- Intensive Care Unit, Hangzhou Hospital of Traditional Chinese Medicine, Hangzhou, China
Background: Acute kidney injury (AKI) is a prevalent and severe medical condition that is frequently observed in the intensive care unit (ICU). Although numerous biomarkers have been identified to predict the prognosis of AKI, the lactate dehydrogenase to albumin ratio [LDH/ALB ratio (LAR)] has not been extensively investigated. The principal objective of this study was to assess the relationship between LAR and all-cause mortality in patients with AKI.
Methods: A total of 6,831 AKI patients were included in this study, divided into survival (n = 5,152) and non-survival groups (n = 1,679). The association between LAR and mortality was examined through restricted cubic spline (RCS) analysis and Cox regression analysis. Subgroup analysis was used to search for interactive factors. Additionally, the prognostic capability of LAR was further evaluated using receiver operating characteristic (ROC) curve analysis.
Results: The LAR was remarkably higher in the non-survival group (p < 0.001). RCS indicated a non-linear correlation between LAR and ICU death (p for non-linearity < 0.001). A LAR of 10.4 was used as the cutoff point to generate the high-LAR and low-LAR subgroups, and the Kaplan–Meier curves revealed that the ICU cumulative survival rate for patients with AKI was significantly lower in the high-LAR group (log-rank p < 0.001). The LAR’s prediction of ICU mortality in AKI patients yielded an area under the ROC curve of 0.65.
Conclusion: Our research suggests that LAR monitoring may be promising as a prognostic marker among patients with AKI. Higher LAR is associated with greater ICU mortality.
1 Introduction
Acute kidney injury (AKI) is a commonly encountered clinical syndrome that is common in hospitalized patients, particularly those who are critically ill with sepsis or who have undergone major surgery. The “Epidemiology of acute kidney injury in critically ill patients” (AKI-EPI) study was an international cross-sectional study that used the complete Kidney Disease: Improving Global Outcomes (KDIGO) criteria to diagnose and stage AKI and showed that AKI occurred in more than 50% of intensive care unit (ICU) patients (1). In addition, the AKI-EPI study as well as others demonstrated that the development of AKI is associated with increased short- and long-term morbidity and mortality (2).
Clinicians may promote early intervention to minimize mortality using clinical signs to predict illness severity and prognosis. Thus, identifying high-risk AKI individuals is essential for timely and effective interventions to improve patient outcomes. In recent years, AKI has received much attention, and acute biomarkers have been investigated in AKI prediction and treatment (3–5), but applying these biomarkers in clinical practice still faces significant limitations (6).
Ischemia–reperfusion injury (I/R-I) is a leading cause of AKI in several disease states; renal tubular epithelial cells (RTECs) first produce energy via glycolysis rather than oxidative phosphorylation, resulting in a substantial conversion of pyruvate to lactate (7). Lactate dehydrogenase (LDH), as a common enzyme involved in energy metabolism in cells, is involved in this process (8, 9). Albumin (ALB), produced by the liver, is essential in various physiological processes, including antioxidation, anti-inflammation, and the regulation of plasma osmolality (10). Podocyte injury can disrupt the integrity of the glomerular filtration barrier, leading to proteinuria and hypoproteinemia (11). Serum albumin has been demonstrated to be correlated with the development of AKI and death after AKI (12, 13).
The LDH/ALB ratio (LAR) is emerging as a novel biomarker for critically ill individuals. While previous research on LAR has been centered around malignant tumors (14, 15), recent evidence suggests its relevance to the prognosis of patients with sepsis, sepsis-associated acute kidney injury (SA-AKI), pneumonia, and acute respiratory distress syndrome (ARDS) (16–19). The current research exploring its association with AKI patient outcomes in the ICU is not extensive. Consequently, this study was designed to examine the relationship between LAR and ICU mortality, with the objective of assessing the predictive value of LAR and providing guidance for clinical management.
2 Materials and methods
2.1 Study population
This was a retrospective observational cohort study that was conducted utilizing the Medical Information Mart for Intensive Care IV (MIMIC-IV, version 3.0) database. MIMIC-IV 3.0 is a comprehensive and freely accessible repository of intensive care data that encompasses over 94,000 ICU admissions at the Beth Israel Deaconess Medical Center in Boston, Massachusetts, from 2008 to 2022.
We obtained authorization to access the database (certification number: 55670126). All protected health information in the MIMIC database has been de-identified, so individual patient consent was not needed.
The definition of AKI was according to the criteria of the KDIGO (20). AKI was defined as follows: Stage 1, increase in serum creatinine (Scr) 1.5–1.9 times baseline or 0.3 g/dL increase in Scr or urine output <0.5 mL/kg for 6–12 h; Stage 2, increase in Scr 2.0–2.9 times baseline or urine output <0.5 mL/kg for 12 h; and Stage 3, Scr greater than three times baseline or urine output <0.3 mL/kg for 24 h.
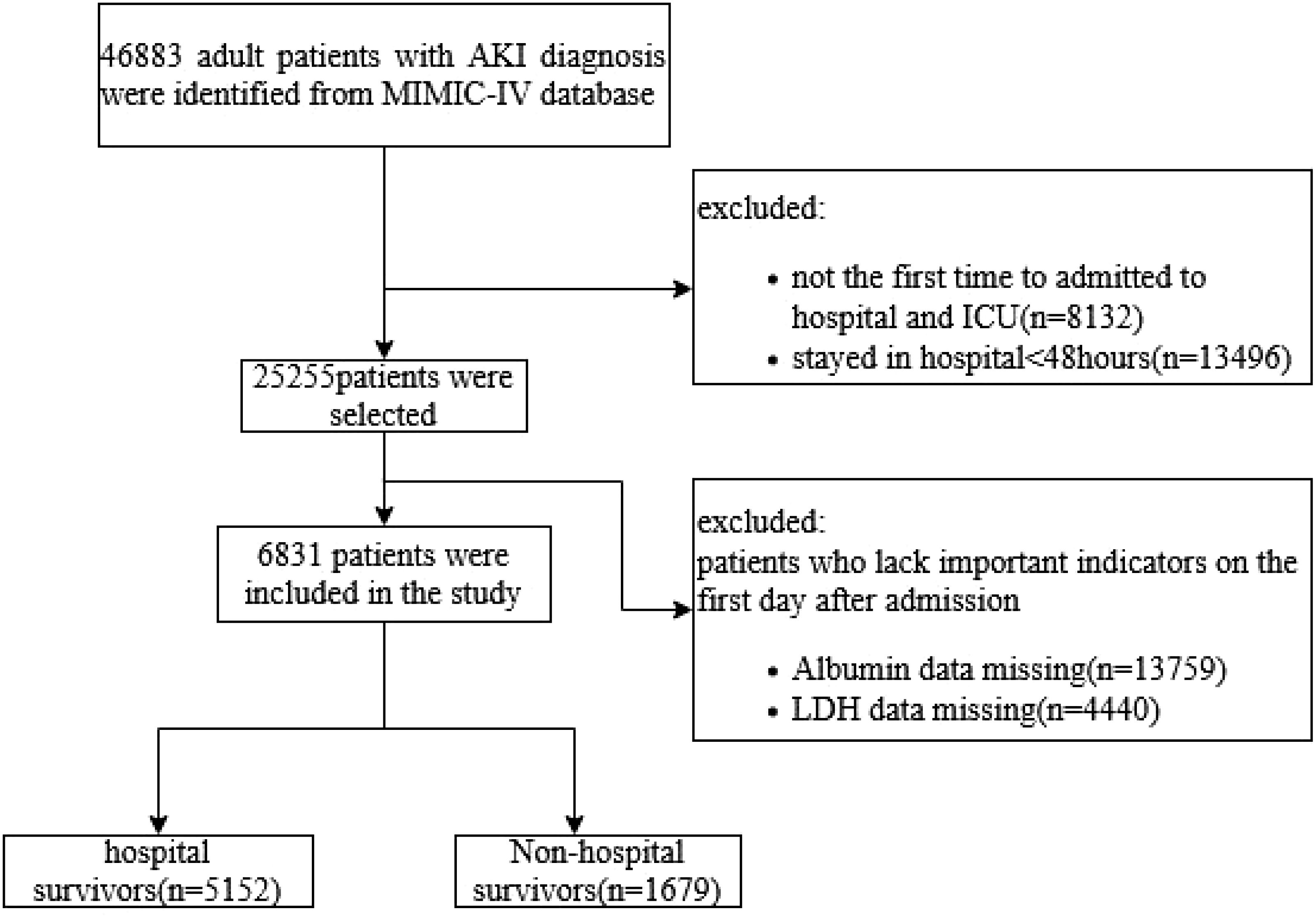
The exclusion criteria were as follows: 1) patients aged <18 years; 2) patients who died within 48 h of ICU admission or stayed in ICU less than 48 h; 3) multiple admissions to the ICU, for whom only data from the first admission were extracted; and 4) patients with missing data for serum albumin or LDH, (Figure 1).
