aiding in the selection of the most appropriate method for personalized TBI management.
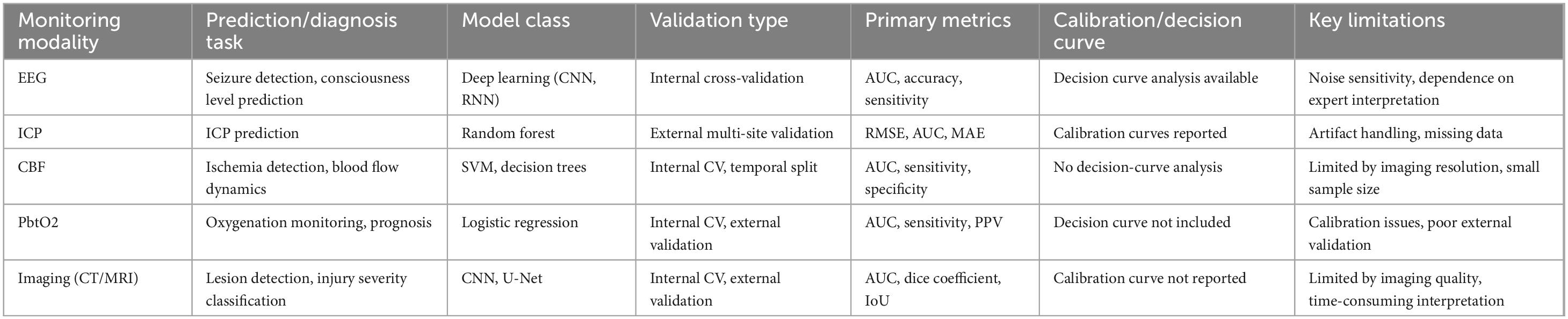
Table 2. Comparison of monitoring modalities, models, and performance metrics in traumatic brain injury (TBI).
3.2 Diagnostic support and decision assistance
Machine learning applications in TBI extend beyond data analysis to provide substantial support for clinical decision-making. Through predictive models and data-driven insights, machine learning assists doctors in formulating more precise diagnostic and treatment plans.
Diagnostic support systems: Based on multimodal data, machine learning models can not only automate diagnostic suggestions for doctors, help identify the severity and type of TBI, but also predict mental state levels. For example, Dabek et al. (2021) studied the potential of various machine learning techniques in predicting the development of psychological issues in patients with first-time mild traumatic brain injury (mTBI). The study found that these technologies could predict mental health risks based on patient data with high accuracy, providing a promising tool for early identification and intervention of psychological problems post-mTBI.
Decision support: By analyzing patient historical data, machine learning can predict the effectiveness of specific treatment plans, helping doctors choose the most appropriate treatment methods. For example, Moyer et al. (2022) developed a predictive model using machine learning techniques that accurately predicts whether moderate-to-severe traumatic brain injury (TBI) patients will require emergency neurosurgery within 24 h post-injury. The model uses key clinical factors for prediction, providing doctors with a rapid decision-making tool to improve patient outcomes (Moyer et al., 2022).
3.3 Real-time monitoring and risk assessment
During TBI treatment, patients’ conditions can change rapidly, making real-time monitoring and risk assessment crucial. Machine learning can analyze multimodal monitoring data in real time to identify early signals of condition changes and guide dynamic adjustments to treatment plans.
Dynamic monitoring systems: Through real-time data flow analysis, machine learning models can continuously monitor patients’ physiological states and automatically detect potential danger signals. For instance, when monitoring ICP, models can identify patterns of abnormal intracranial pressure elevation and alert doctors to take intervention measures. Ye et al. (2022) proposed a machine learning-based method for continuously predicting intracranial pressure changes in traumatic brain injury patients. By analyzing clinical data and training models, the study showed that this method could accurately predict short- and long-term changes in intracranial pressure, providing a new tool for non-invasive intracranial pressure monitoring and improving clinical management. The model’s overall performance includes an average accuracy of 94.62%, sensitivity of 74.91%, specificity of 94.83%, and a root mean square error of approximately 2.18 mmHg (Ye et al., 2022).
Risk assessment tools: Machine learning models can identify risk factors associated with complications and mortality by analyzing patients’ multimodal data. For example, Amorim et al. (2019) developed a model predicting early TBI mortality in low- and middle-income countries (LMIC) using machine learning methods. This model predicts early mortality risk based on key clinical features, providing a tool for early identification of high-risk TBI patients in LMIC regions and improving treatment and survival rates (Amorim et al., 2019).
During TTM, it is important to address the potential trade-offs between ICP and PbtO2. In some cases, PbtO2 (brain tissue oxygen pressure) may decrease during cooling, particularly when ventilation settings are suboptimal (e.g., latent over-ventilation). This phenomenon can result in hypoxia despite maintaining ICP control, and thus, balancing both ICP and PbtO2 becomes critical for effective neuroprotection. To guide clinicians, the following 4-step control heuristic can be proposed:
1. Prioritize ICP control: Keep ICP below a specified threshold (e.g., ICP < 20 mmHg) while maintaining PbtO2 within the target range (e.g., PbtO2 ≥ 20 mmHg).
2. Adjust ventilation if PbtO2 drops: If PbtO2 decreases below the target, first consider adjusting ventilation parameters (e.g., PaCO2 or FiO2) to optimize brain oxygenation, before raising body temperature.
3. Guardrails for shivering: If shivering is detected, consider sedation or muscle relaxants to prevent shivering induced ICP spikes and ensure the target temperature range is maintained.
4. Rewarming considerations: During the rewarming phase, carefully monitor both ICP and PbtO2. Rebound ICP is a common concern, and rewarming rates should be personalized, ideally predicted by machine learning models that take into account patient-specific physiological data.
Machine learning can significantly aid in this process by predicting the risk of PbtO2 drops or ICP elevation based on real-time monitoring data. For example, a machine learning model could analyze ICP, PbtO2, EtCO2, and other critical variables to forecast potential crises, helping clinicians to take proactive actions such as adjusting ventilation or cooling rates. By integrating multiple monitoring modalities, machine learning models can offer personalized recommendations, optimizing both ICP control and PbtO2 maintenance, and improving the safety and efficacy of TTM.
3.4 Personalized medicine and prognosis prediction
Personalized medicine is the future direction of TBI management (Savulich et al., 2018), with machine learning showing enormous potential in this area. By analyzing patient-specific physiological and pathological features, machine learning can tailor treatment and management plans for each patient.
Personalized treatment plan development: Machine learning can analyze multimodal monitoring data to identify different patients’ response patterns to treatments, thus creating personalized treatment plans for precise treatment and discharge planning (Azad et al., 2022). For instance, Satyadev et al. (2022) successfully developed a model predicting the discharge destination of traumatic brain injury patients using machine learning techniques. The model identified key factors affecting discharge outcomes from clinical data and showed high predictive accuracy, aiding healthcare professionals in making more precise decisions for patient rehabilitation paths and resource allocation (Satyadev et al., 2022). Their study developed a machine learning model to predict three-class discharge disposition in TBI patients (n = 5,292) using 84 features, including vital signs, demographics, and injury details. The random forest model demonstrated the best performance, with a weighted average area under the receiver operating characteristic curve (AUC) of 0.84 (95% CI 0.81–0.87) and a weighted average precision-recall AUC of 0.85 (95% CI 0.82–0.88). These models can improve patient triage and treatment, particularly for mild and moderate TBI cases.
Prognosis prediction models: By combining historical and multimodal monitoring data, machine learning models can predict patients’ long-term prognosis and recovery progress. This not only helps doctors assess treatment effectiveness but also provides more accurate prognosis information for patients and their families. Courville et al. (2023) conducted a systematic review and meta-analysis to assess the performance and accuracy of various machine learning algorithms in predicting TBI patient clinical outcomes. Despite limitations such as sample size and data quality, these algorithms generally showed potential in predicting TBI outcomes, providing valuable decision support tools for medical decisions (Courville et al., 2023).
To provide a clearer overview of the performance of different machine learning approaches applied in TBI management, we further summarized key models, their data modalities, validation methods, and performance metrics in Table 3. This comparison highlights both the potential and the limitations of each model, emphasizing the heterogeneity of current evidence and the necessity of rigorous external validation before clinical deployment.
