- Department of Hearing and Speech Sciences, Program in Neuroscience and Cognitive Science, University of Maryland, College Park, MD, United States
Introduction: Training outcomes for verb naming impairment in post-stroke aphasia are limited in their generalization to untrained verbs, and little is known about neuroplasticity associated with verb naming impairment. This is a proof-of-concept study that examined if manipulability (action involving a specific hand shape) is a conceptual feature of verbs.
Methods: Individual differences in verb naming outcomes and associated neuroplastic changes following training to produce manipulable verbs were compared using FMRI in a case series of two persons with post-stroke agrammatic aphasia who had a verb deficit.
Results: Following 12 sessions of training, trained verb naming improved while untrained manipulable and non-manipulable verb naming was unchanged. Functional magnetic resonance imaging of verb naming showed that correct verb naming recruited unlesioned areas of the verb network recruited by neurotypical speakers and a network of compensatory regions including the bilateral perisylvian and subcortical regions. The participant with a larger training effect size showed post-training upregulation in these compensatory regions while the participant with the modest effect size mostly showed a downregulation. In both participants, unlesioned regions of the neurotypical verb network showed a downregulation following verb training.
Discussion: The findings provided limited support for verb manipulability as a conceptual feature. The study also supported prior research on showing that a more effective response to intervention is associated with increased re-engagement of pre-existing networks associated with successful naming.
1 Introduction
Post-stroke aphasia persists chronically in one-third of stroke survivors (Flowers et al., 2016). To date, speech-language therapy aimed at re-training language is the most effective treatment option for persons with aphasia (PWA) (Brady et al., 2016) compared to other approaches (Elsner et al., 2019; Small, 1994). Yet, speech-language pathologists (SLP) find it challenging to select language stimuli and make predictions about treatment and generalization outcomes (Cheng et al., 2020) because of considerable individual heterogeneity in behavioral and neural outcomes (Kristinsson et al., 2020, 2022; Menahemi-Falkov et al., 2022; Schevenels et al., 2020). Methodological heterogeneity across brain imaging studies examining treatment-induced neural plasticity has also contributed to the unclear picture of how treatment impacts brain activity (Simic et al., 2023; Wilson and Schneck, 2021). Mechanisms of treatment-induced neural plasticity are particularly imprecise for verb (or action) naming difficulties in aphasia (De Aguiar et al., 2016; Hickin et al., 2020). This study investigates two fundamental questions about individual variability in the context of manipulable verbs in aphasia: the effect of conceptual overlap between trained and untrained stimuli on verb naming outcomes and neural plasticity, and patterns of treatment-induced recruitment in the brain's verb network (Faroqi-Shah et al., 2018).
1.1 Manipulability as a potential semantic concept
Verbs are not only syntactically complex, but also vary widely in their semantic properties. One of the earliest and most thorough analysis of verbs classified verbs based on their correspondence between semantic and syntactic properties (Levin, 1993). For example, verbs which share the meaning component of “manner of motion” (such as travel, run, walk), behave similarly also in terms of subcategorization (I traveled/ran/walked, I traveled/ran/walked to London, I traveled/ran/walked five miles). Verb naming accuracies across verb classes have shown that some persons with aphasia are more vulnerable to certain classes than others, including “change of state” (e.g., melt, boil: Breedin et al., 1998, N = 6), “contact” (e.g., hit, tap, slap: Kemmerer, 2003, N = 2) and “instrument” (e.g., cut, sweep: Jonkers and Bastiaanse, 1996, N = 13). However, these patterns have been relatively weak and inconsistent across individuals. A larger group study focused on the semantic feature of manipulability, which is associated with a specific hand shape (Arévalo et al., 2007). Manipulable verbs are those that generate a specific hand gesture when speakers are asked to mime the verb (e.g., erase, pour, squeeze) and manipulable nouns are those that can be held in the hand (e.g. pencil, scissors, knife). In a group of 21 PWA, Arévalo et al. (2007) found that manipulable verbs (and nouns) were especially difficult to name and read compared to non-manipulable verbs (e.g., run, blow, listen). In contrast, non-brain damaged control participants showed the opposite pattern of poorer naming with non-manipulable items. This double dissociation suggests that manipulability could be a semantic feature of verbs. However, further research is needed to verify the semantic status of verb manipulability.
In this study, verb training outcomes are used as a way to test the semantic status of manipulability. In aphasiology, generalization to untrained items following training in PWA has been used as a method to better understand and test theories of language representation (e.g., Kiran, 2008; Li and Kiran, 2023; Marangolo et al., 2012; Thompson and Shapiro, 1995). For example, Kiran (2008) found that training of bird names (e.g., penguin, ostrich) generalized to other untrained bird names (robin, sparrow) due to shared semantic features across trained and untrained nouns. If manipulability is a neurally instantiated verb concept, then we can make two predictions about training outcomes and neural activity in PWA. First, training of verb naming using manipulable verbs would strengthen the neural network associated with this concept, and generalize to improved naming of untrained manipulable verbs. This generalization to manipulable verbs would be larger than any improvement in untrained non-manipulable verbs. The second prediction is that manipulable and non-manipulable verbs would show distinct patterns of brain activity in PWA. The next section highlights what is known about the neural representations of verbs.
1.2 The “verb network”
Studies of the neural representation of verbs have yielded a wide range of findings (Crepaldi et al., 2013). Faroqi-Shah et al. (2018) conducted an activation likelihood meta-analysis of probabilistic cytoarchitectonic maps of extant neuroimaging studies of verb processing in neurologically healthy (neurotypical) adults. Verbs (subtracted from Baseline) activated a left-dominant peri-Sylvian network of regions consisting of large frontal clusters (Brodmann's areas [BA] 9/46, 6, 47), two superior-middle temporal clusters (BA 21, 22), and fusiform gyrus (BA 37) (Faroqi-Shah et al., 2018). In this study, we refer to this distribution as the neurotypical verb network. Key terms used in this study are listed in Table 1. The neurotypical verb network regions are identified in Supplementary Figure 1. When subtracted from noun processing (Verbs minus Nouns), the largest cluster was in the left mid-temporal cortex (BA 21), followed by a left frontal cluster extending into the insula (BA 44/45, BA 13), and two small clusters in the right temporal (BA 21) and middle frontal (BA 6) regions. The largest region of the neurotypical verb network is the left frontal cortex, which has been associated with a variety of verb processes including morphological transformation and verb semantics (Caspers et al., 2010; Sahin et al., 2006; Shapiro et al., 2005, 2006). The left mid-temporal region is associated with action verbs (Bedny et al., 2008; Bedny and Caramazza, 2011; Peelen et al., 2012) and domain general action concepts (Caspers et al., 2010; Watson et al., 2013). This region is also associated with grammatical properties of verbs (Hernández et al., 2014; Papeo and Lingnau, 2015). While this was not found in the activation likelihood meta-analysis, a few studies have identified the bilateral parietal cortex for specific action plans, such as when general verbs are compared to more specific action verbs (e.g., clean vs. wipe) (van Dam et al., 2010), and left parietal cortex activation for verbs with more complex (two- vs. one-) argument structures (De Ouden et al., 2009; Thompson et al., 2013).
Some research has suggested that semantically distinct verb classes may be processed in neuroanatomically distinct brain regions. For example, different neural patterns were found for verbs within the semantic classes of “action”, “motion”, “contact”, “change of state” and “instrument” (Kemmerer et al., 2008). Studies have also found unique representations for verbs associated with specific body parts, such that mouth-verbs like smiling activate sensorimotor cortex associated with the mouth (Hauk et al., 2004; Kemmerer et al., 2012; Riccardi et al., 2019). However, other authors have criticized the interpretation of such findings (Caramazza et al., 2014) or have found conflicting findings (De Zubicaray et al., 2013). For example, De Zubicaray et al. (2013) found that verbs as well as non-words that sounded like verbs activated the motor cortex, showing no unique activation for action verbs. Additionally, meta-analyses have not found exclusive association between action verbs and motor cortex (Giacobbe et al., 2022; Solana et al., 2024), and that prior studies of action-motor associations were underpowered with a publication bias (Solana and Santiago, 2022). Thus, there is a need to further examine the semantic properties of verbs and their neural instantiation.
1.3 Behavioral and neural outcomes of verb naming treatment in PWA
The most recent meta-analysis of verb treatment outcomes of individual participant data revealed that trained verbs improved in 80% of PWA, while untrained verbs improved in only 15% of PWA (Hickin et al., 2020). It has been proposed that generalization outcomes of untrained verbs could be improved if there was semantic overlap between untrained verbs and the verbs used in training (Faroqi-Shah and Graham, 2011). Generalization to semantically related untrained items has been found for nouns in several studies (Kiran, 2008; Li and Kiran, 2023; Sandberg and Kiran, 2014). However, improvements in untrained semantically related verbs have not been found in the two studies that examined this (Faroqi-Shah and Graham, 2011; Li and Kiran, 2023). One challenge in facilitating improvements in untrained verbs is our limited understanding of the semantic constitution of verbs (Kable et al., 2002; Van Valin, 2006). Consequently, the stimuli being selected to test generalization to so-called “semantically” related verbs may not be truly representative of the actual semantic network of verbs. As argued earlier, manipulability holds promise as a semantic feature. But it awaits empirical validation, both in behavioral and neural realms. Another way in which generalization to untrained verbs may be improved is by targeting multiple aspects of verb retrieval, such as their use in sentence contexts, verb argument relationships, as well as semantic and phonological practice (Thompson et al., 2013). The verb training used in the present study incorporates both these suggestions: overlap of manipulability between training and untrained verbs as well as a novel total verb therapy protocol.
Few studies have specifically examined neural plasticity of verb training in PWA using functional magnetic resonance imaging (fMRI) (Durand et al., 2018; Thompson et al., 2013). Durand et al.'s verb naming treatment (N = 2) focused on sensorimotor attributes of actions such as observing, mimicking, and creating mental images of the action. Following training, there was a decrease in activation (downregulation) of regions used in naming and an increase in activation (upregulation) of sensorimotor regions. Thompson et al.'s (2013) verb naming treatment (N = 4) focused on the argument structure of verbs. Following training, there was upregulation in regions engaged by neurotypical speakers for verb naming, in contrast to Durand et al.'s findings.
