- 1Rheumatology Unit, Department of Medical and Surgical Specialties, University of Pisa, Pisa, Italy
- 2Department of Clinical and Experimental Medicine and Department of Mathematics, University of Pisa, Pisa, Italy
- 3Orthopaedic and Traumatologic Clinic, University of Pisa, Pisa, Italy
- 4Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy
- 5Rheumatology Unit, Department of Medical and Surgical Specialties, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy
Introduction: The timely administration of anti-osteoporotic medications (AOM) to patients with fragility fractures (FF) reduces the imminent refracture risk. European studies found a wide osteoporosis treatment gap, but Italian cohort-based data are lacking.
Materials and methods: We aimed to assess the entity of the osteoporosis treatment gap in an Italian cohort, i.e., the percentage of patients not treated within 2 months from the index fragility fractures (FF) and the time delay in anti-osteoporotic medications (AOM) administration, and its impact on refracture risk. We retrospectively collected the clinical histories of 500 randomly selected osteoporotic patients with FFs referred to our Fracture Liaison Service. We identified those who had AOM prescribed within 2 months from the index FF (“early treatment” group) and those who had not (“untreated” group). Refracture occurrence was retrospectively assessed in both groups, followed by a survival and risk analysis.
Results: Forty-one patients were excluded for missing data. Out of 459 patients, 374 (81.5%) received AOM therapy more than 2 months after the index FF, with a median delay of 24 months (IQR 52) (range 3–312; mean 47 months). The log-rank test showed that the “untreated” group was significantly more prone to refracture than “early treatment” (78% vs. 48%, respectively; p = 0.0001). Cox regression revealed a 44% lower probability of refracture in the “early treatment” group.
Discussion: In this study, 81.5% of individuals had their first AOM prescription after a median time of 24 months after the index FF, resulting in higher refracture risk. Preventive strategies to reduce the osteoporosis treatment gap should be implemented.
1 Introduction
Osteoporosis (OP) is a progressive skeletal disease characterized by reduced bone mass and deterioration of bone architecture. It is prevalent especially among postmenopausal women and the elderly population. OP is the most frequent cause of atraumatic or low-energy fractures, also called fragility fractures (FFs), and therefore is often associated with disability and low quality of life, along with increased premature mortality rates (1). Comprehensive data showed that in 2019, more than 23 million people were at high risk of FFs in the European Union (EU), with a lifetime risk of 33% for women and at least 15% for men. Moreover, an increase of 24.8% in the number of FFs was estimated to occur in the EU between 2019 and 2034, mirroring the expected increase in the elderly population by 11.4% in the same timeframe. OP is a major and increasingly relevant economic burden for healthcare systems across Europe; the total direct cost of incident FFs in the EU amounted to €56.9 billion in 2019, after an increase of 64% from 2010 despite the lack of increment in pharmacological costs (2).
Medical management of FFs is aimed at secondary prevention of subsequent fractures and includes pain control, surgical intervention, physical therapy, and nowadays numerous anti-osteoporotic medications (AOM) comprising both antiresorptive (oral or intravenous bisphosphonates, denosumab, and selective estrogen receptor modulators) and anabolic agents (parathyroid hormone receptor agonists and romosozumab) (3). It is undisputed that a patient with a prior FF has an increased risk for subsequent FFs and that such risk is highest immediately after an FF (4). Thus, timely AOM prescription after an FF is vital to limit refracture occurrence (5, 6). However, the gap between patients eligible for AOM treatment and the number of patients treated with AOMs, which is referred to as the OP treatment gap, is still very high in recent reports from several countries (6–12) and in some cases has even increased over the past decades (13). Recent estimates suggested that untreated women with FFs increased from 10.6 million to 14 million between 2010 and 2019 in the EU (14).
As for Italy, the scorecard for osteoporosis in Europe (SCOPE) showed that it was the country with the highest prevalence of OP (6.3% of the population) in the EU and had a crude incidence of FFs amounting to 20.6/1,000 individuals aged 50 or more. Notably, Italy is projected to have the second-largest number of FFs—approximately 666,000—in the EU in 2034. The estimated OP treatment gap for postmenopausal women in Italy was approximately 71% in 2019 (2). In 2019, the total direct cost for FFs amounted to €9.45 billion and accounted for approximately 6% of the national healthcare expenditure, the second highest proportion across the EU. The number of FF-related deaths exceeded that for diseases such as lung cancer (14).
