- 1Department of Cardiology and Vascular Medicine, Universitas Indonesia Hospital, Depok, Java, Indonesia
- 2Kalideres Regional General Hospital (RSUD Kalideres), Jakarta, Indonesia
- 3Dharmais National Cancer Center Hospital, Jakarta, Indonesia
- 4Lavalette Hospital, Malang, Indonesia
- 5Haji Batu Mother & Child Hospital, Batu City, Indonesia
- 6Faculty of MedicineMuhammadiyah University of Malang, Malang, Java, Indonesia
- 7Nurussyifa General Hospital, Kudus, Indonesia
- 8Duta Mulya General Hospital, Bekasi, Java, Indonesia
- 9Altius Hospitals Harapan Indah, Bekasi, Java, Indonesia
- 10EKA Grand Family Mother and Child Hospital (RSIA EKA Grand Family), Tangerang, Banten, Indonesia
- 11Prembun Regional Hospital (RSUD Prembun), Kebumen, Java, Indonesia
- 12Faculty of Medicine, Jenderal Soedirman University, Purwokerto, Java, Indonesia
- 13Faculty of Medicine, Maranatha Christian University, Bandung, Java, Indonesia
- 14Faculty of Medicine, Tanjungpura University, Pontianak, Kalimantan, Indonesia
- 15Siloam Hospital, Jember, Indonesia
- 16Faculty of Medicine, Sam Ratulangi University, Manado, Sulawesi, Indonesia
- 17Division of Psychosomatic and Palliative Medicine, Department of Internal Medicine, Cipto Mangunkusumo National General Hospital (RSCM), Jakarta, Indonesia
- 18Faculty of MedicineSultan Agung Islamic University, Semarang, Java, Indonesia
- 19Faculty of Medicine, Indonesian Christian University, Jakarta, Indonesia
- 20Faculty of Medicine, Gadjah Mada University, Yogyakarta, Indonesia
- 21Dr. Sardjito Hospital, Yogyakarta, Indonesia
- 22Faculty of Medicine, UPN Veteran Jakarta, Jakarta, Indonesia
- 23Faculty of Medicine, Sultan Agung Islamic University, Semarang, Java, Indonesia
- 24Division of Endocrinology, Metabolism and Diabetes, Department of Internal Medicine, Cipto Mangunkusumo National General Hospital (RSCM), Jakarta, Indonesia
- 25Aisyiyah Klaten General Hospital (RSU Aisyiyah Klaten), Klaten, Java, Indonesia
- 26Faculty of Medicine, Brawijaya University, Malang, Java, Indonesia
- 27Faculty of Medicine, Maulana Malik Ibrahim State Islamic University, Malang, Indonesia
- 28Faculty of Medicine and Science, Universitas Katolik Indonesia Atma Jaya, Jakarta, Indonesia
Background: Chronic kidney disease (CKD) affects nearly 10% of the global population and often progresses silently to end-stage renal disease, requiring dialysis or transplantation. Hypertension, prevalent in both adults and children, is a key driver of CKD progression. Acute kidney injury (AKI), particularly sepsis-associated AKI (S-AKI), poses a critical risk for long-term renal dysfunction, especially in patients with pre-existing CKD. S-AKI, defined by abrupt renal function decline during sepsis or septic shock, can accelerate CKD progression, yet its risk factors and outcomes across pediatric and adult populations remain incompletely characterized.
Objective: Aims to systematically evaluate existing research on the relationship between Risk Factors for CKD and Septic Shock with Hypertension in Adults and Children.
Methods: A systematic literature search was conducted using PubMed, Google Scholar, and the Cochrane Library for studies published between 2004 and 2024. Search terms included “chronic kidney disease,” “septic shock,” “hypertension,” and “acute kidney injury.” After applying PRISMA-based screening and eligibility criteria, 9 studies were included for qualitative synthesis.
Results: A total of 762 articles were identified through database searching. After screening and eligibility assessment, 9 studies were included in the final synthesis. The findings revealed that both CKD and hypertension are significant independent risk factors for S-AKI and septic shock. Preexisting albuminuria, uncontrolled blood pressure, advanced age, and diabetes mellitus were frequently associated with poor outcomes. Several studies highlighted the role of MPP and fluid resuscitation strategies in preventing AKI progression in septic patients. In pediatric populations, a history of AKI was strongly associated with new-onset hypertension and subsequent CKD development, increasing vulnerability to severe septic complications.