2.2 Data extraction
In this study, the information was extracted using the Post-gresSQL software (version 13.7.2) and Navicat Premium software (version 16) through the execution of a Structured Query Language (SQL). Comorbidities were determined according to the 9th and 10th Edition Clinical revision codes.
Data extracted from the MIMIC-IV database on the first 24 h of ICU admission included the following:
1. Demographic variables: age, sex, and race.
2. Vital signs: respiratory rate (RR), heart rate, mean arterial pressure (MAP), and oxygen saturation levels (SpO2) were recorded on the first day of admission.
3. Comorbidities: myocardial infarction (MI), congestive heart failure (CHF), cerebrovascular disease, chronic obstructive pulmonary disease (COPD), severe liver disease, diabetes, metastatic solid tumor, and chronic kidney disease (CKD).
4. Laboratory tests were performed within the initial 24 h of ICU admission, including white blood cell (WBC) count, platelet count, serum potassium level, hemoglobin level, blood glucose level, serum urea nitrogen level [blood urea nitrogen (BUN)], Scr level, LDH, ALB, and other laboratory markers. If a variable was measured multiple times within the previous 24 h, the mean value was used.
5. Acute Physiology Score III (APSIII), Charlson Comorbidity Index (CCI), and Sequential Organ Failure Assessment (SOFA) score were used to assess the severity of illness upon admission.
6. Medical interventions during hospitalization include the administration of vasoactive drugs and antibiotics, the first 24-h urine output (UO), the use of continuous renal replacement therapy (CRRT) and CRRT mode, and the AKI stage during hospitalization.
7. The duration of ICU stay, the overall length of hospital stay, and LAR were calculated using the LDH (U/L)/ALB (g/L) ratio.
2.3 Groups and outcomes
Patients were classified based on survival outcomes during the ICU stay into two groups: the survival group and the death group. To mitigate potential bias between these groups, propensity score matching (PSM) analysis was conducted. The PSM analysis employed a 1:1 nearest-neighbor matching algorithm with a caliper width of 0.05 to ensure close matching of the pairs (Supplementary Figures S1A, B).
The primary outcome of the present study was ICU mortality, and the secondary endpoints were in-hospital mortality and within 30 and 90 days after admission to the ICU.
2.4 Statistical analysis
Normal distribution was assessed using the Shapiro–Wilk test. Continuous variables were presented as mean ± standard deviation (SD) for variables with normal distribution or as median [interquartile range (IQR)] for variables without normal distribution and compared using either Student’s t-test or the Mann–Whitney U test, respectively. Categorical variables were presented as numbers and percentages (%) and were compared using the chi-square test. LAR was examined as both a continuous variable and a categorical variable. Multivariate Cox proportional hazards models were constructed to study the association between LAR and ICU mortality, and the hazard ratio (HR) of mortality was calculated. In the multiple regression analysis models, adjusted covariates were selected based on the difference in baseline characteristics among the groups in which p < 0.05 or p < 0.001. Furthermore, restricted cubic spline (RCS) was performed to determine the linear and non-linear relationships between the LAR value and the risk of mortality. Subgroup analysis was performed to verify the robustness of the initial results and to find potential interactive factors.
Data analysis was conducted using the Stata software version 14.0 and the Rstudio programming language. A two-tailed p-value of less than 0.05 was considered to indicate statistical significance.
3 Results
3.1 Population and baseline information
According to the inclusion and exclusion criteria, a total of 6,831 patients with AKI were included in the current study (Figure 1). Baseline characteristics of the patients are shown in Table 1. The average age of the participants was 64.73 ( ± 16.54) years, and men constituted 57.84% of the study population. It was observed that the death group exhibited higher mean values of BUN, Scr, potassium, LAR level, SOFA score, APSIII score, and CCI (all p < 0.001). It also had a higher incidence of sepsis (88.45% vs. 73.37%, p < 0.001). The patients in the death group were more likely to have a history of cerebrovascular (19% vs. 16.36%, p = 0.013), chronic kidney disease (12.03% vs. 7.01%, p < 0.001), severe liver disease (19.24% vs. 11.20%, p < 0.001), and solid tumor (11.73% vs. 5.94%, p < 0.001). However, no statistical differences were found in the history of MI, CHF, COPD, and diabetes (all p > 0.05).
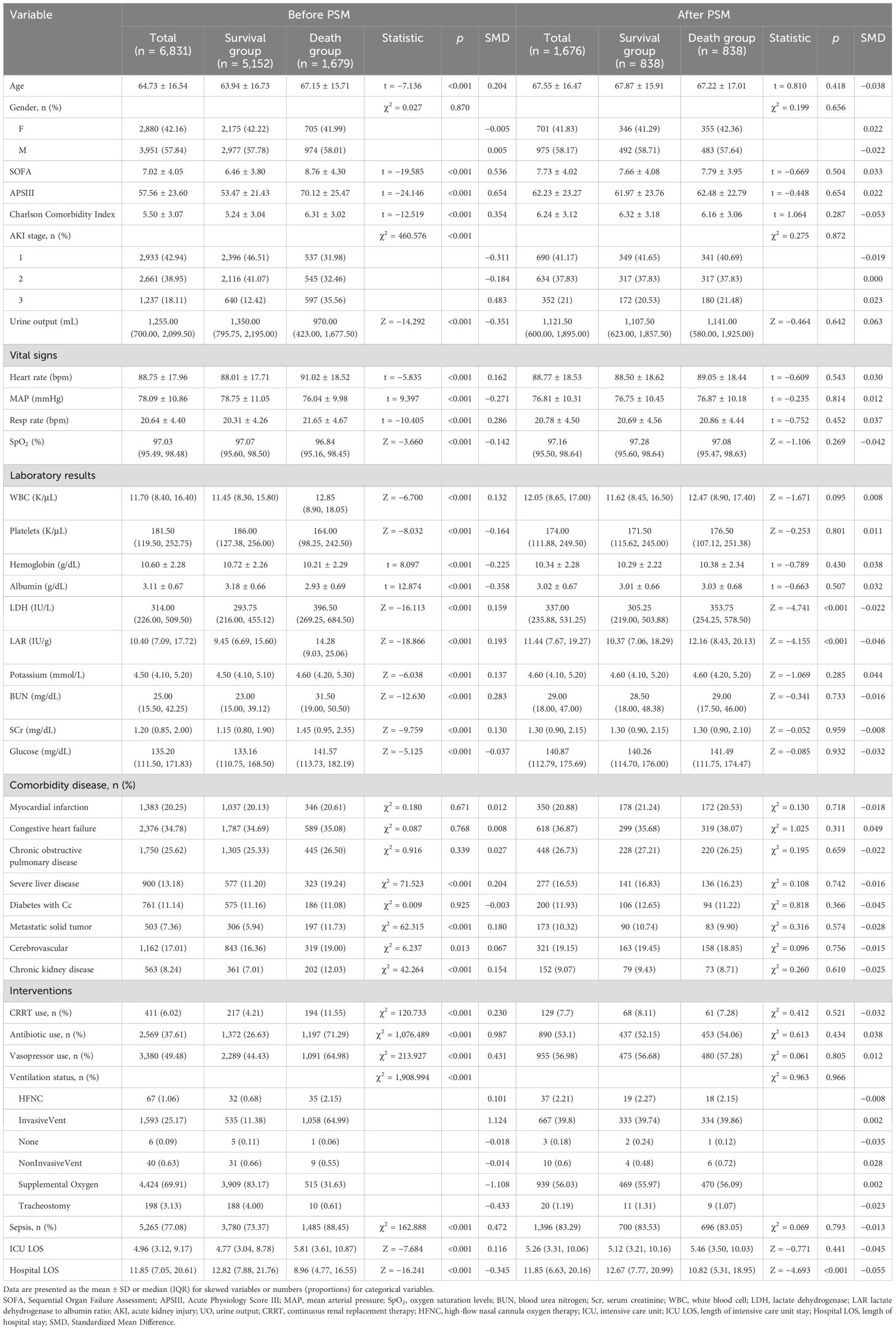
Table 1. Baseline characteristics of patients in the survival and death groups before and after propensity score matching (PSM).
