- Department of Orthopedics, Fuzhou Second General Hospital, Fujian Provincial Clinical Medical Research Center for Trauma Orthopedics Emergency and Rehabilitation, Fuzhou, China
Background: This study aimed to examine the evolving trends in the global burden of smoking-attributable low back pain (LBP) from 1990 to 2021 and predicted disease burden until 2035.
Methods: Using Global Burden of Disease (GBD) 2021 data, we analyzed years lived with disability (YLDs) from smoking-attributable LBP across 204 countries. We assessed trends by sex, age, and region using estimated annual percentage change (EAPC) and projected future burden via Bayesian age-period-cohort (BAPC) modeling.
Results: In 2021, smoking-attributable LBP caused 8.82 million (95% UI 5.18–13.13) YLDs globally, with an age-standardized YLD rate (ASYR) of 102.0 per 100,000 (EAPC = −1.26, 95% CI −1.28 to −1.24). The burden showed marked geographic variation, being highest in Eastern Europe (ASYR: 194.0, 95% UI 115.5–290.1) and lowest in Western Sub-Saharan Africa (ASYR: 32.7, 95% UI 18.3–50.7). And high SDI regions recorded the highest ASYR (173.8, 95% UI 101.7–261.1), 3.2-fold higher than low SDI regions (54.6, 95% UI 31.3–83.6) in 2021. Middle SDI regions demonstrated the most significant ASYR reduction (percentage change in ASYR: −33.8%, 95% UI −36.3 to −31.7) from 1990 to 2021. Males consistently bore 62.7% of the burden, with peak incidence occurring at ages 60–64 (ASYR: 294.2, 95% UI 162.0–480.3). Projections indicate the ASYR will decline to 82.7/100,000 by 2035, representing a 45.9% reduction from 1990 levels.
Conclusion: While global ASYR trends show improvement, persistent disparities by sex, age, and region underscore the need for targeted interventions. Priority should be given to: (1) male-focused smoking cessation programs, (2) workplace interventions for middle-aged populations, and (3) enhanced tobacco control policies in high-burden regions.
Introduction
Low back pain (LBP) represents a global public health crisis, affecting approximately 619 million people worldwide in 2020 and accounting for 69.0 million years lived with disability (YLDs) requiring rehabilitation (1). Beyond its substantial health impacts, LBP imposes severe socioeconomic consequences, including work productivity losses, reduced economic output, and increased healthcare expenditures (2, 3). In Brazil alone, LBP-related costs reached US$500 million annually during 2012–2016, with productivity losses constituting 79% of this burden (4). As the leading cause of global disability (1, 5), LBP has been increasingly linked to modifiable risk factors, particularly combustible tobacco use. We explicitly define “smoking” as the use of traditional combustible products (cigarettes, pipes, cigars, hookahs), excluding electronic nicotine delivery systems (ENDS), heated tobacco products (HTPs), and secondhand smoke exposure (5, 6). This definition aligns with GBD 2021 methodology and reflects distinct pathophysiological mechanisms for combustible vs. non-combustible nicotine products in spinal degeneration. Mounting evidence demonstrates that smoking accelerates intervertebral disc degeneration through multiple synergistic pathways: (1) Nicotine-induced vasoconstriction reduces capillary blood flow to vertebral endplates, impairing nutrient diffusion to disc cells (7, 8); (2) Reactive oxygen species generated by cigarette smoke trigger mitochondrial dysfunction and extracellular matrix degradation via MMP-3/9 upregulation (9–11); (3) Carbon monoxide exposure diminishes oxygen-carrying capacity, exacerbating disc hypoxia (12); (4) Epigenetic modifications (e.g., METTL14-mediated m6A methylation) dysregulate critical anabolic genes like ACAN and COL2A1 (8, 13, 14). These molecular alterations collectively accelerate disc dehydration, annular fissuring, and height loss—key histopathological substrates of LBP. However, despite these established biological mechanisms, the global burden of smoking-attributable LBP remains poorly quantified.
The global burden of disease (GBD), injuries, and risk factors Study 2021 provides the most comprehensive epidemiological assessment of disease burden worldwide (15). While GBD has identified smoking as a significant risk factor for LBP, current tobacco control frameworks predominantly focus on cardiopulmonary outcomes, largely neglecting nicotine's detrimental effects on spinal health. This oversight is particularly concerning given projections of a 36% increase in LBP prevalence by 2050, driven largely by aging populations (1). Existing research has three critical limitations: First, socioeconomic disparities in smoking-related LBP remain uncharacterized. Second, gender-specific burden patterns influenced by occupational exposures and hormonal factors are poorly understood. Third, the paradoxical phenomenon of declining age-standardized rates alongside rising absolute disability in developing nations requires explanation.
To address these knowledge gaps, we conducted the first comprehensive analysis of smoking-attributable LBP burden across 204 countries from 1990 to 2021. Utilizing advanced GBD 2021 methodologies—including frontier analysis and Bayesian age-period-cohort modeling—we integrate geospatial, demographic, and socioeconomic determinants to: quantify the global burden of smoking-attributable LBP; identify development-stage specific vulnerabilities; propose targeted policy interventions. Our findings provide crucial evidence for refining tobacco control strategies and advancing progress toward Sustainable Development Goal 3.4 (reducing non-communicable disease mortality) (5, 6).
Methods
Data source and definition
The GBD 2021 study incorporated 100,983 data sources, such as vital registration systems, verbal autopsies, censuses, household surveys, specific disease registries, and health service contact data, to calculate disability—adjusted life years (DALYs), years of life lost (YLLs), and YLDs for 371 diseases and injuries. This study adopted complex statistical models, like MR-BRT [meta-regression—Bayesian, regularized, trimmed; details described elsewhere (15)] and Disease Modeling Meta-Regression 2.1 (DisMod-MR 2.1), to systematically adjust epidemiological data, addressing biases from differences in data sources, definitions, and measurement methods. It ensured the internal consistency of estimates across regions, ages, sexes, and years. Since GBD 2019, the GBD location hierarchy has covered all World Health Organization (WHO) Member States. Unless stated otherwise, the data involve 204 countries and territories without duplication (15).
The definition of low back pain was pain from the lower margin of the 12th ribs to the lower gluteal folds, with or without pain in one or both lower limbs, lasting at least 1 day. The reference ICD-10 codes for low back pain were M54.3 (sciatica), M54.4 (lumbago with sciatica), and M54.5 (low back pain), while the ICD-9 code was 724 (low back pain). Each data source was given a unique identifier and included in the Global Health Data Exchange (GHDx). Members of the core Institute for Health Metrics and Evaluation (IHME) research team for this topic had full access to the underlying data used to generate the estimates in this paper (1, 16). As for smoking scenario definitions, the GBD reference case was a person who uses any smoking product daily or occasionally, or has used any in the past. This included cigarettes, pipes, cigars, and hookahs, but excluded smokeless tobacco, electronic cigarettes, vaporized products, and heated tobacco products (5, 6). Risks from chewing tobacco and secondhand smoke, listed as other risk factors in GBD 2021, were not included in this study.
Analytical modeling employed DisMod-MR 2.1 with age-threshold constraints (≥30 years onset) to derive incidence estimates, generating 95% uncertainty intervals (UIs) through post-convergence bootstrap resampling (1,000 iterations; 2.5th–97.5th percentiles). Disability weight stratification—validated via U.S. Medical Expenditure Panel Surveys—categorized low back pain into six severity tiers (with/without radiculopathy; http://links.lww.com/BRS/C597). YLD computation integrated age-sex-location-year specific prevalence rates with comorbidity-adjusted disability weights, following GBD's mortality-neutral framework where fatal opioid-related pathways are attributed exclusively to substance use disorders. This attribution protocol maintains DALY-YLD equivalence for non-fatal conditions, thereby quantifying burden exclusively through YLD metrics per GBD comparative risk assessment standards (1, 17).
Additionally, socio-demographic index (SDI) was employed as a composite development metric, derived through geometric mean aggregation of three normalized indicators: age-standardized total fertility rate (<25 years), population-level educational attainment (≥15 years), and lag-adjusted per capita income (Supplementary Table S1). Nations were stratified into development quintiles using IHME-validated thresholds: low (0–0.4658), low-middle (0.4658–0.6188), middle (0.6188–0.7120), high-middle (0.7120–0.8103), and high SDI (0.8103–1.0000). This quintile stratification enables comparative analysis of burden differentials across socioeconomic strata, providing granular insights into development-health outcome gradients (15, 18).
All data used in this study were sourced from the GBD 2021 with explicit permission for academic reuse granted through the IHME Data Access Protocol (https://creativecommons.org/licenses/by-nc-nd/4.0/). We comply with all terms specified in the GHDx Terms of Use (https://ghdx.healthdata.org/terms; Supplementary Material 1). This secondary analysis adheres to IHME's data redistribution policies, including proper source attribution and prohibition of commercial use. Ethical compliance was ensured through secondary analysis of fully anonymized population-level data obtained from publicly accessible GBD repositories, constituting non-human subjects research as defined by Declaration of Helsinki Article 32 (2013 revision). Institutional Review Board exemption was granted under 45 CFR 46.104(d) (4) guidelines for retrospective analyses of de-identified aggregate data, obviating individual consent requirements while maintaining WHO ethical standards for global health research.
Statistics
To evaluate the burden of smoking-attributable LBP, both the YLD rate (per 100,000) and case count (total cases) were used, with 95% uncertainty intervals (UI) provided. The estimated annual percentage change (EAPC) was applied to summarize age—standardized rate (ASR) trends. A regression line was fitted to the natural logarithm of the ASR ratio [y = α + βx + ɛ, where y = ln(ASR), x = calendar year]. EAPC was calculated as 100 × [exp(β) − 1], with its 95% confidence interval (CI) from the linear regression model. ASRs were considered increasing if both the EAPC and its 95% CI lower limit were >0; decreasing if both the EAPC and its 95% CI upper limit were <0; otherwise, stable (19). All analyses used appropriate statistical models (p < 0.05 as statistically significant). Detailed methods for specific analyses are in later sections. Analyses and visualizations were done via the WHO's Health Equity Assessment Toolkit and R 4.4.2 implementation (ggplot2 packages).
Cross-country inequality analysis
Health inequality assessment employed WHO-recommended composite metrics—the Slope Index of Inequality (SII) for absolute disparity quantification and Concentration Index (CI) for relative inequality evaluation (20), applied to age-standardized YLD rates of smoking-attributable low back pain across 204 nations (1990–2021). SII computation utilized rank-ordered socioeconomic positioning, regressing YLD rates against SDI-based population quintile midpoints through robust MM-estimation regression—a heteroscedasticity-consistent approach demonstrating >50% breakdown point for outlier resistance, substantially outperforming ordinary least squares in handling cross-country heterogeneity. CI derivation implemented Lorenz curve integration, mapping cumulative YLD proportions against population-ranked SDI distributions to quantify systematic socioeconomic gradients. Temporal inequality dynamics were assessed via stratified mixed-effects modeling, controlling for healthcare access differentials and demographic transition patterns, with analytical rigor ensured through R's MASS package implementation featuring bias-corrected standard errors and asymptotic efficiency under non-normal distributions.
