- 1Division of Medical Oncology, Institute of Oncology Ljubljana, Ljubljana, Slovenia
- 2Department of Nephrology, University Medical Center Ljubljana, Ljubljana, Slovenia
- 3Division of Nephrology, Cooper University Hospital and Cooper Medical School of Rowan University, Camden, NJ, United States
- 4Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
- 5Renal Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 6Weill Cornell Medical College, New York, NY, United States
Background: Percutaneous renal biopsy (PRB) provides valuable information to guide treatment decisions in patients with metastatic renal cell carcinoma (mRCC) who develop acute kidney injury (AKI) after systemic anticancer therapy (SACT). The rising incidence of renal cell carcinoma (RCC) and the substantial impact of SACT on overall survival suggest a higher prevalence of RCC patients with reduced nephron mass and a solitary kidney (SK) requiring PRB for AKI. However, safety data on SK biopsies are scarce, and the potential for dialysis-requiring complications may deter clinicians.
Methods: This retrospective case series reports the safety of 13 PRBs in 12 mRCC patients with reduced nephron mass who developed AKI during SACT as well as six PRBs in six patients with metastatic solid malignancies and AKI, which developed during SACT.
Results: Eleven biopsies in mRCC patients and five biopsies in patients with metastatic solid malignancies were uneventful. One patient with mRCC experienced a major bleeding event due to an arteriovenous (AV) fistula seven days post-procedure, while another mRCC patient developed macrohematuria within 24 hours. In the group of patients with metastatic solid malignancies, one patient experienced a small perinephric hematoma during the observational period. Despite the small sample size, individual chart reviews and direct management of adverse events allowed assessment of the association between biopsy and complications.
Conclusion: Until further data become available, a longer observation period is recommended for these patient cohorts compared to the general population. Further studies are needed to develop consensus guidelines for PRB in mRCC patients with reduced nephron mass.
1 Introduction
Percutaneous renal biopsy (PRB) is the gold standard for assessing renal parenchymal disease. An accurate histologic diagnosis is key to guiding further decision making in patients with metastatic renal cell carcinoma (mRCC) and acute kidney injury (AKI) who have received various systemic anticancer therapy (SACT), such as immune checkpoint inhibitors (ICIs) and vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKI) (1, 2).
Up to 82% of patients with advanced renal cell carcinoma (RCC) undergo total or partial nephrectomy, resulting in reduced nephron mass before starting sequential SACT for metastatic disease (3–6). Furthermore, approximately 50% of patients with synchronous advanced disease who begin SACT with a tumor in place receive cytoreductive nephrectomy during their first-line therapy, followed by continued SACT (7). Localized clear cell renal cell carcinoma (ccRCC) patients frequently undergo radical nephrectomy before adjuvant therapy (up to 92%), and approximately 26% subsequently receive VEGFR-TKI or ICIs due to disease progression (8). The rising incidence of RCC, coupled with the substantial impact of ICI-based SACT on overall survival (OS) in both mRCC patients and localized disease patients, suggests a higher prevalence of RCC patients with reduced nephron mass who may require PRB due to AKI associated with SACT (9, 10).
Data on the safety of solitary kidney (SK) biopsies are limited, leading to clinician hesitance due to potential complications requiring nephrectomy. However, foregoing PRB can delay appropriate treatment or result in unnecessary initiation of corticosteroid therapy for suspected ICI induced interstitial nephritis, potentially impacting OS (2, 11).
While PRB of native kidneys is generally considered a low-risk procedure in the general population, bleeding complications remain a significant concern. Advanced age, diabetes, anemia, metastatic disease, liver disease, and pre-existing AKI all increase the risk of complications during native PRB (12–14). Although the incidence of major bleeding events or death is similar between SK and native kidney biopsies in the general population, the potential consequences of an ablative procedure to manage major bleeding in an SK patient can lead to dialysis dependence. Whether patients with a SK receiving SACT face a similar risk remains unclear. The increased risk of hypertension and bleeding associated with SACT further raises safety concerns about PRB in this patient group. To our knowledge, no published reports describe PRB use for AKI evaluation in patients receiving SACT for mRCC with reduced nephron mass.
2 Methods
2.1 Patient population
This retrospective analysis was performed at the Institute of Oncology Ljubljana and the University Medical Centre Ljubljana, Slovenia, and Cooper University Hospital, Camden, New Jersey, USA. Individual chart reviews analyzed complications associated with PRBs performed between 2018 and 2023 for the evaluation of AKI in mRCC patients with reduced nephron mass and non-mRCC PRBs were performed at least seven days after AKI detection. All patients received SACT with VEGFR-TKIs or ICIs before AKI development, and SACT was withheld for at least seven days before PRB. During the same period, we gathered data on complications of PRBs in patients with metastatic solid cancers who experienced AKI as an adverse event (AE) during SACT. One of those patients had a SK while the others did not have reduced renal parenchymal mass.
2.2 Definitions
Reduced nephron mass was defined as a condition following total or partial nephrectomy or the presence of a primary tumor. AEs, including AKI, proteinuria, hypertension, and anemia, were graded according to the Common Terminology Criteria for Adverse Events, version 5.0. Minor bleeding complications were defined as macroscopic hematuria or hematoma not requiring blood products. Major bleeding complications were defined similarly to other studies: the need for blood products, radiologic or surgical intervention, intensive care unit admission, loss of parenchymal functional mass, or death (15, 16).
