- 1Cardiovascular Research Center, Massachusetts General Hospital, Boston, MA, United States
- 2Department of Cardiology, Larnaca General Hospital, Larnaca, Cyprus
- 3Medical School, European University Cyprus, Nicosia, Cyprus
- 4Department of Emergency Medicine, Massachusetts General Hospital, Boston, MA, United States
- 5Division of Health Science, Warwick Medical School, University of Warwick, Coventry, United Kingdom
- 6Healthcare Transformation Lab, Massachusetts General Hospital, Boston, MA, United States
- 7Cardiac Surgical Intensive Care Unit, Massachusetts General Hospital, Boston, MA, United States
- 8Cardiology Division, Cardiac Arrhythmia Service, Massachusetts General Hospital, Boston, MA, United States
- 9Broad Institute, Massachusetts Institute of Technology, Cambridge, MA, United States
Background: Artificial intelligence (AI)-based models can augment clinical decision-making, including prediction, diagnosis, and treatment, in all aspects of medicine.
Research questions: The current systematic review aims to provide a summary of existing data about the role of machine learning (ML) techniques in predicting in-hospital cardiac arrest, life-threatening ventricular arrhythmias, and respiratory arrest.
Methods: The study was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) framework. PubMed, Embase, and Web of Science without any restriction were searched to extract relevant manuscripts until October 20, 2023. Additionally, the reference list of all potential studies was searched to identify further relevant articles. Original publications were regarded as eligible if they only recruited adult patients (≥18 years of age), employed AI/ML algorithms for predicting cardiac arrest, life-threatening ventricular arrhythmias, and respiratory arrest in the setting of critical care, used data gathered from wards with critically ill patients (ICUs, cardiac ICUs, and emergency departments), and were published in English. The following information was extracted: first author, journal, ward, sample size, performance and features of ML and conventional models, and outcomes.
Results: ML algorithms have been used for cardiac arrest prediction using easily obtained variables as inputs. ML algorithms showed promising results (AUC 0.73–0.96) in predicting cardiac arrest in different settings, including critically ill ICU patients, patients in the emergency department and patients with sepsis, they demonstrated variable performance (AUC 0.54–0.94) in predicting respiratory arrest in COVID-19 patients, as well as other clinical settings.
Conclusion: ML algorithms have shown promising results in predicting in-hospital cardiac and respiratory arrest using readily available clinical data. These algorithms may enhance early identification of high risk patients and support timely interventions, thereby reducing mortality and morbidity rates. However, the prospective validation of these algorithms and their integration into clinical workflows need further exploration.
Introduction
Approximately 200,000 in-hospital cardiac (CA) and respiratory arrests (RA) occur annually in US hospitals (1, 2); survival is ∼25%, and has improved only moderately over recent decades (3–5). Identification of patients at risk for adverse events leading to CA has been key to improving outcomes. Despite numerous efforts, including early warning scores and rapid response protocols (6–11), recognizing high-risk patients remains a limiting step in providing pre-emptive care. Detection of patient deterioration typically occurs during clinical examination or vital sign measurements at varying intervals (12, 13), depending on hospital and intensive care unit (ICU) policy (14), which leaves significant potential for unnoticed patient deterioration (15, 16).
Given the potential culmination in mortality and serious neurological sequelae, timely detection of clinical deterioration is essential (17). While current risk-stratification tools, such as Early Warning Score (EWS) based methods, have aided in clinical decision-making, they are limited in accuracy, sensitivity, and user dependency (18). Accordingly, further improvements in the performance of predictive tools are warranted for better clinical judgment regarding in-hospital patient safety (19, 20) (Figure 1).
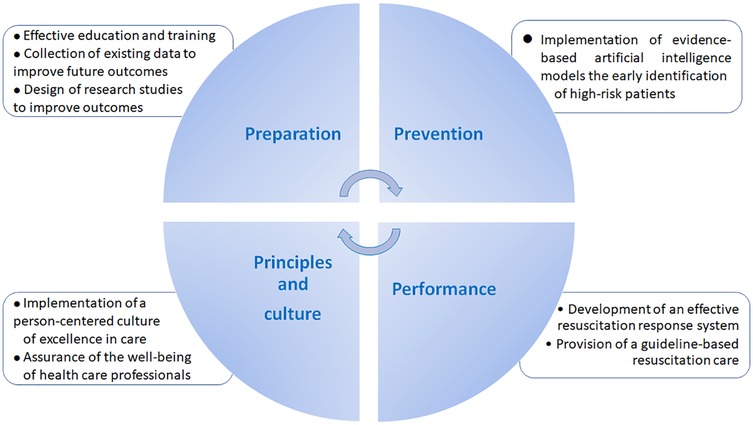
Figure 1. Practical measures to improve the outcomes of cardiac and respiratory arrest in clinical practice.
Artificial intelligence (AI)-based models can facilitate clinical decision-making (21–28) via handling of complex massive datasets (29–31). Considering the growing number of AI-based algorithms developed for predicting life-threatening events (32–34), the current systematic review aims to assess the role of machine learning (ML) algorithms in predicting cardiac arrest, life-threatening ventricular arrhythmias, and RA, in in-hospital, critically ill patients.
Methods
The current systematic review study was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) framework. This review was not registered and no protocol was prepared.
Eligibility criteria
This review focuses on peer-reviewed articles that applied AI/ML methods to predict the occurrence of cardiac arrest, life-threatening ventricular arrhythmias (ventricular fibrillation, ventricular tachycardia, asystole, pulseless electrical activity), and RA in critical care settings. Original publications were regarded as eligible if they only recruited adult patients (≥18 years of age), employed AI/ML algorithms for predicting the above-mentioned adverse events, used data gathered from wards with critically ill patients (ICUs, cardiac ICUs, and emergency departments), and were published in English. Publications were excluded if they used data from general hospital wards. Apart from original articles, other journal manuscript types were excluded. Studies involving animals, in vitro, and in vivo research projects were also excluded. Out-of-hospital cardiac arrest patients were not included in this review.
Search strategy
The research databases, including PubMed, Embase, and Web of Science, without any restriction, were used to extract relevant manuscripts until October 20, 2023. Moreover, the reference list of all potential studies was scrutinized and searched for additional articles. An advanced search strategy was conducted, structured around three groups of terms: critical care settings, artificial intelligence/machine learning, and cardiac or RA. Each group was searched using both exploded Emtree terms and keywords in titles, abstracts, and keyword fields. Terms within each group were combined using OR, and the three groups were combined using AND, ensuring retrieval of articles containing terms from all groups. Results were limited to publication types “Article”, “Article in Press”, and “Preprint”. A detailed search strategy is included in the Online Supplement.
Data extraction
First, the identified citations from each database were uploaded into Endnote 20 and duplicates were eliminated. Two independent authors (AG, GB) screened the titles and abstracts of the remaining papers. Then, the selected full-text articles were reviewed according to the eligibility criteria in the same manner. Disagreements at any step were settled through discussion. The following information was extracted: first author name, journal, ward, sample size, performance and features of ML and conventional models, and outcomes.
A brief description of the reported AI/ML models in this manuscript is provided in the Online Supplement.
Quality assessment
Risk of bias and quality assessment were performed using the QUADAS-2 tool. Two categories, risk of bias and concerns regarding applicability, were assessed in the three domains of patient selection, index test, and reference standard. With the former being assessed in the domain of flow and timing, as well. For assessing the risk of bias, the following criteria were applied for each of the four domains: (1) when the answer to all questions is “yes”, the overall bias risk of the domain is “low”; (2) when the answer to more than one question is “no”, bias risk was definitely identified, and the overall bias risk of the domain is “high”; (3) deemed “unclear” when the data reported is insufficient to make a judgment; (4) when any domain is high risk, the overall bias risk score is “high”; (5) only when the bias risk of one domain is unclear, the overall bias risk of the study is “unclear”.