Frontier analysis
Frontier analytical framework was implemented to quantify theoretically achievable minimum burdens of smoking-attributable low back pain (LBP) across socioeconomic development spectra, employing age-standardized YLD rate (ASYR) as the burden metric and Socio-demographic Index (SDI) as the development proxy. Diverging from conventional parametric regression, this nonparametric approach captures context-specific determinants through locally estimated scatterplot smoothing (LOESS) with adaptive bandwidth optimization (span = 0.3–0.5), generating smoothed frontier trajectories that represent epidemiologically optimal performance benchmarks. Methodological rigor was ensured via 1,000-round bootstrap resampling to derive bias-corrected mean ASYR estimates per SDI decile, addressing small area estimation uncertainties. Effective disparity metrics were calculated as absolute vertical distances between observed 2021 ASYR values and their corresponding frontier potentials, quantifying nation-specific improvement capacity while controlling for development-stage heterogeneity. This dual-phase approach—first establishing stochastic frontiers through locally weighted polynomial regression, then computing performance gaps via Monte Carlo simulation—overcomes limitations of linear parametric models in modeling complex SDI-ASYR associations, particularly critical for identifying overperforming/underperforming health systems across development continua (18, 21).
Bayesian age-period-cohort (BAPC) analysis
The BAPC model was selected for disease burden projection based on its demonstrated efficacy in managing multidimensional, sparse datasets with inherent complexity—a hallmark characteristic of GBD epidemiological research. This methodology extends conventional generalized linear modeling through Bayesian hierarchical frameworks, enabling simultaneous quantification of temporal effects (age, period, cohort) with continuous temporal smoothing via second order random walk priors. The resultant posterior probability distributions demonstrate enhanced predictive accuracy through adaptive regularization of parameter estimates. Computational innovations in the BAPC framework include Integrated Nested Laplace Approximation (INLA) algorithms for marginal posterior distribution estimation. This approach circumvents computational bottlenecks associated with traditional Markov chain Monte Carlo (MCMC) approaches while maintaining numerical precision—particularly advantageous for large-scale population health analyses. These computational advantages enhance model stability and facilitate reliable long-term forecasting of epidemiological patterns through time series analysis. Our application leveraged the validated BAPC R package (version 0.0.36), integrating IHME demographic projections with GBD 2021 morbidity data. This configuration enables holistic assessment of smoking-attributable low back pain trajectories through key model assumptions of: (1) Continuity of current trends: projections assume that observed historical trends (1990–2021) in smoking prevalence and associated LBP burden will continue linearly through 2035, without accounting for potential discontinuities (e.g., unprecedented tobacco control policies or emergence of new risk factors); (2) Demographic consistency: future population structures are derived from UN population prospects, assuming stable fertility/mortality patterns; (3) Risk exposure stability: the population attributable fractions (PAFs) for smoking remains constant relative to historical exposure-disease relationships, implying no substantial changes in smoking's pathophysiological impact over time; (4) Intervention neutrality: the model does not explicitly incorporate the timing or intensity of public health interventions (e.g., tobacco taxes, smoking bans) unless their effects are already embedded in historical trend data. While the model intrinsically captures period effects (e.g., policy impacts manifested during 1990–2021), it does not prospectively simulate intervention scenarios. The rate of decline in smoking prevalence is implicitly captured through the period and cohort effects calibrated to GBD 2021 data. The model's extensive validation in epidemiological research, particularly for age-structured population analyses, ensures methodological rigor in capturing complex interaction effects critical for public health planning (18, 22).
Results
The YLDs trend of smoking-attributable LBP at the global level
From 1990 to 2021, the absolute number of YLDs due to smoking-attributable LBP increased by 30.1% globally, rising from 6,784,850.3 (95% UI 4,068,101.6–10,067,705.8) to 8,823,842.7 (95% UI 5,183,695.1–13,132,647.2). Notably, this increase exhibited significant gender disparity, with males consistently demonstrating higher burden than females. However, the ASYR displayed an inverse trend, decreasing by 33.4% from 153.2 (95% UI 91.4–226.6) to 102.0 (95% UI 60.0–152.1) per 100,000 population (EAPC = −1.26, 95% CI −1.28 to −1.24, Table 1).
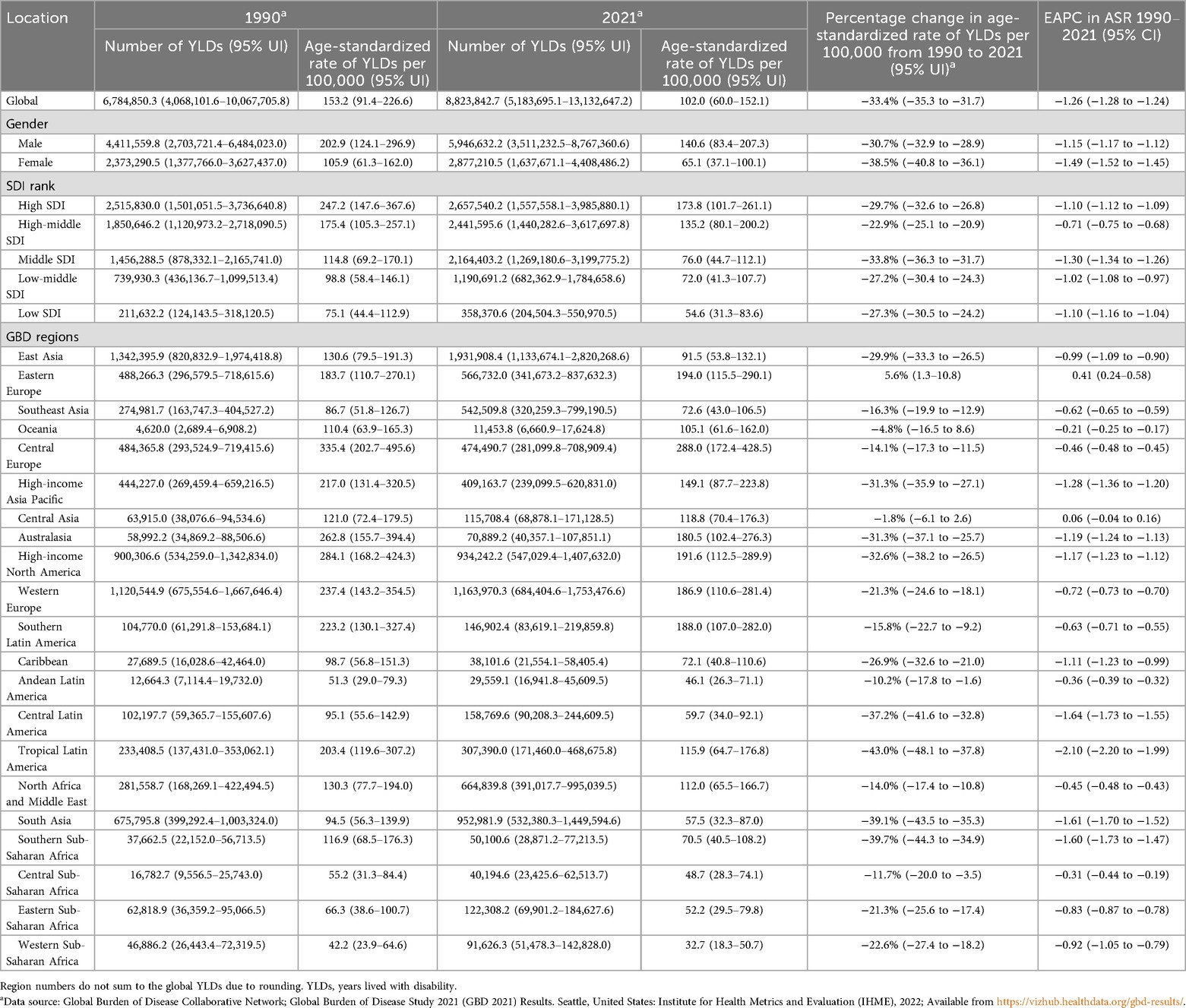
Table 1. YLDs, age-standardised rates of YLDs per 100,000 population in 1990 and 2021, and percentage change between 1990 and 2021 as well as EAPC from 1990 to 2021 for low back pain attributable to smoking globally, by genders, SDI and GBD regions.
The YLDs trend of smoking-attributable LBP at the regions level
Regional analysis revealed striking socioeconomic gradients. In 2021, high SDI regions recorded the highest ASYR at 173.8 (95% UI 101.7–261.1) per 100,000, 3.2-fold higher than low SDI regions (54.6, 95% UI 31.3–83.6). Among the 21 GBD regions, Eastern Europe demonstrated the most severe burden (194.0, 95% UI 115.5–290.1), contrasting with Western Sub-Saharan Africa where rates were lowest (32.7, 95% UI 18.3–50.7).
Quantitative analysis revealed a significant positive correlation between EAPC and baseline ASYR (r = 0.2217, p < 0.001; Figure 1A). Socioeconomic stratification demonstrated paradoxical patterns, with middle SDI quintiles achieving the most rapid ASYR decline (EAPC = −1.30, 95% CI −1.34 to −1.26), while high-middle SDI regions showed attenuated progress (EAPC = −0.71, 95% CI −0.75 to −0.68)—a disparity particularly accentuated in female populations (Figures 1B,C). Geospatial analysis identified substantial heterogeneity: Tropical Latin America led ASYR reductions (EAPC = −2.10, 95% CI −2.20 to −1.99), followed by Central Latin America (EAPC = −1.64, 95% CI −1.73 to −1.55). Of particular concern, Eastern Europe exhibited sustained increases (EAPC = 0.41, 95% CI 0.24–0.58), while Central Asia demonstrated epidemiological stagnation (EAPC = 0.06, 95% CI −0.04 to 0.16)—trends predominantly driven by female demographic patterns. Notably, male-specific analyses revealed universal declines across all 21 GBD regions (Figure 1D). The observed positive EAPC-ASYR correlation (r = 0.22) suggests regions with higher baseline burdens face greater implementation challenges in controlling smoking-attributable low back pain.