2.3 Patient preparation
Informed written consent was obtained from all patients after discussing the risks and benefits of PRB. All patients underwent a thorough evaluation, including complete blood count, metabolic panel, and coagulation profile tests. Anemia was corrected according to protocol recommendations, which included a minimum hemoglobin level of 10 g/dl. Kidney ultrasound was performed pre-biopsy to visualize kidney anatomy and rule out obstruction as a cause of AKI.
Platelet aggregation inhibitors (aspirin, clopidogrel, non-steroidal anti-inflammatory drugs) were discontinued at least seven days prior to PRB unless contraindicated by vascular risk factors (17). In high-risk patients, a specialist consultation determined the appropriateness of antithrombotic therapy, potentially including low-dose aspirin (≤100 mg) (18).
For patients on anticoagulation, warfarin was discontinued five days prior to PRB, with an INR target of <1.5. Unfractionated heparin bridge therapy was used if needed and the infusion ceased six hours prior to PRB and resumed 12 hours post-biopsy. Patients receiving low-molecular-weight heparin received their last dose 12 hours prior to PRB, with anticoagulation restarted 12 hours later. Hypertensive patients had to maintain blood pressures below 150/90 mmHg before PRB.
2.4 Procedure
All PRBs were performed under ultrasound guidance by a skilled nephrologist, following the local biopsy protocol. Patients were positioned prone with a pillow under the upper abdomen to facilitate slight trunk flexion. Either a 16- or 18-gauge needle was used.
2.5 Post-biopsy monitoring
Post- procedure, patients remained supine position and refrained from strenuous activity. Pulse, blood pressure, and urine output were monitored regularly during a 24-hour observation period. Kidney ultrasound was performed to detect hematoma formation, and urine was monitored for gross hematuria. Hemoglobin concentrations were monitored as required by local protocol. Patients were discharged home 24 hours post-procedure if no complications were observed.
2.6 Statistical analysis
Descriptive statistics were used throughout the analysis. Categorical variables (patient characteristics and comorbidities, procedural details) were analyzed using absolute numbers and percentages. Due to the small sample size, numerical variables were summarized as medians with interquartile ranges (IQR; 25th and 75th percentiles). Microsoft Excel for Mac version 16.25 was used for data analysis.
3 Results
3.1 Patient characteristics
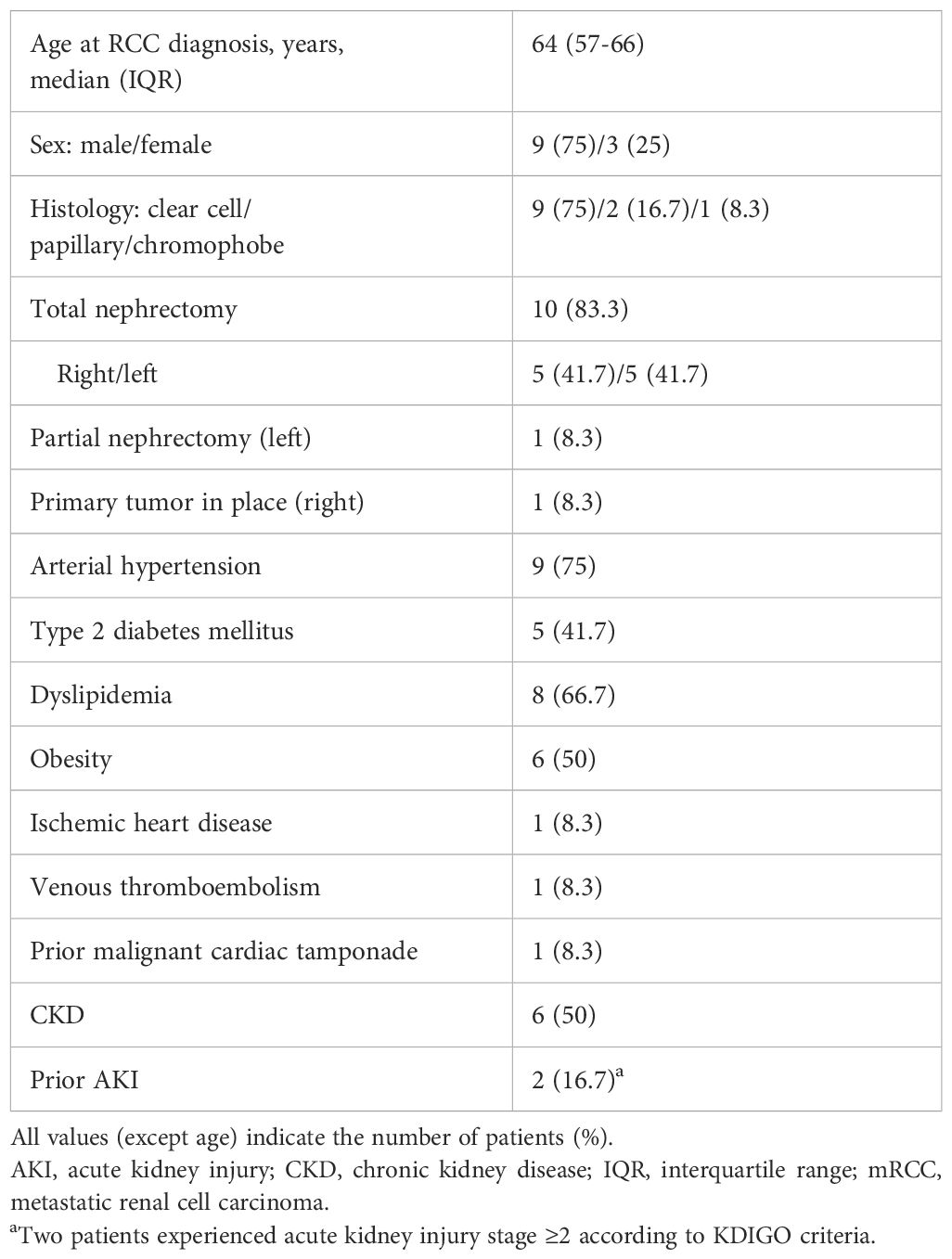
Thirteen PRBs were performed on 12 patients with mRCC and reduced nephron mass who developed AKI during SACT. Most patients (9/12) had ccRCC, and ten had previously undergone total nephrectomy. The baseline characteristics of the mRCC patients, including comorbidities, are shown in Table 1.