The survivors typically had shorter ICU stays (4.77 vs. 5.81, p < 0.001); less use of CRRT (4.21% vs. 11.55%, p < 0.001), antibiotics (26.63% vs. 71.29%, p < 0.001), and vasopressors (44.43% vs. 64.98%, p < 0.001); more urine output (1,350 vs. 970 mL, p < 0.001); and significantly higher levels of albumin, platelets, and hemoglobin.
After PSM, a total of 838 pairs were successfully matched, achieving well-balanced baseline demographic characteristics between groups. It was observed that the death group also exhibited higher values of LAR (12.16 vs. 10.37, p < 0.001).
3.2 Association between LAR and mortality
A restricted cubic spline curve was employed in order to flexibly visualize and analyze the association between LAR and ICU mortality in patients with AKI in Figure 2A (p for non-linear <0.001).
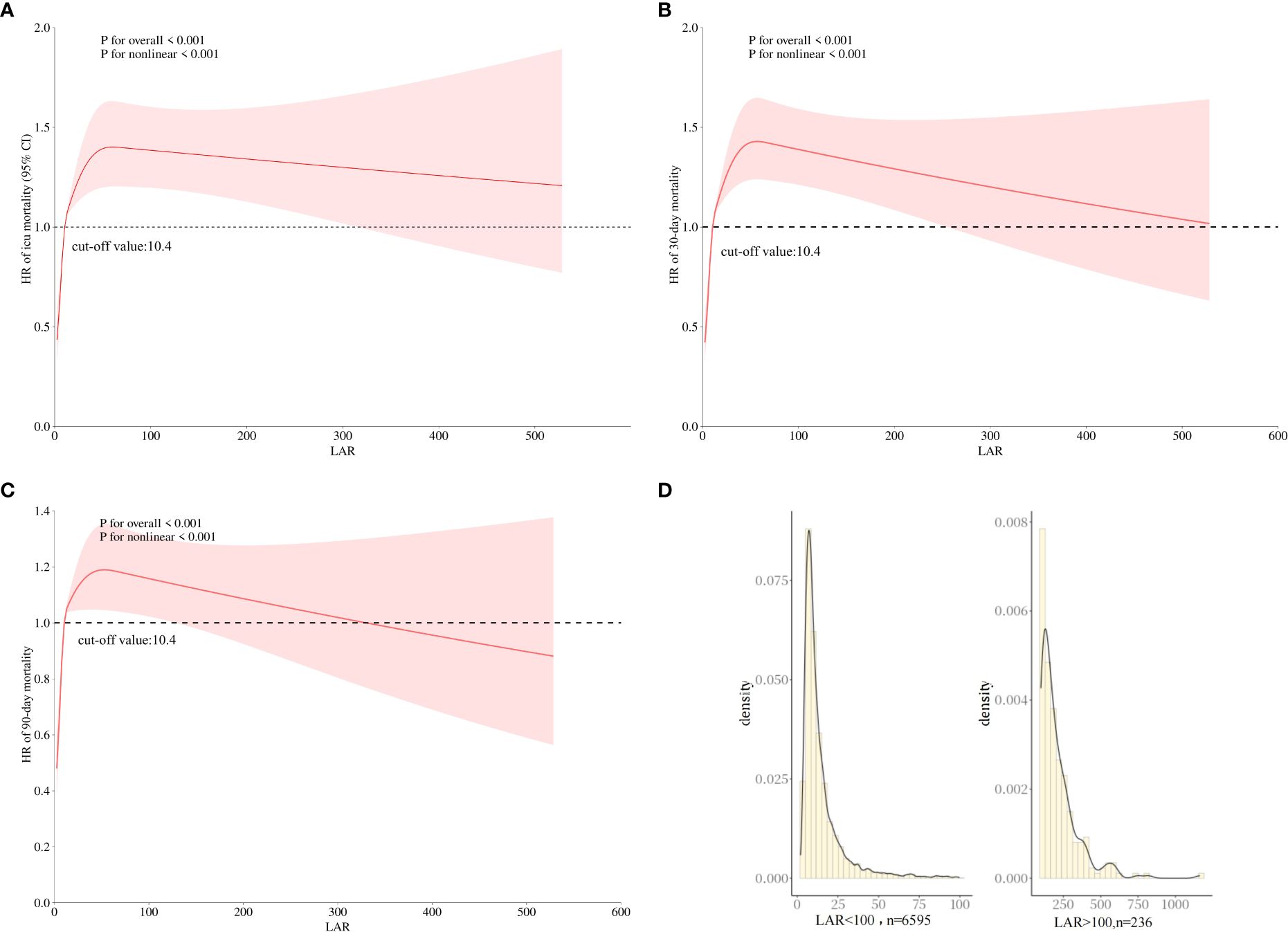
Figure 2. The association between LAR and the HR of ICU (A) and 30-day (B) and 90-day (C) mortality using restricted cubic spline analysis. (D) The distribution of LAR values. HR, hazard ratio; LAR, lactate dehydrogenase to albumin ratio; ICU, intensive care unit.
In Figure 2A, when LAR is less than 10.4 IU/g, HR is less than 1. When LAR is greater than 10.4, HR is greater than 1, and LAR is positively correlated with HR. In addition, similar linear associations were also observed in the analysis of 30-day mortality and 90-day mortality (Figures 2B, C). Therefore, a LAR of 10.4 was used as the cutoff point to generate the high-LAR and low-LAR subgroups for the subgroup analysis. Although an elevated LAR > 100 was inversely associated with mortality, this finding was observed only in a very small subset of samples. Figure 2D shows the distribution of LAR values, which indicates that the majority of the cohort (over 97%) fell within the 0–100 range, suggesting limited reliability of the association at extreme LAR levels.
To evaluate cumulative survival at different levels of LAR, we generated survival curves for patients with AKI by stratifying based on the high and low LAR levels. The Kaplan–Meier analysis showed that patients with lower LAR levels had a significantly higher ICU survival probability (p < 0.001) (Figure 3A). In addition, similar results were observed in the 30-day and 90-day survival curves (Figures 3B, C).