Typically, a post-training downregulation of neural activity has been associated with increased efficiency or automatization of the language process. An upregulation, in contrast, is interpreted as increased cognitive effort that may occur from engagement of new brain regions or compensatory strategies (Thompson et al., 2013). A study that compared post-training neural activation with treatment outcomes (N = 26) found that responders (those who improved significantly) were more likely to show upregulation compared to non-responders (Johnson et al., 2019). A recent systematic review of 33 studies of treatment-induced neural plasticity in anomia (documenting mostly noun training) found that upregulation was more commonly reported across studies, particularly in the left supramarginal gyrus and left/bilateral precunei (Simic et al., 2023). Downregulation was less frequently reported following therapy, and typically was noted in bilateral superior temporal and right middle frontal gyri in individual data. A meta-analysis of 86 non-treatment studies found that recruitment of left hemisphere language regions (and possibly right temporal activity) was associated with better language performance in aphasia (Wilson and Schneck, 2021). Overall, both studies concluded that (as yet) there is no robust pattern of neural plasticity in PWA.
A key finding in both verb training studies (Durand et al., 2018; Thompson et al., 2013) and the systematic review (Simic et al., 2023) was extensive individual variability in treatment-induced neural changes. This variability is not surprising, given differences in lesion extent and hypoperfusion patterns across participants. Verb-related neural plasticity could be better understood by identifying the network of regions recruited for successful verb naming by each aphasic person prior to verb training, which we refer to as the compensatory verb network (Table 1). The term compensatory network has been used to refer to the altered neural response pattern following a neurological lesion (e.g., Hallam et al., 2018; van Hees et al., 2014). An individual-level region of interest (ROI) comparison of compensatory vs. neurotypical verb networks will inform how verb naming is achieved by persons with aphasia (PWA) prior to- and in response to- verb training. Of particular interest is the extent to which engagement of unlesioned regions of neurotypical verb network is associated with successful verb naming. We hypothesized that greater recruitment of the neurotypical verb network would be associated with better verb naming outcomes, for both trained items and generalization to untrained items.
1.4 The present study
To address the limited understanding of verb semantics and mechanisms underlying verb naming training outcomes in PWA, this proof-of-concept study sought to investigate two unresolved issues: the effect of conceptual overlap (i.e., manipulability) between trained and untrained stimuli on verb naming outcomes, and how the compensatory verb network compares with the neurotypical verb network Faroqi-Shah et al., (2018). Given that response to treatment may potentially impact neural change (Johnson et al., 2019), we focused on individual variability across two PWA who had a verb impairment and were in the chronic phase (post 6-months). Using a single case experimental design, each individual was trained for 12 sessions on a personalized set of 20 manipulable verbs. Naming of trained and untrained manipulable and non-manipulable verbs was assessed before and after training behaviorally and using fMRI. For the FMRI task, naming of action videos was compared with matched non-iconic signs from American Sign Language which did not have a verb label (see Methods Section 2.7 for protocol details).
Three research questions were posed. The first research question is a proof-of-concept, testing whether verb manipulability is a trainable semantic feature in PWA. This research question was addressed by examining the efficacy of Total Verb Therapy (TVT) on trained and untrained manipulable and non-manipulable verbs. Based on prior findings on trained words in aphasia (e.g., Hickin et al., 2020), we predicted improvement of trained verbs (Hypothesis 1a). The critical support for manipulability as a conceptual feature comes from comparing generalization effects across untrained manipulable and non-manipulable verbs. If naming of untrained manipulable verbs, but not untrained non-manipulable verbs improves after manipulable verb training (Hypothesis 1b), then we can conclude that manipulability is a likely semantic feature of verbs (Faroqi-Shah and Graham, 2011). The second research question asked which brain regions are utilized for correct verb naming in persons with damage to (parts of) the neurotypical verb network. We hypothesized that this compensatory verb network would consist of unlesioned parts of the neurotypical verb network (Thompson et al., 2013), ancillary regions identified in the Verb-minus-Noun contrast by (Faroqi-Shah et al. 2018), and right hemisphere homologs of the neurotypical verb network (Hypothesis 2a). Further, if manipulability is neurally instantiated, there would be unique patterns of activation for correctly named manipulable vs. non-manipulable verbs (Hypothesis 2b). The third research question investigated training-induced neural plasticity across the two participants for trained and untrained verbs. We hypothesized that following training, the neural activation for trained verbs would approximate the compensatory verb network of each participant because this network is already recruited for verbs that are correctly named before training (Hypothesis 3a). Given the likelihood of individual variability in aphasia treatment outcomes, we expected differences in neural plasticity across the two participants and hypothesized that the PWA with a larger post-training effect size would show a greater recruitment of the neurotypical verb network as well as a greater upregulation of brain activity (Johnson et al., 2019; Simic et al., 2023) (Hypothesis 3b).
2 Methods
The study was performed with ethics approval from the University of Maryland, College Park. Participants provided informed consent prior to participation, in accordance with ethical standards set forth by the Declaration of Helsinki. They received monetary compensation for participating in the study. The Single-Case Reporting Guideline In BEhavioural Interventions (SCRIBE, Tate et al., 2016) protocol was followed in reporting the verb training outcomes. The SCRIBE checklist is given in Appendix A.
2.1 Overall design
The timeline of language assessments, training, and FMRI data collection is illustrated in Figure 1. The study followed a multiple baseline across behaviors single case experimental design (MB-SCED). SCEDs (also known as N-of-1-trials) utilize multiple study phases, a Baseline phase, where pre-training data are obtained, followed by a Training phase, where treatment is administered and performance data are obtained, and finally a measurement phase when treatment is withdrawn (Post-training) (Tate et al., 2016). Multiple baselines (MB) refer to the two types of verbs (manipulable and non-manipulable) whose performance is measured across the study phases. During the Baseline phase, participants' overall language profile was assessed and an FMRI scan was conducted during which participants named videos of actions. Categorization of verbs into training and generalization verbs for training and FMRI was based on each individual's verb naming performance in the Baseline phase (further explained in Section 2.3). The Training phase included 12 (almost) daily sessions of 1 h each over a period of 2 (or 3) weeks, and a mid-point naming assessment of all verbs following the 6th session. The treatment duration of 12 h was based on the significant treatment gains of a prior action verb naming treatment study which provided 10 h of treatment (Marangolo et al., 2012). The Post-training phase included one session each of language assessment and FMRI within 4 days of the last training session.

Figure 1. The timeline of the study showing the three phases of the study (baseline, training, and post-training) and the activities conducted at each phase.
2.2 Participants
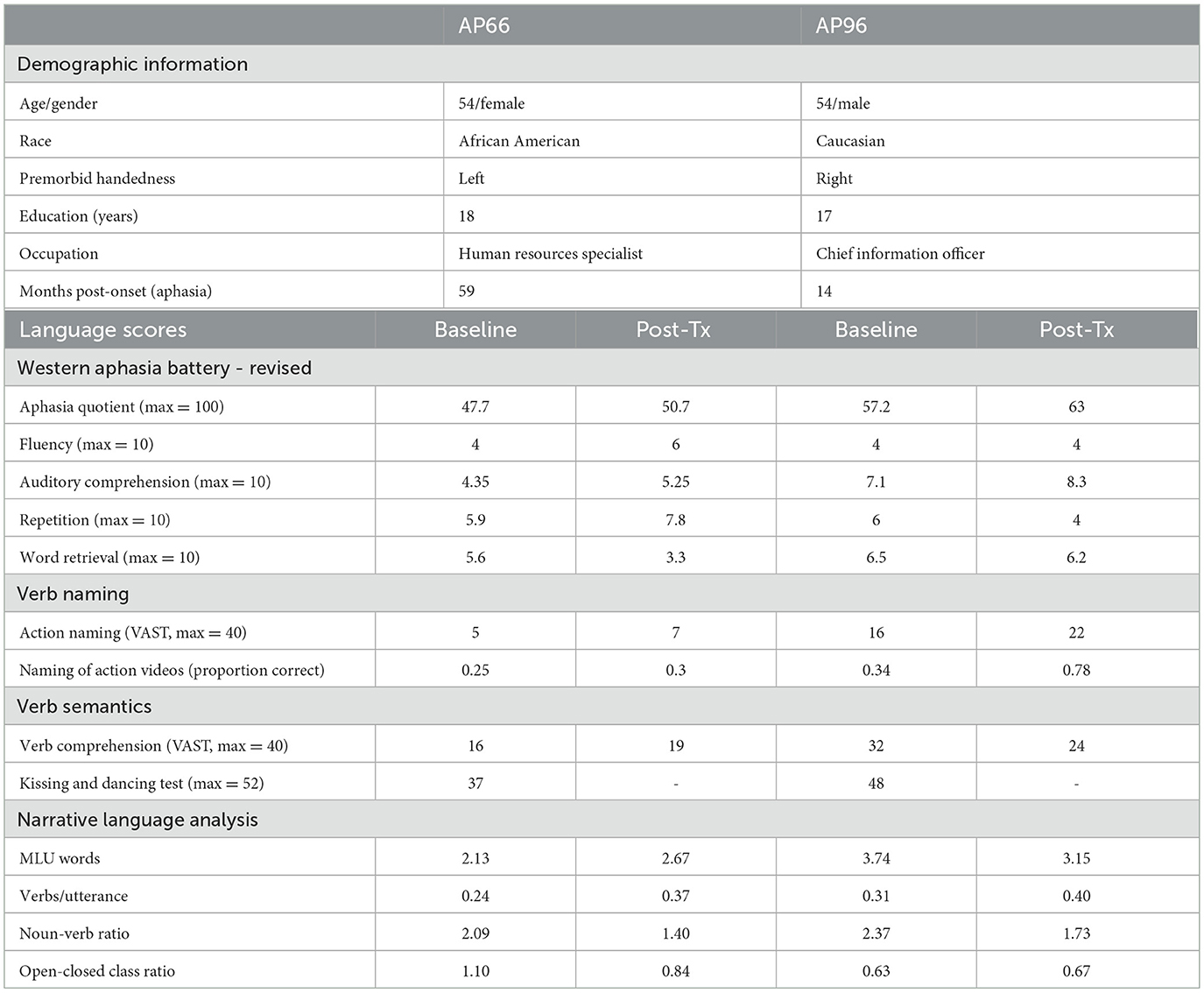
Two participants with aphasia are reported in this study, AP66 and AP96. These two participants are part of a cohort of seven PWA with a verb deficit who participated in an efficacy study of TVT (Faroqi-Shah, 2018) and also received two fMRI scans. Participants were recruited consecutively based on meeting eligibility criteria without any randomization. Demographic details are given in Table 1. Both participants had developed aphasia consequent to a single left hemisphere ischemic cerebrovascular accident of the middle cerebral artery territory. They were also diagnosed with right-sided hemiparesis. AP66 was also diagnosed with depression following the stroke. They were at least 1-year post onset, were native speakers of English, had at least high school education, and had no pre-morbid history of psychiatric, neurological, cognitive, or speech-language conditions. Both participants passed binaural pure tone audiometric screening at 500, 1,000, and 2,000 Hz at 25 dBHL (ANSI, 1969) and passed a vision screen (at least 20/40 corrected or uncorrected vision and the absence of spatial neglect and visual field deficits). Both participants underwent 2–4 months of physical, occupational, and speech therapy following their stroke, which is the standard care available to persons with aphasia in the United States. Neither of the participants were engaged in other speech-language therapy services for the duration of this study or had been part of any other funded treatment research. Both participants have been reported in two other studies (Faroqi-Shah et al., 2020, 2023).
