- 1Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 2USERN Office, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 3Fertility and Infertility Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 4Department of Tissue Engineering, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 5Department of Mathematics and Physics “E. De Giorgi”, University of Salento, Lecce, Italy
Medical science is striving to find new solutions to treat various diseases. Tissue engineering with a great potential to develop tissues and even organs from synthetic and biological materials, open a new gate toward absolute treatments. Although in tissue engineering as a subtype of regenerative medicine, decellularized tissues are new, promising way to fill the previous methods gaps. Outside of the biological aspects, artificial intelligence (AI) and machine learning (ML) are applied to tissue engineering. Decellularization is a very important area where AI supports protocols and ensures the process is repeated identically each time. It also greatly assists in monitoring the extracellular matrix (ECM) to ensure it remains intact. Nonetheless, the use of AI in tissue engineering is not fully discussed in scientific articles. Although based on the tissue used for decellularization these features could vary, to optimize decellularization we need new method to reach high accuracy. In these current days, Pericardium, a double-layered membrane around the heart of mammalians, as a natural ECM has been utilized in cardiac surgery for many years. However, the use of decellularized pericardium as a scaffold for tissue engineering has gained significant attention in recent times, due to its retention strength, flexibility, supports for cell growth and differentiation, etc. That altogether put it among the top choices for tissue engineering and regenerative medicine. In this review we aim to cover the different decellularization methods, application of decellularized pericardium, commercial products that are available and challenges and future direction of this potent therapy.
1 Introduction
Tissue engineering has recently become a ground-breaking area of regenerative medicine, offering patients with organ failure or tissue damage fresh hope. The potential for transforming healthcare and enhancing patient outcomes is enormous when it comes to the ability to grow functional tissues and organs in the lab. Utilizing decellularized tissues like pericardium is one of the promising strategies in tissue engineering. To construct functional tissues, tissue engineering combines scaffolds, cells, and chemical agents with physiological activity. It emerged from the development of biomaterials. Tissue engineering aims to produce functional structures that restore, protect, or improve injured tissues or complete organs (1–6).
In 2021, Barbolescu and et al, given that gold standard methods for scaffold evaluation are lacking and scaffold destruction is required, developed an artificial intelligence-based method using deep convolutional neural networks (DCNN) to accurately identify the different stages of decellularization, which allows for precise determination of the completion time of the process. Apart from the tissue engineering approach, ML and deep learning can support increasing the quality of histological analysis and DNA removal (7).
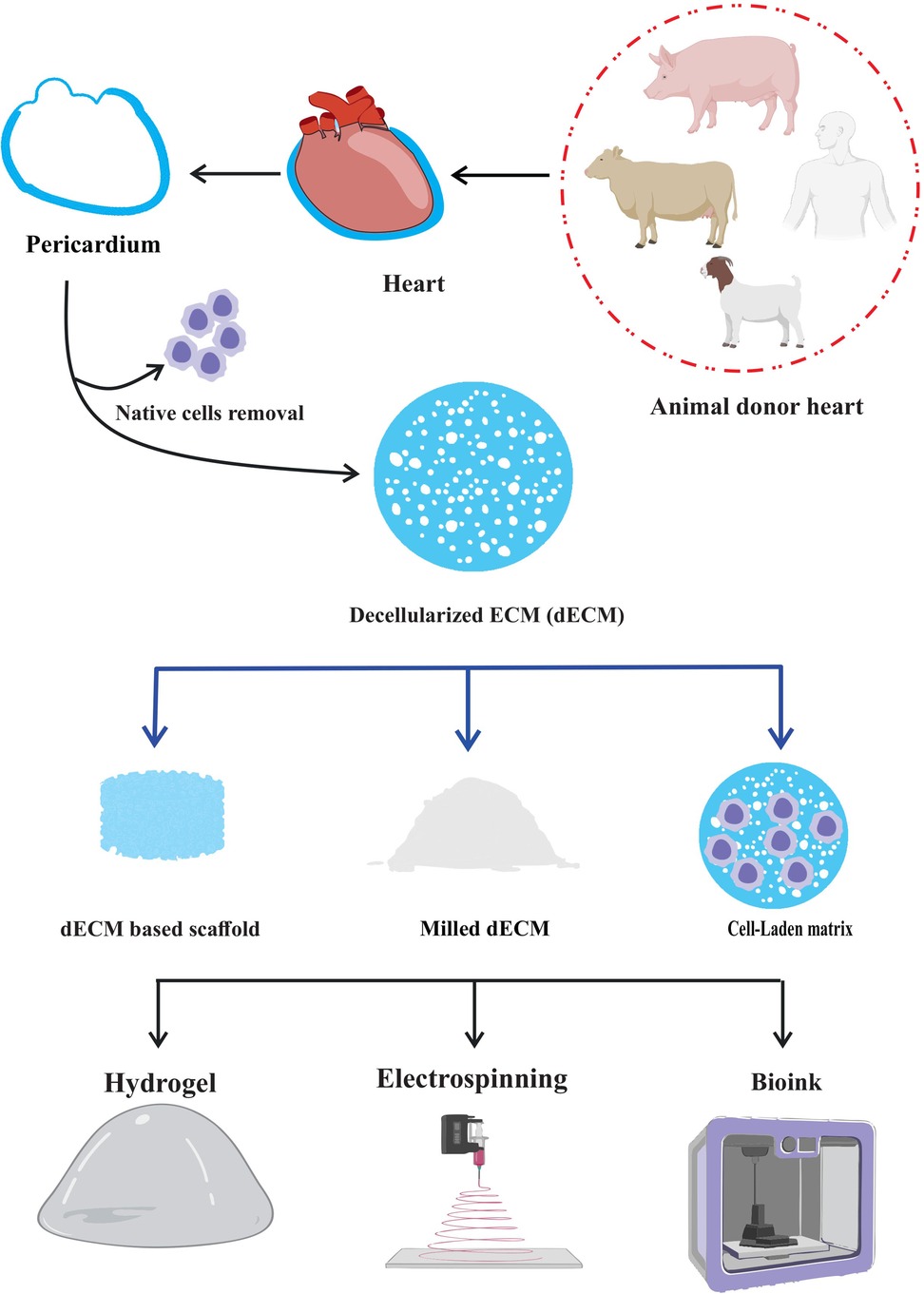
The pericardium is a thin, double-layered membrane that cover the heart, and has special qualities that make it a prime target for tissue engineering. The ECM remains after decellularization procedure, and it keeps its structural integrity and bioactive molecules while removing immunogenicity and potential dangers of disease transmission (8–10). When decellularized and employed as a hydrogel, the decellularized pericardium can generate a three-dimensional environment that promotes tissue growth. As a bioink, it provides a customized platform for three-dimensional (3D) bioprinting, enabling the exact deposition (11) of cells and ECM to replicate genuine tissue architecture. Also, electrospinning decellularized pericardium can create fibrous scaffolds that mimic the ECM's nanofibrous structure, offering a large surface area for cell contact and nutrition exchange. These applications demonstrate the potential of decellularized pericardium in tissue engineering and regenerative therapy (8, 12, 13).
Comparing decellularized pericardium to synthetic materials, which are frequently utilized in tissue engineering, reveals significant advantages. Because of the near resemblance between its natural makeup and that of native tissues, it offers the ideal milieu for cell adhesion, proliferation, and differentiation. It also possesses superior mechanical qualities including tensile strength and elasticity, which guarantee the stability and functionality of designed tissues (14, 15). The use of decellularized pericardium in tissue engineering has produced encouraging outcomes in several domains. Researchers have successfully used this biomaterial to create viable replacements for harmed or diseased tissues in a variety of fields, from orthopedics to cardiovascular regeneration. Scientists can direct cellular behavior, improve tissue regeneration, and minimize immunological rejection by seeding patient-specific cells onto the decellularized scaffold (8, 16–19).
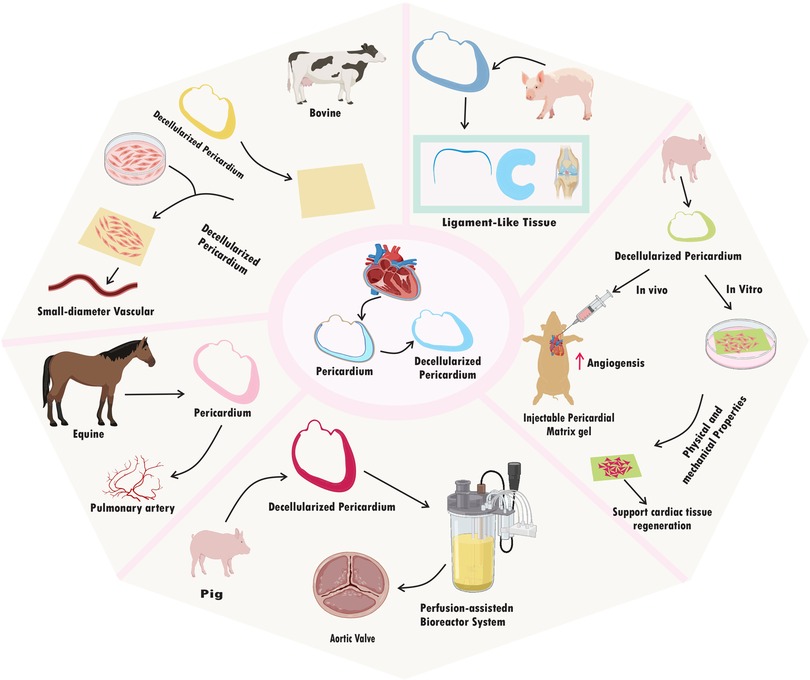
Decellularized pericardium can also be easily modified to suit the needs of other tissues or organs. To improve its mechanical qualities, it can be molded into certain geometries, mixed with other biomaterials, or added growth factors for specific biological responses. Due of its versatility, it can be used to build intricate, 3D structures that closely resemble the structure of natural tissues (20–22). The use of decellularized pericardium has enormous promise for tackling important problems in regenerative medicine as research in this area develops. This cutting-edge strategy offers a workable answer for individualized treatment and better patient care, from replacing broken heart valves to mending bone deformities or even creating bioartificial organs (23–25). Here, we discuss the most cutting-edge methods for decellularizing pericardium and highlight recent developments in their use in a variety of tissue engineering disciplines and limitation of decellularization method in this era. Understanding this method's possible advantages and disadvantages may help us develop more potent treatments that make use of nature's own building blocks for repairing damaged tissues and regaining normal physiological function (Figure 1).
