- 1Department of Linguistics, University of Salzburg, Salzburg, Austria
- 2Department of Kinesiology, University of Salzburg, Salzburg, Austria
- 3Department of Speech, Language and Hearing Sciences, University of Alabama, Tuscaloosa, AL, United States
- 4Department of Linguistics, Purdue University, West Lafayette, IN, United States
- 5Centre for Cognitive Neuroscience (CCNS), University of Salzburg, Salzburg, Austria
Introduction: The manuscript presents an experimental investigation into the linguistic and motor control mechanisms underlying grammatical marker production in Austrian Sign Language (ÖGS). It focuses on the cross-linguistically attested phonological parameter of hand articulator tension and its role as a grammatical marker for adjective intensification.
Method: By combining advanced methods, including motion capture and electromyography (EMG), the study allows for a multimodal analysis of grammatical marker production in ÖGS. The experimental data were recorded from six proficient ÖGS signers, each producing fifteen adjectives (based on a set varied in phonological parameters of root forms) in intensified and non-intensified forms. Motion capture data were analyzed in terms of the kinematics of hand and arm movements [velocity, acceleration, as well their temporal distribution such as time to peak deceleration and spatiotemporal index (STI)]; EMG data of muscle activation in forearm and upper-arm flexor and extensor muscles were analyzed both separately and in active combination (using mean and median band-specific EMG and co-contraction indices as measures).
Results: Results revealed significant differences between intensified and non-intensified forms, with intensified adjectives showing higher co-contraction indices in forearm and upper-arm muscles and later deceleration patterns within signs. These findings demonstrate that articulator tension is a quantifiable grammatical marker of intensification, reflected in distinct biomechanical control patterns.
Discussion: This study advances understanding of the neural and motor correlates of sign language production by operationalizing the biophysical basis of grammatical markers. It highlights the linguistic control of biomechanical articulator features, advancing models of language production. Individual variations observed in intensified adjective production suggest further avenues for research into signing styles, language proficiency, and language acquisition. In addition to its linguistic contributions, the manuscript proposes a novel methodological approach for studying variables at the intersection of linguistics, neuroscience, and kinesiology. This work offers practical applications for sign language teaching, language acquisition research, and cross-modal investigations into human language systems, contributing to a broader understanding of linguistic communication as a multimodal phenomenon.
1 Introduction
The question regarding the relationship between grammatical features and their expression in articulatory motion in sign languages has long been of interest to linguists and cognitive scientists (Klima and Bellugi, 1979; Boutet and Garcia, 2009; Tyrone et al., 2010), as it lies at the intersection of language, cognition, and biomechanics. Understanding the cognitive mechanisms underlying sign language production requires accounting for the modality-specific interaction between motor control and linguistic processing (Malaia and Wilbur, 2019). In this work, we use a multimodal analysis (motion capture combined with electromyography/EMG) to examine a well-established grammatical marker in sign languages—adjective intensification—in Austrian Sign Language (ÖGS). Our focus is on the linguistic parameter of “tension,” as expressed in hand motion and neural control (as inferred from EMG).
Although the linguistic relevance of the movement of manual and nonmanual sign articulators, along with the dynamic properties of these modulations, has been noted since the early days of linguistic research on sign languages (e.g., Klima and Bellugi, 1979; Wilbur, 1979, 1987, 1994, 1999; Wilbur and Schick, 1987), less is known about (a) which kinematic variables (e.g., velocity, acceleration, or duration) are used to indicate specific grammatical markings and (b) which arm muscles are involved in executing the movement modulations that express linguistically relevant distinctions. In this experiment, we combined motion capture and EMG analysis to operationalize the linguistic marker of tension and investigate the linguistic control of motion in ÖGS.
To put the question in perspective, it is useful to revisit the origin of the term “tense” in the sign language literature. In both spoken and signed languages, tension has been recognized as a perceptual correlate of linguistic function, particularly in marking grammatical, prosodic, or affective contrasts. The term initially referred to a perceptual-level phenomenon, as there were no techniques available for quantifying production. In spoken language, this may manifest as changes in vocal fold tension (e.g., “creaky” phonation). Although frequently referenced descriptively in linguistics and speech pathology literature, tension has remained a perceptual category: reliably noted by native users and linguists, but not formally quantified or grounded in physiological mechanisms. In spoken languages, it has been observed that in addition to increasing tension in the vocal channel, methods of increasing tension may include the addition of beat gestures that may replace an intensifier in a sentence (cf. Khatin-Zadeh et al., 2023). In sign language research, Klima and Bellugi (1979) used the term to describe various sign “modulations,” including: (1) adding stress 1; (2) rapid, tense movement for intensification; (3) a sharp (rapid, tense) movement incompatible with repetition; (4) a single, tense rapid movement for “dark blue” derived from “blue;” (5) successive repetitions with increasing tension and sharper movements for “redder and redder;” (6) a thrustlike motion combining brief tension (in forearm muscles) with a lax, soft handshape termed the “thrust” modulation for the “susceptative aspect;” and (7) a tiny, tense, uneven movement made as rapidly as possible and iterated, termed the “tremolo” modulation for the “incessant aspect.” Sorting through these descriptions is essential for teaching sign learners the correct muscle actions needed to convey accurate messages. From a linguistic perspective, we can identify the following standard functions:
1. Prosodic stress marking (see Wilbur, 2022)
2. Adjectival intensification (cold - very cold)
3. Adjectival derivation (blue - dark blue)
4. Augmentative reduplication (redder and redder)
5. Predicate adjective aspectual modification (“thrust” for “susceptative”)
6. Verb aspectual modification (“tremolo” for “incessant”)
Unlike non-communicative motion (e.g., a reaching movement), which typically follows a bell-shaped velocity profile with gradual acceleration to a mid-movement peak and symmetric deceleration to rest, sign language production is modulated by linguistic constraints (Blumenthal-Dramé and Malaia, 2019). Sign language motion is driven primarily by the need to encode grammatical and semantic content across multiple scales of motion, as opposed to end-point attainment, as reaching motion may be. Prior research has shown that sign language motion has higher information density than everyday movement, as evidenced by greater fractal complexity (Malaia et al., 2018; Borneman et al., 2018). Notably, the visual form of verbs in, for example, ASL systematically reflects argument structure distinctions (such as transitivity) even for native viewers, indicating that sign language encodes grammatical structure in motion in a perceivable and learnable way (Bradley et al., 2022; Bradley and Wilbur, 2023). Experimental data from EMG and motion capture further confirm that sign language motion exhibits distinct timing and muscular activation profiles depending on linguistic features such as differences in verb type (Krebs et al., 2023).