A survey carried out by the Italian Region of Tuscany showed that in 2018, out of 2,964 patients with a recent vertebral FF and out of 6,972 cases of femoral FF, only 10.8% and 4.6% were prescribed AOM therapy within 90 days after the FF, respectively; the proportion of patients who performed a dual-energy X-ray absorptiometry (DXA) scan and went to a OP consultation within 90 days from the FF was even lower, at approximately 1% (15). To our knowledge, no Italian cohort-based data concerning the OP treatment gap have ever been published in the scientific literature, nor the time delay in AOM prescription for fractured patients has ever been quantified. Therefore, we carried out a pilot retrospective study aimed at measuring the OP treatment gap using local data collected in the Fracture Liaison Service (FLS) of the Pisa University Hospital in Tuscany, Italy. We also investigated whether the OP treatment gap affected the risk of refracture in the population herein described.
2 Materials and methods
2.1 Study design and data collection
We conducted a monocentric, retrospective, observational, and longitudinal study using data from a cohort of 500 adult patients of both sexes followed at the FLS of the Pisa University Hospital and with a history of primary OP- or secondary OP-related major FFs, i.e., fractures at the vertebrae, hip, proximal humerus, wrist, and pelvis, which occurred spontaneously or were caused by a fall from standing height or by low-intensity trauma. Our FLS is a multidisciplinary outpatient clinic specializing in the diagnosis, treatment, and rehabilitation of patients with vertebral FFs and is made up of rheumatologists, orthopedic surgeons, and physiatrists of the Pisa University Hospital. All patients followed at our FLS have a history of at least one vertebral FF, either as first FF or as refracture—or both.
We gathered data based on the information collected during visits that took place between January 2014, coinciding with the beginning of the FLS activity, and December 2023. For each patient, an accurate medical history is performed at the time of the first visit. Firstly, at the time of retrospective analysis, we collected patient demographics, weight, height, body mass index (BMI), smoking status, medications including glucocorticoids and psychotropic drugs (a risk factor for falls), comorbidities [expressed with the Charlson Comorbidity Index (16)], family history of FFs (considered positive if a patient's parent ever had vertebral or femoral FFs), and previously performed DXA scans. Due to the focus of our FLS on patients with vertebral FFs, we considered T-scores only at the total hip on DXA scans in consideration of the known artifacts caused by vertebral FFs on T-scores at L1–L4.
Secondly, we retrieved information on each patient's previous history of FF(s), specifically the month, year, and site of the first (index) FF. FFs were considered only if patients had either radiologic documentation for image review or a hospital or emergency department discharge letter demonstrating the diagnosis of FF. The patients' age and FRAX value (17) following the index FF were retrieved.
Thirdly, we assessed whether patients had ever taken AOM therapy after the index FF. Our definition of AOM included only drugs with proven anti-refracture efficacy and available in Italy in compliance with reimbursement policies established by Nota 79 provided by the Italian Medicines Agency (AIFA) (18) during the 2014–2023 period, i.e., alendronate, ibandronate, risedronate, zoledronate, raloxifene, bazedoxifene, denosumab, and teriparatide. AOM history was ascertained with previous medical documentation retrieved at the first visit, or, if not available, by self-report by the patient.
Incompleteness of any of the previous data resulted in patient exclusion from the definitive cohort.
Our endpoints were to assess the OP treatment gap, i.e., the percentage of patients with FF(s) who had not received AOM treatment within 2 months from the index FF, and the time delay in AOM prescription. The cutoff of 2 months was chosen with the assumption that it would be rational for a patient with OP-related FFs to receive a timely AOM treatment, considering that, as previously mentioned, the risk of subsequent FF is highest immediately after an index FF.
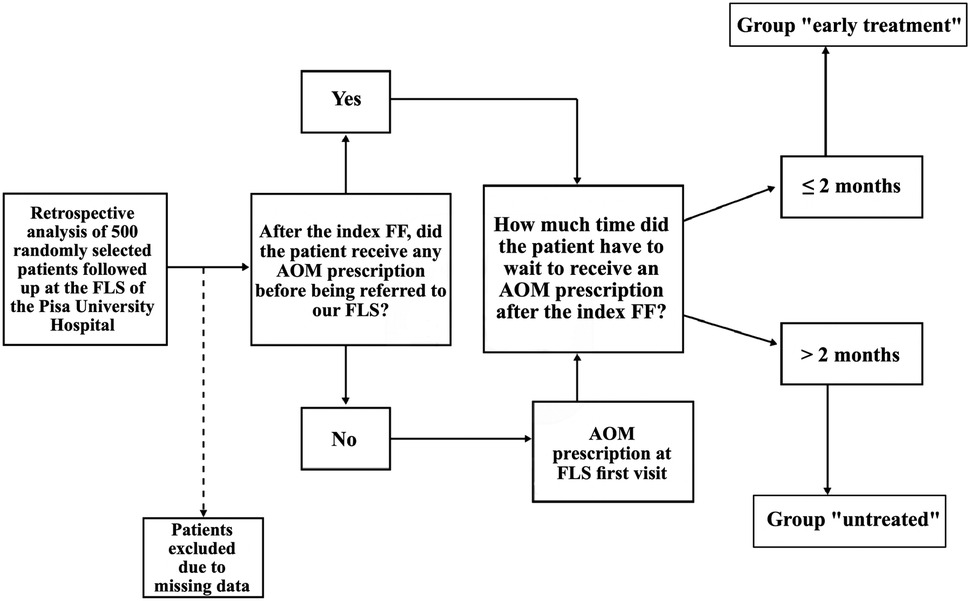
With regards to this endpoint, at the time in which patients came to our FLS for the first time, we assessed whether patients ever received an AOM prescription after the index FF. If AOM therapy had been prescribed elsewhere after their index FF, we measured the time elapsed between the index FF and AOM prescription. By contrast, if they had never been prescribed any AOMs, patients were prescribed the most appropriate AOM at the first visit at our FLS, and then we measured the time elapsed between the index FF and the day of the visit. In this way, we identified two groups of patients: those who received their first AOM prescription, either elsewhere or at our FLS, within 2 months from the index FF (“early treatment” group), and those who did not (“untreated” group), either because they started taking AOM treatment after 2 months from the index FF, or because they were never treated before being referred to our FLS and two months from the index FF had already elapsed (Figure 1).
