- 1Department of Renal Medicine, Raja Isteri Pengiran Anak Saleha Hospital, Bandar Seri Begawan, Brunei
- 2PAPRSAB Institute of Health Sciences, Universiti Brunei Darussalam, Bandar Seri Begawan, Brunei
- 3Department of Renal Medicine, King Fahad General Hospital, Jeddah, Saudi Arabia
- 4Department of Metabolism, Digestion and Reproduction, Imperial College, London, United Kingdom
- 5Imperial College Healthcare NHS Trust, London, United Kingdom
Continuous glucose monitoring (CGM) is used more frequently among patients with chronic kidney disease (CKD), including those undergoing haemodialysis and peritoneal dialysis. However, there is a lack of information and evidence regarding CGM use in kidney transplantation (KT). Dysglycaemia is commonly observed in the transplant setting; often complicated by impaired kidney function with fluctuating glomerular filtration rates and competing influences of diabetogenic immunosuppressants, perioperative surgical stress and transplant-related complications. This narrative review, the first of its kind, examines the utility, accuracy, efficacy and clinical outcomes of CGM in KT patients. It also addresses specific transplant-related issues that may necessitate future CGM usage and highlights knowledge gaps to inform future research directions.
Background
Globally, diabetes mellitus (DM) is the most prevalent cause of chronic kidney disease (CKD) and end stage kidney disease (ESKD) (1). Correspondingly, approximately 20-40% of all patients with DM will be affected by Diabetic Kidney Disease (DKD) (2). The incidence and prevalence of DM-related ESKD are increasing annually, in parallel with trends of DM; likely through the effects of urbanization (3). Amongst kidney transplant (KT) patients, the incidence of post-transplant diabetes mellitus (PTDM) ranges from 15-30% (4). Control of DM in CKD patients is often challenging due to the complex interplay between insulin metabolism and aberrant renal gluconeogenesis through metabolic acidosis, uraemia and chronic inflammation (5). In KT recipients, glycaemic control is further complicated by additional factors such as the diabetogenic effects of immunosuppressive therapy, surgical stress, and an increased susceptibility to infections.
Traditionally, glycated haemoglobin (HbA1c) is often regarded as the yardstick measure for long-term glycaemic control, but is fraught with intricacies regarding applicability and accuracy within the CKD population due to altered red blood cell turnover and anaemia (4). Furthermore, HbA1c provides no information about the frequency and the burden of hypoglycaemia or glucose variability. The Dialysis Outcomes and Practice Pattern Study (DOPPS) reported a U-shaped relationship between HbA1c and all-cause mortality, with increased risk of mortalities by 38% and 21% in dialysis patients with HbA1c >9% and < 7%, respectively, compared to patients with HbA1c of 7-8% (6). The 2022 Kidney Disease Improving Global Outcome (KDIGO) guideline recommended an optimal HbA1c range between 6.5-8.0% for non-dialysis patients with DKD, with higher HbA1c targets for more advanced stages of CKD (7). For KT patients with diabetes, the recommended target HbA1c is 7.0-7.5% and to specifically avoid Hba1c of < 6.0%, especially in patients with advanced CKD stages of KT (8). However, HbA1c levels can be less reliable in KT recipients, especially in the early post-transplant period (typically first three months), due to immediate changes in red blood cell turnover and dynamic fluid shift post-transplant. While HbA1c remains a valuable tool for long-term glycaemic assessment, its interpretation in this specific context requires caution, and alternative methods like continuous glucose monitoring (CGM) might be considered.
Continuous Glucose Monitoring (CGM) has revolutionized the surveillance and management of DM over the past decade (9). The use of CGM in people with insulin-treated diabetes has been shown to improve HbA1c, increase time spent in the target glucose range of 3.9 to 10 mmol/L, reduce risk of hypoglycaemia, and improve patient-reported outcome measures (10, 11). A recent meta-analysis encompassing 26 randomized controlled trials demonstrated that the use of CGM was associated with significant improvements in HbA1c levels and reductions in glycaemic medication requirements among patients with type 2 diabetes mellitus (T2DM) (12). Nonetheless, several caveats persist, including uncertainties regarding CGM accuracy due to discrepancies between interstitial and plasma glucose measurements, the influence of extreme temperatures and altered tissue perfusion, as well as potential complications related to sensor insertion, such as local inflammation and infection. A key advantage of CGM in CKD patients is the better assessment of true glycaemia control beyond the accepted paradigm of HbA1c and fasting blood sugar (FBS). CGM -metrics like time in range (TIR), time below range (TBR), time above range (TAR), glucose variability (GV), coefficient variation (CV), and glucose management indicator (GMI) provide a better overall assessment of glycaemia, particularly in situations when glycaemic control is unstable and dynamic. The recent KDIGO clinical practice guideline has recommended CGM to guide self-management when HbA1c is discordant with measured readings or to help prevent hypoglycaemia through predictive trends (7).
Previous reviews have focused on CGM research in CKD patients, including haemodialysis (HD) and peritoneal dialysis (PD) patients (13–15). A recent consensus report on CGM in CKD identified three main goals of management: encourage greater use of CGM in CKD patients, evaluate usage in ESKD patients and allow equitable access (16). The report also acknowledged that there is limited literature on CGM usage in KT, with recommendations to determine the accuracy of CGM and its impact on PTDM (16). This is the first-ever review on CGM usage in KT patients. The objectives of this review are to evaluate the benefits of CGM in KT patients with diabetes, discuss unique transplant issues that render the utility of CGM and identify knowledge gaps that may guide future research directions. In addition, it also enables the evaluation of CGM evidence in selected CKD and non-KT populations and extrapolate its applicability to KT patients.
Challenges of glycaemic control in advanced CKD and transplant patients
Management of glycaemic control in CKD patients can be challenging with a high risk of hypoglycaemia and hyperglycaemia. HbA1c assessment is often influenced by anaemia, use of erythropoiesis agents and chronic inflammation (15). Other surrogate glycaemic markers like glycated albumin and fructosamine are affected by uraemia and abnormal albumin metabolism (15). Assessment of glycaemic control in the dialysis population is also complicated by unpredictable daily fluid volume shift, dialysers’ removal of drug and glucose and exposure to glucose-containing dialysate (5).
Many pharmacological, biochemical and surgical factors can influence dysglycaemia in KT. Glycaemic assessment using HbA1c may not accurately reflect the true glycaemic burden during the early post-transplant period (typically the first three months) due to several confounding factors, including recent blood transfusions, increased endogenous erythropoietin production from the newly functioning graft, fluctuating glomerular filtration rates, and the use of high-dose corticosteroids and calcineurin inhibitors (CNI) (17). The correlation between actual glucose levels and HbA1c measurements generally improves beyond the first three months post-transplant, coinciding with stabilization of graft function and reduced variability in haematologic and glycaemic parameters.
