- 1Department of Kinesiology and Physical Education, Northern Illinois University, Dekalb, IL, United States
- 2Division of Allied Health and Communicative Disorders, Northern Illinois University, Dekalb, IL, United States
Concussions pose significant health risks across the lifespan, with most symptoms typically resolving within four weeks in otherwise healthy adults. However, emerging research suggests that individuals with a history of concussion—even after receiving medical clearance—may face an increased risk of subsequent upper- and lower-extremity musculoskeletal injuries. Additionally, prior concussions have been linked to an elevated risk of osteoarthritis later in life. The 6th Consensus Statement on Concussion in Sport highlights the urgent need for further research into recovery determinants and the long-term neurodegenerative consequences of concussion. This mini-review explores the potential neurodegenerative sequelae following concussion and examines the role of neuromuscular exercise interventions in mitigating these effects. By addressing these concerns, such interventions may help reduce concussion-related injury risks and enhance long-term health outcomes.
1 Introduction
Concussions are a significant public health concern and result in complex health consequences that may persist for life (1–7). These injuries are caused by traumatic force to the brain and result in a variety of signs and symptoms, including headaches, cognitive decline, emotional instability, changes to sleep patterns, and biomechanic alterations (6). Concussions are believed to create functional disturbances that result in the observed signs and symptoms (6). Recent research has shown a correlation between previous concussions and lower extremity musculoskeletal injuries (2–4), and upper extremity musculoskeletal injuries (7), and another group of literature has explored the elevated risk of osteoarthritis following concussion (5). The 6th Consensus Statement on Concussion in Sport highlights a critical need for further investigation into the factors that determine recovery and the long-term neurodegenerative consequences of concussion (6). In response to this call, this mini-review explores the potential neurodegenerative outcomes associated with concussion and assesses how neuromuscular exercise interventions may mitigate these effects as a means to incite future research in this area. This mini-review aims to drive future research innovation and clinical application by highlighting the current state of research in this area; the subsequent research may lead to strategies that could reduce the risk of concussion-related injuries and promote better long-term health outcomes for individuals who have sustained a concussion.
2 Historical context of coordination, balance, and dual-task concussion testing
Underreporting of concussion symptoms is well-documented and may increase the risk of subsequent injury (8). This risk is further exacerbated when clinicians rely heavily on singular tests or symptom scores only (6). Regarding postural control and balance, these symptoms were potentially previously overlooked due to the lack of sensitive and specific clinical tools. For example, the modified Balance Error Scoring System (mBESS) is intended to quantify balance impairment and implemented as one of many clinical metrics to assess coordination and balance progression, as described in the Sport Concussion Assessment Tool 6th Edition (SCAT6) and The Sports Concussion Office Assessment Tool 6th Edition (SCOAT6) (9, 10). The mBESS may only identify gross postural instability within the first three days of a concussion, with sensitivity and specificity decreasing to 0.10 and 0.66, respectively, after 72 h (11, 12). The low sensitivity and moderate specificity may limit the mBESS's identification of coordination and balance changes throughout the course of recovery. A systematic review and meta-analysis revealed that individuals who sustained a concussion yet were deemed recovered needed to be challenged physically and cognitively to detect balance and gait alterations with research-grade equipment (13).
The 6th International Conference on Concussion in Sport Conference released the most recent Consensus Statement on Concussion in Sport in 2023 (6). This document outlines the importance of individualized rehabilitation in order to negate any lingering symptoms after medical clearance (6). Part of this strategy involves adapting the tandem gait test to the clinical evaluation of concussion to identify concussion-related motor disturbances (6). Tandem gait has been shown to have superior sensitivity and specificity to the mBESS. During acute concussion, single-task tandem gait has been reported to have a moderate sensitivity of 0.63 and a specificity of 0.61 (14). Conversely, dual-task tandem gait has been shown to have moderate to high sensitivity and specificity of 0.85 and 0.72, respectively (15). Despite showing greater sensitivity and specificity to concussion-related motor disturbances, tandem gait has limitations. The main outcome measure of this test is time to completion, which involves patients walking a total of six meters with tandem gait while the clinician records the time of the patient to complete the task. Therefore, by only using time as the outcome measure, clinicians may be unable to observe and quantify concussion-related coordination and balance deficits accurately. Unaddressed coordination and balance deficits following concussion may plausibly contribute to neurodegenerative changes over time. The next section will examine the potential neuromuscular alterations at the motor unit level in the context of recovery determinants and the long-term sequelae of post-concussion neurodegenerative effects.
