- 1Neurosurgery Department, N.V. Sklifosovsky Research Institute for Emergency Medicine, Moscow, Russia
- 2Pirogov Russian National Research Medical University (Pirogov Medical University), Moscow, Russia
Objective: We present a case of a condition that is most likely to be complex regional pain syndrome (CRPS) type 1 in a young male patient with an atypical presentation.
Case description: A 32-year-old male patient admitted to the outpatient department reported slow progressive complaints that included foot weakness, abnormal posture, edema, and temperature and skin discoloration of the affected leg. A wide range of instrumental studies revealed little-to-no abnormalities that could explain the symptoms. Thus, the diagnosis of exclusion remains CRPS. However, the patient did not experience pain, which is necessary for the diagnosis of CRPS.
Discussion: There are several cases in the literature describing the condition that is very similar to CRPS, but without pain syndrome. Since CRPS is a rare condition, and the exact mechanisms of its pathogenesis are not fully understood. It is not possible to conclude whether these cases represent an atypical manifestation of CRPS or a similar condition with different underlying pathophysiology. CRPS should be included in the differential diagnosis in cases where all other clinical features of CRPS, except pain, are present.
Introduction
Complex regional pain syndrome (CRPS) is a condition characterized by the coexistence of prolonged and disproportionate to primary trauma pain and autonomic disorders (1). Afflicted persons living with CRPS experience abnormal pain, which places a heavy physical and psychological burden on them (2). Other obligatory symptoms include sensory abnormalities, changes in skin color, temperature, hair and nail growth, sweating and/or swelling of limbs despite the fact that any body part can be affected (3). The condition is remarkably rare, affecting 6.28–26.2 per 100,000 person-years. Several risk factors have been associated with the development of CRPS, including injuries—particularly to the foot—smoking, poor peripheral circulation, diabetes, autoimmune disorders, and a history of nerve damage (2). CRPS is more commonly diagnosed around the age of 40–50, but it can occur at any age, and women are most likely to be affected (4). There are two main types of CRPS: more prevalent CRPS type1 related to any soft tissue trauma, and CRPS type 2 related to an injury of a specific nerve, though both types are treated similarly (1). Also, there have been described cases with no history of previous trauma at all, in which the patients developed the same disabling conditions (5).
The pathophysiology of CRPS is still not fully understood. It is considered to involve simultaneous malfunctioning of both central and peripheral nerve systems (3). CRPS is characterized by a cascade of complex interactions, such as inappropriate tissue inflammatory response to injury, abnormal sensitization of the peripheral and central nervous systems along with accompanying autoimmune and autonomic dysfunction. It is also thought that hereditary and psychological factors may contribute to the development of CRPS (4).
The Budapest Criteria, which have remained relevant for many years, are the gold standard for the clinical diagnosis of CRPS (2, 6). The diagnosis is made clinically because no single confirmatory test can support CRPS (3). A history of recent trauma and a thorough examination can give clues. Nerve conduction studies (NCS) and electromyography (EMG) can detect most nerve injuries associated with CRPS type 2, but the results of these studies would be normal for CRPS type 1, although they are extremely difficult to perform in patients with severe pain syndrome. Ultrasound or magnetic resonance imaging (MRI) may reveal underlying nerve and tissue damage, or help rule out other conditions whose symptoms may mimic CRPS.
Case presentation
A 32-year-old male patient was referred to a neurophysiology laboratory for electrodiagnostic evaluation to assess a suspected nerve injury. The case history revealed that six months before the presentation, the patient had sustained a left lower extremity injury secondary to an anti-tank mine explosion during military engagement. Notably, the injury occurred without shrapnel contamination or any penetrating trauma. Immediately following the incident, the patient reported no acute neurological or vascular abnormalities, and no swelling in the affected limb. However, after a two-week observation period in a field hospital, he developed progressive weakness and sensory loss in the left foot, which manifested in the absence of pain. Subsequently, he observed persistent hypothermia and intermittent cyanotic discoloration of the left leg distal to the knee.
Initial therapeutic interventions included self-prescribed food supplements, neuromuscular electrical stimulation and acupuncture, none of which yielded clinical improvement. Three months post-injury, the patient was admitted to a specialized outpatient clinic for comprehensive neurological and vascular assessment (Figure 1A). The patient reported no history of chronic diseases and had not been under continuous medical care previously.

Figure 1. Timeline of clinical evolution (A) and left calf and foot of the patient at examination time (B) six months after the injury.
Neurological assessment
The patient was alert and fully oriented in person, place, and time with normal speech. The neurological assessment revealed muscle weakness with MRC 1 score in the left foot of both dorsiflexion and plantar flexion, the patellar reflexes were symmetric and brisk, but the ankle jerk was absent on the left. There were no signs of any type of sensory loss and pain. Also, there was a pathological positioning with the foot pointed downward and inward. The gait was impaired; the patient dragged his affected leg in a semicircle and used a cane to keep the balance.
