- 1Hughston Clinic, Columbus, GA, United States
- 2TriStar Skyline Medical Center, Nashville, TN, United States
- 3Augusta University, Augusta, GA, United States
Purpose: Intradural spinal arachnoid cysts (SACs) and spinal arachnoid webs (SAWs) are rare extramedullary lesions in the spine. We aimed to assess the relative incidence of the two entities while also better assessing the similarities and differences in imaging appearance and clinical presentation.
Methods: A retrospective analysis was performed of the incidence, imaging features, and clinical presentation of SACs and SAWs at a single institution from 2015 to 2021.
Results: There were 12 cases of SACs and 9 cases of SAWs. The incidence of cysts was .050% and the incidence of webs was .037%. The clinical presentation was similar in the two groups, with the most common signs and symptoms being back pain followed by leg pain. Other signs of myelopathy were common in both groups including absent or diminished reflexes, decreased sensation, and decreased strength. The locations of the cysts were: 1 cervical (8%), 6 thoracic (50%), 5 lumbar (42%). All of the cervical and thoracic cysts caused cord compression. All 9 webs caused focal cord compression (scalpel sign).
Conclusion: SACs and SAWs are both rare lesions that we believe have a similar incidence, with cysts being only a little more common in our cohort. As they both often present with signs and symptoms of myelopathy, imaging findings are more useful in distinguishing between the two entities. The location of the cyst and the appearance of mass effect on the cord are helpful, as webs cause a focal compression, the scalpel sign, while cysts cause broader and smoother compression.
Introduction
Spinal arachnoid cysts (SACs) are rare but well described benign cysts that are most often intradural (1). They can be primary or can be secondary to insults such as surgery, trauma, or infection (2). SACs can be seen in any part of the spine but are most common in the thoracic spine. SACs can cause cord compression, syringomyelia, and abnormal increased T2 signal in the cord (3, 4). Patients can be asymptomatic but clinical presentations often include back pain, radiculopathy, and myelopathic signs such as decreased or absent reflexes (5). SACs are sometimes treated conservatively but are often treated with surgery, with good surgical outcomes (2, 5, 6). Primary cysts have better surgical outcomes than secondary cysts (2).
Spinal arachnoid webs (SAWs) are more recently described entities that occur exclusively in the dorsal aspect of the thoracic spine. They are characterized by an intradural band of arachnoid tissue in the subarachnoid space that results in mass effect on the posterior cord (7–9). On MR imaging, SAWs exhibit a characteristic “scalpel sign”, a dorsal indentation on the cord due to the web (10). The web itself is usually not seen. The scalpel sign distinguishes SAWs from the c-shaped indentation seen in spinal cord herniation (11, 12).
Like SACs, SAWs can also result in syringomyelia and increased T2 cord signal abnormality. Patients with SAWs also often present with back and leg pain and myelopathic signs (9). SAWs are sometimes treated conservatively, with definitive treatment coming from surgical techniques including fenestration and complete excision of the aberrant intradural membrane (13, 14).
To date, no study concurrently analyzing SACs and SAWs exists in the literature. We assess the relative incidence of SACs and SAWs as well as their overlapping and unique imaging features, signs, and symptoms.
Methods
This retrospective analysis first received institutional review board approval. A PACS search was performed for all instances of cervical spine MRIs, cervical CT myelograms, thoracic spine MRIs, thoracic CT myelograms, lumbar spine MRIs, and lumbar CT myelograms over the time period from January 2015 through the end of December 2021. The MRI exams were performed without intravenous contrast, with and without contrast, or only with contrast. The reports for each of these exams were reviewed for mention of possible arachnoid cysts or webs.
All examinations and reports were reviewed in the NovaPACS system. The cases were all reviewed by a radiologist (R.S.) and an orthopedic spine surgeon (D.P.) with 12 years and 14 years of experience, respectively. The positive cases were agreed upon by consensus. When possible, operative reports were used as evidence of positive cases.