3 Concussion-related long-term neurodegenerative effects
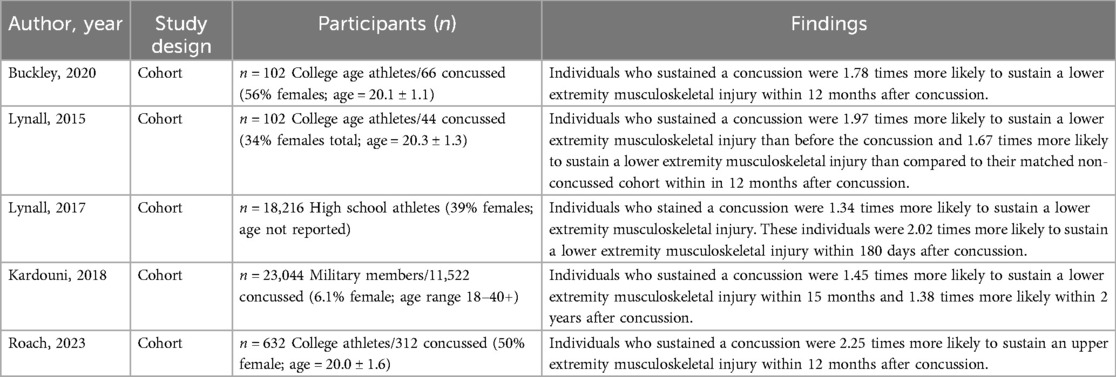
Concussion-related deficits to coordination, balance, and gait may have been previously dismissed due to their potentially innocuous nature and minimal impact on the individual's gross motor function (13). However, individuals who have sustained a concussion are known to be at a greater risk of musculoskeletal injury, dexterity limitations, and disability (1–5, 7). For example, Kardouni and colleagues demonstrated that concussed individuals were at a 45% greater risk of lower extremity musculoskeletal risk 15 months following injury in a military sample (1). Similarly, Lynall and colleagues found that high school and college athletes were at an increased risk of lower extremity musculoskeletal injury following concussion (2, 4). Roach and colleagues found that individuals who sustained a concussion were at 1.84–2.25 times the risk of upper extremity musculoskeletal injury within a year following concussion (7). This was further confirmed by Buckley and colleagues, who found individuals who sustained a concussion were 1.78 times more likely to sustain a subsequent musculoskeletal injury (3). More details on these studies can be found in Table 1.
A history of concussion has been implicated in developing osteoarthritis and osteoarthritis-related disability later in life as well (5, 16). Lynall and colleagues observed that individuals with a history of concussion, even without a subsequent lower extremity musculoskeletal injury, were at a higher risk of developing osteoarthritis compared to those with no concussion history (5). This risk was notably elevated among individuals under 55 years of age who had experienced both a concussion and a subsequent lower extremity musculoskeletal injury (5). These findings suggest that independent of direct musculoskeletal injury—which is known to increase osteoarthritis risk—concussion alone may contribute to long-term neurodegenerative changes that heighten osteoarthritis susceptibility. Given that osteoarthritis often leads to chronic disability and reduced quality of life, these results indicate that concussion could be a contributing factor to physical disability in young and middle-aged adults (5, 16).