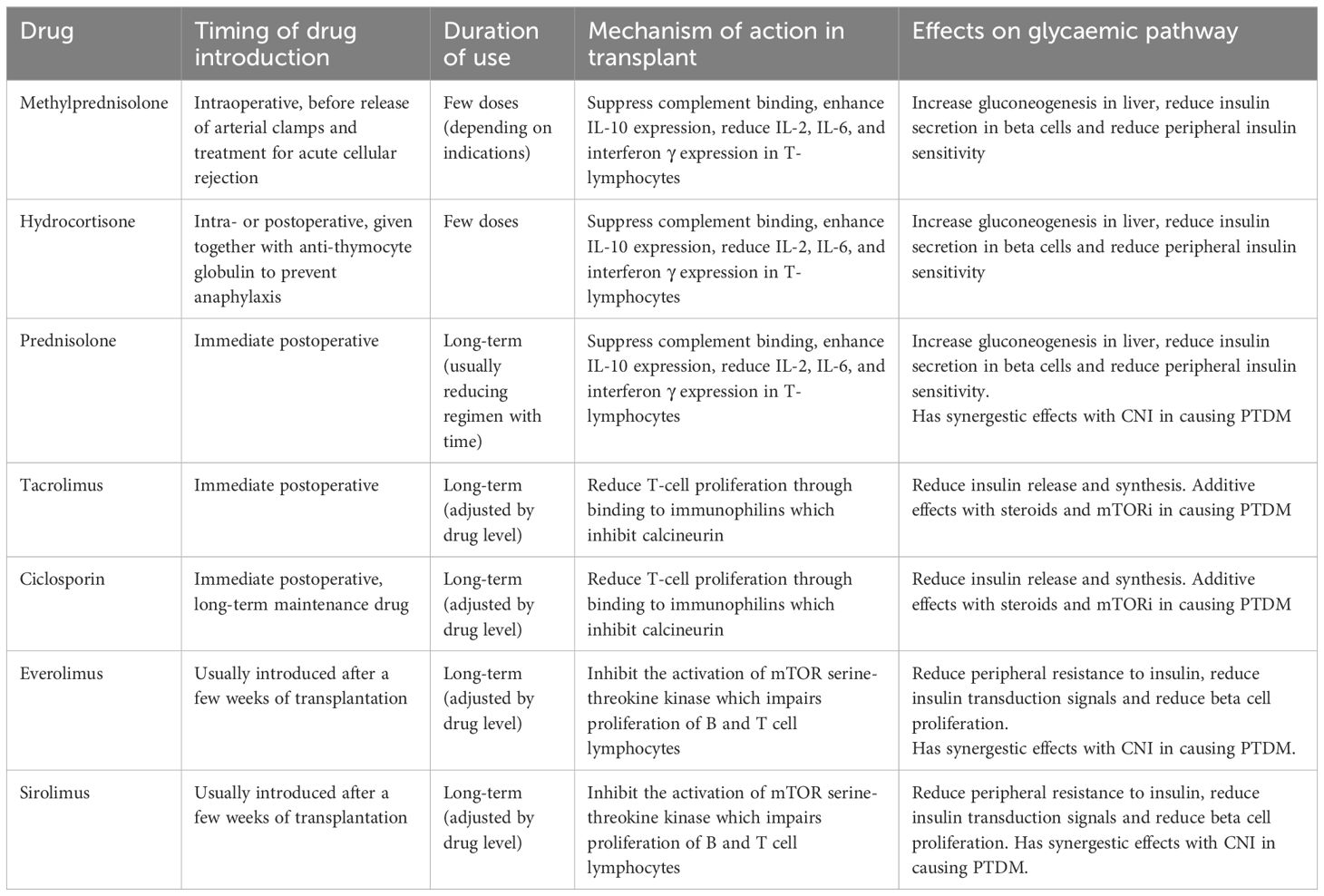
In experimental studies with mice, the administration of tacrolimus is associated with immediate impaired oral glucose tolerance test response, which indicate a propensity for postprandial hyperglycaemia (17). Methylprednisolone and prednisolone have a peak transient glycaemic action of 4–6 hours after administration, through the development of insulin resistance (18). Treatment with CNI and SGLT-2 may also predispose patients to diabetic ketoacidosis (DKA), even in those without a prior diagnosis of DM (19). Mammalian target of rapamycin inhibitor (mTORi), like everolimus and sirolimus, reduces peripheral resistance to insulin and insulin transduction signals leading to long-term dysregulated glucose control (20). The combination of CNI and mTORi is known to have a potent synergetic effect in the development of PTDM, compared to monotherapy (21). Similarly, steroids with CNI are also observed to have enhanced diabetogenic effects on patients (22). Table 1 summarises the effects of transplant medications on glycaemic control.
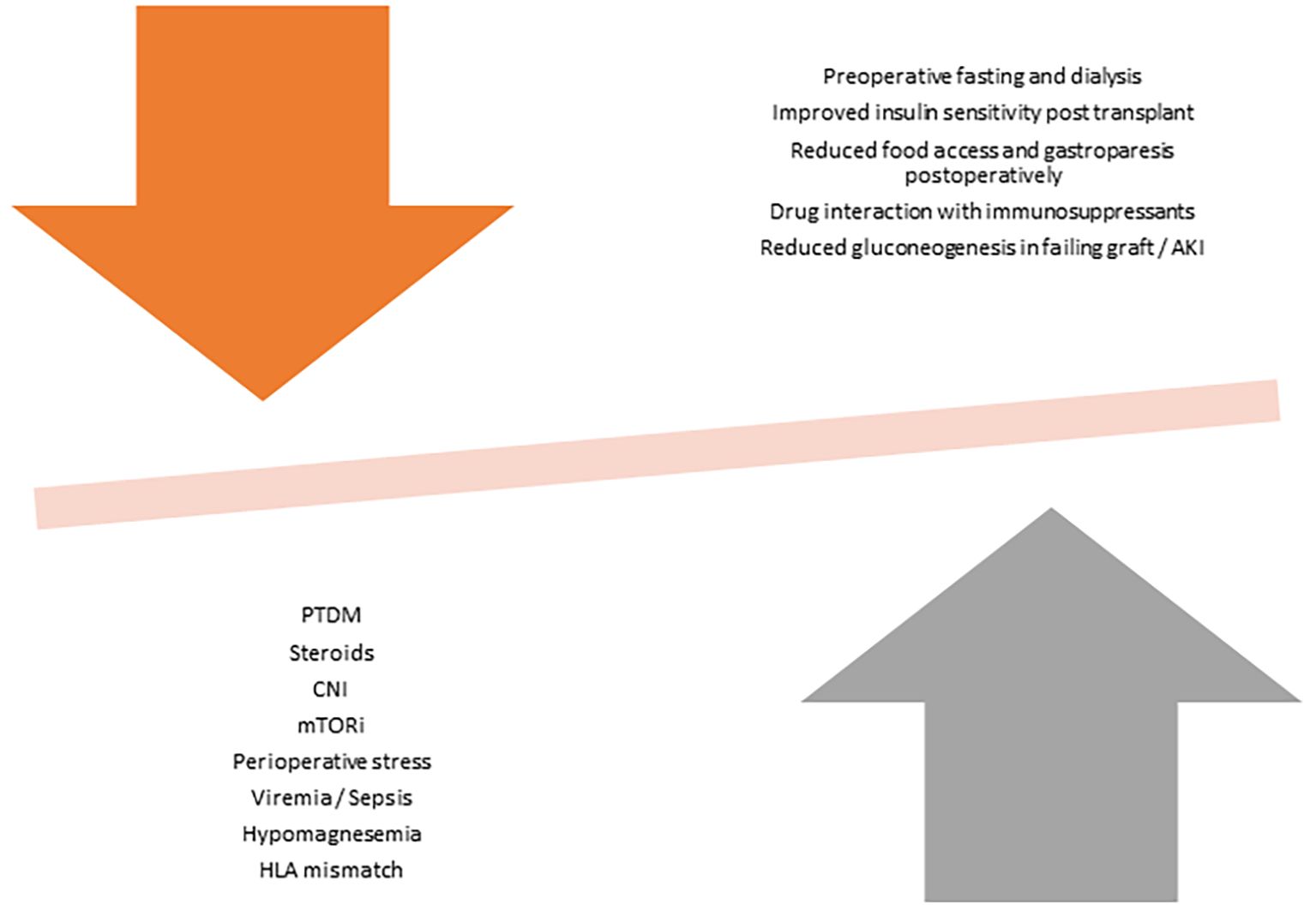
Non-pharmacological factors like surgical stress and pain can cause hyperglycaemia through secretion of cytokines and stress hormones that can affect insulin function (23). Improvement of kidney function in the post-operative phase improves insulin clearance, which may unmask preexisting hyperglycaemic tendencies (23). Acute or chronic hypomagnesemia, often seen in KT patients, has also been identified as an independent risk factor for PTDM through its role as an intracellular cofactor for glucose transport between membranes, glucose oxidation and insulin-mediated tyrosine kinase pathways (24). Asymptomatic Cytomegalovirus infection can cause impaired beta cell antiviral defence in the pancreas and increase risk of PTDM (25). Other transplant-related factors that increases risk of PTDM include male gender, HLA matching characteristics, hepatitis C infections and deceased donor kidney (16). Figure 1 demonstrate the competing influences of KT-related factors on hyperglycaemia and hypoglycaemia.
Use of CGM in advanced CKD (including transplant) and dialysis
The shortcomings of HbA1c as a glycaemic indicator in CKD patients provide opportunities for CGM to improve and complement existing measures. Amongst patients with ‘burnt-out’ diabetes in ESKD, CGM is reported to be superior to Hba1c and fructosamine in demonstrating undiagnosed hyperglycaemia and GV (26). The correlation between HbA1c and CGM metrics decreases with advanced stages of CKD (27), showing a good relationship between HbA1c and TAR and TIR, but not with TBR and hypoglycaemia (15). Glycaemic variability (GV), which has been shown to influence long-term adverse microvascular outcomes (28), has a poor correlation with HbA1c in both CKD (15) and non-CKD (29) populations. International consensus recommends that clinical targets for CGM data interpretation for high-risk patients include > 50% TIR (target range 3.9–10 mmol/l), < 1% TBR (< 3.9 mmol/l), and <10% TAR (>13.9 mmol/l) (30). However, these targets have not been validated in patients with CKD, and long-term hard outcomes data are still unavailable (16).