The median baseline estimated glomerular filtration rate (eGFR; CKD-EPI) before SACT initiation was 60.5 mL/min/1.73 m² (IQR, 42-70). One patient began SACT with an eGFR of 15 mL/min/1.73 m². Table 2 details SACT regimens, AKI characteristics at PRB, and related AEs. In six cases of PRB, patients had grade (G)2 AKI. Two patients had G3 AKI, which was related to ICIs. Four patients had G3 proteinuria, which was linked to VEGFR-TKI therapy in three patients. Six patients had proteinuria ≥1 g/day, and two had clinical evidence of nephrotic syndrome. Three patients treated with ICIs also experienced extra-renal serious AEs related either to the immunotherapy itself or to the associated therapy.
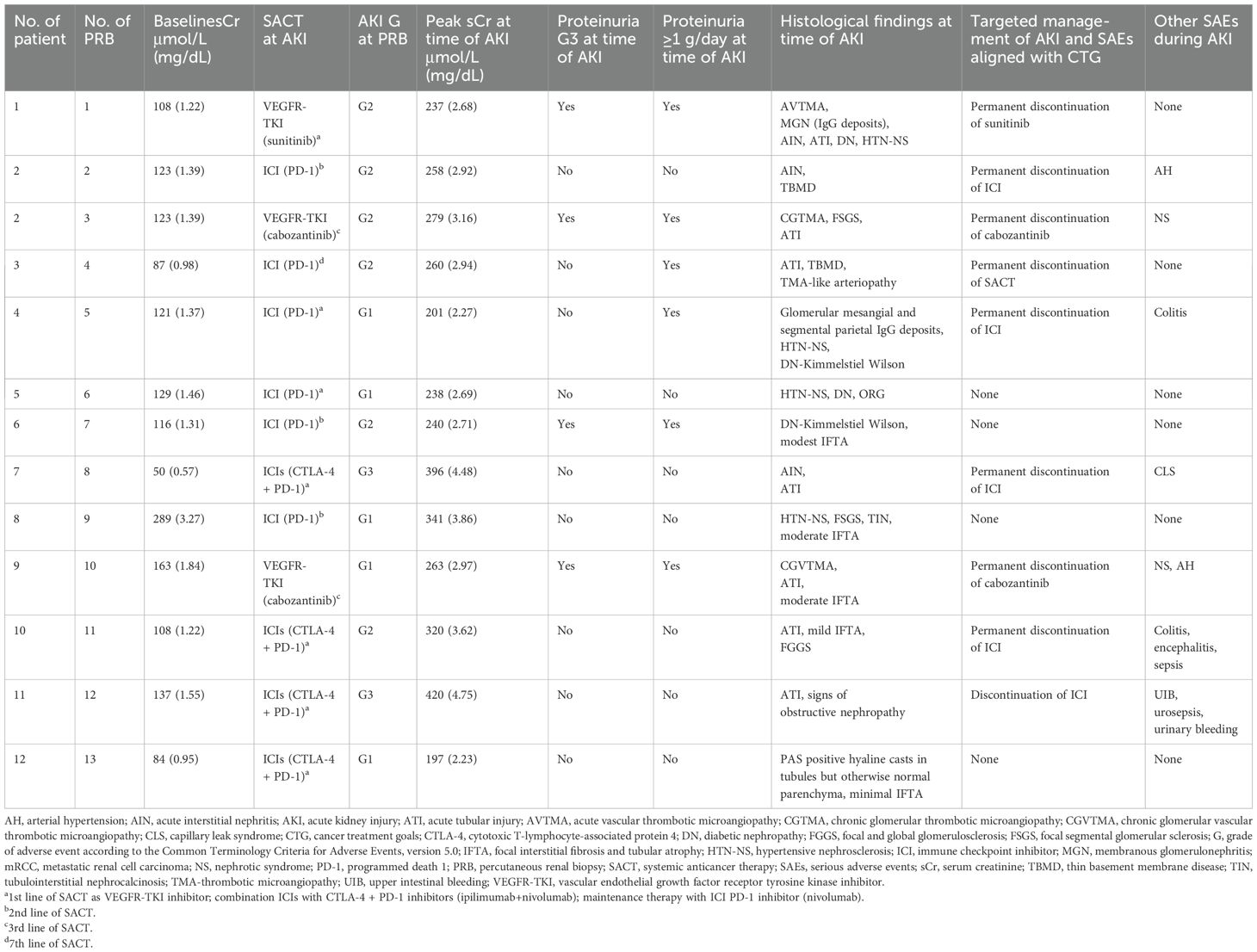
Table 2. Baseline SCr before sequential SACT, AKI-related characteristics at time of biopsy, and related measures in patients with mRCC (n=12).