There currently exists a dearth of knowledge surrounding the mechanisms and variables that influence the relationship between concussion and risk factors for long-term neurodegenerative effects, such as musculoskeletal injury risk and osteoarthritis development. While there are likely numerous potential contributors, concussion-related subclinical neuromuscular adaptations may provide some insight. Osteoarthritis can develop as a result of biomechanical changes to limb movement, which can be caused by neuromuscular adaptations, such as altered motor unit recruitment or changes in the timing of motor unit firing (17). These adaptations can gradually change how forces are distributed across the joint surface, leading to increased cartilage wear and progression of osteoarthritis (17). The relationships between neuromuscular adaptations and osteoarthritis (in addition to other mobility decrements) have been documented following peripheral injuries (16), but relatively little research exists on the impact of neuromuscular adaptations at the motor unit level following concussion.
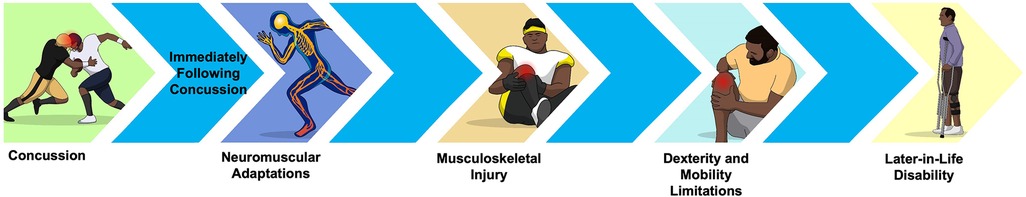
It is hypothesized that neuromuscular adaptations may become evident during physically and cognitively demanding tasks, as seen in documented balance and gait deficits post-concussion (13). These adaptations may represent early indicators of concussion-related neurodegenerative effects. As illustrated in Figure 1, subclinical neuromuscular adaptations may lead to biomechanic changes, including increased center of pressure (COP) sway and more conservative gait strategies (13). Over time, these changes could heighten the risk of musculoskeletal injury (16, 18), which, in turn, elevates the risk of diminished dexterity later in life—ultimately increasing susceptibility to osteoarthritis and related disability (18). It is important to note that the directional arrows in Figure 1 are theoretical and not indicative of causation but serve as a conceptual framework suggesting how neuromuscular adaptations could contribute to osteoarthritis-related disability over time. This framework underscores the importance of further research into motor unit level neuromuscular adaptations post-concussion to understand recovery. The next section will discuss the current state of research on concussion-related subclinical neuromuscular adaptations, which may underlie the neurodegenerative effects just discussed.
4 Concussion-related subclinical neuromuscular adaptations
One of the primary neuromuscular adaptions affected by concussions is impaired proprioception and postural control. Concussions can disrupt the brain's ability to integrate sensory information from various sources, such as the visual, vestibular, and somatosensory systems, leading to poor coordination, increased postural sway, and gait deficits (13). These observed changes may be from subclinical adaptations in the frontal and parietal lobes of the brain, which result in alterations to neuromuscular control at the motor unit level (13, 19). The central nervous system's neural pathways responsible for coordinating muscle activity may be disrupted or altered, leading to abnormal muscle firing sequences, reduced muscle strength, and compensatory movement strategies. The relationship between abnormal muscle activation patterns and poor performance implies that concussion-related biomechanic changes may be amplified with the demands of sensory processing tasks. Several studies have theorized that subclinical neuromuscular adaptations following concussion are associated with subsequent injury incidence (13, 20, 21). To our knowledge, only one study has examined neuromuscular adaptions at the motor unit level post-concussion. Jain and colleagues utilized electromyography (EMG) during gait tasks to find that adolescents who had suffered a concussion exhibited significantly higher instantaneous mean frequency, as indicated by the first functional principal component, in the tibialis anterior, biceps femoris, and semitendinosus muscles during both single-task and dual-task conditions compared to uninjured adolescents (20). These findings suggest that concussed adolescents experience inefficient recruitment of motor units, lasting beyond two weeks after the injury, irrespective of whether they are performing a single task or a dual task involving a secondary task (20).
