- 1School of Engineering, RMIT University, Melbourne, VIC, Australia
- 2Electrical Engineering, Politeknik Negeri Bandung, Bandung, Indonesia
- 3Electrical Engineering, Universitas Surabaya, Surabaya, Indonesia
- 4Bolton Clarke Research Institute, Melbourne, VIC, Australia
- 5Department of Medicine, University of Melbourne, Parkville, VIC, Australia
- 6Australian Centre for Accelerating Diabetes Innovations (ACADI), Department of Medicine, University of Melbourne, Melbourne, VIC, Australia
- 7Department of Endocrinology, Austin Health, Heidelberg, VIC, Australia
Introduction: Early and accurate detection of diabetes-related foot ulcers (DFU) that may become chronic is essential to prevent long-term disability, amputation, and mortality. Various non-invasive imaging techniques have been developed to detect and monitor DFU progression, but none have yet been widely adopted in clinical practice. This review summarizes current advancements in non-invasive image techniques for DFU wound healing prediction and identifies research directions to support clinical translation.
Methods: A systematic, multi-disciplinary review was conducted focusing on three imaging methods: photographic, hyperspectral, and thermal imaging. Articles published between July 2014 and July 2024 were searched across five databases: PubMed, Scopus, CINAHL, Embase, and Web of Science. The search was limited to English-language, peer-reviewed journal articles. The review followed PRISMA guidelines and applied the CASP quality appraisal tool.
Results: The initial search identified 2,937 articles, of which 22 studies met the inclusion criteria, including 17 original studies (9 medical and 8 engineering) on DFU healing prediction using imaging techniques and 5 relevant review articles.
Discussion: Each imaging method offers specific benefits and faces unique limitations: photographic imaging is user-friendly but lighting-sensitive; thermal imaging reflects inflammation but requires multimodal integration; hyperspectral imaging provides biochemical insight but is costly and less portable. Visual and thermal imaging, in particular, demonstrate strong potential for early and real-time prediction when combined with machine learning/deep learning. These methods offer portability, ease of use, and potential for automated analysis on a single device, making them suitable for clinical and community settings. However, challenges such as standardization and integration complexity remain. Continued research with larger datasets and improved validation is needed to enhance clinical readiness.
1 Introduction
Diabetes mellitus is a chronic metabolic disorder characterized by elevated blood glucose levels, which can lead to several complications if not optimally managed. Among these, diabetes-related neuropathic foot ulcers pose a substantial public health concern (1–3). Recent reports show that the lifetime risk of developing a diabetes-related foot ulcer (DFU) among individuals with diabetes ranges from 19% to 34% (4). This number highlights the importance of preventive care and regular monitoring of DFUs as an integrative treatment in diabetes care. DFUs are a leading cause of non-traumatic lower limb amputation and significantly increase the risk of infection, reduce quality of life, and impose a substantial economic burden on both healthcare systems and people living with diabetes (5, 6).
The most common cause of foot ulcers in people living with diabetes is trauma in an insensate foot due to peripheral neuropathy (7). Currently, an estimated 20% of DFUs do not heal in the expected trajectory, increasing the risk of complications such as limb amputations (4). The current best clinical practice to identify whether a wound will become chronic, i.e., a wound that will not heal within 12 weeks, is based on the area of the wound being reduced by half in the first four weeks. However, there are several issues with this method. It requires waiting 4 weeks to ascertain wound healing ability, thereby delaying intervention and increasing the risk of complications developing. Further, wound area measurement is generally undertaken by tracing the wound using acetate, necessitating physical contact with the wound and increasing the risk of infection. In addition, a recent review of the literature has suggested that this approach has little evidence to support it (8).
Conventional imaging methods like Magnetic Resonance Imaging (MRI), Computed Tomography (CT), and Positron Emission Tomography (PET) are still used to check for infection or bone damage in DFU, but they are impractical for routine outpatient use or for predicting DFU healing trajectories (9). The limitations of these images include radiation exposure (in CT and PET), long scan times, and limited availability (10–13). Recent advancements in non-invasive imaging technologies have increased options to predict wound healing, with researchers reporting on various imaging techniques for assessing and monitoring DFUs. These include visual [Red Green Blue (RGB)] imaging, thermal imaging, and hyperspectral imaging modalities. While the focus of these technologies may vary, the main aim is to enable clinicians to accurately assess DFUs, including predicting the ability to heal in the normal trajectory. The results reported show that these imaging techniques, when used with analysis algorithms and software, were more accurate when compared to traditional paper-based documentation of wound assessment methods, which are based on measuring change in the area of the wound, and thus have the potential to enhance clinical care (14). While the research outcomes are very positive, none have yet been integrated into standard clinical practice. It is also not evident which of the imaging modalities are most suitable to be used in clinical, community, and home care services, and what the limitations are reported in the literature.
In clinical studies, advancements in nanoparticles and drug delivery systems (15–17) have been developed in chronic wound care. Raghunanth et al. (18), demonstrated a promising oral delivery system for insulin using chitosan-coated solid lipid nanoparticles (Ch-Ln-SLNs) with piperine, which significantly improved glycemic control and could potentially influence DFU healing. Sarma et al. (19) reviewed the use of electrospun nanofibers for wound dressing that supports controlled drug release and tissue regeneration for chronic wound therapy. Ahikiriza et al. (20) highlighted the therapeutic potential of Ugandan natural products for managing diabetes. Additionally, imaging techniques, such as thermal and hyperspectral imaging can non-invasively monitor the local wound response to such therapies by detecting changes in perfusion, oxygenation, and inflammation. The integration of advanced nanoparticles and imaging technologies like thermal and hyperspectral imaging could enhance diagnostic accuracy and therapeutic monitoring in chronic wounds, including diabetic foot ulcers.
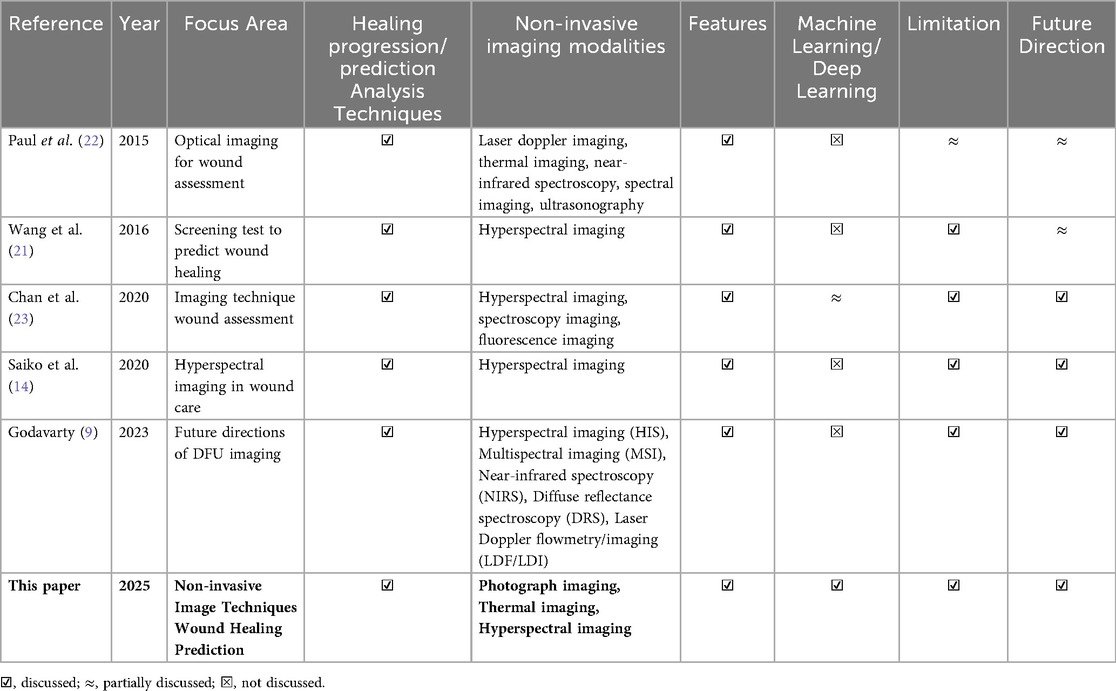
Existing studies primarily focus on broad imaging techniques but lack a targeted exploration of the potential to predict DFU wound healing outcomes in those technologies where this could be an effective application. A systematic review and meta-analysis of tools for predicting wound healing in DFUs by Wang et al. (21) reported that most studies up to October 2011 relied on transcutaneous oxygen measurement (TcPO2) and ankle-brachial index (ABI) as prognostic tools from hyperspectral imaging analysis. In 2015, Paul et al. (22) reviewed optical imaging technologies for wound assessment and highlighted the potential areas for future exploration. More recently, Chan et al. (14) briefly reviewed progress in wound assessment, imaging, and monitoring systems but did not specifically address DFU healing prediction. Another recent review by Saiko et al. (23) investigated 32 years of research on the use of Hyperspectral Imaging (HSI) in wound care, including DFU applications, but did not compare different imaging modalities for predictive effectiveness. Similarly, a recent narrative review by Godavarty et al. (9) provided an extensive overview of conventional and optical imaging techniques for DFU, including a novel spatiotemporal near-infrared (NIR) based approach. However, the review was not systematic and did not identify the most suitable imaging modality for DFU wound healing prediction. Overall, none of these reviews offer a comparative framework to identify a suitable path that will result in the adoption of imaging technologies to predict wound healing of DFU. Table 1 presents a comparative summary of our systematic review against previous literature.
This systematic, multi-disciplinary review aims to identify what is the most feasible imaging technique that can be used for predicting the healing status of DFU in clinical, community, and home care settings with a focus on three primary imaging methods: visual imaging (RGB), hyperspectral imaging, and thermal imaging. The review will address the following research questions:
• What are the strengths and weaknesses of the imaging techniques for predicting the healing status of diabetes-related foot ulcers?
• How do different wound characteristics captured by non-invasive imaging compare for developing healing prediction models?
• Is research in this field mature for the into clinical practice?