The median patient age at PRB was 68 years (IQR, 60-72), the median serum creatinine was 2.17 mg/dL (IQR, 2.04-2.75), and the median hemoglobin level was 10.8 g/dL (IQR, 10.6-11.8). Platelet counts were within normal limits. One patient required a red blood cell transfusion before PRB, following standard protocol.
Table 3 displays clinical characteristics and laboratory values at PRB of mRCC patients and patients with metastatic solid malignancies. Table 4 lists medications administered prior to PRB that could increase the risk of bleeding.
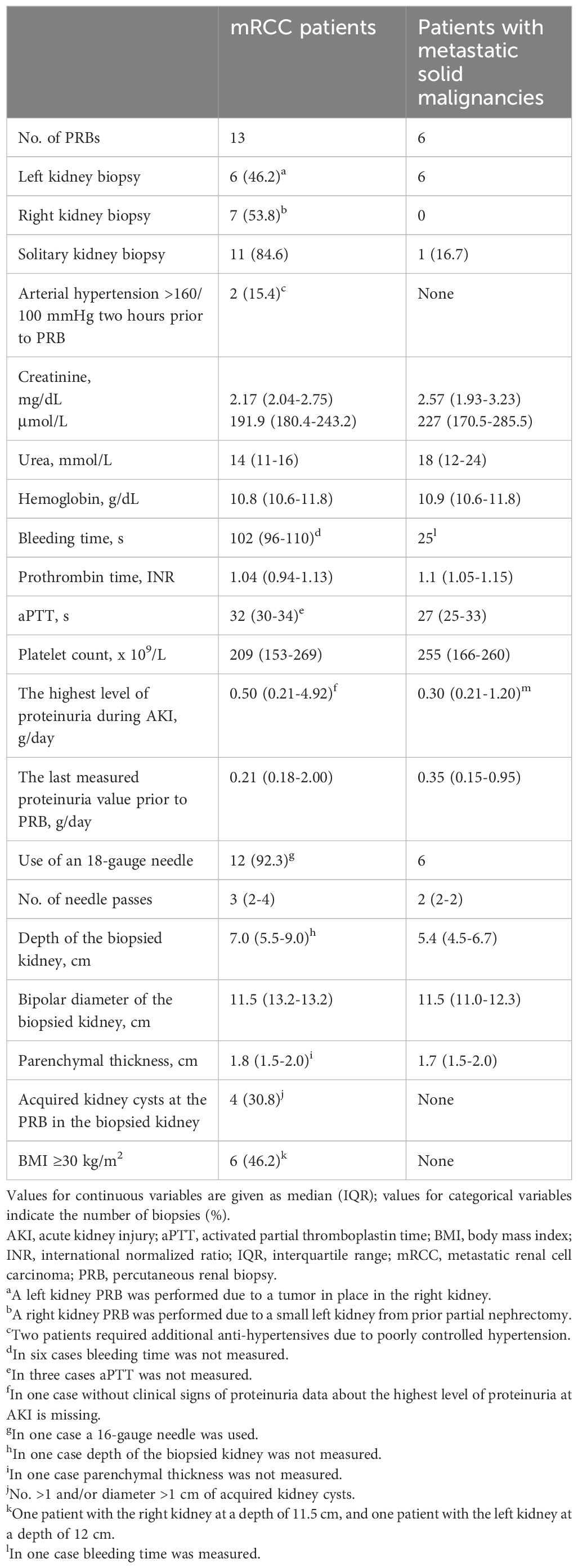
Table 3. Clinical characteristics and laboratory values at PRB in mRCC patients (n=13) and patients with metastatic solid malignancies (n=6).