2.2.1 Neuroanatomical lesion
Both participants received T1 and T2-weighted structural MRIs using a Siemens Trio 3T scanner with 1.0 mm resolution. Each participant's lesion boundaries were manually traced by the 2nd author on these structural scans using MRICron (Rorden et al., 2007). Their lesions are shown in Figure 2. In Table 2, each participant's lesion is compared with the neurotypical verb network (Faroqi-Shah et al., 2018). Both participants' lesions spanned perisylvian regions of the left hemisphere. Both participants' lesions included portions of primary motor cortex (“precentral gyrus”). Their lesions encompassed all the regions of the neurotypical verb network (IFG, MFG, MTG) with the exception of fusiform gyrus, which was spared in both participants. There was considerable overlap in the lesioned regions even outside the neurotypical verb network. However, AP66′s lesion (49.5 cc) was larger than that of AP96 (24.2cc), occupying a greater volume in the frontal, temporal, parietal and occipital lobes.

Figure 2. Horizontal slices showing each participant's lesion in standard space (MNI-152). AP66 left panel, AP96 right panel.

Table 2. Lesion extent of each participant (Harvard-Oxford Atlas) along with the fronto-temporal regions of the verb network (Faroqi-Shah et al., 2018) in the left column.
2.2.2 Language assessment
Language assessments focused on determining an overall aphasia profile and the nature of verb impairment (Table 1). The profile and severity of aphasia was assessed using a standardized diagnostic battery (Western Aphasia Battery-Revised, WAB-R, Kertesz, 2006). Narrative language samples were elicited using a complex picture (cookie theft picture, Goodglass et al., 2001), a story retell task (Cinderella story), and a personal narrative (story of their stroke). Single word reading was screened using the written word-to-picture matching portion of the Reading subtest of the WAB-R. Apraxia of speech was assessed using Apraxia Battery for Adults, 2nd edition (Dabul, 2000). Both participants showed a profile of Broca's aphasia of moderate severity (as per the WAB-R) with negligible-to-mild apraxia of speech (as per Apraxia Battery for Adults). As seen in Table 3, their narrative language showed reduced production of verbs (high noun:verb ratio) and fragmented utterances (low mean length of utterance [words]), consistent with a profile of agrammatic language production (see Table 1) (per the criteria noted in Hsu and Thompson, 2018).
Verb abilities. Verb naming was assessed using 131 action videos developed for this study (described under Section 2.3) and the Action Naming portion of the Verb and Sentence Test (VAST, Bastiaanse et al., 2010). During the Baseline phase, verb naming using the 131 action videos naming was administered thrice, at least 1 week apart. Access to semantic representations of verbs was assessed using the Verb Comprehension section of the VAST (Bastiaanse et al., 2010) and the Kissing and Dancing Test (KDT, Bak and Hodges, 2003). Both of these standardized measures have been validated for assessing verb semantics in aphasia (Bak and Hodges, 2003; Bastiaanse et al., 2010). The Baseline scores in Table 1 show that both participants were severely impaired in naming verbs. For verb comprehension, both participants scored above chance on the KDT (2-choice task, chance level is 50%, 26/52) and VAST (4-choice task, chance level is 25%, or 10/40).
Inclusionary criteria. The participants had to fulfill multiple eligibility criteria which were established prior to participant recruitment. The main criterion was a verb naming impairment, defined as less than 60% accuracy on the VAST action naming subtest and action naming videos of this study. Additionally, they had to have a relatively stable baseline of verb naming, defined as less than 25% difference in total accuracy across the three action video administrations. Finally, they had to have adequate comprehension abilities to follow the study requirements, defined as a composite comprehension score of at least 4 (out of 10) on the WAB-R (Kertesz, 2006). Both participants met these inclusionary criteria. Across all measures, AP66′s language abilities are more severely impaired than those of AP96.
2.3 Stimuli for verb naming, training, and FMRI
Three-second action videos (with no sound) were filmed for 131 verbs, all depicting an imageable human action. All videos consist of one or two actors depicting an action with real scenarios (e.g., knocking on a door, pedaling a bicycle, etc.). These videos were shown to 10 native English speaking college students for norming of naming. All videos elicited a verb label with more than 60% name agreement. As noted in Table 1, Manipulable verbs (N=61) had a hand action, either with or without a tool, and were defined as those which generate a specific hand gesture when speakers are asked to mime the verb (Arévalo et al., 2007) (e.g., erasing, knocking, cutting). Non-manipulable verbs (N = 70) depicted an action that could be performed with the face (e.g. smiling), leg (e.g., pedaling), or whole body (e.g., running). Illustrative examples of the videos are provided in Figure 3. The entire list of stimuli is provided in Appendix B.

Figure 3. Examples of video stimuli used in the study (A) a manipulable verb - chop; (B) a non-manipulable verb – sit, and (C) sign. Videos of signs were only used as the control condition for the FMRI component of the study.
Each participant's naming accuracy across the three Baseline elicitations was used to identify verbs that were consistently named accurately (correct verbs) and those that were named incorrectly in at least two out of three administrations (incorrect verbs). A verb was scored as correct if the label provided by the participant corresponded with the target verb name (as determined by the naming norms from 10 neurotypical adults). Twenty incorrectly named manipulable verbs were identified as Training verbs. Each participant's training verbs are identified in Appendix B. All other verbs (N = 111) were classified as generalization verbs for the purpose of measuring training outcomes. Depending on the type of action, these verbs were labeled as Generalization-Manipulable and Generalization-Non-Manipulable.
A subset of the verbs was used as stimuli in the FMRI scans. As noted in Table 1, verbs were grouped into four categories, based on whether they were correctly or incorrectly named during Baseline assessments1, as described above, as well as whether they were manipulable or non-manipulable verbs. Thus, the four categories of verbs used in the FMRI study were: correct manipulable (CM), incorrect manipulable (IM), correct non-manipulable (CNM), and incorrect non-manipulable (INM). Each participant's stimuli in the FMRI study corresponded to their individual performance. Based on individual performance, the specific verbs in each category differed for each participant. There were 16 verbs in each of the four categories. The incorrect manipulable (IM) verb list mostly consisted of training verbs for that participant, and thus represents trained verbs. The incorrect non-manipulable (INM) verb list consisted of untrained verbs, and thus represent untrained verbs. In addition, 16 videos of non-iconic signs that had no straightforward verb label were used from a prior study (Redcay and Carlson, 2015). These sign videos had similar visual complexity as the verb videos and served as a baseline for subtraction.
2.4 Verb training
Action videos of the 20 training verbs (for each participant) were used. All training was conducted on a computer, either in person or via videoconferencing. The training was conducted by the first author (who is a SLP) and research assistants trained by the first author. Each session began with naming probes of treatment verbs. Naming probes were transcribed and scored during the session. Following this, any one Training verb video was presented and the steps of the TVT protocol were followed. The TVT protocol steps were repeated for other verbs (presented in random order). The treatment approach (which was developed by the first author) sought to activate and strengthen the verbs' representations along multiple dimensions: syntactic, semantic, and phonological. The TVT protocol consists of four steps, administered in the same order for each verb: naming and repetition, sentence production, semantic feature generation, and intermittent recall. The first training step involves strengthening lexical-phonological representation by engaging in verb naming and repetition. Verb repetition also fosters errorless learning (i.e., production without errored naming attempts) and improves participant engagement by reducing the frustration associated with incorrect naming (Cahana-Amitay et al., 2011). Errorless learning was used only during the first six sessions. The syntactic dimension (argument structure) is strengthened by asking participants to construct sentences using the verbs. The semantic dimension is targeted by having participants complete a semantic feature analysis chart of the verbs that identified relevant verb arguments (following Wambaugh and Ferguson, 2007). After cycling through five verbs with each of the above steps, the participant was encouraged to recall the five verbs that had just been practiced. This was done to promote retrieval induced consolidation by testing for forward learning (Pastötter and Bäuml, 2014). Verbs were practiced in random order. Cueing and guidance was significantly reduced during the last 6 sessions to raise the level of challenge for the participant, and hence promote learning (Schuchard and Middleton, 2018). There was no homework involved.
As per the Rehabilitation Treatment Specification System (RTSS, Fridriksson et al., 2022; Hart et al., 2019), the target of training was naming of training and generalization verbs, the ingredients of the TVT include phonological, syntactic and semantic practice, as well as errorless learning and retrieval induced reconsolidation. The mechanisms of action include the repeated practice of verb naming with semantic feature generation and sentence production for each trained verb during the training phase.
2.5 Assessment of verb naming outcomes
Verb naming scores were transcribed and scored by the tester during the session (no blinding). There were three verb outcomes: training, Generalization - Manipulable, Generalization – Non-Manipulable, and there were three study phases: Baseline (3 measures), Training, and Post-Training. For the first research question, change in verb accuracy between the Baseline (n = 3 sessions) and Post-Training (n = 1 session) phases was assessed using two measures that are commonly used in aphasiology for SCED: effect size (Beeson and Robey, 2006) and Tau-U (Lee and Cherney, 2018). Effect size was calculated using standardized mean difference (SMD) using Beeson and Robey's (2006) formula ([Post-Training Accuracy – Baseline Mean Accuracy]/Baseline standard deviation) and the magnitude of effect size was interpreted using their values for aphasiology. To calculate Tau-U, scores from the Baseline and Post-training were entered into a web application (Tarlow, 2016). Neither participant had a slope in the Baseline phase (AP66: Tau-U = 0.00, p = 1.46; AP96: Tau-U =.82, p = 0.54). Hence baseline correction was not applied.
2.6 Training fidelity and scoring reliability
To document fidelity of the TVT protocol administration, a research assistant or the first author observed four sessions of each participant (30% of total training duration) and checked that each training step in the protocol was followed. The protocol fidelity was 100%. Scoring reliability for verb naming probes was obtained by a research assistant who watched the videorecorded sessions. Scoring reliability between the original and reliability scorers was 95% (intraclass correlation coefficient = 0.98).
2.7 Brain imaging
Structural and functional magnetic resonance imaging scans were obtained during the same session, at two time points (Baseline and Post-training), as illustrated in Figure 1. The experimental task for FMRI involved oral naming of 3-second action videos presented on a visual display. As noted in Section 2.3, the videos fell into five categories: correct and incorrect manipulable (CM, IM) and non-manipulable (CNM, INM) actions as well as Signs. The videos were presented in three “runs” (or blocks) with a break in-between runs. Participants were asked to either name the action if they knew its label, or say “pass” if they could not name the action video. Thus all non-iconic ASL signs and any verbs that could not be named were responded with “pass”. Participants were given practice with this FMRI task on a computer prior to brain imaging.