2 Methodology
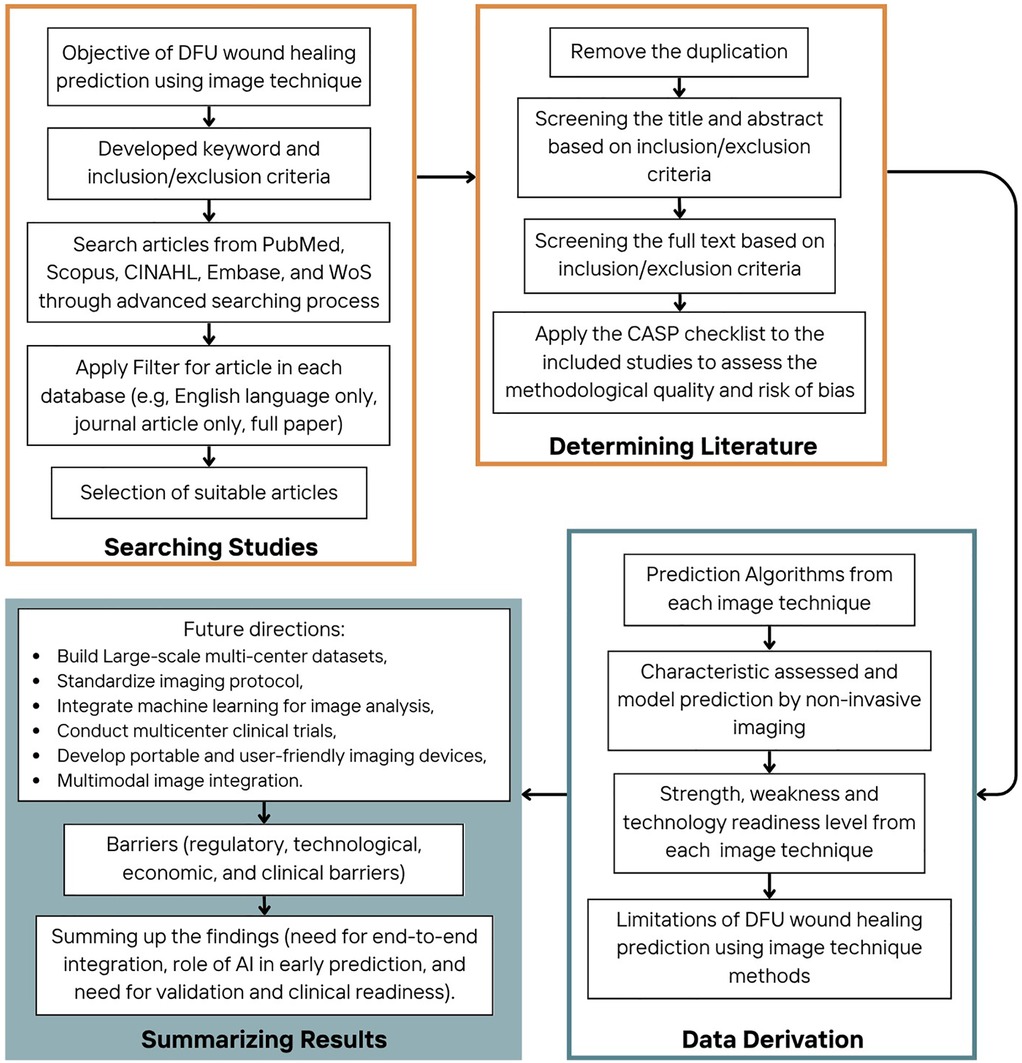
The process for evaluating DFU wound healing prediction using non-invasive image techniques followed a structured approach, divided into four main stages: searching strategy, determining literature, data derivation, and summarizing results, as can be seen in Figure 1. A list of acronyms used in this paper is given in Table 2.
2.1 Searching strategy
In this stage, the primary objective was to identify relevant studies on DFU wound healing prediction using imaging techniques. PRISMA guidelines were used to identify journal articles published in the last decade, (July 2014 to July 2024) that reported wound healing prediction algorithms utilizing imaging techniques. Publications were obtained from five online databases that included engineering and clinical journals (Web of Science, Scopus, CINAHL, Embase, and PubMed) using an advanced search process. The following search terms, developed in consultation with the university librarian, were used; (“diabetic foot” OR “diabetic foot ulcers” OR “diabetic-related foot ulcers” OR “neuropathic diabetic foot ulcer”) AND (“imaging technique” OR “imaging system” OR “hyperspectral imaging” OR “thermal imaging” OR “photographic imaging”) AND (“prediction” OR “predicting” OR “predict”) AND (“healing status” OR “healing time” OR “healing”). Only English-language journal articles were included; non-English studies were excluded due to resource limitations for accurate translation. Preprints and unpublished data were not considered, as filters were applied during the initial database search to include only peer-reviewed, published journal articles. This approach ensured that all included studies went through formal scientific review, reduced the risk of bias associated with unverified or incomplete findings.
2.2 Determining literature
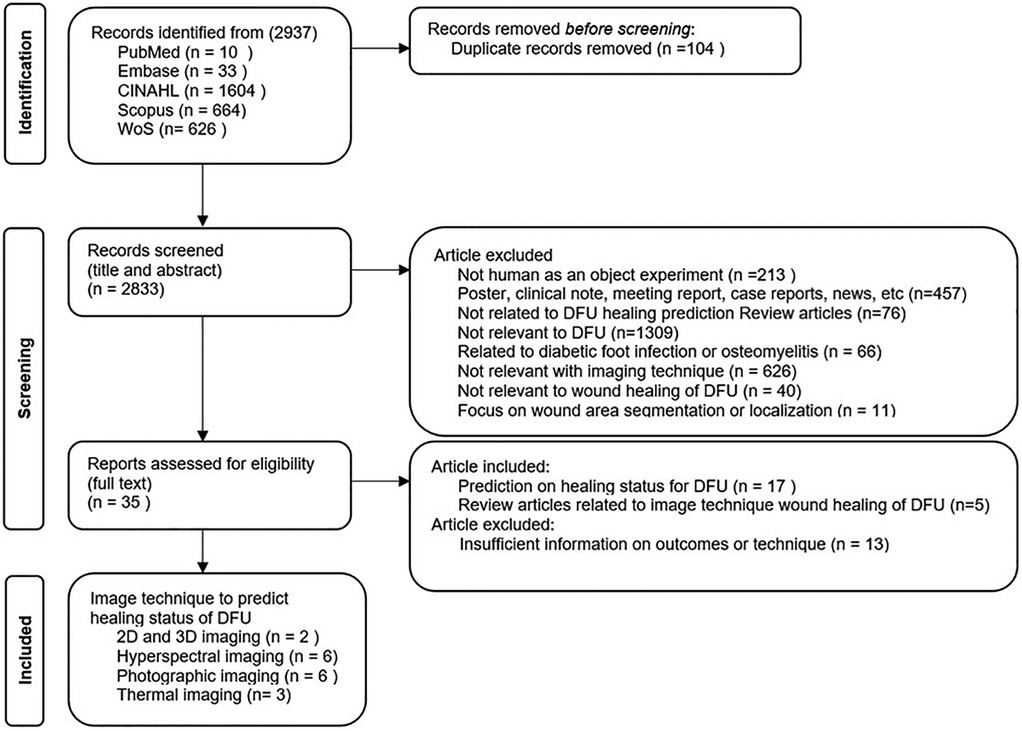
After collecting the studies, the next stage involved screening the articles. A PRISMA flowchart is shown in Figure 2, which was used to identify the articles. EndNote (Clarivate, UK) was used to identify and remove duplicates. After the removal of 104 duplicates, the searching process identified 2,833 publications to be considered for the screening step.
The screening process was conducted in two steps. In the first step, the title and abstract of each study were reviewed by two reviewers (NNS, NDP) to generate a list of relevant studies based on the inclusion and exclusion criteria. Grey literature, including posters, clinical notes, meeting reports, case reports, and news articles (n = 457), was excluded to maintain consistency in quality assessment and ensure inclusion of studies that went through the peer review process. The exclusion criteria included; studies that did not involve human subjects (n = 213); studies that not related to DFU healing prediction review articles (n = 76); studies not relevant to DFU (n = 1,309); those focused on diabetic foot infections or osteomyelitis (n = 66); studies that did not utilize imaging techniques (n = 626); those unrelated to DFU wound healing or healing prediction (n = 40); that focused on wound area segmentation or localization only (n = 11).
If the information from the title and abstract is insufficient to decide on the inclusion or exclusion of a study, the full text was reviewed. The second step involved a full-text review by the two reviewers (NNS, NDP) to obtain the final list of studies for data extraction. The inclusion criteria were: (1) original articles focused on predicting the healing status of diabetic foot ulcers (DFU) using imaging techniques (n = 17) and (2) review articles discussing imaging methods relevant to DFU wound healing (n = 5). Studies with insufficient information on outcomes or imaging methodology were excluded (n = 13). Any conflicts between the two reviewers were resolved by input from a third reviewer (QN).