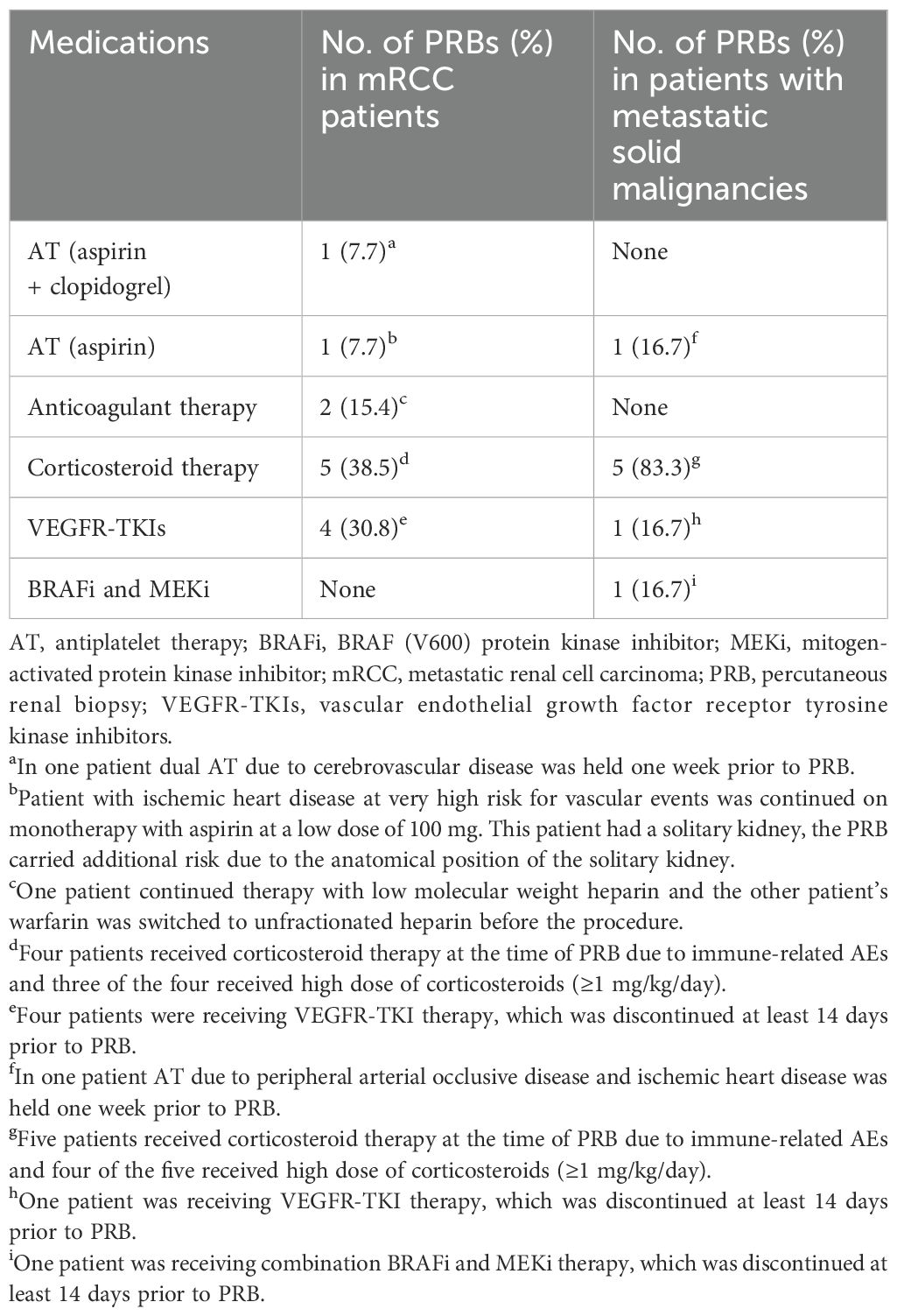
Table 4. Use of medications prior to PRB that may increase the risk of bleeding in mRCC patients (n=13) and patients with metastatic solid malignancies (n=6).
Six PRBs were performed in six patients with metastatic solid cancers who had developed AKI during SACT. Two patients had melanoma, two had urothelial cancer, one had thyroid cancer, and one had gastric cancer. Except for one patient with a SK following right nephrectomy for upper urinary tract carcinoma, none of the patients met the criteria for reduced renal mass. All patients with metastatic solid cancer began SACT with eGFR >50 mL/min/1.73 m².
Among patients with metastatic solid cancers undergoing PRB, two had G4 AKI, one of them had a SK, and both underwent acute hemodialysis prior to biopsy. Three patients experienced G3 AKI, and one patient had G2 AKI. A patient with thyroid cancer developed nephrotic syndrome during VEGFR-TKI therapy, with peak proteinuria at 6.85 g/day during G3 AKI. One patient required a red blood cell transfusion before PRB.
In the cohort of six patients with metastatic solid malignancies, the median patient age at PRB was 63 years (IQR, 53.5–65.5), the median hemoglobin level was 10.9 g/dL (IQR, 10.6–11.8), and the median platelet count was 255 x 109/L (IQR, 166-260).
3.2 Complications related to PRB
One mRCC patient with a SK and grade 3 AKI (due to ICIs) developed an arteriovenous (AV) fistula, detected by post-procedure kidney ultrasound. Seven days later, genitourinary hemorrhage caused hemodynamic instability requiring emergent AV fistula coiling and a red blood cell transfusion. The patient’s course was further complicated by upper gastrointestinal bleeding and urosepsis. Prior to biopsy, this patient had not received antithrombotic therapy but had been exposed to high-dose corticosteroids. Despite the severity of the complications, the patient fully recovered.
Another mRCC patient with a SK, grade 1 AKI and nephrotic syndrome due to cabozantinib required a red blood cell transfusion pre-biopsy, having remained on aspirin. Gross hematuria developed during the observation period, but no intervention was necessary. No other patients experienced procedure-related complications.
During the observational period, a patient with metastatic urothelial cancer, who had both kidneys, experienced a minor bleeding event after a post-procedure ultrasound revealed a 15 mm perirenal hematoma in the left kidney. However, no further measures were necessary. The patient had been exposed to high-dose corticosteroids due to AEs prior to biopsy. Antiplatelet therapy with low-dose aspirin had been discontinued seven days prior to the procedure.
4 Discussion
This study assessed PRB safety in mRCC patients with reduced nephron mass receiving SACT. This is, to our knowledge, the first safety analysis of PRB in this specific population. Although clinicians are often hesitant to perform PRB in patients with a SK, there are an increasing number of individuals with mRCC and a SK who require a comprehensive evaluation for AKI or proteinuria. Due to the incidence and prevalence of high-grade AKI in this patient population, there is a critical need for an accurate and timely biopsy evaluation to avoid a negative impact on planned treatment goals.