The same scanning procedure was used for Baseline and Post-training scans. All scans were conducted using a 3T Siemens Trio Scanner. A sagittal T1 weighted MPRAGE sequence (TR = 2,350 ms, TE 1 = 2.15 ms, flip angle = 7°, T1 = 1,200 ms, slice thickness = 1.00 mm) was used to acquire structural images used in the imaging analyses. For functional scans, blood oxygen level dependent (BOLD) responses were measured with an echoplanar imaging sequence (TR = 1,000 ms, TE = 31.0 ms, flip angle = 90°, slice thickness = 2.20 mm). The BOLD response during the 4-s viewing and planning period following the start of the video was analyzed.
FSL (FMRIB Software Library, Jenkinson et al., 2012) software was used for image processing and analysis. Non-brain voxels were removed using Opti-BET tools for brain extraction, accounting for lesions. The same lesion mask was used for pre- and post-therapy scans (Lutkenhoff et al., 2014). Additional image pre-processing included application of standard motion correction with MCFLIRT, spatial smoothing using a 5 mm Gaussian kernel and high pass filtering (100 Hz). An interleaved slice-timing correction was applied for functional scans. Images were normalized to MNI-152 space (Fonov et al., 2009).
For each of the Baseline and Post-training runs, a first-level analysis of the BOLD response for each participant used a general linear model with regressors for each condition described in Section 2.3 (CM, CNM, IM, INM, and Signs) convolved with a double-gamma hemodynamic response function. Standard motion parameters and temporal derivatives were applied. Two kinds of second-level analysis were performed. We used a fixed-effects analysis to average each participant's available Baseline runs (3 runs for each participant) and post-therapy runs (3 runs AP96, 2 runs AP66). We also compared Baseline and Post-training scans using a fixed-effects analysis with pre-therapy scans coded as 1 and post-therapy scans as −1 for each participant. For all analyses, to evaluate significant voxels, we applied cluster correction with Z-threshold = 3.1 and p-threshold < 0.05. For the second level analyses, we applied a pre-threshold MNI152 T1 2 mm brain mask. Whole brain analyses refer to the second-level results. We did not exclude the lesion areas from analyses in order to capture changes in peri-lesional tissue and to perform neurotypical verb network ROI analyses (see below).
The critical comparisons for the second and third research questions are presented in Table 4. For the second research question, the Baseline FMRI data was analyzed for identifying the compensatory verb network ((CM + CNM) > Signs) and differences in neural activation for manipulable vs. non-manipulable verbs (CM > CNM and CNM > CM). To analyze how each participant's activation compared with the neurotypical verb network, we created a set of ROIs using the MNI coordinates of the weighted center in each neurotypical verb network ROI in Faroqi-Shah et al. (2018) (Supplementary Figure 1). Following conventions from the previous literature (e.g., Sharer and Thothathiri, 2020; Skipper-Kallal et al., 2017), FSL tools (fslmaths) were used to create a 5 mm sphere around each weighted center point and each ROI was binarized (Skipper-Kallal et al., 2017). Each of the 5 mm ROIs spanned 81 voxels. Overlap analyses between participants' compensatory verb networks and between compensatory and neurotypical verb networks used fslmaths tools to multiply images of interest.
For the third research question, the critical comparisons examined changes in neural activation for trained verb naming between the Baseline and Post-training sessions (increased activation/upregulation after therapy: Post (IM > Signs) > (IM > Signs), decreased activation/downregulation after therapy: Baseline (IM > Signs) > Post (IM > Signs), and activity when naming trained verbs became more like (approximated) activity of the baseline compensatory verb network identified in the second research question (Baseline (CM > IM) > Post (CM > IM)). We examined the same critical contrasts for untrained verbs (INM and CNM). To test training-induced changes in neural activity for verb naming, we used FSL's featquery tool to determine mean activation and standard deviation of activations across voxels in the neurotypical verb network ROIs for each participant's Baseline > Post-training difference in activation for naming trained (IM > Signs) and untrained (INM > Signs) verbs. Because the value of each voxel represents the parameter estimates for the Baseline scan minus the Post-training scan, positive difference scores reflect a decrease in activation following treatment (downregulation) and negative difference scores reflect an increase in activation following treatment (upregulation). A two-tailed, one sample t-test was conducted for each contrast (trained, untrained) in each ROI for each participant to determine if the Baseline-Post-training difference was significantly different than 0 (no change). With Bonferroni correction to account for a family-wise error rate of 0.05 over 24 comparisons, the significance threshold was α < 0.002.
3 Results
There were no procedural changes to the a priori design or any adverse events during the study.
3.1 Verb training outcomes
The dosage of verb training was comparable across the two participants. They received a total of 520 (AP66) and 500 (AP96) minutes of practice over 12 training sessions (that is an average of 8.5 treatment hours)2, and practiced an average of 15 (AP66) and 17 (AP96) verbs per session. Verb naming accuracies of each participant across each study phase are plotted in Figure 4. The raw accuracy data is provided at the Open Science Framework link for this project: https://osf.io/vrbsg. AP66 improved by 42% from baseline (8%) to post-training (50%) on trained verbs (SMD = 2.89; Tau-U = 0.80, SE = 0.35, p = 0.07). AP96 improved by 66% (14% at Baseline, 80% at post-Training) on trained verbs (SMD = 21.71; Tau-U = 0.91, SE = 0.25, p = 0.06). It is noteworthy (for research question 3) that AP96 had a much larger magnitude of improvement on trained verbs compared to AP66 (see also Figure 4).

Figure 4. Verb naming accuracies for each verb category during each study phase for AP66 (top) and AP96 (bottom). All verbs were assessed at Baseline, the 6th training session (mid-point), and at Post-training. Naming probes for training verbs were conducted at the start of each training session. Tx-Man = Training Verbs; Gen-Man = Generalization Manipulable Verbs; Gen-NonMan = Generalization Non-Manipulable verbs.
Untrained manipulable verb accuracy showed negligible changes for AP66 (16% at Baseline, 24% at post-Training; SMD = 0.95; Tau-U = 0.43, SE = 0.52, p = 0.38) and AP96 (57% at Baseline, 55% at post-Training; SMD = −0.49; Tau-U = −0.78, SE = 0.36, p = 0.08). Untrained non-manipulable verb scores also showed negligible changes (AP66: 24% at Baseline, 29% at post-Training, SMD = 0.87; Tau-U = 0.27, SE = 0.56, p = 0.66; AP96: 39% at Baseline, 46% at post-Training, SMD = 0.55; Tau-U = 0.26, SE = 0.56, p = 0.66). For additional statistical analyses, generalized linear mixed effects analysis results are provided in Supplementary Table 1. Critically, for the first research question, that was improvement in trained verbs (Hypothesis 1a), but no generalization advantage for manipulable verbs compared to non-manipulable verbs (Hypothesis 1b). The result from these two participants does not support that manipulability is a potential semantic feature of verbs.
3.2 fMRI results
The activation clusters for the contrasts are given in Table 5 and the results are illustrated in Figures 5–7. For ease of reporting, we list only clusters with 100 voxels or more in Table 5. Tables with all significant clusters can be found in the Supplementary Table 2.

Figure 5. (A) Baseline compensatory verb network for AP96 (blue) and AP66 (yellow). Significant clusters (>100 voxels, Cluster correction, Z>3.1, P < 0.05) for contrasts comparing all correct verbs and signs ([CM + CNM]>Signs). CM, correct manipulable; CNM, correct non-manipulable; Signs, signs (control condition). Left panel, left hemisphere; right panel, right hemisphere. Only brain surface is shown. For both participants, lighter colors indicate higher z statistic values. (B) Overlap in baseline compensatory verb network for AP96 and AP66 (green; brain shown as transparent, superior view to view all clusters). Anterior shown at top of image.
3.2.1 Compensatory verb network
For the second research question regarding each person's compensatory verb network, we identified the regions with greater activation for correctly naming verbs (manipulable and non-manipulable) than signs during the Baseline scan (Table 4). Each participant's compensatory network is shown in Figure 5A and the regions are listed in Table 5A. As is evident from Figure 5A, AP66 and AP96 had overall different patterns of compensatory verb network activity. However, there were some overlapping locations, which are illustrated in Figure 5B and listed in Table 5A. Both participants' compensatory verb networks included activation in canonical language production regions in the left hemisphere (pars triangularis/middle frontal gyrus, insular cortex, and middle temporal gyrus; Figure 5A) and right hemisphere homologs of these regions (middle frontal gyrus; Figures 5A, B). There was also overlap between participants' compensatory verb networks in regions less typically associated with language, including cortical (bilateral frontal pole, right orbitofrontal cortex, and superior frontal gyrus) and subcortical regions (bilateral caudate nuclei, and right cerebellum; Figures 5A, B). Both participants had activations in some of the same anatomical regions although the stereotaxic coordinates did not directly overlap (left and right fontal pole, left and right middle frontal gyrus, left and right putamen, left insula, left middle temporal gyrus, right anterior cingulate gyrus). Participants' compensatory verb network had some overlap with the neurotypical verb network ROIs. However, the overlap was limited, with less than 30% of the voxels in any ROI for either participant (Hypothesis 2a).
Besides these similarities, AP96 (blue in Figure 5A) showed a much more extensive network of regions compared to AP66 (yellow in Figure 5A), with larger activation clusters in bilateral frontal and parietal regions, left hemisphere sensorimotor cortices and precuneus, and right hemisphere temporal regions. AP66′s network uniquely included portions of left middle frontal gyrus, middle temporal gyrus, lateral occipital cortex, and putamen (Figure 5A, Table 5A; Supplementary Table 2).
3.2.2 Manipulability
As shown in Tables 5B, C, there were no differences in the representation of manipulable and non-manipulable verbs for either participant (Hypothesis 2b). There was one small cluster for AP66 that did not meet the 100-voxel threshold (see Supplementary Table 2).
3.2.3 Training-induced neural plasticity
The results of the whole brain analysis comparing Baseline and Post-Training activity are illustrated in Figure 6 and listed in Tables 5D–I. The verb network ROI analyses results are illustrated in Figure 7. The three whole-brain contrasts of interest (upregulation, downregulation, and approximation to the compensatory verb network) for trained and untrained verbs are presented below. Results of the verb-network ROI analyses are then discussed.