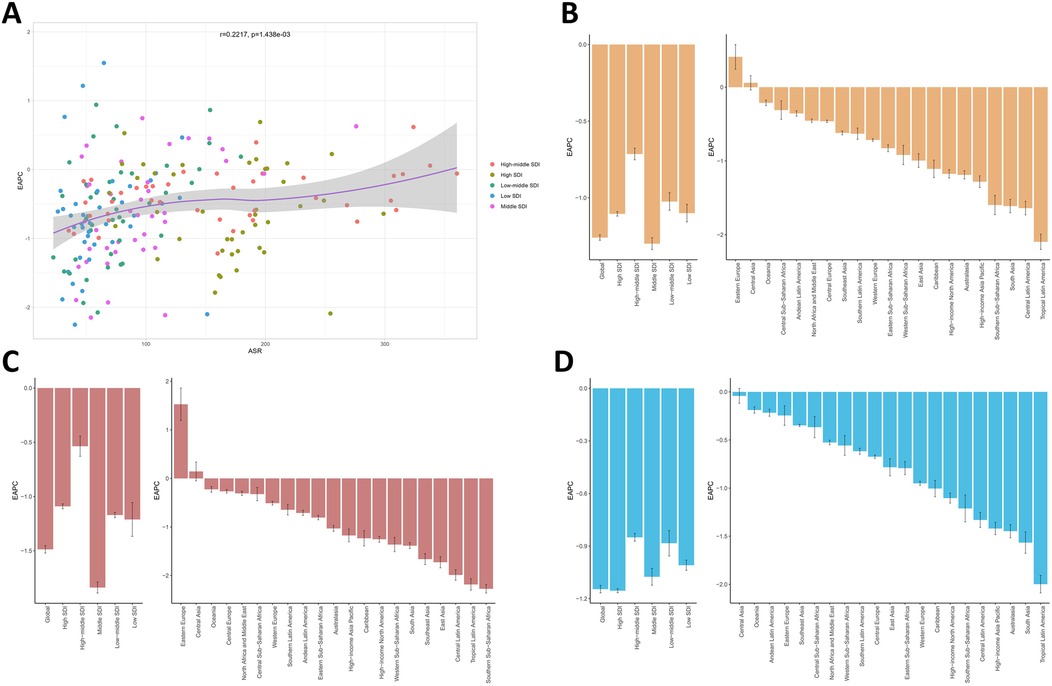
Figure 1. (A) The association between EAPC and ASYR. (B) EAPC in the burden of smoking-attributable LBP in 5 SDI regions and 21 GBD regions. EAPC in the burden of smoking-attributable LBP for female (C) and male (D) at the regions level. EAPC, estimated annual percentage change; ASYR, age-standardized years lived with disability rate; LBP, low back pain; SDI, socio-demographic index; GBD, global burden of disease.
The YLDs trend of smoking-attributable LBP in the nations
A total of 204 countries and territories were included in the GBD 2021 study. As evidenced in Figure 2A, the burden exhibited geographic concentration within European countries, particularly in Eastern Europe where exceptional rates were observed: Montenegro (ASYR: 360.3; 95% UI 213.0–535.1), Republic of Serbia (ASYR: 337.9; 95% UI 200.8–501.0), Bosnia and Herzegovina, North Macedonia, Hungary, Republic of Croatia, and Republic of Bulgaria all demonstrated ASYR exceeding 300.0 per 100,000 (Supplementary Table S2). Figure 2B highlights divergent temporal patterns in ASYR trends from 1990 to 2021. While 167 nations (81.7%) showed favorable declines, 37 countries (18.1%) exhibited concerning upward trajectories, most prominently the Islamic Republic of Afghanistan (EAPC = 1.55; 95% CI 1.29–1.81) and Republic of Mali (EAPC = 1.22; 95% CI 1.11–1.32). Conversely, the most substantial reductions emerged in Republic of Madagascar, United Mexican States, Federative Republic of Brazil, Federal Democratic Republic of Nepal, Kingdom of Denmark, and Republic of the Union of Myanmar, all with EAPCs below −2.00 (Figure 2B, Supplementary Table S3). Of particular note, absolute YLD counts surged over 300% in five nations, led by State of Qatar (775.9% increase) and United Arab Emirates (767.6%). In stark contrast, only 19 countries achieved absolute reductions, with Republic of Bulgaria (−24.3%), Kingdom of Denmark (−22.8%), and Republic of Latvia (−21.5%) demonstrating the most significant declines (Figure 2C, Supplementary Table S2).
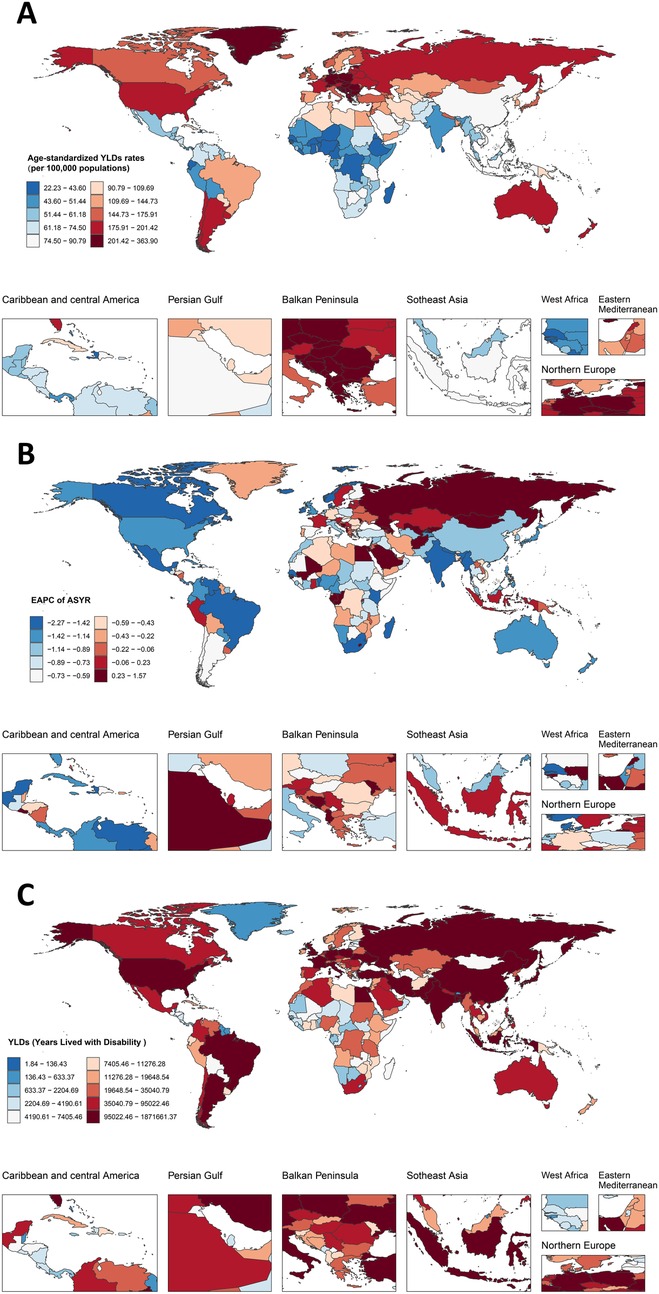
Figure 2. The burdens of LBP associated with smoking among 204 countries and territories in 2021. (A) Age-standardized YLDs rates of LBP associated with smoking. (B) EAPC for LBP attributable to smoking in different countries and regions from 1990 to 2021. (C) The number of YLDs of LBP associated with smoking. LBP, low back pain; EAPC, estimated annual percentage change.
Gender-specific trends in smoking-attributable LBP burden
Figure 3 delineates gender-stratified trends in both YLD rates and absolute numbers for smoking-attributable LBP from 1990 to 2021. Notably, ASYR demonstrated consistent declines across all SDI regions for both genders, while the number of YLDs exhibited moderate increases. Among females, ASYR decreased by 38.5% from 105.9 per 100,000 (95% UI 61.3–162.0) in 1990 to 65.1 (95% UI 37.1–100.1) in 2021. Concurrently, the number of YLDs increased from 2,373,290.5 (95% UI 1,377,766.0–3,627,437.0) to 2,877,210.5 (95% UI 1,637,671.1–4,408,486.2). For males, ASYR showed a 30.7% reduction, declining from 202.9 per 100,000 (95% UI 124.1–296.9) to 140.6 (95% UI 83.4–207.3). Correspondingly, the number of YLDs rose from 4,411,559.8 (95% UI 2,703,721.4–6,484,023.0) to 5,946,632.2 (95% UI 3,511,232.5–8,767,360.6). Analysis of the 21 GBD regions revealed persistent gender disparities, with only Southern Latin America, Tropical Latin America and Australasia demonstrating higher ASYR among females compared to males—a pattern consistent with observations from three decades prior. Over the three-decade study period, females demonstrated faster improvement in ASYR trends (EAPC = −1.49; 95% CI −1.52 to −1.45) compared to males (EAPC = −1.15; 95% CI −1.17 to −1.12). This gender disparity in burden reduction may reflect differential patterns in smoking prevalence, healthcare access, or occupational exposures.
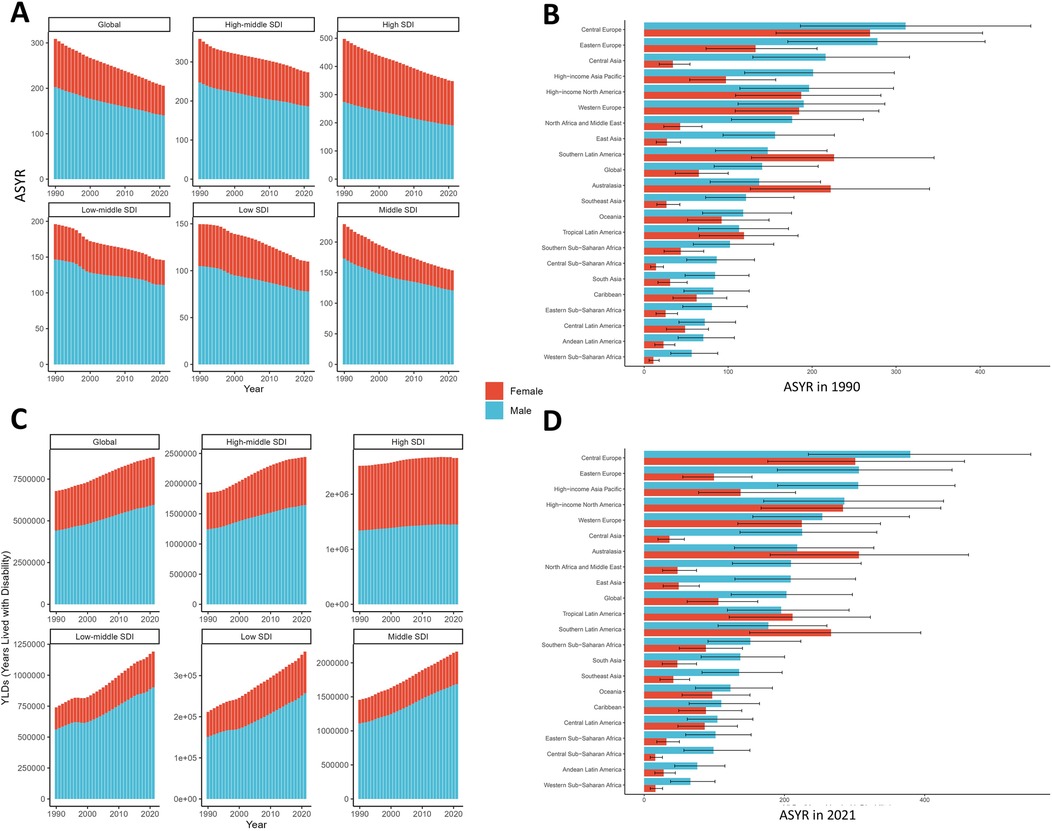
Figure 3. Gender-specific trends of ASYR (A) and YLDs (C) for smoking-attributable LBP at 5 SDI regions from 1990 to 2021. Gender -stratified patterns of ASYR for smoking-attributable LBP in 1990 (B) and 2021 (D) at 21 GBD regions. ASYR, age-standardized years lived with disability rate; LBP, low back pain; SDI, socio-demographic index; GBD, global burden of disease.