A cross-sectional study in Australia reported that nephrologists are less likely to perform PRBs in SK patients (19). However, limited data exist regarding PRB complications in SK patients. One prospective registry showed that eight of nine SK patients underwent successful PRB, with gross hematuria occurring in only one patient (20). A large retrospective study (n>118,000) showed a high red blood cell transfusion rate (26%), although not all complications were directly attributed to biopsy. Cohort heterogeneity regarding cancer diagnosis and staging limited meaningful subgroup analysis of SK patients (21).
AV fistula complications post-PRB vary widely (0.7-15%), though hemodynamic instability is rare. The difference in complication rates compared to other studies could be because Sosa-Barrios et al. used routine post-biopsy ultrasound and Doppler imaging by nephrologists trained in ultrasound (22). In contrast, another multicenter study showed that 26% of biopsies were performed by non-nephrologists without routine post-biopsy imaging, resulting in a 5% major complication rate and a 22% increased risk per additional needle pass. This study also suggested a protective effect of high proteinuria (odds ratio 0.95 per additional g/day) against major complications, with a median proteinuria of 2.4 g/day and 38.5% of patients exhibiting nephrotic-range proteinuria (15). In our cohort, both patients experiencing bleeding complications had two passes; one patient required four passes yet experienced no complications.
In our cohort of mRCC patients, five of 13 biopsies (38.4%) showed proteinuria ≥1 g/day; four (30.8%) had nephrotic range proteinuria; and two patients exhibited clinical evidence of nephrotic syndrome. Median proteinuria before PRB was 0.21 g/day (IQR, 0.18-2.0), and the highest median during AKI was 0.5 g/day (IQR, 0.21-4.92). The difference likely resulted from VEGFR-TKI withdrawal prior to biopsy. In the cohort of six patients with metastatic solid malignancies, the median value of proteinuria before PRB was 0.35 g/day (IQR, 0.15-0.95). Only one patient experienced proteinuria ≥1 g/day with a peak level of 6.85 g/day detected prior to PRB, along with nephrotic syndrome due to the VEGFR-TKI.
A meta-analysis showed a higher rate of red blood cell transfusions with 14-gauge (2.1%) versus 16-gauge (0.4%) or 18-gauge (0.6%) needles (13). Sixteen-gauge needles represent a reasonable compromise, and needles smaller than 16-gauge should be avoided (23). We used 18-gauge needles for all three patients with bleeding complications (two with mRCC and one with metastatic solid cancer), neither of whom had obvious procedural or anatomical risk factors.
The increased bleeding risk in cancer patients treated with certain VEGFR-TKIs (sunitinib, bevacizumab, sorafenib) necessitates careful consideration of anticoagulant risk factors (24, 25). In our cohort, VEGFR-TKI therapy was discontinued at least two weeks prior to PRB. Existing guidelines generally recommend discontinuing antithrombotic therapy 7–10 days before elective PRB, but in urgent cases or high-risk cardiovascular patients, low-dose aspirin monotherapy (≤100 mg) may be considered safe (17). One patient in our cohort, on aspirin monotherapy (100 mg), experienced gross hematuria. A meta-analysis showed no significant association between aspirin use and serious bleeding complications, although the definition of aspirin exposure varied across studies (26).
A prospective observational study of native kidney biopsies in patients with eGFR <30 mL/min/1.73 m² found a 5.6% major complication rate, excluding SK patients. Patients with eGFR <15 mL/min/1.73 m² had a significantly higher rate of hematomas ≥2 cm (27). Our cohort of mRCC patients had a median baseline eGFR of 60.5 mL/min/1.73 m² (IQR, 42-70) with one SK patient beginning SACT at 15 mL/min/1.73 m². This patient, however, experienced no complications. Among six patients with metastatic solid malignancies nobody began SACT with eGFR <50 mL/min/1.73 m².
Well-controlled hypertension does not appear to increase biopsy risk (28) while uncontrolled hypertension (≥160/100 mmHg) is a relative contraindication (17). Two mRCC patients required antihypertensive medication before PRB due to blood pressures exceeding 160/90 mmHg but did not experience post-biopsy bleeding complications.
In the cohort of mRCC patients, eight of thirteen (61.5%) biopsies were performed in patients with G2 or higher AKI (including two G3 cases), at least seven days after peak creatinine levels. In the cohort of patients with metastatic solid malignancies, two required acute hemodialysis due to AKI; one patient with SK remained on renal replacement therapy during the period of PRB. A systematic review and meta-analysis of over 100,000 native kidney biopsies showed that hospitalized patients with AKI have a higher risk of post-biopsy complications (29). A single-center study of patients with acute kidney disease, primarily AKI, also demonstrated increased risk in hospitalized patients, excluding those with renal malignancy. In that study, 8% of hospitalized patients with acute kidney disease required red blood cell transfusions, and 2% needed interventions to stop bleeding, compared to none in the non-hospitalized group (30). In our cohorts, neither patient experiencing bleeding complications was hospitalized, and all underwent elective biopsies at least seven days post AKI onset.