Local status
The left leg below the knee was colder than the right. It was also slightly hypotrophic and hypotonic, and the foot was slightly swollen and discolored. The discoloration was more obvious when the patient remained seated with both feet on the floor for some time-in this case the affected foot became cyanotic (Figure 1B).
Diagnostic assessment
Routine laboratory tests revealed no abnormalities, including tests for inflammatory processes such as C-reactive protein and erythrocyte sedimentation rate. The patient presented with one-side foot weakness with no significant sensory disturbances but with obvious vasomotor abnormalities, which necessitated a differential diagnosis in order to exclude CNS disorders, radiculopathy and peripheral nerve damage and also vascular insufficiency.
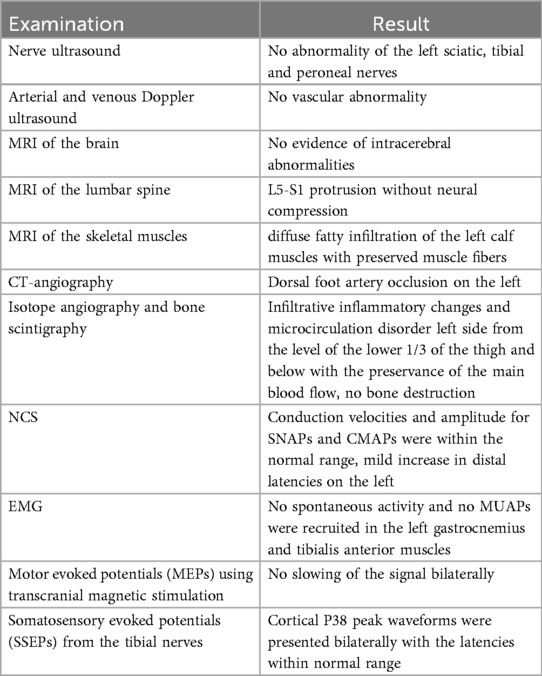
The results of the imaging and electrodiagnostic studies are summarized in Table 1.
MRI of the skeletal muscles revealed diffuse fatty infiltration of the left calf muscles with preserved muscle fibers, which may be consistent with post-contusion changes (Figure 2).

Figure 2. MRI scans: coronal T2-weighted images (A) and axial short tau inversion recovery (STIR) images (B) showing diffuse, abnormally high linear signal and reduced muscle volume in the affected leg (red arrows).
Isotope angiography and bone scintigraphy showed infiltrative inflammatory changes and microcirculation disorder left side from the level of the lower 1/3 of the thigh and below with the preservance of the main blood flow. It also showed no bone destruction. The right leg remained unaffected (Figure 3).

Figure 3. A decreased soft tissue uptake in the left thigh, left calf and left foot visible on the anterior (A) and posterior (B) views on the technetium-99m hydroxydiphosphonate (Tc-99m HDP) bone scan. A well-noted atypical position of the left foot.
Multimodal electrodiagnostic studies were performed using the Keypoint® G4 EMG/NCS/EP Workstation. NCS showed no abnormalities, except for a mild increase in distal latencies on the left due to lower temperature of the affected leg. The conduction velocities were within the normal range, as was the amplitude for both sensory nerve action potentials (SNAPs) and compound muscle action potentials (CMAPs). EMG did not reveal any kind of spontaneous activity as well as no motor unit action potentials (MUAPs) were recruited in the left gastrocnemius and tibialis anterior muscles. However, the EMG assessment of the left biceps femoris revealed no presence of spontaneous activity, with normal MUAP recruitment during muscle activation. Additionally, motor evoked potentials (MEPs) using transcranial magnetic stimulation and somatosensory evoked potentials (SSEPs) from the tibial nerves were performed. MEPs revealed no slowing of the signal. SSEPs were not considered to be abnormal since cortical P38 peak waveforms were presented bilaterally with the latencies within normal range (Figure 4). Interpeak difference is a result of lower temperature of the affected leg regarding the fact that low limb temperature can increase latencies in cortical and spinal components (7).

Figure 4. Left (A) and right (B) tibial nerve SSEP shows evidence of bilateral ascending somatosensory signal to the primary somatosensory cortex (peak P38).
The patient presented with a history of left lower limb trauma and reported experiencing vasomotor, trophic, sudomotor and motor disturbances in the affected extremity, which correlated with objectively confirmed clinical findings. An extensive diagnostic workup was conducted to exclude alternative etiologies prior to confirming a diagnosis of CRPS. Notably, the clinical presentation lacked pain features.