Age-specific burden of smoking-attributable LBP
Figure 4 presents age- and gender-stratified patterns of ASYR and absolute numbers for smoking-attributable LBP in 1990 and 2021. The ASYR demonstrated a characteristic inverted U-shaped distribution, peaking in the 60–64 age group (294.2 per 100,000; 95% UI 162.0–480.3) before declining. Notably, this pattern remained consistent across genders and study years. In 2021, ASYR increased progressively from 119.4 per 100,000 (95% UI 62.1–199.8) in the 30–34 age group to its peak in the 60–64 age group, then declined to 84.2 per 100,000 (95% UI 42.8–142.2) in the 95+ age group. Similarly, the number of YLDs followed an age-dependent trajectory, peaking earlier in the 50–54 age group (1,172,149.2; 95% UI 620,540.3–1,912,838.3) before decreasing sharply to 4,586.9 (95% UI 2,334.5–7,752.3) in the 95+ age group. Remarkably, the structural patterns of age-specific burden distribution showed minimal variation over the three-decade study period.
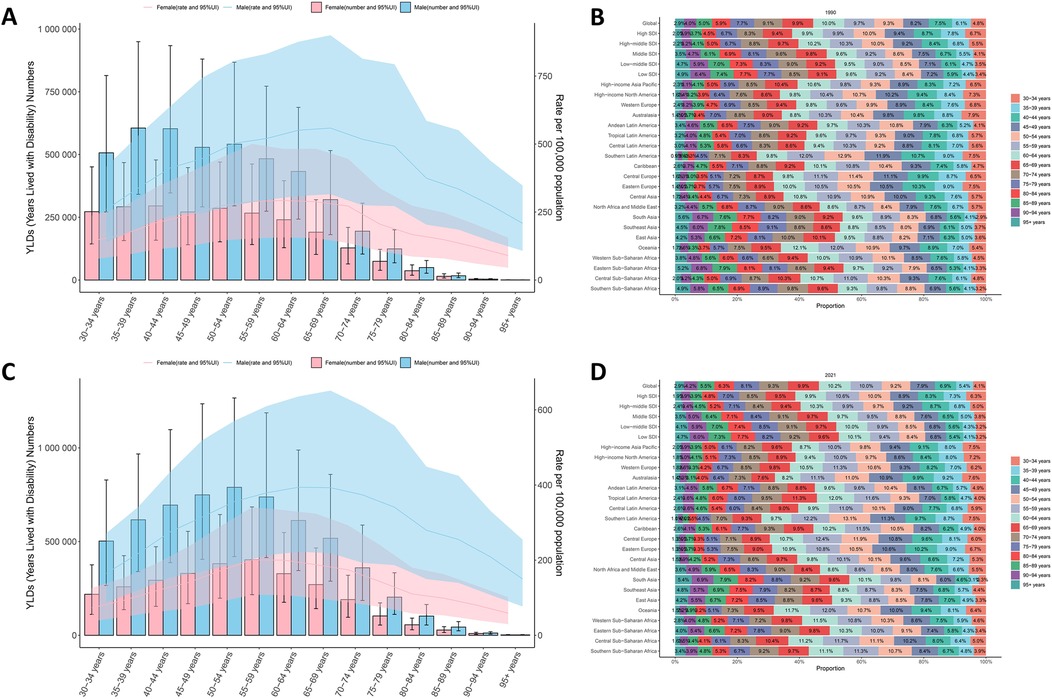
Figure 4. Trends in the number and age-standardized rates of YLDs of smoking-attributable LBP worldwide from 1990 (A) to 2021 (C) Age-stratified patterns of ASYR for smoking-attributable LBP in 1990 (B) and 2021 (D) at regional level. Error bars represent 95% uncertainty intervals (UIs) of the numbers. Shading indicates the 95% UI of the rate. ASYR, age-standardized years lived with disability rate; LBP, low back pain.
Analysis of smoking-attributable LBP burden across socioeconomic strata revealed a characteristic inverted U-shaped relationship with SDI. The burden escalated progressively in regions with SDI < 0.80, peaked at SDI 0.80, and subsequently declined in higher SDI settings. Notably, Central Europe and Oceania demonstrated higher-than-expected burden levels, while Western Sub-Saharan Africa, Andean Latin America, and High-income Asia Pacific exhibited lower-than-projected values (Figure 5A). This socioeconomic patterning was recapitulated at national level, with Montenegro, Republic of Kiribati, and Federal Democratic Republic of Nepal showing excess burden, contrasting with below-expected rates in Federal Democratic Republic of Ethiopia, Federal Republic of Nigeria, and Republic of Singapore (Figure 5B). Crucially, no significant association emerged between EAPC and SDI values (p > 0.5, Figure 5C), indicating that temporal trends in smoking-attributable LBP burden operate independently of baseline socioeconomic development levels.
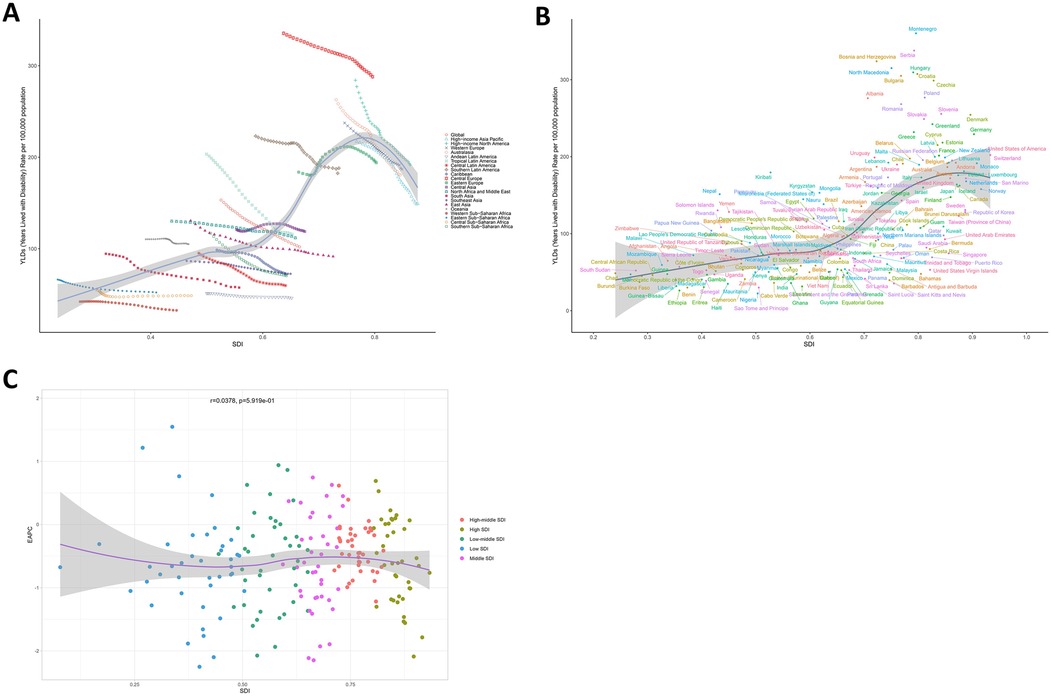
Figure 5. Socioeconomic determinants of smoking-attributable LBP burden. (A) ASYR patterns across 21 GBD regions and globally, stratified by SDI in 2021. (B) National-level ASYR distribution across 204 countries and territories, categorized by SDI in 2021. (C) Association between EAPC and baseline SDI in 2021. ASYR, age-standardized years lived with disability rate; LBP, low back pain; SDI, socio-demographic index; EAPC, estimated annual percentage change.
Persistent socioeconomic disparities in smoking-attributable LBP burden were evident across the study period (Figure 6). Visual analysis of SDI-LBP burden gradients revealed a positive exposure-response relationship, with higher SDI regions demonstrating disproportionately elevated YLD rates. Quantitatively, the SII decreased marginally from 147.06 (95% CI: 121.04–173.08) YLDs/100,000 in 1990 to 123.36 (102.37–144.35) in 2021, indicating modest progress in absolute inequality reduction. Concentration Index analysis demonstrated stable relative inequality patterns, decreasing from 0.24 (0.20–0.27) to 0.23 (0.20–0.25) over three decades—persistent pro-rich inequality despite improved estimation precision (narrowing CIs). Notably, significant CI overlap (1990 vs. 2021) suggests temporal stability in socioeconomic gradients of LBP burden distribution.
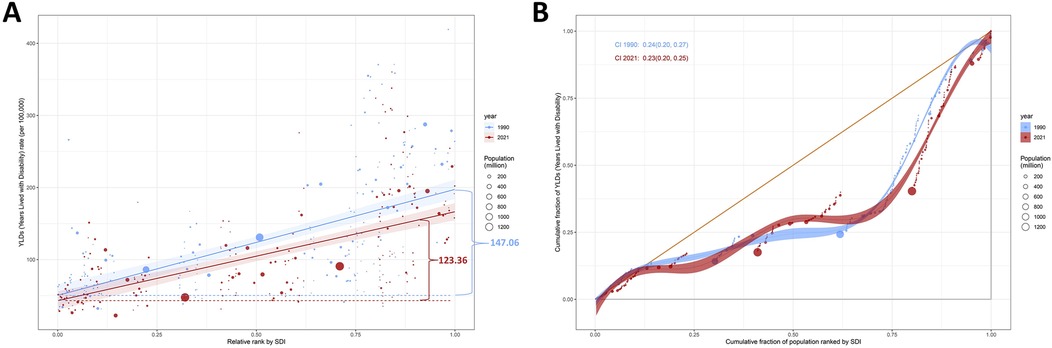
Figure 6. Health inequality regression curves and concentration curves for the ASYR of smoking-attributable LBP (A,B) worldwide, 1990 and 2021. Panels A illustrate the slope index of inequality, depicting the relationship between SDI and ASYR for each condition, with points representing individual countries and territories sized by population. Panels B present the concentration index, which quantifies relative inequalities by integrating the area under the Lorenz curve, aligning YLDs distribution with population distribution by SDI. Blue represents data from 1990, and red represents data from 2021. ASYR, age-standardized years lived with disability rate; LBP, low back pain; SDI, socio-demographic index.
Frontier analysis
Frontier analysis spanning 1990–2021 identified substantial cross-national heterogeneity in achieving smoking-attributable LBP burden control relative to socioeconomic development levels. Fifteen nations demonstrated critical improvement potential, with effective performance gaps ranging 337.51–219.41—predominantly Eastern European countries (Montenegro, Serbia, Bosnia and Herzegovina, North Macedonia, Hungary, Croatia, Bulgaria, Czechia, Poland, Albania, Romania, Slovenia, Slovakia) and high-income regions (Denmark, Greenland) exhibiting disproportionately elevated ASYR compared to socioeconomic peers. Notably, low-SDI nations (<0.5) including Somalia, Niger, Ethiopia, Benin, and Nigeria achieved effective burden control despite resource constraints, outperforming development-stage expectations. Conversely, high-SDI countries (>0.8) such as Czechia, Poland, Slovenia, and Denmark manifested unanticipated performance deficits, suggesting underutilized prevention capacities given their advanced healthcare infrastructures (Figure 7). This inverse efficiency pattern highlights structural mismatches between socioeconomic development and smoking-related disease control effectiveness.