2 Different pericardium decellularization approaches
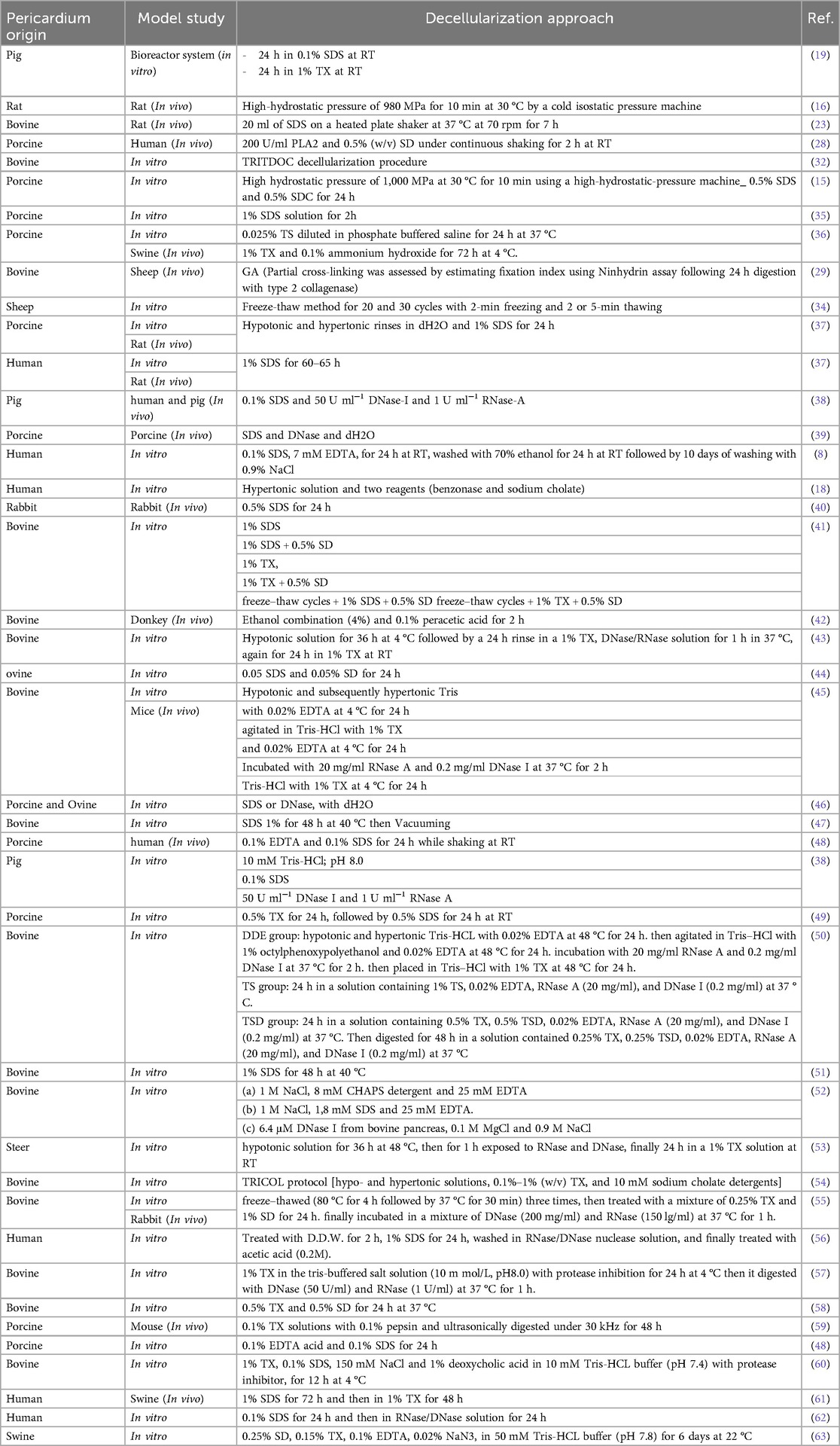
In numerous investigations, several pericardium decellularization techniques have been investigated. A perfusion-assisted bioreactor system employing pig pericardium as the tissue source is one such technique. In an in vitro model research, the pericardium underwent a decellularization treatment that included 24 h at room temperature (RT) in 0.1% (w/v) sodium dodecyl sulphate (SDS) and 24 h in 1% (v/v) Triton X-100 (TX) (19). Overall, SDS and TX are the most common materials used for decellularization. But the point is about the timings, temperature, shaker round per minute, washing methods, etc. that this process take place (26, 27).
Another study used an in vitro cell culture model to treat porcine pericardium with SDS. The decellularization procedure involves treating the tissue for 24 h with 0.5% SDS and 0.5% SDC after exposing it to high hydrostatic pressure of 1,000 MPa at 30 °C for 10 min using a high-hydrostatic pressure machine (15). In vivo research using human participants and swine pericardium as the tissue source has been done to investigate the effects of PLA2 and sodium deoxycholate (SD) treatment combined. The pericardium was treated with 200 U/ml PLA2 and 0.5% (w/v) SD as part of the decellularization process, which took place over the course of 2 h at RT with constant shaking (20). High hydrostatic pressure is another potential technique, uses to decellularized rat pericardium in a study, have present special features. A cold isostatic pressure machine was used to subject the pericardium to a high-hydrostatic pressure of 980 MPa for 10 min at 30 °C in an in vivo rat model study (28). Another decellularization procedure that has been investigated for swine pericardium is hypotonic and hypertonic rinses that is used with deionized water (dH2O) and subsequent treatment with 1% SDS in phosphate-buffered saline (PBS) for 24 h, for both in vitro and in vivo experiments (29).
TRITDOC (TRIton-X100 and TauroDeOxyCholic acid) decellularization procedure, introduced as a holistic approach to heart valve tissue engineering (30) which has evaluation during time (31). In this procedure Pericardial patches were treated with alternating hypo- or hypertonic solutions, TX (0.1%–1%), and 4 mM sodium taurodeoxycholate. The extractions were performed in a degassed solution with 10 mM sodium ascorbate and 5 mM ethylenediaminetetraacetic acid (EDTA) under N2 atmosphere and continuous stirring. The residual nucleic acids were digested with 1,500 U cm−2 of the non-specific endonuclease Benzonase™ at 37 °C for 24 h. The solutions were sterilized using Filtropur filters with a pore size of 0.20 μm. TBP was decellularized and kept at 4 °C in an antibiotic/antimycotic solution containing 3 U ml−1 penicillin, 3 mg ml−1 streptomycin, and 2.5 μg ml−1 Amphotericin B (30–32).
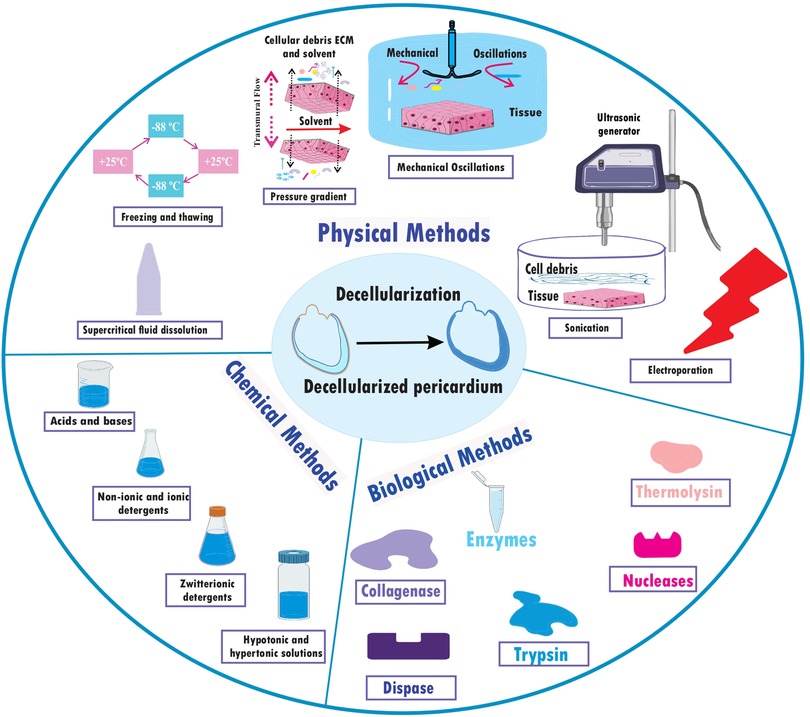
A fixative-free decellularization technique has been examined in an in vivo investigation employing swine pericardium as the tissue source, including participants from both the pig and human species. This procedure made use of a hypotonic (10 mM Tris-HCl; pH 8.0) detergent solution containing 0.1% (w/v) SDS and 50 U ml−1 DNase-I and 1 U ml−1 RNase-A, and it doesn’t glutaraldehyde (GA) as frequency used fixative for histological processes (33). Finally, non-chemical methods of decellularization that are non-toxic and may work faster could provide a suitable mechanism for decellularization. Freeze-thawing is one of the methods which shows to be successful. Freezing and thawing tissue for 20–30 cycles, each lasting 2–5 min was enough to cause noticeable tissue decellularization (34). These numerous techniques give multiple ways to successfully decellularize pericardial tissues from various animal sources, and they can offer insightful information for creating tactics that will work for upcoming application (26) (Table 1) (Figure 2).
3 Mechanical properties
In tissue engineering applications, the mechanical characteristics of decellularized tissues are extremely important. Several significant characteristics of decellularized pericardium have been identified. And here we’re going to discuss some of the most effected ones during decellularization approaches. Tensile Strength and Modulus of Elasticity (Young's modulus) (Figure 3).
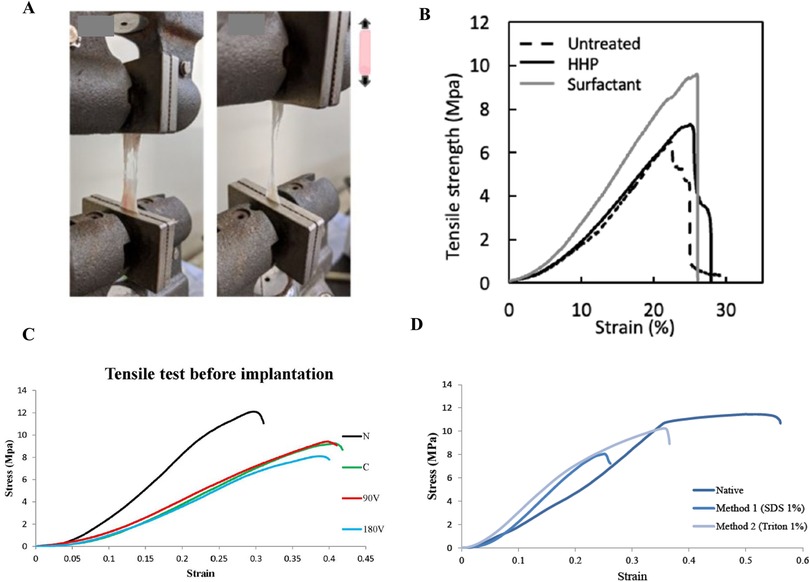
Figure 3. Mechanical test, (A) shows how we access and measure the tensile strength, (B) (15), depicts typical stress-strain curves for the untreated pericardium, high hydrostatic pressurization (HHP)-decellularized pericardium, and surfactant-decellularized pericardium, (C) (49) shows strain measurements of vacuumed decellularized pericardium before and after implantation, and (D) (82) illustrates the stress-strain curves for decellularized tissue in native, SDS-treated, and TX-treated pericardium. Various studies show that tensile strength (Young's modulus) decreases in decellularized tissues compared to native tissue. The type of detergent and the decellularization method have a significant effect on reducing the mechanical properties. As can be seen, the HHP method and the use of surfactants have a higher Young's modulus than the SDS method.
Tensile strength gauges a material's capacity to withstand pulling or stretching forces without breaking. The decellularized pericardium's capacity to preserve structural integrity under tension is determined by its tensile strength (39). The elastic modulus, sometimes referred to as Young's modulus, gauges a material's stiffness or rigidity. It explains how much a material deforms when a force is applied and how much it straightens out again when the force is removed. Decellularized pericardium's elastic modulus is an indicator of its capacity to withstand mechanical stress and keep its shape (19).
The impact of decellularization on tensile strength and elasticity has been investigated in various research; While the general trend suggests that decellularization does not significantly affect the ultimate tensile strength compared to native tissue (65, 66). Here are some specific findings and comparisons that can show the alternations. Studies comparing decellularized and glutaraldehyde-fixed pericardium reveal distinct differences. GA fixation generally increases stiffness and reduces elasticity. The following data illustrate that while GA treatment results in higher tensile strength, it also leads to significantly higher elongation at break, indicating reduced elasticity.