The patient was discharged with no improvement. However, he was prescribed physical therapy and ankle-foot orthosis in order to improve gate quality and foot positioning. This significantly changed the condition for the better. Not only he managed to walk better, but also his emotional condition improved. Besides he was recommended to attend long-term psychotherapy.
Discussion
In 1993 in a prospective study of CRPS carried out by Veldman et al. (8) of 829 patients 7% did not experience pain, although pain remained among the most common and invariable symptoms along with color differences, temperature differences, paresis and increase complaints after exercise.
In 2003, Eisenberg and Melamed (9) presented 5 cases of patients who developed a full clinical picture of CRPS subsequent to the trauma with the exception of pain. Interestingly, 4 out of 5 patients had a nerve or nerve root injury confirmed with NCS and EMG suggesting that CRPS type 2 could be painless as well. The authors even suggested a special term for this condition—complex regional painless syndrome (CRPLS).
Kumar et al. (10) in 2009 described two cases of CRPS-like changes in the absence of pain in patients after hip surgery. The authors pointed out to relationship between the symptoms and the period of non-weight bearing. In these cases, abnormal sensitization of the peripheral and central nervous systems due to lack of proprioception alongside with an increase in flow mediated dilatation of arteries and a decrease in venous capacitance might contribute to the development of CRPS-like symptoms.
In order to study clinical subtypes of CRPS beyond the classical dichotomy CRPS 1 and 2 subtypes, Bruehl et al. (11) divided 113 patients into 3 separate groups by their clinical presentations: the first group with the prevalence of the vasomotor and motor/trophic changes, the second one with more prominent pain/sensory symptoms and the third was characterized by the presence in all symptom categories such as pain/sensory, vasomotor, sudomotor/edema, and motor/trophic. Based on the study results the authors offered the following CRPS subtypes: a relatively limited syndrome in which vasomotor signs predominate, a relatively limited syndrome in which neuropathic pain/ sensory abnormalities predominate, and a florid CRPS. Regarding the presentation of pain, the patients with so-called florid CRPS displayed significantly fewer signs of pain/ sensory dysfunction than the patients with a relatively limited syndrome which was associated with nerve injury and neuropathic pain. However, the authors noted that reliable pain rating data were not available for analysis, so it is unclear if there were differences in pain severity between the groups.
Even though since 2004, when the Budapest criteria were first developed, there have been made several changes and adaptations, the presence of continuing pain, disproportionate to the inciting event has always been a prerequisite. For patients who have never met the diagnostic criteria fully, there is a special diagnosis of CRPS Not Otherwise Specified.
However, there is no ultimate list of tests and studies that should be performed in case of suggested CRPS. Different diagnostic algorithms suggest variety of options including plain radiography or bone scintigraphy, quantitative sensory testing, bone mineral density, MRI, computed tomography, skin biopsy, three-phase bone scintigraphy, quantitative sudomotor axon reflex, laser Doppler flowmetry, NCS, and EMG (4). The aim of evaluation is to exclude other vascular disorders and range of neuropathies that could cause similar symptoms.
In the present case, comprehensive neurophysiological assessment played a key diagnostic role, despite the patient's atypical clinical manifestations. NCS and EMG provided crucial insights into the functional integrity of peripheral motor and sensory axons, revealing no evidence of axonal degeneration or demyelination. MEPs and SSEPs confirmed preservation of corticospinal tract integrity and dorsal column-medial lemniscus pathway function, respectively, thus ruling out central nervous system lesions. These findings demonstrated intact neural transmission across both central and peripheral pathways, effectively excluding neuropathies or spinal cord lesions as primary etiologies. Concurrently, vascular evaluation has become an important diagnostic component, given the prevalence of vasomotor abnormalities (e.g., temperature asymmetry, cyanotic skin coloration) and motor deficits in the clinical picture. Non-invasive vascular studies, including Doppler ultrasonography and isotope angiography, were employed to assess peripheral blood flow dynamics and identify potential microvascular dysfunction or autonomic dysregulation. The integration of neurophysiological and vascular data proved instrumental in narrowing the differential diagnosis, distinguishing between post-traumatic neurovascular complications (e.g., complex regional pain syndrome), non-compressive mononeuropathies, and ischemia-mediated tissue injury. This multimodal approach highlights the need to synthesize functional neurodiagnostic findings with hemodynamic profiling to elucidate the pathophysiological interplay between neuronal integrity and vascular homeostasis in blast injury without direct tissue penetration.
It should be mentioned though that the latest studies of CRPS are dedicated to the possibility of using different biomarkers for diagnosing and prognostication of CRPS. Thus, the systematic review by Lopes et al. (12) demonstrates the key role of cytokines (most frequently IL-6 and TNF-α), autoantibodies and immune cell infiltration that may contribute in CRPS diagnostic assessment.