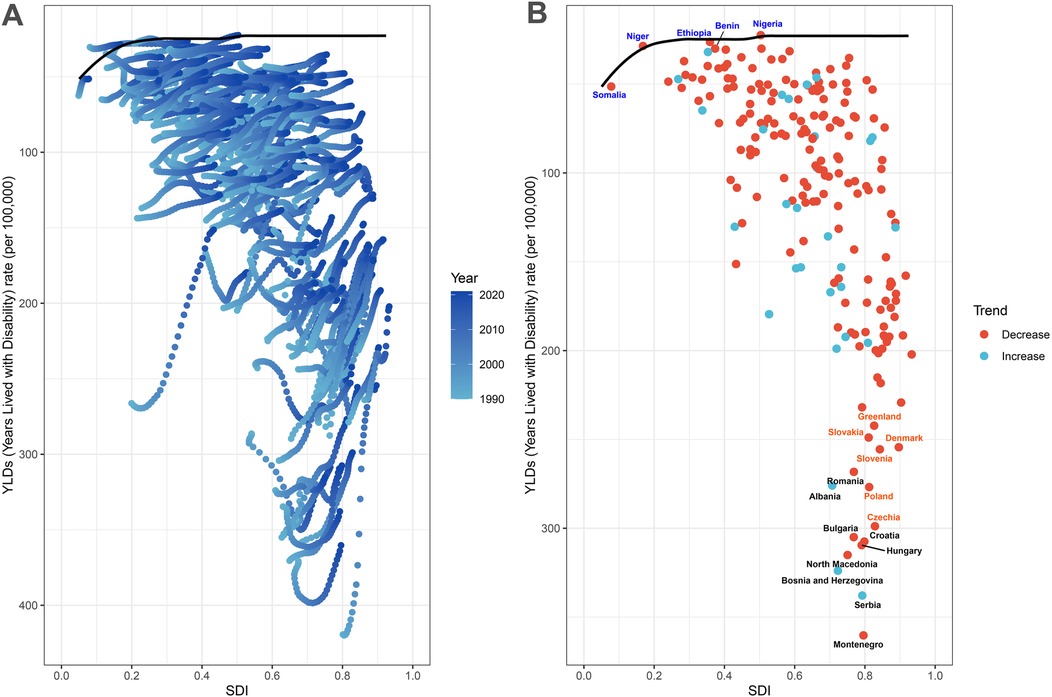
Figure 7. Frontier analysis results. (A) Illustrates leading-edge analysis based on ASYR and SDI from 1990 to 2021. Color levels range from light blue (1990) to dark blue (2021). The solid black line depicts the boundary. (B) Illustrates the leading-edge analysis based on ASYR and SDI in 2021. Solid black lines represent borders and dots represent countries and regions. The top 15 countries and territories with the largest effective differences are marked in black. Examples of border countries with low SDI (<0.5) and low effective variance are marked in blue, and examples of countries and regions with high SDI (>0.8) and relatively high effective variance are marked in red. Red dots indicate a decline in ASYR, while blue dots indicate an increase between 1990 and 2021. ASYR, age-standardized YLDs rate; SDI, socio demographic index.
YLDs projections for smoking-attributable LBP in 2022–2035
Projections based on BAPC modeling of GBD data (1990–2021) indicate substantial reductions in ASYR for smoking-attributable LBP across all age groups through 2035 (Figures 8A,B). Globally, ASYR is projected to decline from 153.2 per 100,000 in 1990 to 82.7 per 100,000 by 2035, while absolute YLD counts demonstrate stabilization trends. The peak burden is anticipated in 2025 at 8,817,075.6 YLDs (95% UI 8,666,320.0–8,967,831.2), representing a 30.0% increase (2,032,225.3 YLDs) from 1990 levels, with subsequent stabilization at 8,676,316.6 (95% UI 8,257,520.9–9,095,112.2) by 2035. Notably, age-stratified projections reveal critical epidemiological transitions (Figures 8C,D). The 45–49 age group emerges as a pivotal transition point, with progressive burden escalation in populations >50 years contrasting with continued declines in younger cohorts (<45 years). This demographic shift underscores the long-term population health impacts of smoking-attributable LBP, particularly in aging populations (Supplementary Table S4).
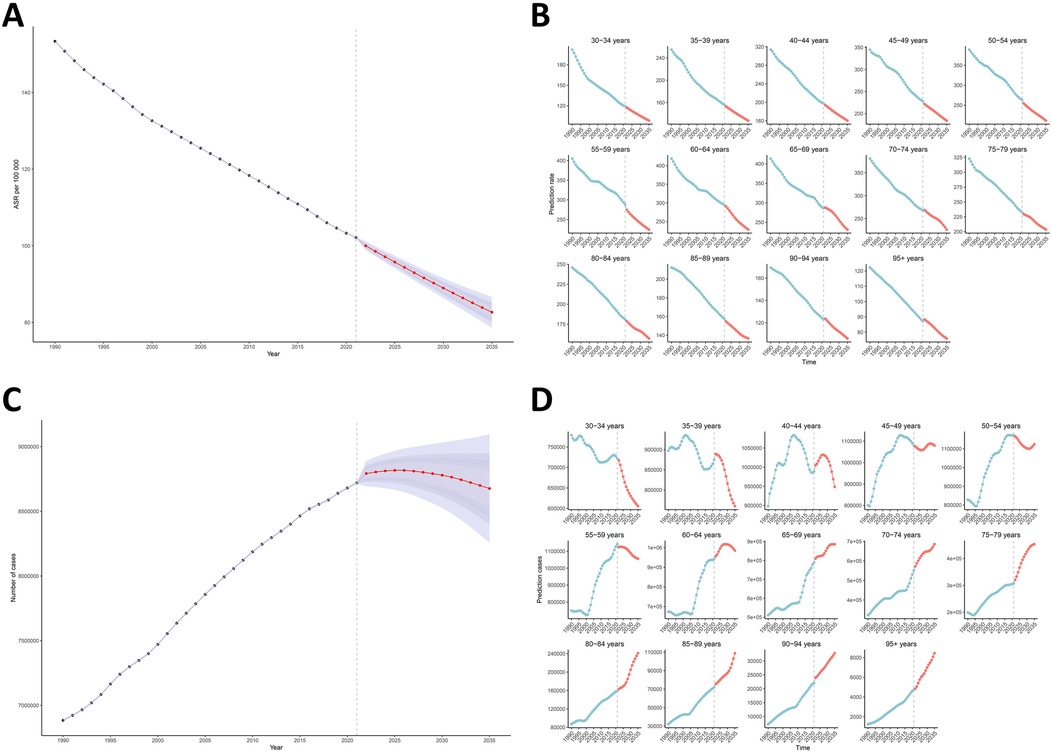
Figure 8. Temporal trends and projections of smoking-attributable LBP burden by age group, 1990–2035. (A,B) Observed and predicted ASYR derived from BAPC modeling. (C,D) The number of YLDs, showing historical trends and future projections. ASYR, age-standardized years lived with disability rate; LBP, low back pain; BAPC, Bayesian age-period-cohort model.
Discussion
This study offers detailed information on the disease burden due to smoking-attributable LBP, including YLDs and ASYR, across 204 countries and territories from 1990 to 2021, using the most recent publicly available model data and methods from GBD study 2021 (15). According to a recent study by the GBD 2021 Low Back Pain Collaborators, in 2020, there were more than half a billion prevalent cases of low back pain worldwide, representing 7·7% of all YLDs and thus the greatest contribution to the world's burden of disability. Globally, 12·5% and 11·5% of YLDs due to LBP were attributed to the lifestyle factors smoking and elevated BMI, respectively (1). Our analysis of the global burden of smoking-attributable LBP reveals critical disparities across age, gender, and geographic regions, with profound implications for clinical practice and public health strategies. Between 1990 and 2021, the absolute number of YLDs attributed to smoking-related LBP increased by 30.1%, while the ASYR decreased by 33.4% (EAPC = −1.26, 95% CI −1.28 to −1.24; Figure 1; Table 1). This paradoxical pattern likely reflects competing forces of population aging (elevating absolute burden) and progressive tobacco control policies (driving relative declines). Persistent male predominance in YLDs (3:1 male-to-female ratio) aligns with global smoking prevalence disparities, though concerning ASYR increases among females in high-SDI regions (e.g., Eastern Europe). Building on the observed trends from 1990 to 2021 and projections to 2035, we contextualize these findings through a multidimensional lens.
Age-specific burden: dual challenges across the lifespan
The global burden of LBP attributable to smoking exhibits an inverted U-shaped age distribution, peaking in the 60–64 age group (ASYR: 294.2 per 100,000; 95% UI 162.0–480.3). The inverted U-shaped burden distribution peaking at 60–64 years mirrors the cumulative nature of smoking-induced disc degeneration. This corresponds to the ∼30-year latency required for: (1) Critical accumulation of advanced glycation end-products (AGEs) from chronic oxidative stress (10, 23); (2) Threshold depletion of proteoglycan content (−40% in smokers vs. nonsmokers) (9, 24); (3) Microvascular rarefaction reaching >50% reduction in endplate capillary density (7). This pattern reflects the cumulative effects of smoking-induced spinal degeneration, characterized by progressive intervertebral disc damage and vascular insufficiency. Foizer et al. (12) demonstrated in a retrospective case-control study (n = 68) that smoking intensity significantly correlates with both the presence (OR = 4.09) and severity of Modic changes (MC), underscoring smoking as a modifiable risk factor for structural spinal pathology. While younger populations (30–34 years) exhibit lower ASYR (119.4 per 100,000; 95% UI 62.1–199.8), projections indicate a marked escalation in LBP burden among individuals aged >50 years (Figure 8), signaling a critical public health challenge for aging societies. This trajectory aligns with the delayed clinical manifestation of smoking-related disc degeneration, exacerbated by age-related comorbidities such as osteoporosis (7, 25, 26). Notably, the reduced ASYR observed in the 95+ age group (84.2 per 100,000; 95% UI 42.8–142.2) likely reflects survivorship bias and competing mortality risks rather than diminished biological susceptibility. Complementing these findings, Ikeda et al. (27) analyzed longitudinal data from 6,467 older adults in the English Longitudinal Study of Aging (ELSA), revealing that sustained smoking cessation over 4 years significantly reduced LBP risk (RR = 0.955), whereas smoking resumption within the same period elevated risk (RR = 1.536). These results emphasize the dual importance of early-life preventive interventions to mitigate smoking-induced LBP and targeted smoking cessation programs for older populations to disrupt disease progression.