The recommendation of the QUADAS-2 tool was followed, and the clinical applicability of each study was scored by evaluating whether it matched the concerns of our review, and rated as “low”, “high”, or “unclear”. An author (XL) independently performed the data extraction and quality assessment. Disagreements were resolved through discussion and independent assessment by another researcher to reach a consensus. The final study quality was classified as low risk of bias, high risk of bias, or unclear (Supplementary Table S1).
Results
Search results
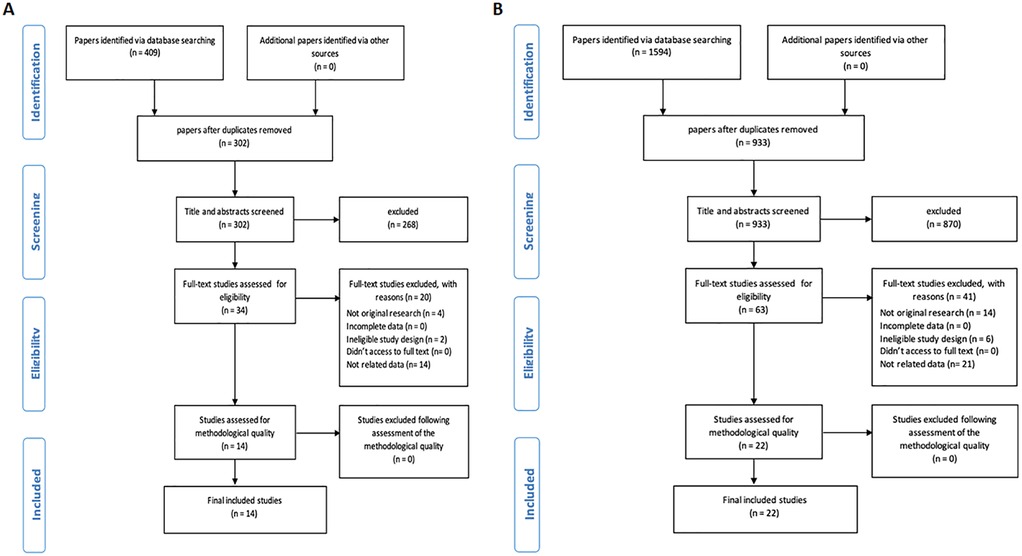
Initially, we obtained 1,594 articles for RA and 409 for CA from three distinct databases, including PubMed, Embase, and Web of Science. Subsequently, we identified and removed duplicates (107 for CA, 661 for RA), leaving us with 302 CA articles and 933 RA articles. Finally, 14 CA studies and 22 RA studies met the inclusion and exclusion criteria and were included in the systematic review (Figures 2A,B, for CA and RA, respectively).
Cardiac arrest
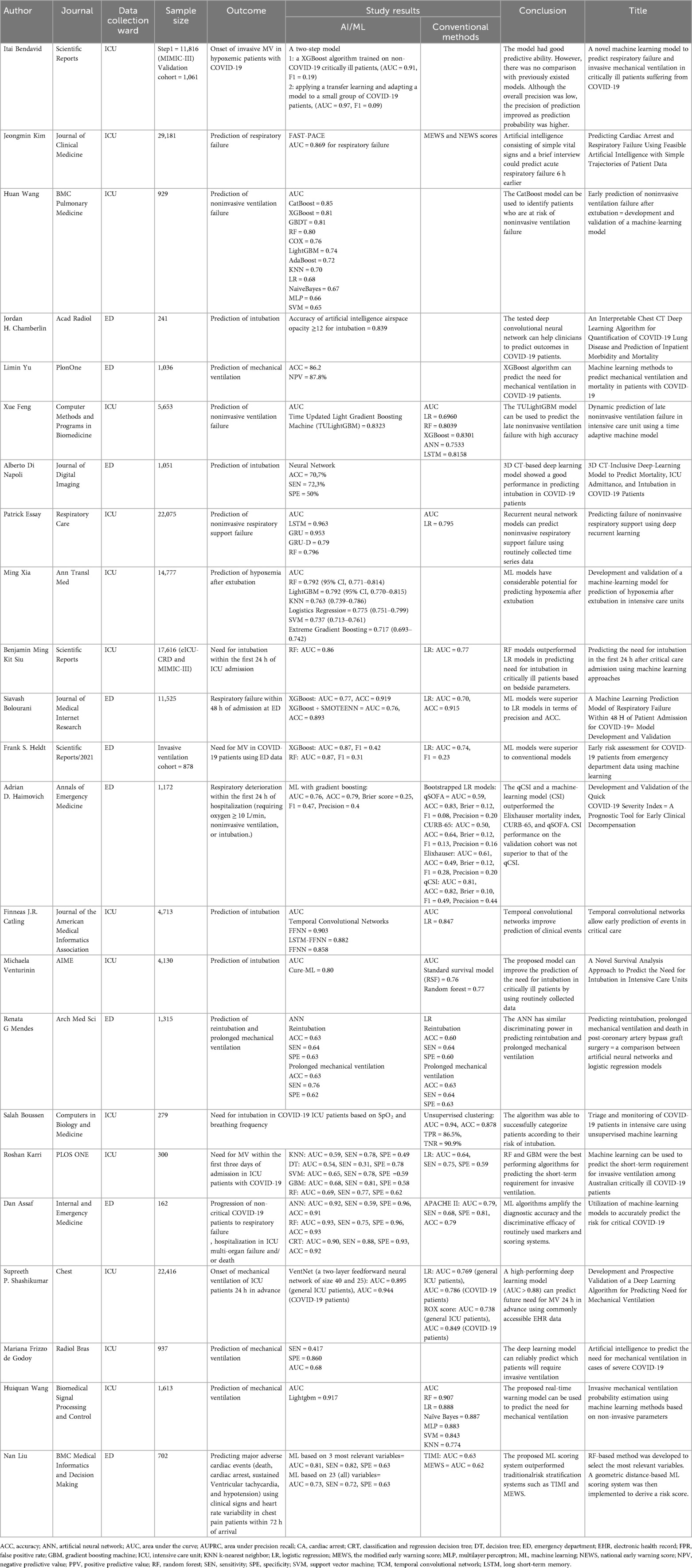
Prediction of cardiac arrest holds great importance in clinical practice in order to activate timely preventive measures. ML algorithms have been used to predict cardiac arrest using easily obtained variables as inputs (Table 1).
Intensive Care Unit Yijing L et al., studied a cardiac arrest prediction index in critically ill ICU patients (35). In this study, bedside vital signs monitoring was used as inputs (heart rate, systolic blood pressure, diastolic blood pressure, mean blood pressure, SpO2, and respiratory rate) (35). The cardiac arrest prediction index predicted 95% of cardiac arrest events. Interestingly, 80% of the cardiac arrest events were identified more than 25 min in advance (35). In a study by Kim J et al., the authors used bedside vital signs, underlying disease, laboratory data, medication, and organ failure to predict cardiac arrest in critically ill patients using ML models (36). The proposed model showed a sensitivity between 0.846 and 0.909, and a specificity between 0.923 and 0.946 (36).
Another deep learning model has been proposed for cardiac arrest prediction in ICU patients using physiological and demographic features. The proposed model outperformed the Modified Early Warning Score (MEWS) and National Early Warning Score (NEWS) scores in cardiac arrest prediction at the tested time intervals (17). Tang Q et al., proposed another deep learning model based on time series of vital signs from electronic health records. In this model, features were captured by an efficient temporal convolutional network and explained using the deep Taylor decomposition theoretical framework. The results showed that the model demonstrated superior CA prediction accuracy compared to the standard NEWS score (37). An artificial neural network (ANN) has been developed to predict ventricular tachycardia 1 h before its onset, using parameters obtained from heart rate variability and respiratory rate variability analysis (38). The ventricular tachycardia prediction model achieved a sensitivity of 88%, specificity of 82%, and an AUC of 0.93 (38).