CGM devices measure glucose readings in interstitial fluid through an electrochemical reaction in the filament sensor located in subcutaneous tissues, which is determined by the dynamic gradient across the blood-interstitial barrier (31). Chronic hypoxia and anaemia in CKD patients interfere with the electrochemical sensing of the oxidase-peroxidase reaction and may explain the elevated Mean Absolute Relative Difference (MARD) values between CGM and capillary blood glucose readings (32, 33). Early sensors routinely had a %MARD greater than 20% but most commercially available sensors in people without advanced CKD or undergoing dialysis now have a %MARD lower than 10%, which indicates significantly improved sensor accuracy (34). Additionally, endogenous substances like urea and uric acid, severe metabolic acidosis and extreme fluid overload are known to interfere with the glucose oxidase reaction in glucometers, may also affect sensor performance (35). MARD values also differ with different venous and capillary comparator analysers: Freestyle Libre 3 (ranges between 9.5-11.6%), Dexcom G7 (9.9-12%) and Medtronic (11.6-16.6%) (36, 37). Dexcom G6 and Freestyle Libre 2 also show excellent correlations between improvements in TIR and Hba1c (10). The UK Diabetes Specialist Nurse Forum has also published detailed technical and accuracy data in their website to facilitate updated comparisons of currently available CGM devices (38).
Amongst patients with advanced kidney impairment on PD, CGM correctly identified 96.5% of hyperglycaemic and 60% of hypoglycaemic events, with alarm detection rates of 94.9% and 100%, respectively. Further, authors found no significant correlations between MARD and BMI, extracellular water, relative hydration index, lean or fat mass, or haemoglobin levels (39). Additionally, de Boer et al. reported that CGM frequently identified hypoglycaemia and hyperglycaemia that may not be clinically evident in dialysis patients (40). A recent study evaluated the accuracy of the Dexcom G6 continuous glucose monitoring (CGM) system in hospitalised patients with diabetes (n=31) undergoing maintenance HD (41). The MARD was approximately 20% overall, with higher values observed during HD sessions (22.0%) compared to non-HD periods (18.2%). In a prospective study, Genua et al. assessed the accuracy of the FreeStyle Libre (FSL) Generation 1 intermittently scanned glucose monitoring system in patients with diabetes mellitus (n=16) undergoing maintenance haemodialysis and reported that the global MARD was 23%, increasing to 29% during HD sessions (42). Avari et al. evaluated the accuracy of two CGM systems, Dexcom G6 and Abbott Freestyle Libre 1, in 40 adults with diabetes undergoing haemodialysis (43). The overall MARD was 22.7% for Dexcom G6 and 11.3% for Freestyle Libre 1. Further, authors recently investigated the accuracy of the latest generation Dexcom G7 CGM system in adults with diabetes undergoing HD (44). Compared to laboratory measurements, the overall MARD for Dexcom G7 was 10.4% (44). Zhang et al, in a meta-analysis, acknowledges that CGM provides valuable insights into glycaemic variability and control, but robust evidence from large, well-designed randomized controlled trials remains limited and interpretation of CGM-derived data in CKD patients should be approached with caution due to potential alterations in glucose metabolism and device accuracy in this population (13).
Kidney transplant donors
Transplant physicians often prevaricate about the ethics of kidney donation in high-risk individuals with DM, prediabetes, family history of DM and gestational DM. The lack of clarity in guidelines have perpetuated concerns about ethics of such practice, given that donors will already have a small risk of kidney disease progression with a singleton kidney. The limitations of current measures to predict future DM or DM-related complications have compounded the concerns about donor selection. International renal guidelines do not have consensus views, and permission to donate are usually left to the discretion of clinicians and local ethics committees. Position statements from international societies are often imprecise and vague. The Organ Procurement and Transplantation Network (OPTN) recently amended policy to allow kidney donations from living donors with DM in 2022 (45). Other leading guidelines made nebulous references to donation from diabetic donors: “only under exceptional circumstances’ (European Best Practice Guidelines) (46), “after a thorough assessment of the lifetime risk.” (British Transplantation Society) (47), “individualization to allow for the consideration of very low-risk individuals with Type 2 DM (T2DM)’ (KDIGO) (48). Their positions on prediabetes are also contentious: “impaired glucose tolerance is not an absolute contraindication to donation” (European Best Practice Guidelines) (46), “individualized based on demographic and health profile in relation to transplant program’s acceptable risk threshold” (KDIGO) (48). The current OPTN guideline enlists eligibility criteria for donation from preexisting type 2 DM patients, where the only objective measure of glycaemic control is “Hba1c < 7% on at least three occasions within the past 2 years” Additionally, for high-risk individuals, including prediabetes, history of gestational diabetes and first-degree relatives of diabetics, GTT or Hba1c is the only required test to justify donation eligibility (45).
Given the potential consequence of developing DM and kidney failure with solitary kidney, there should arguably be more rigorous measures to screen donors. In a 10-year follow up study, Chandran et al. showed that risk of prediabetics developing DM was higher in kidney donors compared to healthy controls (15.6% vs 2.2%) (49). Independent to DM risks, prediabetic patients are also prone to glomerular hyperfiltration (50) and development of microalbuminuria (51), although overt risk in developing CKD have not been elucidated. However, for context, a previous study involving 8280 donors with 1826 with impaired fasting glucose at the time of donation, there was no difference in patient survival and kidney failure rates in the two groups of patients (52).
The use of CGM as a screening and predictive tool for development of future DM could be valuable in the donor selection process. CGM can display GV through graphical display and project progression from prediabetes to advanced diabetes, through analysis of CV and TIR (53). Important salient features that can also be detected by CGM, which can predict onset of T2DM in prediabetic patients, are postprandial hyperglycaemia and overnight rise in blood glucose (dawn phenomenon) (54). When used in tandem with demographic factors, CGM metrics can also detect disease progression and classifying risk of Type 1 DM (T1DM), potentially defining eligibility for prevention trials (55). Machine learning technology can also enhance detection of T1DM in high-risk individuals through self-administered CGM home testing (56). Similarly, predictive potential for T2DM can be harnessed through CGM-generated glucose curves to predict muscle-insulin-resistance and β-cell-deficiency sub-phenotypes and stratify individuals with glucose dysregulation (57). Research have also highlighted that CGM can enhance self-monitoring behaviour and increase exercise adherence in prediabetics (58), which would support a preventative role for CGM in such donors.
Preoperative, Intraoperative and intensive care usage of CGM
CGM experience within the preoperative, intraoperative and critical care phase of KT is limited. However, evidence from non-CGM studies have indicated benefits of intensified glycaemic control on outcome measures in both pre- and intra-operative phase. Amongst 2872 diabetic patients who underwent KT, poor pretransplant glycaemic control (through HbA1c measurements) was associated with decreased patient survival (59). Another study with 832 patients admitted for solid organ transplantation (561 KT and 12 KT with other organs), elevated admission glucose level amongst non-DM patients was associated with increased 30-days mortality (60). Amongst 680 liver transplantation patients, intraoperative hyperglycaemia was independently associated with postoperative infection and mortality (61).
Amongst studies utilizing CGM in KT, Hagerf et al. reported the use of CGM in 65 critically ill patients after abdominal surgery (13 of which had concomitant kidney transplant surgery with pancreas, islet cells and liver transplant) through an infraclavicular sensor placement. The study showed that CGM was safe, feasible and reliable, with promising potential to improve glycaemic management (62). Another study assessing usage of CGM in prediabetes patients during elective abdominal surgery showed acceptable and consistent accuracy during the operation with MARD of 12.7% compared to reference capillary glucose readings. Amongst recent KT patients, in a randomized controlled trial, who were intensively treated with intravenous insulin regimen versus standard subcutaneous insulin regimen, the former group had less delayed graft function (18% vs 24%), although the results were not significant (63). A prospective randomized controlled trial to evaluate the efficacy of intraoperative CGM in predicting adverse outcomes in liver transplantation is currently being conducted (64), but a previous study on liver transplantation have reported that incidence of post-operative graft dysfunction was higher in recipients with intraoperative CV of > 28% than in recipients in low CV (10.8% vs 0%, p < 0.05) (65). In cardiac surgery, Rangasami et al. demonstrated that GV through measurements of CV and blood glucose risk index values have negative prognostic outcomes for major adverse events (66).