Mildly Cross-linked Decellularized Bovine Pericardium (29):
Tensile Strength: 13.14 ± 4.32 MPa
% Strain at Max Load: 47.11 ± 20.4%
Young's Modulus: 50.08 ± 18.9 MPa
Elongation at Break: 61.2 ± 19.7%
Commercially Available Glutaraldehyde-Treated Bovine Pericardium (29):
Tensile Strength: 16.7 ± 6.3 MPa
% Strain at Max Load: 71.19 ± 7.8%
Young's Modulus: 47.46 ± 15.76 MPa
Elongation at Break: 86.33 ± 9.03%
In another study they conduct direct comparisons between native and decellularized pericardium, often shows no statistically significant differences in tensile strength or elastic modulus. As the following data confirm, the differences in the numbers were not significant (P value >0.05), supporting the theory that decellularization can preserve the native mechanical integrity. Studies on porcine pericardium also confirm these findings.
Native Bovine Pericardium (NBP) (32):
Elastic Modulus: 51.4 ± 4.6 MPa
Ultimate Tensile Strength: 17.3 ± 0.8 MPa
Decellularized Bovine Pericardium (TBP) (32):
Elastic Modulus: 48.7 ± 4.9 MPa
Ultimate Tensile Strength: 15.3 ± 1.2 MPa
Another important aspect in this section is the impact of decellularization Methods; Different decellularization protocols can have varying effects. While some studies using high hydrostatic pressure or surfactant methods; they found no significant differences in ultimate tensile strength, failure strain, and elastic modulus, and even others observed changes in Young's modulus depending on the specific detergent (e.g., SDS) concentration. One study on porcine pericardium treated with SDS showed the following (35):
Native Pericardium: Young's moduli of 36.23 ± 3.2 MPa
0.5% SDS: Young's moduli of 13.66 ± 0.9 MPa and 26.81 ± 3.8 MPa
1% SDS: Young's moduli of 12.17 ± 5.5 MPa and 36.26 ± 2.9 MPa
So, these results suggest that SDS treatment can influence the elastic modulus (increase in SDS concentration decrease the elasticity), although the differences were not always statistically significant.
Other mechanical characteristics:
Distensibility is an important mechanical property of tissues, which researcher for evaluating their works, assess it through methods like confined-flow perfusion, biaxial inflation, and pressure-volume curves. These curves could provide insights into tissue stiffness, and to make their interpretations easy, you should know that higher slopes indicating increased resistance to deformation. But pressure–volume (P–V) curve analysis goes further than this. In most of the studies decellularized pericardium shows similar behavior to native tissue, while GA-treated tissue exhibits higher curve slopes, which as explained present as having a significantly higher tissue stiffness of both elastin and collagen fibers. While decellularization can reduce GAG content and potentially increase thickness, its overall impact on distensibility varies depending on the specific method and the tissue source. As you noticed, understanding these factors is critical for optimizing tissue engineering strategies and developing biocompatible pericardial grafts (38, 67, 68).
Permeability is defined as the ability of a material to allow fluids to pass through. It's a factor that play a role in the success of pericardial tissue engineering. Various methods, including perfusion tests and molecular transport studies, can be used to test this property of our product. Decellularization procedures, particularly TX, enhance permeability significantly by disrupting cell-matrix interactions, this helps cell seeding, nutrient/oxygen transport, and overall recellularization efficiency. Increased thickness can also contribute to higher permeability. Maintaining adequate mechanical strength and preventing excessive fluid flow remain should always be considered for successful tissue engineering applications (19, 23, 69).
Stress-Strain Behavior is another property for decellularized tissue which is tested by Uniaxial Tensile Loading (UTL) Test. Decellularized pericardium typically demonstrate a J-shaped stress-strain curve. These curves were generated to determine the biomechanical properties. The elastic modulus, representing the slope of the stress-strain curve, was measured at low (E1) and high (E2) strain (E1 reflects tissue resistance due to elastin fibers, while E2 reflects resistance from collagen fibers). Based on the study and final application of decellularized tissue, this test may be necessary (8, 19, 24).
Suture retention strength is a crucial factor for successful surgical grafts. It's being evaluated in a composite material made of decellularized ECM and a synthetic polymer (the maximum force required to pull a suture through the material). dECM appears to have significantly greater suture retention strength than native tissues (independent of the polymer's crosslinking method). Although the retention decreased with enzymatic degradation of the matrix, highlighting the importance of the decellularized matrix component and the method used for decellularization. This superior strength suggests a reduced risk of graft failure at the surgical connection site, reveal as a critical advantage for in vivo applications (23, 44).
Circumferential stress, a measure of a vessel's resistance to outward pressure. In a study worked on a decellularized porcine right coronary artery, it evaluated in ring-shaped dECM + PPF grafts using mechanical testing. Rather than the addition of the synthetic polymer (PPF) greatly dictated the graft's circumferential strength in this study, increasing UV exposure time leads to an increase in stress resistance (from 2.24 ± 0.99 MPa to 4.20 ± 2.05 MPa between 15 and 45 min). Notably, enzymatic degradation, mimicking in vivo breakdown, led to increased circumferential strength, likely due to the inherent properties of the PPF. This suggests that the dECM contributes to the graft's mechanical integrity even after some degradation (38, 70).
According to these, Decellularization effectively preserves many key biomechanical characteristics, while it also greatly depends on decellularization method and source of the tissue.
4 Application of decellularized pericardium in regeneration
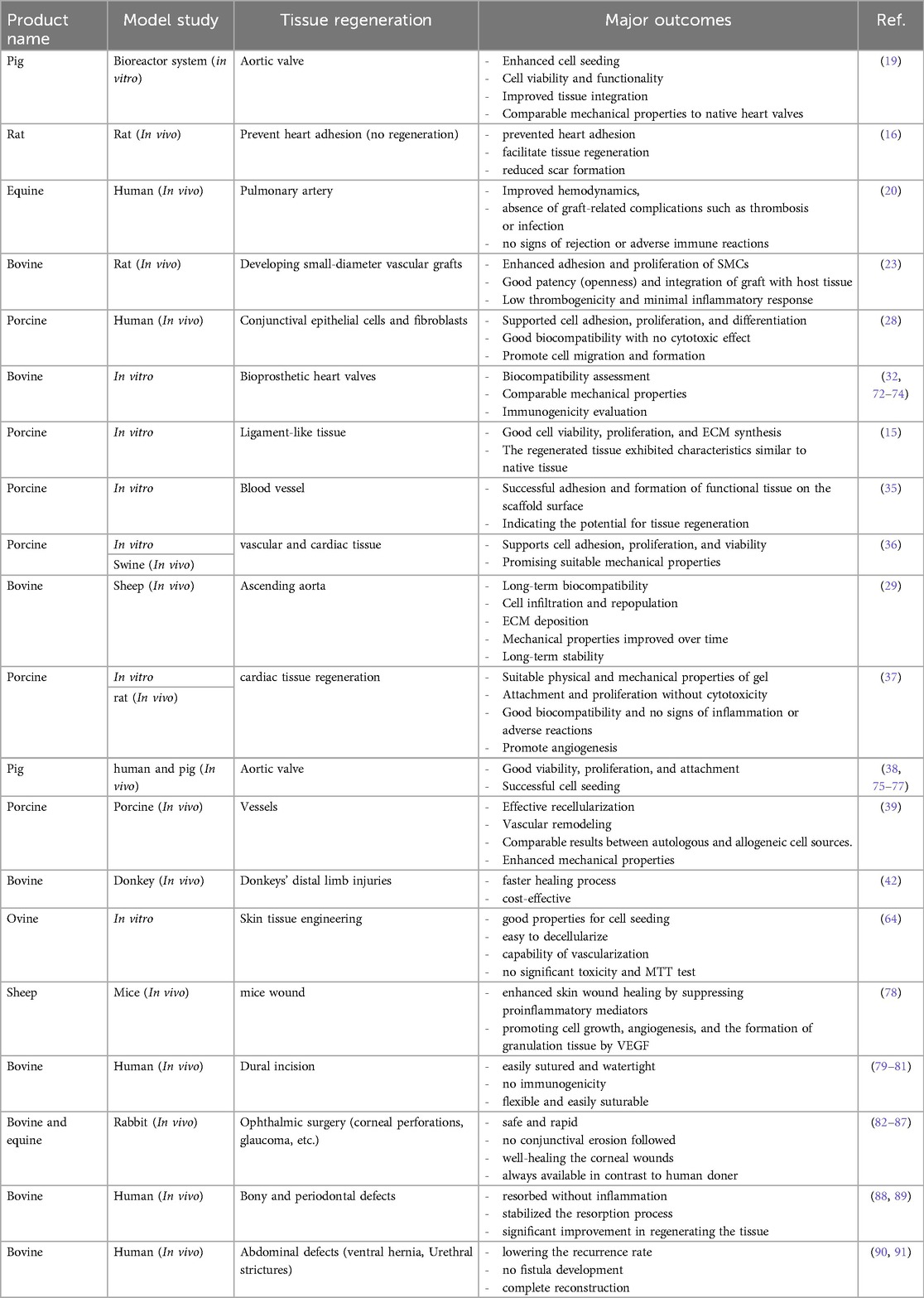
In numerous models and applications, the decellularized pericardium has demonstrated encouraging potential for tissue regeneration. A perfusion-assisted bioreactor system was used to regenerate the aortic valve in a model study utilizing pig pericardium. Enhanced cell seeding with uniform distribution and aortic valve cell adhesion to decellularized scaffolds were the main findings of this investigation. The seeds showed functional traits typical of mature heart valve cells and persisted in viability. Additionally, the planted cells displayed enhanced ECM integration, suggesting potential for tissue remodeling and regeneration. The synthetic structures also showed mechanical characteristics like those of natural heart valves, indicating feasibility for implantation in the future (19). The goal of a different study that used rat pericardium was to prevent cardiac adhesion. In the rat model, the application of fibrin-coated pericardial ECM successfully prevented heart adhesion. In addition, when compared to control groups that received no ECM therapy, this strategy promoted tissue regeneration and decreased scar formation (28). Research into tissue regeneration in the pulmonary artery using equine pericardium has been conducted. Positive results were seen in the first single-center trial with Matrix PatchTM, which involved 10 patients. Improvements in hemodynamics and a lack of graft-related problems such thrombosis or infection were seen. During the follow-up period, no indications of rejection or harmful immunological reactions were found (37).
It has been investigated to create small-diameter vascular grafts using pericardium from cows. Pericardial tissue was successfully decellularized while maintaining the content and structure of the ECM. Rat smooth muscle cells (SMCs) exhibited increased adhesion and proliferation in in vitro experiments when grown on a decellularized pericardial ECM scaffold, demonstrating biocompatibility. Studies conducted in vivo further confirmed its promise as a small-diameter vascular graft, demonstrating high patency and tissue integration, low thrombogenicity, and little inflammatory reaction (32). In an in vitro experiment using human conjunctival epithelial cells and fibroblasts, crosslinked decellularized swine pericardium demonstrated promise in promoting cell adhesion, proliferation, and differentiation. The substance showed excellent biocompatibility and had no harmful effects on the test cells. Additionally, it encouraged cell migration and the development of multilayered epithelial structures that resembled natural conjunctival tissue (20).
The interaction between human umbilical vein endothelial cells (HUVECs) with both decellularized bovine pericardial patches and commercial bioprosthetic heart valves was examined in an in vitro comparative study employing bovine pericardium. The two materials’ mechanical attributes, including tensile strength and elasticity, were contrasted. Additionally, to assess the likelihood of immunological reactions, an analysis of the immune response elicited by both materials was done (23). In an in vitro cell culture model, the porcine pericardium has also been investigated for ligament-like tissue regeneration. Cell survival, proliferation, and ECM production in fibroblasts grown on a scaffold made from decellularized pericardium were all good. The regenerated tissue displayed traits, such as collagen synthesis and alignment, that were comparable to those of natural ligaments. Mechanical testing revealed that the tissue's strength was on par with that of native ligaments (15).