Figure 6. Treatment effects for trained verbs for AP96 (top) and AP66 (bottom). The left panels show changes in trained verbs relative to Signs. Activation increases/upregulation (Post > Baseline) are indicated in red and activation decreases/downregulation (Baseline > Post) are indicated in blue. The right panels show approximation of trained verbs to each participant's compensatory verb network (green). Significant voxels reflect cluster correction, z > 3.1, p < 0.05). IM = incorrect manipulable (trained), CM, correct manipulable; signs, signs (control).

Figure 7. Mean parameter estimates for the Pre>Post contrast between activation for trained verbs (Incorrect Manipulable > Signs) and untrained/generalization verbs (Incorrect Non-manipulable > Signs) for each 5 mm spherical ROI in the verb network (Faroqi-Shah et al., 2018) and for each participant. Each panel (A–F) represents one ROI: (A) left middle frontal gyrus (BA6), (B) left inferior frontal gyrus (BA47), (C) left inferior frontal gyrus (BA9/46), (D) left middle temporal gyrus (BA21), (E) left superior temporal gyrus (BA22), (F) left fusiform gyrus (BA37). Positive values represent larger Baseline values and smaller values post-therapy (decrease in activation/downregulation over treatment). Negative values represent smaller Baseline values and larger values post-therapy (increase in activation/upregulation over treatment). Error bars represent standard error. Asterisks represent significant results for one-sample t-tests (FWER = 0.05, with Bonferroni correction).
3.2.3.1 Trained verbs
The whole brain analysis showed that neither participant had significant increases in the difference between correct and incorrect manipulable verbs over the course of training (Hypothesis 3a). For AP66 showed that, following training, there was no post-training approximation toward the compensatory verb network (Figure 5 lower right; Table 5F; Supplementary Table 2). While AP66 had no changes in activation relative to the compensatory verb network, there were changes outside of this network. There was relatively more downregulation than upregulation following treatment (Figure 5 lower left; Tables 5D, E; Supplementary Table 2). Upregulation was found in left orbito- and medial frontal cortex, and right frontal pole. The extent of downregulation was much more widespread compared to AP96. This included significant decreases in bilateral frontal and temporal regions as well as right parietal regions (Table 5E; Supplementary Table 2). There were significant decreases in subcortical regions including the right brain stem, and bilateral thalami and cerebellum.
AP96 showed a pattern of relatively more upregulation than downregulation for the whole brain analysis (Hypothesis 3b). AP96 showed upregulation primarily in right hemisphere frontal, temporal, and parietal regions (frontal pole, precentral gyrus, temporal pole, and supramarginal gyrus). There were also increases in activation in the left temporal pole. There was downregulation in perilesional tissue in left precentral gyrus (Figure 5, upper left; Tables 5D, E; Supplementary Table 2). A smaller difference in activation between correct and incorrect manipulable verbs Post-training than at Baseline (i.e., activation for incorrect verbs became more like activation for correct verbs after therapy) was found in the right hemisphere frontal, parietal, temporal, and occipital regions. There was also one significant cluster in the left supramarginal gyrus (Figure 5, upper right; Table 5F; Supplementary Table 2).
The blue bars in Figure 7 show changes in the verb network ROIs for trained verbs following training. Positive parameter estimates indicate a post-training downregulation. Both AP96 and AP66 showed a downregulation in two ROIs: left middle frontal (BA6) and fusiform (BA37) gyri. AP96 also had a downregulation in left STG/MTG (BA22) and an upregulation in left inferior frontal gyrus (BA47). In contrast, AP66 had an upregulation in the IFG, extending dorsally (BA9/46), and in the left MTG (BA21).
3.2.3.2 Generalization verbs
As outlined in Section 3.1, there was no change in the naming accuracy of generalization verbs following manipulable verb training and no generalization advantage for manipulable verbs. Following training, AP66 showed no Post-training upregulation for generalization verbs. There was downregulation in the left putamen and right precentral gyrus (Tables 5G, H; Supplementary Table 2). AP66 showed no approximation toward the compensatory verb network for non-manipulable verbs (i.e., a decrease in the difference between correct and incorrect non-manipulable verbs) (Table 5I; Supplementary Table 2).
AP96 also showed no upregulation for untrained verbs. For AP96, there was downregulation of neural activity in the left hemisphere parietal and occipital regions, as well as the cerebellum (Tables 5G, H; Supplementary Table 2). Following training, AP96 also had a significant decrease in the difference between correct and incorrect non-manipulable verbs in left and right hemisphere frontal and parietal regions. In the left hemisphere this contrast had significant decreases in precentral gyrus, postcentral gyrus, and superior frontal gyrus. In the right hemisphere this contrast had decreases in middle frontal gyrus, and precentral gyrus (Table 5I; Supplementary Table 2). This shows approximation toward the compensatory verb network, meaning that in these regions the neural activity in response to naming initially incorrect verbs became more like the neural activity in response to successful verb naming.
In the verb network ROIs (green bars in Figure 7), both participants showed the same pattern of downregulation in three regions: MFG (BA6), posterior IFG extending dorsally (BA9/46), and Fusiform (BA37). It is noteworthy that the MFG/Fusiform downregulation mimicked the pattern for trained verbs AP96 additionally showed downregulation in the STG/MTG (BA21) and in Left MTG (BA22). In BA47, the two participants showed opposing patterns, with an activation decrease for AP66 and activation increase for AP96.
4 Discussion
The present study used manipulable verbs as a test case to examine fundamental questions about verb representation in persons with aphasia. The first question explored the effect of semantic overlap between trained and untrained stimuli on verb naming outcomes. Second, we investigated the compensatory verb network of each participant and its relationship with the neurotypical verb network. The third research question investigated individual variability in the neuroplastic response to verb training. Using a single case experimental design, this study found improvements in trained verb naming whose magnitude was larger for AP96. There was no generalization to untrained verbs, irrespective of manipulability. The compensatory verb network recruited by each participant to correctly name (a few) verbs prior to training included unlesioned areas of the neurotypical verb network and bilateral perisylvian, frontal, and subcortical regions. Following verb training, both participants showed a downregulation of the neurotypical verb network for trained and untrained verbs. Beyond this common downregulation, there were several post-training individual differences. AP96 showed a much larger extent of upregulation overall and an approximation toward his compensatory verb network. In contrast, AP66, who had a smaller treatment effect size, predominantly showed downregulation of brain activity with no approximation toward her compensatory verb network. The implications of these findings are presented in the following sections.
4.1 Verb training outcomes and manipulability
The finding of successful learning of trained verbs with Total Verb Therapy provides efficacy data for this new treatment approach. The verb training outcomes are consistent with prior research which showed that 80% of PWA improve on trained verbs (Hickin et al., 2020). The 12-session (average of 8.5 h) dosage of the present study was much lower than the median dosage of 20 treatment hours reported in PWA treatment research (Brady et al., 2022; Cavanaugh et al., 2021). In fact, some studies provide as much as 60 hours of treatment (e.g., Kendall et al., 2015).
There were notable individual differences in treatment outcomes although both participants had an overall similar aphasia profile (agrammatic Broca's aphasia with verb deficit). The session-to-session data in Figure 4 show AP96 showing steeper (faster) acquisition and AP66 showing a more gradual learning trajectory. Likewise, AP96 had a large treatment effect while AP66′s effect size was small. While AP96 had a shorter time post onset of stroke than AP66, this may not have played a significant role in the prognosis because both participants had chronic aphasia and there is evidence that time post stroke does not impact treatment gains within the chronic phase (Moss and Nicholas, 2006). The better prognosis of AP96 can be attributed to three factors. First, AP96 had higher initial language and verb scores (Table 3), and aphasia severity is generally considered the single strongest predictor of response to impairment-based therapy (Fridriksson and Hillis, 2021; Kristinsson et al., 2022; Nakagawa et al., 2019). Secondly, AP66 had a larger lesion size than AP96 (Table 2, Figure 2). Larger lesions are more likely to impact a greater number of language network nodes, as well as domain-general systems supporting language processing. Studies have found poorer treatment-induced prognosis in PWA with larger lesion volumes (Goldenberg and Spatt, 1994) although the lesion site and extent of connectivity also play a critical role in prognosis (Kristinsson et al., 2022; Yourganov et al., 2016). Relatedly, the fMRI results showed that AP96 recruited a more widespread compensatory verb network at baseline than AP66 and re-recruited this network for trained verbs following training. Other studies have similarly found that baseline cortical activity and re-recruitment of language regions following training to predict prognosis (Marcotte et al., 2013; Yourganov et al., 2016). Understanding of factors that affect response to TVT can be improved by examining differences in FMRI patterns of AP96 and AP66 and with future research on TVT efficacy with larger numbers of participants.
The lack of improvement in untrained verbs following TVT is also consistent with prior research showing lack of generalizability in verb training (De Aguiar et al., 2016; Faroqi-Shah and Graham, 2011; Hickin et al., 2020). Moreover, there was no generalization advantage for untrained manipulable verbs compared to non-manipulable verbs. Based on the assumption that generalization occurs to semantically related lexical items (as observed for noun naming in numerous studies, e.g., Kiran, 2008; Sandberg and Kiran, 2014), an obvious explanation is that manipulability is not a viable semantic feature of verbs. This interpretation is further supported by the absence of significant differences in neural activation patterns for manipulable and non-manipulable verbs in these two verb impaired PWA (Tables 5B, C). While both the treatment outcomes and neural activation patterns cast doubt on manipulability as a semantic feature, further research is needed to confirm this interpretation by integrating complementary evidence from neurotypical speakers and PWA. Given that manipulable verbs are a subset of hand-action verbs, it is noteworthy that verb-impaired PWA have shown preserved priming effects between hand-verbs in a prior study (Faroqi-Shah et al., 2010; replicating findings in neurotypical speakers by Bergen et al., 2010). If hand-verbs do co-activate other hand-verbs (and by extension manipulable verbs), then it is plausible that such spread of activation occurred during TVT. However, the overall amount of practice in the present study could have been insufficient to produce generalization to untrained manipulable verbs. While the relationship between treatment dosage and generalization outcomes is not well understood, a recent meta-analysis found that greatest gains in standardized language assessments were associated with >20 to 50 h of intervention (Brady et al., 2022). One could argue that trained verbs need to be repeatedly retrieved with high criterion accuracy of 90–100% and automaticity before generalization to untrained items can be achieved (Hickin et al., 2020). Future research examining generalization effects of manipulable verb TVT with increased dosage could provide a further test of manipulability as a semantic feature.