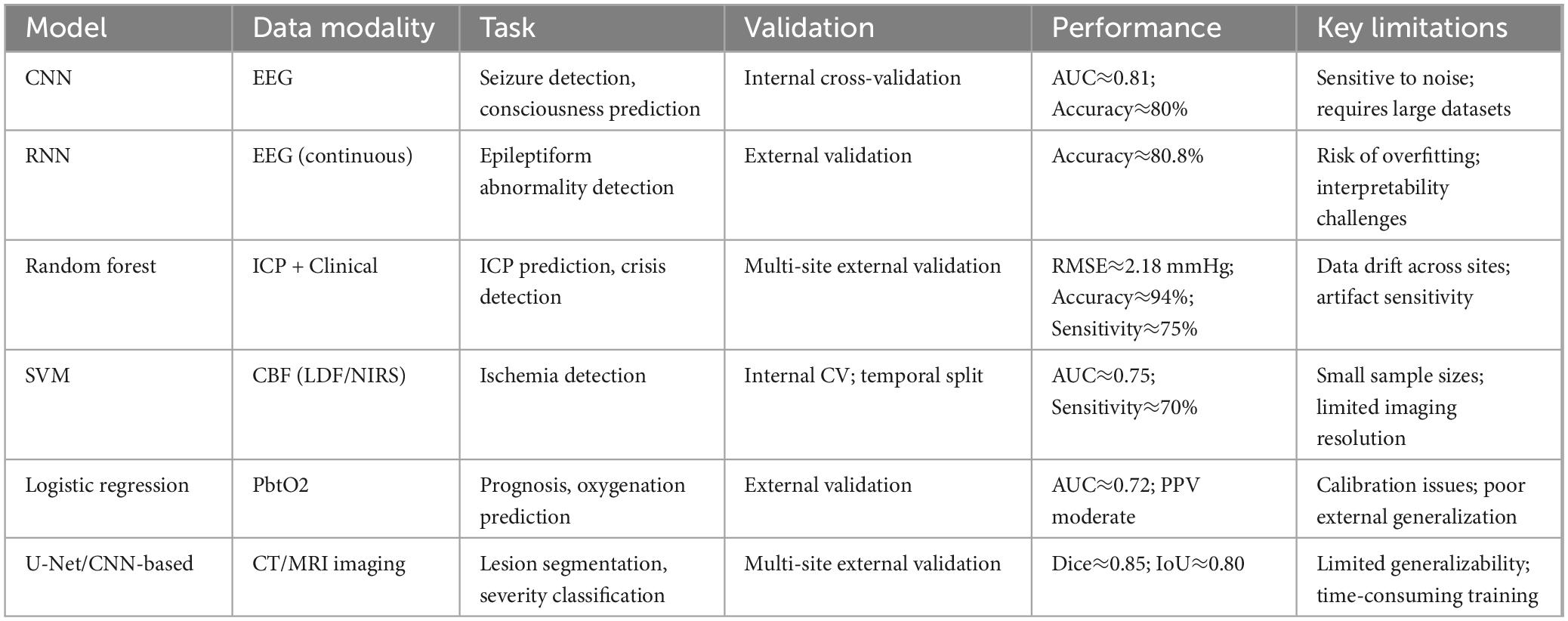
Table 3. Comparative performance of machine learning models applied in traumatic brain injury (TBI) management.
4 Hypothermic neuroprotection and other treatment strategies
Targeted Temperature Management (TTM), also known as Therapeutic Hypothermia (TH), is a cornerstone of neuroprotective strategies. Hypothermic neuroprotection and other treatment strategies play a crucial role in TBI management by improving patient outcomes and reducing mortality and morbidity. These strategies include hypothermic neuroprotection, pharmacological treatments, and rehabilitation training, which will be discussed in detail below along with their potential integration with machine learning technologies (Andresen et al., 2015; Hong et al., 2022; Kawakita et al., 2024) (as shown in Figure 3).
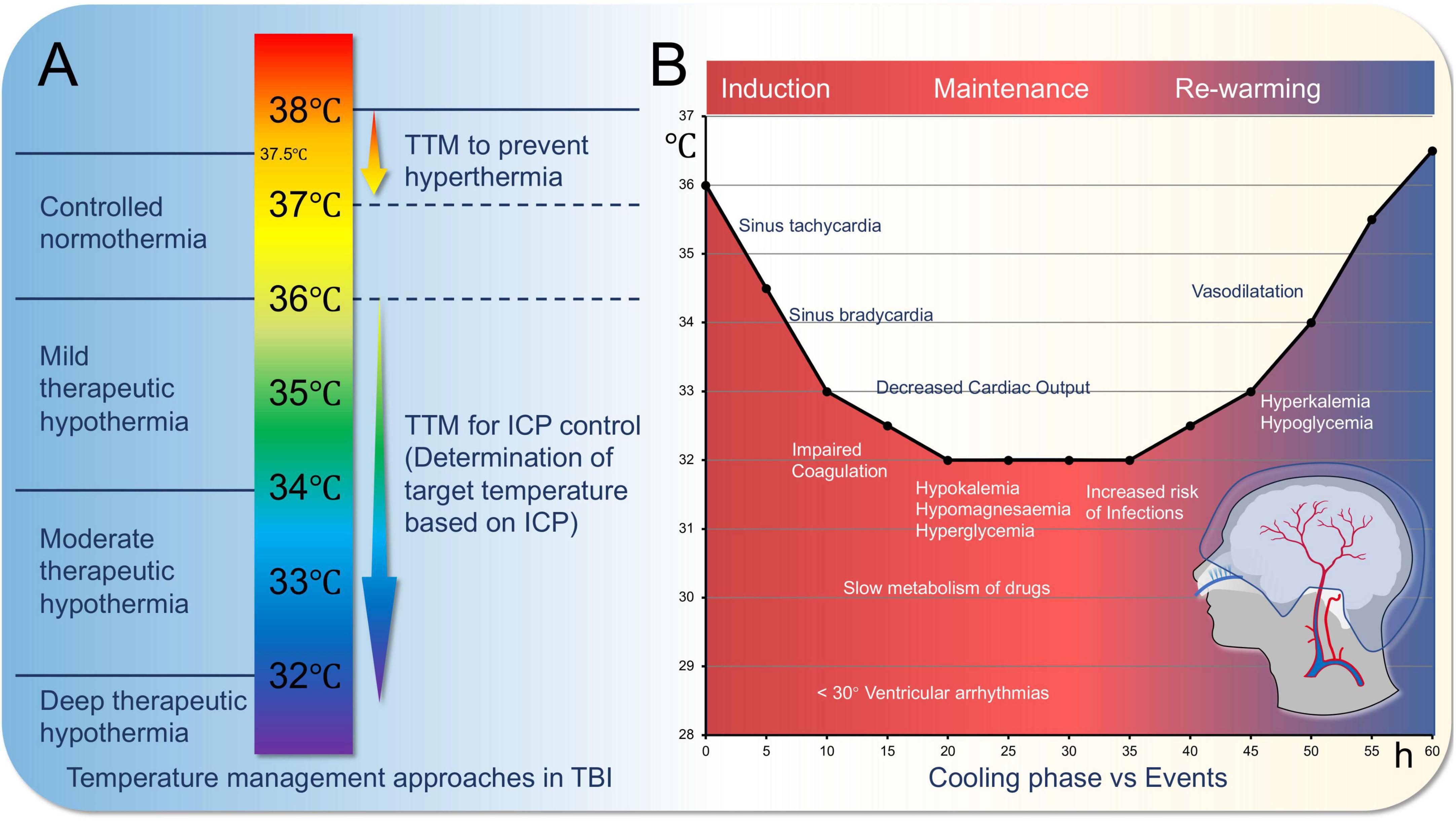
Figure 3. Targeted temperature management for traumatic brain injury (TBI). (A) Variations in the approach to temperature management in severe traumatic brain injury. Conventional therapeutic hypothermia is depicted on the left, while the concept of therapeutic hypotherm (TTM) for severe TBI is illustrated on the right. (B). Cooling phase vs. events.
4.1 Basic concepts and processes of hypothermic neuroprotection
Hypothermic neuroprotection, as a treatment strategy with significant neuroprotective effects, has gained widespread attention in TBI management. Its basic principle is to reduce the patient’s body temperature to slow down metabolic processes after brain injury, thereby reducing energy consumption and oxidative stress in brain cells (Parranto et al., 2025): lowering metabolic rate and restoring oxygen supply-demand balance. It reduces excitotoxicity, limits inflammation, prevents ATP depletion, reduces free radical production, and prevents intracellular calcium overload, avoiding apoptosis. Methods include external cooling methods such as ice blankets, ice packs, alcohol baths, cold water immersion, cold saline gastric lavage, helmet devices, and internal methods such as infusion of cold saline via central venous catheter or direct reduction of blood temperature (Andresen et al., 2015). Cooling treatment parameters should be adjusted according to the patient’s GCS (Glasgow Coma Scale) score. For patients with a GCS score of 3–5, a faster cooling rate may be required to minimize neural damage, while patients with higher GCS scores (e.g., 6–8) may not need as aggressive cooling treatment. The target temperature should be maintained between 32 °C and 35 °C, but the specific temperature range and duration of treatment should be further personalized based on the patient’s physiological state.
In TBI, hypothermic treatment can reduce brain edema, inhibit inflammatory responses, and decrease free radical production, all of which contribute to neurofunctional recovery. Hypothermic treatment is mainly divided into induction phase, maintenance phase, and rewarming phase, with physiological changes occurring at each stage (Jo, 2022). Additionally, it is worth noting that for severe traumatic brain injury (sTBI) patients, therapeutic hypothermia may require extended treatment time (Hui et al., 2021).
4.2 Expert consensus on hypothermic neuroprotection
In 2024, the ESICM/NACCS (European Society of Intensive Care Medicine/Neurocritical Care Society) consensus on best practices for targeted temperature management post-TBI provides guidelines for managing patients to optimize outcomes (Lavinio et al., 2024). Key recommendations include: (1). Indications: Initiate targeted temperature management (TTM) for severe TBI patients (Glasgow Coma Scale score ≤ 8) who do not comply with post-resuscitation orders. (2). Target Temperature: Maintain 32 °C and 35 °C (89.6–95°F) for at least 48 h to minimize secondary brain injury and improve neurological outcomes. (3). Initiation and Duration: Start TTM within 6 h of injury; duration should be personalized based on clinical response and evolving brain injury. (4). Methods: Use surface cooling devices, intravascular cooling catheters, or other methods to achieve and maintain target temperature. (5). Monitoring and Complications: Continuously monitor temperature and neurological status; manage complications such as hypotension, electrolyte imbalances, infections, and coagulopathy during TTM. (6). Multimodal Approach: TTM should be part of a multimodal approach to managing severe TBI, which may include surgical interventions, pharmacological treatments, and rehabilitation strategies tailored to individual patient needs. These consensus recommendations aim to standardize and improve the treatment and care of severe TBI patients by providing evidence-based guidelines for targeted temperature control, potentially improving outcomes and reducing morbidity associated with secondary brain injury.