Emergency department
Another topic of interest is the prediction of in-hospital CA in patients who presented to the emergency department. In this setting, a ML model has been implemented using triage data. The authors showed that Random Forest outperformed other ML models (Gradient Boosting and Extra Trees classifier), achieving an AUC of 0.931 (39). Interestingly, although the difference in AUC between each ML model and logistic regression was not significant, ML models performed significantly better than the NEWS scoring system (39). An ML algorithm has also been proposed to predict critical care outcomes, including CA, in patients with chest pain presenting to the emergency department (40). Specifically, a LASSO regression model was developed using easily obtained features. The proposed model significantly outperformed the HEART, GRACE, and TIMI scores achieving an AUC of 0.953 (95% CI: 0.922–0.984) (40). Liu N et al., aimed to identify the most relevant variables for predicting major adverse cardiac events including CA, in patients presented to the emergency department (41). The authors used a novel random forest-based method to select the most relevant variables while a geometric distance-based ML scoring system was implemented to derive the risk score. The use of three variables (systolic blood pressure, the mean electrocardiographic RR interval and the mean instantaneous heart rate) demonstrated good performance in predicting adverse events (AUC: 0.812), outperforming the model using 23 variables (AUC: 0.736), and the conventional TIMI (AUC: 0.637) and MEWS (AUC: 0.622) scores (41).
An ML model incorporating heart rate variability was proposed to predict CA in critically ill patients presenting to the emergency department (42). The results showed that the ML model outperformed the conventional methods in predicting CA within 72 h, with an AUC of 0.781 compared to 0.680 for MEWS (42). ML models developed on triage data have also been proposed to predict in-hospital CA or ICU admissions in patients visiting the emergency department (43). The proposed model demonstrated better sensitivity and accuracy in predicting critical outcomes compared to the assessments made by emergency physicians (43).
Sepsis
ML models have been implemented for the prediction of CA in patients with sepsis. In this setting, the best results were obtained using a stacking algorithm and multivariate dataset (44). The proposed model predicted the arrest incidence with an accuracy and sensitivity of over 70%, up to 6 h earlier. Although ML algorithms outperformed the conventional methods (APACHE II and MEWS scoring variables) for determining the patients' health status, higher sensitivity and specificity are needed for implementation in clinical practice (44). Baral S et al., proposed a deep learning algorithm to reduce the false alarm rates and increase the sensitivity of the previous models for CA prediction in patients with sepsis (45). Specifically, a hybrid model using a multilayer perceptron and enhanced bidirectional Long Short-Term Memory (LSTM) was proposed to handle baseline features and time-series vital signs (45). Compared to the state-of-the-art algorithms, the proposed model improved accuracy, sensitivity, specificity, and AUC, while reducing the false alarm rates.
Respiratory arrest
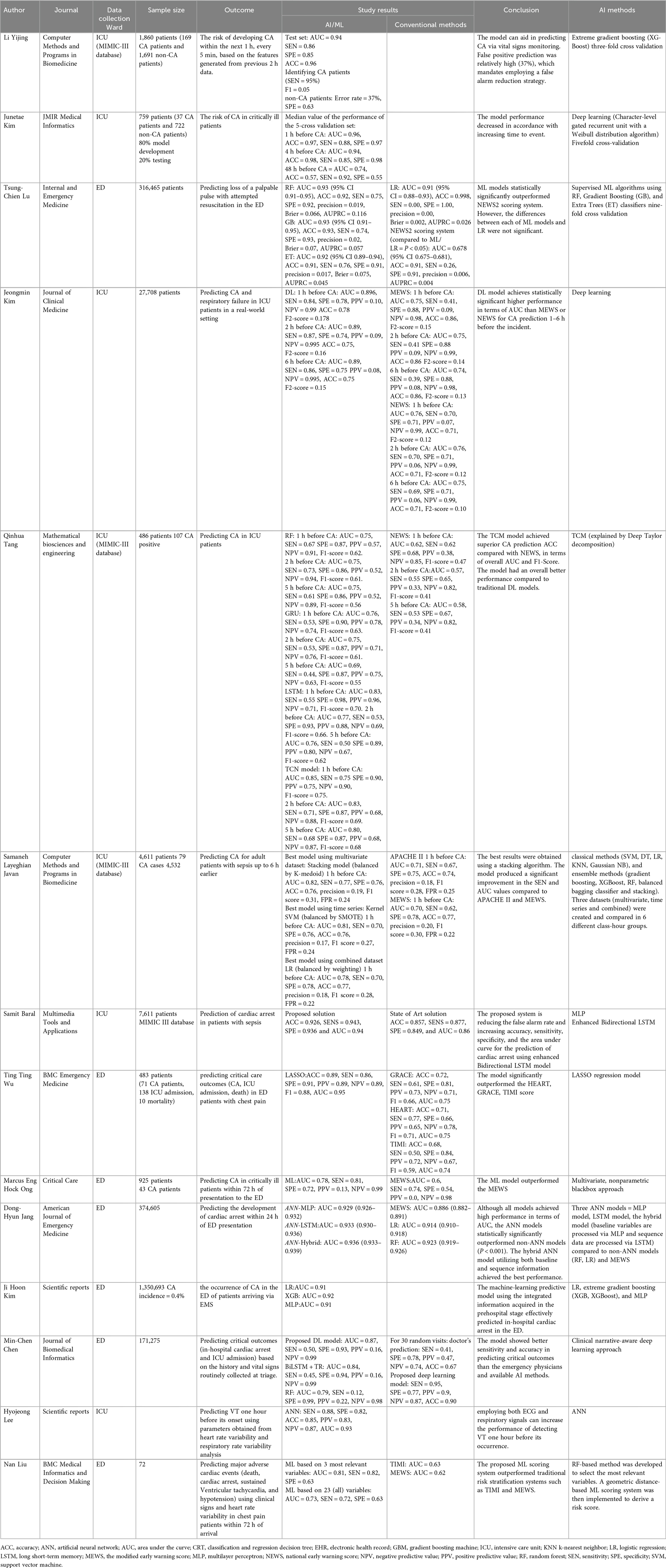
Prediction of RA and the need for mechanical ventilation can help clinicians identify high-risk patients and implement timely preventive measures (Table 2).
COVID-19
The random forest classifier, decision tree classifier, logistic regression, K-nearest neighbors classifier, support vector machine, and gradient boosted machine have been used for the prediction of invasive ventilation in COVID-19 patients admitted to the ICU (46). The random forest and Gradient boosted machine showed the best performance, achieving mean AUCs of 0.69 and 0.68, respectively (46). In the same setting, commonly used clinical variables (heart rate, oxygen saturation, respiratory rate, FIO2, and pH) were used as inputs in a deep learning model for the prediction of mechanical ventilation in hospitalized patients and in those with COVID-19 (47). The proposed model showed good performance (AUC > 0.88) in predicting those needing mechanical ventilation 24 h in advance (47). In addition, a two-step model has been used for the prediction of respiratory failure and invasive mechanical ventilation in critically ill patients suffering from COVID-19 (48). An Extreme Gradient Boosting (XGBoost) algorithm was trained on data from the MIMIC-III database to predict if a patient would require invasive mechanical ventilation within the next 6, 12, 18 or 24 h. The proposed two-step model showed good performance in both the general ICU population and COVID-19 patients (48).