In this study, we focus on adjectival intensification as the test case for the operationalization of tension as a grammatical marker. In general, the intensification of adjectives in sign languages is expressed through the modification of manual and non-manual components.2 For American Sign Language (ASL), Wilbur et al. (2012) report that intensified adjectives are produced by an increased tension of the manual and facial articulators. The manual movement is modified by adding a path movement (if none) and/or enlarging the movement trajectory. They also observe a delayed release of the onset of sign movement. Many of the intensified adjectives produced with such a delayed release show a hold of the hands in space prior to movement onset. If the sign involves a change in mouth position, this occurs with the onset of hand movement. The non-manual modifications marking adjectival intensification involve a frown on the face and a head tilt (Wilbur et al., 2012). Schlenker and Lamberton (2021) also report that a brow raise can be used to intensify adjectives in ASL.
In ÖGS, adjectival intensification is marked by modulating the manual movement such that intensified adjectives are longer in duration than non-intensified signs. Adjectives may also be modulated in space (e.g., larger or smaller sign movement) to indicate intensification. Additionally, specific non-manual markings co-occurring with adjective signs may indicate intensification. The non-manuals observed in the context of adjectival intensification in ÖGS include raised or furrowed eyebrows, wide-open or squinted eyes, various forms of mouth gestures, enhanced mouthing, and head nods (Krebs and Fenkart, 2024).
This study aims to fill that gap in quantification of tension as a marker by operationalizing it as co-contraction of agonist and antagonist muscles: a well-documented biomechanical strategy used to increase joint stiffness and movement precision without necessarily changing the outward form of the movement (Gribble et al., 2003; Enoka and Duchateau, 2015). Co-contraction allows signers to modulate the internal force of a sign while maintaining its shape and trajectory, effectively layering grammatical information onto a given lexical form. This is particularly salient in the case of adjective intensification, where the same sign can be modified to convey grammatical intensification through increased muscular engagement rather than additional motion.
Experimental methods, such as motion capture and electromyography (EMG), provide a valuable framework for analysis of sign language production, as they allow us to quantify both articulatory dynamics (which are also relevant to perception) and motor control that governs the production of grammatical markers. For example, in a previous report, we presented motion capture data from four Deaf ÖGS signers producing pairs of adjectives (intensified vs. non-intensified; e.g., cold vs. very cold), showing that intensified adjectives are longer in duration compared to non-intensified forms (Krebs et al., 2024a). An analysis of the phonological structure of the tested adjective signs suggested that the longer duration in intensified adjectives appears to be related to the size of the sign and the velocity of hand motion, but not to sign repetition. The data also revealed individual differences among the signers, which might be interpreted as personal signing styles [cf. Bigand et al., 2020; see also Xavier, 2013, which describes intra- and inter-subject variation in expressing meaning intensification through the doubling of the number of hands in Brazilian Sign Language (Libras)]. The present study expands on interdisciplinary work at the intersection of linguistics and kinesiology by examining additional kinematic variables corresponding to the grammatical marking of adjective intensification and presenting corresponding EMG data. While previous EMG studies on sign language production have primarily used this method for developing automatic sign language recognition or translation systems (e.g., Zhuang et al., 2017; Savur and Sahin, 2016; Galea and Smeaton, 2019; Tateno et al., 2020; Gu et al., 2022; for a review, see Ben Haj Amor et al., 2023), our research employs EMG combined with motion capture to investigate the grammatical structure of ÖGS (as previously shown by Krebs et al., 2023). To the extent that a sign viewer is expected to perceive these distinctions visually for meaningful purposes, we hypothesize that different muscle activations, as reflected in the EMG recordings, will help us understand how these distinct messages are produced.
We use surface EMG to operationalize the linguistic control of hand motor units in sign language in the context of muscle activation. Our previous research on ÖGS has shown that telic verb signs (verbs with a defined endpoint) exhibit higher muscular activity in upper arm muscles compared to atelic verbs (verbs without a defined endpoint), demonstrating that different types of movements in sign language require distinct patterns of muscle activation (Krebs et al., 2023). The analysis of motor activation in sign language differs somewhat from the analysis of motor control in sports, as muscles contain different types of motor units (slow, fast fatigue-resistant, and fast fatigable), which are recruited depending on the task. In sign language, proficient movement is fast (Borneman et al., 2018; Bosworth et al., 2019), finely spatially coordinated (Wilbur, 1987), but does not involve strong muscle contractions. Since the level of muscle activation (measured by EMG) is related to the force required to produce movement (Enoka and Duchateau, 2015), our analysis focuses on EMG signals in the lower frequency range, which have been shown to be more sensitive to contractions below 30% of maximal contraction (Roman-Liu and Konarska, 2009).
Importantly, while such tension is visually perceptible to fluent signers, our study is focused on sign language production: on how signers encode grammatical distinctions through neuromuscular control. In the overall communication chain, it is the signer's responsibility to provide linguistic cues and the perceiver's task to interpret them. Although we do not assess perception directly here, the production-side evidence presented in this study offers strong grounding for future research on how observers recognize and interpret such grammatical cues.
2 Material and methods
2.1 Participants
Six Deaf signers (4 F) were included in the analysis (Age M = 55, SD = 9, range 40–64). All participants were born deaf or lost their hearing early in life (4 participants were born deaf, one lost her hearing between 0–3 years and another signer lost her hearing around 4 years of age). All of the participants who took part in the study were fluent ÖGS signers, used ÖGS as their first language in daily life, are members of the Deaf community, are trained ÖGS instructors, and have been associated with our research for many years. Five participants self-reported as right-handed; one self-reported as left-handed.
2.2 Materials and design
Each participant produced a list of 102 signs. The critical stimuli comprised 15 adjectives in non-intensified form (e.g. sweet), and the same 15 adjectives in intensified form (e.g., very sweet). The tested adjectives are: HOT, COLD, BIG, SMALL, OLD, YOUNG, RICH, POOR, FAR, NEAR, SHORT, LONG, SWEET, SOUR, and THIN. The additional signs functioned as filler material including verbs (36 telic such as ARRIVE and 36 atelic verb such as WRITE). The stimuli were presented in a power point presentation, whereby a written gloss of each sign was presented on a separate slide. The stimuli were elicited in pseudo-randomized order, such that no sign type appeared more than two times in a row. To eliminate potential order effects, every other participant was presented with the list in the reversed order. One participant returned to repeat all 102 signs one week later (also in reversed order) to estimate reproducibility of any observed differences.
