- 1Department of Biomedical Engineering, ZetrOZ Systems, LLC, Trumbull, CT, United States
- 2Department of Biopsychology, The University of California, Santa Barbara, CA, United States
- 3Department of Biomedical Engineering, The University of Connecticut, Storrs, CT, United States
- 4Department of Pharmacy, Compound Solutions, Inc., Monroe, CT, United States
Background: Sustained Acoustic Medicine (SAM) is a non-invasive long-term wearable device that delivers localized long duration high-frequency continuous ultrasound. SAM's biomechanical and diathermic stimuli enhance local circulation and oxygenation, accelerate tissue healing, and alleviate pain. The sonophoresis effects of SAM further improve transdermal drug delivery. Diclofenac is a topical Nonsteroidal anti-inflammatory drug for treating localized musculoskeletal (MSK) pain. Its efficacy is significantly dependent on skin porosity. This study aims to determine the diathermic effects of SAM and diclofenac on localized blood circulation.
Methods: Sixty-four healthy participants were randomly assigned to four groups (Active SAM group: n = 32, Placebo SAM group: n = 32): (A) Coupling gel + placebo SAM), (B) Coupling gel + active SAM, (C) 2.5% Diclofenac gel + placebo SAM, and (D) 2.5% Diclofenac gel + active SAM. Both forearms were treated with a placebo and active SAM devices for 1 h. The blood flux (perfusion units, PU) and temperature (degrees centigrade) change were recorded at 10 min intervals for 60 min using high-laser-power Doppler flowmetry. Blood circulation and temperature were recorded and reported (Clinical trial Identifier: NCT06510062).
Results: SAM increased blood flow significantly over 60 min by 19.2 PU (p < 0.0001) with coupling patch and 18.6 PU with 2.5% diclofenac patch (p < 0.0001) vs. placebo. Surface level tissue temperature increased by Δ2.4°C (p < 0.0001) with gel coupling patch and Δ2.2°C (p < 0.0001) with 2.5% diclofenac patch vs. placebo ultrasound treatment (p < 0.0001). There was no significant difference between standard coupling ultrasound gel and 2.5% diclofenac gel in blood flow and temperature. SAM provided a significant temperature increase at 20 min and a circulation increase at 10 min, which remained for the duration of the 60 min. All participants completed the study with no adverse events.
Conclusion: SAM treatment significantly increases local blood circulation after 10 min, increases temperature after 20 min, and sustains the effects of SAM's stimulation. The 2.5% diclofenac gel does not affect SAM's biological effects to increase local circulation. The study concludes that the application of diclofenac does not affect the diathermic properties of SAM exposure while enhancing the efficacy of diclofenac delivery through sonophoresis.
Clinical Trial Registration: identifier NCT06510062.
Introduction
Musculoskeletal (MSK) disorders, which affect approximately 1.71 billion people globally, are a leading cause of disability and economic burden, with their prevalence steadily increasing due to the aging population. These conditions account for an estimated 0.2% of the global gross domestic product (GDP) (1, 2). In the U.S. alone, the annual cost of treating MSK disorders exceeds $125 billion (3). Beyond the economic impact, MSK disorders are linked to cardiovascular diseases, chronic depression, insomnia, and a diminished quality of life.
Blood circulation plays an essential role in treating MSK disorders. Enhanced blood flow (hyperemia) improves tissue oxygenation, metabolic activity, and the clearance of damaged tissue (4). Studies have shown that increased tissue temperature correlates with localized hyperemia (5–7) Low-intensity ultrasound (US) generates acoustic mechanical waves, inducing mechanical and thermal forces that travel through a medium. The propagation of acoustic waves through biological tissue induces tissue, cellular, molecular, and genetic responses. The biomechanical forces induce the alignment of the extracellular matrix, activating transmembrane integrin, ion channels, receptors, and downstream pathways (6, 8). US has been shown to enhance endothelial nitric oxide (eNOS) synthase activity, increasing localized production of nitric oxide, which is known to increase blood flow, smooth muscle relaxation, and vasodilation (9, 10). US increases localized muscle temperature, leading to increase blow flow Long-duration continuous ultrasound serves as an effective modality to stimulate localized mechanical and thermal effects, promoting vasodilation, cellular proliferation, inflammation resolution, tissue regeneration, and pain relief (11–16).
Sustained Acoustic Medicine (SAM) is an FDA-approved, noninvasive, wearable medical device delivering localized ultrasound therapy. SAM operates at 3 MHz and 132 mW/cm2, providing 18,700 joules of energy over a four-hour treatment duration (17, 18). The therapy induces vigorous diathermic effects (>4°C over local temperature), increasing localized tissue temperature, accelerating healing, and alleviating MSK pain (7, 19–23). SAM's acoustic ultrasound waves generate biomechanical forces, compression and rarefaction, which form localized cavitation bubbles in the skin and enhance its permeability (24). This diametric effect loosens the extracellular matrix, further improving transdermal drug delivery (25, 26).
Diclofenac sodium, a cyclooxygenase (COX1 and COX2) inhibitor, is a widely used non-steroidal anti-inflammatory drug (NSAID) for treating chronic inflammatory and degenerative MSK conditions (27–29). The inhibition of COX1 suppresses the expression of prostaglandin E2, a key modulator of inflammation and pain. COX2 upregulates inflammation and tissue degradation by activating inflammatory pathways (27, 30, 31). Additionally, diclofenac modulates cytokine expression, decreasing levels of pro-inflammatory mediators such as tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β), further contributing to its analgesic and anti-inflammatory properties (27, 29, 32). However, oral diclofenac often results in systemic side effects, including gastrointestinal, cardiovascular, and neurological complications (29). Topical formulations of diclofenac are safer with limited efficacy due to poor skin permeability (33). This single-blind, placebo-controlled study aims to evaluate the impact of SAM-induced hyperemia and diathermic effects on the transdermal delivery of 2.5% diclofenac ultrasound gel compared to standard ultrasound gel.
Materials and methods
Participants
Sixty-seven (n = 67) healthy adults (male and female) between the ages of 18 to 50 were screened by a board-certified medical officer. Individuals were excluded if they did not meet age criteria, were pregnant or nursing, had a history of hypertension or cardiovascular disorders, had recently had coronary artery bypass graft surgery (within the past six months), had known allergic reactions to diclofenac or other NSAIDs, or had prior treatment for MSK disorders. Following this screening process, 64 participants were enrolled in the study.