Our second endpoint was to compare the “early treatment” group and the “untreated” group in terms of refracture risk and refracture-free survival. Refracture was defined as the first clinically evident major osteoporotic FF (vertebral, hip, proximal humerus, wrist, or pelvis) occurring after the index FF. Refracture was considered only if either radiologic documentation for image review or a hospital or emergency department discharge letter demonstrating the diagnosis were available. In the case of refracture, we collected the month and year of occurrence, its site, and the time elapsed from the index FF.
For patients identified as “early treatment,” we investigated the occurrence of refracture after AOM prescription by retrieving documentation from each FLS follow-up visit (either every 6 or 12 months, according to the monitoring needs of the specific AOM taken by the patient) until the last available visit; in case a patient of the “early treatment” group did not have at least one follow-up visit, the patient was excluded from the survival analysis. For patients identified as “untreated,” we investigated the occurrence of refracture in the period between the index FF and the date of the first AOM prescription; therefore, for the “untreated” group, the period after the AOM prescription was not assessed for refracture occurrence. For any patient, independent of group, the date of the first refracture would correspond to the end of the retrospective analysis. The occurrence of a second (or subsequent) refracture was not investigated or considered in this study.
We carried out a survival analysis with the data obtained on refracture occurrence. Since AOM treatment prescription for patients in the “early treatment” group did not always coincide with the immediate timeframe around the index FF, this could lead to a potential immortal time bias in our analysis. We employed a landmark analysis (19) to counter this issue. Because patients of the “early treatment” group started taking AOM treatment within 2 months from the index FF, the landmark (i.e., time zero of the survival analysis) was moved from the index FF date to the end of a 2-month (62 days) “ascertainment window” for both groups. Since for patients of the “untreated” group we did not consider the period after AOM prescription, moving the landmark as previously explained was sufficient to solve a potential immortal time bias issue. Patients of the entire cohort who had the first refracture during the 2-month “ascertainment window” (i.e., within 2 months from index FF) were excluded from the survival analysis (Figure 2).
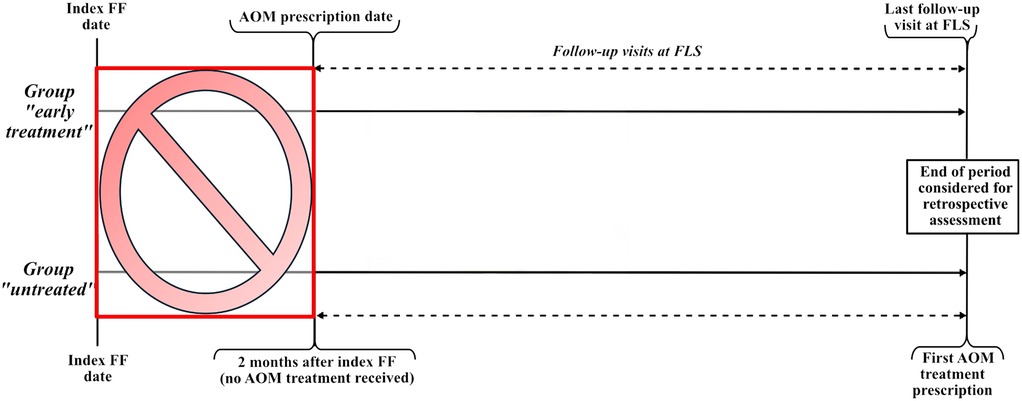
Figure 2. Graphics illustrating the follow-up periods of the “early treatment” and “untreated” groups that were set in the survival analysis to address and minimize immortal time bias. Patients who had a refracture during a 2-month “ascertainment window” (the red box) were excluded from the survival analysis. Follow-up for refracture started at the end of the “ascertainment window,” i.e., at the AOM prescription date for the “early treatment” group and at 2 months from the index FF for the “untreated” group.