A 3D CT-based deep learning model has also been proposed for the prediction of COVID-19 outcomes, including the need for intubation (49). The prediction results improved when laboratory data were included, while the model accuracy decreased when CT images were excluded (49). A deep convolutional neural network (dCNN) was evaluated to predict inpatient outcomes, including intubation associated with COVID-19 pneumonia (50). Airspace opacity scoring systems, defined by the extent of airspace opacity in each lobe on chest CT scans, were estimated using the deep learning algorithm and used to predict clinical outcomes. Τhe tested algorithm was found to be highly predictive of inpatient outcomes, including intubation (50). De Godoy MF et al., studied the role of CT imaging, assessed by dCNN, in predicting the need for mechanical ventilation in the setting of COVID-19 (51). The high specificity exhibited by the model enabled it to predict which patients may need mechanical ventilation due to COVID-19 infection (51). Bussen S et al., used an unsupervised ML algorithm (the Gaussian mixture model) to predict intubation in COVID-19 patients (52). The algorithm achieved an accuracy of 87.8% for intubation recognition using simple parameters (breathing frequency and SpO2) (52). In addition, XGBoost and Categorical Boosting (CatBoost) algorithms demonstrated high accuracy in predicting the need for mechanical ventilation in COVID-19 patients, using vital signs and demographics for initial triage, in the emergency department (53). In another study, XGBoost and Random Forest outperformed Logistic regression in predicting mechanical ventilation in COVID-19 patients using electronic health records data, in the emergency department (54). Similarly, another study showed that the XGBoost model had the highest mean accuracy for predicting respiratory failure within 48 h of a patient's admission for COVID-19 (55). XGBoost outperformed SMOTEENN XGBoost, Logistic regression, and the Modified Early Warning Score (55). Easily obtained variables were used as inputs including the type of oxygen delivery used in the emergency department, patient age, the Emergency Severity Index level, respiratory rate, serum lactate, and demographic characteristics. In another study, Haimovich AD et al., showed that a bedside ML model (quick COVID-19 Severity Index) that employed 3 variables (respiratory rate, pulse oximetry, and oxygen flow rate), the COVID-19 Severity Index can be used to predict critical respiratory illness in COVID-19 patients (56). These models outperformed the quick Sequential [Sepsis-related] Organ Failure Assessment, CURB-65 and Elixhauser scores. Furthermore, another study showed that ML models (Neural Network, Random Forest, and Classification and Regression Decision Tree) outperformed conventional tools, including the APACHE II score in predicting critical COVID-19 based on clinical parameters on admission (57).
Different clinical settings
Kim J et al., proposed an artificial intelligence model to predict acute respiratory failure 1 h, 2 h, 4 h, and 6 h prior to its occurrence using physiological signatures and past medical history (17). The AUC of this model was 0.869 for respiratory failure 6 h before occurrence. Additionally, the model outperformed the MEWS and NEWS scores (17). Xia M et al., used supervised ML algorithms to predict hypoxemia after extubation in the ICU (58). The authors found that from the tested algorithms (logistic regression, random forest, K-nearest neighbors, support-vector machine, XGBoost, Light Gradient Boosting Machine (LightGBM)), random forest, and Light Gradient Boosting Machine showed the best performance in hypoxemia prediction (58).
ML techniques have been used to predict intubation within 24 h using commonly available bedside and laboratory variables taken at critical care admission. Random forest and logistic regression exhibited good performance for intubation prediction (AUC = 0.86 and 0.77 respectively) (59). Recurrent Neural Network models have been developed to predict the failure of noninvasive respiratory support using time series data (60). The authors showed that a Long-short term memory model had the highest accuracy and AUC compared to a Gated Recurrent Unit and a Gated Recurrent Unit with Trainable Decay (60). In another study, an ML (CatBoost) model was developed to predict noninvasive ventilation failure after extubation (61); fifteen parameters (mechanical ventilation duration, RR, urine output, GCS, mean airway pressure, temperature, age, heart rate, glucose, time from extubation to NIV, mean blood pressure, input volume, SpO2, PaO2, and pH) were used as inputs. The authors showed that the proposed model showed better performance compared to the RF, LR, XGBoost, KNN, Naïve Bayes, Light GBM, SCM, AdaBoost, and MLP (61). Furthermore, a temporal convolutional network-feedforward neural network outperformed the LSTM, feedforward neural networks, and logistic regression in predicting intubation in the critical care setting (62).
An ML algorithm has been used to predict reintubation, prolonged mechanical ventilation and death in patients undergoing coronary artery bypass surgery (63). Specifically, an artificial neural network showed good performance in predicting these outcomes, with no difference compared to the logistic regression model (63). Another novel model for predicting intubation in critically ill patients (64), using data collected within the first hours of admission in the ICU, outperformed the standard clinical benchmarks (64). Recently, a real-time warning algorithm for the prediction of invasive mechanical ventilation in ICU patients was developed (65). The proposed algorithm used seven ML models (LightGBM, Random Forest, Naive Bayes, Neural Networks, Logistic regression, Support Vector Machines, K-Nearest Neighbor), exhibiting improved performance compared to traditional adjustment risk algorithms (65). Interestingly, the model using only non-invasive parameters provided excellent predictive performance, compared to the model using both non-invasive and invasive parameters (65). The Time Updated Light Gradient Boosting Machine model has also been proposed to predict late noninvasive ventilation failure (66), showing better performance in comparison with common models (logistic regression, random forest, LightGBM, XGBoost, artificial neural network, and LSTM) (66).
Implications for clinical practice
The integration of AI/ML models into acute care settings carries significant implications for transforming clinical practice, moving towards more proactive and precise patient management.
Augmented Clinical Decision-Making and Early Intervention AI/ML models offer a substantial opportunity to augment clinician decision-making, particularly for initial risk stratification and triage in high-volume environments like emergency departments. By providing early warnings of impending CA or RA, these models can broaden the “diagnostic and therapeutic window” for intervention, allowing clinicians to initiate preventive measures well before overt deterioration. This proactive approach represents a marked improvement over current reactive responses, which often occur after a critical event has already begun.
Potential for Reduced Morbidity and Mortality The core clinical benefit derived from these models lies in their ability to identify high-risk patients, prompting timely interventions that could significantly reduce in-hospital morbidity and mortality associated with CA/RA. This translates directly to improved patient safety and better overall outcomes, as critical resources and attention can be directed to those most in need, precisely when it matters most.
Enhanced Monitoring and Proactive Care The seamless integration of AI/ML with streaming vital signs and EHR can enable continuous, intelligent monitoring. This capability allows for the detection of subtle physiological shifts indicative of worsening disease, often missed by intermittent manual checks. Such a system moves clinical practice from periodic, interval-based assessments to a more dynamic, real-time surveillance system, fostering a culture of pre-emptive care where interventions are initiated before a full-blown crisis develops.
Necessity of Clinician Education and Workflow Integration For successful implementation, it is crucial that clinicians receive adequate education on how to effectively use and interpret these AI/ML models, “as labeled”. This implies the need for intuitive user interfaces that present complex AI predictions in an understandable format, clear guidelines on alert interpretation, and thoughtful integration into existing clinical workflows to ensure seamless adoption and avoid disruption to established care processes. Without proper training and integration, even the most accurate models may not achieve their full clinical potential.
Addressing Regulatory and Ethical Considerations Prior to widespread clinical adoption, a robust framework must be established to regulate critical issues such as liability for AI-driven decisions, standardized adverse event reporting mechanisms, protocols for system upgrading and maintenance, and stringent cybersecurity measures to protect sensitive patient data. These considerations are foundational for building trust among clinicians and patients and ensuring the responsible and equitable deployment of AI in healthcare.