The decellularization procedure successfully eliminated cellular components while retaining the ECM structure in an in vitro cell culture investigation utilizing swine pericardium. Compared to other experiments, HUVECs successfully attached and created functional endothelium monolayers on the scaffold surface in a short amount of time. Human mesenchymal stem cells (hMSCs) also showed development into cells that resembled smooth muscles, suggesting the possibility of tissue regeneration (35). Another study found that a porcine pericardium-based nanomaterial-tissue patch had promising mechanical properties suited for cardiovascular applications. The patch, maintained cell adhesion, proliferation, and survival with success in vitro, indicating its viability as a viable treatment for and vascular repair (36).
An experiment employing an animal model and bovine pericardium revealed long-term biocompatibility with little inflammatory reaction even after long-term implantation. The patch shape facilitated fresh ECM deposition by host cells as well as incremental host cell infiltration and repopulation over time, indicating tissue regeneration. The patches exhibited long-term stability and endurance throughout the study period, maintaining their structural integrity and functionality (38). The physical, mechanical, biocompatibility, and angiogenesis-promoting characteristics of an injectable pericardial matrix gel were studied to create an injectable scaffold that could assist heart tissue regeneration. When injected into rat hearts, the gel demonstrated good biocompatibility and encouraged the growth of new blood vessels, demonstrating its potential to enhance tissue regeneration (29). According to a study that used decellularized pig pericardium as a scaffold for aortic valve interstitial cells (VICs), both pig and human VICs demonstrated good viability, proliferation, and adhesion. This implies that the fixative-free decellularization technique efficiently eliminates cellular components while maintaining the integrity of the ECM, enabling successful cell seeding (16).
The effective recellularization of decellularized pericardial scaffolds with autologous or allogeneic cells was also seen in a pig carotid artery model, as well as signs of vascular remodeling in response to recellularized patches and matrices. These results imply a great potential for decellularized pericardium to be used in tissue regeneration, particularly in the setting of blood vessels and cardiac tissue (39). All things considered, this research show the various uses of decellularized pericardium in tissue regeneration across various models, tissues, and organs. The research shows that it has the potential to improve cell seeding, promote cell viability/functionality/integration with ECM, maintain mechanical properties like native tissue, prevent adhesion and scar formation, improve hemodynamics, support cell adhesion/proliferation/differentiation, promote tissue regeneration and remodeling, and exhibit biocompatibility with minimal immune response. Decellularized pericardium has the potential to be a flexible biomaterial for a range of regenerative medicine applications, such as ligament-like tissue regeneration, vascular graft creation, conjunctival tissue repair, and more. To fully explore its potential and maximize its usage in clinical settings, additional study in both the laboratory and the clinic is required (71) (Table 2) (Figure 4).
5 Artificial intelligence (AI) and machine learning (ML) intersection with decellularization in tissue engineering
AI and ML are the most significant approaches in the scope of tissue engineering particularly in areas of decellularization, protocol optimization, histological analysis as well as ECM characterization. These computational methods can provide high accuracy, Standardization in biomedical application with the lowest experimental cost (92).
5.1 AI in decellularization processes
Decellularization is used to preserve the ECM, allowing it to function as a framework for regeneration by removing cellular components from tissues. Meanwhile, the AI models that have excited researchers are especially good at identifying the underlying factors that affect different treatments. These models examine enzyme levels, blood flow rates through tissues and appreciations of how long someone is exposed to something. Supervised learning models in particular have been employed to optimize detergent formulations and assess tissue integrity following decellularization. Deep learning has also been employed to assess decellularization efficacy through imaging and chemical data, which dramatically reduces the need for costly trial-and-error experimentation (93). Moreover, the use of feedback systems allows for the adjustment of characteristics used in the decellularization process for real-time monitoring throughout the entire process, further enriching AI's role in decellularization. Predictive modeling can allow certain key structural components to remain preserved, minimize damage to the equipment, allow the consistency and integrity of the tissue not to be compromised, and will make them more viable for use in biomedical applications (94).
5.2 Optimization of decellularization protocols
Historically, decellularization protocols have involved a significant amount of trial and error to determine optimal conditions and really nail everything down. This enables the optimization of these protocols by way of AI sifting through massive datasets to determine the optimal parameters. One aspect of AI, reinforcement learning, can potentially be employed to dynamically change variables such as temperature, pressure, and exposure duration to enhance ECM preservation. Plus, AI simulations allow researchers to organize situations before entering lab work this allows them to predict what results will be and all but remove the use of materials or other assets. AI-driven computational fluid dynamics (CFD) models have been used to simulate perfusion decellularization, allowing for the identification of optimal flow rates and exposure times to achieve full cellular removal. These simulations are useful because they allow us to sort out protocols in a gentle way, without too much guess and check in the chemistry lab (7, 95).
5.3 AI for histological analysis and ECM characterization
While decellularized tissues have many potential applications, detailed tissue analysis is critical to determining the quality of the decellularized tissues. With the advent of AI-assisted image processing tools, automatic segmentation, classification, and quantification of tissue structures have recently transformed studies of the tissue. Convolutional neural networks (CNNs) excel at identifying residual cellular material and evaluating ECM quality. AI-assisted histological analysis provides consistency between different observers, thereby minimizing the possibility of subjective discrepancies in manual evaluations (96). AI is also instrumental in ECM characterization through the integration of various data sources, including biochemical assays, mechanical testing and microscopic imaging. This is where ML models come in handy, in correlating ECM composition to its functional properties. In other words, making sure decellularized scaffolds retain their mechanical strength and biochemical signatures that are conducive to transplantation (97, 98).
6 Pericardium commercial products
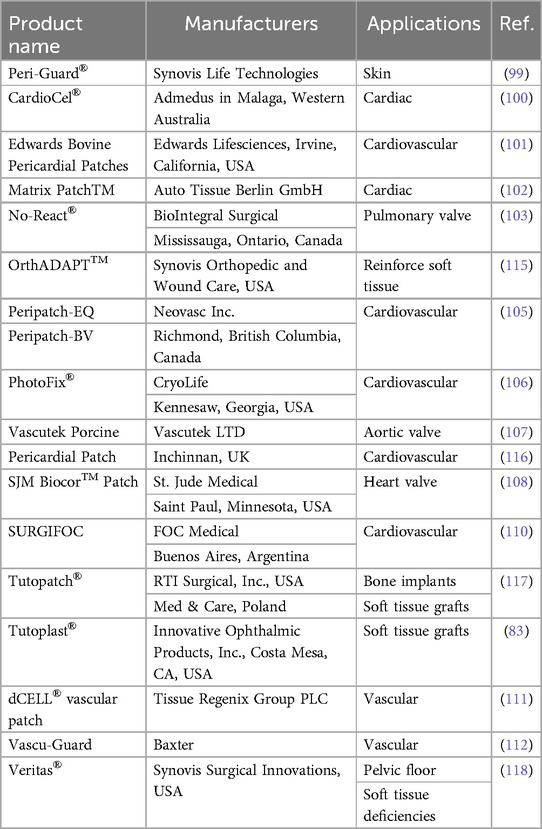
Products made from decellularized pericardium that are readily accessible on the market are frequently employed for soft tissue repair and rebuilding in a variety of surgical procedures. Regarding heart tissue repair and reconstruction as well as other cardiovascular and vascular surgeries, these products provide a secure and efficient solution. Peri-Guard®, produced by Synovis Life Technologies, is one of the most well-known products in this category. A decellularized pericardial patch called Peri-Guard® was created especially for repairing and reconstructing soft tissue. In surgical operations, it is frequently utilized to repair injured tissues and speed up healing (99). CardioCel®, made by Admedus in Malaga, Western Australia, is another noteworthy item. A decellularized bovine pericardial patch known as CardioCel® is frequently used in cardiovascular surgery. It is a vital tool for cardiac surgeons since it is very successful at mending and rebuilding heart tissues. Excellent biocompatibility and durability are provided by this product (100). Edwards Bovine Pericardial Patches are provided by Edwards Lifesciences, a company situated in Irvine, California, USA. These patches undergo decellularization to eliminate cellular matter and are also made from bovine pericardium. They are suitable for a variety of heart repair treatments due to their strength and flexibility, which are well known for them (101).
Equine pericardium serves as the raw material for Matrix PatchTM production by Auto Tissue Berlin GmbH. This decellularized patch is frequently used in cardiovascular procedures and has great handling qualities (102). No-React®, manufactured by BioIntegral Surgical, is made from decellularized pig pericardium. This product is renowned for its great biocompatibility and low immunogenicity (103). Peri-Guard by Baxter International Inc., with headquarters in Deerfield, Illinois, uses pericardium from cows. It goes through a process called GA-crosslinking to improve its toughness and mechanical qualities (104). Peripatch-EQ and Peripatch-BV are provided by Neovasc Inc., a Canadian company based in Richmond, British Columbia. These patches are made from the pericardium of horses and cows, respectively. Due to their good handling characteristics, they are frequently employed in cardiovascular procedures (105).
The pericardium of cows is used to make PhotoFix®, which is produced by CryoLife. Decellularization is used on this product to keep the ECM intact while removing cellular components. It is renowned for being flexible and simple to use (106). The pig pericardium used to make Vascutek pig Pericardial Patch is produced by Vascutek LTD., which has its headquarters in Inchinnan, UK. It receives a GA-crosslinking procedure to improve its mechanical qualities (107). Bovine pericardium is the source of the SJM BiocorTM Patch by St. Jude Medical, which has outstanding biocompatibility and longevity (108). Although it also uses bovine pericardium, the SJM Pericardial Patch with EnCapTM AC Technology by St. Jude Medical integrates cutting-edge technology for better tissue integration (109).
Both bovine and porcine pericardium are used in the production of SURGIFOC by FOC Medical in Buenos Aires, Argentina. It receives a GA-crosslinking procedure to improve its mechanical qualities (110). The Tissue Regenix Group PLC's dCELL® vascular patch, which is made from pig pericardium, has outstanding biocompatibility and improved tissue regeneration capacities (111).
Baxter's Vascu-Guard is made from bovine pericardium and offers superior handling and improved tissue integration features (112). Products made from decellularized pericardium that are sold commercially are essential for soft tissue restoration and repair during a variety of surgical procedures. In the realm of cardiovascular surgery, these tools give doctors secure and efficient ways to repair damaged tissues, improving patient outcomes (113, 114) (Table 3).
7 Challenges, limitations, and future directions
Decellularized pericardium is so promising as a scaffold for tissue engineering applications. Before these techniques be widely used in clinical practice, there are several restrictions and difficulties that must be overcome. The short lifespan is one of the main drawbacks of decellularized pericardium in tissue engineering techniques. The long-term pressures and strains necessary for permanent tissue replacement may be beyond their ability to bear, even though they can offer temporary support. Additionally, using tissues taken from animals may cause the receiver to have an immunological reaction, which could result in rejection or other difficulties. Furthermore, there aren't enough clinical studies on the security and effectiveness of these treatments and using decellularized pericardium may not be appropriate for all tissue types or applications (15, 23, 119).
Another approach includes Determining successful tissue decellularization is challenging due to limitations in current evaluation methods. Traditional techniques, such as DNA quantification and histological staining, require tissue damage, hindering their use in practical applications. However, the traditional methods are time-consuming and requires constant attention. To overcome these obstacles, ML to precisely identify the completion of the decellularization process developed an innovative, automated system can be utilized. This system eliminates the need for tissue damage or continuous monitoring (120, 121).