2.2 Sample size calculation and statistical analysis
Considering the results of the previously mentioned survey carried out by the region of Tuscany (15) and that no references in the previous literature could drive a specific target sample size, we designed a pilot study on 500 patients and calculated the post hoc statistical power with the “PowerSurvEpi” package of R to verify the robustness of the results.
Based on the results of the Shapiro–Wilk test, continuous data were described as median and interquartile range (IQR) in case of non-Gaussian distribution, or as mean and standard deviation (SD) in case of Gaussian distribution. Categorical variables were expressed as numbers and proportions related to the available data. The groups were compared using the Chi-squared test and the Mann–Whitney U-test for qualitative and quantitative variables, respectively. Missing data were not imputed. p-value was set at 0.05 level.
A Cox regression was carried out to model the survival time. The Cox regression model included group assignment (“early treatment” vs. “untreated”) as the primary covariate. We verified the proportional hazards assumption through visual inspection of log–log survival plots. The model assumed independence of observations, non-informative censoring, and proportional hazards over time.
The Kaplan–Meier method was then applied, with a log-rank test, divided by group.
Statistical elaborations were performed using the STATA 15.1 software package (StataCorp) and R 4.4.2 for MacOS (Apple Inc.).
3 Results
Five hundred subjects were randomly selected for our retrospective study. Forty-one were excluded due to missing data; therefore, 459 patients (414 women, 90.2%) made up the definitive cohort. According to our definition of AOM treatment, only two patients among the 500 who were randomly selected had been prescribed appropriate AOM therapy (alendronate in both cases) elsewhere before the first FLS visit; due to incomplete data regarding their medical history, they were among the 41 excluded patients. Therefore, all 459 patients in the definitive cohort had never received AOM treatment by the time of the first FLS visit.
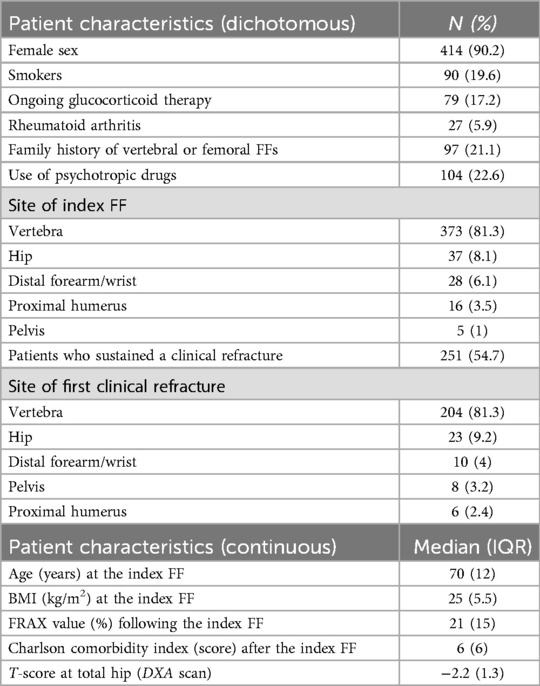
The median age at the index FF was 70 years (IQR 12). The index FFs were most frequent at the vertebrae (n = 373; 81.3%), followed by the hip (n = 37; 8.1%), the distal forearm or wrist (n = 28; 6.1%), the proximal humerus (n = 16; 3.5%), and the pelvis (n = 5; 1%). The remaining patient characteristics are summarized in Table 1.
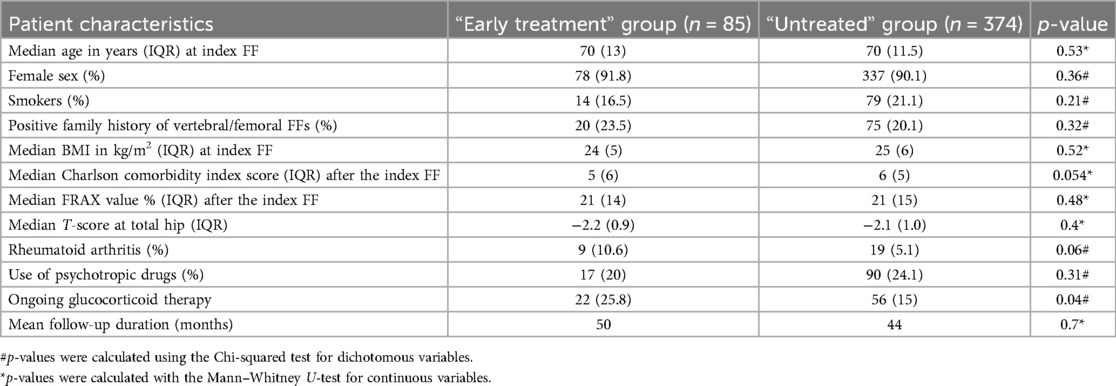
All 459 patients received their first AOM prescription after the index FF at the first FLS visit. Based on the retrospectively retrieved time between index FF and AOM prescription date, we observed that 374 (81.5%) patients did not receive AOM therapy within 2 months from the index FF. In these patients, identified as the “untreated” group, the median time delay between the index FF and first AOM prescription was 24 months (IQR 52 months) (range 3–312; mean 47 months). The remaining 85 (18.5%) patients received the first AOM prescription within 2 months from the index FF and thus were identified as the “early treatment” group. The two groups were comparable in terms of all considered variables except for ongoing glucocorticoid therapy which was significantly more frequent in the “early treatment” group (Table 2).