These changes in muscle recruitment patterns can place additional strain on muscles, tendons, and joints, heightening the risk of overuse injuries or aggravating existing musculoskeletal conditions (21). Other post-concussion symptoms, such as dizziness and headache, along with signs like delayed reaction time and impaired visual-motor integration, may further increase injury risk by slowing responses to external stimuli or obstacles (22). As illustrated in Figure 1, if these neuromuscular adaptations become ingrained in movement patterns, they may lead to lifelong changes that contribute to neurodegenerative effects over time. Therefore, it is essential to conduct more research to uncover the underlying mechanisms of persistent neuromuscular adaptations after a concussion to contribute to the determinants of recovery (such as a diagnostic test) and understand the long-term neurodegenerative effect sequelae. Additional EMG studies, in particular, may be needed to address this gap. Identifying these mechanisms could support the development of evidence-based therapeutic interventions targeting concussion-related subclinical neuromuscular adaptations, potentially reducing the risk of neurodegenerative effects linked to concussion. The next section will draw parallels from various neuromuscular populations to examine potential exercise intervention frameworks to improve concussion-related neuromuscular adaptations.
5 Theoretical exercise intervention framework to improve neuromuscular adaptations
Interventions after concussion have traditionally been reserved for individuals who have persistent and severe symptoms lasting more than two to four weeks post-injury (23). The severe and persistent symptoms require a multimodal assessment approach involving professionals from various disciplines, such as physicians, athletic training, physical therapy, psychology, ophthalmology, audiology, and exercise physiology, targeting various aspects, including sports, neurorehabilitation, vestibular therapy, and cognitive rehabilitation (24). The rehabilitation interventions employed, in practice, are likely to incorporate theoretical principles from motor control, cognitive, and psychological sciences to treat acute symptoms. However, evidence-based interventions that can be implemented across healthcare domains to treat persistent concussion-related symptoms after medical clearance are lacking. This deficit of interventions may be placing individuals who experience concussion-related subclinical neuromuscular adaptations at a greater risk of neurodegenerative effects (13, 20, 21).
Current concussion return-to-activity protocols place a large emphasis on the presence of symptoms and symptom reduction to facilitate return (6). This approach, as explained by the 6th International Conference on Concussion in Sport, does allow for minimal symptom exacerbation with learning and exercise but overall ensures that patients are symptom-free before resuming activities (9). While this method effectively addresses immediate symptoms, it may not adequately account for the neuromuscular adaptations that persist post-concussion, potentially leaving patients vulnerable to further complications in both the short and long-term. Exercise interventions have been shown to be effective in decreasing neuromuscular symptoms and neurodegenerative effects in a variety of populations, yet there is limited research on their effects post-concussion. To facilitate this future research, an exercise intervention framework that decreases concussion-related neuromuscular adaptations should be explored so that the risk of concussion-related neurodegenerative effects is decreased. Given that concussion affects cognitive and physical performance, a therapeutic framework may need to include cognitive and physical components.
Research connecting outcomes from coordination and balance assessments in concussion evaluations to subsequent neuromuscular adaptations is limited. Current return-to-play guidelines prioritize restoring patients to baseline function, often leading to discharge without further examination of potential neuromuscular adaptations. Protocols should address the link between deviations in coordination and balance from baseline and the emergence of neuromuscular adaptations. Previous studies indicate that early aerobic exercise can effectively reduce post-concussion symptoms compared to no exercise intervention (25, 26). While symptom reduction may be achieved with aerobic exercise alone, a more comprehensive intervention framework may be necessary to address concussion-related neuromuscular adaptations. For individuals who continue to exhibit these adaptations, targeted interventions could play a critical role in minimizing the risk of longer-term neurodegenerative effects.