2.3 Data collection and analysis
2.3.1 3D-motion capture
Body kinematics of the trunk, head, and upper extremities including hands were recorded using a custom-built marker set (see Figure 1), and a 12-camera infrared motion capture system (Qualisys AB, Göteborg, Sweden) with a sampling frequency of 300 Hz. A 2D-Video (150 Hz, Qualisys AB, Göteborg, Sweden) of the participant's frontal plane was recorded simultaneously, and time-locked to motion capture data. Marker trajectories were low-pass filtered using a second-order, zero-lag Butterworth filter with a cutoff frequency of 25 Hz. Segment positions and orientations were determined using an inverse kinematics algorithm (V3D; C-Motion, Rockville, MD, USA). Joint centers of the wrist, elbow, and shoulder were defined as virtual landmarks at 50 percent of the line between the lateral and medial joint markers. The velocity of the wrist joint center (vertical component) of the dominant hand was used to define the onset (v>0.1 m/s) and offset (v <0.1 m/s) of hand movement. The dominant hand in sign language production is the one that is used for signing one-handed signs. In two-handed asymmetric signs the dominant hand executes the primary movement and the second (non-dominant) hand functions as a place of articulation. For statistical analysis, each sign was evaluated individually, and the dominant hand data for each signer and sign were used.
All signers started their hand/arm movement from the same resting position with the arms at the sides of the body. The start and end of the sign phase was visually set by a skilled signer using 2D video recording time-aligned to motion capture data. Sign onset was defined as the video frame when the target handshape reached the target location from where the sign movement started (Wilbur and Malaia, 2008). The sign offset was defined as the video frame when the handshape or the hand orientation of the sign changed or when the hand moved away from the final position. The complete sign was divided into 3 phases: the preparation phase (hand movement onset–start sign), the sign phase (start sign–end sign), and the down phase (end sign—hand movement offset). The present analysis focused exclusively on the sign phase.
The wrist position data were exported from V3D to MATLAB (2024b, The Mathworks Inc., Natick, MA). After trimming to sign phase, sign duration was extracted and then the position data were time-normalized to 100 points using spline interpolation. The first and second derivatives were calculated using MATLAB function “gradient” to obtain velocity and acceleration, respectively (see Figure 2). Velocity data were transformed using the Euclidean norm to obtain absolute speed, from which the median and peak speed (m/s) were labeled. Resultant acceleration was also calculated, from which peak deceleration (m/s2) and the time to peak deceleration (0 − 100% of sign) were extracted. Movement entropy was calculated from the speed vector using the function “SampEn” with a 0.2 tolerance (Lee, 2025). Finally, a spatiotemporal index (STI; velocity and acceleration) was calculated to estimate movement variability by calculating the standard deviation (SD) at every other time point (50 SDs) and then summing all SDs into a scalar value (Howell et al., 2009).
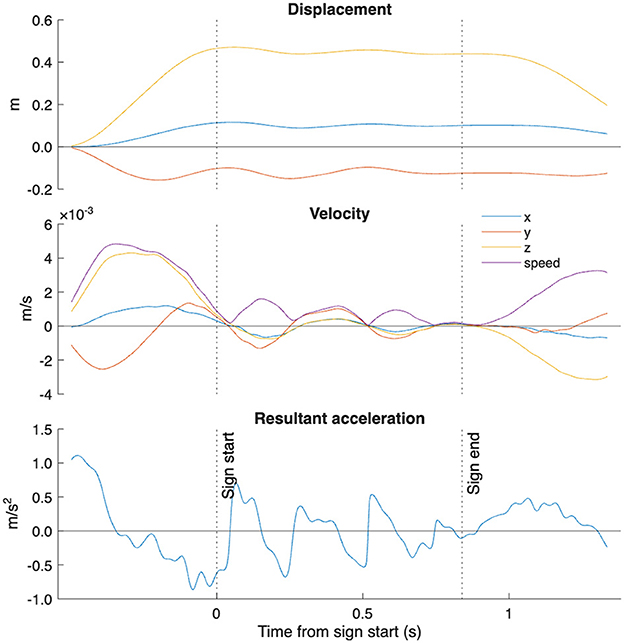
Figure 2. Example motion capture processing steps performed for one sign (“sehr weit”) and participant. Sign start and end manually labeled as detailed in methods. Abbreviations: m, meters; s, seconds.
2.3.2 Electromyography
In biomechanics and kinesiology, surface EMG is frequently used as a non-invasive technique to measure electric signal on the surface of the muscle. It is particularly valuable for identifying the start and end of muscle activation, as well as the relative magnitude of contractions, including intermuscular coordination patterns (Schwameder and Dengg, 2021).
The EMG analysis was performed using EMG sensors (Ultium(TM) EMG, Noraxon, Scottsdale, AZ, USA) connected to surface electrodes (Ambu blue, 30 × 22 mm, Ag/AgCl). Data were collected simultaneously with the kinematic analysis through the Qualisys Track Manager (Qualisys AB, Göteborg, Sweden). EMG data were collected at 2,000 Hz. EMG signals were recorded from four arm muscles: m. extensor digitorum, m. flexor digitorum, m. biceps brachii and m. triceps brachii of the dominant arm. EMG electrodes were placed on the participant's skin, which was prepared beforehand (by shaving and disinfecting the skin to remove skin scales, hair, and skin oil to get the best possible EMG signal) at specific anatomical places (e.g., thickest part of the muscle of interest) according to the recommendations of SENIAM (http://www.seniam.org/).
Participants performed maximum voluntary contraction (MVC) procedures according to best practices (Burden, 2010) by contracting against a fixed object in standardized positions (wrist 0, elbow 90 degrees flexion). They were given strong verbal encouragement to push maximally for three seconds. MVC estimation was performed as it allows for more accurate comparison of activation levels between adjacent muscles.
EMG data were post processed using MATLAB according to best practice recommendations (Muceli and Merletti, 2024). Raw EMG data were high-pass filtered at 10 Hz, low-pass filtered at 300Hz then notch filtered at 50 Hz to remove power line intereference. Next it was rectified and smoothed using root mean square with a moving window of 100 data points (0.05 s). MVC was extracted from the highest 1s average in accordance with current best practices (Burden, 2010). Sign phase EMG was trimmed and normalized to MVC.
A co-contraction index was calculated to approximate the degree of activation between agonist and antagonist muscles in the dominant hand (e.g., upper arm biceps and triceps) (Li et al., 2021). The formula of Rudolph et al. (2000) was adapted to consider that certain sign expressions had alternating agonist muscles within the sign. Thus, at each sample point the following formula was used:
Where emgl is the EMG signal with a lower amplitude and emgh higher. The mean value across the sign was retained as a discrete representation of co-contraction for each sign.