At the time of the index FF and before arrival at our FLS, out of 374 patients in the “untreated” group, 187 (50%) received vitamin D supplementation, 25 (6.7%) received calcium + vitamin D supplementation, and 201 (53.4%) were prescribed intramuscular clodronate, a non-nitrogen-containing bisphosphonate which is not included among drugs listed in Nota 79 (18). The persistence of clodronate treatment was very low, with 8% of the patients still on therapy after 1 year from the first prescription.
In our cohort, 251 (54.7%) patients had a refracture. Approximately 91% (n = 228) of these patients belonged to the “untreated” group. The most frequent sites of refracture were the vertebrae (n = 204; 81.3%), followed by the hip (n = 23; 9.2%), the distal forearm or the wrist (n = 10; 4%), the pelvis (n = 8; 3.2%), and the proximal humerus (n = 6; 2.4%).
For the second endpoint of the study, we carried out a survival analysis. To address a potential immortal time bias, 12 patients (11 of whom belonged to the “untreated” group and one belonged to the “early treatment” group) who had a refracture during the previously mentioned 2-month “ascertainment window” were excluded. Therefore, the final survival analysis included 447 individuals (363 in the “untreated” group and 84 in the “early treatment” group). The comparability of the two groups (Table 2) was unaffected by the exclusion of the 12 patients (data not shown). The log-rank test showed that patients in the “untreated” group were significantly more prone to refracture as opposed to patients in the “early treatment” group (78% vs. 48%, respectively; χ2 = 19.1; p = 0.0001). The Cox regression analysis revealed a 44% lower probability of refracture in the “early treatment” group compared with the “untreated” group [hazard ratio (HR) 0.56; 95% CI: 38%–73%; p = 0.004].
Despite the numerical imbalance between the two groups, the statistical robustness of our results was supported by a post hoc power analysis; considering the overall HR, the power was 95.5% (95% CI: 95.0–95.7%).
The Kaplan–Meier estimator showed that, for patients in the “early treatment” group, the median time between AOM treatment prescription and first clinical refracture was 41 months (IQR 47 months), whereas for patients in the “untreated” group, the median time between the end of the 2-month “ascertainment window” and the first clinical refracture was 26 months (IQR 50 months), over a considered follow-up time of 120 months (Figure 3).
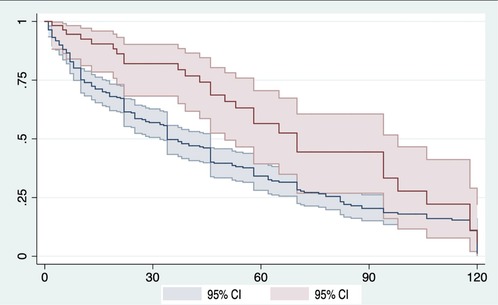
Figure 3. Kaplan–Meier survival curve comparing the “early treatment” (red line) and “untreated” (blue line) groups. To address the potential immortal time bias, the landmark (time zero) corresponds to the end of the 2-month “ascertainment window.” Refer to text and Figure 2 in Materials and methods for further details. The event corresponds to the first clinical refracture. In the graph, the x-axis represents the time elapsed from the landmark, expressed in months, and the y-axis represents the refracture-free survival, expressed in decimals. CI, confidence interval.
4 Discussion
To our knowledge, our retrospective study represents the first description of the entity of the OP treatment gap in the catchment area of an Italian FLS and of its impact on refracture recurrence.
Our first finding is that only 18.5% of the patients living in the catchment area of our hospital's FLS received an AOM prescription within 2 months from the index FF (“early treatment” group). This finding is in accordance with a recent study which reported that only 20% of 915 patients recruited at eight Austrian centers were prescribed AOM treatment at the time of the index FF (8).