Research from populations with neuromuscular conditions may provide insight when considering a theoretical exercise intervention framework. Research in individuals with Parkinson's disease (27), multiple sclerosis (28), and osteoarthritis (29) has demonstrated the efficacy of neuromuscular rehabilitation programs in improving motor function, reducing injury risk, and enhancing overall quality of life. These programs often incorporate principles from motor control and cognitive sciences, utilizing a combination of physical exercises and cognitive-motor interference tasks to challenge and retrain the neuromuscular system. Rosenfeldt and colleagues found dual-task gait training improved gait performance in individuals with Parkinson's disease, with tasks having the greatest complexity had superior benefits (27). Elwishy and colleagues found that dual-task balance exercises improved balance and quality of life in individuals with multiple sclerosis (28). Hiyama and colleagues found that dual-task walking exercises improved motor and cognitive performance in people with osteoarthritis (29). Collectively, these articles show the benefit of dual-tasking to improve motor performance, which may decrease the risk of future injury.
Dual-task exercises have been utilized to examine the effects of acute concussion and as a tool to improve initial recovery before medical clearance (6). However, it is unclear if the benefits of dual-task training continue after the initial stages of recovery, such as the non-contact and contact phases of a return to activity protocol (6). As there is a paucity of diagnostic tools to quantify subclinical neuromuscular alterations following concussion, it is reasonable to speculate that the lack of targeted neuromuscular interventions is contributing to concussion-related neurodegenerative effects. Comparatively, dual-task research in neuromuscular conditions is conducted when there is a clinical change in motor performance. This research is generally conducted in older adults with lower cognitive-motor interface thresholds than their younger counterparts and with significant changes to walking and balance. Therefore, further research is needed to identify the specific physical and cognitive tasks most relevant to a typically younger and healthier population, such as those who sustain a concussion, to develop effective intervention strategies.
6 Practical recommendations
Given the evidence that indicates concussions have the potential to cause subclinical neuromuscular adaptations, such as altered motor unit recruitment and muscle activation, clinicians should consider changes to their return-to-activity protocols. Current return-to-activity protocol recommendations utilize the mBESS and tandem gait test to measure motor control deficits and symptom scores as the standard for progression from activities of daily living to sport- or work-specific activity, with little emphasis on the movement mechanics (6). The mBESS can lose its clinical utility within 72 h after injury (11, 12), and the tadem gait test does not quantify movement deficits, which may limit a clinician's ability to identify these deficits (6). Clinicians may want to consider adding commercially available tools, such as force plates or wearable sensors, to quantify motor control and postural sway as objective measures of the motor control deficits.
Additionally, by only using symptom scores and not examining movement mechanics, clinicians may overlook neuromuscular deficits, which put the individual at risk for injury and long-term neurodegenerative consequences. Clinicians could consider adding objective neuromuscular tests (e.g., EMG testing during dual-task gait or balance testing) to clinical decision-making to identify residual impairment and promote targeted rehabilitation strategies. A potentially clinically feasible tool may be the Landing Error Scoring System, which may be capable of identifying faulty movement mechanics to detect the risk of musculoskeletal injuries (30). However, more research is needed to identify clinically feasible methods to recognize neuromuscular adaptations post-concussion.
Clinicians may also consider incorporating dual-task training into their return-to-activity protocol. This type of training, which has been successfully applied in populations with Parkinson's disease, multiple sclerosis, and osteoarthritis, challenges both motor and cognitive systems and may help with cognitive-motor integration to reduce concussion-related neuromuscular adaptations. There are various dual-task paradigms that clinicians may utilize (31). Clinicians may consider adding cognitive tasks that are relevant to the individual who sustained the concussion while incorporating them with functional and performance-specific activities. More research is needed to examine evidence-based rehabilitation interventions to improve neuromuscular adaptations following concussion.