The following Table 4 summarizes the use of therapeutic hypothermia across different TBI populations, outlining the target temperature range, timing and duration of cooling, primary outcomes, complications, and the net signal of the treatment’s effectiveness. It also highlights key caveats associated with each treatment approach, offering insights into the complexities of managing TBI with hypothermic neuroprotection. By reviewing the data from various studies on severe and mixed TBI, the table helps contextualize the varying responses to therapeutic hypothermia, guiding personalized treatment strategies for optimal patient care.
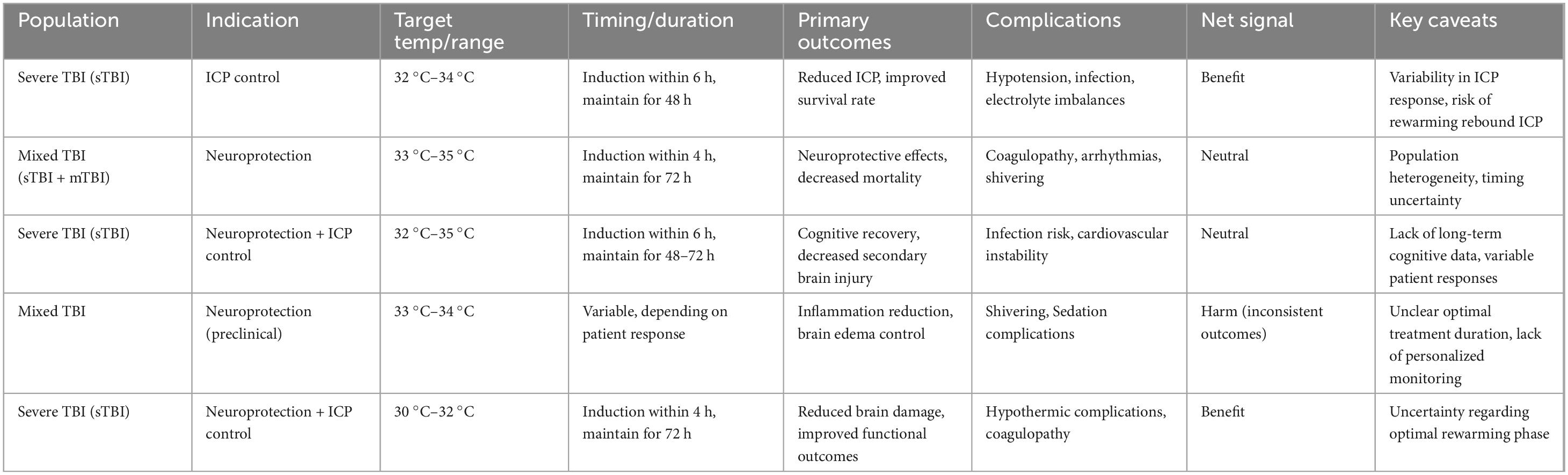
Table 4. Overview of therapeutic hypothermia parameters and outcomes in traumatic brain injury (TBI).
4.3 Necessity and potential approaches of machine learning in hypothermic brain protection
In clinical applications, hypothermic brain protection faces challenges such as determining the optimal cooling timing, temperature range, and duration. These parameters may vary among patients, necessitating personalized implementation of hypothermia therapy. For instance, Targeted Temperature Management (TTM) combined with brain tissue oxygen pressure (PbtO2) monitoring plays a crucial role in TBI treatment. TTM may reduce intracranial pressure by lowering body temperature, but its effect on PbtO2 can vary between patients, sometimes even causing a decrease in PbtO2, possibly related to latent overventilation during cooling. Therefore, TTM and PbtO2 monitoring need to be personalized and adjusted based on the patient’s specific condition and risk assessment (Cujkevic-Plecko et al., 2023). Machine learning can play a significant role in this process by analyzing large amounts of multimodal monitoring data to identify personalized optimal treatment parameters. Additionally, hypothermia therapy may lead to complications such as increased infection risk and arrhythmias, so close monitoring of the patient’s physiological status is essential. Machine learning can identify potential risks early through real-time data analysis, guiding clinical intervention decisions to improve the safety and efficacy of hypothermic brain protection. Machine learning can optimize hypothermic treatment parameters by analyzing multimodal monitoring data such as ICP (intracranial pressure) and PbtO2 (brain tissue oxygen pressure). For instance, when ICP exceeds a certain threshold, machine learning models can suggest increasing the cooling rate or adjusting the target temperature based on changes in PbtO2 to ensure adequate oxygenation of brain tissue.
Machine learning applications in brain injury hypothermia therapy are an emerging and promising field. Although still in the research stage, it has shown some potential applications and advantages:
1. Prediction and personalization of treatment: Machine learning can analyze large datasets, including clinical, imaging, and physiological data, to predict a patient’s response to hypothermia therapy. This helps doctors create personalized treatment plans to improve outcomes.
2. Optimization of treatment strategies: By analyzing patient data under different treatment strategies, machine learning can help determine the best hypothermia parameters (e.g., cooling rate, target temperature), thus optimizing the treatment plan and improving success rates.
3. Prediction of complications and patient outcomes: Machine learning can identify risks of complications and predict long-term outcomes such as survival rates and neurological recovery.
4. Imaging analysis support: Machine learning has potential in analyzing brain imaging data. It can automatically identify types, locations, and severity of brain injuries, providing more accurate diagnostic and treatment recommendations.
5. Real-time monitoring and feedback: Combining real-time monitoring technology with machine learning can analyze physiological parameters and feedback data, helping adjust the implementation of hypothermia therapy to ensure efficacy and patient safety.
Despite its early stage and need for extensive clinical validation, machine learning shows promise in improving treatment strategies and personalized medicine. As technology and data accumulate, machine learning is expected to play a greater role in providing more effective treatment and care solutions for patients.
The following Table 5 summarizes key aspects of the validation process, data handling methods, performance stability, and clinical utility for various machine learning models applied to TBI monitoring, including EEG, ICP, CBF, PbtO2, and imaging. It provides an overview of the validation techniques used, such as cross-validation, temporal splits, and multi-site external validation, as well as how missing data and potential data leakage were addressed. Additionally, the table highlights the performance stability and clinical utility of these models, shedding light on their current state and limitations for practical application in TBI management.
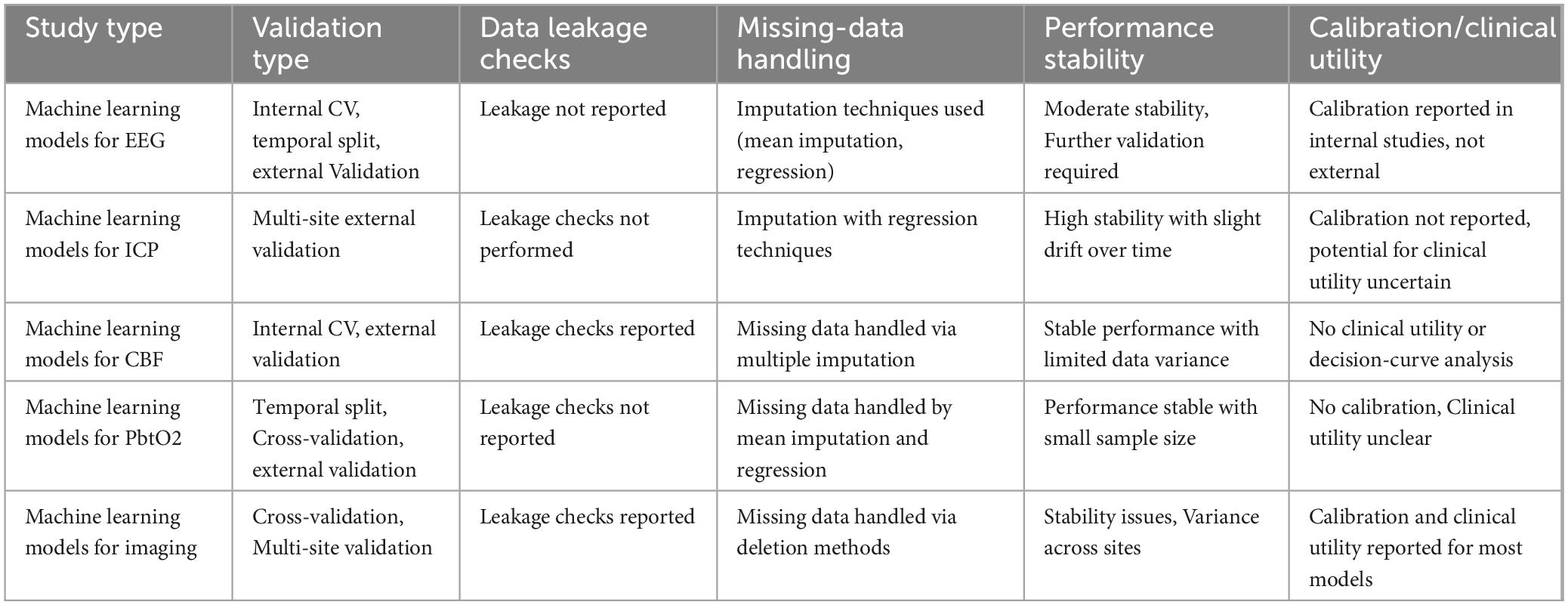
Table 5. Performance evaluation and validation of machine learning models in traumatic brain injury (TBI) monitoring.