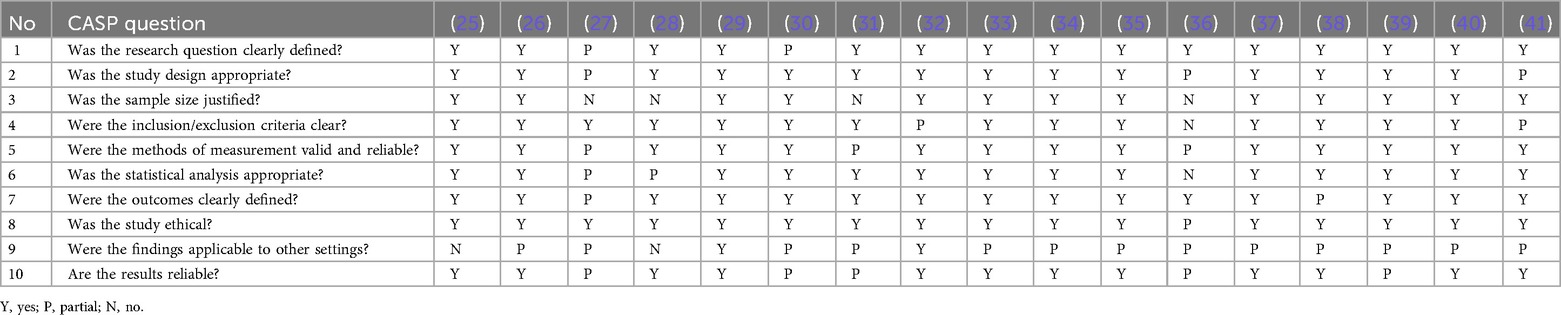
After the two-stage screening process, as shown in Figure 2, 22 publications (17 journal articles and 5 systematic reviews) were identified. Nine articles were from clinical journals, eight were from engineering journals, and five were review articles, all of which were from the clinical domain. The CASP (Critical Appraisal Skills Programme) checklist for appraising quantitative studies was used for a quality checklist of included articles as shown in Table 3. Fifteen articles met the criteria for high-quality studies, while two met fewer criteria. No studies were removed based on quality because all were regarded as giving valuable insight.
While only 17 studies were included out of 2,937 screened, this targeted selection allowed for a more in-depth evaluation of advanced image modalities. This focused inclusion ensured that each selected study contributed valuable insights into the prediction of DFU wound healing using non-invasive imaging techniques. This approach aligns with the PRISMA guidelines, which emphasize the importance of methodological rigor in systematic reviews, thus enhancing the reliability and depth of our findings.
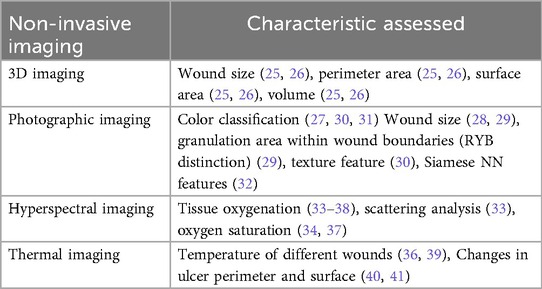
From the review, we identified four main categories of wound healing prediction using non-invasive imaging techniques: (a) 3D imaging (25, 26), (b) visual (RGB) imaging (27–32), (c) hyperspectral imaging (33–38), and (d) thermal imaging (39–41). While 3D imaging was not included in the search keywords, it appeared in the results and hence has been included in this study due to the visibility of this device for use in clinical, community, and home care services.
2.3 Data Derivation
In this stage, valuable data were extracted from the selected articles. The prediction algorithms used in each non-invasive imaging technique were identified in Section 3, the characteristics for wound healing were assessed in Section 4, and the prediction models were described in Section 5. Additionally, the strengths, weaknesses, and technological readiness levels of each imaging technique were evaluated in Section 6.
2.4 Summarizing Results
The final stage involved summarizing the findings from the data derivation process. Key insights were drawn, including future directions for research, open research issues, and the barriers that were faced in clinical practice (Sections 7 and 8). This stage provided a comprehensive summary of findings and future investigations of DFU wound healing management, especially for wound healing prediction using non-invasive imaging techniques.
3 Prediction algorithms
Wound healing prediction algorithm based on image technique can be considered according to each different image modality.
3.1 Healing prediction using 3D imaging
Measuring the volume of the wound, which includes the depth of the wound has been proposed for better evaluation of the wounds. Two studies reported the use of 3D imaging techniques in DFUs (25, 26). This prediction technique typically follows a series of steps to evaluate wound healing, beginning with capturing the wound image using a specialized 3D camera system. One study involved an adhesive optical target for calibration (26).
Multiple images were captured from multiple angles after wound debridement, and these images were then combined into a single 3D model by the software. The clinicians then manually traced the wound area on the monitor for precise measurements to calculate various parameters such as wound area, volume, and depth. The wound measurements were compared from baseline to predict wound healing progression.
These measurements were analyzed using statistical models such as linear regression, which correlates the changes in wound dimensions with time to healing. Prognostic indicators, such as the healing slope and regression analysis, determined whether the wound was healing as expected or stagnating. One study (25) used measurement from 2D imaging as a comparison, while another (26) used the complete closure of the wound (100% skin regrowth) as a reference.
3.2 Healing prediction using visible light imaging
Visible light photographic imaging, generally performed using RGB (Red, Green, Blue) imaging, is one of the imaging techniques that closely resembles traditional assessment in clinical practice due to its focus on measuring physical appearance such as length, width, depth, edge, and peri-wound skin to monitor the wound healing progression (42, 43).
In the list of included articles, six studies focused on using photographic imaging techniques to predict wound healing progression in DFU patients (27–32). The algorithms for predicting healing in DFUs mostly involve four main processes: image acquisition, preprocessing, segmentation, and classification. The algorithm steps used for predicting the healing progression are described below.
3.2.1 Image acquisition
The first step requires capturing the visual light images of the DFU with a controlled image acquisition protocol (31); for example, the wounds were taken at baseline and on days 3, 7, and 14 (29), with the distance between the camera and the wound, camera properties, and the ambient light conditions well described. While the use of smartphone cameras (27, 28) provides ease of data capture, one study proposed a specialized image capture box designed to ensure the consistency of lighting and angles in pictures captured at different time (27).
3.2.2 Pre-processing
After capturing the image, it is necessary to pre-process the images to improve the quality, handle missing pixels or reduce the size, as required for the application. In the reviewed papers, images were pre-processed by decompressing into 24-bit bitmap files, down-sampled to reduce resolution and processing time, and then applied Gaussian smoothing to remove noise (27, 31). In a multi-step approach study (30) that combined clinical data with image processing techniques, the k-nearest neighbor algorithm was used to handle missing data collection, while non-numeric features were discretized using methods such as one-hot encoding (30).
3.2.3 Segmentation
Image segmentation is the process of separating the image into separate segments, often to identify objects or regions of interest. In the segmentation process reported by Wang et al. (27), a mean-shift segmentation algorithm was used to divide the image into homogeneous regions, followed by region fusion to address over-segmentation. The foot outline was detected using skin color thresholds in the CIELAB color space, the guidelines for color representation based on human perception. The wound boundary was identified through connected component analysis (27). In another study, the wound area (WA), granulation area (GA) based color, and the calculation of the granulation index (GI) as the percentage of GA relative to the total wound area were traced and measured using ImageJ software (29). Clinicians also conducted manual segmentation to collect features using image editing software (30). For skin segmentation using the YCbCr color space, morphological operators were applied to refine the image, and a 25 cm2 reference square was used for pixel-to-real-area calibration (31).
3.2.4 Classification of wound
The classification of wound area has been used to differentiate between color, size, and tissue type. Five approaches were reported for the process. K-means clustering algorithms were used to divide the segmented wound area into red, yellow, and black tissue, representing healthy, slough/infected, and necrotic tissues, respectively (27). Statistical analysis with SPSS was used to assess the significance of wound size changes and compare the efficacy of different treatments (29). Third, a Random Forest and Support Vector Machine was trained for predictive analytics (30). Fourth, using RGB pixel values and a feedforward multilayer perceptron (or artificial neural network), the ulcer tissues were classified as granulated, dilacerated/fibrin, or necrotic (31). Fifth, a Siamese Neural Network (SNN) was used to assess DFU progression over time by computing distances from each class anchor point (none, infection, ischemia, both, and healthy) and generated a table with figures and a radar chart (32).
3.3 Healing prediction using hyperspectral imaging
Hyperspectral imaging (HSI) is a technique that determines the high-resolution spectrum of each pixel across the electromagnetic spectrum of interest. Six studies used HSI to predict the progression of DFU healing (33–38). As the first step, the eligible patients were properly screened based on inclusion criteria (35, 37, 38), for example, by abstaining from smoking or caffeine (33). To reduce noise and normalize attenuation values, the calibration of a push-broom camera across a wavelength range of 430–750 nm was applied in the study conducted by Yang et al. (34). Hyperspectral imaging data, corrected for light scattering (33), was processed to calculate tissue oxygenation levels (33), with skin temperature and the ankle-brachial pressure index (ABPI) recorded as baseline measurements (33). In another study, baseline assessments included transcutaneous oxygen pressure (TcpO2), ankle-brachial and toe-brachial indexes, and HSI, which evaluated tissue parameters such as oxygen saturation, hemoglobin index, near-infrared perfusion, and water content (37). The HSI was also used to quantify peri-wound oxyhemoglobin (OxyHb), deoxyhemoglobin (DeoxyHb), and oxygen saturation (O2Sat) in two studies (36, 38). Photonics-based algorithms were used in one study (36) and included the combination of HSI and thermal imaging applied to capture data on biomarkers including OxyHb, DeoxyHb as well as foot temperature patterns to compute the oxygenation metrics Oxygen saturation (SpO2) and Tissue oxygen saturation (StO2).
Monitoring of wound healing progression was carried out at 12 weeks (33, 38) and 24 weeks (33, 37), while other studies conducted monitoring over a 3-week period to assess changes in wound size, perfusion [e.g., transcutaneous oxygen levels (36), hyperspectral imaging], and cytokine levels (35). Using these features, wounds were classified as “healers” or “non-healers,” and a predictive model was created to estimate the likelihood of wound healing. In another study (37) healing was defined as complete epithelization without drainage sustained for at least 10 days within 24 weeks.
3.4 Healing prediction using thermal imaging
Thermal imaging records the infrared or near-infrared radiation of the object to assess its thermal condition. It is a non-invasive technique that detects temperature variations on the surface of an object. In the prediction of wound healing of DFU, in the study by (28) thermal images were segmented into isothermal patches to analyze the wound boundaries and the areas according to the temperature distribution (39). The assessment parameters include the temperature of the wound bed, the area of the isothermal patch on the wound bed, the area of the isothermal patch surrounding the wound and the number of isolated isothermal patches within the wound region. The physical area of the wound bed was determined from color images (39).