Participants were recruited through flyers, social media, word of mouth, and collaborations with local physician practices. All volunteers underwent physical examinations, reviewed the Case Report Form (CRF), and informed consent with the research assistant and medical director for eligibility confirmation before enrollment.
Study design
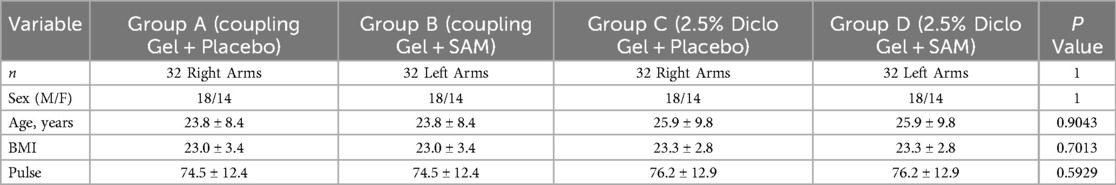
A voluntary, randomized, single-site, placebo-controlled study was conducted at ZetrOZ Systems (Trumbull, CT). Participants were randomized with a random number generator. The study was registered on ClinicalTrials.gov (Identifier: NCT06510062) and received approval from the Advarra Institutional Review Board (IRB) (Protocol ID: Pro00080714). All the participants signed a written informed consent form, and the study adhered to Good Clinical Practice (GCP) guidelines. Sixty-four (n = 64) healthy participants, male and female, (Table 1) were randomly assigned to four groups (n = 32): A) Coupling gel + placebo SAM), B) Coupling gel + active SAM, C) 2.5% Diclofenac gel + placebo SAM, and D) 2.5% Diclofenac gel + active SAM (Figure 1). All the female participants were not post-menopausal. For groups A and C, placebo treatments were administered to the left forearm, while active SAM treatments were applied to the right forearm in a contralateral design. For groups B and D, placebo treatments were administered to the right forearm, while active SAM treatments were applied to the left forearm (Figure 2). The treatment side was selected by flipping a coin.
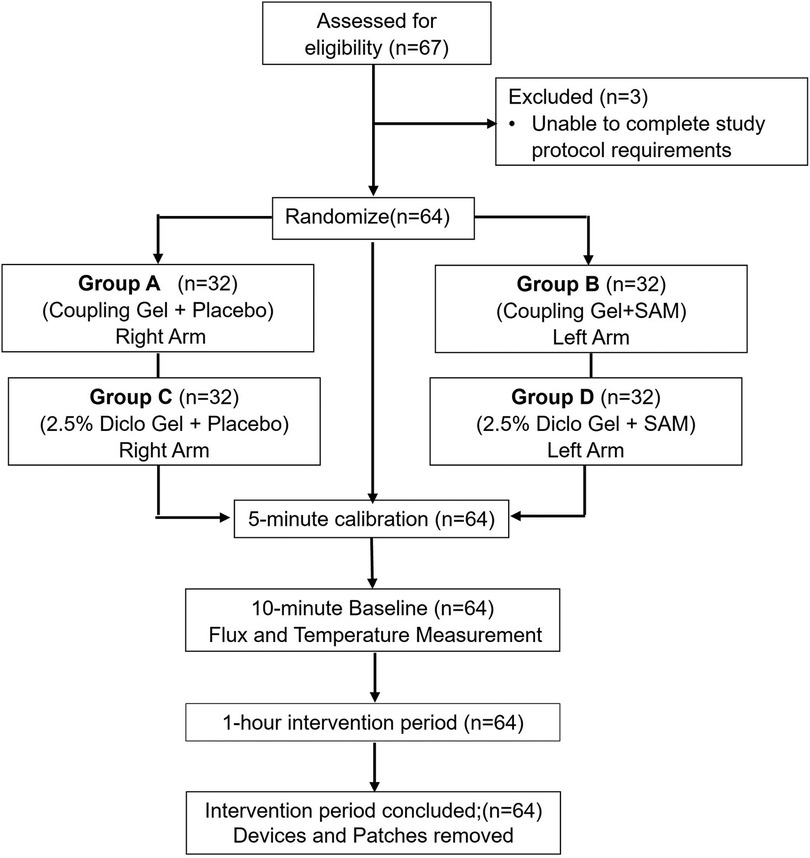
Figure 1. CONSORT flow diagram of study inclusion/exclusion test subjects, randomization, calibration, baseline, and intervention period.