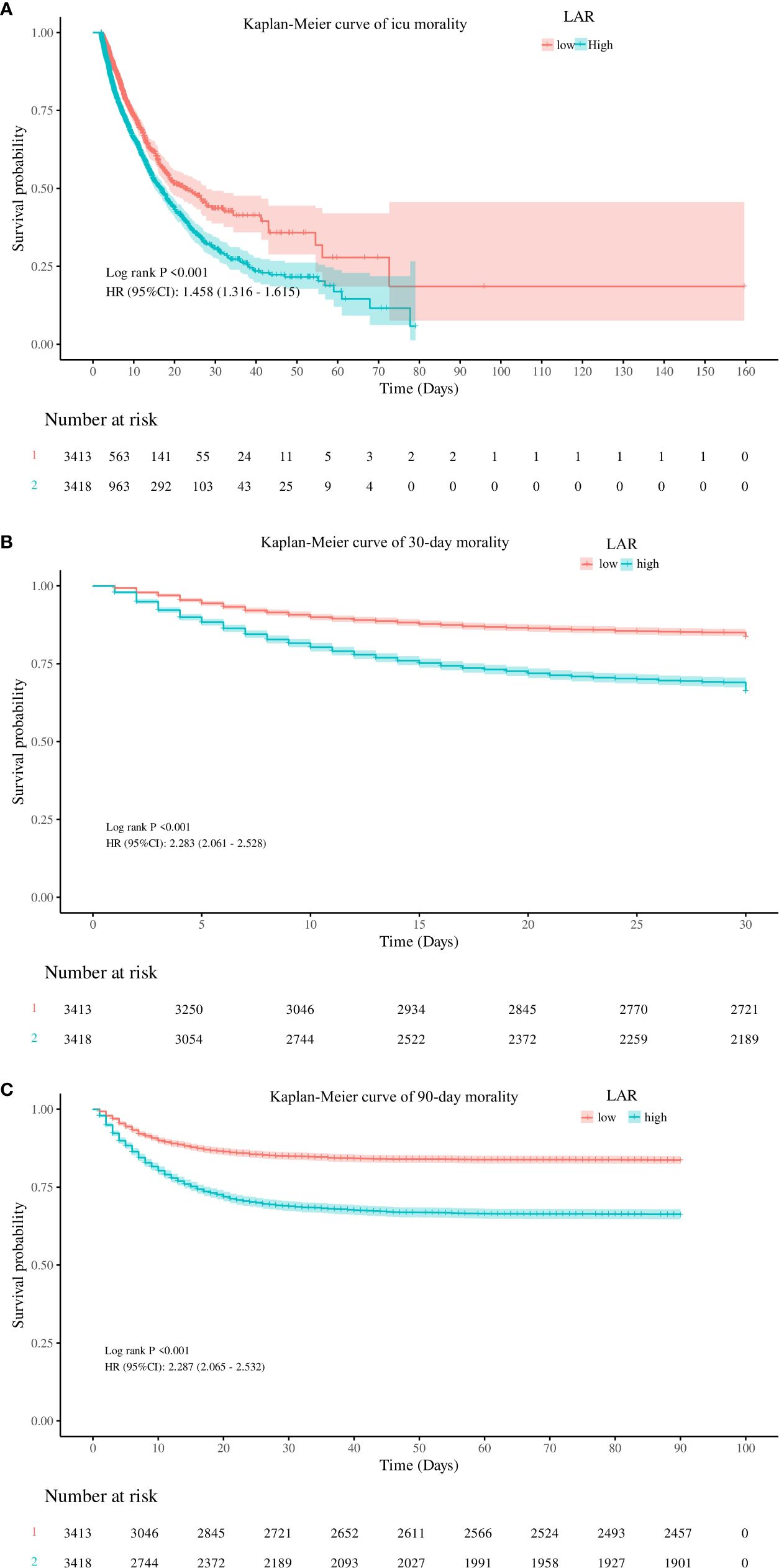
Figure 3. Kaplan–Meier curves for ICU stay (A) and 30-day (B) and 90-day (C) accumulative survival rates stratified by the high and low groups of LAR. ICU, intensive care unit; LAR, lactate dehydrogenase to albumin ratio.
3.3 LAR categories and clinical outcomes
Patients were further divided into four categories according to the IQR of the LAR value (Q1–Q4 categories). The crude outcomes of those four subgroups are shown in Table 2. Table 2 reveals a gradual escalation in SOFA and APSIII scores across the Q1 to Q4 subgroups, and all pairwise comparisons demonstrated statistical significance (p < 0.001). Regarding clinical outcomes, the Q1 to Q4 subgroups exhibited an increasing trend in the proportion of CRRT utilization, ICU length of stay (LOS), and hospital LOS. CRRT utilization increased from 2.69% to 11.59%, ICU LOS extended from 4.13 (2.88, 7.22) days to 6.12 (3.56, 11.74) days, and hospital LOS rose from 10.02 (6.58, 16.74) days to 13.99 (7.50, 23.07) days (p for trend <0.001). Mortality rates in the Q4 group were markedly elevated (in-hospital, 38.41%; 30 days, 40.69%; 90 days, 48.24%) compared to lower quartiles (all p < 0.001).
After performing a multi-factor logistic regression analysis (Supplementary Table S1), we developed and used four multivariable models to identify significant correlations between the different LAR categories and hospital mortality. Table 3 displays the HRs and 95% confidence intervals (CIs) for the models. Crude model was without any adjustments. Model 1 was adjusted for age, gender, and race; Model 2 was built upon Model 1 and further adjusted for APSIII, CCI, and comorbidities including MI, CHF, cerebrovascular disease, severe liver disease, and solid tumor. Model 3 encompassed all covariates from Model 2 and also included adjustments for the laboratory tests, first 24-h urine output, and interventions such as antibiotics, vasopressors, and CRRT use.
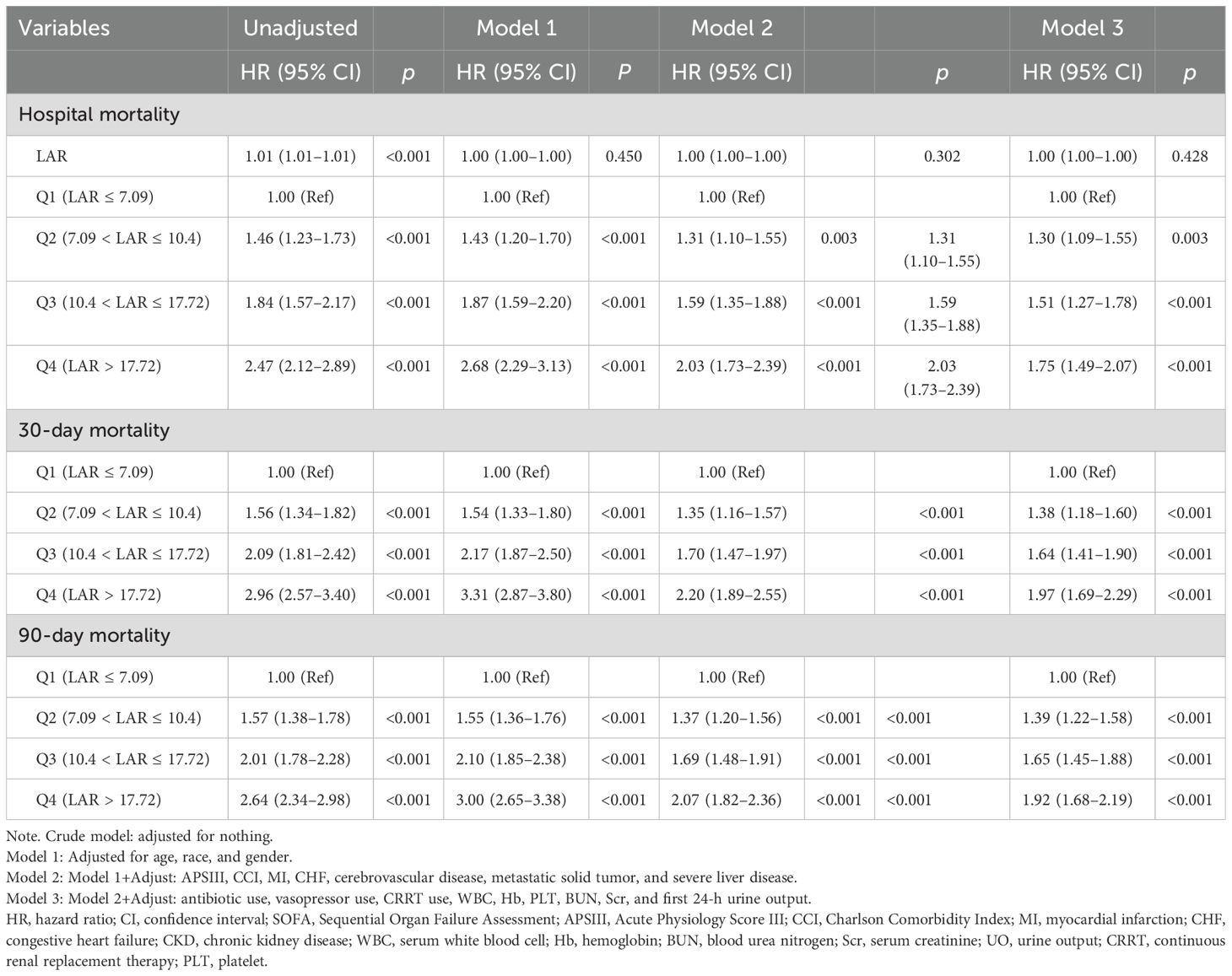
Table 3. Cox proportional hazards regression analysis of LAR categories and clinical outcomes in patients with AKI.
Table 3 shows that when LAR was a continuous variable in one original model, each IU/g increase in LAR was significantly associated with a 1% increase in in-hospital mortality (HR, 1.001, p < 0.001) but lost statistical significance in adjusted models (p = 0.45 in Model 1, p = 0.30 in Model 2, and p = 0.43 in Model 3).