Recommendations for future research
While the potential of AI/ML in acute care is evident, several critical areas require focused future research to facilitate their successful and safe translation into routine clinical practice.
Rigorous Prospective Validation and Demonstration of Clinical Utility A paramount recommendation is the urgent need for rigorous prospective evaluation of AI/ML models. While retrospective studies have shown considerable promise, future research must move beyond these to large-scale prospective clinical trials that confirm efficacy in real-world settings. Crucially, these trials must demonstrate a tangible impact on clinical endpoints such as patient mortality, reduced length of stay, or decreased incidence of adverse events. Studies must explicitly show how these approaches translate into “actionable care pathways and workflows” that demonstrate clear clinical utility, rather than merely improved statistical prediction.
Standardization of Datasets and Platforms A significant challenge identified is the “lack of uniform datasets and of parameters employed by the proposed AI/ML algorithms”, which currently hinders the assessment of their generalizability and comparability across different institutions. Future research should focus on developing standardized data collection protocols and creating standardized platforms for reporting predictions to clinicians, ensuring interoperability and facilitating broader adoption. Such standardization would enable more robust multi-center studies and foster a collaborative environment for AI development and validation.
Improving Model Specificity to Mitigate Alarm Burden While high sensitivity is highly desirable for life-threatening conditions to ensure no critical event is missed, the specificity of a model must also be high for implementation in clinical practice. A low specificity leads to a high burden of false alarms, which can significantly increase clinician workload, induce stress, and potentially lead to alarm fatigue and desensitization. This desensitization could paradoxically result in missed true events, undermining the very goal of patient safety. The inherent tension between maximizing sensitivity (to avoid missing a critical event) and achieving high specificity (to minimize false alarms) in life-threatening conditions presents a profound ethical and practical dilemma for AI in healthcare. Clinicians are ethically bound to prioritize patient safety, meaning they will naturally lean towards higher sensitivity in predictive tools for conditions like cardiac or RA. However, the consequence of high sensitivity without commensurate specificity is an increased rate of false positives. A high burden of false alarms results in increased workload and stress for healthcare providers and eventually alarm fatigue. Prioritizing the optimization of the balance between sensitivity and specificity to ensure practical utility and avoid clinician burnout necessitates interdisciplinary research involving not just AI developers but also human factors specialists, such as clinicians and healthcare administrators, to design systems that are both statistically effective and clinically usable, perhaps through adaptive alerting systems or tiered alert levels.
Addressing Data Quality, Noise, and Ground-Truth Labeling Real-world clinical data often suffer from “noise” and variability in quality, with some studies reporting valid data for as little as half of the monitoring time. Future research must develop robust methods for handling incomplete or noisy data to ensure model reliability in diverse clinical environments. Furthermore, accurate “ground-truth labels” are fundamental for effective AI/ML algorithm training, and current methods like natural language processing for label generation can be prone to errors, while semi-supervised models remain in the research phase.
Ethical AI Development and Governance Beyond technical performance, future AI/ML models must be developed with explicit consideration of ethical principles, including equity, accuracy, transparency, interpretability, accountability, data privacy, and cybersecurity (32, 33). These considerations are not merely regulatory hurdles but foundational requirements for building trust and ensuring the responsible and equitable integration of AI into clinical care. Furthermore, research into explainable AI and fairness in algorithms will be crucial to address these concerns.
Larger Sample Sizes and Generalizability The current body of evidence largely comprises studies with “relatively small sample sizes”, which limits the generalizability of their findings. Future research must prioritize larger-scale, multi-center studies to validate model performance across diverse patient populations and clinical environments, ensuring robust and generalizable results that can be applied broadly.
Systemic Redesign for Actionable Care Pathways The repeated emphasis on the need for AI models to translate into “actionable care pathways and workflows” signifies that the objective extends far beyond merely developing a technically superior predictive algorithm. An AI model, no matter how accurate, is an inert tool if its predictions do not seamlessly integrate into and actively inform clinical decision-making and subsequent actions. This implies a need for a fundamental redesign of existing clinical processes, rather than simply overlaying AI on top of current practices. For example, an early warning from an AI system must trigger a predefined, efficient, and well-rehearsed response protocol involving specific roles, responsibilities, and interventions. This necessitates interdisciplinary research and development involving not only AI specialists but also clinical workflow experts, engineers, healthcare administrators, and even policy-makers. The ultimate success of AI in healthcare will hinge on its ability to catalyze and support these systemic changes, transforming predictive insights into tangible improvements in patient care delivery and outcomes.
Discussion
In-hospital CA and RA are catastrophic complications of any admission. It is estimated that between 1 and 5 of every 1,000 admissions yearly will result in CA and RA (67), while the survival rate for in-hospital CA remains between 23% and 24% (2, 3, 68). However, efforts to develop early warning scores of deterioration aiming to activate rapid response protocols (6–11), should recognize that there is only a limited time-window to provide pre-emptive care. Retrospective reviews frequently show that signs of deterioration are unobserved or overlooked by medical staff (12, 13). Continuous telemetry monitoring is routine in the ICU and some non-ICU units (69, 70), yet CAs and RAs are still frequent.
To assess whether current developments on ML models can improve outcomes in predicting CA and RA, a systematic search of PubMed, Embase, and Web of Science was conducted. The search strategy focused on critical care settings, AI/ML techniques, and cardiac or RA outcomes. The selection process is detailed in Figures 2A,B, resulting in 14 CA and 22 RA studies included for analysis.
Improving not just survival but also the quality of care for in-hospital CA patients requires a comprehensive set of programs and actions, such as, first, plans and preparation for CA and RA, second, delivery of high-quality, guideline-based resuscitation, third, continuous evaluation and improvement itself within a culture of person-centered care, and fourth, the potential for AI to assist in the prediction and prevention of CA. Although the prediction of cardiac and RA could reduce in-hospital morbidity and mortality, further studies are needed to confirm this in clinical practice. Identification of high-risk patients especially in the emergency department is of great importance (Figure 3). Furthermore, enhanced monitoring and early preventive measures may help identify high-risk hospitalized patients, prevent adverse clinical outcomes, and thus reduce morbidity and mortality. This systematic review shows that ML models may be used for the prediction of both cardiac and RA in the emergency department and in the ICU. Furthermore, the retrospective studies show that the proposed models have a good prediction performance using easily obtained variables. Interestingly, in the prospective studies, although it is not clearly mentioned, the results of the AI/ML prediction models were not shared with the attending physicians, and therefore they did not influence clinical outcomes.
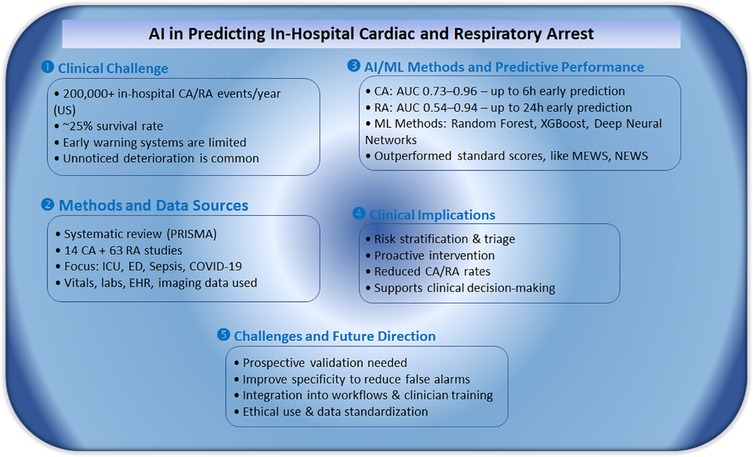
Figure 3. Summary of key findings from a systematic review on AI-based prediction of in-hospital cardiac and respiratory arrest.