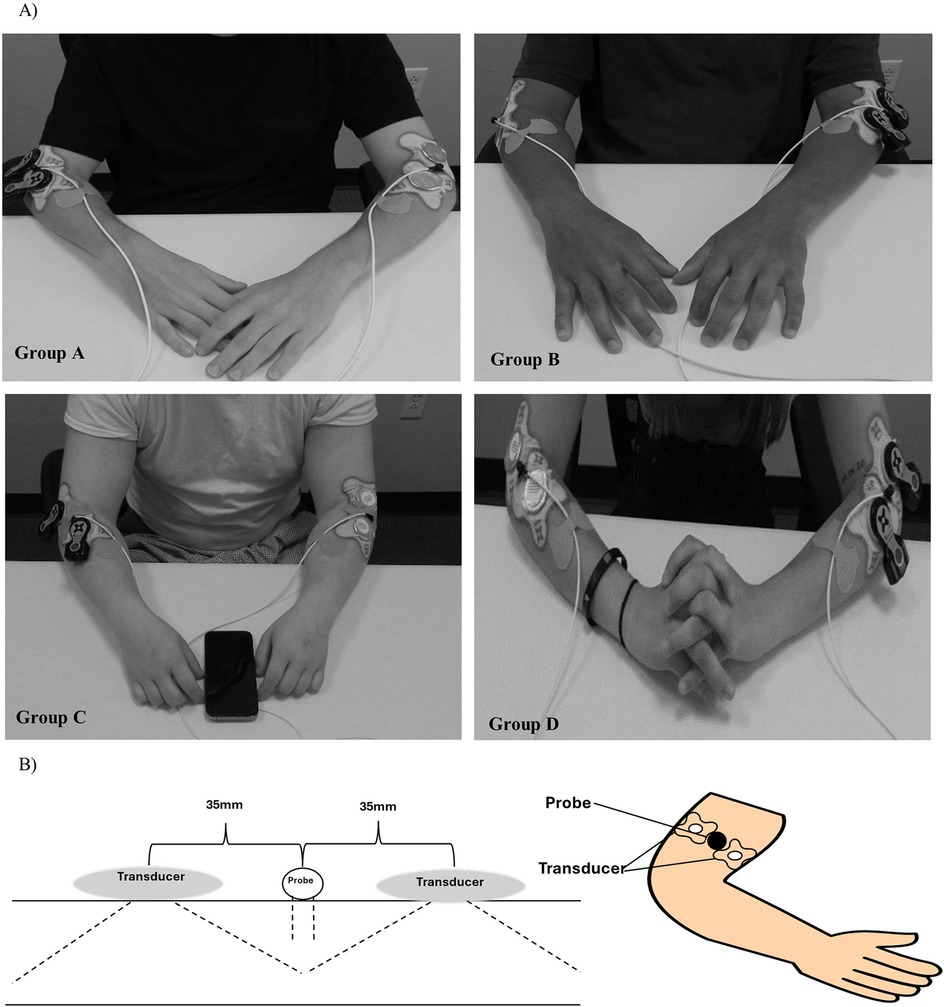
Figure 2. Sustained acoustic medicine (SAM) stimulation setup: (A) application to lateral forearm position for all four study groups (A–D), (B) graphic illustration of SAM x1 and probe positioning.
Participants were instructed to refrain from strenuous exercise and only indulge in day-to-day activities. The study was conducted during daily working hours (9 am—5 pm EST) in a well-controlled environment at 20°C. Participants were seated comfortably in an upright position for 15 min before and throughout the 60 min stimulation period (Figure 2). A high-power Laser Doppler Flowmetry (LDF) probe was positioned 2.5 cm distal to the elbow, between the two SAM x1 transducers, to monitor changes in blood circulation and skin temperature during stimulation. Patches were placed over the cables 5 cm equidistant from the probe's laser location to minimize any excess movement from the LDF device. Participants were checked every 15 min to ensure comfort and were permitted to watch pre-selected television shows or listen to music during the procedure. Each participant received $100 compensation at the completion of the study.
Continuous data acquisition was performed using the LDF software provided by the manufacturer during a 5 min calibration period, a 10 min baseline period, and a subsequent 60 min stimulation period. Measurements were recorded at 10 min intervals throughout the entire baseline and stimulation periods for analysis. No data was analyzed during the 5 min calibration period.
Instrumentation
The study utilized the dual SAM x1 device and four ultrasound coupling patches (two per forearm) to deliver active or placebo ultrasound treatment for one hour per subject. All the devices were new and calibrated prior to application. The patches were prefilled with 3 ml of coupling gel containing either standard coupling ultrasound gel or 2.5% diclofenac gel (Compounded Solutions, Monroe, CT).
A high-power Laser Doppler Flowmetry (LDF) system (VMS-LDF2-HP, Moor Instruments, UK), connected to non-invasive skin probes (VP2-V2-HP), was employed to measure real-time circulation and skin temperature. The system operated with a 785 nm, 20 mW laser, and probes were calibrated with polystyrene latex particle suspensions before each session, following manufacturer instructions. The LDF system continuously recorded data during the 1 h, 15 min protocol.
Circulation was quantified as flux perfusion units (PU), representing the red blood cell movement rate at 1–3 mm below the skin's surface, alongside temperature in degrees Celsius. Data was analyzed using moorVMS-PC research software and exported into Excel for biostatistical analysis.
This study, supported by Minority Health and Health Disparities (Project ID: MD015912), assessed the mechanotransduction performance of the wireless SAM x1 device (model SA551) with diclofenac and aqueous coupling gels.
Statistical analysis
A statistical analysis was conducted to evaluate differences in blood flow and temperature between placebo and active treatment arms using coupling and 2.5% diclofenac gel. The primary factor analyzed was the SAM stimulation (active or placebo), with gel type (water-based or diclofenac gel) considered as an interaction factor. Raw data was aggregated, normalized in Microsoft Excel, and analyzed by an independent biostatistician using R programming.
Data analysis included baseline and treatment intervals measured every 10 min. A two-way ANOVA was employed to compare group means and assess the independent and interaction effects of SAM active or placebo treatment and aqueous or 2.5% diclofenac gel type. A post hoc test was used to determine p-values for the two-way ANOVA data. Descriptive statistics for demographic data were calculated using chi-square tests for categorical variables and t-tests for the means between the active and placebo groups at the 10 min intervals.
Statistical significance was defined as p < 0.05, with results presented as mean ± standard deviation (SD) unless stated otherwise. Confidence intervals (95%) and p-values were reported to provide additional statistical context. Graphical representations, including error bars denoting standard error of the mean (SEM), and data tables were generated using R and Excel.
Results
Subject enrollment demographics
Sixty-four participants were selected for the study after physical examination and randomly assigned to four groups (n = 32), applying for a contralateral study design. The demographic data shows no significant differences between sex, age, BMI, and pulse rate before the start of the stimulation (Table 1).