In addition, when the LAR was used as a categorical variable (categorized by quartiles), elevated LAR was identified as an independent risk factor for 30-day and in-hospital mortality in all models (all HR > 1, p < 0.001). The risk of mortality tended to increase steadily with increasing LAR levels from Q2 (HR, 1.46, 95% CI 1.23–1.73, p < 0.001 for in-hospital mortality; HR, 1.56, 95% CI 1.34–1.83, p < 0.001 for 30-day mortality) to Q4 (HR, 2.47, 95% CI 2.12–2.89, p < 0.001 for in-hospital mortality; HR, 2.96, 95% CI 2.57–3.40, p < 0.001 for 30-day mortality) in unadjusted model, with the Q1 category (LAR ≤ 7.09 IU/g) as a reference.
After controlling for age, sex, race, APSIII, CCI, comorbidities, laboratory tests and interventions, the adjusted HRs for the Q2–Q4 groups compared to Q1 were 1.30 (HR, 1.30, 95% CI 1.09–1.55, p < 0.001), 1.51 (HR, 1.51, 95% CI 1.27–1.78, p < 0.001), and 1.75 (HR, 1.75, 95% CI 1.49–2.07, p < 0.001). Investigation of mortality after 30 and 90 days yielded comparable findings.
3.4 Receiver operating characteristic analysis
The clinical value of LAR in predicting mortality was determined by receiver operating characteristic (ROC) curve analysis. As shown in Figure 4, LAR showed a moderate predictive ability for ICU mortality [area under the curve (AUC) 0.65, 95% CI 0.64–0.67]. LAR had a better predictive ability than urine output (AUC 0.62, 95% CI 0.60–0.63) and Scr (AUC 0.58, 95% CI 0.56–0.59), but less than APSIII (AUC 0.70, 95% CI 0.68–0.71) and SOFA (AUC 0.66, 95% CI 0.64–0.67).
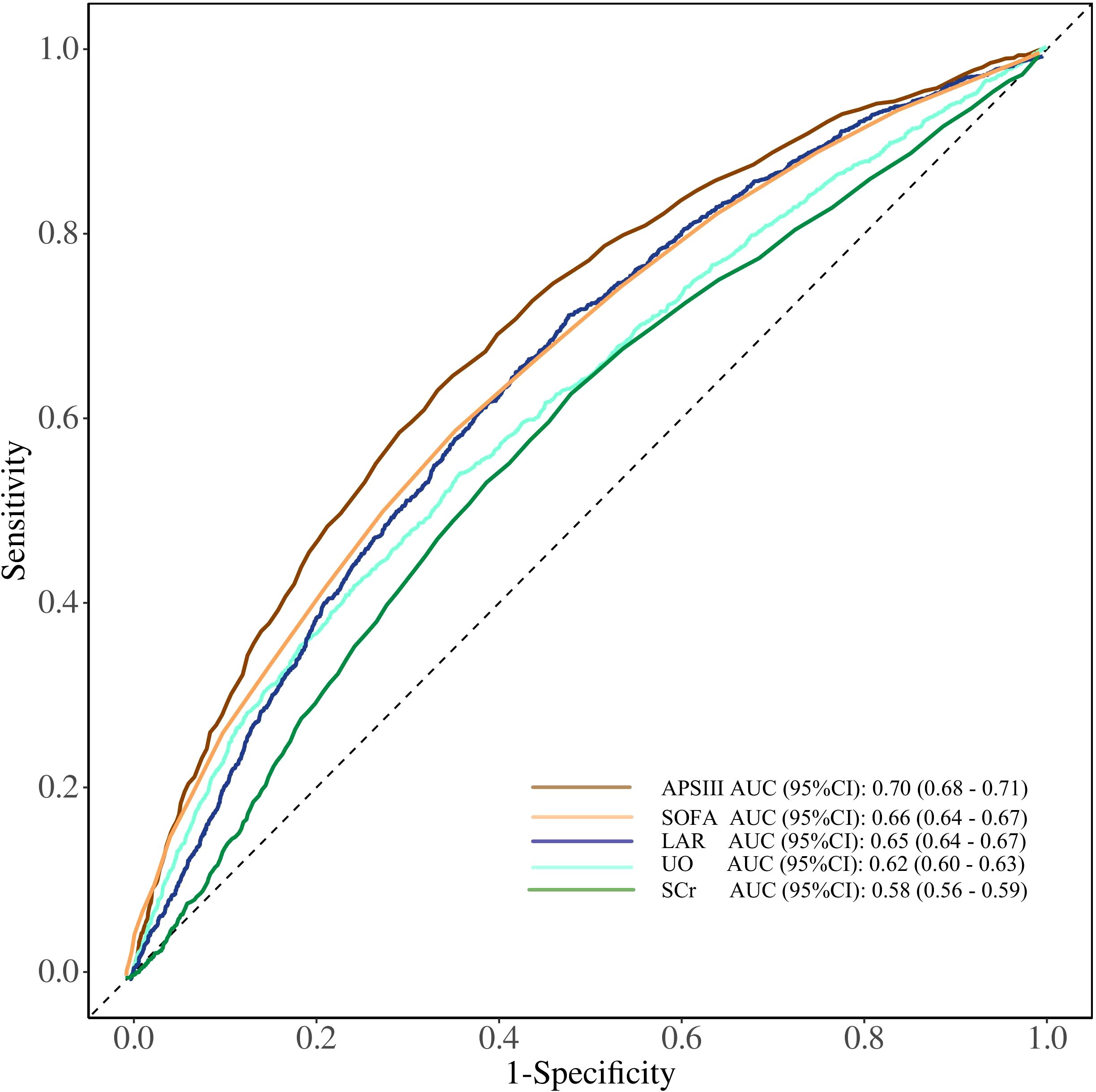
Figure 4. ROC curves of LDH/ALB ratio, SOFA, APSIII score, UO, and Scr for predicting ICU mortality in patients with AKI. SOFA, Sequential Organ Failure Assessment; APSIII, Acute Physiology Score III; UO, urine output; Scr, serum creatinine; LDH/ALB ratio, lactate dehydrogenase to albumin ratio; ROC, receiver operating characteristic; ICU, intensive care unit.
3.5 Subgroup analysis and post-hoc analysis
To explore the differences in the predictive value of LAR among different subgroups, we performed subgroup analysis as shown in Figure 5. The higher LAR was consistently associated with increased hospital mortality in different subgroups, including AKI Stage 1 to 3, age <65 or ≥65 years, SOFA <7 or ≥7, APSIII<54 or ≥54, female or male, with or without MI, CHF, solid tumor, cerebrovascular disease, sepsis, CKD, and use of antibiotics.
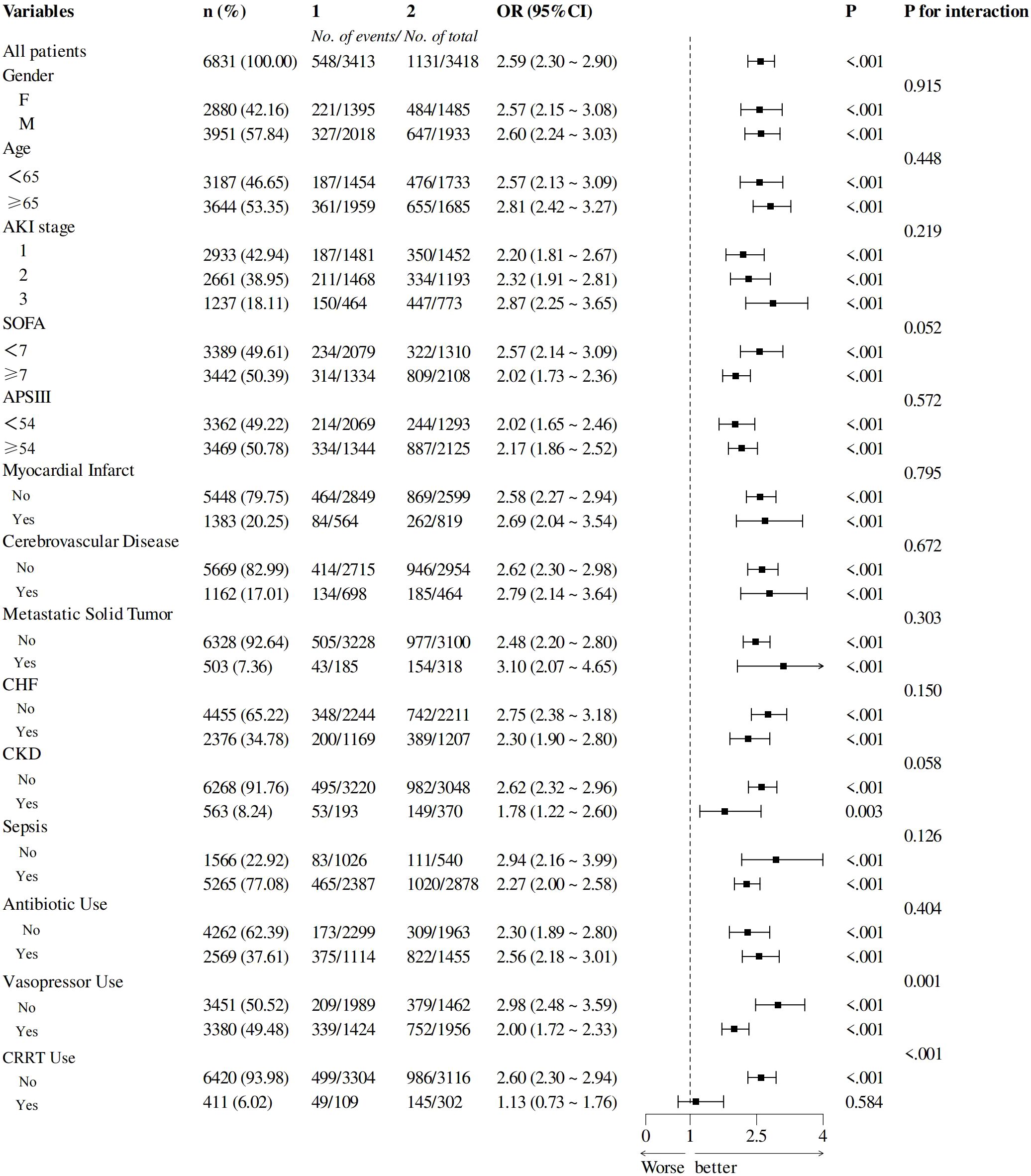
Figure 5. Subgroup analyses of the LDH/ALB ratio in patients with AKI. LDH/ALB, lactate dehydrogenase/albumin; AKI, acute kidney injury.