Historically, patients were observed for 24 hours post-biopsy to monitor for procedure-related complications (31). An earlier study showed that 77% of complications were apparent by eight hours and 100% of serious complications by 24 hours (32). While this study used real-time ultrasound, not all used automated biopsy needles (32). In his retrospective analysis, Abuelo recommended a 6–8-hour observation period while other studies suggest 8–12 hours for uncomplicated biopsies, but 24 hours for high-risk patients or those living far from a hospital (17, 33).
Based on our findings and previous studies, we recommend 24-hour post-biopsy observation for this specific patient population. Routine post-biopsy ultrasound is also recommended. The patient with a major complication (hemodynamically significant AV fistula) had no other significant risk factors. The patient with gross hematuria was on low-dose aspirin monotherapy due to cardiovascular risk. In a patient with small perinephric hematoma, low-dose heparin monotherapy due to ischemic heart disease and peripheral artery disease was withdrawn seven days before the PRB. While some studies suggest an increased risk of complications with aspirin use, this is not consistently observed (17, 26).
Our retrospective analysis has several strengths. Chart review and direct AE management allowed assessment of the causal relationship between biopsy and complications. The homogeneous studied cohort (mRCC, reduced nephron mass, AKI during SACT) allowed meaningful comparison to other similar studies, though the small sample size limits generalizability.
5 Conclusions
Renal biopsy is essential when histopathology could significantly impact treatment, but it is challenging in SK patients due to potential complications. This study, using a homogeneous mRCC cohort in a real-world clinical setting, reveals a potential link between PRB and safety outcomes. Given the lack of safety data, a longer post-PRB observation period (24 hours) is prudent for mRCC patients with reduced nephron mass and AKI receiving SACT. Patients should be fully informed about the limited safety data. Due to high survival rates in mRCC patients, the associated nephrotoxic risks of SACT, and the potential for biopsy-related complications, large prospective observational studies are needed. Consensus guidelines for PRB in this patient population are crucial to standardize care and improve outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institute of Oncology Ljubljana. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The study is a retrospective analysis.
Author contributions
TM: Conceptualization, Methodology, Investigation, Project administration, Formal analysis, Writing – original draft, Writing – review & editing. VS: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. VP: Investigation, Writing – review & editing. MA: Investigation, Writing – review & editing. JO: Investigation, Writing – review & editing. EJ: Supervision, Writing – original draft, Writing – review & editing.
Funding
The authors declare no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Aleša Rakar, MD, MSC, for her assistance with data interpretation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Perazella MA. Kidney biopsy should be performed to document the cause of immune checkpoint inhibitor–associated acute kidney injury: Commentary. Kidney360. (2020) 1:166–8. doi: 10.34067/kid.0001072019
2. Moss EM and Perazella MA. The role of kidney biopsy in immune checkpoint inhibitor nephrotoxicity. Front Med (Lausanne). (2022) 9:964335. doi: 10.3389/fmed.2022.964335
3. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. (2019) 380:1116–27. doi: 10.1056/NEJMoa1816714
4. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. (2018) 378:1277–90. doi: 10.1056/NEJMoa1712126
5. Powles T, Burotto M, Escudier B, Apolo AB, Bourlon MT, Shah AY, et al. Nivolumab plus cabozantinib versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended follow-up from the phase III randomised CheckMate 9ER trial. ESMO Open. (2024) 9:102994. doi: 10.1016/j.esmoop.2024.102994
6. Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. (2021) 384:1289–300. doi: 10.1056/NEJMoa2035716
7. Dragomir A, Nazha S, Tanguay S, Breau RH, Bhindi B, Rendon RA, et al. Outcomes of cytoreductive nephrectomy for patients with metastatic renal cell carcinoma: real world data from Canadian centers. Eur Urol Focus. (2022) 8:1703–10. doi: 10.1016/j.euf.2021.10.004
8. Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang YH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. (2021) 385:683–94. doi: 10.1056/NEJMoa2106391
9. Motzer RJ, McDermott DF, Escudier B, Burotto M, Choueiri TK, Hammers HJ, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. (2022) 128:2085–97. doi: 10.1002/cncr.34180
10. Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, et al. Overall survival with adjuvant pembrolizumab in renal-cell carcinoma. N Engl J Med. (2024) 390:1359–71. doi: 10.1056/NEJMoa2312695
11. Verheijden RJ, van Eijs MJM, May AM, van Wijk F, and Suijkerbuijk KPM. Immunosuppression for immune-related adverse events during checkpoint inhibition: an intricate balance. NPJ Precis Oncol. (2023) 7:41. doi: 10.1038/s41698-023-00380-1