The sequences performed for the spinal MRIs in general include: axial T2 weighted images, sagittal T1 weighted images, sagittal T2 weighted images, and sagittal STIR images. The CT exams included axial 2.5 mm with 2.0 mm coronal and sagittal reconstructions.
The MRI scans were performed on one of the following magnets: GE 450W 1.5T, GE Signa Echospeed 1.5T, GE Optima MR 360 1.5T, Philips Achieva 1.5T, Esoate S-scan.25T. All of the CT myelograms were performed on a GE Lightspeed 64-slice VCT.
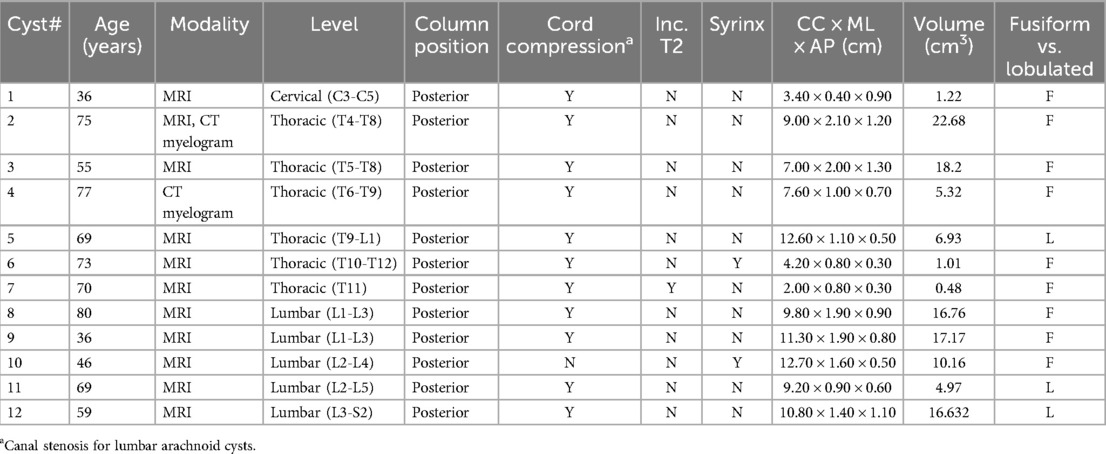
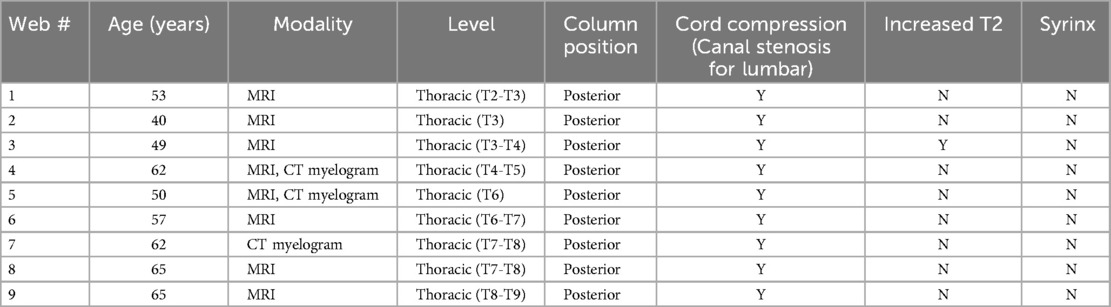
The 22 confirmed cases were assessed for location including primarily cervical, thoracic, or lumbar, and anterior vs. posterior position in the spinal canal. The lesions were categorized as cysts or webs, with webs exhibiting the scalpel sign and cysts consisting of cystic lesions. The cord was assessed for compression, syringomyelia, and increased T2 signal. The cysts were assessed for morphology: fusiform vs. lobulated. Each of the cysts was measured in three planes. For the lumbar cysts beyond the cord, the presence or absence of spinal canal stenosis was determined.
The clinical features of the lesions were assessed by a review of the patient's medical record in Athena.