Successful tissue engineering depends on achieving correct integration between the decellularized pericardium and host tissue yet fostering this integration can be difficult (36). Getting adequate cell seeding and integration into the decellularized pericardium is one of the main problems. Technically challenging tasks include stimulating cell adhesion, proliferation, and differentiation while ensuring uniform cell dispersion across the scaffold. Additionally, for nutrient delivery, waste elimination, and general tissue viability, significant vascularization is required. However, the decellularized pericardium's capacity to maintain long-term tissue life can be constrained by the difficulty of encouraging blood vessel ingrowth (35). Converting encouraging in vitro findings into clinical applications is another difficulty. Extensive preclinical research, proof of safety and efficacy in animal models, and ultimately human clinical trials are required to successfully translate this technology from laboratory research to clinical practice. Additionally, there may be difficulties in terms of time, resources, and adherence to safety regulations when scaling up the production of these patches to satisfy clinical demands and regulatory criteria (19).
Cost-effectiveness is another crucial factor. It is necessary to weigh prospective benefits in terms of patient outcomes and healthcare costs against the costs associated with development, production, and implementation. Additionally, it can be difficult to obtain enough viable aortic valve interstitial cells (AVICs) from both pig and human sources. It can be challenging to get reliable findings because of the variability in these cells’ availability and quality. For tissue engineering to be successful, it is also essential to guarantee the viability of AVICs while they are being seeded (16, 122).
Another difficulty is obtaining consistent results across several studies and samples. The technique's reproducibility may be impacted by variations in cell behavior, tissue quality, and other elements. Additionally, it could be challenging to compare the outcomes of various research due to the lack of established protocols for decellularization, recellularization, and assessment techniques. Finally, studies examining tissue engineering approaches based on decellularized pericardium have several disadvantages. These include small sample sizes, bias potential, and quick assessments. Furthermore, not all cellular residues from the decellularization procedure utilized to remove cellular components from the bovine pericardial patches may have been eliminated, which could possibly cause an immunological reaction in the recipient. Despite these difficulties, tissue engineering approaches based on decellularized pericardium show significant potential for enhancing patient outcomes in cardiac surgery and other applications. To thoroughly assess their potential advantages and hazards as well as to address the difficulties involved in implementing them in clinical practice, more study is required (71, 123–125).
In tissue engineering and regenerative medicine, a hotly debated question refers to the route that decellularized pericardium will take in the future. Decellularized pericardium has demonstrated a great potential as a scaffold for several procedures, such as conjunctival repair, vascular grafts, and tissue engineering of heart valves (71, 125, 126). Thousand possibilities to improve future of regenerative medicine, are now investigating in multiple area. One, is Enhancing the cell seeding procedure onto decellularized pericardium. When Amadeo et al. introduce the perfusion-assisted bioreactor technique, aortic valve cells seeding onto decellularized animal pericardium become more than a dream and open its way in the future (19). When contemplating the clinical use of decellularized pericardial patches, long-term healing results are critical. To understand the endurance and stability of decellularized bovine pericardial patches throughout time, Umashankar et al. examined the long-term healing of these patches (38). Santoro et al. also looked at the viability of utilizing aortic valve interstitial cells from pigs or humans on fixative-free decellularized animal pericardium. This study offers important insights on cell integration and compatibility with the scaffolding (16). Not only these revolutions could happen in cardiovascular surgery, but also hydrogels are the way to treat diseases, deliver drugs, heal wounds, etc. For all kind of problems from cardiovascular to musculoskeletal. Decellularized pericardial matrix gel in injectable form has also been created as prospective heart tissue engineering scaffolds (29). These injectable gels have benefits like minimally invasive delivery and capacity to conform to uneven heart shapes. Although decellularization methods are advance nowadays, but they are not perfect yet. Finding ways for decellularization that can improve mechanical characteristics, is one of the focus points. For instance, Kimicata et al. evaluated the usage of (polypropylene fumarate) biohybrid in combination with decellularized pericardial ECM for small-diameter vascular graft applications. The study's findings suggested its potential as a synthetic graft substitute by demonstrating superior mechanical characteristics and biocompatibility (32). This also can be followed by strategies that could encourage cell adhesion and growth, offering a viable remedy for ocular surface reconstruction. As showed by Crosslinked decellularized swine pericardium was investigated by Chen et al. as a substrate for conjunctival restoration (20). Additionally, in a pig carotid artery model, Chlupac et al. investigated vascular remodeling utilizing clinically used patches and recellularized decellularized pericardial matrices with autologous or allogeneic cells. According to the results, both autologous and allogeneic cell-seeded matrices exhibited favorable remodeling traits, pointing to their potential for use in vascular tissue engineering (39).
This article show how decellularized pericardium will be used in the future in a variety of biomedical domains, including cardiovascular tissue engineering and regenerative medicine. To fully realize the promise of this biomaterial, additional study is required to optimize production processes, improve mechanical qualities, increase long-term durability, and assess clinical outcomes.
8 Discussion
This review aimed to introduce and make the selection of decellularization methods simple for researchers by providing a complete list. But one question remains; which approach is the best? Surprisingly our answer is “we don’t know!” because it all depends on your study design, application, and purpose of your decellularized pericardium; you need better mechanical properties, better biocompatibility, better preservation of ECM or just it's important for you to cells completely being removed for your tissue? So, here we’re going to make it even more simple for you. Choosing the best approaches at first need a deep dive in the advantages and disadvantages of each method. Using Detergents (SDS, Triton X-100, SD, etc.) as one of the most common methods for decellularization have benefits like an effective removal of cells which make them to be used frequently and they are also inexpensive. But on the other side, they Can be harsh and potentially damage the ECM if not used carefully (about their concentration, timing and temperature) but when used correctly, they even can preserve collagen, elastin, and glycosaminoglycans (GAGs). And they may also require extensive washing to remove residual detergents that can damage our study model (especially important for the animal and human trials) (41, 127).
Another method for decellularization that were discussed was “enzymes” (DNase, RNase, PLA2, etc.). using enzymes to dissolve and remove exact components that we need, become popular within researchers to conduct exact experiments. These agents can effectively remove DNA and RNA, potentially preserving more delicate ECM components, so, it would be a great chose for situations that need the ECM to be finely preserved. But as an unwritten role, the more delicate work needs more money. They may also require optimization of enzyme concentration and incubation time which need an exact expert researcher (128, 129). One of the first methods that researchers find out that it could work; Physical Methods [High Hydrostatic Pressure (HHP), Freeze-Thawing, etc.]. they potentially have less damage to ECM compared to chemicals. But they require specialized equipment and, they may not be as effective in complete cell removal for thicker tissues, and they need other methods by their side (15, 41). Finally combined methods which discussed completely. Based on the agents that each methods contain they could have various pros and cons, but on the overall view, they can enhance decellularization efficacy while minimizing damage to the ECM, as the purpose of these methods (32).
As mentioned in Table 3, pericardium has been used as a suitable ECM source in repair, especially in cardiovascular tissues. Choosing the right pericardium source can have a great impact on the mechanical, biological, and functional properties of the final scaffold, which may also vary its application. Human pericardium is the best choice due to its structural similarity and high biocompatibility, but the challenges in using this tissue have limited its application, including limited availability compared to animal sources. However, despite the decellularization process in this tissue, it can be suitable for human grafts (130, 131). Bovine pericardium is one of the most widely used sources in the fabrication of heart valves and other tissue engineering scaffolds. The dense collagen structure increases resistance to mechanical stresses. However, it requires optimization processes in the decellularization process to reduce immunogenicity. Porcine pericardium is more like human pericardium and has found widespread use in tissue engineering. Due to the fibrous arrangement of collagen, it is more flexible than bovine pericardium (132, 133). Some studies have also reported interesting results comparing equine and bovine pericardium, pointing to the better use of equine pericardium because it is more flexible in cardiac applications (134).
Based on these comparisons we could conclude some point that may help researcher to choose the best method based on their work.
• For preserving mechanical properties while achieving decellularization: HHP combined with mild chemical treatments appears promising. HHP can disrupt cells while causing less damage to the ECM than harsh chemicals.
• For effective removal of cellular components: A combination of detergents like SDS and TX is highly effective.
• For cardiovascular applications: The TRITDOC method has been developed as a holistic approach to heart valve tissue engineering with a focus on ECM preservation and removal of cellular components and is a more advanced method compared to just using SDS/TX. Perfusion-assisted bioreactors are also very effective in seeding cells into decellularized scaffolds in cardiovascular applications.
• For applications where toxicity is a concern: Freeze-thawing is a good option. This is because it avoids the use of chemical agents.
• For applications requiring preservation of delicate ECM components or where harsh chemicals are a concern: methods involving enzymes or physical methods (or a combination) may be preferred.
Conclusion
Tissue engineering's investigation of decellularized pericardium has opened exciting new possibilities for the creation of cutting-edge biomedical products. Decellularized pericardium has several qualities that make it an attractive option for tissue engineering applications, including biocompatibility and biomechanical integrity. We highlight its efficaciousness in promoting cell adhesion, proliferation, and differentiation, thereby aiding in the development of functional tissue constructs. Even with the significant advancements this analysis points out, we nevertheless need to recognize the information gaps that remain. Subsequent investigations ought to concentrate on streamlining decellularization processes, improving scaffold characteristics, and investigating novel approaches to augment integration with host tissues. To confirm the safety and effectiveness of decellularized pericardium-based constructions in practical applications, including long-term biocompatibility tests and clinical trials research are necessary. In conclusion, the use of decellularized pericardium in tissue engineering is a rapidly developing field that has the potential to revolutionize regenerative medicine in near future with further research.
Author contributions
PS: Investigation, Validation, Writing – original draft. MK: Investigation, Writing – original draft. EG: Investigation, Software, Validation, Writing – original draft. MR: Investigation, Writing – original draft. LR: Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank the Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran. This study was carried out under the approval code IR.KUMS.REC.1403.090 at Kermanshah University of Medical Sciences, Kermanshah, Iran.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ECM, extracellular matrix; SDS, sodium dodecyl sulphate; SD, sodium deoxycholate; PBS, phosphate-buffered saline; SMC, smooth muscle cell; HUVEC, human umbilical vein endothelial cell; EDTA, ethylenediaminetetraacetic acid, hMSC, human mesenchymal stem cell; VIC, valve interstitial cell; AVICs, aortic valve interstitial cells; RT, room temperature; TX, Triton X-100; dH2O, deionized water; PLA2, phospholipase A2; TRITDOC, TRIton-X100 and TauroDeOxyCholic acid; DNase, Deoxyribonuclease; RNase, Ribonuclease; Tris, Trisaminomethane; HCL, Hydrochloric acid; NaN3, Sodium azide; D.D.W., double distilled water; CHAPS, ((3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate); TS, Trypsin; TSD, Triton X-100 and sodium-deoxycholate; GA, glutaraldehyde; AI, artificial intelligence; ML, machine learning.