A review of 22 studies reported high technical reliability within the intraoperative setting but highlighted concerns about accuracy (67). Perez-Gulman et al. reported electrocautery interferences, common patterns of signal loss and negative bias during surgery; but within intensive care setting, CGM use was helpful to guide insulin delivery when regular point of care tests were impractical especially in situations when hourly POC tests were required for decision-making in the sickest patients that required continuous insulin infusion (68). Mannitol, used intraoperatively as osmotic diuretic and transurethral irrigation fluids, was identified as possible interferent for the Eversense CGM sensor to falsely high readings (69).
Postoperative usage of CGM
Hyperglycaemia occurs in about 80-90% of patients in the first days to week following KT (70). Many non-CGM studies have acknowledged that this pattern is associated with increased rates of acute rejections, high infection rates and mortality (71). Early post-transplant hyperglycaemia (within the first 45 days), is an independent risk factor for hospital readmissions, worst graft function, acute rejection and future PTDM development (72, 73). Whilst immediate poor glycaemic control was associated with poor graft outcomes, strict glycaemic control (HbA1c < 7.7%) may conversely also be detrimental (74). The need to regulate glycaemic control over a tight therapeutic range lends towards the application of rigorous CGM metrics to complement existing measures for postoperative surveillance.
Studies utilizing CGM metrics have already demonstrated interesting patterns beyond traditional HbA1c-centric studies. In a randomized controlled trial, Jandowitz et al. in 2023 showed that patients on CGM have significantly lower rates hyperglycaemic (≥ 10mmol/l) episodes and median glucose levels without increasing the number of hypoglycaemic (≤ 4.4 mmol/l) episodes, compared to those with finger-stick glucose monitoring in the first 5 days of transplant (75). Shin et al, using CGM pre- and post-operatively in KT patients for two weeks, showed that CGM has superior predictive potential for PTDM over capillary blood glucose monitoring (76). Male patients with a higher postoperative TAR of 10mmol/l were also identified as a significant risk factor (66). Another study, specifically in non-diabetic patients, demonstrated that hyperglycaemia is a constant feature in the immediate post-operative phase, with 19% of patients developing PTDM after a mean follow up of 72 months (77).
Comparing kidney and liver transplant patients, CGM showed that KT patients have greater glycaemic excursions and GV in the immediate post-op phase (78). The mean amplitude of glycaemic excursion and mean absolute glucose levels were also higher in KT patients (79). Amongst combined organ procedures, Dadlani et al. in 2019 compared simultaneous kidney pancreas (SPK), kidney after pancreas (PAK) and pancreas only transplant, with CGM providing a viable and reliable way of monitoring glycaemic control 3–6 weeks post-transplant (79). Mittal et al. also showed that amongst 22 SPK patients, CGM correlated well with post-operative OGTT and was easier to perform and provided 24-hour data that can help decision making (80).
Long-term usage and predictive AI modelling of CGM
Good glycaemic control has been demonstrated to achieve better long-term outcomes through Hba1c-driven studies (74). Amongst CGM studies, Aouad et al. reported the evolution of glycaemic control over 0, 3 and 6 months in 28 patients post KT demonstrating a distinct pattern of afternoon and evening hyperglycaemia (81). Pasti et al. confirmed these findings in a cohort of paediatric patients whereby those with IGT tend to have “lowest glucose” level and less hypoglycaemic episodes, whereas glucose variability tends to improve with time after KT (82). Amongst long-term transplant patients, Werzowa et al. identified that existing DM patients had significant GV compared to PTDM patients, indicating potential pathophysiological differences between the two groups (83). Jakubowska et al. reported that CGM had a positive effect of KT patients with pre-existing DM or PTDM on perception of quality of health (84). However, Kurnikowski et al. showed that there was no difference in long term control amongst patients on CGM and continuous subcutaneous insulin therapy against standard of care basal insulin therapy amongst KT patients in a study with follow up of up to two years (85).
Artificial intelligence, through the use of machine learning, has been used in KT specifically with radiological and pathological evaluation of allograft, prediction of graft survival, optimizing dose of immunosuppression and prediction of graft function (86). Elefteriadis et al. reported the predictive potential of CGM for PTDM in non-diabetic KT patients at varying time points after the operation (87). The study showed good concordance with oral glucose tolerance test (OGTT) from day 8 to day 90, yielding high sensitivity/specificity especially those achieving high % TAR threshold (87). Aouad et al. also reported that the magnitude of hyperglycaemia and variability in transplant patients 3–6 months post-transplant is also predictive towards the development of PTDM during follow up through the use of the Glycaemic Risk Assessment Diabetes Equation score (81). In PD, the use of CGM alongside multinomics approach through PD fluid effluent can influence diabetic management through individualizing diet, medication and dialysis management (88). To date, there are machine-learning driven AI algorithms that are trained to interpret complex datasets from CGM tracings to predict the development of diabetic retinopathy and nephropathy, which can potentially be used for early diagnosis of complications and prediction of outcomes (89).
CGM in acute kidney injury and the failing transplant graft
AKI frequently occurs in KT patients from a myriad of reasons, including sepsis and acute rejection (90). This review did not identify any studies investigating the utility of CGM in patients with AKI, but many studies have indicated that intensive surveillance of glycaemic control can improve outcomes. Diabetic patients have higher infection and rejection risks, on top of inherent propensity for DKA and hypoglycaemia (91). Patients with greater variability in Hba1c (HVS) are reported to have an increased risk of AKI, with a hazard ratio of 1.4 for HVS of 0-20% against 80-100% (92).
A failing transplant graft will typically show severe dysglycaemia through the effects of acidosis, uraemia and inflammation (5). The use of immunosuppressive regimens can contribute to increased GV owing to their variable pharmacodynamic effects, particularly in the context of reduced renal reserve. Corticosteroids diminish insulin sensitivity (18), while CNI induces pancreatic β-cell toxicity (17), together promoting an erratic hyperglycaemic pattern. Conversely, prolonged steroid exposure may result in iatrogenic adrenal insufficiency, leading to unpredictable hypoglycaemic episodes (93). In addition, fluctuating CNI levels in the setting of a failing graft may further impair eGFR and alter the renal clearance of oral hypoglycaemic agents. Furthermore, true glycaemic assessment is also impaired by the discordance between CGM and Hba1c readings at advanced stages of CKD (27). Frequent infections, rejection episodes, anti-rejection rescue therapy and frequent hospital admissions necessitate the need for tighter glycaemic surveillance. The 2020 KDIGO guideline has recommended the use of GMI from CGM to evaluate glycaemic control in patients with CKD4-5, in situations where Hba1c may not be reliable (7). Stathi et al. reported a case series with the use of automated insulin delivery system with CGM in SPK patients with failing graft function to tighten glycaemic variability and reduce hypoglycaemia, in order to protect residual pancreatic and kidney graft function (94).