Results
Over the period of January 2015-December 2021, there were 22 positive cases of spinal arachnoid cysts and webs (22 studies in 21 unique patients—two of the patients had both an MRI and a CT myelogram) out of a total of 23,372 MRI exams and 768 CT myelograms. There were 12 SACs and 9 SAWs. The incidence of SACs was .050% and the incidence of SAWs was .037%.
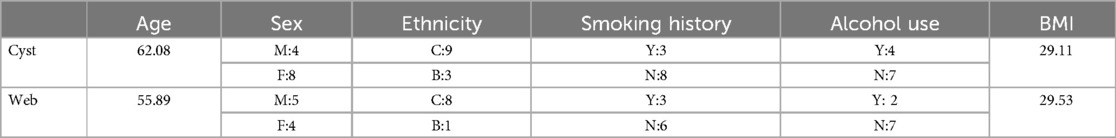
The mean age of the SACs was 62.1 years old (range of 36–80). There were 4 males and 8 females. The locations of the SACs were: 1 cervical (8%), 6 thoracic (50%), 5 lumbar (42%). All 12 SACs were posterior. All 12 SACs were intradural. The largest SAC by volume was 9.0 × 2.2 × 1.2 cm (CC × ML × AP), 7.56 cm3. The smallest SAC by volume was 2.0 × 0.8 × 0.3 cm (CC × ML × AP), 0.16 cm3. 9 of 12 SACs were fusiform and 3 were lobulated.
Of the cervical and thoracic SACs, 6 of 6 caused cord compression. 1 of 6 (17%) caused increased T2 signal in the cord. 1 of 6 (17%) caused syringomyelia. Of the lumbar SACs, 4 of 5 (80%) caused canal stenosis. 1 of 5 (20%) lumbar SACs resulted in syringomyelia.
All 9 SAWs were by definition seen posteriorly in the thoracic spine and caused cord compression. There were 5 males and 4 females. The mean age of the patients was 55.9 years old (range of 40–65). Of the 7 SAWs identified on MRI, 1(14.3%) caused increased T2 signal in the cord. 0 of 9 SAWs caused syringomyelia.
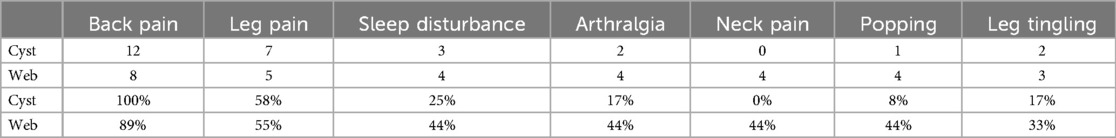
The most common symptoms in patients with SACs were: back pain (12/12, 100%), leg pain (7/12, 58%), and sleep disturbance (3/12, 25%). The most common physical exam findings in patients with SACs were: back tenderness (5/12, 42%), absent or diminished reflexes (5/12, 42%), decreased strength (3/12, 25%), and decreased sensation (3/12, 25%). The average body mass index (BMI) was 29.1.
The most common symptoms in patients with SAWs were: back pain (8/9, 89%), leg pain (5/9, 56%), and sleep disturbance, arthralgia, popping, and neck pain (each 4/9, 44%). The most common physical exam findings in patients with SAWs were: back tenderness (5/9, 56%), decreased flexion (4/9, 44%), decreased sensation and absent or decreased reflexes (each 2/9, 22%). The average BMI was 29.5. (see Tables 1–5 for summary of Results).
Discussion
SACs and SAWs are both rare entities that we believe have a similar incidence, with cysts being only a little more common in our experience. This refutes the notion that webs are extremely rare. In their research, Ben Ali et al. wondered whether SAWs were extremely rare or just being under-diagnosed/underreported (9). We believe the latter to be true. Spinal arachnoid webs are likely underreported in the literature, perhaps reported as cysts in many of the studies before the description of the scalpel sign (10).
The age of presentation of the two groups was similar, both occurring on average in late middle age. Cysts were found twice as often in women, while webs had a slight male predominance. Both groups were of similar average weight (BMI of 29).