Next, the power spectral density estimate was obtained from the sign phase raw EMG signals using the function “periodogram”. Then, the mean and median frequency were extracted and the power in five distinct frequency bands was estimated (6–15, 16–25, 26–60, 61–75, and 76–140 Hz). This approach was selected as Roman-Liu and Konarska (2009) demonstrated its sensitivity and specificity to comparing different muscle contractions below 30% maximal contraction.
2.4 Statistical analysis
All participants in the study were proficient and fluent signers of Austrian Sign Language (ÖGS). As language competence is an individual, and not group-level trait, each signer's data represent a valid realization of linguistic production, rather than a noisy approximation of a shared norm. Therefore, the focus of the analysis is on the systematic patterns within and across individual signers. This approach aligns with established practices in linguistic and psycholinguistic research (Davis et al., 2014).
Given the known distributional properties of EMG and motion capture data (Borneman et al., 2018), we employed non-parametric statistical tests (Wilcoxon signed-rank tests) for within-subject comparisons. Non-parametric methods offer a more robust analysis framework under these conditions and are widely adopted in neuromuscular and kinematic research for this reason.
Data are presented as mean ± standard deviation unless otherwise stated. Kinematic and EMG variables for all adjective pairs (intensified and non-intensified) were compared using paired non-parametric Wilcoxon sign-rank tests. Cohen's d effect sizes with confidence intervals were calculated using MATLAB's “meanEffectSize” function with “robust” and “paired” settings, which utilizes 20% trimmed means, pooled 20% Winsorized variance, and bootstrapped confidence intervals. Alpha was set at α = 0.05 (Algina et al., 2005).
3 Results
A total of 90 adjective pairs were collected, from which three were excluded due to missing data. Table 1 contains descriptive data for all participants, as well as results of statistical comparisons. An example comparison for one adjective pair is displayed in Figure 3. Intensified adjectives had an average 0.21 s longer sign duration versus non-intensified pairs (p < 0.001, d = 0.38 [0.22,0.54]). The time to peak deceleration occurred about 2% later in the intensified forms (p = 0.01, d = 0.43 [0.18,0.66]).
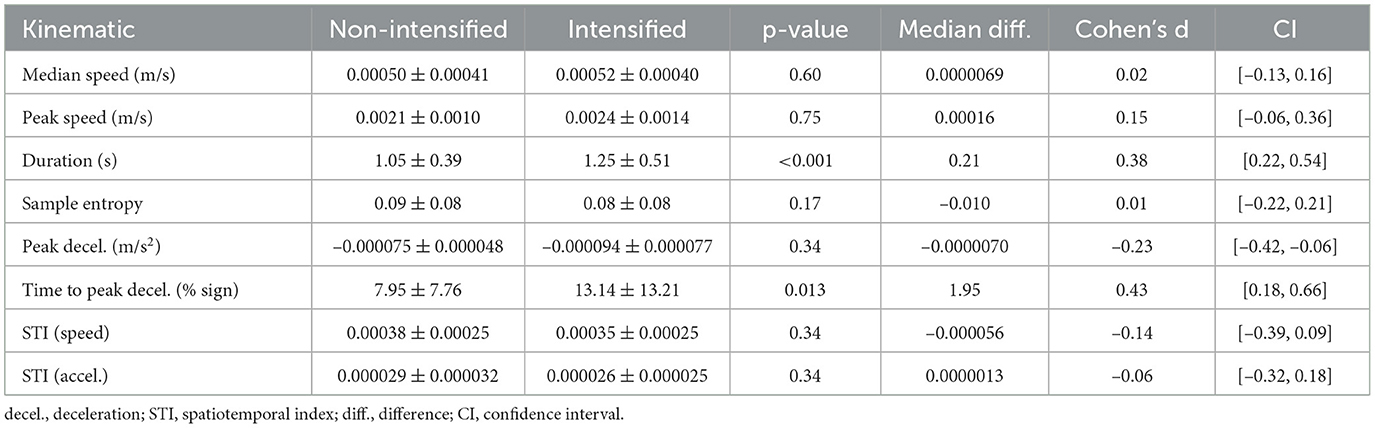
Table 1. Kinematic measures comparing non-intensified and intensified adjectives between all participants.
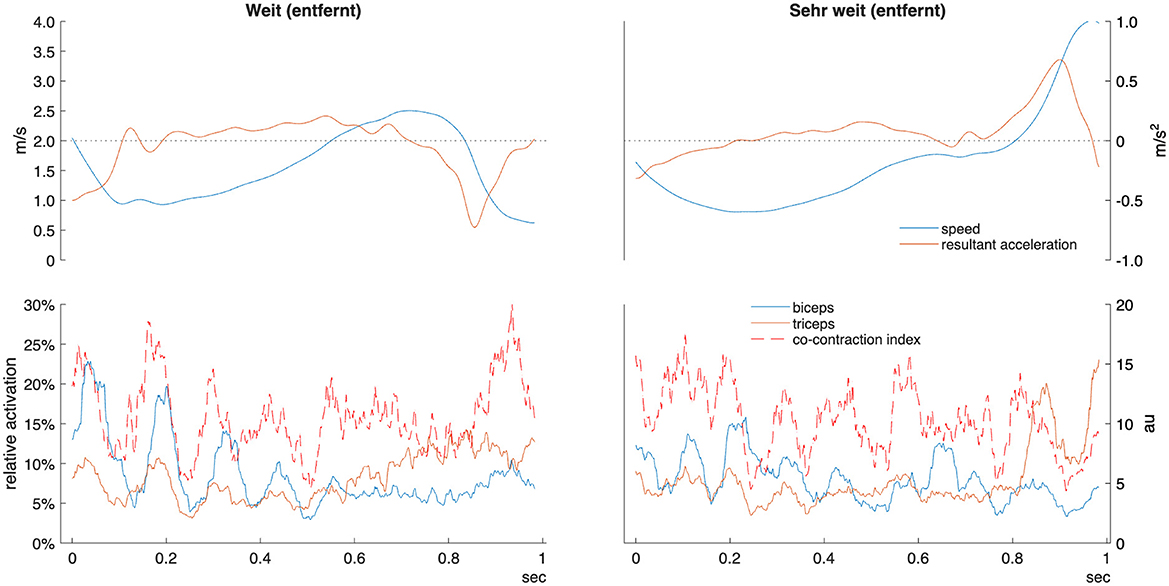
Figure 3. Example kinematic and EMG data for one adjective pair “weit” (far) and “sehr weit” (very far) in one participant. Sign time displayed from manual start and end labels as detailed in methods. Relative activation indicates percentage of maximal voluntary contraction. Co-contraction index units are arbitrary (au). m/s, meters per second.