Moreover, 81.5% of the cohort received the first AOM prescription after the index FF with a median delay of 24 months, with some patients receiving AOM therapy for the first time >10 years after the index FF. These data are worrisome since it is known that the risk of refracture is time-dependent, with an imminent risk that is highest in the first 2 years after the index FF (20). Although the patients in the “untreated” group did not receive AOM therapy, we observed that many had been identified as osteoporotic in the months after the index FF; as a matter of fact, before the first visit in our FLS, a considerable proportion had been treated with calcium, vitamin D supplementation, and/or intramuscular clodronate, a non-nitrogen-containing bisphosphonate not included in Nota 79 (18). Although a randomized, double-blind, placebo-controlled trial in women unselected for osteoporosis showed that 3-year oral daily 800 mg clodronate reduced the incidence of any clinical FF by 20% with respect to placebo, hip FF incidence was unaffected, and vertebral FF incidence was not specifically addressed (21). To the best of our knowledge, no randomized controlled trial has been carried out to test the ability of either oral or intramuscular clodronate to prevent FF recurrence, i.e., secondary FF prevention, in an osteoporotic population.
Our second finding is that AOM prescription within 2 months from the index FF reduced the risk of refracture by 44%. Thus, our data provide evidence of the importance of timely and efficacious AOM prescription following FFs.
Despite continuous advancements in OP diagnosis and treatment over the decades, it is evident that still too many osteoporotic patients with FFs are not offered timely AOM treatment. This phenomenon has been recently defined as a global “crisis” because of its disastrous consequences in terms of both refracture rate and economic burden on society (22). It is worrisome to observe that the OP treatment gap is widening in several countries over time, as reported in a recent study from Switzerland (13), showing that this worldwide problem is not being addressed in the manner it deserves. Several elements have been suggested to inflate the treatment gap. It is possible that there are not as many OP specialists as it is needed considering the population aging currently occurring worldwide (23). Furthermore, healthcare providers may incorrectly consider OP an inevitable degenerative process of the bone that would not benefit from any treatment (24), and others may ignore or disregard the latest treatment guidelines. Importantly, the fear of treatment-related side effects, in particular the osteonecrosis of the jaw following treatment with bisphosphonates and denosumab, may lead to omission of AOM prescription by physicians and avoidance of AOM treatment by patients, despite the evidence that serious adverse events occur only in a small minority of AOM-treated patients as opposed to the large-scale benefits that AOM therapy has been shown to yield in FF prevention (25). Last, but not least, the absence of an effective action by those in charge of clinical governance at either regional or national level may play a role. Potential strategies to reduce the OP treatment gap may include the creation of FLS and Bone Units, i.e., of departments aimed at capturing the largest possible proportion of FFs to promptly prescribe AOM treatment.
Our study has limitations within which our results should be interpreted. Firstly, the retrospective design of the study did not allow the systematic collection of data such as the specific reason(s) behind the lack of timely AOM prescription in the “untreated” group, and thus we could not draw any overall conclusion on this aspect. Nevertheless, all patients with missing data were excluded from the final analysis to compensate for the retrospective design, still resulting in a sample size that proved more than sufficient for high statistical power and a definitive cohort with comprehensive and uniform data. Furthermore, we acknowledge that the retrospective nature of the study may introduce potential confounding variables. However, we addressed this by comparing baseline characteristics between groups (Table 2), which showed comparable distributions (except for glucocorticoid use), and by collecting comprehensive clinical data including several risk factors for osteoporosis among the considered variables. While we did not perform multivariable adjustments due to the pilot nature of the study, the groups were well-balanced for most potential confounders. Future prospective studies should include multivariable Cox regression to adjust for all potential confounders, such as the number of falls in the previous 12 months, which may not be effectively monitored with retrospective studies.
Secondly, the low proportion of male patients did not allow us to conclude on any inter-gender differences regarding the impact of the OP treatment gap. Due to the limited number of male patients (n = 45, 9.8%), with only seven males in the “early treatment” group, formal sex-stratified analyses would have been underpowered and potentially misleading. Descriptive data showed refracture rates of 35.9% vs. 60.7% in females (“early treatment” vs. “untreated”) and 14.3% vs. 47.4% in males, suggesting potential sex differences. However, with only one refracture event among males receiving early treatment, these estimates are highly unstable. This represents an important area for future investigation in larger multicenter studies with adequate power to detect sex-specific effects, particularly given the known differences in bone metabolism between sexes.
Another limitation of our study could be that our FLS is addressed specifically at patients who had a history of at least one vertebral FF, either as index FF or refracture (or both). Therefore, we acknowledge that the results of our pilot study may not be generalizable to the entire fractured population and that a nationwide multicentric study, involving more accurate data from a more uniform population with regard to FF sites, is warranted to overcome this issue. Nevertheless, vertebrae are the most common site of FF in osteoporotic patients (3), and our cohort still featured a relevant proportion of patients (18.7% and 18.8%) who had their index FF and first clinical refracture, respectively, at a non-vertebral site (hip, wrist, proximal humerus, and pelvis). Furthermore, we focused on clinically evident FFs, vertebral or non-vertebral, that have come under medical attention. From this point of view, we believe that the treatment gap in the real world is even wider than what we found in this study, since many vertebral FFs are often not diagnosed.