While ML algorithms show a promising performance in predicting in-hospital cardiac and RA, the integration of these models into clinical workflows remains a significant challenge. Practical considerations include integration with the electronic health record systems, ensuring data interoperability, and adequate staff training to effectively utilize the predictions from these models to improve clinical-decision outcomes. However, further research is needed to understand the real-world barriers to designing and implementing ML tools in clinical practice.
Limitations
Most of the included studies were of relatively small sample size, and therefore the results should be interpreted with caution. There was also substantial heterogeneity across studies in terms of study design, ML methodologies, and data sources, which may affect the comparability and generalizability of the results. In clinical practice, the quality of data that are required as inputs cannot be identical. Although AI systems have been shown to improve accuracy over traditional diagnostic systems, albeit with a broad range of accuracy, prospective studies on the clinical validation of these models for forecasting clinical deterioration are important, yet they are relatively sparse. The specificity of a model must be high for implementation in clinical practice. A low specificity will lead in a high burden of false alarms that will increase the workload and stress of healthcare providers. Furthermore, prospective studies are needed not only to further establish the accuracy and generalizability of these approaches, but also their translation to actionable care pathways, which can demonstrate clinical utility.
Conclusions
ML algorithms show promising results for the prediction of in-patient cardiac and RA using easily obtained variables as inputs. If successfully implemented in clinical practice, the ML models could identify high-risk patients and reduce mortality and morbidity. However, further validation and the design of clinical trials will determine the efficacy of the ML models in each clinical setting.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
GT: Data curation, Investigation, Writing – original draft. GB: Data curation, Investigation, Writing – original draft. AG: Data curation, Investigation, Writing – original draft. JZ: Data curation, Investigation, Writing – original draft. SB: Writing – original draft. EI: Writing – original draft. VD: Writing – original draft. JS: Writing – original draft. AA: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. AAA is partly funded by the Institute of Precision Medicine (17UNPG33840017) of the American Heart Association, the RICBAC Foundation, NIH grants 1 R01 HL135335-01, 1 R01 HL161008-01, 1 R21 HL137870-01, 1 R21EB026164-01 and 3R21EB026164-02S1.
Acknowledgments
AAA was partly supported by a Grant-in-Aid (#15GRNT23070001) from the American Heart Association (AHA), the Institute of Precision Medicine (17UNPG33840017) from the AHA, the RICBAC Foundation, NIH grants 1 R01 HL135335-01, 1 R01 HL161008-01, 1 R21 HL137870-01, 1 R21EB026164-01 and 3R21EB026164-02S.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmedt.2025.1681059/full#supplementary-material
References
1. Merchant RM, Yang L, Becker LB, Berg RA, Nadkarni V, Nichol G, et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. (2011) 39:2401–6. doi: 10.1097/CCM.0b013e3182257459
2. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American heart association. Circulation. (2017) 135:e146–603. doi: 10.1161/CIR.0000000000000485
3. Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS, et al. Trends in survival after in-hospital cardiac arrest. N Engl J Med. (2012) 367:1912–20. doi: 10.1056/NEJMoa1109148
4. McGrath RB. In-house cardiopulmonary resuscitation–after a quarter of a century. Ann Emerg Med. (1987) 16:1365–8. doi: 10.1016/S0196-0644(87)80420-1
5. Kolte D, Khera S, Aronow WS, Palaniswamy C, Mujib M, Ahn C, et al. Regional variation in the incidence and outcomes of in-hospital cardiac arrest in the United States. Circulation. (2015) 131:1415–25. doi: 10.1161/CIRCULATIONAHA.114.014542
6. Ko RE, Kwon O, Cho KJ, Lee YJ, Kwon JM, Park J, et al. Quick sequential organ failure assessment score and the modified early warning score for predicting clinical deterioration in general ward patients regardless of suspected infection. J Korean Med Sci. (2022) 37:e122. doi: 10.3346/jkms.2022.37.e122
7. Haegdorens F, Monsieurs KG, De Meester K, Van Bogaert P. An intervention including the national early warning score improves patient monitoring practice and reduces mortality: a cluster randomized controlled trial. J Adv Nurs. (2019) 75:1996–2005. doi: 10.1111/jan.14034
8. Bell D, Baker J, Williams C, Bassin L. A trend-based early warning score can be implemented in a hospital electronic medical record to effectively predict inpatient deterioration. Crit Care Med. (2021) 49:e961–7. doi: 10.1097/CCM.0000000000005064
9. Saab A, Khalil A, Jammal C, Saikali M, Lamy M, B J. Early prediction of all-cause clinical deterioration in general wards patients: development and validation of a biomarker-based machine learning model derived from rapid response team activations. J Patient Saf. (2022) 18:578–86. doi: 10.1097/PTS.0000000000001069
10. Yazdanyar A, Greenberg MR, Chen Z, Li S, Greenberg MR, Buonanno AP, et al. A customized early warning score enhanced emergency department patient flow process and clinical outcomes in a COVID-19 pandemic. J Am Coll Emerg Physicians Open. (2022) 3:e12783. doi: 10.1002/emp2.12783
11. Hyun DG, Lee SY, Ahn JH, Huh JW, Hong SB, Koh Y, et al. Mortality of patients with hospital-onset sepsis in hospitals with all-day and non-all-day rapid response teams: a prospective nationwide multicenter cohort study. Critical Care. (2022) 26:280. doi: 10.1186/s13054-022-04149-z
12. Franklin C, Mathew J. Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event. Crit Care Med. (1994) 22:244–7. doi: 10.1097/00003246-199402000-00014
13. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. (1990) 98:1388–92. doi: 10.1378/chest.98.6.1388
14. DeVita MA, Smith GB, Adam SK, Adams-Pizarro I, Buist M, Bellomo R, et al. “Identifying the hospitalised patient in crisis”–a consensus conference on the afferent limb of rapid response systems. Resuscitation. (2010) 81:375–82. doi: 10.1016/j.resuscitation.2009.12.008
15. Hravnak M, Devita MA, Clontz A, Edwards L, Valenta C, Pinsky MR. Cardiorespiratory instability before and after implementing an integrated monitoring system. Crit Care Med. (2011) 39:65–72. doi: 10.1097/CCM.0b013e3181fb7b1c
16. Edelson DP. A weak link in the rapid response system. Arch Intern Med. (2010) 170:12–3. doi: 10.1001/archinternmed.2009.466
17. Kim J, Chae M, Chang HJ, Kim YA, Park E. Predicting cardiac arrest and respiratory failure using feasible artificial intelligence with simple trajectories of patient data. J Clin Med. (2019) 8:1336. doi: 10.3390/jcm8091336
18. Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the national early warning score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. (2013) 84:465–70. doi: 10.1016/j.resuscitation.2012.12.016