Forearm and upper arm co-contraction were higher in intensified adjective forms (p = 0.01, d = 0.26 [0.10 , 0.47] and p < 0.001, d = 0.30 [0.15, 0.55], respectively) (see Figure 4). Although mean and median power frequencies had negligible differences (p = 0.07–0.92), there was greatly elevated activity in the intensified forms within the wrist extensors (d = 0.27–0.36), four of five bands in the biceps (d = 0.18–0.33), and four of five in the triceps (d = 0.51–0.67) (see Figure 5).
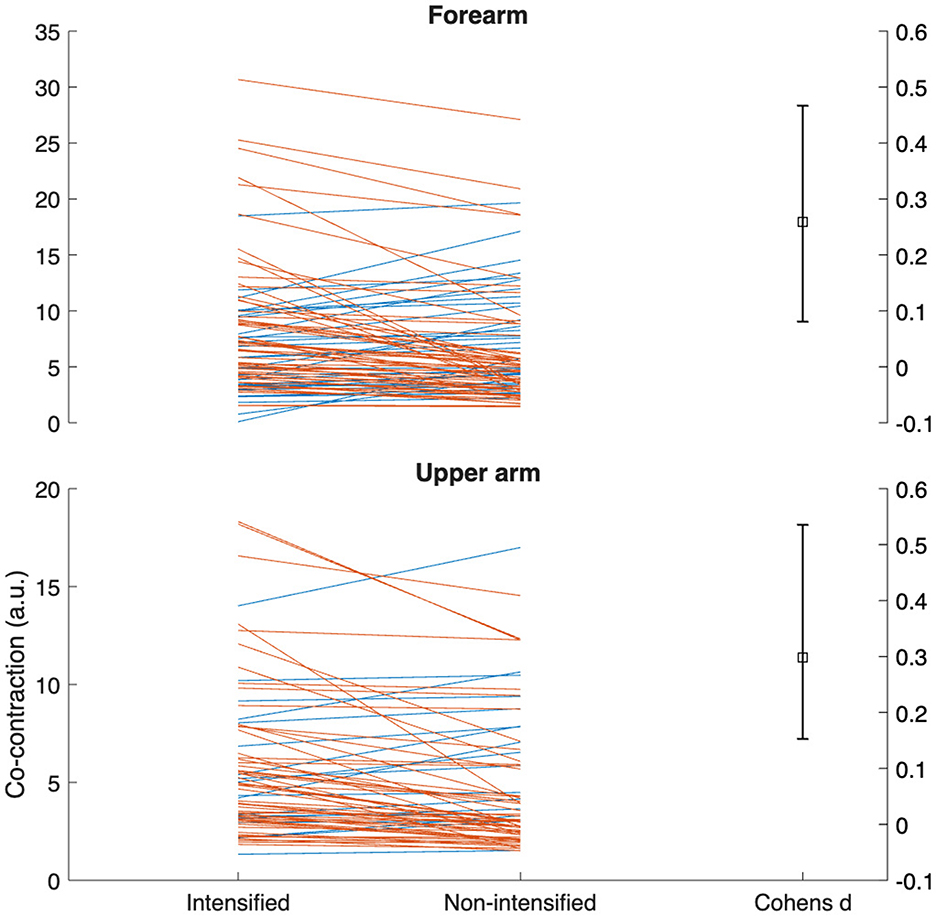
Figure 4. Gardner-Altman plots of the differences in forearm and upper arm co-contraction between intensified and non-intensified adjective pairs. Each line represents one participant/adjective pair for the dominant signing arm among all participants. Orange lines represent adjective pairs with greater co-contraction in the intensified form, while blue lines the opposite. a.u., arbitrary units.
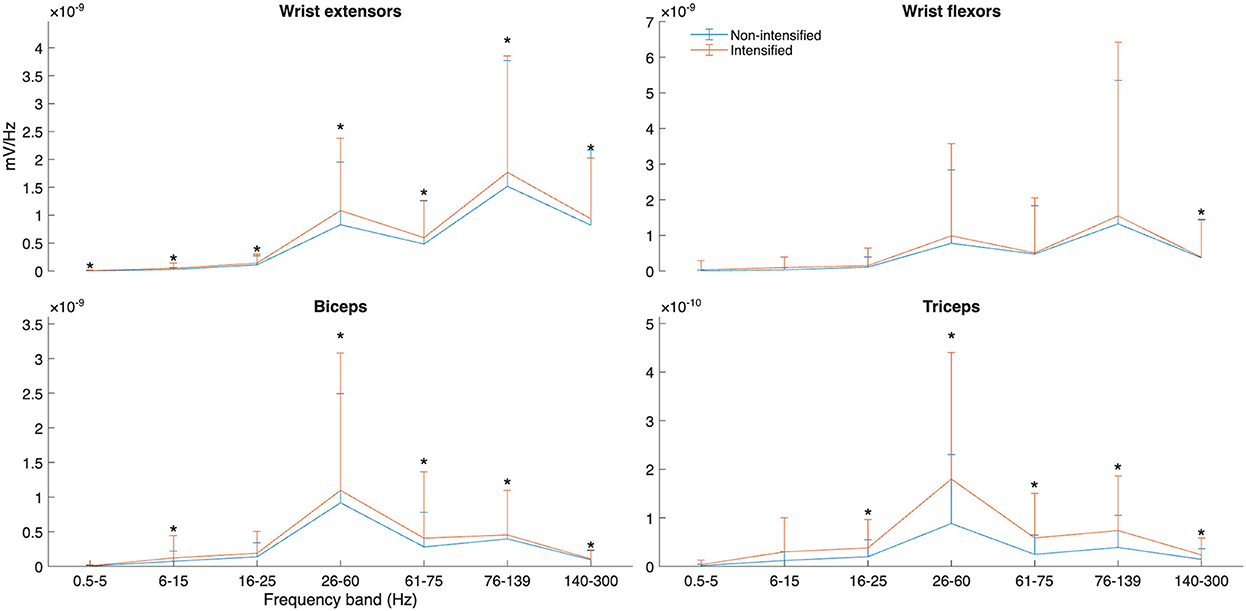
Figure 5. Power spectral density (PSD) of selected muscles and frequency bands for intensified and non-intensified adjectives between all participants. Significant differences between paired data at each band are marked with *.