There was an interaction between the use of vasopressors, CRRT, and LAR on hospital mortality (p for interaction = 0.001, <0.001), and the predictive value of LAR was less pronounced in these patients.
In the post-hoc analysis, the relationship and predictive value between LAR and hospital mortality in patients with or without CRRT use were further determined. Supplementary Figure S2 illustrates the distribution of LAR across different subgroups. Figure 6a shows that a significant positive linear relationship between the LAR and hospital mortality still existed in patients without CRRT use (p for non-linear <0.001). However, it did not hold true for AKI patients with CRRT use. As shown in Figure 6b, there was no significant trend between LAR and hospital mortality in AKI patients’ CRRT use (p for non-linear = 0.079).
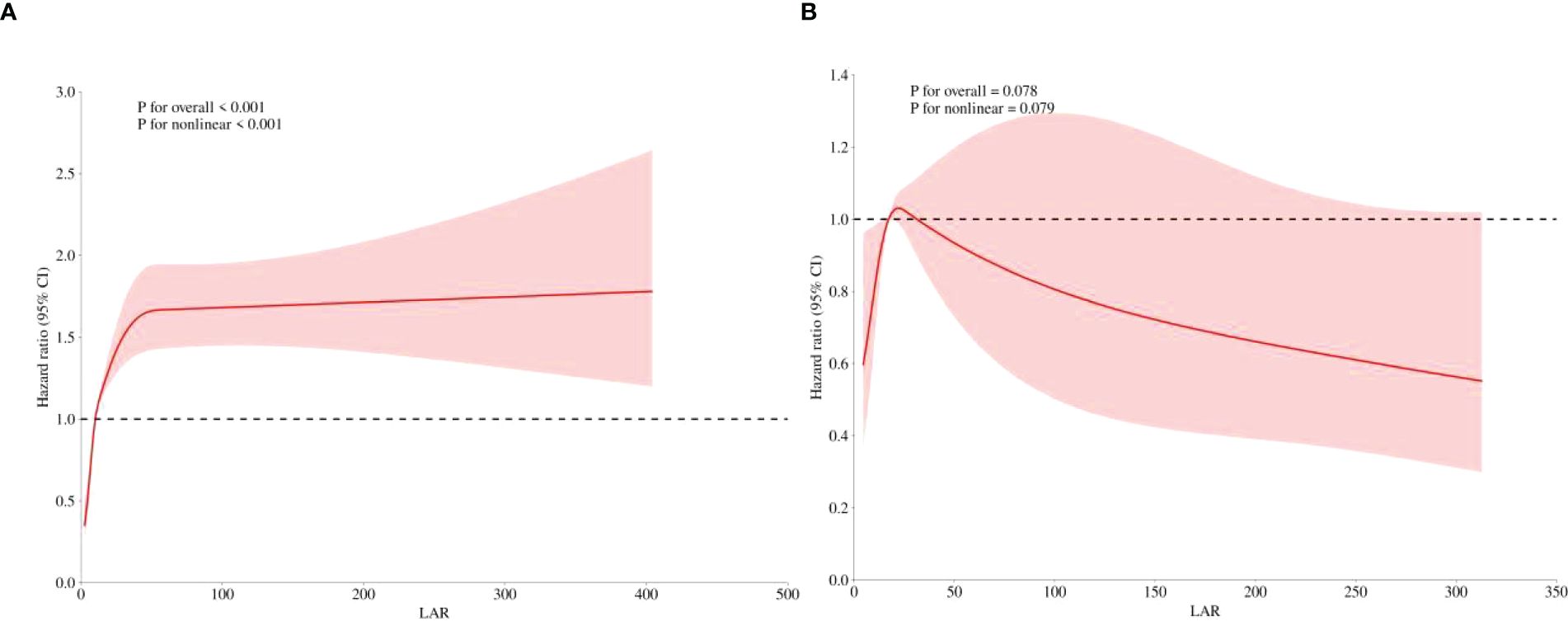
Figure 6. Post-hoc analysis. Restricted cubic spline showed the association between the LAR and the hospital mortality was further investigated in the non-CRRT (A) and CRRT cohorts (B) of patients with AKI. LAR, lactate dehydrogenase to albumin ratio; CRRT, continuous renal replacement therapy; AKI, acute kidney injury.
4 Discussion
4.1 Key findings
In this retrospective cohort study, we utilized data from the large-scale MIMIC-IV database to explore the association between the LDH/ALB ratio and the risk of death in AKI patients admitted to the ICU. The current study’s findings suggested that higher levels of the LDH/ALB ratio are associated with increased mortality in critically ill patients with AKI. Notably, even after accounting for possible confounding variables, this association is still statistically significant. Multivariate regression analyses exhibited the independent prognostic significance of the LDH/ALB ratio for ICU all-cause mortality; this association was constant across subgroup analyses and sensitivity analyses, demonstrating the robustness of the finding. Additionally, the current study also highlights the significant association of the LDH/ALB ratio with in-hospital mortality, length of ICU, and 90-day mortality among critically ill patients with AKI.
4.2 Relation with previous evidence
Even though the pathogenesis of AKI is still not well recognized, it is often a complex condition regularly implicated by hemodynamic instability, sepsis, and medication toxicity. Regardless of the specific cause, some distinct pathophysiologic processes, including endothelial dysfunction, oxidative stress (OS), changes in microcirculation, and intrarenal inflammation, occur concurrently and in order (21, 22). Inflammation is a major contributor to AKI pathophysiology. Septic shock can cause inflammatory responses inducing AKI and a chronic microinflammatory state, and oxidative stress also exists in diabetes mellitus, chronic kidney disease, and malignancy mediated by inflammatory cytokines. Inflammatory factors and oxidative stress can directly promote apoptosis and further aggravate renal impairment. LDH, as a marker of inflammatory factors and oxidative stress, is involved in the apoptosis of renal tubular epithelial cells. In certain malignancies, elevated levels of LDH correlate with disease activity, tumor proliferation rate, and cellular breakdown, and thus, the combination of these mechanisms may render an individual more susceptible to AKI. It can be speculated that the effects of inflammation, oxidative stress, and apoptosis induced by certain diseases themselves may lead to an enhanced association between LAR and the development of AKI.