References
1. Hubbell JA. Tissue and cell engineering. Curr Opin Biotechnol. (2004) 15(5):381–2. doi: 10.1016/j.copbio.2004.08.015
2. Banfi G, Corsi MM. Regenerative medicine. J Biol Regul Homeost Agents. (2011) 25(2 Suppl):S1.22051165
3. Pecha S, Eschenhagen T, Reichenspurner H. Myocardial tissue engineering for cardiac repair. J Heart Lung Transplant. (2016) 35(3):294–8. doi: 10.1016/j.healun.2015.12.007
4. Vunjak-Novakovic G. Tissue engineering of the heart: an evolving paradigm. J Thorac Cardiovasc Surg. (2017) 153(3):593–5. doi: 10.1016/j.jtcvs.2016.08.057
5. Khademhosseini A. Experimental approaches to tissue engineering. J Vis Exp. (2007) 7:272. doi: 10.3791/272
6. Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, et al. Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int. (2018) 2018:2495848. doi: 10.1155/2018/2495848
7. Barbulescu GI, Buica TP, Goje ID, Bojin FM, Ordodi VL, Olteanu GE, et al. Optimization of complete rat heart decellularization using artificial neural networks. Micromachines (Basel). (2022) 13(1):79. doi: 10.3390/mi13010079
8. Wollmann L, Suss P, Mendonça J, Luzia C, Schittini A, Rosa G, et al. Characterization of decellularized human pericardium for tissue engineering and regenerative medicine applications. Arq Bras Cardiol. (2019) 113(1):11–7. doi: 10.5935/abc.20190094
9. Taylor AJ. The pericardium. Introduction. J Cardiovasc Comput Tomogr. (2013) 7(1):1–2. doi: 10.1016/j.jcct.2013.01.007
10. Birnbaum Y, Uretsky BF. How electrically silent is the pericardium? Heart. (2022) 108(18):1428–9. doi: 10.1136/heartjnl-2021-320728
11. De Santis MM, Alsafadi HN, Tas S, Bölükbas DA, Prithiviraj S, Da Silva IAN, et al. Extracellular-matrix-reinforced bioinks for 3D bioprinting human tissue. Adv Mater. (2021) 33(3):e2005476. doi: 10.1002/adma.202005476
12. Zhou Z, Huang Y, Liu H, Zhao G. 3D bioprinting of modified mannan bioink for tissue engineering. STAR Protoc. (2022) 3(3):101585. doi: 10.1016/j.xpro.2022.101585
13. Alizadeh M, Rezakhani L, Taghdiri Nooshabadi V, Alizadeh A. The effect of Scrophularia striata on cell attachment and biocompatibility of decellularized bovine pericardia. Cell Tissue Bank. (2022) 23(2):261–9. doi: 10.1007/s10561-021-09939-3
14. Mallis P, Michalopoulos E, Dimitriou C, Kostomitsopoulos N, Stavropoulos-Giokas C. Histological and biomechanical characterization of decellularized porcine pericardium as a potential scaffold for tissue engineering applications. Biomed Mater Eng. (2017) 28(5):477–88. doi: 10.3233/BME-171689
15. Suzuki M, Kimura T, Yoshida Y, Kobayashi M, Hashimoto Y, Takahashi H, et al. In vitro tissue reconstruction using decellularized pericardium cultured with cells for ligament regeneration. Polymers (Basel). (2022) 14(12):2351. doi: 10.3390/polym14122351
16. Funamoto S, Hashimoto Y, Kishida A, Negishi J. A fibrin-coated pericardial extracellular matrix prevented heart adhesion in a rat model. J Biomed Mater Res B Appl Biomater. (2019) 107(4):1088–94. doi: 10.1002/jbm.b.34201
17. Khazaei M, Alizadeh M, Rezakhani L. Resveratrol-loaded decellularized ovine pericardium: ECM introduced for tissue engineering. Biotechnol Appl Biochem. (2023) 71:387–401. doi: 10.1002/bab.2547
18. Montagner G, Barbazza A, Lugas AT, Terzini M, Serino G, Bignardi C, et al. Decellularized cryopreserved human pericardium: a validation study towards tissue bank practice. Cell Tissue Bank. (2023) 25:401–10. doi: 10.1007/s10561-023-10072-6
19. Amadeo F, Boschetti F, Polvani G, Banfi C, Pesce M, Santoro R. Aortic valve cell seeding into decellularized animal pericardium by perfusion-assisted bioreactor. J Tissue Eng Regen Med. (2018) 12(6):1481–93. doi: 10.1002/term.2680
20. Murin P, Weixler VHM, Kuschnerus K, Romanchenko O, Lorenzen V, Nordmeyer J, et al. Pulmonary artery augmentation using decellularized equine pericardium (matrix patch™): initial single-centre experience. Eur J Cardiothorac Surg. (2021) 60(5):1094–101. doi: 10.1093/ejcts/ezab183
21. Xiao Z, Zhang E, Li Y, Chen H, Lai Y. Immunogenicity of acellular bovine pericardium in vivo. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. (2005) 22(6):1203–5.16422100
22. Bozso SJ, Kang JJH, El-Andari R, Boe D, Hedtke H, Moon MC, et al. Recellularized bovine pericardium with autologous mesenchymal stem cells reduces immune activation. Xenotransplantation. (2022) 29(6):e12774. doi: 10.1111/xen.12774
23. Kimicata M, Allbritton-King JD, Navarro J, Santoro M, Inoue T, Hibino N, et al. Assessment of decellularized pericardial extracellular matrix and poly(propylene fumarate) biohybrid for small-diameter vascular graft applications. Acta Biomater. (2020) 110:68–81. doi: 10.1016/j.actbio.2020.04.013
24. Jannasch A, Rix J, Welzel C, Schackert G, Kirsch M, König U, et al. Brillouin confocal microscopy to determine biomechanical properties of SULEEI-treated bovine pericardium for application in cardiac surgery. Clin Hemorheol Microcirc. (2021) 79(1):179–92. doi: 10.3233/CH-219119
25. Dohmen PM, da Costa F, Lopes SV, Vilani R, Bloch O, Konertz W. Successful implantation of a decellularized equine pericardial patch into the systemic circulation. Med Sci Monit Basic Res. (2014) 20:1–8. doi: 10.12659/MSMBR.889915
26. Daniele E, Ferrari B, Rassu N, Ben-Nun J, Bosio L, Barbaro V, et al. Comparison of human amniotic membrane decellularisation approaches for hESC-derived RPE cells culture. BMJ Open Ophthalmol. (2022) 7(1):e000981. doi: 10.1136/bmjophth-2022-000981
27. Grauss RW, Hazekamp MG, Oppenhuizen F, van Munsteren CJ, Gittenberger-de Groot AC, DeRuiter MC. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg. (2005) 27(4):566–71. doi: 10.1016/j.ejcts.2004.12.052
28. Chen F, Deng J, Luo L, Zhu Y, Dong Y, Yang Y, et al. Crosslinked decellularized porcine pericardium as a substrate for conjunctival reconstruction. Stem Cells Int. (2022) 2022:7571146. doi: 10.1155/2022/7571146
29. Umashankar PR, Sabareeswaran A, Shenoy SJ. Long-term healing of mildly cross-linked decellularized bovine pericardial aortic patch. J Biomed Mater Res B Appl Biomater. (2017) 105(7):2145–52. doi: 10.1002/jbm.b.33755
30. Buratto E, Gastaldello A, Dal Lin C, Tarzia V, Naso F, Bottio T, et al. Structural, morphological and hydrodynamic characterisation of taurodeoxycholate decellularised porcine heart valves: a holistic approach to heart valve tissue engineering. Heart Lung Circ. (2011) 20:S229. doi: 10.1016/j.hlc.2011.05.563
31. Iop L, Bonetti A, Naso F, Rizzo S, Cagnin S, Bianco R, et al. Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PLoS One. (2014) 9(6):e99593. doi: 10.1371/journal.pone.0099593
32. Aguiari P, Iop L, Favaretto F, Fidalgo CM, Naso F, Milan G, et al. In vitro comparative assessment of decellularized bovine pericardial patches and commercial bioprosthetic heart valves. Biomed Mater. (2017) 12(1):015021. doi: 10.1088/1748-605X/aa5644
33. Santoro R, Consolo F, Spiccia M, Piola M, Kassem S, Prandi F, et al. Feasibility of pig and human-derived aortic valve interstitial cells seeding on fixative-free decellularized animal pericardium. J Biomed Mater Res B Appl Biomater. (2016) 104(2):345–56. doi: 10.1002/jbm.b.33404
34. Belikov NV, Pushkarev AV, Tsiganov DI, Khaydukova IV, Gafarova ER, Korneev AA, et al. Freeze-thaw sheep pericardium decellularization without detergents: a pilot study. Materialia. (2023) 32:101909. doi: 10.1016/j.mtla.2023.101909
35. Filova E, Steinerova M, Travnickova M, Knitlova J, Musilkova J, Eckhardt A, et al. Accelerated in vitro recellularization of decellularized porcine pericardium for cardiovascular grafts. Biomed Mater. (2021) 16(2):025024. doi: 10.1088/1748-605X/abbdbd
36. Ostdiek AM, Ivey JR, Hansen SA, Gopaldas R, Grant SA. Feasibility of a nanomaterial-tissue patch for vascular and cardiac reconstruction. J Biomed Mater Res B Appl Biomater. (2016) 104(3):449–57. doi: 10.1002/jbm.b.33410
37. Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ, Hu DP, Christman KL. Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering. Tissue Eng Part A. (2010) 16(6):2017–27. doi: 10.1089/ten.tea.2009.0768
38. Santoro R, Consolo F, Spiccia M, Piola M, Kassem S, Prandi F, et al. Feasibility of pig and human-derived aortic valve interstitial cells seeding on fixative-free decellularized animal pericardium. J Biomed Mater Res B Appl Biomater. (2016) 104(2):345–56. doi: 10.1002/jbm.b.33404
39. Chlupac J, Matejka R, Konarik M, Novotny R, Simunkova Z, Mrazova I, et al. Vascular remodeling of clinically used patches and decellularized pericardial matrices recellularized with autologous or allogeneic cells in a porcine carotid artery model. Int J Mol Sci. (2022) 23(6):3310. doi: 10.3390/ijms23063310
40. Kajbafzadeh AM, Tafti SHA, Khorramirouz R, Sabetkish S, Kameli SM, Orangian S, et al. Evaluating the role of autologous mesenchymal stem cell seeded on decellularized pericardium in the treatment of myocardial infarction: an animal study. Cell Tissue Bank. (2017) 18(4):527–38. doi: 10.1007/s10561-017-9629-2
41. Li N, Li Y, Gong DJ, Xia CP, Liu XH, Xu ZY. Efficient decellularization for bovine pericardium with extracellular matrix preservation and good biocompatibility. Interact Cardiovasc Thorac Surg. (2018) 26(5):768–76. doi: 10.1093/icvts/ivx416
42. Albahrawy M, Abouelnasr K, Mosbah E, Zaghloul A, Abass M. Biostimulation effect of platelet-rich fibrin augmented with decellularized bovine pericardium on full-thickness cutaneous wound healing in donkeys (equus asinus). BMC Vet Res. (2023) 19(1):166. doi: 10.1186/s12917-023-03733-x
43. Ariganello MB, Simionescu DT, Labow RS, Lee JM. Macrophage differentiation and polarization on a decellularized pericardial biomaterial. Biomaterials. (2011) 32(2):439–49. doi: 10.1016/j.biomaterials.2010.09.004
44. Mogaldea A, Theodoridis K, Goecke T, Tudorache I, Haverich A, Cebotari S, et al. Assessment of cytocompatibility and mechanical properties of detergent-decellularized ovine pericardial tissue. Int J Artif Organs. (2019) 42(11):628–35. doi: 10.1177/0391398819850583
45. Yang M, Chen CZ, Shu YS, Shi WP, Cheng SF, Gu YJ. Preseeding of human vascular cells in decellularized bovine pericardium scaffold for tissue-engineered heart valve: an in vitro and in vivo feasibility study. J Biomed Mater Res B Appl Biomater. (2012) 100B(6):1654–61. doi: 10.1002/jbm.b.32734
46. Matejka R, Konarík M, Stepanovská J, Lipensky J, Chlupác J, Turek D, et al. Bioreactor processed stromal cell seeding and cultivation on decellularized pericardium patches for cardiovascular use. Appl Sci Basel. (2020) 10(16):5613. doi: 10.3390/app10165613
47. Alizadeh M, Rezakhani L, Khodaei M, Soleimannejad M, Alizadeh A. Evaluating the effects of vacuum on the microstructure and biocompatibility of bovine decellularized pericardium. J Tissue Eng Regen Med. (2021) 15(2):116–28. doi: 10.1002/term.3150