Another potential limitation may arise from the fact that, for the “early treatment” group, only clinical FFs were considered during the retrospective assessment for refracture, since we do not regularly carry out serial dorsolumbar spine X-rays to search for possible clinically silent vertebral refractures.
In conclusion, the osteoporosis treatment gap in this cohort of patients was wide, with 81.5% of the patients not receiving appropriate and timely AOM treatment after the index FF, with a median delay of 24 months in AOM prescription. When prescribed within 2 months from the index FF, AOM therapy can reduce the occurrence of clinical refracture by 44% and proportionally reduce the economic burden on the health system. Since the elderly population and the annual number of OP-related FFs in Italy are expected to increase (2), it is of utmost importance to address the OP treatment gap to avoid an even more amplified impact in the future. Our pilot, local study is intended to prompt larger studies at a national level and to persuade health authorities to prioritize the implementation of strategies aimed at reducing the OP treatment gap.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethical Committee of Area Vasta Toscana Nord-Ovest (CEAVNO). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GDM: Writing – original draft, Writing – review & editing, Data curation, Methodology, Investigation, Conceptualization. MM: Writing – original draft, Writing – review & editing, Formal analysis, Methodology. VB: Investigation, Writing – review & editing. DA: Investigation, Writing – review & editing. AM: Writing – review & editing, Investigation. MiM: Investigation, Writing – review & editing. MaM: Writing – review & editing, Supervision. MMa: Writing – review & editing, Supervision, Methodology, Investigation, Data curation, Writing – original draft, Conceptualization.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maffi M, De Mattia G, Mazzantini M. Osteoporosis and gut microbiota, radiofrequency echographic multispectrometry and machine learning: one year in review 2023. Clin Exp Rheumatol. (2023) 41(7):1377–83. doi: 10.55563/clinexprheumatol/ma4l1t
2. Kanis JA, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos. (2021) 16(1):82. doi: 10.1007/s11657-020-00871-9
3. Tai TW, Tsai YL, Shih CA, Li CC, Chang YF, Huang CF, et al. Refracture risk and all-cause mortality after vertebral fragility fractures: anti-osteoporotic medications matter. J Formos Med Assoc. (2023) 122(Suppl 1):S65–73. doi: 10.1016/j.jfma.2023.04.004
4. van Geel TA, Huntjens KM, van den Bergh JP, Dinant GJ, Geusens PP. Timing of subsequent fractures after an initial fracture. Curr Osteoporos Rep. (2010) 8(3):118–22. doi: 10.1007/s11914-010-0023-2
5. Banefelt J, Åkesson KE, Spångéus A, Ljunggren O, Karlsson L, Ström O, et al. Risk of imminent fracture following a previous fracture in a Swedish database study. Osteoporos Int. (2019) 30(3):601–9. doi: 10.1007/s00198-019-04852-8
6. Adachi JD, Brown JP, Schemitsch E, Tarride JE, Brown V, Bell AD, et al. Fragility fracture identifies patients at imminent risk for subsequent fracture: real-world retrospective database study in Ontario, Canada. BMC Musculoskelet Disord. (2021) 22(1):224. doi: 10.1186/s12891-021-04051-9
7. Briot K, Grange L, Cortet B, Feron JM, Chauvin P, Coulomb A, et al. Real-world care for individuals aged over fifty with fractures in France: evidence for a wide care gap-the EPIFRACT study. Joint Bone Spine. (2020) 87(5):467–73. doi: 10.1016/j.jbspin.2020.04.007
8. Malle O, Borgstroem F, Fahrleitner-Pammer A, Svedbom A, Dimai SV, Dimai HP. Mind the gap: incidence of osteoporosis treatment after an osteoporotic fracture - results of the Austrian branch of the international costs and utilities related to osteoporotic fractures study (ICUROS). Bone. (2021) 142:115071. doi: 10.1016/j.bone.2019.115071
9. Iki M, Fujimori K, Nakatoh S, Tamaki J, Ishii S, Okimoto N, et al. Delayed initiation of anti-osteoporosis medications increases subsequent hip and vertebral fractures in patients on long-term glucocorticoid therapy: a nationwide health insurance claims database study in Japan. Bone. (2022) 160:116396. doi: 10.1016/j.bone.2022.116396