Research direction
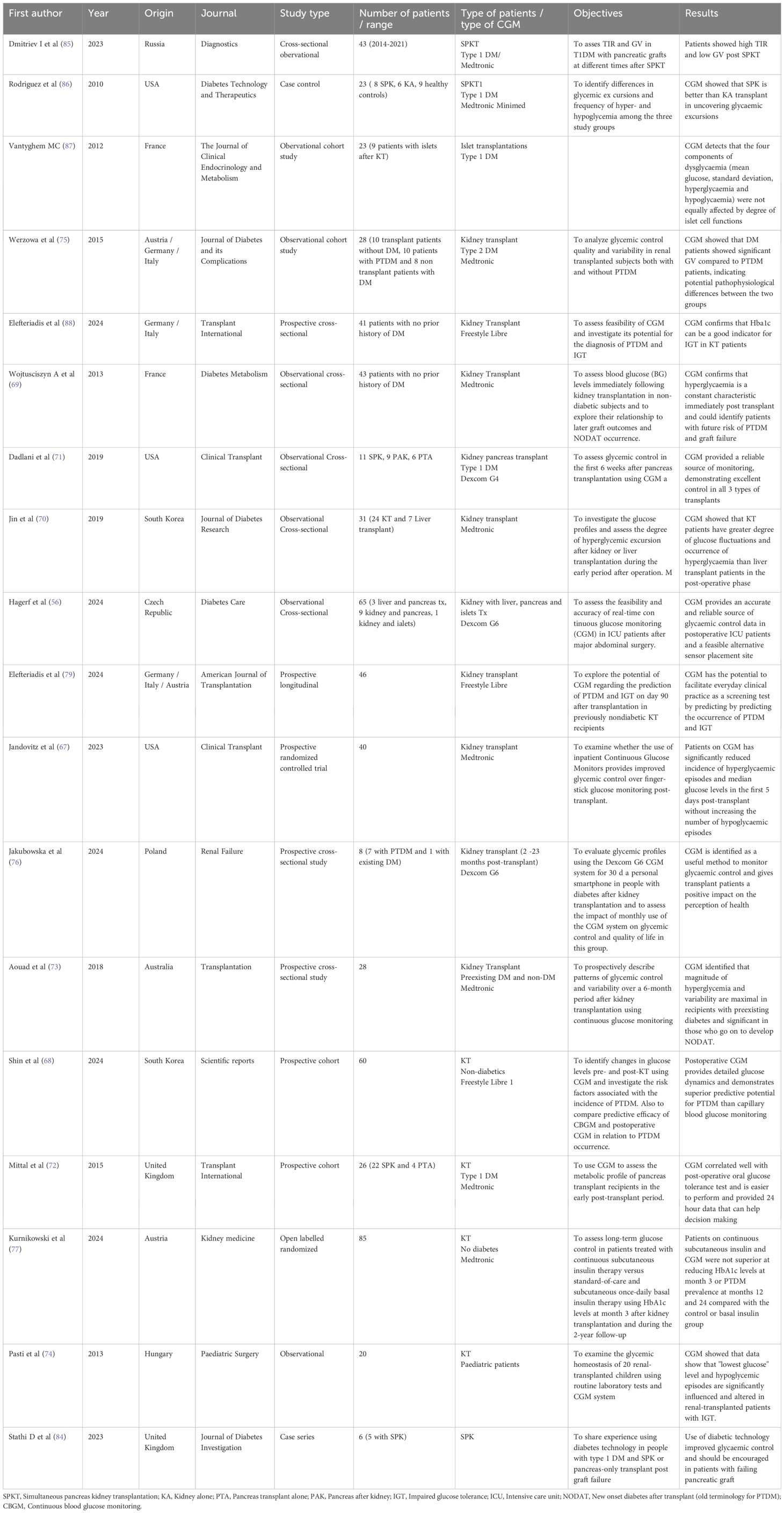
There is a paucity of published research on CGM usage in KT patients, with only 18 papers identified in the literature. (Table 2) (62, 75–85, 87, 94–98) More research in specific domains is needed to elucidate our understanding on this subject.
Determination of pattern in different KT phases
CGM-metrics should be used to evaluate clinical end-points like PTDM, acute and chronic rejections, delayed graft function, graft survival, CMV and BK infections and graft survival. Patient-reported outcomes like quality of life and psychosocial modifications will be particularly useful in failing grafts and complex patients, especially those with combined pancreas transplants.
Determination of accuracy
Similar to research in dialysis population, more studies should be done to determine accuracy of CGM in transplant patients, especially within acute settings in operating theatres and intensive care.
Donor and recipient assessment
Donor assessment in high-risk patients can also be improved with CGM to predict future DM. Augmentation of pre-transplantation glycaemic control in recipients and its impact on post-transplantation outcomes can be explored. Prediabetic donors and recipients may also benefit from long-term CGM usage to mitigate risks and promote behavioural changes.
Predictive algorithm
Machine-learning technology can be used to evaluate patients’ glycaemic risk profile and tailor individualised immunosuppressive regimen. More research can be done to improve existing predictive algorithms for PTDM.
Medical education and training
There is a need to improved current understanding and knowledge of clinicians and transplant patients on CGM-metrics for glycaemic assessment and evaluate ways to incorporate CGM education to transplant and nephrology curriculum. Barriers to adoption of CGM amongst varied healthcare professionals includes lack of training/education and limited exposure (99).
Improving access to CGM
Equity of access to CGM can be improved through more research to produce affordable alternatives (100). Additional, prioritized access can be given to selected KT population especially during high-risk periods where there is added values in utilizing CGM-metrics to improve clinical management or when alternate means of glycaemic surveillance is deemed difficult.
Conclusion
CGM heralds a new era in DM management and surveillance, with the potential to influence short- and long-term management in both KT donors and recipients. In pre-transplant settings, CGM can complement traditional tests to determine suitability of high-risk donors for donation. There is scope for using CGM during the intraoperative and postoperative phase of the transplant, to deliver target-driven glycaemic control and prevent transplant-related complications. Additionally, CGM can circumvent hypoglycaemia and improve glycaemic variability in patients with failing grafts. More research should be done to determine accuracy and utility of CGM, harness the potential of predictive algorithms, improve patients’ and clinician’s understanding and enable equity of access to patients.
Author contributions
JT: Formal analysis, Data curation, Project administration, Writing – review & editing, Conceptualization, Writing – original draft, Methodology. MK: Writing – review & editing. PA: Writing – review & editing. LL: Writing – review & editing, Methodology, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alicic RZ, Rooney MT, and Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. (2017) 12:2032–45. doi: 10.2215/CJN.11491116
2. Gheith O, Farouk N, Nampoory N, Halim MA, and Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. (2016) 5:49–56.
3. Sabanayagam C and Lim CC. Kidney failure trends in people with diabetes: the looming epidemic. Lancet Reg Health West Pac. (2021) 12:100173. doi: 10.1016/j.lanwpc.2021.100173
4. Rossi MR, Mazzali M, and de Sousa MV. Post-transplant diabetes mellitus: risk factors and outcomes in a 5-year follow-up. Front Clin Diabetes Healthc. (2024) 5:1336896. doi: 10.3389/fcdhc.2024.1336896
5. Galindo RJ, Beck RW, Scioscia MF, Umpierrez GE, and Tuttle KR. Glycemic monitoring and management in advanced chronic kidney disease. Endocr Rev. (2020) 41:756–74. doi: 10.1210/endrev/bnaa017
6. Ramirez SP, McCullough KP, Thumma JR, Nelson RG, Morgenstern H, Gillespie BW, et al. Hemoglobin A(1c) levels and mortality in the diabetic hemodialysis population: findings from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Diabetes Care. (2012) 35:2527–32. doi: 10.2337/dc12-0573
7. Rossing P, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, Khunti K, et al. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: an update based on rapidly emerging new evidence. Kidney Int. (2022) 102:990–9. doi: 10.1016/j.kint.2022.06.013
8. Group KDIGOKTW. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. (2009) 9:S1–155. doi: 10.1111/j.1600-6143.2009.02834.x
9. Lin R, Brown F, James S, Jones J, and Ekinci E. Continuous glucose monitoring: A review of the evidence in type 1 and 2 diabetes mellitus. Diabetes Med. (2021) 38:e14528. doi: 10.1111/dme.14528
10. Leelarathna L, Evans ML, Neupane S, Rayman G, Lumley S, Cranston I, et al. Intermittently scanned continuous glucose monitoring for type 1 diabetes. N Engl J Med. (2022) 387:1477–87. doi: 10.1056/NEJMoa2205650
11. Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. (2017) 317:371–8. doi: 10.1001/jama.2016.19975
12. Seidu S, Kunutsor SK, Ajjan RA, and Choudhary P. Efficacy and safety of continuous glucose monitoring and intermittently scanned continuous glucose monitoring in patients with type 2 diabetes: A systematic review and meta-analysis of interventional evidence. Diabetes Care. (2024) 47:169–79. doi: 10.2337/dc23-1520