48. Megerle K, Woon C, Kraus A, Raghavan S, Pham H, Chang J. Flexor tendon sheath engineering using decellularized porcine pericardium. Plast Reconstr Surg. (2016) 138(4):630E–41E. doi: 10.1097/PRS.0000000000002459
49. Morticelli L, Magdei M, Tschalaki N, Petersen B, Haverich A, Hilfiker A. Generation of glycans depleted decellularized porcine pericardium, using digestive enzymatic supplements and enzymatic mixtures for food industry. Xenotransplantation. (2021) 28(6):e12705. doi: 10.1111/xen.12705
50. Yang M, Chen CZ, Wang XN, Zhu YB, Gu YJ. Favorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valves. J Biomed Mater Res B Appl Biomater. (2009) 91B(1):354–61. doi: 10.1002/jbm.b.31409
51. Alizadeh M, Rezakhani L, Soleimannejad M, Sharifi E, Anjomshoa M, Alizadeh A. Evaluation of vacuum washing in the removal of SDS from decellularized bovine pericardium: method and device description. Heliyon. (2019) 5(8):e02253. doi: 10.1016/j.heliyon.2019.e02253
52. Giannini C, Terzi A, Fusaro L, Sibillano T, Diaz A, Ramella M, et al. Scanning x-ray microdiffraction of decellularized pericardium tissue at increasing glucose concentration. J Biophotonics. (2019) 12(10):e201900106. doi: 10.1002/jbio.201900106
53. Ariganello MB, Labow RS, Lee JM. In vitro response of monocyte-derived macrophages to a decellularized pericardial biomaterial. J Biomed Mater Res A. (2010) 93A(1):280–8. doi: 10.1002/jbm.a.32554
54. Zouhair S, Aguiari P, Lop L, Vasquez-Rivera A, Filippi A, Romanato F, et al. Preservation strategies for decellularized pericardial scaffolds for off-the-shelf availability. Acta Biomater. (2019) 84:208–21. doi: 10.1016/j.actbio.2018.10.026
55. Bai M, Zhang T, Ling T, Zhou Z, Xie H, Zhang W, et al. Guided bone regeneration using acellular bovine pericardium in a rabbit mandibular model: in vitro and in vivo studies. J Periodontal Res. (2014) 49(4):499–507. doi: 10.1111/jre.12129
56. Jalili-Firoozinezhad S, Rajabi-Zeleti S, Marsano A, Aghdami N, Baharvand H. Influence of decellularized pericardium matrix on the behavior of cardiac progenitors. J Appl Polym Sci. (2016) 133(14). doi: 10.1002/app.43255
57. Zhu D, Jin L, Wang X, Xu L, Liu T. Combined anticalcification treatment of bovine pericardium with decellularization and hyaluronic acid derivative. Biomed Mater Eng. (2014) 24(1):741–9. doi: 10.3233/BME-130862
58. Mathapati S, Bishi DK, Venugopal JR, Cherian KM, Guhathakurta S, Ramakrishna S, et al. Nanofibers coated on acellular tissue-engineered bovine pericardium supports differentiation of mesenchymal stem cells into endothelial cells for tissue engineering. Nanomedicine (Lond). (2014) 9(5):623–34. doi: 10.2217/nnm.13.76
59. Wang X, Wang K, Zhang W, Qiang M, Luo Y. A bilaminated decellularized scaffold for islet transplantation: structure, properties and functions in diabetic mice. Biomaterials. (2017) 138:80–90. doi: 10.1016/j.biomaterials.2017.05.033
60. Pagoulatou E, Triantaphyllidou IE, Vynios DH, Papachristou DJ, Koletsis E, Deligianni D, et al. Biomechanical and structural changes following the decellularization of bovine pericardial tissues for use as a tissue engineering scaffold. J Mater Sci Mater Med. (2012) 23(6):1387–96. doi: 10.1007/s10856-012-4620-8
61. Gálvez-Montón C, Bragós R, Soler-Botija C, Díaz-Güemes I, Prat-Vidal C, Crisóstomo V, et al. Noninvasive assessment of an engineered bioactive graft in myocardial infarction: impact on cardiac function and scar healing. Stem Cells Transl Med. (2017) 6(2):647–55. doi: 10.5966/sctm.2016-0063
62. Rajabi-Zeleti S, Jalili-Firoozinezhad S, Azarnia M, Khayyatan F, Vahdat S, Nikeghbalian S, et al. The behavior of cardiac progenitor cells on macroporous pericardium-derived scaffolds. Biomaterials. (2014) 35(3):970–82. doi: 10.1016/j.biomaterials.2013.10.045
63. Tedder ME, Liao J, Weed B, Stabler C, Zhang H, Simionescu A, et al. Stabilized collagen scaffolds for heart valve tissue engineering. Tissue Eng Part A. (2009) 15(6):1257–68. doi: 10.1089/ten.tea.2008.0263
64. Alizadeh M, Rezakhani L, Alizadeh A. Characterization of the decellularized ovine pericardium for skin tissue engineering. J Shahrekord Univ Med Sci. (2020) 22(4):173–80. doi: 10.34172/jsums.2020.28
65. Kanda H, Oya K, Irisawa T, Goto M. Tensile strength of ostrich carotid artery decellularized with liquefied dimethyl ether and DNase: an effort in addressing religious and cultural concerns. Arab J Chem. (2023) 16(4):104578. doi: 10.1016/j.arabjc.2023.104578
66. Jang WS, Kim YJ, Kim S-H. Effects on tensile strength and elasticity after treatment with glutaraldehyde, solvent, decellularization and detoxification in fresh bovine pericardium. Korean J Thorac Cardiovasc Surg. (2010) 43:1–10. doi: 10.5090/kjtcs.2010.43.1.1
67. Sheridan WS, Duffy GP, Murphy BP. Mechanical characterization of a customized decellularized scaffold for vascular tissue engineering. J Mech Behav Biomed Mater. (2012) 8:58–70. doi: 10.1016/j.jmbbm.2011.12.003
68. Roy S, Silacci P, Stergiopulos N. Biomechanical properties of decellularized porcine common carotid arteries. Am J Physiol Heart Circ Physiol. (2005) 289(4):H1567–76. doi: 10.1152/ajpheart.00564.2004
69. Li Y, Zhang Y, Zhang G. Comparative analysis of decellularization methods for the production of decellularized umbilical cord matrix. Curr Issues Mol Biol. (2024) 46(7):7686–701. doi: 10.3390/cimb46070455
70. Kostelnik C, Hohn J, Escoto-Diaz CE, Kooistra JB, Stern M, Swinton DE, et al. Small-diameter artery decellularization: effects of anionic detergent concentration and treatment duration on porcine internal thoracic arteries. J Biomed Mater Res B Appl Biomater. (2022) 110(4):885–97. doi: 10.1002/jbm.b.34969
71. Hussein K, Korossis S, Iop L. Editorial: tissue and organ decellularization strategies in regenerative medicine; recent advances, current translational challenges, and future directions. Front Bioeng Biotechnol. (2023) 11:1201041. doi: 10.3389/fbioe.2023.1201041
72. Shomura Y, Okada Y, Nasu M, Koyama T, Yuzaki M, Murashita T, et al. Late results of mitral valve repair with glutaraldehyde-treated autologous pericardium. Ann Thorac Surg. (2013) 95(6):2000–5. doi: 10.1016/j.athoracsur.2013.02.024
73. García-Rinaldi R. Tricuspid anterior leaflet replacement with autologous pericardium and polytetrafluoroethylene chordae, followed by edge-to-edge repair. Tex Heart Inst J. (2007) 34(3):310–2.
74. Deutsch O, Bruehl F, Cleuziou J, Prinzing A, Schlitter AM, Krane M, et al. Histological examination of explanted tissue-engineered bovine pericardium following heart valve repair. Interact Cardiovasc Thorac Surg. (2020) 30(1):64–73. doi: 10.1093/icvts/ivz234
75. Kawase I, Ozaki S, Yamashita H, Uchida S, Nozawa Y, Matsuyama T, et al. Aortic valve reconstruction of unicuspid aortic valve by tricuspidization using autologous pericardium. Ann Thorac Surg. (2012) 94(4):1180–4. doi: 10.1016/j.athoracsur.2012.05.016
76. Ozaki S, Kawase I, Yamashita H, Uchida S, Nozawa Y, Takatoh M, et al. Reconstruction of bicuspid aortic valve with autologous pericardium—usefulness of tricuspidization. Circ J. (2014) 78(5):1144–51. doi: 10.1253/circj.CJ-13-1335
77. Ozaki S, Kawase I, Yamashita H, Uchida S, Nozawa Y, Takatoh M, et al. A total of 404 cases of aortic valve reconstruction with glutaraldehyde-treated autologous pericardium. J Thorac Cardiovasc Surg. (2014) 147(1):301–6. doi: 10.1016/j.jtcvs.2012.11.012
78. Khazaei M, Rahmati S, Khazaei MR, Rezakhani L. Accelerated wound healing with resveratrol-loaded decellularized pericardium in mice model. Cell Tissue Bank. (2024) 25(1):245–53. doi: 10.1007/s10561-023-10117-w
79. Baharuddin A, Go BT, Firdaus MN, Abdullah J. Bovine pericardium for dural graft: clinical results in 22 patients. Clin Neurol Neurosurg. (2002) 104(4):342–4. doi: 10.1016/S0303-8467(02)00029-X
80. Laun A, Tonn JC, Jerusalem C. Comparative study of lyophilized human dura mater and lyophilized bovine pericardium as dural substitutes in neurosurgery. Acta Neurochir (Wien). (1990) 107(1-2):16–21. doi: 10.1007/BF01402607
81. Filippi R, Schwarz M, Voth D, Reisch R, Grunert P, Perneczky A. Bovine pericardium for duraplasty: clinical results in 32 patients. Neurosurg Rev. (2001) 24(2-3):103–7. doi: 10.1007/PL00012392
82. Akbari MR, Mirmohammadsadeghi A, Mahmoudzadeh R, Veisi A. Management of thyroid eye disease-related strabismus. J Curr Ophthalmol. (2020) 32(1):1–13. doi: 10.1016/j.joco.2019.10.002
83. Ashena Z, Holmes C, Nanavaty MA. Pericardium patch graft for severe corneal wound burn. J Curr Ophthalmol. (2021) 33(3):342–4. doi: 10.4103/joco.joco_195_20
84. Wishart PK, Choudhary A, Wong D. Ahmed glaucoma valves in refractory glaucoma: a 7-year audit. Br J Ophthalmol. (2010) 94(9):1174–9. doi: 10.1136/bjo.2009.165357
85. Quaranta L, Riva I, Floriani IC. Outcomes of using a sutureless bovine pericardial patch graft for ahmed glaucoma valve implantation. Eur J Ophthalmol. (2013) 23(5):738–42. doi: 10.5301/ejo.5000260
86. Gupta M, Puri P, Rennie IG. Use of bovine pericardium as a wrapping material for hydroxyapatite orbital implants. Br J Ophthalmol. (2002) 86(3):288–9. doi: 10.1136/bjo.86.3.288
87. Koay AC, Yew YH, Ngo CT, Loo VP, Intan G, Chua CN. The use of preserved bovine pericardium for emergency temporizing graft in corneal perforation. Med J Malaysia. (2008) 63(5):421–2.19803308
88. Stavropoulos A, Chiantella G, Costa D, Steigmann M, Windisch P, Sculean A. Clinical and histologic evaluation of a granular bovine bone biomaterial used as an adjunct to GTR with a bioresorbable bovine pericardium collagen membrane in the treatment of intrabony defects. J Periodontol. (2011) 82(3):462–70. doi: 10.1902/jop.2010.100331
89. Rothamel D, Schwarz F, Fienitz T, Smeets R, Dreiseidler T, Ritter L, et al. Biocompatibility and biodegradation of a native porcine pericardium membrane: results of in vitro and in vivo examinations. Int J Oral Maxillofac Implants. (2012) 27(1):146–54.22299091
90. Limpert JN, Desai AR, Kumpf AL, Fallucco MA, Aridge DL. Repair of abdominal wall defects with bovine pericardium. Am J Surg. (2009) 198(5):e60–5. doi: 10.1016/j.amjsurg.2009.01.027
91. Mandal TK, Dhanuka S, Choudhury S, Mukhopadhyay BC, Kayal A, Majhi TK, et al. Tissue engineered indigenous pericardial patch urethroplasty: a promising solution to a nagging problem. Asian J Urol. (2020) 7(1):56–60. doi: 10.1016/j.ajur.2019.05.001
92. Bagherpour R, Bagherpour G, Mohammadi P. Application of artificial intelligence in tissue engineering. Tissue Engineering Part B: Reviews. (2025) 31(1):31–43. doi: 10.1089/ten.teb.2024.0022
93. Bonciog D-D, Ordodi VL, Lascu M-R, Mâţiu-Iovan L. eds. Automation of decellularization process using artificial neural networks. 2023 17th International Conference on Engineering of Modern Electric Systems (EMES) (2023). IEEE.