7 Conclusions and future directions
Research has consistently demonstrated that concussions can have lasting harmful effects, contributing to subsequent musculoskeletal injuries, an increased risk of osteoarthritis, and related long-term disability. The 6th Consensus Statement on Concussion in Sport highlights an urgent need for further investigation into the determinants of recovery and the long-term neurodegenerative sequelae of concussion. This mini-review aimed to address these research gaps by exploring the existing literature on concussion-related neurodegenerative outcomes, examining potential contributing factors such as neuromotor adaptations at the motor unit level, and discussing rehabilitation strategies. This information can be utilized to drive future research and clinical applications to improve the long-term quality of life for individuals who have sustained a concussion.
Moving forward, more research may be essential to uncover the mechanisms underlying well-documented balance and gait alterations in concussed individuals. As early research has shown that individuals who sustain a concussion are at a greater risk of impaired neuromotor control (20), it is important that research attempts to understand the frequency of this impaired neuromotor control. Additionally, specific areas for future investigation include identifying objective diagnostic tests for neuromuscular adaptations and determining the thresholds at which these adaptations may lead to neurodegenerative changes. Developing precise diagnostic tools could allow clinicians to detect neuromuscular adaptations earlier and more accurately. Furthermore, targeted intervention studies are needed to evaluate the effectiveness of specific rehabilitative exercises in mitigating neurodegenerative risks, with a focus on restoring motor control and coordination. Future research should also examine the long-term benefits of incorporating neuromuscular and cognitive rehabilitation into current post-concussion protocols to minimize the likelihood of osteoarthritis and other disabling conditions. Ultimately, a better understanding of these mechanisms could lead to interventions that not only reduce the neurodegenerative effects of concussion but also improve the quality of life and mobility in the affected population, setting a foundation for optimized, long-term care.
Author contributions
TW: Writing – original draft, Writing – review & editing. MK: Writing – original draft, Writing – review & editing. NG: Writing – original draft, Writing – review & editing. MW: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kardouni JR, Shing TL, McKinnon CJ, Scofield DE, Proctor SP. Risk for lower extremity injury after concussion: a matched cohort study in soldiers. J Orthop Sports Phys Ther. (2018) 48(7):533–40. doi: 10.2519/jospt.2018.8053
2. Lynall RC, Mauntel TC, Pohlig RT, Kerr ZY, Dompier TP, Hall EE, et al. Lower extremity musculoskeletal injury risk after concussion recovery in high school athletes. J Athl Train. (2017) 52(11):1028–34. doi: 10.4085/1062-6050-52.11.22
3. Buckley TA, Howard CM, Oldham JR, Lynall RC, Swanik CB, Getchell N. No clinical predictors of postconcussion musculoskeletal injury in college athletes. Med Sci Sports Exercise. (2020) 52(6):1256. doi: 10.1249/MSS.0000000000002269
4. Lynall RC, Mauntel TC, Padua DA, Mihalik JP. Acute lower extremity injury rates increase after concussion in college athletes. Med Sci Sports Exercise. (2015) 47(12):2487–92. doi: 10.1249/MSS.0000000000000716