Albumin is an indicator of inflammation and systemic nutritional status, studies have shown that albumin has a renoprotective mechanism, and hypoproteinemia is considered an independent risk factor for the prognosis of AKI (23–25). In addition, there is a negative relationship between albumin levels and levels of neutrophil gelatinase-associated lipocalin (NGAL), which is commonly associated with inflammation and kidney injury and is recognized as a biomarker for renal function and damage (26, 27). LDH is not only a metabolite but also a prognostic biomarker for immune surveillance. Renal tissue damage caused by inflammation, oxidative stress, and ischemic/hypoxic events during AKI may lead to the release of intracellular LDH into the serum (7). LDH has been shown to be associated with in-hospital mortality in critically ill patients with acute kidney injury (28). In our present study, we found that patients in the death group had higher LDH and lower albumin than those in the survival group.
The LDH/ALB ratio, which combines factors of oxidative stress, inflammation, and nutritional status, may provide more comprehensive prognostic information than the separate predictive values of LDH or albumin. Recent studies have investigated the clinical value of LDH in combination with albumin. Guan XY et al. (16), in a study of individuals with sepsis, suggested a non-linear relationship between the LDH/ALB ratio and the risk of ICU mortality and indicated that an elevated LDH/ALB ratio (≥10.57) was a significant predictor of all-cause mortality among ICU patients with sepsis. In another study of sepsis, Fang XP, et al. (17) found a positive linear correlation between LAR and the development of sepsis-associated AKI (SA-AKI), and they indicated that LAR 12 h before and after the diagnosis of sepsis is an independent risk factor for the development of SA-AKI in patients with sepsis.
Our study showed that LAR ≥ 10.4 was a significant predictor of all-cause mortality among ICU patients with AKI. To mitigate the effects of heart and liver tissue injuries on the predictive accuracy of LAR, we controlled for several variables including coronary heart disease, heart failure, severe liver disease, solid tumor, and cerebrovascular disorders evaluated in our multi-factor logistic regression analysis. Nevertheless, elevated LAR was identified as an independent risk factor for 30-day, 90-day, and in-hospital mortality in both crude and adjusted models.
Urine output and creatinine are traditional indicators reflecting renal function and can also be used to predict the prognosis of AKI patients (29). Our study further revealed that in predicting in-ICU mortality among AKI patients, LAR demonstrated a better predictive ability than urine output and creatinine by ROC curve analysis, ranking second only to SOFA and APS III scores. This finding further confirms that LAR can serve as a reliable biomarker for predicting AKI prognosis.
In our study, patients were stratified based on sepsis status, and analyses were performed in subgroup analysis, which showed that whether sepsis was present or not, LAR still demonstrated a good correlation with the prognosis of patients with kidney injury. It may be attributed to the fact that a majority of patients with kidney injury experience protein leakage as well as ischemia and hypoxia. Albumin, serving as a critical component in maintaining plasma colloid osmotic pressure, plays a pivotal role in regulating fluid balance and tissue perfusion, thereby mitigating the progression of acute kidney injury (23).
In our subgroup analysis, the predictive effect of LAR on in-hospital mortality showed no significant interaction with the presence of CKD, which contrasts with previous findings. Specifically, in the study by Deng et al. (30), the prognostic value of the LDH/ALB ratio was more pronounced in the non-CKD patient subgroup. Interestingly, in studies investigating the predictive value of the LDH/ALB ratio for sepsis-associated acute kidney injury (SAKI), Fang et al. (17) found that the LDH/ALB ratio lost its prognostic utility for SAKI progression in patients with CKD, whereas it remained significant in non-CKD patients. They proposed that this discrepancy may be attributed to podocyte injury-induced proteinuria, which substantially reduces serum albumin levels and reduces the physiological reserve of LDH in the cell. In our study, even though the LDH/ALB ratio demonstrated a more pronounced predictive effect on in-hospital mortality in non-CKD patients compared to the CKD group (OR 2.62 vs. 1.78), this difference did not reach statistical significance (p for interaction = 0.058).
Furthermore, our subgroup analysis showed that there was an interaction among the use of vasopressors, CRRT, and LAR on hospital mortality, and in post-hoc analysis, the association between LAR and in-hospital mortality was further examined in AKI patients with and without CRRT using restricted cubic splines (RCS). The prognostic value of LAR on hospital mortality was still robust in the non-CRRT subgroup but disappeared in the CRRT subgroup. We speculated that the possible mechanism may be due to the reduction of serum albumin levels, which diminishes the predictive power of the LDH/ALB ratio. Our findings serve as a reminder that CRRT use should not be ignored when using the LDH/ALB ratio to predict mortality.
4.3 Strengths and limitations
This research possesses several notable strengths. First, the data utilized in this study were obtained from the well-established MIMIC-IV 3.0 database, which was a high-quality intensive care database with a substantial sample size, enhancing the accuracy and reliability of our findings. The MIMIC-IV database version 3.0 was updated in July 2024 with three additional years of patient data (2020–2022). While the number of ICU admissions increased by less than 30%, the corresponding chart event table volume grew by nearly 50%, indicating that the newly added patient data exhibit finer granularity compared to previous records. Additionally, we employed rigorous statistical techniques, including PSM analysis and detailed subgroup analyses, to avoid the potential influence of confounding factors on the outcomes of our study.
This study had several limitations. First, this is a retrospective cohort study, and the lack of information regarding AKI etiologies in the database restricts the comprehensiveness and level of detail in the study. Therefore, we cannot accurately determine the time of all AKI occurrences, which may affect our accurate assessment of the relationship between the LDH/ALB ratio and the time of AKI occurrence.
Second, we excluded some participants with missing LDH and albumin data, which may lead to selection bias and limit the generalizability of the study results. There was an absence of data regarding interventions during the initial stabilization phase; for instance, the administration of albumin could potentially result in reduced LAR levels and improved survival outcomes, representing an unmeasured confounding variable, which may have a certain impact on the promotion of the study conclusions.
Third, we only extracted data on admission, and we could not detect the fluctuations of LDH or albumin, and inflammatory and immune response indicators during hospitalization. As dynamic change indicators, their values at a single point in time may not accurately reflect the true situation of patients. The absence of this information may limit our comprehensive understanding of the overall condition of the patient, thereby affecting the in-depth analysis of the relationship between the LAR and the occurrence of AKI. Trajectory analysis to identify the different trajectory groups of LAR would be an optional method to solve this problem. Future studies are needed to address these issues, which will be of high value.
5 Conclusion
Our research suggests that LAR monitoring may be promising as a prognostic marker among patients with AKI. Higher LAR is associated with greater ICU mortality.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://physionet.org/content/mimiciv.
Ethics statement
The studies involving humans were approved by the Massachusetts Institute of Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The review boards of the Massachusetts Institute of Technology evaluated and authorized the experiments involving human volunteers. According to national law and institutional regulations, written informed consent to participate in this study was not required.
Author contributions
JG: Data curation, Formal analysis, Writing – original draft, Funding acquisition, Conceptualization, Validation, Writing – review & editing. HC: Conceptualization, Writing – review & editing, Data curation, Investigation, Supervision, Validation, Funding acquisition. YW: Data curation, Formal analysis, Writing – original draft, Funding acquisition, Investigation. CX: Data curation, Formal analysis, Investigation, Writing – review & editing. YJ: Data curation, Writing – original draft, Formal analysis, Supervision, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Zhejiang Provincial Medical and Health Science and Technology Program (2024KY222).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneph.2025.1583913/full#supplementary-material
Supplementary Figure/Image S1 and S2 | Showed The PSM analysis which employed a 1:1 nearest-neighbor matching algorithm with a caliper width of 0.05 to ensure close matching of the pairs.
Supplementary Figure/Image S3 | Showed the distribution of LAR across different subgroups (1 represents the crrt group and 0 represents the non-crrt group).
SUPPLEMENTARY TABLE 1 | A multi-factor logistic regression analysis.
Abbreviations
AUC, area under the curve; AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; APSIII, Acute Physiology Score III; CCI, Charlson Comorbidity Index; CRRT, continuous renal replacement therapy; LAR (LDH/ALB ratio) lactate dehydrogenase to albumin ratio; LDH, lactate dehydrogenase; ICU, intensive care unit; IQR, interquartile range; MIMIC IV, The Medical Information Mart for Intensive Care IV; KDIGO, Kidney Disease Improving Global Outcomes; RCS, restricted cubic spline; ROC, receiver operating characteristic; BUN, blood urea nitrogen; Scr, serum creatinine; SD, standard deviation; SOFA, Sequential Organ Failure Assessment; WBC, serum white blood cell; RR, respiratory rate; HR, heart rate; MAP, mean arterial pressure; SpO2, oxygen saturation levels; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; PSM, propensity score matching; UO, urine output; HFNC, high-flow nasal cannula oxygen therapy; CHF, congestive heart failure; CKD, chronic kidney disease.