In another study (40), the combination of thermal imaging and computerized planimetry was used to first monitor the temperature change indicative of metabolic activity and inflammation, and second to measure wound surface area and wound perimeter. This study integrated these measurements to evaluate the effect of Hyperbaric Oxygen Therapy (HBOT) (40).
Deep learning-based methodology was used in the combination of thermal and visible imaging (41). Experts annotated the fused image as an input to the Mask R-CNN model for precise wound segmentation. Weekly images were analyzed to calculate metrics (39–41) including ulcer-to-foot area ratio and mean temperature distribution. These metrics were correlated with clinician-provided ground truth data to track the healing progression.
4 Wound characteristics assessed by non-invasive imaging
The literature describes three methods for assessing the healing condition of wounds: physiological, geometric, and surface assessment.
4.1 Physiological assessment
Evaluating the physiological aspects of wounds by hyperspectral imaging is essential for forecasting healing trajectory. This assessment concentrates on internal tissue properties and physiological states. Wound healing is a complex and dynamic process that involves four connected stages: blood clotting (hemostasis), inflammatory response, tissue growth (proliferation), and tissue repair (44–46). A proper understanding of the four stages of wound healing is essential for the physiological assessment of wounds (47, 48).
4.1.1 Tissue oxygenation
This parameter is one of the critical predictors of healing status for DFUs in HSI due to its fundamental role in wound repair processes (33–38). Adequate oxygen, a key component for cellular respiration, is important for various stages of wound healing (49, 50). Higher tissue oxygenation levels correlate with better wound healing outcomes in diabetic patients, whereas hypoxia impairs healing (49). Measurements of wound oxygenation, such as transcutaneous oxygen measurements (TcpO2), can guide treatment planning and predict healing potential (50).
4.1.2 Tissue hemoglobin
The tissue hemoglobin index serves as a predictor of healing status in DFUs in HSI due to its ability to assess microvascular oxygenation and perfusion, which are crucial factors in wound healing (37). The cutaneous tissue hemoglobin oxygenation can be quantified from hyperspectral imaging technology. The technology generates anatomically relevant tissue oxygenation maps that correlate with wound healing potential (49). Studies have shown that higher oxyhemoglobin levels and oxygen saturation in the peri-wound area are associated with better healing outcomes (49, 51).
4.1.3 Temperature
Temperature monitoring of DFUs is a predictor of healing status due to its ability to reflect local blood supply, tissue damage, and inflammatory processes (39). Higher wound temperatures typically indicate infection or inflammation, while decreased temperatures suggest impaired healing (52, 53).
4.2 Geometrical assessment
Geometric wound assessment is an important predictor of healing status for DFUs, including the shape, wound size, perimeter, surface area, and volume measurements (25, 26, 43, 54, 55). These quantitative metrics provide objective and reproducible data that can help clinicians monitor wound progression over time, evaluate treatment efficacy, and predict healing outcomes (56, 57). The linear nature of wound margin advance (WMA) over time, calculated as the change in wound area divided by wound perimeter, has been shown to be an effective predictor of healing in DFUs (56). Medical professionals can use this linear pattern to estimate the total healing time based on assessments undertaken at 4–5 weekly intervals. This capability makes it a valuable asset for timely intervention and fine-tuning treatment approaches (56). Studies have shown that a 40% reduction in wound surface area within the first two to three weeks of treatment predicts healing within 12–24 weeks (57), though there is scant evidence to support per cent area reduction in isolation as a surrogate for complete DFU healing in routine clinical practice (8).
4.3 Surface assessment
An analysis of surface features, textures, and segmentation may be used for understanding wound structure and valuable predictor of healing status in DFUs (58). They provide objective, quantifiable data on wound progression, including color classification (27, 30, 31), granulation area (RYB distinction) (29), texture feature (30), siamese neural network features (32), Mask R-CNN for wound segmentation (41), changes in ulcer perimeter and surface (40) and scattering analysis for detecting wound bed and surrounding tissue (33).
5 Characteristic wound healing prediction model
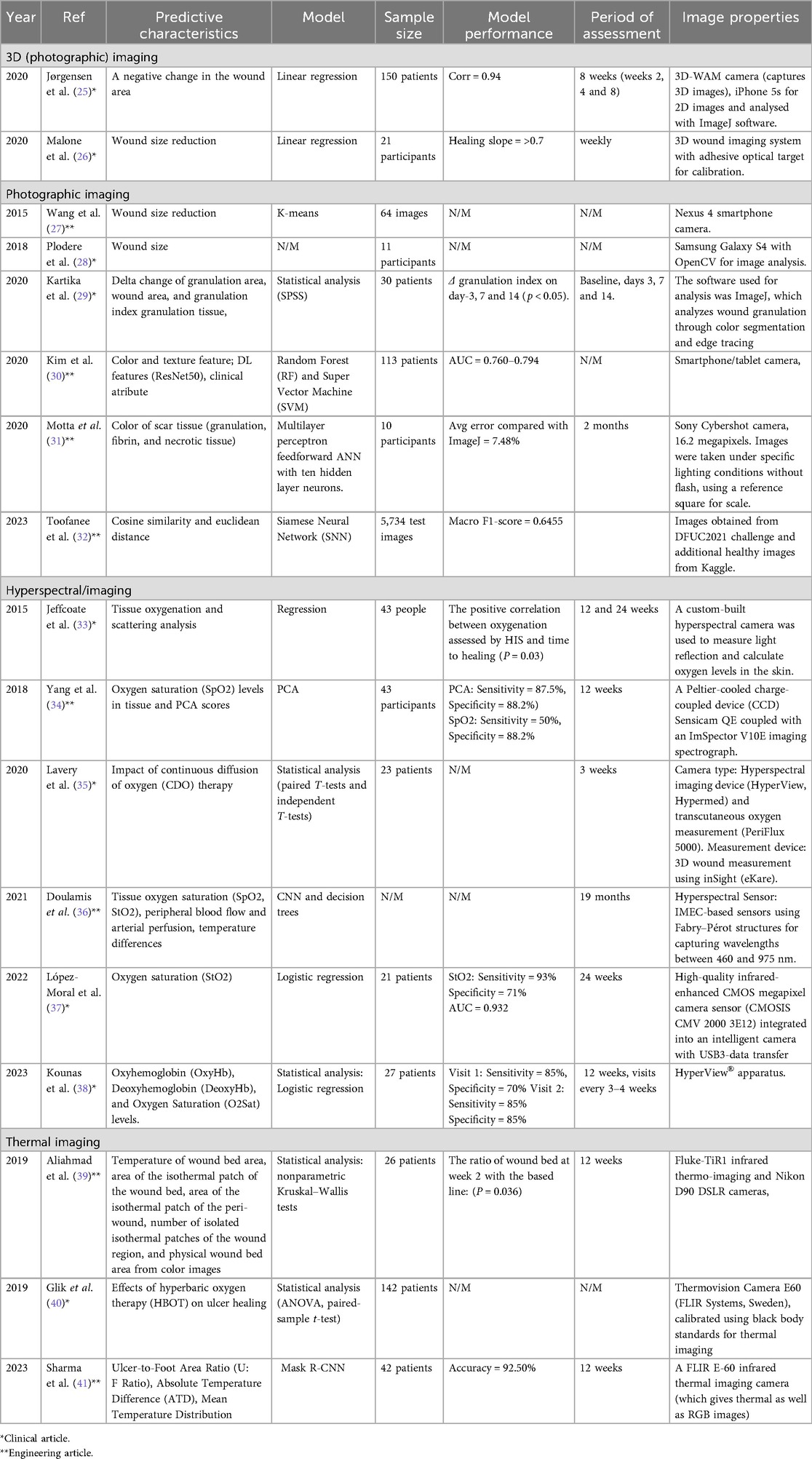
Over the past ten years, various studies have utilized prediction models with non-invasive imaging techniques. According to the list of articles that met the inclusion criteria of the current review, nine out of seventeen papers were published in clinical journals, while the remaining eight appeared in engineering journals. According to the list of articles that met the inclusion criteria, the clinical journals predominantly used statistical approaches as the foundation for prediction tasks, although some studies still relied on manual analysis by clinicians to validate healing progression. In engineering journals, most studies used machine learning strategies to predict healing progression. Table 4 shows the characteristic wound healing prediction model assessed by non-invasive imaging.
5.1 Statistical model analysis
Statistical methods were selected based on the assumption that the healing of DFUs follows a linear pattern in wound area reduction (25) (26, 54, 59, 60). Jorgensen et al. (25) compared the changes in 2D and 3D area measurements using statistical approaches. Linear regressions were used to estimate the mean changes from each patient, with the negative values as healing (decreasing in size) and positive values as nonhealing (increasing in size). The paired Wilcoxon rank test was used to test the zero median difference of the slope between 3D and 2D area measurements. The Bonett-Price approach was then used to provide the confidence interval from that median difference and the Spearman correlation coefficient was used to calculate the correlations between changes in 2D and 3D area measurements (25). Data from 150 wounds revealed that while 2D and 3D area measurements changed in the same direction, the magnitude of changes in 3D measurements was consistently greater than those observed in 2D measurements (25).
Malone et al. (26) investigated the relationship between mean wound healing measurement variables obtained from five 3D wound measurements from 21 participants using linear regression and Pearson's correlation coefficient. The performance analysis metrics were the linear healing slope and statistical significance, with a p-value of 0.0001 and an R-value greater than 0.70, respectively.
Kartika et al. (29) proposed an alternative statistical analysis approach for assessing the wound healing process in DFUs. The researchers quantified wound area, granulation area, granulation index, and respective temporal changes using ImageJ software, a free web-based software that allows easy editing, display, and analysis of images. The statistical analysis was subsequently performed to evaluate the healing progression using SPSS version 20. There were statistically significant differences in granulation index on day 3, day 7, and day 14 (p < 0.05).