10. McCloskey E, Rathi J, Heijmans S, Blagden M, Cortet B, Czerwinski E, et al. The osteoporosis treatment gap in patients at risk of fracture in European primary care: a multi-country cross-sectional observational study. Osteoporos Int. (2021) 32(2):251–9. doi: 10.1007/s00198-020-05557-z
11. Skjødt MK, Ernst MT, Khalid S, Libanati C, Cooper C, Delmestri A, et al. The treatment gap after major osteoporotic fractures in Denmark 2005–2014: a combined analysis including both prescription-based and hospital-administered anti-osteoporosis medications. Osteoporos Int. (2021) 32(10):1961–71. doi: 10.1007/s00198-021-05890-x
12. Fardellone P, Barnieh L, Quignot N, Gusto G, Khachatryan A, Kahangire DA, et al. Exploring the treatment gap among patients with osteoporosis-related fractures in France. Arch Osteoporos. (2022) 17(1):29. doi: 10.1007/s11657-021-01041-1
13. Lippuner K, Moghadam BY, Schwab P. The osteoporosis treatment gap in Switzerland between 1998 and 2018. Arch Osteoporos. (2023) 18(1):20. doi: 10.1007/s11657-022-01206-6
14. Willers C, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, et al. Osteoporosis in Europe: a compendium of country-specific reports. Arch Osteoporos. (2022) 17(1):23. doi: 10.1007/s11657-021-00969-8
15. Percorso Assistenziale ed Organizzativo per la Gestione e la Prevenzione Secondaria delle Fratture da Fragilità Maggiori. Decisione Comitato Tecnico Scientifico n. 18 del 06/08/2020. Regione Toscana: Organismo Toscano per il Governo Clinico, Settore qualità dei servizi e reti cliniche, Direzione Diritti di Cittadinanza e Coesione sociale (2020). Available at: https://www.nbst.it/779-delibera-1253-15-settembre-2020-fratture-fragilita-maggiori-percorso-assistenziale-toscana.html
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40(5):373–83. doi: 10.1016/0021-9681(87)90171-8
17. Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. (2008) 19(4):385–97. doi: 10.1007/s00198-007-0543-5
18. Agenzia Italiana del Farmaco. Nota 79. Available at: https://www.aifa.gov.it/documents/20142/1728074/nota-79.pdf (Accessed June 14, 2023).
19. Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int. (2018) 31(2):125–30. doi: 10.1111/tri.13081
20. Roux C, Briot K. Imminent fracture risk. Osteoporos Int. (2017) 28(6):1765–9. doi: 10.1007/s00198-017-3976-5
21. McCloskey EV, Beneton M, Charlesworth D, Kayan K, de Takats D, Dey A, et al. Clodronate reduces the incidence of fractures in community-dwelling elderly women unselected for osteoporosis: results of a double-blind, placebo-controlled randomized study. J Bone Miner Res. (2007) 22(1):135–41. doi: 10.1359/jbmr.061008
22. Khosla S, Cauley JA, Compston J, Kiel DP, Rosen C, Saag KG, et al. Addressing the crisis in the treatment of osteoporosis: a path forward. J Bone Miner Res. (2017) 32(3):424–30. doi: 10.1002/jbmr.3074
23. Lewiecki EM. Osteoporosis. In: Camacho P, editor. Metabolic Bone Diseases. Switzerland AG: Springer Nature (2019). p. 1–13. doi: 10.1007/978-3-030-03694-2_1
24. Alami S, Hervouet L, Poiraudeau S, Briot K, Roux C. Barriers to effective postmenopausal osteoporosis treatment: a qualitative study of patients’ and practitioners’ view. PLoS One. (2016) 11:e0158365. doi: 10.1371/journal.pone.0158365
Keywords: osteoporosis, treatment gap, fragility fracture, Fracture Liaison Service/FLS, refracture risk, Italy, Europe, prevention
Citation: De Mattia G, Manca ML, Bottai V, Antognetti D, Menconi A, Maffi M, Mosca M and Mazzantini M (2025) Osteoporosis treatment gap and risk of refracture: a pilot retrospective study on a cohort of patients referred to a Fracture Liaison Service in Italy. Front. Musculoskelet. Disord. 3:1620506. doi: 10.3389/fmscd.2025.1620506
Received: 29 April 2025; Accepted: 4 June 2025;
Published: 23 June 2025.
Edited by:
Enwu Liu, Flinders University, AustraliaReviewed by:
Nikita Nirwan, Jamia Hamdard University, IndiaMei Dong, Inner Mongolia Medical College, China
Copyright: © 2025 De Mattia, Manca, Bottai, Antognetti, Menconi, Maffi, Mosca and Mazzantini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maurizio Mazzantini, bWF1Lm1henphbnRpbmlAZ21haWwuY29t
 Giammarco De Mattia
Giammarco De Mattia Maria Laura Manca
Maria Laura Manca Vanna Bottai3
Vanna Bottai3 Maurizio Mazzantini
Maurizio Mazzantini