4.4 Clinical uncertainties and challenges in the application of hypothermic therapy for traumatic brain injury
4.4.1 Theoretical basis of hypothermic therapy vs. clinical uncertainties
Theoretical basis of hypothermic therapy: Hypothermic therapy (Targeted Temperature Management, TTM) is based on the principle of reducing brain temperature to slow down metabolic processes, thereby reducing energy consumption and oxidative stress. The main mechanisms are:
1. Metabolic Suppression: By lowering body temperature, the metabolic rate of brain cells is slowed, reducing energy consumption and preventing cell death and tissue necrosis due to energy deficiency.
2. Reduction of ICP (Intracranial Pressure): Lowering the temperature may help reduce brain edema, thus lowering ICP. This can alleviate secondary brain injury caused by elevated ICP by reducing cerebral blood flow.
3. Suppression of Inflammatory Response: Cooling reduces the activity of the immune system, inhibiting inflammation and minimizing neuronal damage caused by inflammatory cell infiltration.
Clinical uncertainties and challenges: Despite the promising theoretical benefits, the clinical application of TTM faces several uncertainties and challenges:
1. Heterogeneity of patient populations: The effectiveness of TTM may vary significantly between different patients. For instance, patients with severe traumatic brain injury (sTBI) may respond differently compared to those with mild traumatic brain injury (mTBI). Age, comorbidities, and other factors contribute to these differences.
2. Timing of treatment: The optimal timing to initiate TTM is still unclear. Research suggests that earlier initiation of cooling may provide better outcomes, but determining the exact window for treatment remains challenging.
3. Rewarming risks: The rewarming phase (the process of gradually raising the body temperature) poses a risk of ICP rebound. If rewarming is not controlled carefully, it could lead to an increase in ICP, exacerbating brain injury.
4. Infection and coagulopathy: Hypothermic therapy may suppress the immune system, increasing the risk of infections. Additionally, hypothermia can impair coagulation, raising the risk of bleeding, which complicates the treatment further.
4.4.2 Rewarming process and ICP rebound analysis
ICP rebound during rewarming: The rewarming process is critical in TTM. While hypothermia helps reduce brain edema and control ICP, it may lead to ICP rebound during the rewarming phase. This rebound could be due to changes in cerebral blood flow and blood-brain barrier permeability as the brain temperature rises. Studies have shown that rewarming too quickly can lead to a sudden increase in ICP, which may further impair brain function and increase secondary injury.
It is essential to control the rewarming rate precisely to avoid a rapid rise in ICP. Research suggests that the ideal rewarming rate should be slow and controlled to minimize ICP fluctuations.
Machine learning’s role in predicting safe rewarming trajectories: Machine learning (ML) could be pivotal in predicting safe rewarming trajectories by analyzing multimodal data from various monitoring systems (such as ICP, PbtO2, EtCO2, MAP, and ventilation settings). By integrating data from these different sources, ML models could help predict potential ICP spikes during rewarming and recommend adjustments to minimize risks.
1. Multimodal Data Integration: ML models can integrate real-time data from EEG, ICP, PbtO2, and other monitoring systems to create a comprehensive patient status model. This could forecast the risk of ICP increase and provide recommendations for adjusting treatment strategies.
2. Personalized Rewarming Strategies: ML could enable the development of personalized rewarming plans for each patient based on their individual response to TTM. For example, if ICP is approaching a critical threshold, the model could suggest adjusting ventilation parameters (e.g., PaCO2 or FiO2) or delay rewarming to ensure safety.
3. Real-Time Monitoring: By continuously monitoring multiple physiological parameters, ML models can detect early signs of ICP rebound and prompt timely interventions, such as adjusting sedation levels or ventilation settings to maintain ICP within a safe range.
4.4 Other treatment strategies
Machine learning plays a crucial role in optimizing treatment strategies for TBI, particularly in drug therapy and rehabilitation training. By analyzing large amounts of patient data, machine learning can facilitate the personalization of treatment plans, optimizing their effectiveness.
4.4.1 Personalized drug dosage prediction
Drug therapy is another crucial component in TBI management, aiming to mitigate secondary pathological processes following brain injury. Commonly used drugs include antioxidants, anti-inflammatory agents, and neuroprotective agents. Antioxidants neutralize free radicals generated during injury, reducing oxidative stress on neurons (Fesharaki-Zadeh, 2022). Anti-inflammatory agents reduce brain edema and inflammation by inhibiting the release of inflammatory mediators (Kalra et al., 2022; Lu et al., 2025). Neuroprotective agents such as creatine (Newman et al., 2023) and adenosine (Bozdemir et al., 2021) help maintain cell membrane integrity and prevent neuronal apoptosis (Tang et al., 2023; Zamanian et al., 2022). Medications may vary based on the disease stage: during the acute phase, tranexamic acid, antiepileptic drugs, hyperosmotic agents, and anesthetics are primary treatments and have proven effective. In later stages, SSRIs, SNRIs, antipsychotics, zolpidem, amantadine, and other drugs are used for neuropsychological issues, while muscle relaxants and botulinum toxin are used for spasticity (Tani et al., 2022).
Although drug therapy shows efficacy in TBI, its use requires careful consideration of individual factors. Machine learning can help optimize drug therapy (Lipponen et al., 2019) by analyzing patient data to predict responses to specific drugs, enabling precision medicine. Moreover, machine learning models can identify potential side effects and guide physicians in risk assessment and treatment adjustments (Fucich et al., 2020). Suppose a severe TBI patient with a GCS score of 5 has an initial ICP of 25 mmHg and a PbtO2 of 12 mmHg. Based on this data, the machine learning model can predict that the cooling rate should be set to a decrease of 0.5 °C per hour, and the target temperature should be adjusted to 33 °C for a duration of 48 hours, to ensure optimal neuroprotective effects.
Machine learning can predict the optimal drug dosage based on a patient’s genotype, particularly genes related to drug metabolism. For example, the metabolism rates of antiepileptic drugs such as phenytoin and carbamazepine are influenced by variations in the CYP450 gene family. By establishing machine learning models, it is possible to predict the most appropriate drug dosage based on a patient’s genetic data, drug concentration, and clinical symptoms, thereby optimizing therapeutic effects. With this personalized prediction, doctors can more accurately adjust drug dosages, minimizing the risks of overdose or insufficient dosage.
4.4.2 Dynamic adjustment of rehabilitation plans
Rehabilitation training is an essential component of recovery for TBI patients, aimed at promoting functional recovery and improving long-term prognosis (Bayley et al., 2023). Physical rehabilitation includes exercise training, balance training, and functional training to enhance motor abilities and daily living functions (Gmelig Meyling et al., 2022). Cognitive rehabilitation addresses common cognitive impairments in TBI patients, such as memory, attention, and executive function deficits, through specialized training to improve cognitive function (Paggetti et al., 2025). Additionally, psychological support is crucial in TBI recovery, helping patients cope with emotional disorders and psychological stress through interventions and supportive therapy, promoting holistic recovery (Howlett et al., 2022). Machine learning can analyze rehabilitation training data, assess training effectiveness, and recommend personalized rehabilitation plans (Appiah Balaji et al., 2023). By tracking patient progress, machine learning models can adjust training plans in real-time to ensure each patient receives the most appropriate rehabilitation program, thereby improving recovery and quality of life.
In rehabilitation training, machine learning can dynamically adjust the intensity and frequency of exercises by analyzing large amounts of data from motion sensors (such as accelerometers and gyroscopes). For instance, machine learning models can monitor patients’ motion data in real-time (e.g., gait, limb movement amplitude) to assess rehabilitation progress. Based on changes in motor abilities, the training plan can be automatically adjusted. By making these dynamic adjustments, machine learning helps optimize the rehabilitation process, preventing overtraining or insufficient training.
4.4.3 Example of machine learning in drug dosage and rehabilitation training
Drug dosage optimization: Take the antiepileptic drug phenytoin as an example. Machine learning can establish personalized drug dosage prediction models using patient genotype data (e.g., CYP450 gene variations), blood drug concentrations, and clinical symptoms. For instance, patients with CYP2C9 gene variants may require lower doses of phenytoin, and machine learning can automatically optimize the dosage based on this information.
Rehabilitation training adjustment: Using motion sensors (such as smart gloves) to monitor hand movements in TBI patients, machine learning models can adjust the intensity and duration of rehabilitation training based on movement amplitude, frequency, and training progress. If the patient’s hand movements recover more slowly, the model can automatically increase the training intensity; conversely, if the patient feels fatigued or limited in movement, the model can reduce the training load accordingly.
To illustrate the practical implementation of multimodal monitoring combined with machine learning in TBI management, we present an integration pipeline in Figure 4. This schematic demonstrates how raw physiological data from various monitoring modalities—such as EEG, intracranial pressure (ICP), cerebral blood flow (CBF), and brain tissue oxygenation (PbtO2)—are first preprocessed, followed by feature extraction and data fusion. Machine learning models then integrate these processed data to enable event prediction, patient stratification, and adaptive optimization of treatment strategies. The outputs are delivered through a decision-support system, providing clinicians with guidance for personalized interventions. Importantly, a feedback loop facilitates continuous refinement, ensuring that treatment personalization adapts dynamically to each patient’s evolving condition.