4.2 Compensatory verb network
This study's second research question asked which brain regions are utilized for successful verb naming in persons with damage to (parts of) the neurotypical verb network. We hypothesized that this compensatory verb network would consist of unlesioned parts of the neurotypical verb network (Faroqi-Shah et al., 2018; Thompson et al., 2013), ancillary regions identified in the Verb-minus-Noun contrast by Faroqi-Shah et al. (2018), and right hemisphere homologs of the neurotypical verb network. As hypothesized, the compensatory verb network included unlesioned regions containing the neurotypical verb network (although not substantially within the verb network ROIs per se) for both participants, notably the left fusiform gyrus and other spared ROIs (middle frontal and superior temporal) (Table 5, Figure 5A). In addition to these verb-specific regions, a broader network of peri-Sylvian language regions was activated bilaterally (middle temporal gyrus, pars triangularis, middle frontal gyrus and insula). Overall, these findings support that persons with aphasia engage pre-existing language networks, and post-stroke language re-organization mainly occurs within these language networks and their right hemisphere homologs (Simic et al., 2023; Stockert et al., 2020; Wilson and Schneck, 2021). Additionally, we found activity in a network of non-language regions (frontal pole, orbitofrontal, superior frontal, and anterior cingulate cortex). One interpretation of these activations is that in persons with a verb deficit, correctly producing (a few) verbs requires extensive cognitive effort, thus over-engaging cognitive networks associated with attention, cognitive control, and self-monitoring. The predominantly superior and midline frontal activation patterns support this interpretation of the non-language neural activations, and is consistent with prior studies that have found similar midline frontal activity in post-stroke aphasia (Brownsett et al., 2014). However, recent meta-analyses of neuroplasticity in aphasia have found inconsistent recruitment of non-language (also called domain general) regions across studies (Wilson and Schneck, 2021). It should be noted that these studies did not separately analyze neural activity associated with correct naming (as done in the present study), and this could explain the difference in non-language neural recruitment across the present study and the meta-analysis of Wilson and Schneck (2021).
In regard to individual differences, AP96 recruited a more extensive compensatory verb network than AP66. This is consistent with prior findings that smaller lesions of the language network promote more extensive recruitment and reorganization of the language network (Kristinsson et al., 2022; Skipper-Kallal et al., 2017). Consistent with prior studies, we propose that AP96′s more extensive compensatory verb network at Baseline heightened his verb learning during the Training phase to a greater extent than AP66 (Marcotte et al., 2013; Yourganov et al., 2016).
In terms of manipulability, no activation differences were found between manipulable (CM, IM) and non-manipulable (CNM, INM) verbs for either participant at Baseline or Post-training. Coupled with the absent post-training verb naming differences between manipulable and non-manipulable verbs, this study does not support manipulability as a neurally instantiated feature of verbs. To our knowledge, this is the first study to examine neural differences for manipulability, and the neural findings need to be interpreted cautiously given that the context of this study is persons with left hemisphere lesions. Overall, this study adds to the body of research on the neural representation of verbs (e.g., Bedny et al., 2008; Caramazza et al., 2014; Crepaldi et al., 2013; De Zubicaray et al., 2013; Giacobbe et al., 2022; Kemmerer et al., 2012; Riccardi et al., 2019; Solana et al., 2024).
4.3 Training induced neural plasticity and individual differences
The third research question investigated training-induced neural plasticity across the two participants for trained and untrained verbs. We hypothesized that post-training activation patterns for trained verbs would approximate the compensatory verb network (i.e., the network engaged in successful verb naming at baseline). Further, we hypothesized that the PWA with a larger post-training effect size would show a greater recruitment of the neurotypical verb network as well as a greater upregulation of brain activity (Johnson et al., 2019; Simic et al., 2023). Both of these hypotheses were supported for trained verbs, and are in line with prior research (Marcotte et al., 2013; Thompson et al., 2013; Yourganov et al., 2016. Following verb training, AP96′s verb naming activations showed an approximation to his compensatory verb network (Table 4 for AP96). This approximation was observed for both manipulable (Baseline (CM > IM) > Post-training (CM > IM)) and non-manipulable (Baseline (CNM > INM) > Post-training (CNM > INM)) verbs. AP96, with the larger magnitude of improvement, showed relatively more upregulation in his compensatory verb network while the participant with the modest effect size mostly showed relatively more downregulation. These individual differences in treatment outcomes suggest that more effective verb training occurs when (1) PWA have a more extensive compensatory verb network at baseline (Johnson et al., 2019; Kristinsson et al., 2022), and (2) increase engagement of this compensatory network during verb training instead of decreasing engagement of this network or recruiting newer/other brain regions. This suggests that the TVT strengthened the pre-existing successful verb naming strategies and neural networks and extended this to the trained verbs for AP96.
There were two other noteworthy results. In both participants, unlesioned regions of the neurotypical verb network (which were also part of the compensatory verb network) showed a downregulation following verb training, for both manipulable and non-manipulable verbs (Figure 6 and Table 5). A similar downregulation following verb training was reported by Durand et al. (2018), but contrasts Thompson et al.'s (2013) finding of upregulation. While the reason for this downregulation across both participants is unclear, other authors have attributed post-training downregulation to automatization or increased efficiency of word retrieval (Thompson et al., 2013). The second interesting finding was the change in neural activation for untrained non-manipulable verbs despite no significant behavioral gains for both participants, including approximation toward the compensatory verb network and downregulation of the neurotypical verb network. While these changes could suggest that neural engagement precedes behavioral change (Warraich and Kleim, 2010), further research is needed to better understand if this change is a reliable neuroplastic pattern.
4.4. Conclusions, limitations, and future directions
This study is an early step in examining the efficacy of TVT, advancing the understanding of verb semantics, and neural plasticity associated with verb deficits and verb training in PWA. This study is along the tradition of small N studies in aphasiology which serve to test initial hypotheses of novel concepts (e.g., Durand et al., 2018 with N = 2 and Thompson et al., 2013 with N = 4). In terms of proof-of concept, TVT showed efficacy for verb learning and there were neuroplastic changes associated with verb learning in both participants. Generalization and FMRI data showed no support for manipulability as a semantic feature of verbs. Individual differences in response to training and neuroplasticity supported prior findings of the role of aphasia severity, lesion volume and baseline compensatory activity on outcomes. There are a few limitations of this study. First, the sample size of this study warrants caution in the conclusions about the efficacy of TVT and related neuroplastic changes. Secondly, as mentioned in the Discussion section, it is an open question whether a longer and more intense intervention would have resulted in improvement in untrained verbs. As for neuroplasticity, having more than one Baseline fMRI scan would have helped to delineate any scan-to-scan variability in brain activity from training-induced changes. The FMRI paradigm did not include untrained manipulable verbs condition that would have allowed us to examine neural plasticity associated with overlap in manipulability (we only had an untrained non-manipulable verbs condition). The very different lesion volumes of the two participants complicated the interpretation of individual differences in neural activity. Future research that includes a larger more diverse group of PWA, variation in treatment dosage, multiple fMRI baselines, treating lesion volume as a co-variate in FMRI analysis, and using additional neuroimaging markers such as structural and functional connectivity would improve our understanding of verb deficits and verb training outcomes in PWA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of University of Maryland, College Park. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YF-S: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KM: Data curation, Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work of supported by University of Maryland, College Park and NIDCD grant R01DC020483 to Yasmeen Faroqi-Shah.
Acknowledgments
We would like to thank Thomas Zeffiro, Donald J Bolger, and Wang Zhan for providing assistance with the FMRI paradigm and Jeremy Purcell for advice on lesion masking. We thank Elizabeth Redcay for contributing sign videos. Funding for this research was provided by the University of Maryland, College Park.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/flang.2025.1560115/full#supplementary-material
Footnotes
1. ^Because the verb stimuli were labeled according to each participant's baseline performance, the categories referred to in FMRI analyses reflect initial behavioral performance rather than whether the verb was named correctly or incorrectly on any particular trial in the scanner.
2. ^Most sessions were 1 h long, in which 10–15 min were utilized for assessing verb naming probes at the beginning of each session (as noted in Section 2.4).