Yang et al. (34) used HSI to capture reflectance spectra from tissue and analyzed them to predict wound healing by examining SpO2 levels. The study compared two approaches: SpO2 measurement and Principal Component Analysis (PCA). The PCA, which reduces dimensionality by identifying principal components that capture relevant features of the data, outperformed SpO2 in predicting the healing by the 12th week. While both methods showed high specificity, PCA achieved higher sensitivity (87.5% vs. 50%) and better overall predictive accuracy, with a positive predictive value of 91.3%. The study demonstrated that PCA, using HSI data, was more effective than traditional SpO2 analysis in predicting the healing of DFUs.
In 2022, Lavery et al. (35) evaluated HSI from 23 wounds to compare continuous variables and healer vs. non-healer status using the paired t-test and independent t-test, respectively. Predictive features were derived from baseline data and weekly trends, focusing on achieving a ≥50% reduction in wound area (healer), improvements in perfusion, and significant changes in cytokine levels.
Kounas et al. (38) employed the two-sample t-test to distinguish between groups, a binary logistic regression analysis comparing healers and non-healers based on oxy-Hb levels. There was a negative correlation between oxyHb levels at Visit 1 and the percentage of ulcer size reduction from Visit 1 to Visit 4 (r = −0.46, p = 0.02), and between oxyHb levels at Visit 2 and the percentage of ulcer size reduction from Visit 2 to Visit 4 (r = −0.65, p = 0.001).
Lopez-Moral et al. (37) applied logistic regression to predict DFU healing status using clinical and imaging-derived features. Key predictors included Transcutaneous oximetry (TcpO2), HSI parameters i.e., Skeletal Muscle Oxygen Saturation (StO2), Thermal Hyperspectral Imager (THI) parameters, Near Infrared Perfusion Index (NIR-PI), Tissue water index (TWI), and vascular indices [i.e., ankle-brachial index (ABI) and toe-brachial index (TBI)]. P-values below 0.05 were considered statistically significant, with a 95% confidence interval (CI). Model performance was assessed via receiver operating characteristic (ROC) curve analysis, identifying optimal cut-off values for each predictor to maximize sensitivity and specificity. TcpO2 demonstrated the highest diagnostic accuracy (AUC = 0.989, p-value = 0.005), followed by StO2 (93% sensitivity and 71% specificity), establishing it as the strongest independent predictor of healing.
In a thermal imaging study, Aliahmad et al. (39) employed the nonparametric Kruskal–Wallis test to investigate the relationship between the healing status of ulcers at week 4 and the ratio of DFU parameters measured in the first two weeks of ulceration (week 2 to week 1 ratio) from the isothermal areas. They defined a timely healing ulcer trajectory as showing more than a 50% reduction in area by week 4, an indirect measure routinely used.
A study conducted by Glik et al. (40) explored the impact of Hyperbaric Oxygen Therapy (HBOT) on wound healing using a combination of planimetry and thermal imaging. Statistical analysis was based on the demographics, age of the treatment group, and the median values and ranges of the collected data. To evaluate relationships between variables, Spearman's rank correlation coefficient was calculated. For normally distributed data, paired-sample t-tests were employed, while Mann–Whitney U or Wilcoxon tests were used for data that did not follow a normal distribution. Levene's test was utilized to assess data normality. When comparing more than two groups, analysis of variance (ANOVA) was applied, with a significance threshold set at p < 0.05.
5.2 Machine learning/deep Learning analysis
Recent advancements in machine learning and deep learning have significantly improved DFU monitoring and healing prediction. Various models have been developed to automate ulcer assessment, optimize treatment strategies, and enhance clinical decision-making. These approaches incorporate techniques like Random Forests, Support Vector Machines, Artificial Neural Networks, Siamese Neural Networks, and Convolutional Neural Networks, each utilizing different imaging modalities and features to provide accurate, non-invasive evaluations of wound healing.
Kim et al. (30) proposed the two machine learning models to predict the results of wound healing: Random Forests (RF) and Support Vector Machines (SVM) with RBF kernel. A total of 208 samples were used, with 25% allocated for testing and 75% used for 3-fold cross-validation training. A randomized grid search with three-fold cross-validation was used to optimize the models on the training set. Two thousand combinations of hyperparameters, such as bootstrapping, tree selection criteria, maximum depth, minimum samples per leaf, minimum samples to split a node, and number of PCA components, were tested for RF.
The PCA components, gamma (kernel coefficient), and C (error term penalty) were among the 2028 combinations tested for SVM. The optimal hyperparameters were chosen for each model from 300 randomly selected combinations to increase efficiency. The area under the receiver operating characteristic (AUROC) curve was used to assess the models' performance. To ascertain feature importance for prediction, a different RF model was trained on clinical and handcrafted image features without PCA. When trained with hand-crafted imaging features alone, both the RF and SVM models performed better than when trained with clinical or deep learning-based features alone (P < 0.05).
Motta et al. (31) developed an Artificial Neural Network (ANN) for classifying wound tissue into granulated, fibrin, and necrotic categories. The ulcer area was manually selected by a professional expert for use during training. A multilayer perceptron feedforward network with ten hidden neurons was employed. The Healing Ulcer Index (HUI), which quantified treatment outcomes, was designed for long-term comparison and to aid in monitoring patients at treatment centers. These indexes were based on changes in the wound area and variations in the proportions of different ulcer tissues.
Toofanee et al. (32) presented a novel framework utilizing Siamese Neural Networks (SNN) to monitor and assess the progression of DFU healing. The process began with data acquisition and preprocessing, including resizing and augmentation to address class imbalances. The framework trained an SNN using EfficientNetV2S and Vision Image Transformers to extract features and implement similarity learning, comparing new DFU images against class anchors—average feature representations for “none,” “infection,” “ischemia,” “both,” and “healthy” categories. During the evaluation, baseline images were compared with subsequent ones, generating radar charts and similarity scores to track healing progress and guide clinical decision-making. The model achieved a Macro F1-score of 0.6455, outperforming others in DFU classification tasks, and was validated by the end user, showing its utility for tracking DFU evolution.
Doulamis et al. (36) proposed a photonics-based device for DFU monitoring that integrates HSI and thermal imaging. This study has used the deep learning framework, including convolutional neural networks (CNNs), to implement the automatic segmentation and classification of pathological regions. The system supports dual configurations: a low-cost in-home version equipped with RGB and low-cost HSI sensors for patient self-monitoring, and a professional edition incorporating high-resolution HSI, near-infrared (NIR) fluorescence, and thermal sensors for clinical use. Data acquisition, processing, and interpretation were managed through an embedded software platform that integrates sensor input with machine learning models to deliver real-time, data-driven diagnostic support.
Sharma et al. (41) employed a deep learning-based approach using Mask R-CNN to evaluate DFU healing. The methodology integrated thermal and visual images through an HSV-based image fusion technique. Mask R-CNN, an instance segmentation model, accurately identifies and delineates the ulcer region, using a Region Proposal Network (RPN) and a refined ROI-Align operation. The model outputs both mask and class predictions independently, optimizing segmentation accuracy. This automated system correlates closely with clinician measurements (92.5% agreement).
The application of machine learning/deep learning in DFU imaging demonstrated the variety of algorithms for specific tasks such as classification and segmentation. Traditional machine learning models like Random Forests and Support Vector Machines used handcrafted features and improved their performance by tuning settings (e.g., randomized grid search) and reducing feature numbers (e.g., PCA). Deep learning approaches, including CNN, SNN, and Mask R-CNN learned features automatically from data, and used techniques like data augmentation to handle small datasets. The performance was measured using AUROC and F1-score, and the results were compared with expert labels as ground truth.
6 Limitation of DFU wound healing prediction using image techniques methods
We have identified five key parameters to compare the technologies reviewed in this study and also the strengths and weaknesses for each non-invasive image techniques, which can be seen in Figure 3 and Table 5 respectively. The information is used as a guide to identify the strengths and limitations of each group of imaging techniques based on the articles included in this study. Based on the review, we have discovered the current limitations in the system reported over the past ten years. These limitations can be grouped into three main areas, as outlined below.
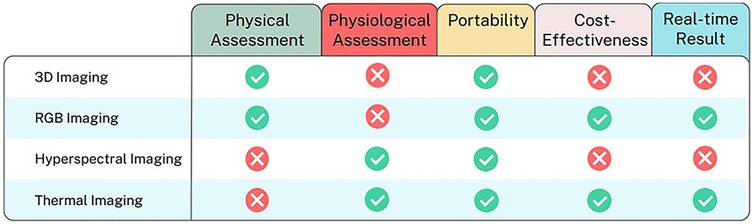
Figure 3. Comparative analysis of image techniques for DFU healing progression analysis across key parameters.
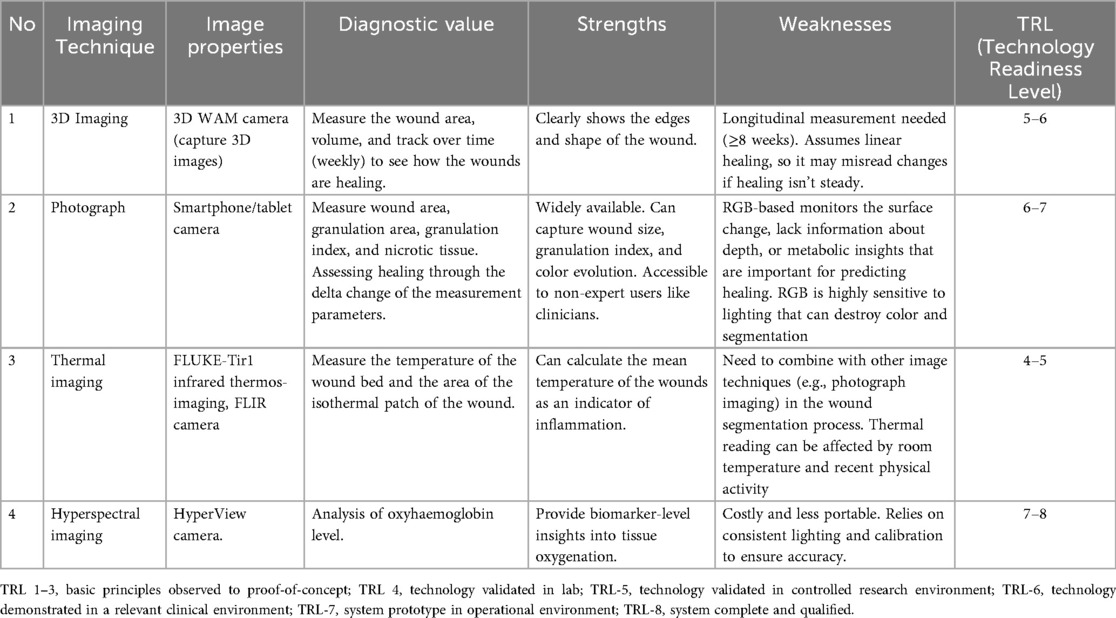
Table 5. The strength, weakness and technology readiness level of image technique for DFU wound healing prediction.