19. Downey CL, Tahir W, Randell R, Brown JM, Jayne DG. Strengths and limitations of early warning scores: a systematic review and narrative synthesis. Int J Nurs Stud. (2017) 76:106–19. doi: 10.1016/j.ijnurstu.2017.09.003
20. Veldhuis LI, Ridderikhof ML, Bergsma L, Van Etten-Jamaludin F, Nanayakkara PW, Hollmann M. Performance of early warning and risk stratification scores versus clinical judgement in the acute setting: a systematic review. Emerg Med J. (2022) 39:918–23. doi: 10.1136/emermed-2021-211524
21. Au-Yeung WM, Sahani AK, Isselbacher EM, Armoundas AA. Reduction of false alarms in the intensive care unit using an optimized machine learning based approach. NPJ Digit Med. (2019) 2:86. doi: 10.1038/s41746-019-0160-7
22. Au-Yeung WM, Sevakula RK, Sahani AK, Kassab M, Boyer R, Isselbacher EM, et al. Real-time machine learning-based intensive care unit alarm classification without prior knowledge of the underlying rhythm. Eur Heart J Digit Health. (2021) 2:437–45. doi: 10.1093/ehjdh/ztab058
23. Bazoukis G, Bollepalli SC, Chung CT, Li X, Tse G, Bartley BL, et al. Application of artificial intelligence in the diagnosis of sleep apnea. J Clin Sleep Med. (2023) 19:1337–63. doi: 10.5664/jcsm.10532
24. Bazoukis G, Stavrakis S, Zhou J, Bollepalli SC, Tse G, Zhang Q, et al. Machine learning versus conventional clinical methods in guiding management of heart failure patients-a systematic review. Heart Fail Rev. (2020) 26(1):23–34. doi: 10.1007/s10741-020-10007-3
25. Bollepalli SC, Sahani AK, Aslam N, Mohan B, Kulkarni K, Goyal A, et al. An optimized machine learning model accurately predicts in-hospital outcomes at admission to a cardiac unit. Diagnostics. (2022) 12:241. doi: 10.3390/diagnostics12020241
26. Bollepalli SC, Sevakula RK, Au-Yeung WM, Kassab MB, Merchant FM, Bazoukis G, et al. Real-time arrhythmia detection using hybrid convolutional neural networks. J Am Heart Assoc. (2021) 10:e023222. doi: 10.1161/JAHA.121.023222
27. Chung CT, Lee S, King E, Liu T, Armoundas AA, Bazoukis G, et al. Clinical significance, challenges and limitations in using artificial intelligence for electrocardiography-based diagnosis. Int J Arrhythmia. (2022) 23:24. doi: 10.1186/s42444-022-00075-x
28. Chung CT, Bazoukis G, Lee S, Liu Y, Liu T, Letsas KP, et al. Machine learning techniques for arrhythmic risk stratification=a review of the literature. Int J Arrhythmia. (2022) 23:10. doi: 10.1186/s42444-022-00062-2
29. Spector-Bagdady K, Armoundas AA, Arnaout R, Hall JL, Yeager McSwain B, Knowles JW, et al. Principles for health information collection, sharing, and use=A policy statement from the American heart association. Circulation. (2023) 148:1061–9. doi: 10.1161/CIR.0000000000001173
30. Sevakula RK, Au-Yeung WM, Singh JP, Heist EK, Isselbacher EM, Armoundas AA. State-of-the-art machine learning techniques aiming to improve patient outcomes pertaining to the cardiovascular system. J Am Heart Assoc. (2020) 9:e013924. doi: 10.1161/JAHA.119.013924
31. Bzdok D, Altman N, Krzywinski M. Statistics versus machine learning. Nat Methods. (2018) 15:233–4. doi: 10.1038/nmeth.4642
32. Armoundas AA, Narayan SM, Arnett DK, Spector-Bagdady K, Bennett DA, Celi LA, et al. Use of artificial intelligence in improving outcomes in heart disease: a scientific statement from the American heart association. Circulation. (2024) 149(14):e1028–50. doi: 10.1161/CIR.0000000000001201
33. Bazoukis G, Hall J, Loscalzo J, Antman EM, Fuster V, Armoundas AA. The inclusion of augmented intelligence in medicine: a framework for successful implementation. Cell Rep Med. (2022) 3:100485. doi: 10.1016/j.xcrm.2021.100485
34. Pearson TA, Vitalis D, Pratt C, Campo R, Armoundas AA, Au D, et al. The science of precision prevention: research opportunities and clinical applications to reduce cardiovascular health disparities. JACC Adv. (2024) 3(1):100759. doi: 10.1016/j.jacadv.2023.100759
35. Yijing L, Wenyu Y, Kang Y, Shengyu Z, Xianliang H, Xingliang J, et al. Prediction of cardiac arrest in critically ill patients based on bedside vital signs monitoring. Comput Methods Programs Biomed. (2022) 214:106568. doi: 10.1016/j.cmpb.2021.106568
36. Kim J, Park YR, Lee JH, Lee JH, Kim YH, Huh JW. Development of a real-time risk prediction model for in-hospital cardiac arrest in critically ill patients using deep learning: retrospective study. JMIR Med Inform. (2020) 8:e16349. doi: 10.2196/16349
37. Tang Q, Cen X, Pan C. Explainable and efficient deep early warning system for cardiac arrest prediction from electronic health records. Math Biosci Eng. (2022) 19:9825–41. doi: 10.3934/mbe.2022457
38. Lee H, Shin SY, Seo M, Nam GB, Joo S. Prediction of ventricular tachycardia one hour before occurrence using artificial neural networks. Sci Rep. (2016) 6:32390. doi: 10.1038/srep32390
39. Lu TC, Wang CH, Chou FY, Sun JT, Chou EH, Huang EP, et al. Machine learning to predict in-hospital cardiac arrest from patients presenting to the emergency department. Intern Emerg Med. (2023) 18:595–605. doi: 10.1007/s11739-022-03143-1
40. Wu TT, Zheng RF, Lin ZZ, Gong HR, Li H. A machine learning model to predict critical care outcomes in patient with chest pain visiting the emergency department. BMC Emerg Med. (2021) 21:112. doi: 10.1186/s12873-021-00501-8
41. Liu N, Koh ZX, Goh J, Lin Z, Haaland B, Ting BP, et al. Prediction of adverse cardiac events in emergency department patients with chest pain using machine learning for variable selection. BMC Med Inform Decis Mak. (2014) 14:75. doi: 10.1186/1472-6947-14-75
42. Ong ME, Lee Ng CH, Goh K, Liu N, Koh ZX, Shahidah N, et al. Prediction of cardiac arrest in critically ill patients presenting to the emergency department using a machine learning score incorporating heart rate variability compared with the modified early warning score. Critical Care. (2012) 16:R108. doi: 10.1186/cc11396
43. Chen MC, Huang TY, Chen TY, Boonyarat P, Chang YC. Clinical narrative-aware deep neural network for emergency department critical outcome prediction. J Biomed Inform. (2023) 138:104284. doi: 10.1016/j.jbi.2023.104284
44. Layeghian Javan S, Sepehri MM, Layeghian Javan M, Khatibi T. An intelligent warning model for early prediction of cardiac arrest in sepsis patients. Comput Methods Programs Biomed. (2019) 178:47–58. doi: 10.1016/j.cmpb.2019.06.010
45. Sharma K, Alsadoon A, Prasad PWC, Al-Dala'in T, Nguyen TQV, Pham DTH. A novel solution of using deep learning for left ventricle detection: enhanced feature extraction. Comput Methods Programs Biomed. (2020) 197:105751. doi: 10.1016/j.cmpb.2020.105751
46. Karri R, Chen YP, Burrell AJC, Penny-Dimri JC, Broadley T, Trapani T, et al. Machine learning predicts the short-term requirement for invasive ventilation among Australian critically ill COVID-19 patients. PLoS One. (2022) 17:e0276509. doi: 10.1371/journal.pone.0276509
47. Shashikumar SP, Wardi G, Paul P, Carlile M, Brenner LN, Hibbert KA, et al. Development and prospective validation of a deep learning algorithm for predicting need for mechanical ventilation. Chest. (2021) 159:2264–73. doi: 10.1016/j.chest.2020.12.009