References
Arévalo, A., Perani, D., Cappa, S. F., Butler, A., Bates, E., Dronkers, N., et al. (2007). Action and object processing in aphasia: from nouns and verbs to the effect of manipulability. Brain Lang. 100, 79–94. doi: 10.1016/j.bandl.2006.06.012
Bak, T. H., and Hodges, J. R. (2003). Kissing and dancing–a test to distinguish the lexical and conceptual contributions to noun/verb and action/object dissociation. Preliminary results in patients with frontotemporal dementia. J. Neuroling. 16, 169–181. doi: 10.1016/S0911-6044(02)00011-8
Bastiaanse, R., Edwards, S., Maas, E., and Rispens, J. (2010). Assessing comprehension and production of verbs and sentences: the verb and sentence test (VAST). Aphasiology 17, 49–73. doi: 10.1080/729254890
Bedny, M., and Caramazza, A. (2011). Perception, action, and word meanings in the human brain: the case from action verbs. Ann. N. Y. Acad. Sci. 1224, 81–95. doi: 10.1111/j.1749-6632.2011.06013.x
Bedny, M., Caramazza, A., Grossman, E., Pascual-Leone, A., and Saxe, R. (2008). Concepts are more than percepts: the case of action verbs. J. Neurosci. 28, 11347–11353. doi: 10.1523/JNEUROSCI.3039-08.2008
Beeson, P. M., and Robey, R. (2006). Evaluating single-subject treatment research: lessons learned from the aphasia literature. Neuropsychol. Rev. 16, 161–169. doi: 10.1007/s11065-006-9013-7
Bergen, B., Chan Lau, T., Narayan, S., Stojanovic, D., and Wheeler, K. (2010). Body part representations in verbal semantics. Memory Cognit. 38, 969–981. doi: 10.3758/MC.38.7.969
Brady, M. C., Ali, M., VandenBerg, K., Williams, L. J., Williams, L. R., and Abo, M. (2022). Dosage, intensity, and frequency of language therapy for aphasia: a systematic review-based, individual participant data network meta-analysis. Stroke 53, 956–967. doi: 10.1161/STROKEAHA.121.035216
Brady, M. C., Godwin, J., Enderby, P., Kelly, H., and Campbell, P. (2016). Speech and language therapy for aphasia after stroke. An updated systematic review and meta-analyses. Stroke 47, e236–e237. doi: 10.1161/STROKEAHA.116.014439
Breedin, S. D., Saffran, E. M., and Schwartz, M. F. (1998). Semantic factors in verb retrieval: an effect of complexity. Brain Lang. 63, 1–31. doi: 10.1006/brln.1997.1923
Brownsett, S. L., Warren, J. E., Geranmayeh, F., Woodhead, Z., Leech, R., Wise, R. J., et al. (2014). Cognitive control and its impact on recovery from aphasic stroke. Brain 137, 242–254. doi: 10.1093/brain/awt289
Cahana-Amitay, D., Albert, M. L., Pyun, S. B., Westwood, A., Jenkins, T., Wolford, S., et al. (2011). Language as a stressor in aphasia. Aphasiology 25, 593–614. doi: 10.1080/02687038.2010.541469
Caramazza, A., Anzellotti, S., Strnad, L., and Lingnau, A. (2014). Embodied cognition and mirror neurons: a critical assessment. Ann. Rev. Neurosci. 37, 1–15. doi: 10.1146/annurev-neuro-071013-013950
Caspers, S., Zilles, K., Laird, A. R., and Eickhoff, S. B. (2010). ALE meta-analysis of action observation and imitation in the human brain. NeuroImage 50, 1148–1167. doi: 10.1016/j.neuroimage.2009.12.112
Cavanaugh, R., Kravetz, C., Jarold, L., Quique, Y., Turner, R., Evans, W. S., et al. (2021). Is there a research-practice dosage gap in aphasia rehabilitation? Am. J. Speech Lang. Pathol. 30, 2115–2129. doi: 10.1044/2021_AJSLP-20-00257
Cheng, B. B. Y., Worrall, L. E., Copland, D. A., and Wallace, S. J. (2020). Prognostication in post-stroke aphasia: how do speech pathologists formulate and deliver information about recovery? Int. J. Lang. Commun. Disord. 55, 520–536. doi: 10.1111/1460-6984.12534
Crepaldi, D., Berlingeri, M., Cattinelli, I., Borghese, N. A., Luzzatti, C., Paulesu, E., et al. (2013). Clustering the lexicon in the brain: a meta-analysis of the neurofunctional evidence on noun and verb processing. Front. Hum. Neurosci. 7:303. doi: 10.3389/fnhum.2013.00303
De Aguiar, V., Bastiaanse, R., and Miceli, G. (2016). Improving production of treated and untreated verbs in aphasia: a meta-analysis [Original Research]. Front. Hum. Neurosci. 10:468. doi: 10.3389/fnhum.2016.00468
De Ouden, D.-B., Fix, S., Parrish, T. B., and Thompson, C. K. (2009). Argument structure effects in action verb naming in static and dynamic conditions. J. Neuroling. 22, 196–215. doi: 10.1016/j.jneuroling.2008.10.004
De Zubicaray, G., Arciuli, J., and McMahon, K. (2013). Putting an “end” to the motor cortex representations of action words. J. Cogn. Neurosci. 25, 1957–1974. doi: 10.1162/jocn_a_00437
Durand, E., Berroir, P., and Ansaldo, A. I. (2018). The neural and behavioral correlates of anomia recovery following personalized observation, execution, and mental imagery therapy: a proof of concept. Neural Plast. 2018:5943759. doi: 10.1155/2018/5943759
Elsner, B., Kugler, J., Pohl, M., and Mehrholz, J. (2019). Transcranial direct current stimulation (tDCS) for improving aphasia in adults with aphasia after stroke. Cochrane Database Syst. Rev. 5:Cd009760. doi: 10.1002/14651858.CD009760.pub4
Faroqi-Shah, Y. (2018). Training outcomes for manipulable verbs in persons with aphasia: implications for verb representation [Academy of Aphasia Conference Abstract]. Front. Hum. Neurosci. doi: 10.3389/conf.fnhum.2018.228.00095
Faroqi-Shah, Y., and Graham, L. E. (2011). Treatment of semantic verb classes in aphasia: acquisition and generalization effects. Clin. Ling. Phonetics 25, 399–418. doi: 10.3109/02699206.2010.545964
Faroqi-Shah, Y., Sebastian, R., and van der Woude, A. (2018). Neural representation of word categories is distinct in the temporal lobe: an activation likelihood analysis. Hum. Brain Mapp. 39, 4925–4938. doi: 10.1002/hbm.24334
Faroqi-Shah, Y., Shi, C., and Goodridge, R. (2023). Short term memory in aphasia: effects of modality and relationship with Western Aphasia Battery-R performance. Aphasiology 37, 1885–1915. doi: 10.1080/02687038.2022.2136482
Faroqi-Shah, Y., Slevc, L. R., Saxena, S., Fisher, S. J., and Pifer, M. (2020). Relationship between musical and language abilities in post-stroke aphasia. Aphasiology 34, 793–819. doi: 10.1080/02687038.2019.1650159
Faroqi-Shah, Y., Wood, E., and Gassert, J. (2010). Verb impairment in aphasia: a priming study of body part overlap. Aphasiology 24, 1377–1388. doi: 10.1080/02687030903515362
Flowers, H. L., Skoretz, S. A., Silver, F. L., Rochon, E., Fang, J., Flamand-Roze, C., et al. (2016). Poststroke aphasia frequency, recovery, and outcomes: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 97, 2188–2201. e2188. doi: 10.1016/j.apmr.2016.03.006
Fonov, V. S., Evans, A. C., McKinstry, R. C., Almli, C. R., and Collins, D. L. (2009). Unbiased nonlinear average age-appropriate brain templates from birth to adulthood, NeuroImage 47:S102. doi: 10.1016/S1053-8119(09)70884-5
Fridriksson, J., Basilakos, A., Boyle, M., Cherney, L. R., DeDe, G., Gordon, J. K., et al. (2022). Demystifying the complexity of aphasia treatment: application of the rehabilitation treatment specification system. Arch. Phys. Med. Rehabil. 103, 574–580. doi: 10.1016/j.apmr.2021.08.025
Fridriksson, J., and Hillis, A. E. (2021). Current approaches to the treatment of post-stroke aphasia. J. Stroke 23, 183–201. doi: 10.5853/jos.2020.05015
Giacobbe, C., Raimo, S., Cropano, M., and Santangelo, G. (2022). Neural correlates of embodied action language processing: a systematic review and meta-analytic study. Brain Imaging Behav. 16, 2353–2374. doi: 10.1007/s11682-022-00680-3
Goldenberg, G., and Spatt, J. (1994). Influence of size and site of cerebral lesions on spontaneous recovery of aphasia and on success of language therapy. Brain Lang. 47, 684–698. doi: 10.1006/brln.1994.1063
Goodglass, H., Kaplan, E., and Barresi, B. (2001). Boston Diagnostic Aphasia Examination, 3rd Edn. Philadelphia: Lippincott Williams and Wilkins.
Hallam, G. P., Thompson, H. E., Hymers, M., Millman, R. E., Rodd, J. M., Lambon Ralph, M. A., et al. (2018). Task-based and resting-state FMRI reveal compensatory network changes following damage to left inferior frontal gyrus. Cortex 99, 150–165. doi: 10.1016/j.cortex.2017.10.004
Hart, T., Dijkers, M. P., Whyte, J., Turkstra, L. S., Zanca, J. M., Packel, A., et al. (2019). A theory-driven system for the specification of rehabilitation treatments. Arch. Phys. Med. Rehabil. 100, 172–180. doi: 10.1016/j.apmr.2018.09.109
Hauk, O., Johnsrude, I., and Pulvermuller, F. (2004). Somatotopic representation of action words in human motor and premotor cortex. Neuron 41, 301–307. doi: 10.1016/S0896-6273(03)00838-9
Hernández, M., Fairhall, S. L., Lenci, A., Baroni, M., and Caramazza, A. (2014). Predication drives verb cortical signatures. J. Cogn. Neurosci. 26, 1829–1839. doi: 10.1162/jocn_a_00598
Hickin, J., Cruice, M., and Dipper, L. (2020). A systematically conducted scoping review of the evidence and fidelity of treatments for verb deficits in aphasia: verb-in-isolation treatments. Am. J. Speech Lang. Pathol. 29, 530–559. doi: 10.1044/2019_AJSLP-CAC48-18-0234
Hsu, C-. J., and Thompson, C. K. (2018). Manual versus automated narrative analysis of agrammatic production patterns: the northwestern narrative language analysis and computerized language analysis. J. Speech Lang. Hear. Res. 61, 373–385. doi: 10.1044/2017_JSLHR-L-17-0185
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., and Smith, S. M. (2012). FSL. NeuroImage, 62, 782–790. doi: 10.1016/j.neuroimage.2011.09.015
Johnson, J. P., Meier, E. L., Pan, Y., and Kiran, S. (2019). Treatment-related changes in neural activation vary according to treatment response and extent of spared tissue in patients with chronic aphasia. Cortex 121, 147–168. doi: 10.1016/j.cortex.2019.08.016
Jonkers, R., and Bastiaanse, R. (1996). The influence of instrumentality and transitivity on action naming in Broca's and anomic aphasia. Brain Lang. 55, 37–39.
Kable, J. W., Lease-Spellmeyer, J., and Chatterjee, A. (2002). Neural substrates of action event knowledge. J. Cogn. Neurosci. 14, 795–805. doi: 10.1162/08989290260138681
Kemmerer, D. (2003). Why can you hit someone on the arm but not break someone on the arm?–a neuropsychological investigation of the English body-part possessor ascension construction. J. Neurolinguist. 16, 13–36. doi: 10.1016/S0911-6044(01)00042-2
Kemmerer, D., Castillo, J. G., Talavage, T., Patterson, S., and Wiley, C. (2008). Neuroanatomical distribution of five semantic components of verbs: evidence from fMRI. Brain Lang. 107, 16–43. doi: 10.1016/j.bandl.2007.09.003
Kemmerer, D., Rudrauf, D., Manzel, K., and Tranel, D. (2012). Behavioral patterns and lesion sites associated with impaired processing of lexical and conceptual knowledge of actions. Cortex 48, 826–848. doi: 10.1016/j.cortex.2010.11.001
Kendall, D. L., Oelke, M., Brookshire, C. E., and Nadeau, S. E. (2015). The influence of phonomotor treatment on word retrieval abilities in 26 individuals with chronic aphasia: an open trial. J. Speech Lang. Hear. Res. 58, 798–812. doi: 10.1044/2015_JSLHR-L-14-0131
Kertesz, A. (2006). Western Aphasia Battery-Revised. San Antonio, TX: Pearson. doi: 10.1037/t15168-000
Kiran, S. (2008). Typicality of inanimate category exemplars in aphasia treatment: further evidence for semantic complexity. J. Speech Lang. Hear. Res. 51, 1550–1568. doi: 10.1044/1092-4388(2008/07-0038)
Kristinsson, S., den Ouden, D. B., Rorden, C., Newman-Norlund, R., Neils-Strunjas, J., Fridriksson, J., et al. (2022). Predictors of therapy response in chronic aphasia: building a foundation for personalized aphasia therapy. J. Stroke 24, 189–206. doi: 10.5853/jos.2022.01102
Kristinsson, S., Thors, H., Yourganov, G., Magnusdottir, S., Hjaltason, H., Stark, B. C. J., et al. (2020). Brain damage associated with impaired sentence processing in acute aphasia. J. Cognit. Neurosci. 32, 256–271. doi: 10.1162/jocn_a_01478
Lee, J. B., and Cherney, L. R. (2018). Tau-U: a quantitative approach for analysis of single-case experimental data in aphasia. Am. J. Speech Lang. Pathol. 27, 495–503. doi: 10.1044/2017_AJSLP-16-0197
Levin, B. (1993). English Verb Classes and Alternations: A Preliminary Investigation. University of Chicago Press.