13. Zhang Y, Singh P, Ganapathy K, Suresh V, Karamat MA, Baharani J, et al. Efficacy of continuous glucose monitoring in people living with diabetes and end stage kidney disease on dialysis: a systematic review. BMC Nephrol. (2024) 25:379. doi: 10.1186/s12882-024-03763-z
14. Bomholt T, Kofod D, Nørgaard K, Rossing P, Feldt-Rasmussen B, and Hornum M. Can the use of continuous glucose monitoring improve glycemic control in patients with type 1 and 2 diabetes receiving dialysis? Nephron. (2023) 147:91–6. doi: 10.1159/000525676
15. Ling J, Ng JKC, Chan JCN, and Chow E. Use of continuous glucose monitoring in the assessment and management of patients with diabetes and chronic kidney disease. Front Endocrinol (Lausanne). (2022) 13:869899. doi: 10.3389/fendo.2022.869899
16. Rhee CM, Gianchandani RY, Kerr D, Philis-Tsimikas A, Kovesdy CP, Stanton RC, et al. Consensus report on the use of continuous glucose monitoring in chronic kidney disease and diabetes. J Diabetes Sci Technol. (2025) 19:217–45. doi: 10.1177/19322968241292041
17. Li Z, Sun F, Zhang Y, Chen H, He N, Song P, et al. Tacrolimus induces insulin resistance and increases the glucose absorption in the jejunum: A potential mechanism of the diabetogenic effects. PloS One. (2015) 10:e0143405. doi: 10.1371/journal.pone.0143405
18. Tamez-Pérez HE, Quintanilla-Flores DL, Rodríguez-Gutiérrez R, González-González JG, and Tamez-Peña AL. Steroid hyperglycemia: Prevalence, early detection and therapeutic recommendations: A narrative review. World J Diabetes. (2015) 6:1073–81. doi: 10.4239/wjd.v6.i8.1073
19. Pham PT. Optimal use of SGLT2 inhibitors in diabetic kidney transplant recipients. Front Nephrol. (2022) 2:1014241. doi: 10.3389/fneph.2022.1014241
20. Popović L and Bulum T. New onset diabetes after organ transplantation: risk factors, treatment, and consequences. Diagnostics (Basel). (2025) 15(3):284. doi: 10.3390/diagnostics15030284
21. Johnston O, Rose CL, Webster AC, and Gill JS. Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol. (2008) 19:1411–8. doi: 10.1681/ASN.2007111202
22. Ponticelli C, Favi E, and Ferraresso M. New-onset diabetes after kidney transplantation. Med (Kaunas). (2021) 57(3):250. doi: 10.3390/medicina57030250
23. Boerner B, Shivaswamy V, Goldner W, and Larsen J. Management of the hospitalized transplant patient. Curr Diabetes Rep. (2015) 15:19. doi: 10.1007/s11892-015-0585-6
24. Huang JW, Famure O, Li Y, and Kim SJ. Hypomagnesemia and the risk of new-onset diabetes mellitus after kidney transplantation. J Am Soc Nephrol. (2016) 27:1793–800. doi: 10.1681/ASN.2015040391
25. Hjelmesaeth J, Sagedal S, Hartmann A, Rollag H, Egeland T, Hagen M, et al. Asymptomatic cytomegalovirus infection is associated with increased risk of new-onset diabetes mellitus and impaired insulin release after renal transplantation. Diabetologia. (2004) 47:1550–6. doi: 10.1007/s00125-004-1499-z
26. Kaminski CY, Galindo RJ, Navarrete JE, Zabala Z, Moazzami B, Gerges A, et al. Assessment of glycemic control by continuous glucose monitoring, hemoglobin A1c, fructosamine, and glycated albumin in patients with end-stage kidney disease and burnt-out diabetes. Diabetes Care. (2024) 47:267–71. doi: 10.2337/dc23-1276
27. Vos FE, Schollum JB, Coulter CV, Manning PJ, Duffull SB, and Walker RJ. Assessment of markers of glycaemic control in diabetic patients with chronic kidney disease using continuous glucose monitoring. Nephrol (Carlton). (2012) 17:182–8. doi: 10.1111/j.1440-1797.2011.01517
28. Subramanian S and Hirsch IB. Diabetic kidney disease: is there a role for glycemic variability? Curr Diabetes Rep. (2018) 18:13. doi: 10.1007/s11892-018-0979-3
29. Vigersky RA and McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. (2019) 21:81–5. doi: 10.1089/dia.2018.0310
30. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. (2019) 42:1593–603. doi: 10.2337/dci19-0028
31. Avari P, Reddy M, and Oliver N. Is it possible to constantly and accurately monitor blood sugar levels, in people with Type 1 diabetes, with a discrete device (non-invasive or invasive)? Diabetes Med. (2020) 37:532–44. doi: 10.1111/dme.13942
32. Rocchitta G, Spanu A, Babudieri S, Latte G, Madeddu G, Galleri G, et al. Enzyme biosensors for biomedical applications: strategies for safeguarding analytical performances in biological fluids. Sensors (Basel). (2016) 16(6):780. doi: 10.3390/s16060780
33. Rodbard D. Characterizing accuracy and precision of glucose sensors and meters. J Diabetes Sci Technol. (2014) 8:980–5. doi: 10.1177/1932296814541810
34. Oliver N, Reddy M, and Leelarathna L. Continuous glucose sensor accuracy: beyond the headline metric. Lancet Diabetes Endocrinol. (2024) 12:934–8. doi: 10.1016/S2213-8587(24)00245-6
35. Ogawa T, Murakawa M, Matsuda A, Kanozawa K, Kato H, Hasegawa H, et al. Endogenous factors modified by hemodialysis may interfere with the accuracy of blood glucose-measuring device. Hemodial Int. (2012) 16:266–73. doi: 10.1111/j.1542-4758.2011.00640.x
36. Eichenlaub M, Waldenmaier D, Wehrstedt S, Pleus S, Link M, Jendrike N, et al. Performance of three continuous glucose monitoring systems in adults with type 1 diabetes. J Diabetes Sci Technol. (2025). doi: 10.1177/19322968251315459
37. Eichenlaub M, Pleus S, Rothenbühler M, Bailey TS, Bally L, Brazg R, et al. Comparator data characteristics and testing procedures for the clinical performance evaluation of continuous glucose monitoring systems. Diabetes Technol Ther. (2024) 26:263–75. doi: 10.1089/dia.2023.0465
38. UK diabetes nurse specialist forum - CGM comparison chart 2025 . Available online at: https://www.diabetesspecialistnurseforumuk.co.uk/new-cgm-comparison-chart (Acceessed November 15, 2025).