The clinical presentation of each group was also similar, with most patients presenting with back pain and many patients presenting with other myelopathic signs including leg pain, leg tingling, altered sensation, and decreased or absent reflexes. Patients also presented with sleep disturbance, sensation of popping in the spine, and decreased flexion.
Absent reflexes was more common in SACs, sensation of popping was more common in SAWs, and decreased flexion was only seen in SAWs. Given the overall clinical similarities between SACS and SAWs, though, the clinical presentation is more helpful in directing the image reader's attention to the possible presence of either entity, rather than useful in distinguishing the two.
In our cohort, SACs were found most commonly in the thoracic spine, but only half of the cases were thoracic. This is in contrast to the reported literature of 80% thoracic. The authors wonder whether some previous literature has included some webs along with cysts, or whether the differences may just be due to the small sample size. When taking into account the SAWs, 15 of 21 cases (71%) of our cases were in the thoracic spine. Our research focused on SAWs and intradural SACS, and so conclusions cannot be reached about extradural SACs, which tend to be less symptomatic (15).
SACs and SAWs are often similar in appearance on MRI and CT myelography. By definition, SAWs have focal cord compression that occurs at a single level. They demonstrate the scalpel sign (Figure 1). The thin web itself was not demonstrated in any of our cases.
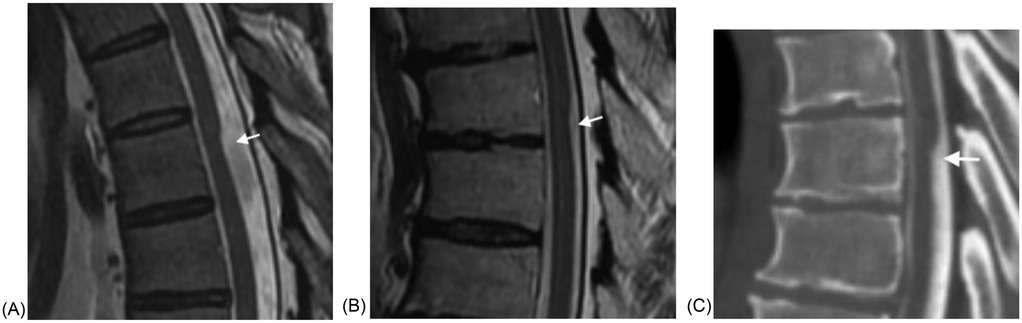
Figure 1. (A) Sagittal T2W MRI demonstrates a scalpel sign of SAW at the T6-T7 level in a 57 year old male with back and leg pain. (B) Sagittal T2W MRI demonstrates a scalpel sign of SAW at the T8-T9 level in a 65 year old male with back pain and absent reflexes and other diminished reflexes. (C) Sagittal CT post myelogram demonstrates a scalpel sign of SAW at T6 in a 50 year old male presenting with back pain, leg pain, and decreased flexion.
In our cohort all of the SACs were posterior and all the ones at the level of the cord caused cord compression. SACs in the thoracic spine, then, can be difficult to distinguish from SAWs, particularly when the cyst walls are not well defined. The most helpful imaging finding is that the cord compression in SAWs is smoother and lacks the sharp angle of the scalpel sign (Figure 2). In addition, in some cases SACs can have more than one site of cord compression (Figure 3). SACs can result in syringomyelia (Figure 4).