Circulation results
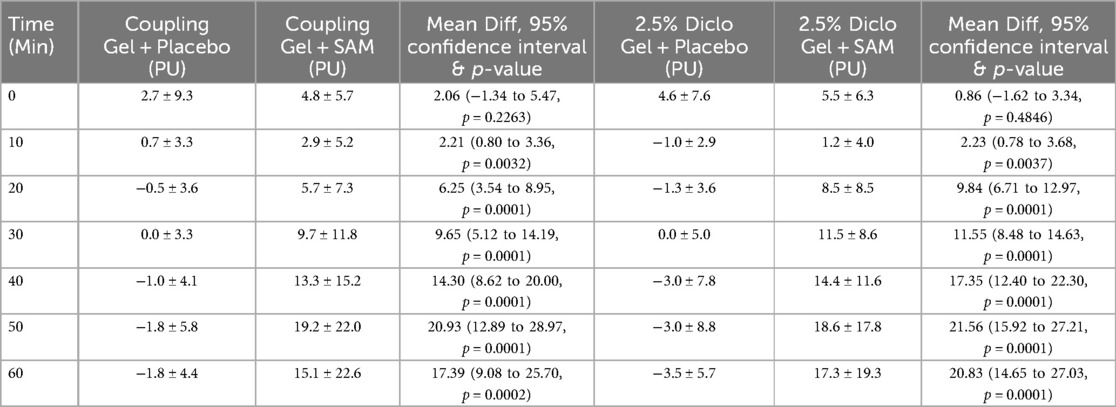
SAM stimulation significantly enhanced circulation and tissue perfusion during 60 min with standard coupling gel or 2.5% Diclofenac gel. With standard coupling gel, circulation increased to a maximum mean difference of 20.93 PU (95% CI: 12.89 to 28.87; p = 0.0001) compared to placebo at 50 min. Similarly, SAM with 2.5% Diclofenac gel achieved a maximum mean difference of 21.56 PU (95% CI: 15.92 to 27.21; p = 0.001) vs. placebo at 50 min (Table 2).
Both groups exhibited statistically significant increases in circulation compared to placebo at 10 min (Coupling Gel: 95% CI: 0.80 to 3.36; p = 0.0032; Diclofenac Gel: 95% CI: 0.78 to 3.68; p = 0.0037), with effects sustained throughout the duration of stimulation (Figure 3). No significant differences were observed between the two active SAM-stimulated groups, while placebo groups without SAM stimulation showed no significant changes in circulation (Figure 3). These findings show that SAM stimulation effectively enhances circulation, irrespective of the coupling medium.
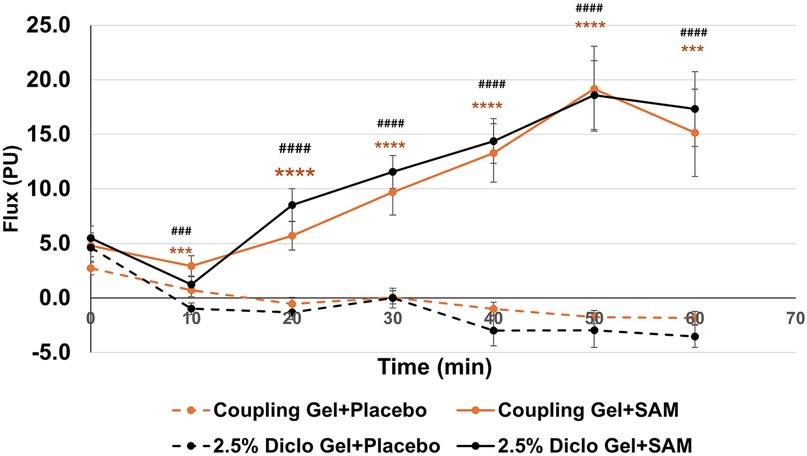
Figure 3. Normalized circulation is below lateral forearm skin averaged at each 10 min segment for baseline and stimulation periods. (*** p < 0.005, **** p < 0.0005 between Coupling Gel + Placebo and Coupling + SAM, ### p < 0.005, #### p < 0.0005 between 2.5% Diclofenac).
Temperature results
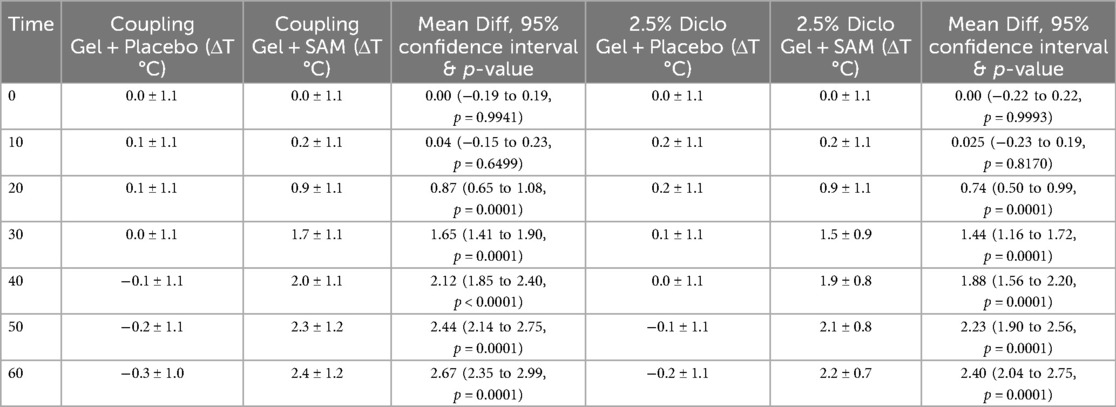
SAM treatment significantly increased tissue temperature over 60 min of stimulation compared to the placebo group. When paired with coupling gel, temperature increased by 2.67°C (95% CI: 2.35 to 2.99, p = 0.0001), while using 2.5% diclofenac gel resulted in a 2.40°C increase (95% CI: 2.04 to 2.75, p = 0.0001) (Table 3). Both SAM-treated groups exhibited a gradual rise in tissue temperature, with significant differences observed after 20 min of stimulation and sustained gradual increases through 60 min. No significant differences were noted between the coupling gel and diclofenac gel groups. The placebo groups did not show temperature change during the 60 min period (Figure 4).