4 Discussion
The present study operationalizes the biophysical bases of grammatical marking of intensified adjectives in ÖGS, and, more broadly, offers a possible measure for the parameter of tension in sign language research. The results demonstrate clear differences in both kinematics and muscle activity between intensified and non-intensified adjective forms in ÖGS. Specifically, the intensified adjectives were characterized by a significantly later time to peak deceleration than non-intensified adjectives with the same root; differences in peak deceleration magnitude did not reach statistical significance. Electromyographic (EMG) data identified significantly increased co-contraction indices in both forearm and upper arm muscles during intensified forms, allowing for greater precision in 3D motion control, with muscular co-contraction (i.e., increased articulatory tension) as the likely source of the observed kinematic differences (cf. Figure 4). Notably, wrist extensor and upper arm muscle activity were increased across multiple frequency bands in intensified adjectives (Figure 5), also supporting the hypothesis that tension is a key marker of grammatical intensification. The findings highlight the relationship between biomechanical and linguistic control of grammatical intensification marker in sign language production, offering first approaches to operationalizing tension as a grammatical and perceptual cue.
The repeated trials of one Deaf signer were analyzed prior to group analysis to qualify the impact of these findings. In this analysis, all kinematic variables were seen to have a smallest worthwhile change (0.2*coefficient of variation; Hopkins, 2000) of <5%, while the smallest worthwhile change in EMG measures ranged between 5%–20%. Rather, this single signer exhibited a high degree of consistency in producing these signs. This indicates that differences greater than these values in the present analysis are likely real, not by chance, and would be observed in repeated observations with other signers.
While there were some differences in deceleration characteristics and spatiotemporal variability, these were likely driven by the larger and more consistent differences in muscle activation: co-contraction (d = 0.26–0.30), forearm extensor (d = 0.27–0.36), and both upper arm muscles' activity were greater (d = 0.18–0.67) in intensified versus non-intensified forms. Wrist flexion and extension results from coordinated activity of the flexor and extensor muscle masses located on the medial and lateral portions of the forearm, respectively. Similarly, elbow movement is primarily controlled by the biceps and triceps. Co-contraction indices such as those investigated by Li et al. (2021) are generally used in kinesiology to estimate joint stiffness in gait and pathologies; however, it also provides a unique basis for movement analysis in manual communication. This is, to our knowledge, the first report using this analytical approach in sign language. Co-contraction may be increased to convey specific elements of intensification such as “tension.” This is corroborated by the known biomechanical consequences of joint stiffness. Alternatively, it could be a reflection of fine motor control; with greater voluntary muscle action, larger motor units in the muscle produce more force that must be counteracted by antagonist muscle groups to produce the target movement accurately. Future work should consider such analyses during natural conversation or to examine learning processes in sign languages. Further variability in articulatory modulation may also arise as a result of individual differences in age of sign language acquisition, which have been shown to have life-long effects on linguistic processing (Krebs et al., 2021).
4.1 Limitations
As in our previous report, we observed individual differences between signers which might be due to differences in signing style (Krebs et al., 2024a; Bigand et al., 2020; Xavier, 2013). For example, although sign duration exhibited consistent differences between intensified and non-intensified forms (p < 0.001, median difference = 0.19s, d = 0.39), within-participant differences ranged from 0.09–0.59s (d = 0.37–0.83). Hence some signers might use timing more than others to express intensification. We observed a similar pattern with forearm co-contraction, as effect sizes ranged from d = –0.27–0.93 within participants. It could be the case that (some) adjectival signs are intensified by cues other than differences in kinematics and manual muscle activation. The intensified adjectives are accompanied by specific non-manual markings which are absent in the non-intensified adjective form. The non-manuals observed in the context of adjective intensification in ÖGS are raised or furrowed eye brows, wide open or squinted eyes, different forms of mouth gesture, an enhanced mouthing or a head nod (Krebs and Fenkart, 2024). Thus, it might be that some signers may intensify some adjectives only by non-manual markings. The non-manual marking alone may convey the linguistic information about intensification and thus may be a sufficient grammatical cue for the perceiving addressee.
The present study focused exclusively on manual articulators. Non-manual signals, such as head motion and non-manual articulator contribution, while important (Krebs et al., 2024b; Malaia et al., 2018), are beyond the scope of this work and were not considered in our analysis.
5 Conclusion
While we quantify tension as a measurable correlate of grammatical adjective intensification in manual articulation in ÖGS, our objective is not to reduce signed language communication to overt motor mappings or embodied gestures. Instead, our approach reflects the view of language as a co-evolved system that leverages the brain's capacity for multiscale information extraction and structured symbolic representation (Malaia et al., 2023). Embodiment may serve as a useful metaphor at the perceptual level, but it is not explanatory at the computational level of language architecture (cf. Borneman et al., 2018). The communicative precision observed in sign language arises from an interaction between articulator control and linguistic structure, not just from general motor expressiveness (Malaia et al., 2018). Our goal in modeling articulator dynamics was to characterize how language systems encode high-density information across temporal and spatial scales. While sequential morphemes added to lexical items do exist in sign languages, a major distinction between signed and spoken languages is the possibility of simultaneous production of multiple levels of morphemic information—in this case, the adjective and its grammatical intensifier morpheme - in signed language. Quantification of linguistic tension as a biomechanial phenomenon—co-contraction with precise timing characteristics of MU control—is one of the spatiotemporal windows into the algorithms underpinning linguistic communication in the visual modality, but certainly not its limit.
In the EMG domain, our results suggest that there is not a single frequency band that reliably captures articulatory tension across all signs, because they vary in articulation and reliance on the specific muscles. Instead, we observed variation across multiple low- to mid-frequency bands, with specific patterns differing between muscle groups (e.g., biceps vs. triceps) and signs. In proficient signers, this appears to reflect coordinated interaction between frequency bands—a characteristic of multi-scale motor control. Skilled sign language production relies on precise modulation of tension, requiring simultaneous recruitment of different motor units with varying firing properties. As a result, motor control is distributed across MU types, particularly in lower frequency bands.
Multimodal research in this domain is critical for advancing sign language linguistics, as it operationalizes expression of grammatical markers in articulator motion, and allows for further cross-linguistic exploration into universal vs. language-specific patterns. By investigating the linguistic motor biomarkers of “tension,” this work contributes to better understanding of how grammatical markers are physically realized in sign languages, and supporting interdisciplinary work at the intersection of linguistics, neuroscience, and kinesiology. From the perspective of kinesiology, this work adds a linguistic control angle to the analysis of motor production. Multimodal experimental analysis of this type provides a testing ground for theoretical models in linguistics, and generates data for advancing computational research into sign language segmentation and recognition, including assistive communication systems (Tyrone, 2015; Kurtoğlu et al., 2021).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by review board of the University of Salzburg. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JK: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Writing – original draft. EH: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. EM: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – original draft. RW: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – original draft. HS: Funding acquisition, Resources, Writing – review & editing. DR: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by the Austrian Science Fund (FWF): ESP 252-G, the Austrian Science Fund (FWF): P 35671, and by the National Science Foundation [grant numbers 1932547, 1734938].