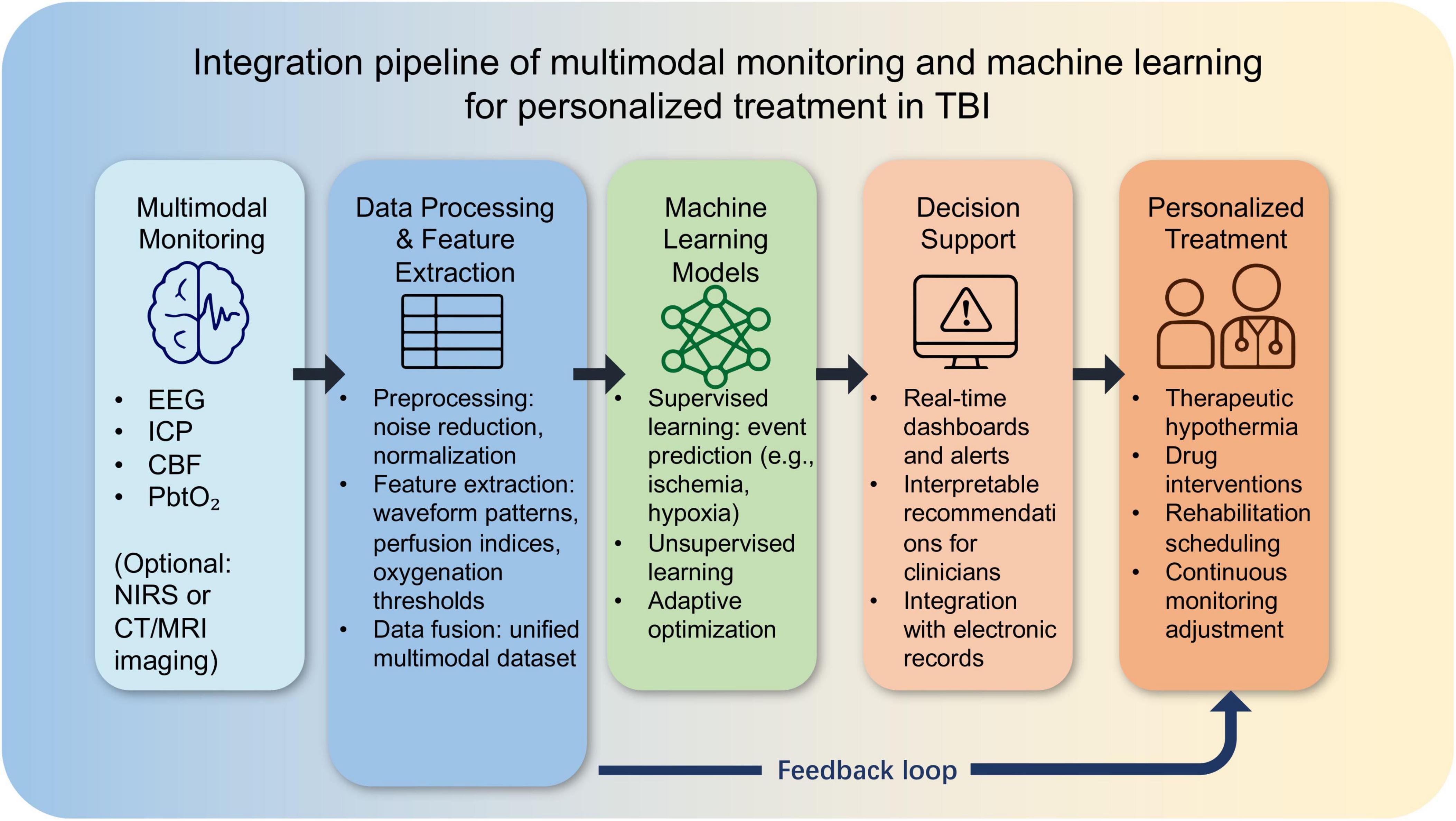
Figure 4. Integration pipeline of multimodal monitoring and machine learning for personalized treatment in traumatic brain injury (TBI).
The following Table 6 outlines the optimal cooling parameters, including target temperature, cooling rate, and maintenance duration, based on the patient’s GCS score. It also highlights the correlation between these parameters and the 6-month Glasgow Outcome Scale (GOS) score, reflecting their potential impact on recovery. This personalized approach aims to improve the efficacy of hypothermic therapy for TBI patients.
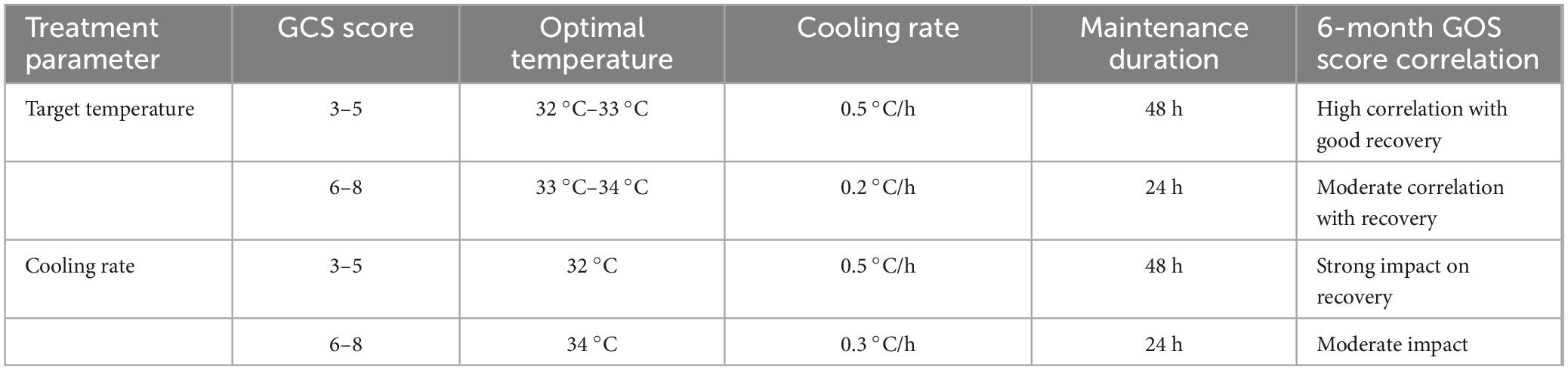
Table 6. Personalized cooling parameters based on GCS score and recovery outcomes in traumatic brain injury (TBI) patients.
5 Discussion
5.1 Current evidence and principal limitations
The integration of multimodal bedside monitoring with ML has emerged as a promising approach to refine TBI management. By leveraging physiological signals such as EEG, ICP, CBF, and PbtO2, ML models have demonstrated potential in detecting seizures, forecasting ICP crises, stratifying risk, and guiding therapeutic interventions including TTM. For instance, recurrent neural networks trained on continuous EEG have achieved accuracies of ∼80% in detecting epileptiform discharges, while deep learning–based approaches for short-term ICP prediction have reported clinically meaningful error margins.
Nevertheless, enthusiasm must be tempered by several limitations. First, evidence from hypothermia trials illustrates the challenges of translating promising physiological mechanisms into consistent clinical benefit. Large-scale RCTs such as Eurotherm3235 and POLAR failed to establish uniform improvements in long-term functional outcomes, suggesting that non-stratified cooling strategies may obscure subgroup-specific benefits. Second, the heterogeneity of multimodal data—arising from differences in acquisition devices, protocols, and patient populations—complicates model generalizability. Small sample sizes further increase the risk of overfitting, with many studies still limited to retrospective, single-center cohorts. Finally, interpretability remains a key barrier: while deep learning can capture non-linear interactions, its “black-box” nature undermines clinical trust, especially for high-stakes interventions. More transparent methods such as logistic regression offer clarity but lack the capacity to model complex physiology. Together, these limitations underscore that while ML-enhanced multimodal monitoring is technically feasible, its clinical utility remains provisional.
5.2 Challenges in clinical translation
Moving from research to bedside deployment requires addressing methodological, operational, and regulatory challenges.
Validation hierarchy: A rigorous stepwise validation pathway is essential: internal cross-validation, temporal split validation, multi-site external validation, silent prospective deployment, and ultimately interventional RCTs. Yet, few published models progress beyond internal validation, raising concerns about reproducibility across centers.
Data governance and multi-center collaboration: FL offers a promising framework for training robust models without sharing raw patient data, thereby circumventing privacy barriers. However, its clinical adoption necessitates standardized data dictionaries, synchronized sampling frequencies, and harmonized labeling criteria across institutions.
Interpretability and human oversight: Clinical decision-support systems must output not only predictions but also interpretable rationales and uncertainty estimates. Approaches such as SHAP-based feature attribution and counterfactual explanations should be integrated as default outputs. When confidence is low, ML systems should trigger a human-in-the-loop fallback, ensuring that responsibility remains with the clinical team.
Safety and workflow integration: To avoid alert fatigue, models should provide prioritized, threshold-based outputs that align with existing clinical response pathways. Continuous performance monitoring and automatic recalibration are equally critical, given device drift and evolving patient populations.
Cost-effectiveness: Multimodal monitoring infrastructures and ML deployment impose substantial costs. Strategies should balance precision against feasibility, for example by developing lightweight models that rely on widely available variables (vital signs, basic ICP, or NIRS indices) in resource-limited settings.
5.3 Future directions and testable proposals
Future research must move beyond proof-of-concept toward rigorous clinical evaluation and real-world implementation. We propose the following priorities:
1. Adaptive, stratified clinical trials for ML-guided TTM. Future RCTs should employ adaptive or sequential multiple assignment randomized trial (SMART) designs, where ML algorithms stratify patients based on multimodal phenotypes and guide individualized cooling parameters. Hypotheses such as whether ML-optimized cooling rates improve ICP control without increasing infection risk can be explicitly tested.
2. Federated, multi-center model development. Large-scale federated learning initiatives should be established, enabling joint model training while preserving patient privacy. Such collaborations would mitigate site-specific biases and accelerate model generalizability.
3. Explainability and uncertainty quantification as regulatory standards. Clinical-grade ML systems must provide interpretable rationales and calibrated confidence intervals as part of routine outputs. For example, a recommendation to adjust temperature targets should be accompanied by explicit features driving the prediction (e.g., rising ICP trend, stable PbtO2, EEG desynchronization).