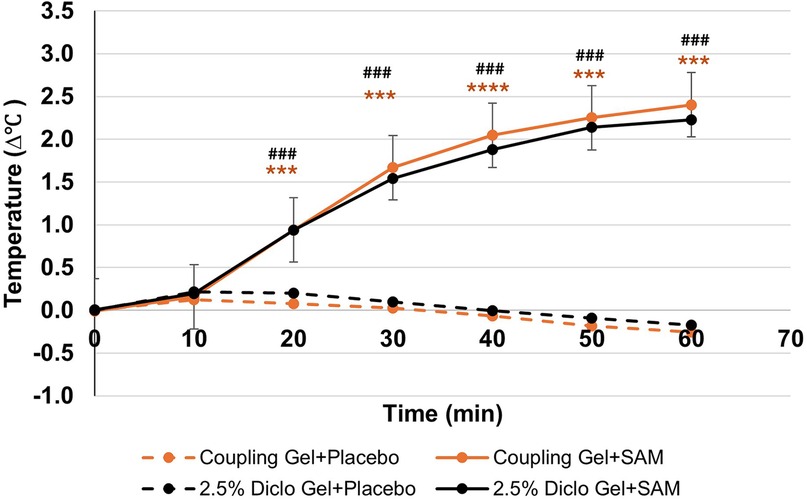
Figure 4. Normalized temperature change at lateral forearm skin averaged at each 10 min segment for baseline and stimulation periods. [*** p < 0.005, **** p < 0.0005 between Coupling Gel + Placebo and Coupling + SAM, ### p < 0.005, #### p < 0.0005 between 2.5% Diclofenac Gel + Placebo and Diclofenac + SAM, Error bar represent (SEM)]. SEM bars are present in placebo groups however they are not visible.
Total cumulative circulation results
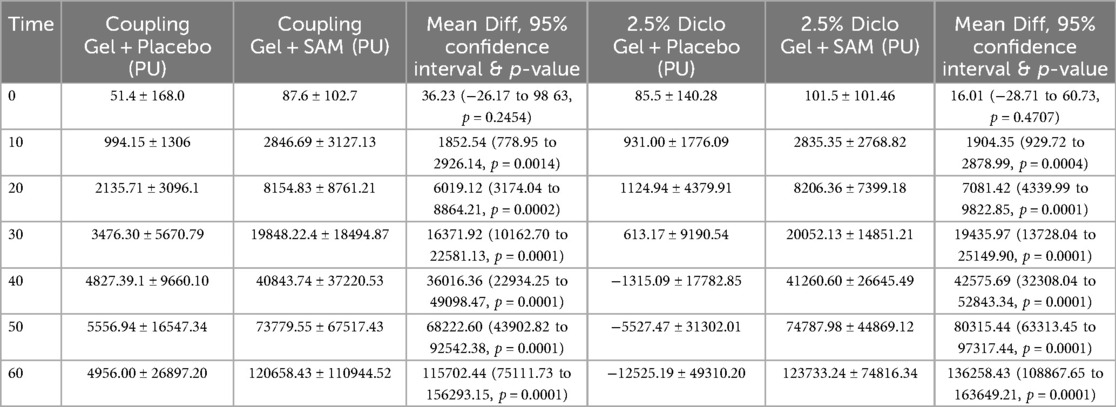
Temporal cumulative circulation showed a significant increase in both the Coupling Gel and 2.5% Diclofenac Gel groups during 60 min of SAM stimulation. Significant changes were observed as early as 10 min (Coupling Gel: 95% CI, 778.95 to 2926.14, p = 0.0014; 2.5% Diclofenac Gel: 95% CI, 929.72 to 2872.99, p = 0.0004) (Table 4). Over the stimulation period, both treatment groups demonstrated a rapid, sustained increase in blood flow, with mean differences of 116,000 PU (SAM + Coupling Gel) and 136,000 PU (SAM + 2.5% Diclofenac Gel) compared to the respective placebo treatments (p = 0.0001). Importantly, both active treatments showed similar sustained blood flow increases with SAM stimulation, whereas placebo groups exhibited no significant cumulative flux change over 60 min (Figure 5).
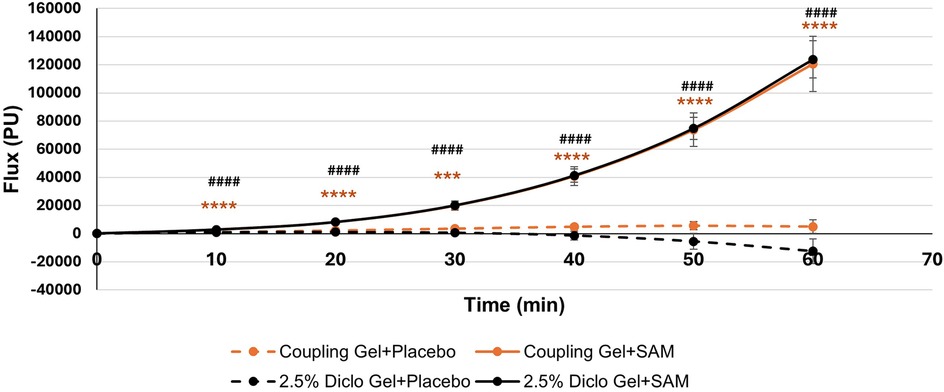
Figure 5. Cumulative circulation changes at the lateral forearm over 60 min treatment period. [*** p < 0.005, **** p < 0.0001 between Coupling Gel + Placebo and Coupling + SAM, ### p < 0.005, #### p < 0.0001 between 2.5% Diclofenac Gel + Placebo and Diclofenac + SA, Error bar represent (SEM)].
Discussion
Sustained Acoustic Medicine (SAM) stimulation significantly improves blood circulation and induces diathermic effects in soft tissue when applied with standard ultrasound coupling gel or 2.5% diclofenac gel during a 60 min session. Importantly, adding diclofenac sodium does not alter SAM's hyperemia or diathermic effects. Placebo groups exhibited no localized blood flow or temperature changes, confirming that these effects are directly attributable to SAM stimulation.
Managing musculoskeletal (MSK) disorders necessitate precise modulation of mechanical stress, angiogenesis, tissue perfusion, and inflammatory responses. These processes involve macrophages, neutrophils, mast cells, and cytokines such as interleukins and tumor necrosis factor-alpha (TNF-α) (34). Adequate blood flow is essential to regulate the treatment by ensuring the delivery of oxygen, nutrients, immune cells, and growth factors. Conversely, impaired blood flow or reduced angiogenesis can disrupt the balance between pro- and anti-inflammatory cytokines and reduce the expression of key angiogenic and vasodilatory mediators such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and endothelial nitric oxide synthase (eNOS) (35, 36), Biomechanical stimulation, localized shear and axial stress promotes the angiogenesis and blood flow by activating VEGF-2, FGF and eNOS (37–39). Mechanotransduction also regulates inflammatory mediators, including TNF-α, IL-1β, and macrophages (38, 40–43).