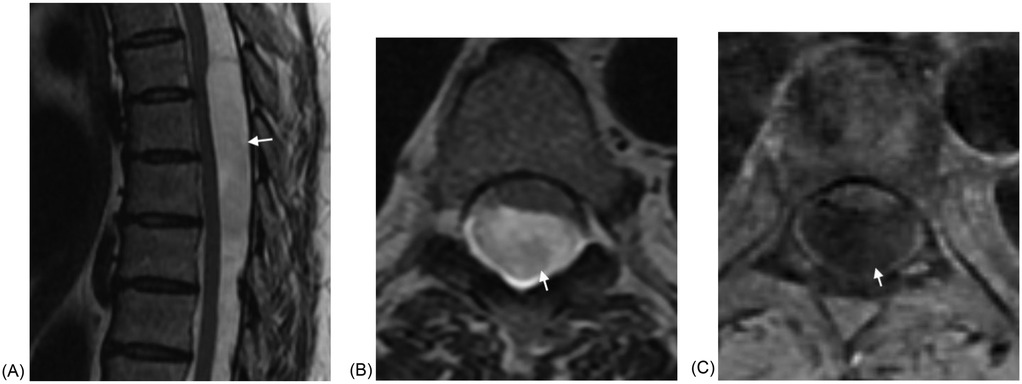
Figure 2. (A) Sagittal T2W MRI demonstrates a large fusiform intradural SAC in the mid thoracic spine with cord compression and anterior displacement of the cord in a 55 year old woman presenting with back pain and leg pain. The lower margin of the cyst is poorly defined. (B) Axial T2W MRI again demonstrates the SAC with resultant cord compression and anterior displacement of the cord. (C) Axial T1WI fat saturated post contrast image demonstrates absent enhancement.
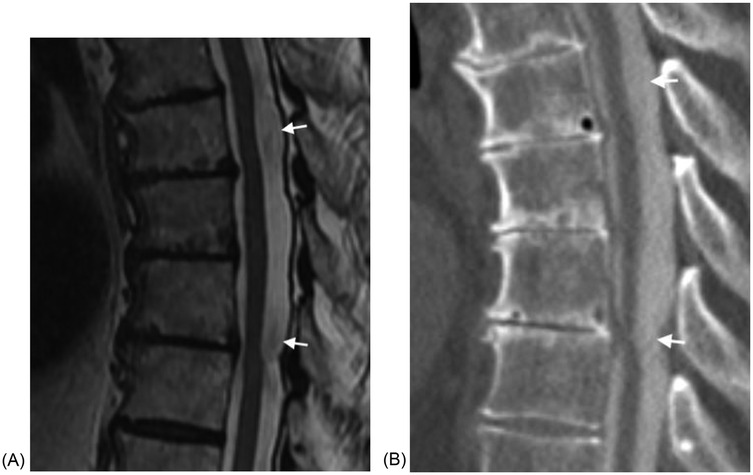
Figure 3. (A) Sagittal T2W MRI demonstrates a large fusiform intradural SAC in the mid thoracic spine with compression of the cord in two locations in a 77 year old male with back pain, leg pain, and absent reflexes. (B) Sagittal CT post myelogram again demonstrates the two sites of cord compression. The cyst fills with intrathecal contrast material.
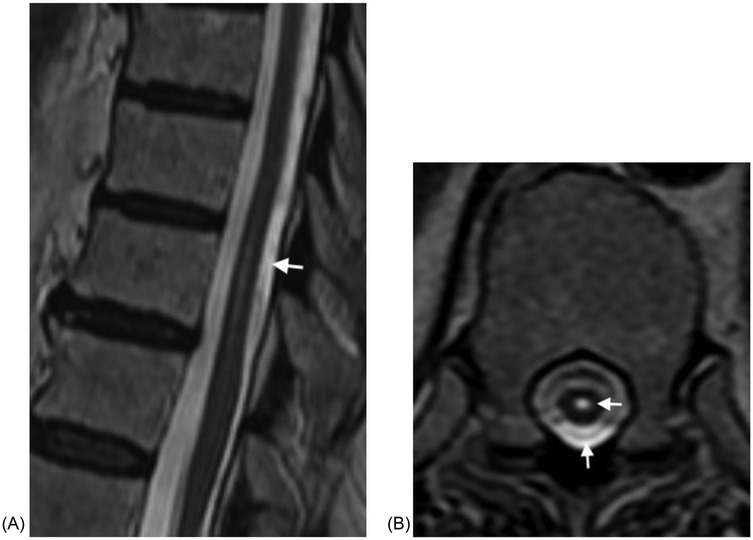
Figure 4. (A) Sagittal T2W MRI demonstrates a fusiform intradural SAC resulting in mild mass effect on the cord at T10 and T11 in a 73 year old woman presenting with back pain, bilateral leg pain, and absent reflexes. (B) Axial T2W MRI at the upper T11 level demonstrates the SAC and small syringomyelia.