4. Model maintenance and drift monitoring. Post-deployment surveillance pipelines are needed to detect covariate drift, label drift, and performance degradation. Automatic recalibration and periodic retraining should be mandated to maintain safety and efficacy.
5. Lightweight models for resource-constrained settings. Developing simplified algorithms that rely on low-cost modalities such as vital signs and NIRS will expand accessibility, ensuring that ML benefits are not limited to high-resource centers.
6. Physiology-informed feature engineering. In addition to end-to-end deep learning, incorporating physiologically interpretable features—such as ICP pulse morphology or dynamic cerebrovascular reactivity indices—can both improve predictive accuracy and facilitate clinical acceptance.
7. Optimizing the role of NIRS. Evidence suggests that raw NIRS values alone provide limited prognostic power; however, derived indices such as cerebrovascular reactivity metrics may add value when integrated into multimodal ML models.
In summary, ML-enabled multimodal monitoring has demonstrated technical feasibility and early promise in TBI management, particularly in enhancing seizure detection, ICP prediction, and personalization of hypothermia protocols. Yet the field remains in an early translational stage, constrained by heterogeneous data, limited external validation, and interpretability challenges. Moving forward, the development of multicenter federated learning frameworks, stratified adaptive trial designs, and interpretable outputs with uncertainty quantification will be critical for bridging the gap between computational potential and bedside utility. Only through such interdisciplinary and methodologically rigorous efforts can ML evolve from experimental tool to a clinically transformative paradigm in TBI care.
6 Conclusion
Traumatic brain injury remains a heterogeneous and complex condition where conventional monitoring and treatment approaches often fail to capture individual variability. The integration of multimodal monitoring with machine learning provides a promising framework to address this challenge, offering opportunities for earlier event prediction, individualized therapeutic adjustment, and data-driven clinical decision support.
Current evidence demonstrates technical feasibility, with encouraging results in tasks such as seizure detection, ICP forecasting, and imaging-based lesion characterization. Yet translation into routine clinical care is limited by small sample sizes, insufficient external validation, and the lack of interpretability in complex models. Large-scale RCTs of hypothermia have further highlighted that “one-size-fits-all” strategies are unlikely to succeed, underscoring the need for stratified, personalized protocols.
Looking ahead, progress will depend on three key pillars: (i) building high-quality, multicenter datasets through federated learning and standardized protocols; (ii) embedding explainability, uncertainty quantification, and robust validation into all clinical-grade ML systems; and (iii) designing adaptive, phenotype-driven clinical trials to test whether ML-guided interventions can yield measurable improvements in outcomes.
In summary, ML-enabled multimodal monitoring represents an emerging paradigm in TBI care. While challenges in validation, interpretability, and deployment remain, the convergence of computational advances, collaborative data sharing, and rigorous clinical evaluation has the potential to transform TBI management from generalized protocols toward truly personalized, precision neurocritical care.
Author contributions
ZW: Conceptualization, Software, Supervision, Writing – original draft, Writing – review & editing. LM: Conceptualization, Resources, Writing – original draft, Writing – review & editing. WC: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This manuscript was supported by the 2024 Shenyang Science and Technology Program, under grant number 24-214-3-08.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AUC, area under the curve; CBF, cerebral blood flow; CNN, convolutional neural network; CT, computed tomography; CV, cross-validation; CVRi, cerebrovascular reactivity index; DBS, deep brain stimulation; EEG, electroencephalography; EtCO2, end-tidal carbon dioxide; GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Scale; ICA, Independent Component Analysis; ICP, Intracranial Pressure; IoU, intersection over Union; LMIC, low- and middle-income countries; MAE, mean absolute error; MAP, mean arterial pressure; ML, machine learning; MRI, magnetic resonance imaging; MSE, Mean Squared Error; NIRS, near-infrared spectroscopy; PbtO2, brain tissue oxygen pressure; PCA, Principal Component Analysis; PPV, Positive Predictive Value; RCT, randomized controlled trial; RMSE, Root Mean Squared Error; RNN, recurrent neural network; ROC, receiver operating characteristic; rSO2, regional oxygen saturation; SVM, Support Vector Machine; TBI, traumatic brain injury; TH, therapeutic hypothermia; TMS, transcranial magnetic stimulation; TTM, targeted temperature management.
References
Acosta, J., Falcone, G., Rajpurkar, P., and Topol, E. (2022). Multimodal biomedical AI. Nat. Med. 28, 1773–1784. doi: 10.1038/s41591-022-01981-2
Amorim, R., Oliveira, L., Malbouisson, L., Nagumo, M., Simoes, M., Miranda, L., et al. (2019). Prediction of early TBI mortality using a machine learning approach in a LMIC population. Front. Neurol. 10:1366. doi: 10.3389/fneur.2019.01366
Andresen, M., Gazmuri, J., Marín, A., Regueira, T., and Rovegno, M. (2015). Therapeutic hypothermia for acute brain injuries. Scand. J. Trauma Resusc. Emerg. Med. 23:42. doi: 10.1186/s13049-015-0121-3
Appavu, B., Burrows, B., Nickoles, T., Boerwinkle, V., Willyerd, A., Gunnala, V., et al. (2021). Implementation of multimodality neurologic monitoring reporting in pediatric traumatic brain injury management. Neurocrit. Care 35, 3–15. doi: 10.1007/s12028-021-01190-8
Appiah Balaji, N., Beaulieu, C., Bogner, J., and Ning, X. (2023). Traumatic brain injury rehabilitation outcome prediction using machine learning methods. Arch. Rehabil. Res. Clin. Transl. 5:100295. doi: 10.1016/j.arrct.2023.100295
Ayasse, T., Gaugain, S., de Roquetaillade, C., Hermans-Didier, A., Kindermans, M., Chousterman, B., et al. (2025). Association between cerebral oxygenation and usual parameters of cerebral perfusion in critically ill patients with acute brain injury. J. Cereb. Blood Flow Metab. 45, 1059–1068. doi: 10.1177/0271678X241310780
Azad, T., Shah, P., Kim, H., and Stevens, R. (2022). Endotypes and the path to precision in moderate and severe traumatic brain injury. Neurocrit. Care 37, 259–266. doi: 10.1007/s12028-022-01475-6
Bai, Y., Lin, Y., and Ziemann, U. (2021). Managing disorders of consciousness: The role of electroencephalography. J. Neurol. 268, 4033–4065. doi: 10.1007/s00415-020-10095-z
Bartnik-Olson, B., Alger, J., Babikian, T., Harris, A., Holshouser, B., Kirov, I., et al. (2021). The clinical utility of proton magnetic resonance spectroscopy in traumatic brain injury: Recommendations from the ENIGMA MRS working group. Brain Imaging Behav. 15, 504–525. doi: 10.1007/s11682-020-00330-6
Barud, M., Dabrowski, W., Siwicka-Gieroba, D., Robba, C., Bielacz, M., and Badenes, R. (2021). Usefulness of cerebral oximetry in TBI by NIRS. J. Clin. Med. 10:2938. doi: 10.3390/jcm10132938
Bayley, M., Janzen, S., Harnett, A., Teasell, R., Patsakos, E., Marshall, S., et al. (2023). INCOG 2.0 guidelines for cognitive rehabilitation following traumatic brain injury: Methods, overview, and principles. J. Head Trauma Rehabil. 38, 7–23. doi: 10.1097/HTR.0000000000000838
Bischof, G., and Cross, D. (2023). Brain trauma imaging. J. Nucl. Med. 64, 20–29. doi: 10.2967/jnumed.121.263293
Bozdemir, E., Vigil, F., Chun, S., Espinoza, L., Bugay, V., Khoury, S., et al. (2021). Neuroprotective roles of the adenosine A3 receptor agonist AST-004 in mouse model of traumatic brain injury. Neurotherapeutics 18, 2707–2721. doi: 10.1007/s13311-021-01113-7
Cobianchi, L., Piccolo, D., Dal Mas, F., Agnoletti, V., Ansaloni, L., Balch, J., et al. (2023). Surgeons’ perspectives on artificial intelligence to support clinical decision-making in trauma and emergency contexts: Results from an international survey. World J. Emerg. Surg. 18:1. doi: 10.1186/s13017-022-00467-3
Courville, E., Kazim, S., Vellek, J., Tarawneh, O., Stack, J., Roster, K., et al. (2023). Machine learning algorithms for predicting outcomes of traumatic brain injury: A systematic review and meta-analysis. Surg. Neurol. Int. 14:262. doi: 10.25259/SNI_312_2023
Cujkevic-Plecko, N., Rodriguez, A., Anderson, T., and Rhodes, J. (2023). Targeted temperature management and PbtO2 in traumatic brain injury. Brain Spine 3:102704. doi: 10.1016/j.bas.2023.102704