Another important point is that pain management plays a major role in the treatment of CRPS and, when successful, can provide significant relief for the patients. Although pain management in CRPS is still a challenging task, there are not so many pharmacological options for other debilitating symptoms such as vasomotor and sudomotor disorders as RCTs show little efficiency of different approaches so far (13, 14). Physical and occupational therapy as well as psychological support remain the milestones of CRPS treatment.
Conclusion
Despite CRPS being a very rare condition, it is important to remember that there is a possibility of its atypical presence with no actual pain syndrome. These paradoxical cases are not relevant to the existing diagnostic criteria, which can lead to delay in starting treatment. Further research into the pathophysiology of CRPS and related conditions is needed to explain the diversity in clinical presentation of this condition.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AA: Visualization, Investigation, Writing – original draft. ES: Writing – review & editing, Investigation. MS: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was sponsored by grants from ANO “Moscow Center for Innovative Healthcare technologies”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Abd-Elsayed A, Stark CW, Topoluk N, Isaamullah M, Uzodinma P, Viswanath O, et al. A brief review of complex regional pain syndrome and current management. Ann Med. (2024) 56(1):2334398. doi: 10.1080/07853890.2024.2334398
3. Alshehri FS. The complex regional pain syndrome: diagnosis and management strategies. Neurosciences (Riyadh). (2023) 28(4):211–9. doi: 10.17712/nsj.2023.4.20230034
4. Kim Y-D. Diagnosis of complex regional pain syndrome. Annals of Clinical Neurophysiology. (2022) 24(2):35–45. doi: 10.14253/acn.2022.24.2.35
5. Abuzied Y, Jaber M, Hafiz M, Al-Hamwy R. Complex regional pain syndrome in a non-traumatic case: a case report. Cureus. (2024) 16(6):e62812. doi: 10.7759/cureus.62812
6. Harden RN, McCabe CS, Goebel A, Massey M, Suvar T, Grieve S, et al. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 5th edition. Pain Med. (2022) 23(Suppl 1):S1–S53. doi: 10.1093/pm/pnac046
7. Muzyka IM, Estephan B. Somatosensory evoked potentials. In: Levin KH, Chauvel P, editors. Handbook of Clinical Neurology. Amsterdam: Elsevier (2019). p. 523–40. doi: 10.1016/B978-0-444-64032-1.00035-7
8. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. (1993) 342(8878):1012–6. doi: 10.1016/0140-6736(93)92877-v
9. Eisenberg E, Melamed E. Can complex regional pain syndrome be painless? Pain. (2003) 106(3):263–7. doi: 10.1016/S0304-3959(03)00290-2
10. Kumar AS, Wong S, Andrew J. Rare case of autonomic instability of the lower limb presenting as painless Complex regional pain syndrome type I following hip surgery: two case reports. J Med Case Rep. (2009) 3:7271. doi: 10.1186/1752-1947-3-7271
11. Bruehl S, Harden RN, Galer BS, Saltz S, Backonja M, Stanton-Hicks M. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain. (2002) 95(1–2):119–24. doi: 10.1016/s0304-3959(01)00387-6
12. Lopes R, Santos A, Gomes T, Ribeiro J, Rodrigues I, Paiva B, et al. An integrative review of potential diagnostic biomarkers for complex regional pain syndrome. J Clin Med. (2025) 14(11):3751. doi: 10.3390/jcm14113751
13. Urits I, Shen AH, Jones MR, Viswanath O, Kaye AD. Complex regional pain syndrome, current concepts and treatment options. Curr Pain Headache Rep. (2018) 22(2):10. doi: 10.1007/s11916-018-0667-7
Keywords: complex regional pain syndrome, atypical presentation, foot weakness, imaging studies, electromyography, vasomotor symptoms
Citation: Arefyeva AP, Seliverstova EG and Sinkin MV (2025) Painless complex regional pain syndrome: a paradoxical case report. Front. Musculoskelet. Disord. 3:1656285. doi: 10.3389/fmscd.2025.1656285
Received: 29 June 2025; Accepted: 19 August 2025;
Published: 5 September 2025.
Edited by:
Ata Murat Kaynar, University of Pittsburgh, United StatesReviewed by:
Hipólito Nzwalo, University of Algarve, PortugalChristian Bohringer, UC Davis Medical Center, United States
Yacoub Abuzied, King Fahd Medical City, Saudi Arabia
Copyright: © 2025 Arefyeva, Seliverstova and Sinkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: A. P. Arefyeva, YW5hcmEyMjAyQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 A. P. Arefyeva
A. P. Arefyeva E. G. Seliverstova
E. G. Seliverstova M. V. Sinkin
M. V. Sinkin