SACS in the lumbar spine can be fusiform or lobulated, and can result in spinal canal stenosis. They can also result in syringomyelia (Figure 5). It should be noted that our data yielded a low rate of syringomyelia in SACs (16.7%). Studies have shown variable rates of syringomyelia in SACs, ranging from 10%–86% (7). None of our webs demonstrated syringomyelia, In the series of Reardon and Raghavan, 7 of 13 patients undergoing MRI (54%) had syringomyelia (10). Cord signal abnormality was only seen in one patient in each group.
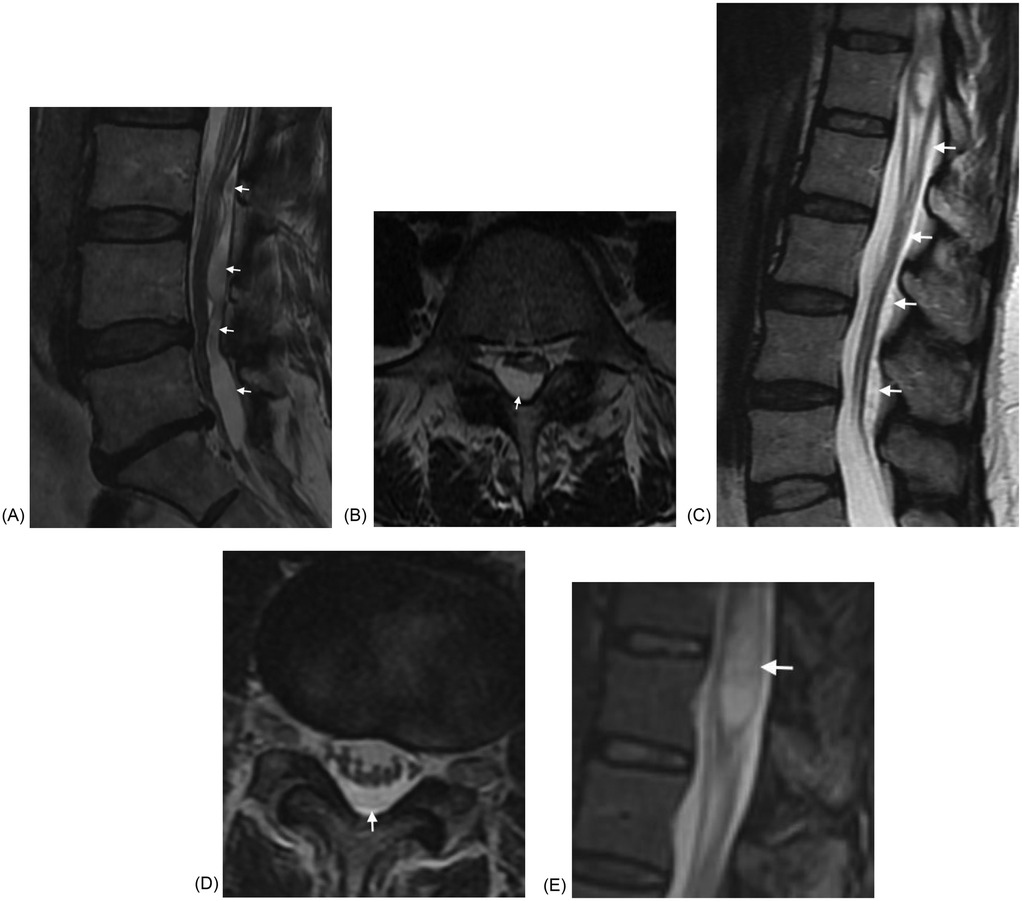
Figure 5. (A) Sagittal T2W MRI demonstrates a lobulated intradural SAC in the lumbar spine causing marked canal stenosis at several levels in a 55 year old woman presenting with back pain and left leg pain. (B) Axial T2W MRI through the L5 level again demonstrates the SAC with resultant canal stenosis. (C) Sagittal T2W MRI demonstrates a fusiform intradural SAC in the upper and mid lumbar spine causing canal stenosis and associated syringomyelia in a 46 woman with back pain, left foot pain, and absent reflexes. (D) Axial T2W MRI at the L3-L4 level demonstrates mild canal stenosis. (E) Sagittal STIR MRI image demonstrating the syringomyelia.