Dabek, F., Hoover, P., Jorgensen-Wagers, K., Wu, T., and Caban, J. (2021). Evaluation of machine learning techniques to predict the likelihood of mental health conditions following a first mTBI. Front. Neurol. 12:769819. doi: 10.3389/fneur.2021.769819
Dewan, M., Rattani, A., Gupta, S., Baticulon, R., Hung, Y., Punchak, M., et al. (2019). Estimating the global incidence of traumatic brain injury. J. Neurosurg. 130, 1080–1097. doi: 10.3171/2017.10.JNS17352
Faghihpirayesh, R., Ruf, S., Rocca, M., Garner, R., Vespa, P., Erdogmus, D., et al. (2021). Automatic detection of EEG epileptiform abnormalities in traumatic brain injury using deep learning. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 302–305. doi: 10.1109/EMBC46164.2021.9630242
Fernando, S., Tran, A., Cheng, W., Rochwerg, B., Taljaard, M., Kyeremanteng, K., et al. (2019). Diagnosis of elevated intracranial pressure in critically ill adults: Systematic review and meta-analysis. BMJ 366:l4225. doi: 10.1136/bmj.l4225
Fesharaki-Zadeh, A. (2022). Oxidative stress in traumatic brain injury. Int. J. Mol. Sci. 23:13000. doi: 10.3390/ijms232113000
Früh, A., Sanchin, A., Vajkoczy, P., and Wolf, S. (2024). Bedside technique for the implantation of licox brain tissue oxygen probes in occipital regions: A technical note. World Neurosurg. 189, 127–131. doi: 10.1016/j.wneu.2024.06.008
Fucich, E., Stielper, Z., Cancienne, H., Edwards, S., Gilpin, N., Molina, P., et al. (2020). Endocannabinoid degradation inhibitors ameliorate neuronal and synaptic alterations following traumatic brain injury. J. Neurophysiol. 123, 707–717. doi: 10.1152/jn.00570.2019
Gmelig Meyling, C., Verschuren, O., Rentinck, I., Engelbert, R., and Gorter, J. (2022). Physical rehabilitation interventions in children with acquired brain injury: A scoping review. Dev. Med. Child Neurol. 64, 40–48. doi: 10.1111/dmcn.14997
Gomez, A., Froese, L., Griesdale, D., Thelin, E., Raj, R., van Iperenburg, L., et al. (2024). Prognostic value of near-infrared spectroscopy regional oxygen saturation and cerebrovascular reactivity index in acute traumatic neural injury: A CAnadian High-resolution traumatic brain injury (CAHR-TBI) cohort study. Crit. Care 28:78. doi: 10.1186/s13054-024-04859-6
Gomez, A., Griesdale, D., Froese, L., Yang, E., Thelin, E., Raj, R., et al. (2023a). Temporal statistical relationship between regional cerebral oxygen saturation (rSO2) and brain tissue oxygen tension (PbtO2) in moderate-to-severe traumatic brain injury: A Canadian high resolution-TBI (CAHR-TBI) cohort Study. Bioengineering 10:1124. doi: 10.3390/bioengineering10101124
Gomez, A., Sainbhi, A., Stein, K., Vakitbilir, N., Froese, L., and Zeiler, F. (2023b). Statistical properties of cerebral near infrared and intracranial pressure-based cerebrovascular reactivity metrics in moderate and severe neural injury: A machine learning and time-series analysis. Intensive Care Med. Exp. 11:57. doi: 10.1186/s40635-023-00541-3
Greener, J., Kandathil, S., Moffat, L., and Jones, D. T. A. (2022). guide to machine learning for biologists. Nat. Rev. Mol. Cell. Biol. 23, 40–55. doi: 10.1038/s41580-021-00407-0
Hartings, J., Andaluz, N., Bullock, M., Hinzman, J., Mathern, B., Pahl, C., et al. (2020). Prognostic value of spreading depolarizations in patients with severe traumatic brain injury. JAMA Neurol. 77, 489–499. doi: 10.1001/jamaneurol.2019.4476
Hong, J., Choi, E., and Park, S. (2022). Selective brain cooling: A new horizon of neuroprotection. Front. Neurol. 13:873165. doi: 10.3389/fneur.2022.873165
Howlett, J., Nelson, L., and Stein, M. (2022). Mental health consequences of traumatic brain injury. Biol. Psychiatry 91, 413–420. doi: 10.1016/j.biopsych.2021.09.024
Hu, L., Yang, S., Jin, B., and Wang, C. (2022). Advanced neuroimaging role in traumatic brain injury: A narrative review. Front. Neurosci. 16:872609. doi: 10.3389/fnins.2022.872609
Hui, J., Feng, J., Tu, Y., Zhang, W., Zhong, C., Liu, M., et al. (2021). Safety and efficacy of long-term mild hypothermia for severe traumatic brain injury with refractory intracranial hypertension (LTH-1): A multicenter randomized controlled trial. EClinicalMedicine 32:100732. doi: 10.1016/j.eclinm.2021.100732
Hwang, S., Kwon, N., Lee, D., Kim, J., Yang, S., Youn, I., et al. (2025). A multimodal fatigue detection system using sEMG and IMU signals with a hybrid CNN-LSTM-attention model. Sensors 25:3309. doi: 10.3390/s25113309
Jo, K. (2022). Target temperature management in traumatic brain injury with a focus on adverse events, recognition, and prevention. Acute Crit. Care. 37, 483–490. doi: 10.4266/acc.2022.01291
Kalra, S., Malik, R., Singh, G., Bhatia, S., Al-Harrasi, A., Mohan, S., et al. (2022). Pathogenesis and management of traumatic brain injury (TBI): Role of neuroinflammation and anti-inflammatory drugs. Inflammopharmacology 30, 1153–1166. doi: 10.1007/s10787-022-01017-8
Kawakita, K., Shishido, H., and Kuroda, Y. (2024). Review of temperature management in traumatic brain injuries. J. Clin. Med. 13:2144. doi: 10.3390/jcm13072144
Kendall, H., Van Kuijk, S., Horst, I. C., Dings, J. T., Aries, M. J., and Haeren, R. H. (2023). Difference between brain temperature and core temperature in severe traumatic brain injury: A systematic review. J. Neurosurg. Sci. 67, 46–54. doi: 10.23736/S0390-5616.21.05519-3
Lavinio, A., Coles, J., Robba, C., Aries, M., Bouzat, P., Chean, D., et al. (2024). Targeted temperature control following traumatic brain injury: esicm/naccs best practice consensus recommendations. Crit. Care 28:170. doi: 10.1186/s13054-024-04951-x
Liang, S., Ti, Y., Li, X., and Zhou, W. (2023). The protective role and mechanism of mild therapeutic hypothermia protection on brain cells. Neuropsychiatr. Dis. Treat. 19, 1625–1631. doi: 10.2147/NDT.S412227
Ling, X., Yang, X., Wang, P., Li, Y., Wen, Z., Wang, J., et al. (2025). Intratumoral and peritumoral heterogeneity based on CT to predict the pathological response after neoadjuvant chemoimmunotherapy in esophageal squamous cell carcinoma. Int. J. Surg. doi: 10.1097/JS9.0000000000003422 Online ahead of print.
Lins, B., Anyaegbu, C., Hellewell, S., Papini, M., McGonigle, T., De Prato, L., et al. (2023). Cannabinoids in traumatic brain injury and related neuropathologies: Preclinical and clinical research on endogenous, plant-derived, and synthetic compounds. J. Neuroinflammation 20, 77. doi: 10.1186/s12974-023-02734-9
Lipponen, A., Natunen, T., Hujo, M., Ciszek, R., Hämäläinen, E., Tohka, J., et al. (2019). In vitro and in vivo pipeline for validation of disease-modifying effects of systems biology-derived network treatments for traumatic brain injury-lessons learned. Int. J. Mol. Sci. 20:5395. doi: 10.3390/ijms20215395
Lu, J., Dai, X., Xi, S., Wang, B., Zhang, P., Fu, X., et al. (2025). Piperine protects against cerebral ischemic injury by regulating the Caspase-1-mediated pyroptosis pathway. Front. Pharmacol. 16:1601873. doi: 10.3389/fphar.2025.1601873
Maas, A., Menon, D., Manley, G., Abrams, M., Åkerlund, C., Andelic, N., et al. (2022). Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 21, 1004–1060. doi: 10.1016/S1474-4422(22)00309-X
Mader, M., Rotermund, R., Lefering, R., Westphal, M., Maegele, M., Czorlich, P., et al. (2021). The faster the better? Time to first CT scan after admission in moderate-to-severe traumatic brain injury and its association with mortality. Neurosurg. Rev. 44, 2697–2706. doi: 10.1007/s10143-020-01456-3
Minta, K., Brinkmalm, G., Al Nimer, F., Thelin, E., Piehl, F., Tullberg, M., et al. (2020). Dynamics of cerebrospinal fluid levels of matrix metalloproteinases in human traumatic brain injury. Sci. Rep. 10:18075. doi: 10.1038/s41598-020-75233-z
Moyer, J., Lee, P., Bernard, C., Henry, L., Lang, E., Cook, F., et al. (2022). Machine learning-based prediction of emergency neurosurgery within 24 h after moderate to severe traumatic brain injury. World J. Emerg. Surg. 17:42. doi: 10.1186/s13017-022-00449-5
Muller, J., Wang, R., Milddleton, D., Alizadeh, M., Kang, K., Hryczyk, R., et al. (2023). Machine learning-based classification of chronic traumatic brain injury using hybrid diffusion imaging. Front. Neurosci. 17:1182509. doi: 10.3389/fnins.2023.1182509
Newman, J., Pekari, T., and Van Wyck, D. (2023). Neuroprotection and therapeutic implications of creatine supplementation for brain injury complications. Med. J. 2023, 31–38.
Paggetti, A., Druda, Y., Sciancalepore, F., Della Gatta, F., Ancidoni, A., Locuratolo, N., et al. (2025). The efficacy of cognitive stimulation, cognitive training, and cognitive rehabilitation for people living with dementia: A systematic review and meta-analysis. Geroscience 47, 409–444. doi: 10.1007/s11357-024-01400-z
Parranto, M., Kung, T., Liddle, L., Khalid, T., Thorkelsson, A., Klahr, A., et al. (2025). A systematic review of depth-dependent cytoprotection with therapeutic hypothermia for cerebral ischemia. Ther. Hypothermia Temp. Manag. doi: 10.1177/21537658251377958 Online ahead of print.