12. Taliercio JJ, McGuire M, and Poggio ED. Biopsying diabetics … How risky is it? Kidney Int Rep. (2021) 7:149–51. doi: 10.1016/j.ekir.2021.12.026
13. Corapi KM, Chen JL, Balk EM, and Gordon CE. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis. (2012) 60:62–73. doi: 10.1053/j.ajkd.2012.02.330
14. Pombas B, Rodríguez E, Sánchez J, Radosevic A, Gimeno J, Busto M, et al. Risk factors associated with major complications after ultrasound-guided percutaneous renal biopsy of native kidneys. Kidney Blood Press Res. (2020) 45:122–30. doi: 10.1159/000504544
15. Andrulli S, Rossini M, Gigliotti G, La Manna G, Feriozzi S, Aucella F, et al. The risks associated with percutaneous native kidney biopsies: a prospective study. Nephrol Dial Transpl. (2023) 38:655–63. doi: 10.1093/ndt/gfac177
16. Anpalahan A, Malacova E, Hegerty K, Malett A, Ranganathan D, Healy HG, et al. Bleeding complications of percutaneous kidney biopsy: Does gender matter? Kidney360. (2021) 2:1308–12. doi: 10.34067/KID.0002432021
17. Hogan JJ, Mocanu M, and Berns JS. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol. (2016) 11:354–62. doi: 10.2215/CJN.05750515
18. Lees JS, McQuarrie EP, Mordi N, Geddes CC, Fox JG, and Mackinnon B. Risk factors for bleeding complications after nephrologist-performed native renal biopsy. Clin Kidney J. (2017) 10:573–7. doi: 10.1093/ckj/sfx012
19. Burke JP, Pham T, May S, Okano S, Ratanjee SK, Thet Z, et al. Kidney biopsy practice amongst Australasian nephrologists. BMC Nephrol. (2021) 22:291. doi: 10.1186/s12882-021-02505-9
20. Mendelssohn DC and Cole EH. Outcomes of percutaneous kidney biopsy, including those of solitary native kidneys. Am J Kidney Dis. (1995) 26:580–5. doi: 10.1016/0272-6386(95)90592-8
21. Al Turk AA, Estiverne C, Agrawal PR, and Michaud JM. Trends and outcomes of the use of percutaneous native kidney biopsy in the United States: 5-year data analysis of the Nationwide Inpatient Sample. Clin Kidney J. (2018) 11:330–6. doi: 10.1093/ckj/sfx102
22. Sosa-Barrios RH, Burguera V, Rodriguez-Mendiola N, Galeano C, Elias S, Ruiz-Roso G, et al. Arteriovenous fistulae after renal biopsy: diagnosis and outcomes using Doppler ultrasound assessment. BMC Nephrol. (2017) 18:365. doi: 10.1186/s12882-017-0786-0
23. Schnuelle P. Renal biopsy for diagnosis in kidney disease: indication, technique, and safety. J Clin Med. (2023) 12:6424. doi: 10.3390/jcm12196424
24. Je Y, Schutz FA, and Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol. (2009) 10:967–74. doi: 10.1016/S1470-2045(09)70222-0
25. Watson N and Al-Samkari H. Thrombotic and bleeding risk of angiogenesis inhibitors in patients with and without Malignancy. J Thromb Haemost. (2021) 19:1852–63. doi: 10.1111/jth.15354
26. Relvas M, Gonçalves J, Castro I, Diniz H, Mendonça L, and Coentrão L. Effects of aspirin on kidney biopsy bleeding complications: a systematic review and meta-analysis (PROSPERO 2021 CRD42021261005). Kidney360. (2023) 4:700–10. doi: 10.34067/KID.0000000000000091
27. Asad RA, Valson AT, Kavitha V, Korula A, Eapen A, Rebekah G, et al. Safety and utility of kidney biopsy in patients with estimated glomerular filtration rate < 30 ml/min/1.73 m2. Nephrol (Carlton). (2021) 26:659–68. doi: 10.1111/nep.13879
28. Peters B, Nasic S, and Segelmark M. Clinical parameters predicting complications in native kidney biopsies. Clin Kidney J. (2019) 13:654–9. doi: 10.1093/ckj/sfz132
29. Poggio ED, McClelland RL, Blank KN, Hansen S, Bansal S, Bomback AS, et al. Systematic review and meta-analysis of native kidney biopsy complications. Clin J Am Soc Nephrol. (2020) 15:1595–602. doi: 10.2215/CJN.18391120
30. Moledina DG, Luciano RL, Kukova L, Chan L, Saha A, Nadkarni G, et al. Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol. (2018) 13:1633–40. doi: 10.2215/CJN.04910418
31. Whittier WL and Korbet SM. Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol. (2004) 15:142–7. doi: 10.1097/01.asn.0000102472.37947.14
32. Marwah DS and Korbet SM. Timing of complications in percutaneous renal biopsy: what is the optimal period of observation? Am J Kidney Dis. (1996) 28:47–52. doi: 10.1016/s0272-6386(96)90129-8
Keywords: acute kidney injury, complications, renal biopsy, renal cell carcinoma, solitary kidney
Citation: Milanez T, Srinivasan V, Premru V, Arnol M, Ocvirk J and Jaimes EA (2025) The safety of percutaneous renal biopsy for acute kidney injury in metastatic renal cell cancer patients with reduced nephron mass. Front. Nephrol. 5:1615779. doi: 10.3389/fneph.2025.1615779
Received: 21 April 2025; Accepted: 14 July 2025;
Published: 06 August 2025.
Edited by:
Jan T. Kielstein, Braunschweig Hospital, GermanyReviewed by:
Amelie Gienapp, Braunschweig Hospital, GermanyHeike Kielstein, Martin Luther University of Halle-Wittenberg, Germany
Copyright © 2025 Milanez, Srinivasan, Premru, Arnol, Ocvirk and Jaimes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomaz Milanez, dG1pbGFuZXpAb25rby1pLnNp
 Tomaz Milanez
Tomaz Milanez Vinay Srinivasan3
Vinay Srinivasan3 Miha Arnol
Miha Arnol Edgar A. Jaimes
Edgar A. Jaimes