39. Ling J, Ng JKC, Lau ESH, Luk AOY, Ma RCW, Vigersky RA, et al. Impact of body composition and anemia on accuracy of a real-time continuous glucose monitor in diabetes patients on continuous ambulatory peritoneal dialysis. Diabetes Technol Ther. (2024) 26:70–5. doi: 10.1089/dia.2023.0349
40. de Boer IH, Anderson LD, Ashford NK, Ayers E, Bansal N, Hall YN, et al. Glycemia assessed by continuous glucose monitoring among people treated with maintenance dialysis. J Am Soc Nephrol. (2025) 36:1798–810. doi: 10.1681/ASN.0000000693
41. Narasaki Y, Kalantar-Zadeh K, Daza AC, You AS, Novoa A, Peralta RA, et al. Accuracy of continuous glucose monitoring in hemodialysis patients with diabetes. Diabetes Care. (2024) 47:1922–9. doi: 10.2337/dc24-0635
42. Genua I, Sánchez-Hernandez J, Martínez MJ, Pujol I, Places J, González C, et al. Accuracy of flash glucose monitoring in patients with diabetes mellitus on hemodialysis and its relationship with hydration status. J Diabetes Sci Technol. (2021) 15:1308–12. doi: 10.1177/1932296820975057
43. Avari P, Pushparatnam R, Leelarathna L, Tan T, Frankel AH, Oliver N, et al. Accuracy of the dexcom G7 continuous glucose monitoring sensors in people with diabetes undergoing hemodialysis (ALPHA-2 study). Diabetes Technol Ther. (2025) 27(5):402–6. doi: 10.1089/dia.2024.0575
44. Avari P, Tang W, Jugnee N, Hersi I, Al-Balah A, Tan T, et al. The accuracy of continuous glucose sensors in people with diabetes undergoing hemodialysis (ALPHA study). Diabetes Technol Ther. (2023) 25:447–56. doi: 10.1089/dia.2023.0013
45. Soliman KM, Daoud A, Posadas Salas MA, Rice T, Uehara G, Shayto R, et al. Accepting living kidney donors with preexisting diabetes mellitus: A perspective on the recent OPTN policy change-july 2022. Clin J Am Soc Nephrol. (2023) 18:127–9. doi: 10.2215/CJN.09460822
46. Abramowicz D, Cochat P, Claas FH, Heemann U, Pascual J, Dudley C, et al. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant. (2015) 30:1790–7. doi: 10.1093/ndt/gfu216
47. Andrews PA and Burnapp L. British transplantation society / renal association UK guidelines for living donor kidney transplantation 2018: summary of updated guidance. Transplantation. (2018) 102:e307. doi: 10.1097/TP.0000000000002253
48. Lentine KL, Kasiske BL, Levey AS, Adams PL, Alberú J, Bakr MA, et al. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation. (2017) 101:S1–S109. doi: 10.1097/TP.0000000000001769
49. Chandran S, Masharani U, Webber AB, and Wojciechowski DM. Prediabetic living kidney donors have preserved kidney function at 10 years after donation. Transplantation. (2014) 97:748–54. doi: 10.1097/01.TP.0000438625.91095.8b
50. Palatini P, Mormino P, Dorigatti F, Santonastaso M, Mos L, De Toni R, et al. Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: the HARVEST. Kidney Int. (2006) 70:578–84. doi: 10.1038/sj.ki.5001603
51. Franciosi M, Pellegrini F, Sacco M, De Berardis G, Rossi MC, Strippoli GF, et al. Identifying patients at risk for microalbuminuria via interaction of the components of the metabolic syndrome: a cross-sectional analytic study. Clin J Am Soc Nephrol. (2007) 2:984–91. doi: 10.2215/CJN.01190307
52. Hebert SA, Murad DN, Nguyen DT, Graviss EA, Adrogue HE, Matas AJ, et al. Outcomes of kidney donors with impaired fasting glucose. Transplantation. (2022) 106:138–46. doi: 10.1097/TP.0000000000003665
53. Rodbard D. Glucose variability: A review of clinical applications and research developments. Diabetes Technol Ther. (2018) 20:S25–S215. doi: 10.1089/dia.2018.0092
54. Xing Y, Wu M, Liu H, Li P, Pang G, Zhao H, et al. Assessing the temporal within-day glycemic variability during hospitalization in patients with type 2 diabetes patients using continuous glucose monitoring: a retrospective observational study. Diabetol Metab Syndr. (2024) 16:56. doi: 10.1186/s13098-024-01269-0
55. Calhoun P, Spanbauer C, Steck AK, Frohnert BI, Herman MA, Keymeulen B, et al. Continuous glucose monitor metrics from five studies identify participants at risk for type 1 diabetes development. Diabetologia. (2025) 68:930–9. doi: 10.1007/s00125-025-06362-1
56. Montaser E, Breton MD, Brown SA, DeBoer MD, Kovatchev B, and Farhy LS. Predicting immunological risk for stage 1 and stage 2 diabetes using a 1-week CGM home test, nocturnal glucose increments, and standardized liquid mixed meal breakfasts, with classification enhanced by machine learning. Diabetes Technol Ther. (2023) 25:631–42. doi: 10.1089/dia.2023.0064
57. Metwally AA, Perelman D, Park H, Wu Y, Jha A, Sharp S, et al. Prediction of metabolic subphenotypes of type 2 diabetes via continuous glucose monitoring and machine learning. Nat BioMed Eng. (2024) 9(8):1222–39. doi: 10.1101/2024.07.20.24310737
58. Bailey KJ, Little JP, and Jung ME. Self-monitoring using continuous glucose monitors with real-time feedback improves exercise adherence in individuals with impaired blood glucose: A pilot study. Diabetes Technol Ther. (2016) 18:185–93. doi: 10.1089/dia.2015.0285
59. Molnar MZ, Huang E, Hoshino J, Krishnan M, Nissenson AR, Kovesdy CP, et al. Association of pretransplant glycemic control with posttransplant outcomes in diabetic kidney transplant recipients. Diabetes Care. (2011) 34:2536–41. doi: 10.2337/dc11-0906
60. Akirov A, Shochat T, and Shimon I. Solid-organ transplant recipients with hyperglycemia on admission face worse outcomes. Am J Manag Care. (2020) 26:163–8. doi: 10.37765/ajmc.2020.42833
61. Park C, Hsu C, Neelakanta G, Nourmand H, Braunfeld M, Wray C, et al. Severe intraoperative hyperglycemia is independently associated with surgical site infection after liver transplantation. Transplantation. (2009) 87:1031–6. doi: 10.1097/TP.0b013e31819cc3e6
62. Voglová Hagerf B, Protus M, Nemetova L, Mraz M, Kieslichova E, Uchytilova E, et al. Accuracy and feasibility of real-time continuous glucose monitoring in critically ill patients after abdominal surgery and solid organ transplantation. Diabetes Care. (2024) 47:956–63. doi: 10.2337/dc23-1663
63. Tripyla A, Herzig D, Joachim D, Nakas CT, Amiet F, Andreou A, et al. Performance of a factory-calibrated, real-time continuous glucose monitoring system during elective abdominal surgery. Diabetes Obes Metab. (2020) 22:1678–82. doi: 10.1111/dom.14073
64. Hermayer KL, Egidi MF, Finch NJ, Baliga P, Lin A, Kettinger L, et al. A randomized controlled trial to evaluate the effect of glycemic control on renal transplantation outcomes. J Clin Endocrinol Metab. (2012) 97:4399–406. doi: 10.1210/jc.2012-1979
65. Duan Y, Li ZZ, Liu P, Cui L, Gao Z, and Zhang H. The efficacy of intraoperatie continuous glucose monitoring in patients undergoing liver transplantation: a study protocol for a prospective randomized controlled superiority trial. Trials. (2023) 24:72. doi: 10.1186/s13063-023-07073-x
66. Rangasamy V, Xu X, Susheela AT, and Subramaniam B. Comparison of glycemic variability indices: blood glucose, risk index, and coefficient of variation in predicting adverse outcomes for patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. (2020) 34:1794–802. doi: 10.1053/j.jvca.2019.12.032
67. Lim HA, Kim M, Kim NJ, Huh J, Jeong JO, Hwang W, et al. The performance of continuous glucose monitoring during the intraoperative period: A scoping review. J Clin Med. (2024) 13(20):6169. doi: 10.3390/jcm13206169
68. Perez-Guzman MC, Duggan E, Gibanica S, Cardona S, Corujo-Rodriguez A, Faloye A, et al. Continuous glucose monitoring in the operating room and cardiac intensive care unit. Diabetes Care. (2021) 44:e50–e2. doi: 10.2337/dc20-2386
69. Lorenz C, Sandoval W, and Mortellaro M. Interference assessment of various endogenous and exogenous substances on the performance of the eversense long-term implantable continuous glucose monitoring system. Diabetes Technol Ther. (2018) 20:344–52. doi: 10.1089/dia.2018.0028
70. Chakkera HA, Knowler WC, Devarapalli Y, Weil EJ, Heilman RL, Dueck A, et al. Relationship between inpatient hyperglycemia and insulin treatment after kidney transplantation and future new onset diabetes mellitus. Clin J Am Soc Nephrol. (2010) 5:1669–75. doi: 10.2215/CJN.09481209
71. Kaplan A, Manela T, Hod T, Ghinea R, Mor E, Tirosh A, et al. Management of early post-transplant hyperglycemia by dedicated endocrine care improves glycemic outcomes. Clin Pract. (2024) 14:1960–9. doi: 10.3390/clinpract14050156
72. Hosseini MS, Nemati E, Pourfarziani V, Taheri S, Nourbala MH, and Einollahi B. Early hyperglycemia after allogenic kidney transplantation: does it induce infections. Ann Transplant. (2007) 12:23–6.
73. Wyzgal J, Paczek L, Ziolkowski J, Pawlowska M, Rowiński W, and Durlik M. Early hyperglycemia after allogenic kidney transplantation. Ann Transplant. (2007) 12:40–5.