Parsa, M., Rad, H., Vaezi, H., Hossein-Zadeh, G., Setarehdan, S., Rostami, R., et al. (2023). EEG-based classification of individuals with neuropsychiatric disorders using deep neural networks: A systematic review of current status and future directions. Comput. Methods Programs Biomed. 240:107683. doi: 10.1016/j.cmpb.2023.107683
Pinggera, D., Luger, M., Bürgler, I., Bauer, M., Thomé, C., and Petr, O. (2020). Safety of early MRI examinations in severe TBI: A test battery for proper patient selection. Front. Neurol. 11:219. doi: 10.3389/fneur.2020.00219
Pyrzowski, J., Kałas, M., Mazurkiewicz-Bełdzińska, M., and Siemiński, M. (2024). EEG biomarkers for the prediction of post-traumatic epilepsy - A systematic review of an emerging field. Seizure 119, 71–77. doi: 10.1016/j.seizure.2024.05.006
Rohaut, B., Calligaris, C., Hermann, B., Perez, P., Faugeras, F., Raimondo, F., et al. (2024). Multimodal assessment improves neuroprognosis performance in clinically unresponsive critical-care patients with brain injury. Nat. Med. 30, 2349–2355. doi: 10.1038/s41591-024-03019-1
Roldán, M., Abay, T., and Kyriacou, P. (2020). Non-invasive techniques for multimodal monitoring in traumatic brain injury: Systematic review and meta-analysis. J. Neurotrauma 37, 2445–2453. doi: 10.1089/neu.2020.7266
Rot, S., Dweek, M., Gutowski, P., Goelz, L., Meier, U., and Lemcke, J. (2020). Comparative investigation of different telemetric methods for measuring intracranial pressure: A prospective pilot study. Fluids Barriers CNS 17:63. doi: 10.1186/s12987-020-00225-0
Satyadev, N., Warman, P., Seas, A., Kolls, B., Haglund, M., Fuller, A., et al. (2022). Machine learning for predicting discharge disposition after traumatic brain injury. Neurosurgery 90, 768–774. doi: 10.1227/neu.0000000000001911
Savulich, G., Menon, D., Stamatakis, E., Pickard, J., and Sahakian, B. (2018). Personalised treatments for traumatic brain injury: Cognitive, emotional and motivational targets. Psychol. Med. 48, 1397–1399. doi: 10.1017/S0033291718000892
Schroder, A., Lawrence, T., Voets, N., Garcia-Gonzalez, D., Jones, M., Peña, J., et al. (2021). A Machine learning enhanced mechanistic simulation framework for functional deficit prediction in TBI. Front. Bioeng. Biotechnol. 9:587082. doi: 10.3389/fbioe.2021.587082
Sconzo, D., Wadhwa, A., Balagurunath, K., Berube, M., Wetsel, Z., Sakthiyendran, N., et al. (2025). Predictors of seizures in postoperative traumatic brain injury patients: A single center retrospective study. Clin. Neurol. Neurosurg. 257:109116. doi: 10.1016/j.clineuro.2025.109116
Shang, P., Zheng, R., Wu, K., Yuan, C., and Pan, S. (2024). New insights on mechanisms and therapeutic targets of cerebral edema. Curr. Neuropharmacol. 22, 2330–2352. doi: 10.2174/1570159X22666240528160237
Shao, A., Zhu, Z., Li, L., Zhang, S., and Zhang, J. (2019). Emerging therapeutic targets associated with the immune system in patients with intracerebral haemorrhage (ICH): From mechanisms to translation. EBioMedicine 45, 615–623. doi: 10.1016/j.ebiom.2019.06.012
Tang, Y., Liu, Y., Zhou, H., Lu, H., Zhang, Y., Hua, J., et al. (2023). Esketamine is neuroprotective against traumatic brain injury through its modulation of autophagy and oxidative stress via AMPK/mTOR-dependent TFEB nuclear translocation. Exp. Neurol. 366:114436. doi: 10.1016/j.expneurol.2023.114436
Tani, J., Wen, Y., Hu, C., and Sung, J. (2022). Current and potential pharmacologic therapies for traumatic brain injury. Pharmaceuticals 15:838. doi: 10.3390/ph15070838
Tas, J., Czosnyka, M., van der Horst, I., Park, S., van Heugten, C., Sekhon, M., et al. (2022). Cerebral multimodality monitoring in adult neurocritical care patients with acute brain injury: A narrative review. Front. Physiol. 13:1071161. doi: 10.3389/fphys.2022.1071161
Tenovuo, O., Diaz-Arrastia, R., Goldstein, L., Sharp, D., van der Naalt, J., and Zasler, N. (2021). Assessing the severity of traumatic brain injury-time for a change? J. Clin. Med. 10:148. doi: 10.3390/jcm10010148
Unadkat, P., Rebeiz, T., Ajmal, E., De Souza, V., Xia, A., Jinu, J., et al. (2025). Neurobiological mechanisms underlying psychological dysfunction after brain injuries. Cells 14:74. doi: 10.3390/cells14020074
Vishwanath, M., Jafarlou, S., Shin, I., Dutt, N., Rahmani, A., Jones, C., et al. (2021). Investigation of machine learning and deep learning approaches for detection of mild traumatic brain injury from human sleep electroencephalogram. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 6134–6137. doi: 10.1109/EMBC46164.2021.9630423
Vu, E., Brown, C., Brady, K., and Hogue, C. (2024). Monitoring of cerebral blood flow autoregulation: Physiologic basis, measurement, and clinical implications. Br. J. Anaesth. 132, 1260–1273. doi: 10.1016/j.bja.2024.01.043
Wang, X., Chen, S., Wang, X., Song, Z., Wang, Z., Niu, X., et al. (2024). Application of artificial hibernation technology in acute brain injury. Neural Regen. Res. 19, 1940–1946. doi: 10.4103/1673-5374.390968
Wu, A., Yong, Y., Pan, Y., Zhang, L., Wu, J., Zhang, Y., et al. (2022). Targeting Nrf2-mediated oxidative stress response in traumatic brain injury: Therapeutic perspectives of phytochemicals. Oxid. Med. Cell. Longev. 2022:1015791. doi: 10.1155/2022/1015791
Wu, X., Tao, Y., Marsons, L., Dee, P., Yu, D., Guan, Y., et al. (2021). The effectiveness of early prophylactic hypothermia in adult patients with traumatic brain injury: A systematic review and meta-analysis. Aust. Crit. Care 34, 83–91. doi: 10.1016/j.aucc.2020.05.005
Yan, C., Mao, J., Yao, C., Liu, Y., Yan, H., and Jin, W. (2022). Neuroprotective effects of mild hypothermia against traumatic brain injury by the involvement of the Nrf2/ARE pathway. Brain Behav. 12:e2686. doi: 10.1002/brb3.2686
Ye, G., Balasubramanian, V., Li, J., and Kaya, M. (2022). Machine learning-based continuous intracranial pressure prediction for traumatic injury patients. IEEE J. Transl. Eng. Health Med. 10:4901008. doi: 10.1109/JTEHM.2022.3179874
Yen, T., Chang, C., Chung, C., Ko, W., Yang, C., and Hsieh, C. (2018). Neuroprotective effects of platonin, a therapeutic immunomodulating medicine, on traumatic brain injury in mice after controlled cortical impact. Int. J. Mol. Sci. 19:1100. doi: 10.3390/ijms19041100
Zamanian, M., Taheri, N., Opulencia, M., Bokov, D., Abdullaev, S., Gholamrezapour, M., et al. (2022). Neuroprotective and anti-inflammatory effects of pioglitazone on traumatic brain injury. Mediators Inflamm. 2022:9860855. doi: 10.1155/2022/9860855
Zeiler, F., Aries, M., Czosnyka, M., and Smielewski, P. (2022). Cerebral autoregulation monitoring in traumatic brain injury: An overview of recent advances in personalized medicine. J. Neurotrauma 39, 1477–1494. doi: 10.1089/neu.2022.0217
Zhang, C., and Ioachimescu, A. (2025). Clinical manifestations and treatment of hypopituitarism due to traumatic brain injury. Best Pract. Res. Clin. Endocrinol. Metab. 39:101996. doi: 10.1016/j.beem.2025.101996
Zhang, K., Cai, L., Song, Y., Liu, T., and Zhao, Y. (2021). Combining external medical knowledge for improving obstetric intelligent diagnosis: Model development and validation. JMIR Med. Inform. 9:e25304. doi: 10.2196/25304
Zhao, X., Lu, M., Yuan, D., Xu, D., Yao, P., Ji, W., et al. (2019). Mitochondrial dysfunction in neural injury. Front. Neurosci. 13:30. doi: 10.3389/fnins.2019.00030
Keywords: traumatic brain injury, multimodal monitoring, machine learning, hypothermic neuroprotection, diagnosis, prognosis
Citation: Wei Z, Meng L and Chong W (2025) Advancements in the application of multimodal monitoring and machine learning for the development of personalized therapeutic strategies in traumatic brain injury. Front. Hum. Neurosci. 19:1695336. doi: 10.3389/fnhum.2025.1695336
Received: 29 August 2025; Accepted: 08 October 2025;
Published: 23 October 2025.
Edited by:
Jin Hong, Nanchang University, ChinaReviewed by:
Siyuan Lu, Nanjing University of Posts and Telecommunications, ChinaMingfeng Cao, Johns Hopkins University, United States
Copyright © 2025 Wei, Meng and Chong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Chong, d2Nob25nQGNtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Zhijing Wei, orcid.org/0000-0003-4596-6901; Lingda Meng, orcid.org/0009-0008-8636-9090
 Zhijing Wei
Zhijing Wei Lingda Meng
Lingda Meng Wei Chong
Wei Chong