94. Wu Y, Ding X, Wang Y, Ouyang D. Harnessing the power of machine learning into tissue engineering: current progress and future prospects. Burns Trauma. (2024) 12:tkae053. doi: 10.1093/burnst/tkae053
95. Shin J, Lee Y, Li Z, Hu J, Park SS, Kim K. Optimized 3D bioprinting technology based on machine learning: a review of recent trends and advances. Micromachines (Basel). (2022) 13(3):363. doi: 10.3390/mi13030363
96. Robles-Bykbaev Y, Naya S, Díaz-Prado S, Calle-López D, Robles-Bykbaev V, Garzón L, et al. An artificial-vision-and statistical-learning-based method for studying the biodegradation of type I collagen scaffolds in bone regeneration systems. PeerJ. (2019) 7:e7233. doi: 10.7717/peerj.7233
97. Azimi SM, Britz D, Engstler M, Fritz M, Mücklich F. Advanced steel microstructural classification by deep learning methods. Sci Rep. (2018) 8(1):2128. doi: 10.1038/s41598-018-20037-5
98. Gao W, Wang C, Li Q, Zhang X, Yuan J, Li D, et al. Application of medical imaging methods and artificial intelligence in tissue engineering and organ-on-a-chip. Front Bioeng Biotechnol. (2022) 10:985692. doi: 10.3389/fbioe.2022.985692
99. Peri-Guard, Synovis Life Technologies Inc. (2020). Available online at: https://advancedsurgery.baxter.com/ (Accessed October 16, 2024).
100. Strange G, Brizard C, Karl TR, Neethling L. An evaluation of Admedus’ tissue engineering process-treated (ADAPT) bovine pericardium patch (CardioCel) for the repair of cardiac and vascular defects. Expert Rev Med Devices. (2015) 12(2):135–41. doi: 10.1586/17434440.2015.985651
101. Edwards. Bovine pericardial patch. Available online at: http://www.edwards.com
102. Auto Tissue Berlin GmbH. Matrix PatchTM. Available online at: http://www.autotissue.de
103. BioIntegral Surgical Inc. No-react patch. Available online at: https://www.biointegral-surgical.com
104. Baxter. Peri-Guard. (2020). Available online at: http://ecatalog.baxter.com/ (Accessed October 16, 2024).
105. Neovasc Inc. News Release—Neovasc Inc. Receives CE Mark for Peripatch-BV Bovine Pericardial Tiussue. (2011). Available online at: http://www.neovasc.com (Accessed October 16, 2024).
106. CryoLife. PhotoFix Decellularized Bovine Pericardium. (2015). Available online at: https://www.cryolife.com (Accessed October 16, 2024).
107. Vascutek OEM. Porcine Pericardial Patch. Available online at: http://oem.vascutek.com
108. St. Jude Medical. SJM BiocorTM Patch Bovine Pericardium Repair Patch. Available online at: https://www.sjmglobal.com
109. St. Jude Medical. SJMTM Pericardial Patch with EnCapTM AC Technology. Available online at: https://www.sjm.com
110. FOC Medical. Bovine Pericardium Patches SURGIFOC. Available online at: http://focmedical.com
111. Tissue Regenix Group PLC. dCELL vascular patch. (2014). Available online at: https://www.tissueregenix.com (Accessed October 16, 2024).
112. Baxter. Vascu-Guard. (2021). Available online at: http://ecatalog.baxter.com (Accessed October 16, 2024).
113. Fitzpatrick LE, McDevitt TC. Cell-derived matrices for tissue engineering and regenerative medicine applications. Biomater Sci. (2015) 3(1):12–24. doi: 10.1039/C4BM00246F
114. Ebrahimi Sadrabadi A, Baei P, Hosseini S, Baghaban Eslaminejad M. Decellularized extracellular matrix as a potent natural biomaterial for regenerative medicine. Adv Exp Med Biol. (2021) 1341:27–43. doi: 10.1007/5584_2020_504
115. Lamas JR, García-Fernández C, Tornero-Esteban P, Lópiz Y, Rodriguez-Rodriguez L, Ortega L, et al. Adverse effects of xenogenic scaffolding in the context of a randomized double-blind placebo-controlled study for repairing full-thickness rotator cuff tears. Trials. (2019) 20(1):387. doi: 10.1186/s13063-019-3504-3
116. Nwaejike N, Mosca R, Hooper TL, Soon SY. Surgical repair of pulmonary vein injury from blunt trauma. Ann R Coll Surg Engl. (2015) 97(3):e34–6. doi: 10.1308/003588414X14055925059714
117. van Hoefen Wijsard M, Haan M, Rietveld E, van Rijn LJ. Donor sclera versus bovine pericardium as patch graft material in glaucoma implant surgery and the impact of a drainage suture. Acta Ophthalmol. (2018) 96(7):692–8. doi: 10.1111/aos.13721
118. Connolly RJ. Evaluation of a unique bovine collagen matrix for soft tissue repair and reinforcement. Int Urogynecol J Pelvic Floor Dysfunct. (2006) 17(Suppl 1):S44–7. doi: 10.1007/s00192-006-0098-6
119. Hu M, Peng X, Zhao Y, Yu X, Cheng C, Yu X. Dialdehyde pectin-crosslinked and hirudin-loaded decellularized porcine pericardium with improved matrix stability, enhanced anti-calcification and anticoagulant for bioprosthetic heart valves. Biomater Sci. (2021) 9(22):7617–35. doi: 10.1039/D1BM01297E
120. Nguyen DT, O’Hara M, Graneli C, Hicks R, Miliotis T, Nyström A-C, et al. Humanizing miniature hearts through 4-flow cannulation perfusion decellularization and recellularization. Sci Rep. (2018) 8(1):7458. doi: 10.1038/s41598-018-25883-x
121. Barbulescu GI, Bojin FM, Ordodi VL, Goje ID, Buica TP, Gavriliuc OI, et al. Innovative biotechnology for generation of cardiac tissue. Appl Sci. (2021) 11(12):5603. doi: 10.3390/app11125603
122. Nordmeyer S, Murin P, Schulz A, Danne F, Nordmeyer J, Kretzschmar J, et al. Results of aortic valve repair using decellularized bovine pericardium in congenital surgery. Eur J Cardiothorac Surg. (2018) 54(6):986–92. doi: 10.1093/ejcts/ezy181
123. Lux M, Haller R, Giere B, Lindner B, Harder M, Mastrobuoni S, et al. Advantages and challenges in processing and quality control of decellularized heart valves. Cell Tissue Bank. (2023) 25(1):43–53. doi: 10.1007/s10561-023-10092-2
124. Şeker Ş, Elçin AE, Elçin YM. Advances in regenerative medicine and biomaterials. Methods Mol Biol. (2023) 2575:127–52. doi: 10.1007/978-1-0716-2716-7_7
125. Spector M. Decellularized tissues and organs: an historical perspective and prospects for the future. Biomed Mater. (2016) 11(2):020201. doi: 10.1088/1748-6041/11/2/020201
126. Yadav S, Khan J, Yadav A. Applications of scaffolds in tissue engineering: current utilization and future prospective. Curr Gene Ther. (2023) 24(2):94–109. doi: 10.2174/0115665232262167231012102837
127. El-Husseiny HM, Mady EA, Kaneda M, Shimada K, Nakazawa Y, Usui T, et al. Comparison of bovine- and porcine-derived decellularized biomaterials: promising platforms for tissue engineering applications. Pharmaceutics. (2023) 15(7):1906. doi: 10.3390/pharmaceutics15071906
128. Perea-Gil I, Uriarte JJ, Prat-Vidal C, Gálvez-Montón C, Roura S, Llucià-Valldeperas A, et al. In vitro comparative study of two decellularization protocols in search of an optimal myocardial scaffold for recellularization. Am J Transl Res. (2015) 7(3):558–73.26045895
129. Stieglmeier F, Grab M, König F, Büch J, Hagl C, Thierfelder N. Mapping of bovine pericardium to enable a standardized acquirement of material for medical implants. J Mech Behav Biomed Mater. (2021) 118:104432. doi: 10.1016/j.jmbbm.2021.104432
130. Straka F, Schornik D, Masin J, Filova E, Mirejovsky T, Burdikova Z, et al. A human pericardium biopolymeric scaffold for autologous heart valve tissue engineering: cellular and extracellular matrix structure and biomechanical properties in comparison with a normal aortic heart valve. J Biomater Sci Polym Ed. (2018) 29(6):599–634. doi: 10.1080/09205063.2018.1429732
131. Vinci MC, Tessitore G, Castiglioni L, Prandi F, Soncini M, Santoro R, et al. Mechanical compliance and immunological compatibility of fixative-free decellularized/cryopreserved human pericardium. PLoS One. (2013) 8(5):e64769. doi: 10.1371/journal.pone.0064769
132. El-Husseiny HM, Mady EA, Kaneda M, Shimada K, Nakazawa Y, Usui T, et al. Comparison of bovine-and porcine-derived decellularized biomaterials: promising platforms for tissue engineering applications. Pharmaceutics. (2023) 15(7):1906. doi: 10.3390/pharmaceutics15071906
133. Zouhair S, Dal Sasso E, Tuladhar SR, Fidalgo C, Vedovelli L, Filippi A, et al. A comprehensive comparison of bovine and porcine decellularized pericardia: new insights for surgical applications. Biomolecules. (2020) 10(3):371. doi: 10.3390/biom10030371
Keywords: pericardium, decellularization, regeneration, extracellular matrix, machine learning
Citation: Shariat Rad P, Khazaei M, Ghanbari E, Rashidi M and Rezakhani L (2025) Recent advances in pericardium extracellular matrix for tissue regeneration, along with a short insight into artificial intelligence. Front. Med. Technol. 7:1503153. doi: 10.3389/fmedt.2025.1503153
Received: 28 September 2024; Accepted: 21 July 2025;
Published: 14 August 2025;
Corrected: 18 August 2025.
Edited by:
Sonia Malik, Université d'Orléans, FranceReviewed by:
Maziar Malekzadeh Kebria, Hamadan University of Medical Sciences, IranUbaid Tariq, Indian Institute of Technology Kanpur, India
Copyright: © 2025 Shariat Rad, Khazaei, Ghanbari, Rashidi and Rezakhani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leila Rezakhani, bGVpbGEucmV6YWtoYW5pQGt1bXMuYWMuaXI=;bGVpbGFfcmV6YWtoYW5pQHlhaG9vLmNvbQ==
 Parand Shariat Rad
Parand Shariat Rad Mozafar Khazaei3,4
Mozafar Khazaei3,4 Elham Ghanbari
Elham Ghanbari Mehdi Rashidi
Mehdi Rashidi Leila Rezakhani
Leila Rezakhani