5. Lynall RC, Pietrosimone B, Kerr ZY, Mauntel TC, Mihalik JP, Guskiewicz KM. Osteoarthritis prevalence in retired national football league players with a history of concussion and lower extremity injury. J Athl Train. (2017) 52(6):518–25. doi: 10.4085/1062-6050-52.2.03
6. Patricios JS, Schneider KJ, Dvorak J, Ahmed OH, Blauwet C, Cantu RC, et al. Consensus statement on concussion in sport: the 6th international conference on concussion in sport-Amsterdam, October 2022. Br J Sports Med. (2023) 57(11):695–711. doi: 10.1136/bjsports-2023-106898
7. Roach MH, Aderman MJ, Ross JD, Kelly TF, Malvasi SR, Posner MA, et al. Risk of upper extremity musculoskeletal injury within the first year after a concussion. Orthop J Sports Med. (2023) 11(5):23259671231163570. doi: 10.1177/23259671231163570
8. Conway FN, Domingues M, Monaco R, Lesnewich LM, Ray AE, Alderman BL, et al. Concussion symptom underreporting among incoming national collegiate athletic association division I college athletes. Clin J Sport Med. (2020) 30(3):203–9. doi: 10.1097/JSM.0000000000000557
9. Echemendia RJ, Brett BL, Broglio S, Davis GA, Giza CC, Guskiewicz KM, et al. Introducing the Sport Concussion Assessment Tool 6 (SCAT6). Br J Sports Med. (2023) 57(11):619–21. doi: 10.1136/bjsports-2023-106849
10. Patricios JS, Schneider GM, van Ierssel J, Purcell LK, Davis GA, Echemendia RJ, et al. Beyond acute concussion assessment to office management: a systematic review informing the development of a sport concussion office assessment tool (SCOAT6) for adults and children. Br J Sports Med. (2023) 57(11):737–48. doi: 10.1136/bjsports-2023-106897
11. King LA, Horak FB, Mancini M, Pierce D, Priest KC, Chesnutt J, et al. Instrumenting the balance error scoring system for use with patients reporting persistent balance problems after mild traumatic brain injury. Arch Phys Med Rehabil. (2014) 95(2):353–9. doi: 10.1016/j.apmr.2013.10.015
12. Buckley TA, Munkasy BA, Clouse BP. Sensitivity and specificity of the modified balance error scoring system in concussed collegiate student athletes. Clin J Sport Med. (2018) 28(2):174–6. doi: 10.1097/JSM.0000000000000426
13. Wood TA, Hsieh KL, An R, Ballard RA, Sosnoff JJ. Balance and gait alterations observed more than 2 weeks after concussion: a systematic review and meta-analysis. Am J Phys Med Rehabil. (2019) 98(7):566–76. doi: 10.1097/PHM.0000000000001152
14. Oldham JR, Difabio MS, Kaminski TW, Dewolf RM, Howell DR, Buckley TA. Efficacy of tandem gait to identify impaired postural control after concussion. Med Sci Sports Exercise. (2018) 50(6):1162–8. doi: 10.1249/MSS.0000000000001540
15. Van Deventer KA, Seehusen CN, Walker GA, Wilson JC, Howell DR. The diagnostic and prognostic utility of the dual-task tandem gait test for pediatric concussion. J Sport Health Sci. (2021) 10(2):131–7. doi: 10.1016/j.jshs.2020.08.005
16. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. (2019) 393(10182):1745–59. doi: 10.1016/S0140-6736(19)30417-9
17. D’souza N, Charlton J, Grayson J, Kobayashi S, Hutchison L, Hunt M, et al. Are biomechanics during gait associated with the structural disease onset and progression of lower limb osteoarthritis? A systematic review and meta-analysis. Osteoarthritis Cartilage. (2022) 30(3):381–94. doi: 10.1016/j.joca.2021.10.010
18. Williams A, Kamper SJ, Wiggers JH, O’Brien KM, Lee H, Wolfenden L, et al. Musculoskeletal conditions may increase the risk of chronic disease: a systematic review and meta-analysis of cohort studies. BMC Med. (2018) 16(1):167. doi: 10.1186/s12916-018-1151-2