Gender disparities: biological and sociocultural drivers
In the context of population-wide burden-of-disease studies on LBP, a consistent gender disparity has been observed, with female generally exhibiting a higher burden than male (1, 17, 19, 21, 28–30). This phenomenon is primarily attributed to several physiological factors specific to female, particularly during and after menopause. The decline in estrogen levels during menopause has been shown to accelerate intervertebral disc degeneration (9), while postmenopausal female face an increased risk of osteoporosis related spinal fractures. Additionally, the perimenopausal period often leads to abdominal weight gain, further contributing to the elevated prevalence of LBP in middle-aged female (21, 29, 31). However, our investigation into the smoking population reveals a distinct pattern, suggesting that these gender-specific risk factors may be modulated or overshadowed by the effects of smoking on LBP development and progression. Our analysis revealed that, in 2021, males accounted for 62.7% of YLDs, while females demonstrating a faster annualized decline in ASYR (EAPC = −1.49 vs. −1.15 in males; Table 1). This paradoxical pattern may stem from multifactorial drivers: (1) behavioral and occupational factors: globally, male smoking prevalence remains 5-fold higher in many regions, compounded by occupational exposures (e.g., manual labor) that amplify biomechanical stress (32). A Brazilian cross-sectional study (n = 600) identified smoking as a predominant LBP risk factor in males, whereas females exhibited stronger associations with ergonomic stressors—a divergence attributed to gender-specific coping strategies (e.g., males more frequently adopt maladaptive approaches like pain catastrophizing) (33); (2) workforce dynamics: while studies from Sweden (34) and the Netherlands (35) confirm males' vulnerability to occupational risks, gender segregation in labor roles may modulate exposure patterns. Females often face dual burdens of occupational and domestic responsibilities, increasing exposure to static postures and repetitive tasks; (3) regional heterogeneity: elevated female ASYR in Southern Latin America and Australasia (Figure 3) suggests region-specific vulnerabilities, potentially mediated by hormonal influences on pain perception and disparities in healthcare access. However, null gender differences observed in Swedish (36), French (37), and Canadian (38) cohorts underscore the complexity of biological-sociocultural interactions. Beyond prevalence differences, molecular studies reveal gender-divergent pathophysiology: (1) Androgen receptors in male disc cells heighten susceptibility to nicotine-induced apoptosis (8, 39); (2) Estrogen's chondroprotective effects in premenopausal females mitigate matrix metalloproteinase activation (9); (3) Male-predominant IL-1β hyperexpression in smoking-associated disc degeneration (10, 40). These findings collectively emphasize that persistent gender disparities arise from intersecting biological susceptibility (e.g., hormonal modulation of nociception) and sociocultural determinants (e.g., occupational segregation, caregiving roles). Targeted interventions must address this duality through gender-specific smoking cessation programs and ergonomic policy reforms.
Geographic inequities: development-health mismatches
This multinational analysis reveals critical geographic disparities in smoking-attributable LBP burden, with Eastern Europe emerging as an alarming epicenter—exemplified by Montenegro's ASYR of 360.3/100,000, nearly 12-fold higher than low-burden nations. The concentration of extreme ASYR (>300/100,000) across Balkan states likely reflects synergistic effects of prolonged tobacco exposure [Russia's male smoking prevalence: 45.6% (5)], aging populations, and inadequate enforcement of workplace ergonomic regulations. Conversely, progress in nations like Brazil (EAPC = −2.11; 95% CI −2.22 to −2.01; Supplementary Table S1) demonstrates the preventive potential of integrated tobacco control and occupational health policies, aligning with their 72.5% male smoking reduction through MPOWER strategies (5). The paradoxical coexistence of declining ASYR and surging absolute YLDs in Gulf states (Qatar: +775.9%) underscores urbanization-driven risks—rapid demographic shifts, sedentary lifestyles, and delayed tobacco control adoption in economically transitioning regions. While 81.7% of countries achieved ASYR reductions, the upward trajectories in 37 nations—particularly conflict-affected areas like Afghanistan (EAPC = 1.55; 95% CI 1.29–1.81)—highlight how healthcare system fragmentation and tobacco industry targeting exacerbate musculoskeletal burdens. The stark gender-neutral progress in Denmark (−22.8% absolute YLDs) vs. female-driven deterioration in Eastern Europe suggests differential policy effectiveness: comprehensive smoking bans and early workplace interventions outperform isolated clinical approaches (1, 5, 6).
The inverted U-shaped relationship between age-standardized years lived with ASYR and the SDI challenges conventional development paradigms, as illustrated in Figure 5. Our analysis of 1990–2021 data reveals a complex pattern: peak burden at SDI 0.80 likely reflects transitional risks during economic acceleration—heightened occupational sedentariness, delayed implementation of tobacco control policies, and increased smoking prevalence among industrial workers. The ASYR of area-level smoking-attributable LBP shows a negative correlation with SDI when SDI exceeds 0.8, while demonstrating a near-positive correlation when SDI falls below 0.8. This pattern aligns with findings from the population-wide LBP burden of disease study (19). The disparities are striking, with high-SDI regions (e.g., Eastern Europe) exhibiting 3.2-fold higher ASYR compared to low-SDI areas (Table 1). This disparity can be attributed to prolonged smoking exposure in aging populations and relatively lax tobacco control policies in these regions. The stagnant socioeconomic inequality indices (SII: 147.06–123.36; Figure 6) over three decades reveal systemic failures in addressing smoking-related musculoskeletal disparities, contrasting with progress in other smoking-attributable diseases—a divergence potentially explained by diagnostic overshadowing of LBP in tobacco control agendas (41–45). Notably, frontier analysis highlights paradoxical efficiency patterns: even countries with advanced healthcare infrastructure, such as Denmark and Slovenia, show unexpected performance gaps (effective difference >200), revealing systemic inefficiencies in translating resources into effective prevention strategies. Conversely, low-SDI nations (e.g., Somalia, Niger) demonstrate relatively effective burden control, potentially due to cultural smoking norms and competing health priorities that may mask LBP disability (Figure 7). The efficiency gap reflects structural path dependencies rather than resource limitations. Eastern Europe's delayed tobacco control adoption (mean 12.4 years post-FCTC ratification) created generational smoking inertia, while fragmented rehabilitation services impede secondary prevention (6, 32). Conversely, high-SDI underperformers like Denmark demonstrate the “specialization paradox” where advanced surgical capacity crowds out preventive investment. Over the past half-century, the evolution of smoking epidemiology reveals that the relatively low tobacco control efficacy in some high-SDI countries may involve the following mechanisms: (1) Historical legacy of the tobacco industry: certain high-income nations (e.g., Austria, Portugal, Chile) still exhibit male smoking rates characteristic of Stage 2 tobacco epidemic (current prevalence 15%–65% without significant decline), reflecting deeply entrenched early tobacco culture (32). Studies indicate these countries reached peak male smoking rates of 60%–70% in the mid-20th century, creating persistent behavioral inertia (46); (2) Policy implementation disparities: while Nordic countries have progressed to Stage 4 (smoking prevalence <25%) through comprehensive measures, France and Portugal remain in Stage 2 (32), with middle-high-income nations like Brazil achieving 70% reduction via advertising bans, health warnings, and taxation, demonstrating policy rigor outweighs economic status (47, 48); (3) Socio-demographic influences: the upper-middle SDI quintile shows paradoxically highest male smoking rates, indicating nonlinear economic-development effects, with emerging economies like China exhibiting transient positive correlation between industrialization and tobacco consumption (5); (4) Cultural specificities: Southern European countries (Spain, France) demonstrate slower female smoking decline (persisting >20% in 2020), markedly higher than Nordic levels, while Pacific Island nations maintain >50% male prevalence due to traditional use (32). Thus, economic development constitutes merely a prerequisite, with policy comprehensiveness (e.g., Brazil's FCTC compliance) and societal consensus (e.g., Nordic smoke-free norms) being pivotal determinants of success.
A particularly interesting finding emerges from the middle SDI region, which, despite facing dual pressures of rising smoking rates and a large industrial workforce, shows the lowest EAPC among the five SDI regions and the most dramatic improvement in smoking-attributable LBP burden. We hypothesize that this improvement may be related to enhanced social conditions benefiting a large population base. The positive correlation between baseline ASYR and EAPC (r = 0.22, p < 0.001) reveals a concerning “disparity amplification” effect, where high-burden regions face disproportionate implementation challenges due to healthcare strain and policy inertia (Figure 1). The absence of SDI-EAPC association (p > 0.5) suggests that smoking-attributable LBP trends are driven more by targeted interventions than passive development gains, emphasizing the need for spine-specific tobacco control strategies (Figure 5). These findings collectively highlight the entrenched nature of smoking-related health inequities, with high SDI populations continuing to bear a disproportionate share of preventable burden (Figure 6). Regional patterns further illuminate this issue, as high smoking prevalence in Southeast Asia (e.g., Indonesia: 58.3% male) and Eastern Europe (e.g., Russia: 45.6% male) correlates strongly with elevated rates of spinal disorders, suggesting a potential pathway for regional disparities in LBP burden (5). Notably, the age-standardized incidence rate (ASIR) of LBP shows positive correlations with both age-standardized disability-adjusted life years rate (ASDR) and SDI in the GBD 2019 study (49). The observed downward trend is encouraging, indicating that LBP burden decreases as socioeconomic development progresses. These findings necessitate context-specific solutions—strengthening tobacco advertising restrictions in high-burden regions, scaling up taxation policies modeled after Brazil's success, and addressing emerging risks in rapidly developing economies through preemptive ergonomic legislation. Future studies should prioritize disentangling smoking's biomechanical effects [e.g., disc hypoxia via nicotine-mediated vasoconstriction (8, 9)] from confounding occupational factors in national burden estimates, particularly given GBD 2021's limited covariates for LBP pathology.
Insights from predictive modelling
The BAPC modeling of GBD data (1990–2021) projects a significant decline in ASYR for smoking-attributable LBP globally, from 153.2 to 82.7 per 100,000 between 1990 and 2035. This reduction aligns with decreasing smoking prevalence trends observed in high-income regions due to strengthened tobacco control policies (e.g., taxation, public health campaigns) (1). However, absolute YLD counts demonstrate a paradoxical stabilization after peaking at 8.8 million in 2025, reflecting the interplay of population aging, growth, and lagged effects of cumulative smoking exposure. Notably, age-stratified analyses reveal a critical epidemiological transition: while younger cohorts (<45 years) exhibit declining burdens—potentially attributable to reduced smoking initiation rates—the >50-year age groups face escalating YLDs. This divergence highlights the long latency of smoking-induced spinal degeneration, where prolonged exposure to tobacco toxins (e.g., nicotine-induced vasoconstriction, oxidative stress) accelerates intervertebral disc degradation and osteoporosis, culminating in LBP later in life (8, 10). The 45–49 age group emerges as a pivotal transition point, likely marking the onset of clinically significant degenerative changes. Regional implications: Stabilizing YLDs post-2025 may mask disparities between high-income countries (declining burdens due to effective tobacco control) and low/middle-income countries (LMICs), where smoking prevalence persists or rises. For instance, Southeast Asia and Eastern Europe—regions with high smoking rates and aging populations—may experience prolonged LBP burdens despite global declines in ASYR (1, 5). While the BAPC model accounts for demographic shifts, it may underestimate regional heterogeneity in smoking cessation trends or healthcare access. Additionally, the analysis assumes linear implementation of tobacco policies, which may not hold in LMICs with fragmented governance. Further studies should integrate spatial-temporal covariates (e.g., policy enforcement indices, socioeconomic gradients) to refine projections. These projections underscore the dual challenge of mitigating smoking-related LBP through preventive measures (e.g., youth anti-smoking programs) while addressing the growing burden in aging populations via targeted rehabilitation and pain management strategies. Policymakers must prioritize region-specific interventions to avert a looming crisis in spinal health, particularly in rapidly aging societies.