74. Kim YC, Shin N, Lee S, Hyuk H, Kim YH, Kim H, et al. Effect of post-transplant glycemic control on long-term clinical outcomes in kidney transplant recipients with diabetic nephropathy: A multicenter cohort study in Korea. PloS One. (2018) 13:e0195566. doi: 10.1371/journal.pone.0195566
75. Jandovitz N, George SJ, Abate M, Kressel AM, Bolognese AC, Lau L, et al. A randomized trial of continuous glucose monitoring to improve post-transplant glycemic control. Clin Transplant. (2023) 37:e15139. doi: 10.1111/ctr.15139
76. Shin J, Jo EA, Woo HY, Cho A, Ko M, Kim S, et al. Perioperative glucose monitoring with continuous glucose monitors identifies risk factors for post-transplant diabetes mellitus in kidney transplant recipients. Sci Rep. (2024) 14:21240. doi: 10.1038/s41598-024-72025-7
77. Wojtusciszyn A, Mourad G, Bringer J, and Renard E. Continuous glucose monitoring after kidney transplantation in non-diabetic patients: early hyperglycaemia is frequent and may herald post-transplantation diabetes mellitus and graft failure. Diabetes Metab. (2013) 39:404–10. doi: 10.1016/j.diabet.2012.10.007
78. Jin HY, Lee KA, Kim YJ, Park TS, Lee S, Park SK, et al. The degree of hyperglycemia excursion in patients of kidney transplantation (KT) or liver transplantation (LT) assessed by continuous glucose monitoring (CGM): pilot study. J Diabetes Res. (2019) 2019:1757182. doi: 10.1155/2019/1757182
79. Dadlani V, Kaur RJ, Stegall M, Xyda SE, Kumari K, Bonner K, et al. Continuous glucose monitoring to assess glycemic control in the first 6 weeks after pancreas transplantation. Clin Transplant. (2019) 33:e13719. doi: 10.1111/ctr.13719
80. Mittal S, Franklin RH, Policola C, Sharples E, Friend PJ, and Gough SC. Early postoperative continuous glucose monitoring in pancreas transplant recipients. Transpl Int. (2015) 28:604–9. doi: 10.1111/tri.12541
81. Aouad LJ, Clayton P, Wyburn KR, Gracey DM, and Chadban SJ. Evolution of glycemic control and variability after kidney transplant. Transplantation. (2018) 102:1563–8. doi: 10.1097/TP.0000000000002155
82. Pasti K, Szabo AJ, Prokai A, Meszaros K, Peko N, Solyom R, et al. Continuous glucose monitoring system (CGMS) in kidney-transplanted children. Pediatr Transplant. (2013) 17:454–60. doi: 10.1111/petr.12106
83. Werzowa J, Pacini G, Hecking M, Fidler C, Haidinger M, Brath H, et al. Comparison of glycemic control and variability in patients with type 2 and posttransplantation diabetes mellitus. J Diabetes Complications. (2015) 29:1211–6. doi: 10.1016/j.jdiacomp.2015.07.014
84. Jakubowska Z and Małyszko J. Continuous glucose monitoring (Dexcom g6) in people with diabetes after kidney transplantation. Ren Fail. (2024) 46:2413007. doi: 10.1080/0886022X.2024.2413007
85. Kurnikowski A, Werzowa J, Hödlmoser S, Krenn S, Paschen C, Mussnig S, et al. Continuous insulin therapy to prevent post-transplant diabetes mellitus: A randomized controlled trial. Kidney Med. (2024) 6:100860. doi: 10.1016/j.xkme.2024.100860
86. Seyahi N and Ozcan SG. Artificial intelligence and kidney transplantation. World J Transplant. (2021) 11:277–89. doi: 10.5500/wjt.v11.i7.27
87. Eleftheriadis G, Naik MG, Osmanodja B, Liefeldt L, Choi M, Halleck F, et al. Continuous glucose monitoring for the prediction of posttransplant diabetes mellitus and impaired glucose tolerance on day 90 after kidney transplantation-A prospective proof-of-concept study. Am J Transplant. (2024) 24:2225–34. doi: 10.1016/j.ajt.2024.07.016
88. Mahdavi S, Anthony NM, Sikaneta T, and Tam PY. Perspective: multiomics and artificial intelligence for personalized nutritional management of diabetes in patients undergoing peritoneal dialysis. Adv Nutr. (2025) 16:100378. doi: 10.1016/j.advnut.2025.100378
89. Scheideman AF, Shao MM, Zelada H, Cuadros J, Foreman J, Sarder P, et al. Machine learning to diagnose complications of diabetes. J Diabetes Sci Technol. (2025) 19(6):1650–70. doi: 10.1177/19322968251365245
90. Palmisano A, Gandolfini I, Delsante M, Cantarelli C, Fiaccadori E, Cravedi P, et al. Acute Kidney Injury (AKI) before and after Kidney Transplantation: Causes, Medical Approach, and Implications for the Long-Term Outcomes. J Clin Med. (2021) 10(7):1484. doi: 10.3390/jcm10071484
91. Yoshida EM, Buczkowski AK, Sirrs SM, Elliott TG, Scudamore CH, Levin A, et al. Post-transplant diabetic ketoacidosis–a possible consequence of immunosuppression with calcineurin inhibiting agents: a case series. Transpl Int. (2000) 13:69–72. doi: 10.1111/j.1432-2277.2000.tb01039.x
92. Xu Y, Dong S, Fu EL, Sjölander A, Grams ME, Selvin E, et al. Long-term visit-to-visit variability in hemoglobin A. Am J Kidney Dis. (2023) 82:267–78. doi: 10.1053/j.ajkd.2023.03.007
93. Chae HH, Ahmed A, Bone JN, Abdulhussein FS, Amed S, Patel T, et al. Adrenal insufficiency in pediatric kidney transplantation recipients. Pediatr Transplant. (2024) 28:e14768. doi: 10.1111/petr.14768
94. Stathi D, Johnston T, Hyslop R, Brackenridge A, and Karalliedde J. Diabetes technology including automated insulin delivery systems to manage hyperglycemia in a failing pancreatic graft: Case series of people with type 1 diabetes and a pancreas kidney or pancreas-only transplant. J Diabetes Investig. (2023) 14:917–20. doi: 10.1111/jdi.14019
95. Dmitriev IV, Severina AS, Zhuravel NS, Yevloyeva MI, Salimkhanov RK, Shchelykalina SP, et al. Continuous glucose monitoring in patients following simultaneous pancreas-kidney transplantation: time in range and glucose variability. Diagnostics (Basel). (2023) 13(9):1606. doi: 10.3390/diagnostics13091606
96. Rodríguez LM, Knight RJ, and Heptulla RA. Continuous glucose monitoring in subjects after simultaneous pancreas-kidney and kidney-alone transplantation. Diabetes Technol Ther. (2010) 12:347–51. doi: 10.1089/dia.2009.0157
97. Vantyghem MC, Raverdy V, Balavoine AS, DeFrance F, Caiazzo R, Arnalsteen L, et al. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (β-score greater than 7) Is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (β-score greater than 3). J Clin Endocrinol Metab. (2012) 97:E2078–83. doi: 10.1210/jc.2012-2115
98. Eleftheriadis G, Naik MG, Osmanodja B, Liefeldt L, Halleck F, Choi M, et al. Continuous glucose monitoring for the diagnosis of post-transplantation diabetes mellitus and impaired glucose tolerance from years one to five after kidney transplantation-A prospective pilot study. Transpl Int. (2024) 37:13724. doi: 10.3389/ti.2024.13724
99. De Block C, Cheng AYY, Christensen TB, Patted URH, and Ginovker A. Healthcare professionals' Knowledge of and attitudes towards the use of time in range in diabetes management: online survey across seven countries. Diabetes Ther. (2023) 14:1399–413. doi: 10.1007/s13300-023-01429-x
Keywords: continuous glucose monitoring, CGM, kidney transplant, kidney failure, diabetes mellitus, post-transplant diabetes mellitus, artificial intelligence
Citation: Tan J, Khalil MAM, Avari P and Leelarathna L (2025) Improving kidney transplant care through the application of continuous glucose monitoring - a narrative review. Front. Nephrol. 5:1630597. doi: 10.3389/fneph.2025.1630597
Received: 18 May 2025; Accepted: 05 November 2025; Revised: 31 October 2025;
Published: 28 November 2025.
Edited by:
PHUONG-THU PHAM, David Geffen School of Medicine, University of California, Los Angeles, United StatesReviewed by:
Chinnarat Pongpruksa, Rajavithi Hospital, ThailandTakeshi Katsuki, Tokyo Saiseikai Central Hospital, Japan
Copyright © 2025 Tan, Khalil, Avari and Leelarathna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jackson Tan, ZHJqYWNrc29udGFuQHlhaG9vLmNvLnVr
 Jackson Tan
Jackson Tan Muhammad Abdul Mabood Khalil3
Muhammad Abdul Mabood Khalil3 Parizad Avari
Parizad Avari