6.1 Limited data for model development
The lack of accessible wound images restricts the amount of data available for training and validating predictive models. As can be seen in Table 6, the sample sizes available in most of the included papers were relatively small, often around twenty, which may not be sufficient for machine learning approaches. Therefore, it is understandable that most of the predictions or measurements of healing were processed statistically. However, large and accessible datasets would be extremely valuable for improving the accuracy of predicting wound healing status in DFUs. Researchers may struggle to create models that generalize well across different populations and clinical scenarios in the absence of a diverse and comprehensive dataset.
6.2 Automated wound segmentation methods
In the clinical environment, efficient computational methods are needed to obtain quick and real-time results, including automated wound segmentation. Manual segmentation makes it unsuitable for the device to be used in the clinical setting, delays the process of analysis, and leads to data without clear standards, as different individuals might produce varying tracing results (25, 30).
6.3 Technical limitations of imaging devices
Technical limitations must be considered before the device can be used in a wide range of clinical settings. Some studies have used complicated tools for data collection that are not portable or feasible for routine use in primary care settings (25, 27, 34, 41). Expensive imaging technologies such as HIS (25, 37) can also prevent many healthcare providers from using advanced tools to assess DFUs, especially since these devices are intended for use not only in clinical environments but also in home care settings.
7 Future directions and open research issues
The future direction of wound diagnostics should focus on supporting healthcare providers while leading towards enabling individuals with wounds to self-monitor. Achieving this would requires a coordinated roadmap that addresses key research gaps across data infrastructure, clinical validation, technology development, and clinical integration. With the growing potential of machine learning and non-invasive imaging, the following roadmap outlines the critical steps to advance predictive wound healing for DFU using non-invasive imaging techniques.
7.1 Build large-scale, multicenter datasets
Currently, there is a lack of large-scale datasets for DFU images. This represents a bottleneck in advancing predictive models for healing. Future efforts should focus on developing centralized, open-access repositories of annotated wound images that are collected from diverse populations and clinical settings (61–63). These repositories would enable researchers to train and test predictive models on a broader set of data, significantly improving the generalizability and robustness (64).
Techniques like synthetic data generation using methods such as generative adversarial networks (GANs), could also be explored to augment existing datasets and overcome data bias. Synthetic wound images have shown promise in enhancing dataset diversity, particularly in addressing class imbalances and rare scenarios (24, 65).
Combining wound imaging with complementary information such as patient demographics, comorbidities, biochemical markers, and treatment history could provide a more holistic understanding of the healing process. Multimodal approaches have been shown to improve model performance in predicting the healing of DFU (66) and chronic wounds (67). This integration would require collaborative efforts across disciplines to standardize data collection protocols and ensure interoperability between datasets.
7.2 Standardize imaging protocol
Standard protocol in non-invasive image technique to predict healing status of DFU is essential to ensure consistent, reliable, and reproducible assessment. Consistent procedures for image acquisition such as lighting, positioning, calibration, environmental/camera control are critical to ensure reliable data across time and clinical settings. Protocol standardization can facilitate clinical implementation by making imaging more interpretable for healthcare providers.
7.3 Integrate machine learning for image analysis
Machine learning methods, especially convolutional neural networks (CNNs), such as U-Net and its variants, have proven highly effective in medical image segmentation and could be adapted for use in predicting DFU healing (68, 69). To enhance clinical adoption, these methods must be computationally efficient, capable of running on standard hardware, and robust to variations in wound appearance caused by lighting, skin tone, and ulcer severity.
In addition, the implementation of explainable AI (XAI) methods in segmentation tools would enhance the transparency and acceptance among clinicians (70). Providing visual overlays or confidence maps could help clinicians validate the automated segmentation results, ensuring the reliability in decision-making processes.
7.4 Conduct multicenter clinical trials
To validate the predictive utility of imaging and AI models, multicenter prospective clinical trials are needed. These should evaluate whether the early prediction of healing status leads to improved patient outcomes, such as earlier intervention or reduced amputation. Trials should also assess how imaging tools perform across different care settings, such as hospitals, community, and home care services.
7.5 Develop portable and user-friendly imaging devices
Future research should focus on developing portable, cost-effective devices that maintain high diagnostic accuracy. Advances in sensor miniaturization, wearable technology, and smartphone-based imaging platforms could provide practical solutions, enabling real-time wound assessment and monitoring in diverse care environments (71, 72). In addition, the integration of user-centered design principles is critical to ensure that these tools are intuitive for non-specialist users, such as general practitioners and caregivers (73, 74). Efforts should also be made to reduce the computational requirements of data analysis algorithms, enabling deployment on low-power devices.
7.6 Enable multimodal imaging integration
Combining multiple image techniques into an integrated framework provides a more comprehensive view of wound healing status by capturing physical, physiological, and biochemical information. These can improve diagnostic capabilities beyond the limitations of individual techniques. Future direction should focus on developing tools that can quickly and accurately analyze the combined imaging features, which make it easier for clinicians to assess wounds and make treatment decisions.
8 Discussion and conclusion
Assessing wound healing progression is crucial in DFU to ensure treatment effectiveness, detect complications early, and guide timely clinical decisions. It helps prevent serious outcomes like infection or amputation and improves patient recovery and care quality. In clinical practice, most clinicians still rely on visual assessment to monitor DFU healing due to simplicity and cost-effectiveness. In recent studies, non-invasive imaging techniques offer safer, affordable, and portable alternatives to conventional methods like MRI, CT, and PET in the healing prediction of DFU. They enable real-time monitoring and early prediction using Machine learning/deep learning analysis, however, they are not yet widely adopted in routine care because of the validity and accessibility.
This systematic review evaluated the current non-invasive imaging techniques used to predict the wound healing status of DFUs. Our results highlight the wound healing classification process, though it remains in the early phases of development and clinical validation. We identified several imaging methods, such as visible light imaging, 3D imaging, hyperspectral imaging, and thermal imaging. Each technique presents its own strengths and faces different problems in development and application. In order to provide a tool that can be used in clinical, home, and community services, several criteria must be met, including a clear data collection protocol; a large number of data samples; a process for extracting information from the wound area, and for classifying the feature information into accurate outputs of healed and non-healed wounds; and the possibility of being developed in real-time analysis in a short time.
While non-invasive imaging techniques hold significant promise for predicting diabetic foot ulcer (DFU) healing, several barriers burden their routine use in clinical practice. Overcoming these obstacles is crucial for successfully translating these promising technologies into everyday patient care.
1. Regulatory barriers: Regulatory approval for new medical technologies presents a significant challenge. The process of approving medical devices and technologies can be time-consuming and costly. Imaging technologies require extensive validation and clinical trials before they can be used in practice. The different standards in clinical validation require multiple rounds of testing that can delay the introduction of promising technologies to be used. Aligning regulations might help speed up approval and reduce the costs.
2. Technological barriers: Challenges such as the lack of standardized image protocols, and the complexity of some technologies (e.g., hyperspectral and 3D imaging) make these tools difficult to integrate into clinical workflows. Additionally, some of these technologies require specialized equipment and expertise that are not universally available. Developing a standardized imaging protocol and improving compatibility with existing healthcare systems may make integration easier.
3. Economic barriers: The high cost of advanced imaging equipment and computational resources needed for data analysis is a significant barrier. Non-invasive imaging technologies can be expensive and may limit their accessibility for use in home or community care. Conducting cost-benefit analyses and improving affordability might help increase accessibility.
4. Clinical barriers: Clinicians may face difficulties in adopting these new technologies due to a lack of training or resistance to change. There is a need for robust clinical validation to demonstrate the reliability and clinical utility of these imaging techniques before they can be widely accepted. Providing comprehensive training programs and demonstrating the clinical benefits might encourage adoption.
This review identifies which imaging techniques are most suitable for routine use in clinical, home care, and community-based care settings for predicting DFU healing outcomes. As the findings were based on only 17 eligible studies, this focused selection allowed for detailed analysis of current imaging techniques, providing valuable insight and highlighting important directions for future research.
Based on our findings, we conclude the following:
1. Need for end-to-end integration: To achieve real-time results, it is necessary to build an end-to-end integrated system, from image acquisition to analysis and output. From this perspective, visual (RGB) imaging and thermal imaging are promising alternatives due to the simplicity and the ability to process data automatically on the same device without human assistance (e.g., through automatic segmentation and classification). These methods are also more portable and user-friendly compared to more complex technologies like hyperspectral imaging or 3D imaging, making them better suited for use in home and community-based settings.
2. Role of AI in early prediction: With the integration of advanced techniques such as machine learning and deep learning, early prediction of healing status is possible (no need to wait up to 4 weeks to ascertain wound healing ability). This can assist clinicians in making more timely and accurate treatment decisions.
3. Need for validation and clinical readiness: While non-invasive imaging techniques have high potential as alternatives to monitor wound healing progress, further validation supported with larger datasets is still needed to ensure the reliability of the results before these techniques can be translated into clinical practice. Additionally, more detailed consideration of the barriers to clinical adoption, such as clinician training, and integration into existing clinical workflow, would be beneficial to fully understand what is required to implement these technologies in routine care.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
NS: Writing – original draft, Validation, Methodology. QN: Writing – review & editing, Methodology, Validation, Conceptualization. NP: Validation, Writing – review & editing. RO: Conceptualization, Writing – review & editing. EE: Supervision, Funding acquisition, Writing – review & editing, Conceptualization. AA: Writing – review & editing. BP: Validation, Writing – review & editing. DK: Conceptualization, Supervision, Methodology, Writing – review & editing, Investigation, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Australian Centre for Accelerating Diabetes Innovation (ACADI), supported by a Medical Research Future Fund (MRFF) grant administered through MTP Connect.