Acknowledgments
Many thanks to all participants taking part in this study. We also want to thank the lab assistants for their help with data collection and data pre-processing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/flang.2025.1632226/full#supplementary-material
Footnotes
1. ^which they describe generally as tension of the muscles and rapid movement but do not distinguish from other uses of tension and speed
2. ^Although there is a sign with the meaning “very” in ÖGS, this sign is usually not used to indicate the intensification of adjectives in ÖGS (a similar observation has been reported in ASL, Wilbur et al., 2012).
References
Algina, J., Keselman, H. J., and Penfield, R. D. (2005). An alternative to Cohen's standardized mean difference effect size: a robust parameter and confidence interval in the two independent groups case. Psychol. Methods 10, 317–328. doi: 10.1037/1082-989X.10.3.317
Ben Haj Amor, A., El Ghoul, O., and Jemni, M. (2023). Sign language recognition using the electromyographic signal: a systematic literature review. Sensors 23:8343. doi: 10.3390/s23198343
Bigand, F., Prigent, E., and Braffort, A. (2020). “Person identification based on sign language motion: insights from human perception and computational modeling,” in Proceedings of the 7th International Conference on Movement and Computing, 1–7. doi: 10.1145/3401956.3404187
Blumenthal–Dramé, A., and Malaia, E. (2019). Shared neural and cognitive mechanisms in action and language: the multiscale information transfer framework. Cogn. Sci. 10:e1484. doi: 10.1002/wcs.1484
Borneman, J. D., Malaia, E., and Wilbur, R. B. (2018). Motion characterization using optical flow and fractal complexity. J. Electron. Imaging 27:051229. doi: 10.1117/1.JEI.27.5.051229
Bosworth, R. G., Wright, C. E., and Dobkins, K. R. (2019). Analysis of the visual spatiotemporal properties of American Sign Language. Vision Res. 164, 34–43. doi: 10.1016/j.visres.2019.08.008
Boutet, D., and Garcia, B. (2009). Compositionnalité morpho-phonétique de la Langue des Signes Française (LSF) et exploration des relations structurales entre paramétres [Morpho-phonetic compositionality in French Sign Language (LSF) and exploration of structural relationships between parameters]. Revue TAL 3, 93–104. Available online at: https://hal.science/hal-00608468v1/file/TAL-2007-48-3-04-Boutet_GarciaV2_1_.pdf
Bradley, C., Malaia, E. A., Siskind, J. M., and Wilbur, R. B. (2022). Visual form of ASL verb signs predicts non–signer judgment of transitivity. PLoS ONE 17:e0262098. doi: 10.1371/journal.pone.0262098
Bradley, C., and Wilbur, R. (2023). Visual form and event semantics predict transitivity in silent gestures: evidence for compositionality. Cogn. Sci. 47:e13331. doi: 10.1111/cogs.13331
Burden, A. (2010). How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. J. Electromyogr. Kinesiol. 20, 1023–1035. doi: 10.1016/j.jelekin.2010.07.004
Davis, H., Gillon, C., and Matthewson, L. (2014). How to investigate linguistic diversity: lessons from the pacific northwest. Language 90, e180–226. doi: 10.1353/lan.2014.0076
Enoka, R. M., and Duchateau, J. (2015). Inappropriate interpretation of surface EMG signals and muscle fiber characteristics impedes understanding of the control of neuromuscular function. J. Appl. Physiol. 119, 1516–1518. doi: 10.1152/japplphysiol.00280.2015
Galea, L. C., and Smeaton, A. F. (2019). “Recognising Irish Sign Language using electromyography,”9D in 2019 International Conference on Content-Based Multimedia Indexing (CBMI) (IEEE), 1–4. doi: 10.1109/CBMI.2019.8877421
Gribble, P. L., Mullin, L. I., Cothros, N., and Mattar, A. (2003). Role of cocontraction in arm movement accuracy. J. Neurophysiol. 89, 2396–2405. doi: 10.1152/jn.01020.2002
Gu, Y., Zheng, C., Todoh, M., and Zha, F. (2022). American Sign Language translation using wearable inertial and electromyography sensors for tracking hand movements and facial expressions. Front. Neurosci. 16:962141. doi: 10.3389/fnins.2022.962141
Hopkins, W. G. (2000). Measures of reliability in sports medicine and science. Sports Med. 30, 1–15. doi: 10.2165/00007256-200030010-00001
Howell, P., Anderson, A. J., Bartrip, J., and Bailey, E. (2009). Comparison of acoustic and kinematic approaches to measuring utterance-level speech variability. J. Speech, Lang. Hear. Res. 52, 1088–1096. doi: 10.1044/1092-4388(2009/07-0167)
Khatin-Zadeh, O., Farsani, D., Hu, J., Eskandari, Z., and Banaruee, H. (2023). Gestural embodiment of intensifiers in iconic, metaphoric, and beat gestures. Behav. Sci. 13:174. doi: 10.3390/bs13020174
Klima, E. S., and Bellugi, U. (1979). The Signs of the Language. Cambridge: Harvard University Press.
Krebs, J., and Fenkart, L. (2024). Einführung in die Grammatik der Österreichischen Gebärdensprache. Das Handbuch [Introduction to the Grammar of Austrian Sign Language. The Handbook]. Verlag Fenkart, Guntramsdorf.
Krebs, J., Fessl, I., Wilbur, R. B., Malaia, E. A., Schwameder, H., Roehm, D., et al. (2023). Event structure reflected in muscle activation differences in Austrian Sign Language (ÖGS) verbs. FEAST 5, 76–87. doi: 10.31009/FEAST.i5.07
Krebs, J., Malaia, E., Wilbur, R. B., Fessl, I., Wiesinger, H.-P., Schwameder, H., et al. (2024a). Motion capture analysis of verb and adjective types in austrian sign language. arXiv preprint arXiv:2405.05161.
Krebs, J., Roehm, D., Wilbur, R. B., and Malaia, E. A. (2021). Age of sign language acquisition has lifelong effect on syntactic preferences in sign language users. Int. J. Behav. Dev. 45, 397–408. doi: 10.1177/0165025420958193
Krebs, J., Wilbur, R. B., Roehm, D., and Malaia, E. A. (2024b). The interaction of syntax, non-manuals, and prosodic cues as potential topic markers in Austrian Sign Language. Sign Lang. Linguist. 28, 1–48. doi: 10.1075/sll.23003.kre
Kurtoğlu, E., Gurbuz, A. C., Malaia, E. A., Griffin, D., Crawford, C., and Gurbuz, S. Z. (2021). ASL trigger recognition in mixed activity/signing sequences for rf sensor-based user interfaces. IEEE Trans. Hum. Mach. Syst. 52, 699–712. doi: 10.1109/THMS.2021.3131675
Lee, K. (2025). Sample entropy. MATLAB Central File Exchange. Available online at: https://www.mathworks.com/matlabcentral/fileexchange/35784-sample-entropy (Accessed January 8, 2025).