SAM provides targeted biomechanical forces (compression and rarefaction), providing the essential mechanical stimulus to upregulate the expression of VEGF, FDF, and eNOS over a long period of time, enhancing the angiogenesis, blood flow and regulating inflammatory responses (6, 20, 44). The diathermic effects of SAM include thermal stimuli that induce vasodilation, improve blood flow, and stimulate cellular metabolism, proliferation, and tissue regeneration (7, 17, 19). Hendren et al. demonstrated significant temperature increases at 1 cm, 2 cm, and 5 cm depths with SAM stimulation, retaining the high temperature above the therapeutic threshold deep into the tissue with little or no adverse effects (45). The biomechanical and thermal modulation of SAM treatment provides the essential stimuli to induce localized angiogenesis and enhanced blood flow to increase oxygen and nutrients and regulate the inflammatory factors to expedite tissue healing.
The regulation of the COX1/COX2 pathway has a crucial role in regulating inflammatory factors and MSK pain. COX1 is essential to retain tissue mucosal integrity and platelet aggregation, and Cox2 expression upregulates the inflammation such as IL-1β, TNF-α contributing to pain and tissue degradation (46). Diclofenac sodium, an anti-inflammatory agent, inhibits PGE2 and cytokines like IL-1β, TNF-α, and VEGF. Diclofenac sodium, a non-selective COX inhibitor, effectively reduces these inflammatory mediators. However, prolonged systemic administration is associated with gastrointestinal and renal side effects (47). Topical diclofenac application improves its safety profile, and its efficacy is limited by the low permeability of the stratum corneum (29, 31, 48). Biomechanical and thermal effects disrupt the skin's lipid bilayers, and losses in the skin's extracellular matrix increase skin porosity (49). Furthermore, US induced cavitation, formation and oscillation of microbubbles, allow the skin to enhance transdermal drug delivery (49–51). SAM long-duration acoustic stimulation enhances skin permeability, facilitating sustained transdermal drug delivery over a long duration. Masterson et al. observed an increase in vitro diclofenac delivery after 4 h of SAM stimulation (52). Madzia et al. reported significant reductions in knee osteoarthritis pain and improved mobility with SAM plus 1% diclofenac sodium (53). Jarit et al. demonstrated that a 4-week intervention using SAM with 2.5% diclofenac gel resulted in 99.3% of patients experiencing pain reduction and 97.8% showing improved function (54).
The study shows little or no significant change in the first 10 min of treatment, there is little change in tissue temperature as body adjust to the SAM stimulation, a significant rise was recorded between after 10 min of stimulation and continued over next 40 min (50 min after the start of treatment) as body start accumulate with the biomechanical and thermal stimulation. The change in blood flux followed the same pattern observed in the increase in the local temperature, exhibiting the relation between the temperature increase and blood circulation. The cumulative flux analysis shows that the quantity of blood flow over time significantly increases in the treated site relative to placebo sites. Placebo-treated sites did not show little or no temperature and flux change, indicating that change in blood circulation was due to the SAM treatment and not systemic physiological changes. The presence of 2.5% diclofenac sodium did not alter SAM's biomechanical or diathermic effects, demonstrating that diclofenac integration does not interfere with the therapeutic ultrasound mechanisms. Both US coupling gel and 2.5% diclofenac gel followed a similar pattern for active and placebo devices, demonstrating that the addition of diclofenac would not affect the SAM thermal and biomechanical effects and making an effective device to be applied for transdermal drug delivery.
Collectively, SAM combined with diclofenac sodium provides a dual-modality approach that leverages mechanical and thermal stimulation alongside pharmacological anti-inflammatory effects, offering a potent and non-invasive strategy for treating MSK disorders. Future research should evaluate the combined efficacy of SAM and topical diclofenac in broader clinical populations, including patients with knee osteoarthritis, low back pain, and other chronic MSK conditions. Furthermore, SAM efficacy with other NSAIDs would be evaluated, and data compared with diclofenac to determine the most effective combination to treat MSK disorders.
Conclusion
Continuous, long-duration ultrasound treatment using the SAMx1 device significantly enhances circulation to localized soft tissues. This study demonstrates that SAM effectively increases tissue perfusion with standard ultrasound coupling gel and 2.5% diclofenac gel. Adding diclofenac did not alter the hyperemic and diathermic effects observed throughout the stimulation period. Future studies will explore SAM's hyperemic and diathermic effects at additional anatomical locations and in populations with specific conditions and MSK disorders.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Advarra Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AS: Writing – original draft, Writing – review & editing. TH: Writing – original draft, Writing – review & editing. ME: Writing – original draft. JZ: Writing – original draft. MR: Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was partially supported by the National Institutes of Minority Health and Health Disparities (Project ID: MD015912).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors AS, TH, ME, JZ, and MR declared their affiliation with the manufacturer of the test device.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen N, Fong DYT, Wong JYH. Health and economic outcomes associated with musculoskeletal disorders attributable to high body mass index in 192 countries and territories in 2019. JAMA Netw Open. (2023) 6(1):e2250674. doi: 10.1001/jamanetworkopen.2022.50674
2. Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the global burden of disease study 2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2021) 396(10267):2006–17. doi: 10.1016/S0140-6736(20)32340-0
3. Gallagher S, Heberger JR. Examining the interaction of force and repetition on musculoskeletal disorder risk: a systematic literature review. Hum Factors. (2013) 55(1):108–24. doi: 10.1177/0018720812449648
4. Garnæs KK, Mørkved S, Tønne T, Furan L, Vasseljen O, Johannessen HH. Mental health among patients with chronic musculoskeletal pain and its relation to number of pain sites and pain intensity, a cross-sectional study among primary health care patients. BMC Musculoskelet Disord. (2022) 23(1):1115. doi: 10.1186/s12891-022-06051-9
5. Juopperi S, Sund R, Rikkonen T, Kröger H, Sirola J. Cardiovascular and musculoskeletal health disorders associate with greater decreases in physical capability in older women. BMC Musculoskelet Disord. (2021) 22(1):192. doi: 10.1186/s12891-021-04056-4
6. Uddin S, Komatsu D, Motyka T, Petterson S. Low-intensity continuous ultrasound Therapies-A systematic review of current state-of-the-art and future perspectives. J Clin Med. (2021) 10(12):2698. doi: 10.3390/jcm10122698
7. Petterson S, Plancher K, Klyve D, Draper D, Ortiz R. Low-intensity continuous ultrasound for the symptomatic treatment of upper shoulder and neck pain: a randomized, double-blind placebo-controlled clinical trial. J Pain Res. (2020) 13:1277–87. doi: 10.2147/JPR.S247463
8. Zhou J, Ning E, Lu L, Zhang H, Yang X, Hao Y. Effectiveness of low-intensity pulsed ultrasound on osteoarthritis: molecular mechanism and tissue engineering. Front Med. (2024) 11:1292473. doi: 10.3389/fmed.2024.1292473
9. Altland OD, Dalecki D, Suchkova VN, Francis CW. Low-intensity ultrasound increases endothelial cell nitric oxide synthase activity and nitric oxide synthesis. J Thromb Haemostasis. (2004) 2(4):637–43. doi: 10.1111/j.1538-7836.2004.00655.x
10. Grange rW, Isotani E, Lau KS, Kamm KE, Huang PL, Stull JT. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol Genomics. (2001) 5(1):35–44. doi: 10.1152/physiolgenomics.2001.5.1.35
11. Rubira APFDA, Rubira MC, Rubira LDA, Comachio J, Magalhães MO, Marques AP. Comparison of the effects of low-level laser and pulsed and continuous ultrasound on pain and physical disability in chronic non-specific low back pain: a randomized controlled clinical trial. Adv Rheumatol. (2019) 59(1):57. doi: 10.1186/s42358-019-0099-z
12. Chung JI, Min BH, Baik EJ. Effect of continuous-wave low-intensity ultrasound in inflammatory resolution of arthritis-associated synovitis. Phys Ther. (2016) 96(6):808–17. doi: 10.2522/ptj.20140559
13. Gallo JA, Draper DO, Brody LT, Fellingham GW. A comparison of human muscle temperature increases during 3-MHz continuous and pulsed ultrasound with equivalent temporal average intensities. J Orthop Sports Phys Ther. (2004) 34(7):395–401. doi: 10.2519/jospt.2004.34.7.395
14. Karnes JL, Burton HW. Continuous therapeutic ultrasound accelerates repair of contraction-induced skeletal muscle damage in rats. Arch Phys Med Rehabil. (2002) 83(1):1–4. doi: 10.1053/apmr.2002.26254
15. Ebadi S, Ansari NN, Henschke N, Naghdi S, van Tulder MW. The effect of continuous ultrasound on chronic low back pain: protocol of a randomized controlled trial. BMC Musculoskelet Disord. (2011) 12:59. doi: 10.1186/1471-2474-12-59
16. Morishita K, Karasuno H, Yokoi Y, MorozumI K, Ogihara H, Ito T, et al. Effects of therapeutic ultrasound on intramuscular blood circulation and oxygen dynamics. J Jpn Phys Ther Assoc. (2014) 17(1):1–7. doi: 10.1298/jjpta.Vol17_001
17. Langer MD, Lewis GK Jr. Sustained acoustic medicine: a novel long duration approach to biomodulation utilizing low intensity therapeutic ultrasound. Proc SPIE Int Soc Opt Eng. (2015):9467.
18. Draper DO. A comparison of traditional ultrasound with, sustained acoustic, medicine (SAM). Am J Biomed Sci & Res. (2019) 51(1):AJBSR.MS.ID.000880. doi: 10.34297/AJBSR.2019.05.000880
19. Rigby JH, Taggart RM, Stratton KL, Lewis GK, Draper DO. Intramuscular heating characteristics of multihour low-intensity therapeutic ultrasound. J Athl Train. (2015) 50(11):1158–64. doi: 10.4085/1062-6050-50.11.03
20. Draper DO. The benefits of long duration ultrasound. Biomed J Sci Tech Res. (2019) 18(4):13728–30. doi: 10.26717/BJSTR.2019.18.003177
21. Draper DO, Castel JC, Castel D. Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J Orthop Sports Phys Ther. (1995) 22(4):142–50. doi: 10.2519/jospt.1995.22.4.142
22. Draper DO, Edvalson CG, Knight KL, Eggett D, Shurtz J. Temperature increases in the human achilles tendon during ultrasound treatments with commercial ultrasound gel and full-thickness and half-thickness gel pads. J Athl Train. (2010) 45(4):333–7. doi: 10.4085/1062-6050-45.4.333
23. Lewis GK Jr., Langer MD, Henderson CR, Ortiz R. Design and evaluation of a wearable self-applied therapeutic ultrasound device for chronic myofascial pain. Ultrasound Med Biol. (2013) 39(8):1429–39. doi: 10.1016/j.ultrasmedbio.2013.03.007
24. Hendrickse P, Degens H. The role of the microcirculation in muscle function and plasticity. J Muscle Res Cell Motil. (2019) 40(2):127–40. doi: 10.1007/s10974-019-09520-2
25. Park D, Park H, Seo J, Lee S. Sonophoresis in transdermal drug deliverys. Ultrasonics. (2014) 54(1):56–65. doi: 10.1016/j.ultras.2013.07.007
26. Park J-H, Lee J-W, Kim Y-C, Prausnitz MR. The effect of heat on skin permeability. Int J Pharm. (2008) 359(1-2):94–103. doi: 10.1016/j.ijpharm.2008.03.032