Public health and policy priorities
The WHO Framework Convention on Tobacco Control (WHO FCTC) outlines comprehensive measures to reduce tobacco use, which can be broadly classified into two categories: (1) supply reduction strategies (including bans on tobacco sales through vending machines, internet platforms, or other technology-based methods, as well as prohibition of single-cigarette or small-packet sales) and (2) demand reduction approaches (comprising tobacco advertising/sponsorship restrictions, public place smoking bans, and taxation policies) (45, 50, 51). However, given the unique pathophysiology and disease burden of smoking-attributable LBP, we propose three targeted optimizations to existing public health strategies: ① Tobacco Control Reinforcement: precision tobacco control integrating osteoporosis screening with smoking cessation in aging high-SDI populations. The 775.9% YLD increase in Qatar and 767.6% YLD increase in United Arab Emirates underscores the urgent need for WHO FCTC aligned policies in rapidly developing nations, including plain packaging laws and vaping regulation (6). ② Health System Re-engineering: high-SDI underperformers (e.g., Denmark, Slovenia) require optimized resource allocation (Figure 7)—redirecting funds from tertiary care to community-based smoking cessation and physiotherapy programs, proven cost-effective in Australia's National LBP Initiative (1). ③ Equity-Focused Surveillance: the SII decline from 147.06 to 123.36 YLDs/100,000 remains insufficient (Figure 6). Progressive taxation of tobacco exports from high-burden Eastern European countries could fund regional preventive networks, modeled on Nigeria's successful tobacco levy for non-communicable disease (NCD) screening (20).
Limitations
This study has several important limitations that should be considered when interpreting the results: First, the GBD data rely on computational models rather than direct measurements, making them susceptible to biases from annual variations in diagnostic criteria for LBP. This challenge is exacerbated by the heterogeneous nature of input data, which incorporate diverse case definitions and recall periods. While regression methods attempt to adjust for these discrepancies, they introduce additional uncertainty and depend heavily on generalizations from a limited number of studies that provide data on different case definitions. The problem is further compounded by the relatively few predictive covariates in GBD 2021 models, as the relationships between LBP prevalence and other potential risk factors remain poorly quantified. Second, substantial variations in data completeness and reporting standards across countries may lead to underestimation of YLD rates associated with LBP, particularly in low-income settings. Key contributing factors include data sparsity, challenges in obtaining representative samples, and restrictive data-sharing policies in some nations. Third, our study population does not represent all age groups because GBD 2021 lacks data on smoking-attributable LBP cases occurring before age 30. While some studies have attempted to impute missing data using PAFs (2), we caution that such approaches risk introducing additional bias. The GBD 2021 Low Back Pain Collaborators themselves acknowledge that many country-level estimates rely on modeled rather than observed data (52). Although ideally all analyses would use standardized, primary-level data from every country, this remains impractical (1). We therefore used the best available GBD population data while anticipating that future iterations (e.g., GBD 2023) may provide more optimized models for improved accuracy. Finally, while our BAPC model explicitly incorporates smoking decline rates and policy timing, it assumes linear implementation of existing interventions. Four unmodeled factors may affect projections: (1) Emergence of next-generation nicotine products (e-cigarettes, heated tobacco); (2) Climate change impacts on occupational patterns; (3) Disruptive healthcare innovations; (4) COVID-19-related impacts on LBP epidemiology. It is noteworthy that several studies have focused on the potential impact of COVID-19 on low back pain (LBP). Studies have indicated that COVID-19 infection can significantly exacerbate pre-existing LBP symptoms, with 90.1% of patients reporting worsened pain following infection (53). Stay-at-home orders led to reduced physical activity and increased sedentary time, resulting in 49% of surveyed individuals reporting new-onset LBP during the pandemic (54), with 24.7% directly attributing it to the confinement measures (55). Furthermore, social surveys revealed a significant association between non-ergonomic home working environments (e.g., poor posture) and LBP incidence (56), and the prevalence of LBP was significantly higher among remote workers compared to commuters (57). Concurrently, a significant decline in LBP-related healthcare utilization was observed during the pandemic; reports indicate a 9.3%–12.4% reduction in new LBP diagnoses in primary care clinics (58). These limitations highlight important areas for methodological improvement in future burden of disease studies.
Conclusion
Our analysis reveals a consistent global decline in smoking-attributable LBP burden from 1990 to 2021, with notable exceptions in select regions. The burden demonstrates significant demographic and socioeconomic patterning, being disproportionately higher among: (1) male populations, (2) middle-aged and older adults (aged ≥50 years), and (3) residents of high-middle and high Socio-demographic Index (SDI) regions. These disparities highlight critical intervention points for targeted tobacco control strategies. For regions exhibiting slower progress in reducing smoking-related LBP burden, we recommend systematic evaluation of successful policy interventions implemented in high-performing nations. Particular attention should be given to evidence-based approaches that have demonstrated effectiveness in comparable socioeconomic contexts, with potential for adaptation and scale-up.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YC: Conceptualization, Writing – review & editing, Data curation, Writing – original draft, Methodology, Visualization. WS: Methodology, Supervision, Writing – review & editing. XL: Visualization, Validation, Writing – original draft. ZW: Writing – review & editing, Data curation, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was supported by the Fujian Provincial Clinical Medical Research Center for First Aid and Rehabilitation in Orthopaedic Trauma (2020Y2014).
Acknowledgments
We acknowledge GBD2021 collaborators whose outstanding contributions have enabled us to complete this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmscd.2025.1619122/full#supplementary-material
Supplementary Material 1 | GBD permission documentation.
Supplementary Table S1 | Global SDI index for 204 countries and territories from 1950 to 2021.
Supplementary Table S2 | Global YLDs of smoking-attributable low back pain by 204 countries and territories.
Supplementary Table S3 | Global EAPC of ASYR for smoking-attributable low back pain by 204 countries and territories.
Supplementary Table S4 | The detailed forecast values of the case numbers and ASRs of YLDs by 2035.
Abbreviations
GBD, global burden of diseases; SDI, socio-demographic index; LBP, low back pain; YLDs, years lived with disability; ASYR, age-standardized YLDs rate; BAPC, Bayesian age-period-cohort; EAPC, estimated annual percentage change; SII, slope index of inequality; CI, concentration index.
References
1. GBD. 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: a systematic analysis of the global burden of disease study 2021. Lancet Rheumatol. (2023) 5:e316–29. doi: 10.1016/S2665-9913(23)00098-X
2. Chen N, Fong DYT, Wong JYH. Health and economic burden of low back pain and rheumatoid arthritis attributable to smoking in 192 countries and territories in 2019. Addiction. (2024) 119:677–85. doi: 10.1111/add.16404
3. The Lancet Rheumatology. The global epidemic of low back pain. Lancet Rheumatol. (2023) 5:e305. doi: 10.1016/S2665-9913(23)00133-9
4. Carregaro RL, Tottoli CR, Rodrigues DDS, Bosmans JE, da Silva EN, van Tulder M. Low back pain should be considered a health and research priority in Brazil: lost productivity and healthcare costs between 2012 and 2016. PLoS One. (2020) 15:e0230902. doi: 10.1371/journal.pone.0230902
5. GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the global burden of disease study 2019. Lancet. (2021) 397:2337–60. doi: 10.1016/S0140-6736(21)01169-7
6. GBD 2021 Tobacco Forecasting Collaborators. Forecasting the effects of smoking prevalence scenarios on years of life lost and life expectancy from 2022 to 2050: a systematic analysis for the global burden of disease study 2021. Lancet Public Health. (2024) 9:e729–44. doi: 10.1016/S2468-2667(24)00166-X
7. Schembri E. Is chronic low back pain and radicular neuropathic pain associated with smoking and a higher nicotine dependence? A cross-sectional study using the DN4 and the Fagerström test for nicotine dependence. Ağrı. (2021) 33:155–167. doi: 10.14744/agri.2021.79836
8. Tu J, Li W, Hansbro PM, Yan Q, Bai X, Donovan C, et al. Smoking and tetramer tryptase accelerate intervertebral disc degeneration by inducing METTL14-mediated DIXDC1 m6 modification. Mol Ther. (2023) 31:2524–42. doi: 10.1016/j.ymthe.2023.06.010
9. Pang H, Chen S, Klyne DM, Harrich D, Ding W, Yang S, et al. Low back pain and osteoarthritis pain: a perspective of estrogen. Bone Res. (2023) 11:42. doi: 10.1038/s41413-023-00280-x
10. Han Z, Chen Y, Ye X. The causality between smoking and intervertebral disc degeneration mediated by IL-1β secreted by macrophage: a Mendelian randomization study. Heliyon. (2024) 10:e37044. doi: 10.1016/j.heliyon.2024.e37044
11. Eser B, Eser O, Yuksel Y, Aksit H, Karavelioglu E, Tosun M, et al. Effects of MMP-1 and MMP-3 gene polymorphisms on gene expression and protein level in lumbar disc herniation. Genet Mol Res. (2016) 15. doi: 10.4238/gmr.15038669
12. Foizer GA, Paiva VCD, Gorios C, Cliquet A, Miranda JBD. Smoking and modic changes in patients with chronic low back pain: a comparative study. Acta Ortop Bras. (2024) 32:e278628. doi: 10.1590/1413-785220243205e278628
13. Harshitha SH, Sibin MK, Chetan GK, Bhat DI. Association of CILP, COL9A2 and MMP3 gene polymorphisms with lumbar disc degeneration in an Indian population. J Mol Neurosci. (2018) 66:378–82. doi: 10.1007/s12031-018-1182-3
14. Perera RS, Dissanayake PH, Senarath U, Wijayaratne LS, Karunanayake AL, Dissanayake VHW. Variants of ACAN are associated with severity of lumbar disc herniation in patients with chronic low back pain. PLoS One. (2017) 12:e0181580. doi: 10.1371/journal.pone.0181580
15. GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2133–61. doi: 10.1016/S0140-6736(24)00757-8