Li, G., Shourijeh, M. S., Ao, D., Patten, C., and Fregly, B. J. (2021). How well do commonly used co-contraction indices approximate lower limb joint stiffness trends during gait for individuals post-stroke? Front. Bioeng. Biotechnol. 8:588908. doi: 10.3389/fbioe.2020.588908
Malaia, E., Borneman, J. D., and Wilbur, R. B. (2018). Information transfer capacity of articulators in American Sign Language. Lang. Speech 61, 97–112. doi: 10.1177/0023830917708461
Malaia, E., and Wilbur, R. B. (2019). Visual and linguistic components of short-term memory: Generalized neural model (GNM) for spoken and sign languages. Cortex 112, 69–79. doi: 10.1016/j.cortex.2018.05.020
Malaia, E. A., Borneman, J. D., Kurtoglu, E., Gurbuz, S. Z., Griffin, D., Crawford, C., et al. (2023). Complexity in sign languages. Linguist. Vang. 9, 121–131. doi: 10.1515/lingvan-2021-0005
Muceli, S., and Merletti, R. (2024). Frequency analysis of the surface EMG signal: Best practices. J. Electromyogr. Kinesiol. 79:102937. doi: 10.1016/j.jelekin.2024.102937
Roman-Liu, D., and Konarska, M. (2009). Characteristics of power spectrum density function of EMG during muscle contraction below 30% MVC. J. Electromyogr. Kinesiol. 19, 864–874. doi: 10.1016/j.jelekin.2008.05.002
Rudolph, K., Axe, M., and Snyder-Mackler, L. (2000). Dynamic stability after ACL injury: who can hop? Knee Surg. Sports Traumatol. Arthros. 8, 262–269. doi: 10.1007/s001670000130
Savur, C., and Sahin, F. (2016). “American Sign Language Recognition system by using surface EMG signal,” in 2016 IEEE International Conference on Systems, Man, and Cybernetics (SMC) (IEEE), 002872–002877. doi: 10.1109/SMC.2016.7844675
Schlenker, P., and Lamberton, J. (2021). Focus and intensification in the semantics of brow raise. Glossa 6:5706. doi: 10.16995/glossa.5706
Schwameder, H., and Dengg, N. (2021). “Elektromyografie (Sportbiomechanik) [electromyography (sports biomechanics)],” in Bewegung, Training, Leistung und Gesundheit [Movement, Training, Performance, and Health] (Springer Verlag), 1–22. doi: 10.1007/978-3-662-53386-4_8-1
Tateno, S., Liu, H., and Ou, J. (2020). Development of sign language motion recognition system for hearing-impaired people using electromyography signal. Sensors 20:5807. doi: 10.3390/s20205807
Tyrone, M. E. (2015). “Instrumented measures of sign production and perception: motion capture, movement analysis, eye-tracking, and reaction times,” in Research methods in sign language studies: A practical guide (Wiley), 89–104. doi: 10.1002/9781118346013.ch6
Tyrone, M. E., Nam, H., Saltzman, E., Mathur, G., and Goldstein, L. (2010). “Prosody and movement in American Sign Language: a task-dynamics approach,” in Speech Prosody 2010-Fifth International Conference. doi: 10.21437/SpeechProsody.2010-144
Wilbur, R. (1979). American Sign Language and Sign Systems. Perspectives in Audiology Series. University Park Press.
Wilbur, R. B. (1987). American Sign Language: Linguistic and Applied Dimensions. Boston: Little, Brown and Co.
Wilbur, R. B. (1994). Foregrounding structures in asl. J. Pragmat. 22, 647–672. doi: 10.1016/0378-2166(94)90034-5
Wilbur, R. B. (1999). Stress in asl: empirical evidence and linguistic issues. Lang. Speech 42, 229–250. doi: 10.1177/00238309990420020501
Wilbur, R. B. (2022). Prosody in sign languages. Hrvatska revija za rehabilitacijska istražvanja 58, 143–174. doi: 10.31299/hrri.58.si.8
Wilbur, R. B., and Malaia, E. (2008). Contributions of sign language research to gesture understanding: what can multimodal computational systems learn from sign language research. Int. J. Semant. Comput. 2, 5–19. doi: 10.1142/S1793351X08000324
Wilbur, R. B., Malaia, E., and Shay, R. A. (2012). “Degree modification and intensification in American Sign Language adjectives,” in Logic, Language and Meaning (Springer), 92–101. doi: 10.1007/978-3-642-31482-7_10
Wilbur, R. B., and Schick, B. S. (1987). The effects of linguistic stress on ASL signs. Lang. Speech 30, 301–323. doi: 10.1177/002383098703000402
Xavier, A. N. (2013). Doubling of the number of hands as a resource for the expression of meaning intensification in Brazilian Sign Language (Libras). J. Speech Sci. 3, 169–181. doi: 10.20396/joss.v3i1.15046
Keywords: Austrian Sign Language, adjectives, intensification, kinematics, muscle activation, motion capture, electromyography
Citation: Krebs J, Harbour E, Malaia EA, Wilbur RB, Schwameder H and Roehm D (2025) Grammatical control of sign language production: EMG and motion capture analysis of adjective intensification in Austrian Sign Language (ÖGS). Front. Lang. Sci. 4:1632226. doi: 10.3389/flang.2025.1632226
Received: 20 May 2025; Accepted: 15 July 2025;
Published: 06 August 2025.
Edited by:
Francesca Peressotti, University of Padua, ItalyReviewed by:
Anne Wienholz, University of Hamburg, GermanyPlinio Almeida Barbosa, State University of Campinas, Brazil
Copyright © 2025 Krebs, Harbour, Malaia, Wilbur, Schwameder and Roehm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julia Krebs, anVsaWEua3JlYnNAcGx1cy5hYy5hdA==
 Julia Krebs
Julia Krebs Eric Harbour
Eric Harbour Evie A. Malaia
Evie A. Malaia Ronnie B. Wilbur
Ronnie B. Wilbur Hermann Schwameder
Hermann Schwameder Dietmar Roehm1,5
Dietmar Roehm1,5