19. Gosselin N, Bottari C, Chen JK, Huntgeburth SC, De Beaumont L, Petrides M, et al. Evaluating the cognitive consequences of mild traumatic brain injury and concussion by using electrophysiology. Neurosurg Focus. (2012) 33(6):E7: 1–7. doi: 10.3171/2012.10.FOCUS12253
20. Jain D, Graci V, Beam ME, Master CL, Prosser LA, McDonald CC, et al. Impaired neuromotor control during gait in concussed adolescents—a frequency analysis. J Appl Biomech. (2023) 40(2):138–46. doi: 10.1123/jab.2023-0126
21. Howell DR, Lynall RC, Buckley TA, Herman DC. Neuromuscular control deficits and the risk of subsequent injury after a concussion: a scoping review. Sports Med. (2018) 48(5):1097–115. doi: 10.1007/s40279-018-0871-y
22. McLoughlin J. Concussion rehabilitation and the application of ten movement training principles. Cureus. (2023) 15(10):1–11. doi: 10.7759/cureus.46520
23. Bennys K, Busto GU, Touchon J. Cumulative effects of subsequent concussions on the neural patterns of young rugby athletes: data from event-related potentials. Res Sports Med. (2023) 32:1–12. doi: 10.1080/15438627.2023.2189594
24. Galeno E, Pullano E, Mourad F, Galeoto G, Frontani F. Effectiveness of vestibular rehabilitation after concussion: a systematic review of randomised controlled trial. Healthcare. (2022) 11:90–106. doi: 10.3390/healthcare11010090
25. Howell DR, Hunt DL, Aaron SE, Meehan WP III, Tan CO. Influence of aerobic exercise volume on postconcussion symptoms. Am J Sports Med. (2021) 49(7):1912–20. doi: 10.1177/03635465211005761
26. Leddy JJ, Haider MN, Ellis MJ, Mannix R, Darling SR, Freitas MS, et al. Early subthreshold aerobic exercise for sport-related concussion: a randomized clinical trial. JAMA Pediatr. (2019) 173(4):319–25. doi: 10.1001/jamapediatrics.2018.4397
27. Rosenfeldt AB, Penko AL, Streicher MC, Zimmerman NM, Koop MM, Alberts JL. Improvements in temporal and postural aspects of gait vary following single- and multi-modal training in individuals with Parkinson’s disease. Parkinsonism Relat Disord. (2019) 64:280–5. doi: 10.1016/j.parkreldis.2019.05.021
28. Elwishy A, Ebraheim AM, Ashour AS, Mohamed AA, El Sherbini AEHE. Influences of dual-task training on walking and cognitive performance of people with relapsing remitting multiple sclerosis: randomized controlled trial. J Chiropr Med. (2020) 19(1):1–8. doi: 10.1016/j.jcm.2019.08.002
29. Hiyama Y, Yamada M, Kitagawa A, Tei N, Okada S. A four-week walking exercise programme in patients with knee osteoarthritis improves the ability of dual-task performance: a randomized controlled trial. Clin Rehabil. (2011) 26(5):403–12. doi: 10.1177/0269215511421028
30. Bisciotti GN, Chamari K, Cena E, Bisciotti A, Bisciotti A, Corsini A, et al. Anterior cruciate ligament injury risk factors in football. J Sports Med Phys Fitness. (2019) 59(10):1724–38. doi: 10.23736/S0022-4707.19.09563-X
Keywords: movement, traumatic brain injury, musculoskeletal, kinesthesis, proprioception
Citation: Wood TA, Kamari M, Grahovec NE and Wilson M (2025) Long-term neurodegenerative sequelae of concussion: implications for musculoskeletal injury risk and neuromuscular interventions. Front. Musculoskelet. Disord. 3:1567611. doi: 10.3389/fmscd.2025.1567611
Received: 27 January 2025; Accepted: 9 June 2025;
Published: 19 June 2025.
Edited by:
Enwu Liu, Flinders University, AustraliaReviewed by:
Frank M. Webbe, Florida Institute of Technology, United StatesKonstantinos Ikonomou, Akeso Sports Medicine, Canada
Copyright: © 2025 Wood, Kamari, Grahovec and Wilson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tyler A. Wood, VFdvb2QxQG5pdS5lZHU=
 Tyler A. Wood
Tyler A. Wood Mahgolzahra Kamari
Mahgolzahra Kamari Nicholas E. Grahovec
Nicholas E. Grahovec Matthew Wilson2
Matthew Wilson2