References
1. Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. (2015) 41:1411–23. doi: 10.1007/s00134-015-3934-7
2. Zarbock A, Weiss R, Albert F, Rutledge K, Kellum JA, Bellomo R, et al. Epidemiology of surgery associated acute kidney injury (EPIS-AKI): a prospective international observational multi-center clinical study. Intensive Care Med. (2023) 49(12):1441–55. doi: 10.1007/s00134-023-07169-7
3. Oh DJ. A long journey for acute kidney injury biomarkers. Ren. Fail. (2020) 42:154–65. doi: 10.1080/0886022X.2020.1721300
4. McMahon BA, Galligan M, Redahan L, Martin T, Meaney E, Cotter EJ, et al. Biomarker predictors of adverse acute kidney injury outcomes in critically ill patients: the Dublin acute biomarker group evaluation study. Am J Nephrol. (2019) 50:19–28. doi: 10.1159/000500231
5. Adam R, Ashley L, Jay LK, and Lui GF. New biomarkers in acute kidney injury. Crit Rev Cl. Lab Sci. (2024) 61:23–44. doi: 10.1080/10408363.2023.2242481
6. Christian S, Hendrik B, Lui F, and Alexander Z. Biomarkers of acute kidney injury: From discovery to the future of clinical practice. J Clin Anesth. (2024) 95:111458. doi: 10.1016/j.jclinane.2024.111458
7. Nielsen PM, Laustsen CH, Bertelsen LB, Qi HY, Mikkelsen E, Kristensen MLV, et al. In situ lactate dehydrogenase activity: a novel renal cortical imaging biomarker of tubular injury? Am J Physiol. Renal. (2017) 312:F465–73. doi: 10.1152/ajprenal.00561.2015
8. Qiao J and Cui L-Y. Multi-Omics techniques make it possible to analyze sepsis-associated acute kidney injury comprehensively. Front Immunol. (2022) 13:905601. doi: 10.3389/fimmu.2022.905601
9. Li JY, Shi XX, Chen ZX, Xu JT, and Chen LM. Renal aerobic glycolysis and local lactate production in AKI. Nephrol Dial Transpl. (2023) 38. doi: 10.1093/ndt/gfad063c_5322
10. De Simone G, di Masi A, and Ascenzi P. Serum albumin: a multifaced enzyme. Int J Mol Sci. (2021) 22(18). doi: 10.3390/ijms221810086
11. Mallipattu SK and He JC. The podocyte as a direct target for treatment of glomerular disease. Am J Physiol Ren. Physiol. (2016) 311:F46–51. doi: 10.1152/ajprenal.00184.2016
12. Hansrivijit P, Yarlagadda K, Cheungpasitporn W, Thongprayoon C, and Ghahramani N. Hypoalbuminemia is associated with increased risk of acute kidney injury in hospitalized patients: a meta-analysis. J Crit Care. (2021) 61:96–102. doi: 10.1016/j.jcrc.2020.10.013
13. Sheng S, Zhang YH, Sheng S, Zhang YH, Ma HK, and Huang Y. Albumin levels predict mortality in sepsis patients with acute kidney injury undergoing continuous renal replacement therapy: a secondary analysis based on a retrospective cohort study. BMC Nephrol. (2022) 23:52. doi: 10.1186/s12882-021-02629-y
14. Zhao R, Liang ZG, Chen KH, and Zhu XD. Nomogram based on inflammatory biomarkers and nutritional indicators for predicting overall survival in locoregionally advanced nasopharyngeal carcinoma. J Inflammation Res. (2022) 15:2971–81. doi: 10.2147/JIR.S366299
15. Shu XP, Xiang YC, Liu F, Cheng Y, Zhang W, and Peng D. Effect of serum lactate dehydrogenase-to-albumin ratio (LAR) on the short-term outcomes and long-term prognosis of colorectal cancer after radical surgery. BMC Cancer. (2023) 23:915. doi: 10.1186/s12885-023-11446-5
16. Guan XY, Zhong L, Zhang JY, Lu JH, Yuan M, Ye LL, et al. The relationship between lactate dehydrogenase to albumin ratio and all-cause mortality during ICU stays in patients with sepsis: A retrospective cohort study with propensity score matching. Heliyon. (2024) 10:e27560. doi: 10.1016/j.heliyon.2024.e27560
17. Fang YP and Zhang Y and Zhang X. The elevated lactate dehydrogenase to albumin ratio is a risk factor for developing sepsis-associated acute kidney injury: a singlecenter retrospective study. BMC Nephrol. (2024) 25:201. doi: 10.1186/s12882-024-03636-5
18. Yan D, Huang QQ, Dai CJ, Ren WW, and Chen SY. Lactic dehydrogenase to albumin ratio is associated with the risk of stroke-associated pneumonia in patients with acute ischemic stroke. Front Nutr. (2021) 8:743216. doi: 10.3389/fnut.2021.743216
19. Zhang FS, Zhang MY, Niu ZG, Sun LN, Kang XH, and Qu YQ. Prognostic value of lactic dehydrogenase-to-albumin ratio in critically ill patients with acute respiratory distress syndrome: a retrospective cohort study. J Thorac Dis. (2024) 16:81–90. doi: 10.21037/jtd-23-1238
20. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012) 120:c179–84. doi: 10.1159/000339789
21. Ostermann M and Liu K. Pathophysiology of AKI. Best Pract Res Clin Anaesthesiol. (2017) 31:305–14. doi: 10.1016/j.bpa.2017.09.001
22. Radi ZA. Immunopathogenesis of acute kidney injury. Toxicol Pathol. (2018) 46:930–43. doi: 10.1177/0192623318799976
23. Wiedermann CJ, Wiedermann W, and Joannidis M. Hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med. (2010) 36:1657–65. doi: 10.1007/s00134-010-1928-z
24. Iglesias J, Abernethy VE, Wang Z, Lieberthal W, Koh JS, and Levine JS. Albumin is a major serum survival factor for renal tubular cells and macrophages through scavenging of ROS. Am J Physiol. (1999) 277:F711–722. doi: 10.1152/ajprenal.1999.277.5.F711
25. MacPhee PJ and Michel CC. Fluid uptake from the renal medulla into the ascending vasa recta in anaesthetized rats. J Physiol. (1995) 487:169–83. doi: 10.1113/jphysiol.1995.sp020869
26. Zhang D and Shi L. Serum lactate dehydrogenase level is associated with in-hospital mortality in critically ill patients with acute kidney injury. Int Urol. Nephrol. (2021) 53:2341–8. doi: 10.1007/s11255-021-02792-z
27. Figueroa SM, Araos P, Reyes J, Gravez B, Barrera-Chimal J, and Amador CA. Oxidized albumin as a mediator of kidney disease. Antioxidants. (2021) 10:404. doi: 10.3390/antiox10030404
28. Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. (2017) 13:241–57. doi: 10.1038/nrneph.2017.2
29. Sutherland, Scott M, Kaddourah A, Soranno DE, Woroniecki RP, Basu RK, et al. Cumulative application of creatinine and urine output staging optimizes the kidney disease: improving global outcomes definition and identifies increased mortality risk in hospitalized patients with acute kidney injury. Crit Care Med. (2021) 49:1912–22. doi: 10.1097/CCM.0000000000005073
Keywords: lactate dehydrogenase, albumin, lactate dehydrogenase to albumin ratio, acute kidney injury, mortality
Citation: Gao J, Chen H, Wu Y, Xu C and Jin Y (2025) Association between lactate dehydrogenase to albumin ratio and ICU mortality in patients with acute kidney injury: a retrospective cohort study. Front. Nephrol. 5:1583913. doi: 10.3389/fneph.2025.1583913
Received: 26 February 2025; Accepted: 28 April 2025;
Published: 02 June 2025.
Edited by:
Ciro Tetta, Unicyte AG, SwitzerlandReviewed by:
Marios Papasotiriou, University of Patras, GreeceAlessandro Domenico Quercia, Nephrology and Dialysis ASLCN1, Italy
Copyright © 2025 Gao, Chen, Wu, Xu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huizhen Chen, aHVpemhlbjE5NzNAMTYzLmNvbQ==
 Jianting Gao
Jianting Gao Huizhen Chen*
Huizhen Chen*