16. Hancock M, Kongsted A. Towards improving the global burden of disease estimates for low back pain. Lancet Rheumatol. (2024) 6:e588–9. doi: 10.1016/S2665-9913(24)00182-6
17. Cheng M, Xue Y, Cui M, Zeng X, Yang C, Ding F, et al. Global, regional, and national burden of low back pain. Spine. (2025) 50:E128–39. doi: 10.1097/BRS.0000000000005265
18. Bai Z, Han J, An J, Wang H, Du X, Yang Z, et al. The global, regional, and national patterns of change in the burden of congenital birth defects, 1990–2021: an analysis of the global burden of disease study 2021 and forecast to 2040. EClinicalMedicine. (2024) 77:102873. doi: 10.1016/j.eclinm.2024.102873
19. Li Y, Zou C, Guo W, Han F, Fan T, Zang L, et al. Global burden of low back pain and its attributable risk factors from 1990 to 2021: a comprehensive analysis from the global burden of disease study 2021. Front Public Health. (2024) 12:1480779. doi: 10.3389/fpubh.2024.1480779
20. Hosseinpoor AR, Bergen N, Schlotheuber A. Promoting health equity: WHO health inequality monitoring at global and national levels. Glob Health Action. (2015) 8:29034. doi: 10.3402/gha.v8.29034
21. Zhang J, Wang B, Zou C, Wang T, Yang L, Zhou Y. Low back pain trends attributable to high body mass index over the period 1990–2021 and projections up to 2036. Front Nutr. (2025) 11:1521567. doi: 10.3389/fnut.2024.1521567
22. Knoll M, Furkel J, Debus J, Abdollahi A, Karch A, Stock C. An R package for an integrated evaluation of statistical approaches to cancer incidence projection. BMC Med Res Methodol. (2020) 20:257. doi: 10.1186/s12874-020-01133-5
23. Zhang Z, Wu O, Ying J, Jin Y, Wang H, Tian H, et al. Regulation of diabetic disc degeneration: the role of AGEAT/miR-204-5p/Mapk4 axis in nucleus pulposus cells’ mitochondrial function and apoptosis. Cell Signal. (2025) 133:111857. doi: 10.1016/j.cellsig.2025.111857
24. Yang W, Yang Y, Wang Y, Gao Z, Zhang J, Gao W, et al. Metformin prevents the onset and progression of intervertebral disc degeneration: new insights and potential mechanisms (review). Int J Mol Med. (2024) 54:71. doi: 10.3892/ijmm.2024.5395
25. Stevans JM, Delitto A, Khoja SS, Patterson CG, Smith CN, Schneider MJ, et al. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw Open. (2021) 4:e2037371. doi: 10.1001/jamanetworkopen.2020.37371
26. Yang Q-H, Zhang Y-H, Du S-H, Wang Y-C, Wang X-Q. Association between smoking and pain, functional disability, anxiety and depression in patients with chronic low back pain. Int J Public Health. (2023) 68:1605583. doi: 10.3389/ijph.2023.1605583
27. Ikeda T, Cooray U, Murakami M, Osaka K. Assessing the impacts of smoking cessation and resumption on back pain risk in later life. Eur J Pain. (2023) 27:973–80. doi: 10.1002/ejp.2139
28. Yang Y, Lai X, Li C, Yang Y, Gu S, Hou W, et al. Focus on the impact of social factors and lifestyle on the disease burden of low back pain: findings from the global burden of disease study 2019. BMC Musculoskelet Disord. (2023) 24:679. doi: 10.1186/s12891-023-06772-5
29. Zhang C, Zi S, Chen Q, Zhang S. The burden, trends, and projections of low back pain attributable to high body mass index globally: an analysis of the global burden of disease study from 1990 to 2021 and projections to 2050. Front Med. (2024) 11:1469298. doi: 10.3389/fmed.2024.1469298
30. Xu S, Chen J, Wang C, Lin Y, Huang W, Zhou H, et al. Global, regional, and national burden of low back pain for adults aged 55 and older 1990–2021: an analysis for the global burden of disease study 2021. BMC Musculoskelet Disord. (2025) 26:81. doi: 10.1186/s12891-025-08326-3
31. Wang YXJ. Menopause as a potential cause for higher prevalence of low back pain in women than in age-matched men. J Orthop Translat. (2017) 8:1–4. doi: 10.1016/j.jot.2016.05.012
32. Dai X, Gakidou E, Lopez AD. Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob Control. (2022) 31:129–37. doi: 10.1136/tobaccocontrol-2021-056535
33. Bento TPF, Genebra CVDS, Maciel NM, Cornelio GP, Simeão SFAP, Vitta Ad. Low back pain and some associated factors: is there any difference between genders? Braz J Phys Ther. (2020) 24:79–87. doi: 10.1016/j.bjpt.2019.01.012
34. Hooftman WE, van der Beek AJ, Bongers PM, van Mechelen W. Is there a gender difference in the effect of work-related physical and psychosocial risk factors on musculoskeletal symptoms and related sickness absence? Scand J Work Environ Health. (2009) 35:85–95. doi: 10.5271/sjweh.1316
35. Aasa U, Barnekow-Bergkvist M, Ängquist K-A, Brulin C. Relationships between work-related factors and disorders in the neck-shoulder and low-back region among female and male ambulance personnel. J Occup Health. (2005) 47:481–9. doi: 10.1539/joh.47.481
36. Björck-van Dijken C, Fjellman-Wiklund A, Hildingsson C. Low back pain, lifestyle factors and physical activity: a population based-study. J Rehabil Med. (2008) 40:864–9. doi: 10.2340/16501977-0273
37. Ono R, Yamazaki S, Takegami M, Otani K, Sekiguchi M, Onishi Y, et al. Gender difference in association between low back pain and metabolic syndrome: locomotive syndrome and health outcome in Aizu cohort study (LOHAS). Spine. (2012) 37:1130–7. doi: 10.1097/BRS.0b013e31824231b8
38. Alkherayf F, Agbi C. Cigarette smoking and chronic low back pain in the adult population. Clin Invest Med. (2009) 32:E360–7. doi: 10.25011/cim.v32i5.6924
39. Saika F, Uta D, Fukazawa Y, Hino Y, Hatano Y, Kishioka S, et al. Androgen receptors expressed in the primary sensory neurons regulate mechanical pain sensitivity. Pain. (2025). doi: 10.1097/j.pain.0000000000003736
40. Ren Q, Chen L, Ma Y, Huang Y, Wang S. Immune microenvironment in intervertebral disc degeneration: pathophysiology and therapeutic potential. Front Immunol. (2025) 16:1563635. doi: 10.3389/fimmu.2025.1563635
41. Zhang L, Tong Z, Han R, Guo R, Zang S, Zhang X, et al. Global, regional, and national burdens of ischemic heart disease attributable to smoking from 1990 to 2019. J Am Heart Assoc. (2023) 12:e028193. doi: 10.1161/JAHA.122.028193
42. Zhai C, Hu D, Yu G, Hu W, Zong Q, Yan Z, et al. Global, regional, and national deaths, disability-adjusted life years, years lived with disability, and years of life lost for the global disease burden attributable to second-hand smoke, 1990–2019: a systematic analysis for the global burden of disease study. Sci Total Environ. (2023) 862:160677. doi: 10.1016/j.scitotenv.2022.160677
43. Jia X, Sheng C, Han X, Li M, Wang K. Global burden of stomach cancer attributable to smoking from 1990 to 2019 and predictions to 2044. Public Health. (2024) 226:182–9. doi: 10.1016/j.puhe.2023.11.019
44. Wang Z, Gu Y, Wang R, He Y, Ge H, Yang Z, et al. The global magnitude and temporal trend of rheumatoid arthritis burden attributable to smoking from 1990 to 2019. Rheumatology. (2024) 63:689–97. doi: 10.1093/rheumatology/kead269
45. Safiri S, Nejadghaderi SA, Abdollahi M, Carson-Chahhoud K, Kaufman JS, Bragazzi NL, et al. Global, regional, and national burden of cancers attributable to tobacco smoking in 204 countries and territories, 1990–2019. Cancer Med. (2022) 11:2662–78. doi: 10.1002/cam4.4647
46. Banks E, Joshy G, Weber MF, Liu B, Grenfell R, Egger S, et al. Tobacco smoking and all-cause mortality in a large Australian cohort study: findings from a mature epidemic with current low smoking prevalence. BMC Med. (2015) 13:38. doi: 10.1186/s12916-015-0281-z
47. Flor LS, Reitsma MB, Gupta V, Ng M, Gakidou E. The effects of tobacco control policies on global smoking prevalence. Nat Med. (2021) 27:239–43. doi: 10.1038/s41591-020-01210-8
48. Almeida L, Szklo A, Sampaio M, Souza M, Martins LF, Szklo M, et al. Global adult tobacco survey data as a tool to monitor the WHO framework convention on tobacco control (WHO FCTC) implementation: the Brazilian case. Int J Environ Res Public Health. (2012) 9:2520–36. doi: 10.3390/ijerph9072520
49. Chen S, Chen M, Wu X, Lin S, Tao C, Cao H, et al. Global, regional and national burden of low back pain 1990–2019: a systematic analysis of the global burden of disease study 2019. J Orthop Translat. (2022) 32:49–58. doi: 10.1016/j.jot.2021.07.005
50. Paraje G, Stoklosa M, Blecher E. Illicit trade in tobacco products: recent trends and coming challenges. Tob Control. (2022) 31:257–62. doi: 10.1136/tobaccocontrol-2021-056557
51. World Health Organization, WHO Framework Convention on Tobacco Control. Protocol to eliminate illicit trade in tobacco products. Geneva: World Health Organization (2013). p. 58. Available online at: https://iris.who.int/handle/10665/80873 (Accessed March 30, 2025).
52. Tamrakar M, Kharel P, Traeger A, Maher C, O’Keeffe M, Ferreira G. Completeness and quality of low back pain prevalence data in the global burden of disease study 2017. BMJ Glob Health. (2021) 6:e005847. doi: 10.1136/bmjgh-2021-005847
53. Sathu S, Kumar R, Maley DK, Eppakayala S, Kashyap A, NynaSindhu A, et al. Increased frequency of low back pain in recent times: does the answer lie in COVID-19? Cureus. (2023) 15:e50021. doi: 10.7759/cureus.50021
54. Hawamdeh M, Altaim TA, Shallan A, Gaowgzeh RA, Obaidat SM, Alfawaz S, et al. Low back pain prevalence among distance learning students. Int J Environ Res Public Health. (2022) 20:342. doi: 10.3390/ijerph20010342
55. Alsayari BM, Alshehri SM, Almulhim AY, Alzakry LM, Alzuraiq AA, Binshalhoub FH, et al. COVID-19 and its impact on back pain in the eastern province of Saudi Arabia. Cureus. (2024) 16:e57475. doi: 10.7759/cureus.57475
56. Minoura A, Ishimaru T, Kokaze A, Tabuchi T. Increased work from home and low back pain among Japanese desk workers during the coronavirus disease 2019 pandemic: a cross-sectional study. Int J Environ Res Public Health. (2021) 18:12363. doi: 10.3390/ijerph182312363
57. Toprak Celenay S, Karaaslan Y, Mete O, Ozer Kaya D. Coronaphobia, musculoskeletal pain, and sleep quality in stay-at home and continued-working persons during the 3-month COVID-19 pandemic lockdown in Turkey. Chronobiol Int. (2020) 37:1778–85. doi: 10.1080/07420528.2020.1815759
Keywords: low back pain, global burden of disease, smoking, burden, years lived with a disability
Citation: Chen Y, Sun W, Lian X and Wang Z (2025) Global perspective on the burden of smoking-attributable low back pain: new insights from the global burden of disease study 2021. Front. Musculoskelet. Disord. 3:1619122. doi: 10.3389/fmscd.2025.1619122
Received: 27 April 2025; Accepted: 25 August 2025;
Published: 5 September 2025.
Edited by:
Maruti Gudavalli, Keiser University, United StatesReviewed by:
Xuefeng Jin, Xiamen University, ChinaDhruvi Modi, Gujarat Adani Institute of Medical Sciences, India
Copyright: © 2025 Chen, Sun, Lian and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yufan Chen, eXVmYW5jaGVuMjAyMkAxNjMuY29t
†ORCID:
Yufan Chen
orcid.org/0009-0002-2962-1006
 Yufan Chen
Yufan Chen Wenjia Sun
Wenjia Sun Xionghan Lian
Xionghan Lian