There are a few limitations of this study. Due to the rarity of both SACs and SAWs, we had a relatively small sample size from which to draw conclusions. In addition, these lesions can be hard to identify and so some lesions may have been missed. The incidence of SACs, in particular, was likely underestimated as they can be very difficult to detect in the absence of cord compression, and these lesions are less likely to result in signs and symptoms. A prospective study where the exams were specifically analyzed for these entities would likely yield a greater number of cases.
Another limitation of our study is the lack of patient outcomes in our cohort. After the diagnosis was made, the patients with both SAWs and SACs were referred to neurosurgery outside of our clinic. As such, we don't know how many of the patients went for surgery, what was seen at the time of surgery, and how the patients fared.
Our study was based on conventional MR imaging techniques and CT myelography. Other imaging sequences and techniques have been used to increase sensitivity. These include constructive interference in steady state (CISS) imaging (16) and cine MRI (17, 18).
In our experience, SACS and SAWs are rare lesions that have similar clinical presentations, usually presenting with myelopathic signs and symptoms, and must be distinguished by their imaging findings. Besides location (all SAWs are thoracic and SACs can be seen anywhere in the spine), the appearance of mass effect on the cord is helpful, particularly in cases of SACs with poorly seen margins. SAWs cause a focal compression, the scalpel sign, while SACS cause broader and smoother compression. Proper recognition of both these entities is important, as multiple studies have shown good outcomes from various surgical techniques.
Data availability statement
The datasets presented in this article are not readily available because data set has medical record numbers and other demographic information. Requests to access the datasets should be directed to Corresponding author.
Ethics statement
The studies involving humans were approved by Hughston Foundation Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
RS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BC: Data curation, Investigation, Writing – review & editing, Methodology. CR: Data curation, Investigation, Methodology, Writing – review & editing. DP: Conceptualization, Data curation, Formal analysis, Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fam MD, Woodroffe RW, Helland L, Noeller J, Dahdaleh NS, Menezes AH, et al. Spinal arachnoid cysts in adults: diagnosis and management. A single-center experience. J Neurosurg Spine. (2018) 29(6):711–9. doi: 10.3171/2018.5.SPINE1820
2. Klekamp J. A new classification for pathologies of spinal meninges-part 2: primary and secondary intradural arachnoid cysts. Neurosurgery. (2017) 81(2):217–29. doi: 10.1093/neuros/nyx050
3. Silbergleit R, Brunberg JA, Patel SC, Mehta BA, Aravapalli SR. Imaging of spinal intradural arachnoid cysts: MRI, myelography and CT. Neuroradiology. (1998) 40(10):664–8. doi: 10.1007/s002340050661
4. Ospina Moreno C, Vela Marín AC, Castán Senar A, Montejo Gañán I, Cózar Bartos M, Marín Cárdenas MA. Radiological diagnosis of spinal arachnoid cysts: a pictorial essay. J Med Imaging Radiat Oncol. (2016) 60(5):632–8. doi: 10.1111/1754-9485.12470
5. Wang MY, Levi AD, Green BA. Intradural spinal arachnoid cysts in adults. Surg Neurol. (2003) 60(1):49–55; discussion 55–6 doi: 10.1016/S0090-3019(03)00149-6