Li, R., and Kiran, S. (2023). Treatment-induced recovery patterns between nouns and verbs in mandarin-english bilingual adults with aphasia. Am. J. Speech Lang. Pathol. 32, 1–18. doi: 10.1044/2023_AJSLP-22-00236
Lutkenhoff, E. S., Rosenberg, M., Chiang, J., Zhang, K., Pickard, J. D., Owen, A. M., et al. (2014). Optimized brain extraction for pathological brains (optiBET). PLoS One 9:e115551. doi: 10.1371/journal.pone.0115551
Marangolo, P., Cipollari, S., Fiori, V., Razzano, C., and Caltagirone, C. (2012). Walking but not barking improves verb recovery: implications for action observation treatment in aphasia rehabilitation. PLoS ONE 7:e38610. doi: 10.1371/journal.pone.0038610
Marcotte, K., Perlbarg, V., Marrelec, G., Benali, H., and Ansaldo, A. I. (2013). Default-mode network functional connectivity in aphasia: therapy-induced neuroplasticity. Brain Lang. 124, 45–55. doi: 10.1016/j.bandl.2012.11.004
Menahemi-Falkov, M., Breitenstein, C., Pierce, J. E., Hill, A. J., O'Halloran, R., Rose, M. L., et al. (2022). A systematic review of maintenance following intensive therapy programs in chronic post-stroke aphasia: importance of individual response analysis. Disabil. Rehabil. 44, 5811–5826. doi: 10.1080/09638288.2021.1955303
Moss, A., and Nicholas, M. (2006). Language rehabilitation in chronic aphasia and time post-onset: a review of single-subject data. Stroke 37, 3043–3051. doi: 10.1161/01.STR.0000249427.74970.15
Nakagawa, Y., Sano, Y., Funayama, M., and Kato, M. (2019). Prognostic factors for long-term improvement from stroke-related aphasia with adequate linguistic rehabilitation. Neurol. Sci. 40, 2141–2146. doi: 10.1007/s10072-019-03956-7
Papeo, L., and Lingnau, A. (2015). First-person and third-person verbs in visual motion-perception regions. Brain Lang. 141, 135–141. doi: 10.1016/j.bandl.2014.11.011
Pastötter, B., and Bäuml, K-. H. T. (2014). Retrieval practice enhances new learning: the forward effect of testing. Front. Psychol. 5:286. doi: 10.3389/fpsyg.2014.00286
Peelen, M., and Romagno, D. A. C. (2012). Independent representations of verbs and actions in left lateral temporal cortex. J. Cognit. Neurosci. 24, 2096–2107. doi: 10.1162/jocn_a_00257
Redcay, E., and Carlson, T. A. (2015). Rapid neural discrimination of communicative gestures. Soc. Cognit. Affect. Neurosci. 10, 545–551. doi: 10.1093/scan/nsu089
Riccardi, N., Yourganov, G., Rorden, C., Fridriksson, J., and Desai, R. H. (2019). Dissociating action and abstract verb comprehension post-stroke. Cortex 120, 131–146. doi: 10.1016/j.cortex.2019.05.013
Rorden, C., Karnath, H. O., and Bonilha, L. (2007). Improving lesion-symptom mapping. J. Cogn. Neurosci. 19, 1081–1088. doi: 10.1162/jocn.2007.19.7.1081
Sahin, N. T., Pinker, S., and Halgren, E. (2006). Abstract grammatical processing of nouns and verbs in Broca's area: evidence from fMRI. Cortex 42, 540–562. doi: 10.1016/S0010-9452(08)70394-0
Sandberg, C., and Kiran, S. (2014). How justice can affect jury: training abstract words promotes generalisation to concrete words in patients with aphasia. Neuropsychol. Rehabil. 24, 738–769. doi: 10.1080/09602011.2014.899504
Schevenels, K., Price, C. J., Zink, I., de Smedt, B., and Vandermosten, M. (2020). A review on treatment-related brain changes in aphasia. Neurobiol. Lang. 1, 402–433. doi: 10.1162/nol_a_00019
Schuchard, J., and Middleton, E. L. (2018). The roles of retrieval practice versus errorless learning in strengthening lexical access in aphasia. J. Speech Lang. Hear. Res. 61, 1700–1717. doi: 10.1044/2018_JSLHR-L-17-0352
Shapiro, K., Moo, L. R., and Caramazza, A. (2006). Cortical signatures of noun and verb production. Proc. Natl. Acad. Sci. U.S.A. 103, 1644–1649. doi: 10.1073/pnas.0504142103
Shapiro, K. A., Mottaghy, F. M., Schiller, N. O., Poeppel, T. D., Flu, M. O., Muller, H. W., et al. (2005). Dissociating neural correlates for nouns and verbs. NeuroImage 24:1058. doi: 10.1016/j.neuroimage.2004.10.015
Sharer, K., and Thothathiri, M. (2020). Neural mechanisms underlying the dynamic updating of native language. Neurobiol. Lang. 1, 492–522. doi: 10.1162/nol_a_00023
Simic, T., Desjardins, M., Courson, M., Bedetti, C., Houzé, B., Brambati, S. M., et al. (2023). Treatment-induced neuroplasticity after anomia therapy in post-stroke aphasia: a systematic review of neuroimaging studies. Brain Lang. 244:105300. doi: 10.1016/j.bandl.2023.105300
Skipper-Kallal, L. M., Lacey, E. H., Xing, S., and Turkeltaub, P. E. (2017). Right hemisphere remapping of naming functions depends on lesion size and location in poststroke aphasia. Neural Plast. 2017, 1–17. doi: 10.1155/2017/8740353
Small, S. L. (1994). Pharmacotherapy of aphasia. A critical review. Stroke 25, 1282–1289. doi: 10.1161/01.STR.25.6.1282
Solana, P., Escámez, O., Casasanto, D., Chica, A. B., and Santiago, J. (2024). No support for a causal role of primary motor cortex in construing meaning from language: an rTMS study. Neuropsychologia 196:108832. doi: 10.1016/j.neuropsychologia.2024.108832
Solana, P., and Santiago, J. (2022). Does the involvement of motor cortex in embodied language comprehension stand on solid ground? A p-curve analysis and test for excess significance of the TMS and tDCS evidence. Neurosci. Biobehav. Rev. 141:104834. doi: 10.1016/j.neubiorev.2022.104834
Stockert, A., Wawrzyniak, M., Klingbeil, J., Wrede, K., Kümmerer, D., Hartwigsen, G., et al. (2020). Dynamics of language reorganization after left temporo-parietal and frontal stroke. Brain 143, 844–861. doi: 10.1093/brain/awaa023
Tarlow, K. R. (2016). Baseline corrected tau calculator. Available online at: http://www.ktarlow.com/stats/tau (Accessed December 10, 2024).
Tate, R. L., Perdices, M., Rosenkoetter, U., Shadish, W., Vohra, S., Barlow, D. H., et al. (2016). The single-case reporting guideline in behavioural interventions (SCRIBE) 2016 statement. Phys. Ther. 96, e1–e10. doi: 10.2522/ptj.2016.96.7.e1
Thompson, C. K., Riley, E. A., den Ouden, D. B., Meltzer-Asscher, A., and Lukic, A. S. (2013). Training verb argument structure production in agrammatic aphasia: behavioral and neural recovery patterns. Cortex 49, 2358–2376. doi: 10.1016/j.cortex.2013.02.003
Thompson, C. K., and Shapiro, L. P. (1995). Training sentence production in agrammatism: implications for normal and disordered language. Brain Lang. 50, 201–224. doi: 10.1006/brln.1995.1045
van Dam, W. O., Rueschemeyer, S-. A., and Bekkering, H. (2010). How specifically are action verbs represented in the neural motor system: an fMRI study. NeuroImage 53, 1318–1325. doi: 10.1016/j.neuroimage.2010.06.071
van Hees, S., McMahon, K., Angwin, A., de Zubicaray, G., Read, S., Copland, D. A., et al. (2014). A functional MRI study of the relationship between naming treatment outcomes and resting state functional connectivity in post-stroke aphasia. Hum. Brain Mapp. 35, 3919–3931. doi: 10.1002/hbm.22448
Van Valin, R. D. (2006). “Some universals of verb semantics,” in Linguistic Universals, eds. R. Mairal and J. Gil (Cambridge: Cambridge University Press), 155–178. doi: 10.1017/CBO9780511618215.008
Wambaugh, J. L., and Ferguson, M. (2007). Application of semantic feature analysis to retrieval of action names in aphasia. J. Rehabil. Res. Dev. 44, 381–394. doi: 10.1682/JRRD.2006.05.0038
Warraich, Z., and Kleim, J. A. (2010). Neural plasticity: the biological substrate for neurorehabilitation. PMR, 2, S208–219. doi: 10.1016/j.pmrj.2010.10.016
Watson, C. E., Cardillo, E. R., Ianni, G. R., and Chatterjee, A. (2013). Action concepts in the brain: an activation likelihood estimation meta-analysis. J. Cogn. Neurosci. 25, 1191–1205. doi: 10.1162/jocn_a_00401
Wilson, S. M., and Schneck, S. M. (2021). Neuroplasticity in post-stroke aphasia: a systematic review and meta-analysis of functional imaging studies of reorganization of language processing. Neurobiol. Lang. 2, 22–82. doi: 10.1162/nol_a_00025
Keywords: aphasia, treatment, intervention, verbs, fMRI, SCED, agrammatism
Citation: Faroqi-Shah Y and Marshall K (2025) Behavioral and neural outcomes of training manipulable verbs in aphasia: a proof-of-concept single subject experimental design. Front. Lang. Sci. 4:1560115. doi: 10.3389/flang.2025.1560115
Received: 13 January 2025; Accepted: 22 September 2025;
Published: 23 October 2025.
Edited by:
Deryk Scott Beal, Holland Bloorview Kids Rehabilitation Hospital, CanadaReviewed by:
Junhua Ding, University of Edinburgh, United KingdomDanielle Kristine Fahey, University of Alabama System, United States
Copyright © 2025 Faroqi-Shah and Marshall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasmeen Faroqi-Shah, eWZzaGFoQHVtZC5lZHU=
 Yasmeen Faroqi-Shah
Yasmeen Faroqi-Shah Kelly Marshall
Kelly Marshall