27. Nair B, Taylor-Gjevre R. A review of topical diclofenac use in musculoskeletal disease. Pharmaceuticals. (2010) 3(6):1892–908. doi: 10.3390/ph3061892
30. Ku EC, Lee W, Kothari HV, Scholer DW. Effect of diclofenac sodium on the arachidonic acid cascade. Am J Med. (1986) 80(4B):18–23. doi: 10.1016/0002-9343(86)90074-4
31. Roth SH, Fuller P. Diclofenac topical solution compared with oral diclofenac: a pooled safety analysis. J Pain Res. (2011) 4:159–67. doi: 10.2147/JPR.S20965
32. Atzeni F, Masala IF, Sarzi-Puttini P. A review of chronic musculoskeletal pain: central and peripheral effects of diclofenac. Pain Ther. (2018) 7(2):163–77. doi: 10.1007/s40122-018-0100-2
33. Hagen M, Baker M. Skin penetration and tissue permeation after topical administration of diclofenac. Curr Med Res Opin. (2017) 33(9):1623–34. doi: 10.1080/03007995.2017.1352497
34. Gallo J, Raska M, Kriegova E, Goodman SB. Inflammation and its resolution and the musculoskeletal system. J Orthop Translat. (2017) 10:52–67. doi: 10.1016/j.jot.2017.05.007
35. Gerwins P, Skoldenberg E, Claesson-Welsh L. Function of fibroblast growth factors and vascular endothelial growth factors and their receptors in angiogenesis. Crit Rev Oncol Hematol. (2000) 34(3):185–94. doi: 10.1016/S1040-8428(00)00062-7
36. Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med. (2004) 36(6):707–17. doi: 10.1016/j.freeradbiomed.2003.11.032
37. Johnson BM, Johnson AM, Heim M, Buckley M, Mortimer B, Berry JL, et al. Biomechanical stimulation promotes blood vessel growth despite VEGFR-2 inhibition. BMC Biol. (2023) 21(1):290. doi: 10.1186/s12915-023-01792-y
38. Hoefer IE, den Adel B, Daemen MJ. Biomechanical factors as triggers of vascular growth. Cardiovasc Res. (2013) 99(2):276–83. doi: 10.1093/cvr/cvt089
39. Gimbrone MA Jr., Anderson KR, Topper JN. Special communicationthe critical role of mechanical forces in blood vessel development, physiology and pathology. J Vasc Surg. (1999) 29(6):1104–51. doi: 10.1016/S0741-5214(99)70252-1
40. Du H, Bartleson JM, Butenko S, Alonso V, Liu WF, Winer DA, et al. Tuning immunity through tissue mechanotransduction. Nat Rev Immunol. (2023) 23(3):174–88. doi: 10.1038/s41577-022-00761-w
41. Diaz MF, Vaidya AB, Evans SM, Lee HJ, Aertker BM, Alexander AJ, et al. Biomechanical forces promote immune regulatory function of bone marrow mesenchymal stromal cells. Stem Cells. (2017) 35(5):1259–72. doi: 10.1002/stem.2587
42. Uddin SMZ, Richbourgh B, Ding Y, Hettinghouse A, Komatsu DE, Qin Y-X, et al. Chondro-protective effects of low intensity pulsed ultrasound. Osteoarthr Cartil. (2016) 24(11):1989–98. doi: 10.1016/j.joca.2016.06.014
43. Huang RB, Eniola-Adefeso O. Shear stress modulation of IL-1beta-induced E-selectin expression in human endothelial cells. PLoS One. (2012) 7(2):e31874. doi: 10.1371/journal.pone.0031874
44. Walters R, Kasik J, Ettel C, Ortiz R. Evaluation of sustained acoustic medicine for treating musculoskeletal injuries in military and sports medicine. Open Orthop J. (2022) 16:1–11. doi: 10.2174/18743250-v16-e221130-2022-8
45. Hendren TF, Yeretzian NR, Bavanasi K. Clinical diathermy performance evaluation of multi-hour sustained acoustic medicine treatment with 2.5% diclofenac ultrasound coupling patch. Int J Phys Med Rehabil. (2023) 11(6):678.37692795
46. Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. (2000) 69:145–82. doi: 10.1146/annurev.biochem.69.1.145
47. Cooper C, Chapurlat R, Al-Daghri N, Herrero-Beaumont G, Bruyère O, Rannou F, et al. Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: what does the literature say? Drugs Aging. (2019) 36(1):15–24. doi: 10.1007/s40266-019-00660-1
48. Altman R, Barkin RL. Topical therapy for osteoarthritis: clinical and pharmacologic perspectives. Postgrad Med. (2009) 121(2):139–47. doi: 10.3810/pgm.2009.03.1986
49. Polat BE, Hart D, Langer R, Blankschtein D. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release. (2011) 152(3):330–48. doi: 10.1016/j.jconrel.2011.01.006
50. Tang H, Wang CCJ, Blankschtein D, Langer R. An investigation of the role of cavitation in low-frequency ultrasound-mediated transdermal drug transport. Pharm Res. (2002) 19(8):1160–9. doi: 10.1023/A:1019898109793
51. Li Y, Chen Z, Ge S. Sonoporation: underlying mechanisms and applications in cellular regulation. BIO Integr. (2021) 2:29–36. doi: 10.15212/bioi-2020-0028
52. Masterson J, Kluge B, Burdette A, Sr GL. Sustained acoustic medicine; sonophoresis for nonsteroidal anti-inflammatory drug delivery in arthritis. Ther Deliv. (2020) 11(6):363–72. doi: 10.4155/tde-2020-0009
53. Madzia A, Agrawal C, Jarit P, Petterson S, Plancher K, Ortiz R. Sustained acoustic medicine combined with A diclofenac ultrasound coupling patch for the rapid symptomatic relief of knee osteoarthritis: multi-site clinical efficacy study. Open Orthop J. (2020) 14:176–85. doi: 10.2174/1874325002014010176
Keywords: sustained acoustic medicine, diathermy, diclofenac, Laser Doppler Flowmetry, blood circulation, transdermal drug delivery, sonophoresis
Citation: Scanzuso A, Hendren T, Egmont M, Zarkar J and Roberge M (2025) Sustained acoustic medicine increases local circulation with a diclofenac delivery patch: a randomized placebo controlled study. Front. Med. Technol. 7:1552294. doi: 10.3389/fmedt.2025.1552294
Received: 7 January 2025; Accepted: 23 June 2025;
Published: 4 July 2025.
Edited by:
Shubhajit Roy Chowdhury, Indian Institute of Technology Mandi, IndiaReviewed by:
Syed Sajid Husain Kazmi, Amity University, IndiaHugo Sanchez, Independent Researcher, Jataí, Brasil
Copyright: © 2025 Scanzuso, Hendren, Egmont, Zarkar and Roberge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Roberge, bWljaGFlbHJvYmVyZ2Uuc2FtQGdtYWlsLmNvbQ==
 Anthony Scanzuso
Anthony Scanzuso Tabitha Hendren1
Tabitha Hendren1 Michael Roberge
Michael Roberge