Acknowledgments
We also acknowledge the first author's doctoral study sponsored the Indonesian Education Scholarship (BPI), the Centre for Higher Education Funding and Assessment (PPPAPT), the Ministry of Higher Education, Science and Technology of the Republic of Indonesia, and the Endowment Fund for Education Agency (LPDP), the Ministry of Finance of the Republic of Indonesia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. (2016) 49(2):106–16. doi: 10.1080/07853890.2016.1231932
2. World Health Organization. Diabetes (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/diabetes (Accessed November 20, 2024).
3. Silva-Tinoco R, Cuatecontzi-Xochitiotzi T, Reyes-Paz Y, Vidal-Santos B, Galíndez-Fuentes A, Castillo-Martínez L. Improving foot ulcer risk assessment and identifying associated factors: results of an initiative enhancing diabetes care in primary settings. Diabet Epidemiol Manag. (2024) 14:100195. doi: 10.1016/j.deman.2023.100195
4. McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care. (2023) 46(1):209–21. doi: 10.2337/dci22-0043
5. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. (2020) 13(1):16. doi: 10.1186/s13047-020-00383-2
6. Wang X, Yuan CX, Xu B, Yu Z. Diabetic foot ulcers: classification, risk factors and management. World J Diabetes. (2022) 13(12):1049–65. doi: 10.4239/wjd.v13.i12.1049
8. Gwilym BL, Mazumdar E, Naik G, Tolley T, Harding K, Bosanquet DC. Initial reduction in ulcer size as a prognostic indicator for complete wound healing: a systematic review of diabetic foot and venous leg ulcers. Adv Wound Care (New Rochelle). (2023) 12(6):327–38. doi: 10.1089/wound.2021.0203
9. Godavarty A, Leiva K, Amadi N, Klonoff DC, Armstrong DG. Diabetic foot ulcer imaging: an overview and future directions. J Diabetes Sci Technol. (2023) 17(6):1662–75. doi: 10.1177/19322968231187660
10. Rogers LC, Frykberg RG, Armstrong DG, Boulton AJ, Edmonds M, Van GH, et al. The charcot foot in diabetes. Diabetes Care. (2011) 34(9):2123–9. doi: 10.2337/dc11-0844
11. Arnon-Sheleg E, Keidar Z. Diabetic foot infection: the role of PET/CT imaging. Curr Pharm Des. (2018) 24(12):1277–86. doi: 10.2174/1381612824666180227095439
12. Nawaz A, Torigian DA, Siegelman ES, Basu S, Chryssikos T, Alavi A. Diagnostic performance of FDG-PET, MRI, and plain film radiography (PFR) for the diagnosis of osteomyelitis in the diabetic foot. Mol Imaging Biol. (2010) 12(3):335–42. doi: 10.1007/s11307-009-0268-2
13. Rubitschung K, Sherwood A, Crisologo AP, Bhavan K, Haley RW, Wukich DK, et al. Pathophysiology and molecular imaging of diabetic foot infections. Int J Mol Sci. (2021) 22(21):11552. doi: 10.3390/ijms222111552
14. Chan KS, Lo ZJ. Wound assessment, imaging and monitoring systems in diabetic foot ulcers: a systematic review. Int Wound J. (2020) 17(6):1909–23. doi: 10.1111/iwj.13481
15. Pattnaik S, Thalluri C, Swain K. Rise of gold nanoparticles as carriers of therapeutic agents. Acta Chim Slov. (2023) 70(4):467–78. doi: 10.17344/acsi.2023.8216
16. Chettupalli AK, Bukke SPN, Rahaman SA, Unnisa A, Adepu M, Kavitha M, et al. Ritonavir loaded solid lipid nanoparticles for oral drug delivery and bioavailability enhancement. Discov Appl Sci. (2025) 7(1):58. doi: 10.1007/s42452-024-06322-1
17. Bukke SPN, Venkatesh C, Bandenahalli Rajanna S, Saraswathi TS, Kusuma PK, Goruntla N, et al. Solid lipid nanocarriers for drug delivery: design innovations and characterization strategies—a comprehensive review. Discov Appl Sci. (2024) 6(6):279. doi: 10.1007/s42452-024-05897-z
18. Raghunath I, Koland M, Saoji SD, Bukke SPN, Deshpande NS, Augustin V, et al. Effects of piperine on intestinal permeation, pharmacodynamics and pharmacokinetics of insulin loaded chitosan coated solid lipid nanoparticles in rats. Sci Rep. (2025) 15(1):22771. doi: 10.1038/s41598-025-05137-3
19. Sarma KN, Thalluri C, Mandhadi JR. Nanofibers in drug delivery systems: a comprehensive scientific review of recent approaches. Int J Pharm Investig. (2024) 14(3):633–46. doi: 10.5530/ijpi.14.3.75
20. Ahikiriza AA, Bukke SPN, Yadesa TM, Nicholas B, Daniel K, Moses K, et al. The potential of natural products in metabolic disease management: a thorough exploration of the case of Uganda. Endocr Metab Immune Disord Drug Targets. (2025) 25:953–9. doi: 10.2174/0118715303320109241014064209
21. Wang Z, Hasan R, Firwana B, Elraiyah T, Tsapas A, Prokop L, et al. A systematic review and meta-analysis of tests to predict wound healing in diabetic foot. J Vasc Surg. (2016) 63(2 Suppl):29S–36.e1-2. doi: 10.1016/j.jvs.2015.10.004
22. Paul DW, Ghassemi P, Ramella-Roman JC, Prindeze NJ, Moffatt LT, Alkhalil A, et al. Noninvasive imaging technologies for cutaneous wound assessment: a review. Wound Repair Regen. (2015) 23(2):149–62. doi: 10.1111/wrr.12262
23. Saiko G, Lombardi P, Au Y, Queen D, Armstrong D, Harding K. Hyperspectral imaging in wound care: a systematic review. Int Wound J. (2020) 17(6):1840–56. doi: 10.1111/iwj.13474
24. Chen Y, Yang X-H, Wei Z, Heidari AA, Zheng N, Li Z, et al. Generative adversarial networks in medical image augmentation: a review. Comput Biol Med. (2022) 144:105382. doi: 10.1016/j.compbiomed.2022.105382
25. Jørgensen LB, Halekoh U, Jemec GBE, Sørensen JA, Yderstræde KB. Monitoring wound healing of diabetic foot ulcers using two-dimensional and three-dimensional wound measurement techniques: a prospective cohort study. Adv Wound Care. (2020) 9(10):553–63. doi: 10.1089/wound.2019.1000
26. Malone M, Schwarzer S, Walsh A, Xuan W, Gannass A, Dickson A, et al. Monitoring wound progression to healing in diabetic foot ulcers using three-dimensional wound imaging. J Diabetes Complicat. (2020) 34(2):107471. doi: 10.1016/j.jdiacomp.2019.107471
27. Wang L, Pedersen PC, Strong DM, Tulu B, Agu E, Ignotz R. Smartphone-Based wound assessment system for patients with diabetes. IEEE Trans Biomed Eng. (2015) 62(2):477–88. doi: 10.1109/TBME.2014.2358632
28. Ploderer B, Brown R, Seng LSD, Lazzarini PA, Van Netten JJ. Promoting self-care of diabetic foot ulcers through a mobile phone app: user-centered design and evaluation. JMIR Diabetes. (2018) 20(10):e10105. doi: 10.2196/10105
29. Kartika RW, Alwi I, Suyatna FD, Yunir E, Waspadji S, Immanuel S, et al. The use of image processing in the evaluation of diabetic foot ulcer granulation after treatment with advanced-platelet rich fibrin + hyaluronic acid. Syst Rev Pharm. (2020) 11(12):519–26.