48. Bendavid I, Statlender L, Shvartser L, Teppler S, Azullay R, Sapir R, et al. A novel machine learning model to predict respiratory failure and invasive mechanical ventilation in critically ill patients suffering from COVID-19. Sci Rep. (2022) 12:10573. doi: 10.1038/s41598-022-14758-x
49. Di Napoli A, Tagliente A, Pasquini E, Cipriano L, Pietrantonio E, Ortis F, et al. 3D CT-inclusive deep-learning model to predict mortality, ICU admittance, and intubation in COVID-19 patients. J Digit Imaging. (2023) 36:603–16. doi: 10.1007/s10278-022-00734-4
50. Chamberlin JH, Aquino G, Schoepf UJ, Nance S, Godoy F, Carson L, et al. An interpretable chest CT deep learning algorithm for quantification of COVID-19 lung disease and prediction of inpatient morbidity and mortality. Acad Radiol. (2022) 29:1178–88. doi: 10.1016/j.acra.2022.03.023
51. de Godoy MF, Chatkin JM, Rodrigues RS, Forte GC, Marchiori E, Gavenski N, et al. Artificial intelligence to predict the need for mechanical ventilation in cases of severe COVID-19. Radiol Bras. (2023) 56:81–5. doi: 10.1590/0100-3984.2022.0049
52. Boussen S, Cordier PY, Malet A, Simeone P, Cataldi S, Vaisse C, et al. Triage and monitoring of COVID-19 patients in intensive care using unsupervised machine learning. Comput Biol Med. (2022) 142:105192. doi: 10.1016/j.compbiomed.2021.105192
53. Yu L, Halalau A, Dalal B, Abbas AE, Ivascu F, Amin M, et al. Machine learning methods to predict mechanical ventilation and mortality in patients with COVID-19. PLoS One. (2021) 16:e0249285. doi: 10.1371/journal.pone.0249285
54. Heldt FS, Vizcaychipi MP, Peacock S, Cinelli M, McLachlan L, Andreotti F, et al. Early risk assessment for COVID-19 patients from emergency department data using machine learning. Sci Rep. (2021) 11:4200. doi: 10.1038/s41598-021-83784-y
55. Bolourani S, Brenner M, Wang P, McGinn T, Hirsch JS, Barnaby D, et al. A machine learning prediction model of respiratory failure within 48 hours of patient admission for COVID-19= model development and validation. J Med Internet Res. (2021) 23:e24246. doi: 10.2196/24246
56. Haimovich AD, Ravindra NG, Stoytchev S, Young HP, Wilson FP, van Dijk D, et al. Development and validation of the quick COVID-19 severity index: a prognostic tool for early clinical decompensation. Ann Emerg Med. (2020) 76:442–53. doi: 10.1016/j.annemergmed.2020.07.022
57. Assaf D, Gutman Y, Neuman Y, Segal G, Amit S, Gefen-Halevi S, et al. Utilization of machine-learning models to accurately predict the risk for critical COVID-19. Intern Emerg Med. (2020) 15:1435–43. doi: 10.1007/s11739-020-02475-0
58. Xia M, Jin C, Cao S, Pei B, Wang J, Xu T, et al. Development and validation of a machine-learning model for prediction of hypoxemia after extubation in intensive care units. Ann Transl Med. (2022) 10:577. doi: 10.21037/atm-22-2118
59. Siu BMK, Kwak GH, Ling L, Hui P. Predicting the need for intubation in the first 24 h after critical care admission using machine learning approaches. Sci Rep. (2020) 10:20931. doi: 10.1038/s41598-020-77893-3
60. Essay PT, Mosier JM, Nayebi A, Fisher JM, Subbian V. Predicting failure of noninvasive respiratory support using deep recurrent learning. Respir Care. (2023) 68:488–96. doi: 10.4187/respcare.10382
61. Wang H, Zhao QY, Luo JC, Liu K, Yu SJ, Ma JF, et al. Early prediction of noninvasive ventilation failure after extubation: development and validation of a machine-learning model. BMC Pulm Med. (2022) 22:304. doi: 10.1186/s12890-022-02096-7
62. Catling FJR, Wolff AH. Temporal convolutional networks allow early prediction of events in critical care. J Am Med Inform Assoc. (2020) 27:355–65. doi: 10.1093/jamia/ocz205
63. Mendes RG, de Souza CR, Machado MN, Correa PR, Di Thommazo-Luporini L, Arena R, et al. Predicting reintubation, prolonged mechanical ventilation and death in post-coronary artery bypass graft surgery: a comparison between artificial neural networks and logistic regression models. Arch Med Sci. (2015) 11:756–63. doi: 10.5114/aoms.2015.48145
64. Venturini M, Van Keilegom I, De Corte W and Vens C. A novel survival analysis approach to predict the need for intubation in intensive care units. In: Michalowski M, Abidi SSR, Abidi S, editors. Artificial Intelligence in Medicine, AIME 2022, Lecture Notes in Computer Science, Vol. 13263. Cham: Springer (2022). pp. 358–64.
65. Pei X, Yu H, Wu Y, Zhou X. Correlation between APACHE II scores and delirium probability of senile severe pneumonia patients undergoing invasive mechanical ventilation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2017) 29:821–4. doi: 10.1007/978-3-031-09342-5_35
66. Feng X, Pan S, Yan M, Shen Y, Liu X, Cai G, et al. Dynamic prediction of late noninvasive ventilation failure in intensive care unit using a time adaptive machine model. Comput Methods Programs Biomed. (2021) 208:106290. doi: 10.1016/j.cmpb.2021.106290
67. Sandroni C, Nolan J, Cavallaro F, Antonelli M. In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Med. (2007) 33:237–45. doi: 10.1007/s00134-006-0326-z
68. Robinson GR II, Hess D. Postdischarge survival and functional status following in-hospital cardiopulmonary resuscitation. Chest. (1994) 105:991–6. doi: 10.1378/chest.105.4.991
69. Drew BJ, Califf RM, Funk M, Kaufman ES, Krucoff MW, Laks MM, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the councils on cardiovascular nursing, clinical cardiology, and cardiovascular disease in the young: endorsed by the international society of computerized electrocardiology and the American association of critical-care nurses. Circulation. (2004) 110:2721–46. doi: 10.1161/01.CIR.0000145144.56673.59
70. Funk M, Winkler CG, May JL, Stephens K, Fennie KP, Rose LL, et al. Unnecessary arrhythmia monitoring and underutilization of ischemia and QT interval monitoring in current clinical practice: baseline results of the practical use of the latest standards for electrocardiography trial. J Electrocardiol. (2010) 43:542–7. doi: 10.1016/j.jelectrocard.2010.07.018
Keywords: machine learning, artificial intelligence, cardiac arrest, respiratory arrest, intensive care unit
Citation: Thambiraj G, Bazoukis G, Ghabousian A, Zhou J, Bollepalli SC, Isselbacher EM, Donahue V, Singh JP and Armoundas AA (2025) Use of artificial intelligence in predicting in-hospital cardiac and respiratory arrest in an acute care environment—implications for clinical practice. Front. Med. Technol. 7:1681059. doi: 10.3389/fmedt.2025.1681059
Received: 6 August 2025; Accepted: 24 September 2025;
Published: 10 October 2025.
Edited by:
Arun H.S. Kumar, University College Dublin, IrelandReviewed by:
Zahra Khosravi, Smurfit Building Beaumont Hospital, RCSI, IrelandSahil Malhotra, American College Dublin, Ireland
Copyright: © 2025 Thambiraj, Bazoukis, Ghabousian, Zhou, Bollepalli, Isselbacher, Donahue, Singh and Armoundas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonis A. Armoundas, YXJtb3VuZGFzLmFudG9uaXNAbWdoLmhhcnZhcmQuZWR1
†These authors have contributed equally to this work
 Geerthy Thambiraj
Geerthy Thambiraj George Bazoukis
George Bazoukis Amir Ghabousian4,†
Amir Ghabousian4,† Jiandong Zhou
Jiandong Zhou Sandeep Chandra Bollepalli
Sandeep Chandra Bollepalli Vivian Donahue
Vivian Donahue Antonis A. Armoundas
Antonis A. Armoundas