6. Minnema A, Shaddy SM, Elder JB, Farhadi HF. Clinical and radiologic outcomes after fenestration and partial wall excision of idiopathic intradural spinal arachnoid cysts presenting with myelopathy. World Neurosurg. (2017) 105:213–22. doi: 10.1016/j.wneu.2017.05.136
7. Kalsi P, Hejrati N, Charalampidis A, Wu PH, Schneider M, Wilson JR, et al. Spinal arachnoid cysts: a case series & systematic review of the literature. Brain Spine. (2022) 2:100904. doi: 10.1016/j.bas.2022.100904
8. Paramore CG. Dorsal arachnoid web with spinal cord compression: variant of an arachnoid cyst? Report of two cases. J Neurosurg. (2000) 93:287–90. doi: 10.3171/spi.2000.93.2.0287
9. Ben Ali H, Hamilton P, Zygmunt S, Yakoub KM. Spinal arachnoid web—a review article. J Spine Surg. (2018) 4:446–50. doi: 10.21037/jss.2018.05.08
10. Reardon MA, Raghavan P, Carpenter-Bailey K, et al. Dorsal thoracic arachnoid web and the “scalpel sign”: a distinct clinical-radiologic entity. AJNR Am J Neuroradiol. (2013) 34:1104–10. doi: 10.3174/ajnr.A3432
11. Schultz R Jr, Steven A, Wessell A, Fischbein N, Sansur CA, Gandhi D, et al. Differentiation of idiopathic spinal cord herniation from dorsal arachnoid webs on MRI and CT myelography. J Neurosurg Spine. (2017) 26:754–9. doi: 10.3171/2016.11.SPINE16696
12. Buntting CS, Ham Y, Teng KX, Dimou J, Gauden AJ, Nair G. Scalpel sign: dorsal thoracic arachnoid web, thoracic arachnoid cyst and ventral cord herniation. Radiol Case Rep. (2022) 17(10):3564–9. doi: 10.1016/j.radcr.2022.06.100
13. Nisson PL, Hussain I, Härtl R, Kim S, Baaj AA. Arachnoid web of the spine: a systematic literature review. J Neurosurg Spine. (2019) 31(2):175–84. doi: 10.3171/2019.1.SPINE181371
14. Laxpati N, Malcolm JG, Tsemo GB, Mustroph C, Saindane AM, Ahmad F, et al. Spinal arachnoid webs: presentation, natural history, and outcomes in 38 patients. Neurosurgery. (2021) 89(5):917–27. doi: 10.1093/neuros/nyab321
15. Ahmed AK, Anisetti B, Huynh T, Agarwal A, Gupta V, Desai A, et al. Clinical and imaging features of spinal extradural arachnoid cysts: a retrospective study of 50 cases. Neuroradiology. (2022) 64(12):2409–16. doi: 10.1007/s00234-022-03042-4
16. Grewal SS, Pirris SM, Vibhute PG, Gupta V. Identification of arachnoid web with a relatively novel magnetic resonance imaging technique. Spine J. (2015) 15(3):554–5. doi: 10.1016/j.spinee.2014.10.023
17. Chang HS, Nagai A, Oya S, Matsui T. Dorsal spinal arachnoid web diagnosed with the quantitative measurement of cerebrospinal fluid flow on magnetic resonance imaging. J Neurosurg Spine. (2014) 20(2):227–33. doi: 10.3171/2013.10.SPINE13395
Keywords: myelopathy, cord compression, back pain, MRI, CT myelogram, arachnoid web, spinal arachnoid cyst
Citation: Stay RM, Cromie BS, Rogers CD and Pahl DW (2025) Intradural spinal arachnoid cysts versus spinal arachnoid webs: a retrospective analysis of incidence, imaging findings and clinical presentation at a single institution. Front. Musculoskelet. Disord. 3:1645555. doi: 10.3389/fmscd.2025.1645555
Received: 12 June 2025; Accepted: 15 July 2025;
Published: 11 August 2025.
Edited by:
Debra Weiner, University of Pittsburgh, United StatesReviewed by:
Benjamin Johnston, Mass General Brigham, United StatesKetan Verma, Virginia Commonwealth University Health System, United States
Copyright: © 2025 Stay, Cromie, Rogers and Pahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rourke M. Stay, cm91cmtlQGFsdW1uaS52aXJnaW5pYS5lZHU=
 Rourke M. Stay
Rourke M. Stay Blake S. Cromie2
Blake S. Cromie2