30. Kim RB, Gryak J, Mishra A, Cui C, Soroushmehr SMR, Najarian K, et al. Utilization of smartphone and tablet camera photographs to predict healing of diabetes-related foot ulcers. Comput Biol Med. (2020) 126:104042. doi: 10.1016/j.compbiomed.2020.104042
31. Motta BC, Marques MP, Guimaraes GA, Ferreira RU, Rosa S. The evaluation of the healing process of diabetic foot wounds using image segmentation and neural networks classification. Int J Biomed Eng Technol. (2022) 38(2):179–92. doi: 10.1504/IJBET.2022.120869
32. Toofanee MSA, Dowlut S, Hamroun M, Tamine K, Duong AK, Petit V, et al. DFU-Helper: an innovative framework for longitudinal diabetic foot ulcer diseases evaluation using deep learning. Appl Sci. (2023) 13(18):10310. doi: 10.3390/app131810310
33. Jeffcoate WJ, Clark DJ, Savic N, Rodmell PI, Hinchliffe RJ, Musgrove A, et al. Use of HSI to measure oxygen saturation in the lower limb and its correlation with healing of foot ulcers in diabetes. Diabetic Med. (2015) 32(6):798–802. doi: 10.1111/dme.12778
34. Yang Q, Sun S, Jeffcoate WJ, Clark DJ, Musgove A, Game FL, et al. Investigation of the performance of hyperspectral imaging by principal component analysis in the prediction of healing of diabetic foot ulcers. J Imaging. (2018) 4(12):144. doi: 10.3390/jimaging4120144
35. Lavery LA, Killeen AL, Farrar D, Akgul Y, Crisologo PA, Malone M, et al. The effect of continuous diffusion of oxygen treatment on cytokines, perfusion, bacterial load, and healing in patients with diabetic foot ulcers. Int Wound J. (2020) 17(6):1986–95. doi: 10.1111/iwj.13490
36. Doulamis A, Doulamis N, Angeli A, Lazaris A, Luthman S, Jayapala M, et al. A non-invasive photonics-based device for monitoring of diabetic foot ulcers: architectural/sensorial components & technical specifications. Inventions. (2021) 6(2):27. doi: 10.3390/inventions6020027
37. López-Moral M, García-Álvarez Y, Molines-Barroso RJ, Tardáguila-García A, García-Madrid M, Lázaro-Martínez JL. A comparison of hyperspectral imaging with routine vascular noninvasive techniques to assess the healing prognosis in patients with diabetic foot ulcers. J Vasc Surg. (2022) 75(1):255–61. doi: 10.1016/j.jvs.2021.07.123
38. Kounas K, Dinh T, Riemer K, Rosenblum BI, Veves A, Giurini JM. Use of hyperspectral imaging to predict healing of diabetic foot ulceration. Wound Repair Regen. (2023) 31(2):199–204. doi: 10.1111/wrr.13071
39. Aliahmad B, Tint AN, Poosapadi Arjunan S, Rani P, Kumar DK, Miller J, et al. Is thermal imaging a useful predictor of the healing Status of diabetes-related foot ulcers? A pilot study. J Diabetes Sci Technol. (2019) 13(3):561–7. doi: 10.1177/1932296818803115
40. Glik J, Cholewka A, Stanek A, Englisz B, Sieroń K, Mikuś-Zagórska K, et al. Thermal imaging and planimetry evaluation of the results of chronic wounds treatment with hyperbaric oxygen therapy. Adv Clin Exp Med. (2019) 28(2):229–36. doi: 10.17219/acem/92304
41. Sharma N, Mirza S, Rastogi A, Mahapatra PK. Utilizing mask R-CNN for automated evaluation of diabetic foot ulcer healing trajectories: a novel approach. Traitement du Signal. (2023) 40(4):1601–10. doi: 10.18280/ts.400428
42. Grey JE, Enoch S, Harding KG. Wound assessment. BMJ (Clin Res Ed). (2006) 332(7536):285–8. doi: 10.1136/bmj.332.7536.285
45. Le DTP, Pham TD. Unveiling the role of artificial intelligence for wound assessment and wound healing prediction. Explor Mede. (2023) 4:589–611. doi: 10.37349/emed.2023.00163
46. Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics. (2020) 12(8):735. doi: 10.3390/pharmaceutics12080735
47. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. (2010) 89(3):219–29. doi: 10.1177/0022034509359125
49. Nouvong A, Hoogwerf B, Mohler E, Davis B, Tajaddini A, Medenilla E. Evaluation of diabetic foot ulcer healing with hyperspectral imaging of oxyhemoglobin and deoxyhemoglobin. Diabetes Care. (2009) 32(11):2056–61. doi: 10.2337/dc08-2246
50. Han S-K. Increasing tissue oxygenation for diabetic wound healing. J Wound Manag Res. (2017) 13(1):2–7. doi: 10.22467/jwmr.2017.00080
51. Khaodhiar L, Dinh T, Schomacker KT, Panasyuk SV, Freeman JE, Lew R, et al. The use of medical hyperspectral technology to evaluate microcirculatory changes in diabetic foot ulcers and to predict clinical outcomes. Diabetes Care. (2007) 30(4):903–10. doi: 10.2337/dc06-2209
52. Houghton VJ, Bower VM, Chant DC. Is an increase in skin temperature predictive of neuropathic foot ulceration in people with diabetes? A systematic review and meta-analysis. J Foot Ankle Res. (2013) 6:31. doi: 10.1186/1757-1146-6-31
53. Liu H, Yan XY, Li GQ, Wang BN, Wang D, Zhang YH, et al. Evaluation of wound temperature monitoring at various anatomical sites in the management of patients with diabetic foot undergoing microcirculation reconstruction. J Orthop Surg Res. (2024) 19(1):776. doi: 10.1186/s13018-024-05278-7
54. Gorin DR, Cordts PR, LaMorte WW, Menzoian JO. The influence of wound geometry on the measurement of wound healing rates in clinical trials. J Vasc Surg. (1996) 23:524–8. doi: 10.1016/S0741-5214(96)80021-8
55. Xu Y, Sun J, Carter RR, Bogie KM. Personalized prediction of chronic wound healing: an exponential mixed effects model using stereophotogrammetric measurement. J Tissue Viability. (2014) 23(2):48–59. doi: 10.1016/j.jtv.2014.04.001
56. Bull RH, Staines KL, Collarte AJ, Bain DS, Ivins NM, Harding KG. Measuring progress to healing: a challenge and an opportunity. Int Wound J. (2022) 19(4):734–40. doi: 10.1111/iwj.13669
57. Yee A, Harmon J, Yi S. Quantitative monitoring wound healing Status through three-dimensional imaging on Mobile platforms. J Am Coll Clin Wound Spec. (2016) 8(1-3):21–7. doi: 10.1016/j.jccw.2017.11.001
58. Alonso MC, Mohammed HT, Fraser RD, Ramirez Garcia Luna JL, Mannion D. Comparison of wound surface area measurements obtained using clinically validated artificial intelligence-based technology versus manual methods and the effect of measurement method on debridement code reimbursement cost. Wounds. (2023) 35(10):E330–8. doi: 10.25270/wnds/23031
59. Lavery LA, Barnes SA, Keith MS, Seaman JW Jr, Armstrong DG. Prediction of healing for postoperative diabetic foot wounds based on early wound area progression. Diabetes Care. (2008) 31(1):26–9. doi: 10.2337/dc07-1300
60. Cardinal M, Eisenbud DE, Armstrong DG. Wound shape geometry measurements correlate to eventual wound healing. Wound Repair Regen. (2009) 17(2):173–8. doi: 10.1111/j.1524-475X.2009.00464.x
61. Armato SG III, Drukker K, Hadjiiski L. AI In medical imaging grand challenges: translation from competition to research benefit and patient care. Br J Radiol. (2023) 96(1150):20221152. doi: 10.1259/bjr.20221152
62. Cassidy B, Hoon Yap M, Pappachan JM, Ahmad N, Haycocks S, O’Shea C, et al. Artificial intelligence for automated detection of diabetic foot ulcers: a real-world proof-of-concept clinical evaluation. Diabetes Res Clin Pract. (2023) 205:110951. doi: 10.1016/j.diabres.2023.110951
63. Ahmad M, Tan M, Bergman H, Shalhoub J, Davies A. The use of artificial intelligence in three-dimensional imaging modalities and diabetic foot disease: a systematic review. JVS Vascular Insights. (2024) 2:100057. doi: 10.1016/j.jvsvi.2024.100057
64. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. (2019) 25(1):44–56. doi: 10.1038/s41591-018-0300-7
65. Sarp S, Kuzlu M, Wilson E, Guler O. WG2AN: synthetic wound image generation using generative adversarial network. J Eng. (2021) 2021(5):286–94. doi: 10.1049/tje2.12033
66. Tehan PE, Burrows T, Hawes MB, Linton C, Norbury K, Peterson B, et al. Factors influencing diabetes-related foot ulcer healing in Australian adults: a prospective cohort study. Diabet Med. (2023) 40(1):e14951. doi: 10.1111/dme.14951
67. Berezo M, Budman J, Deutscher D, Hess CT, Smith K, Hayes D. Predicting chronic wound healing time using machine learning. Adv Wound Care. (2021) 11(6):281–96. doi: 10.1089/wound.2021.0073
68. Ronneberger O, Fischer P, Brox T. U-Net: convolutional networks for biomedical image segmentation. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2015 (2015); Cham: Springer International Publishing.
69. Isensee F, Jaeger PF, Kohl SAA, Petersen J, Maier-Hein KH. nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat Methods. (2021) 18(2):203–11. doi: 10.1038/s41592-020-01008-z
70. Tjoa E, Guan C. A survey on explainable artificial intelligence (XAI): toward medical XAI. IEEE Trans Neural Netw Learn Syst. (2021) 32(11):4793–813. doi: 10.1109/TNNLS.2020.3027314
71. Kabir MA, Samad S, Ahmed F, Naher S, Featherston J, Laird C, et al. Mobile apps for wound assessment and monitoring: limitations, advancements and opportunities. J Med Syst. (2024) 48(1):80. doi: 10.1007/s10916-024-02091-x
72. Wang C, Sani ES, Gao W. Wearable bioelectronics for chronic wound management. Adv Funct Mater. (2022) 32(17):2111022. doi: 10.1002/adfm.202111022
73. De Vito Dabbs A, Myers BA, Mc Curry KR, Dunbar-Jacob J, Hawkins RP, Begey A, et al. User-centered design and interactive health technologies for patients. Comput Inform Nurs. (2009) 27(3):175–83. doi: 10.1097/NCN.0b013e31819f7c7c
Keywords: diabetes-related foot ulcer (DFU), imaging techniques, prediction, healing, systematic review
Citation: Sari NN, Ngo QC, Pah ND, Ogrin R, Ekinci E, Hourani A, Polus B and Kumar DK (2025) Non-invasive imaging techniques for predicting healing status of diabetic foot ulcers: a ten-year systematic review. Front. Med. Technol. 7:1648973. doi: 10.3389/fmedt.2025.1648973
Received: 17 June 2025; Accepted: 16 September 2025;
Published: 29 September 2025.
Edited by:
Lutz Schomburg, Charité University Medicine Berlin, GermanyReviewed by:
Sarad Pawar Naik Bukke, Kampala International University Western Campus, UgandaAbhijit Rayate, Maharashtra Institute of Medical Sciences, India
Copyright: © 2025 Sari, Ngo, Pah, Ogrin, Ekinci, Hourani, Polus and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nemuel D. Pah, bmVtdWVscGFoQHN0YWZmLnViYXlhLmFjLmlk
 Nila N. Sari
Nila N. Sari Quoc C. Ngo
Quoc C. Ngo Nemuel D. Pah
Nemuel D. Pah Rajna Ogrin
Rajna Ogrin Elif Ekinci
Elif Ekinci Akram Hourani
Akram Hourani Barbara Polus
Barbara Polus Dinesh K. Kumar
Dinesh K. Kumar