Conclusion: CKD and hypertension significantly increase the risk of septic complications and worsen renal outcomes, particularly in patients with fluid management challenges. Early identification of high-risk patients, individualized hemodynamic targets, and tailored fluid resuscitation strategies are critical in reducing morbidity and mortality. Special attention is needed in pediatric patients with limited nephron reserve, where long-term surveillance and early intervention may improve outcomes.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php, identifier PROSPERO (CRD420251146866).
Introduction
Chronic kidney disease (CKD) affects 9.1% of the global population and often progresses silently to end-stage renal disease, requiring costly interventions like dialysis or transplantation (1). CKD is defined by structural or functional renal abnormalities lasting over three months (2). Hypertension significantly contributes to CKD progression; hypertensive men have a 2.14-fold and women a 1.54-fold increased risk of developing CKD (3). In children, hypertension is rising, affecting approximately 4% of those under 19 years, with a steadily increasing prevalence over recent decades. Unlike in adults, pediatric hypertension is defined using blood pressure percentiles adjusted for age, sex, and height, making it essential to consider these variables when establishing management thresholds and hemodynamic targets such as mean arterial pressure (MAP).
Acute kidney injury (AKI), common in critically ill and hospitalized children, is a notable risk factor for pediatric hypertension and subsequent CKD. High-risk groups include those with sepsis, cardiac surgery, malignancies, or nephrotoxin exposure. AKI often leads to long-term damage including acute kidney disease (AKD) and CKD. In adults, CKD is strongly associated with cardiovascular disease due to overlapping risk factors like hypertension and diabetes. Pediatric CKD also shows high hypertension prevalence, and early control via RAAS blockade and ambulatory BP monitoring is crucial (4–7).
Sepsis-associated acute kidney injury (S-AKI) is defined as a sudden decline in renal function occurring during sepsis or septic shock, characterized by an increase in serum creatinine or reduction in urine output as per KDIGO criteria. In patients with pre-existing CKD, this represents a superimposed acute insult that can accelerate kidney disease progression. Sepsis and septic shock are leading causes of mortality and are implicated in 25–75% of AKI cases globally. Septic AKI, once seen as transient, is now linked to progression toward CKD and end-stage kidney disease. In septic shock, blood pressure regulation is critical; current guidelines recommend targeting a MAP ≥65 mmHg, or 80–85 mmHg in chronic hypertensives, though vasopressors pose additional risks (8–13).
This study aims to identify risk factors and correlations between CKD and septic shock in both pediatric and adult populations.
Methods
Study design
We conducted a systematic scoping review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. The protocol for this review was prospectively registered on PROSPERO (Registration ID: CRD420251146866). The objective was to synthesize evidence on risk factors for CKD, hypertension, and their associations with S-AKI and septic shock in both adult and pediatric populations.
Overview of the systematic literature review process
The methodology employed in this research is the Systematic Literature Review (SLR), aimed at identifying, assessing, and interpreting all relevant research findings related to the Risk Factors for CKD and Septic Shock with Hypertension in Adults and Children. The SLR process follows the PRISMA guidelines, which consists of the following stages:
a. Identification: In this stage, a literature search is conducted to gather articles, journals, and other documents relevant to the research topic. The search is performed through electronic databases such as Google Scholar, PubMed, and Cochrane using predetermined keywords.
b. Screening: After the identification stage, the search results are screened to remove duplicates and irrelevant articles. Articles that do not meet the inclusion criteria or are outside the scope of the research are eliminated at this stage.
c. Eligibility: Articles that pass the screening stage are then evaluated for eligibility based on the established inclusion and exclusion criteria. Articles that do not provide sufficient data or are not relevant to the research focus are also eliminated at this stage.
d. Inclusion: Articles that meet all criteria are included for further analysis. This stage results in a final list of literature that will be analyzed in depth in the research.
Eligibility criteria (PECO framework)
● Population (P): Adults and children with pre-existing CKD and/or hypertension who developed sepsis or septic shock.
● Exposure (E): Presence of CKD, hypertension, albuminuria, and hemodynamic variables (mean arterial pressure [MAP], mean perfusion pressure [MPP]).
● Comparator (C): Patients without CKD or hypertension (where reported).
● Outcome (O):
● Primary Outcome: Incidence of S-AKI, defined as AKI occurring in the context of sepsis or septic shock based on KDIGO criteria.
● Secondary Outcomes: Progression to AKD, CKD progression, need for renal replacement therapy (RRT), in-hospital and 90-day mortality, and hemodynamic outcomes.
Search strategy
A comprehensive search was conducted in PubMed/MEDLINE, Cochrane Library, and Google Scholar. The final search was performed on March 15, 2024. Equivalent search terms were adapted for Cochrane and Google Scholar. We included studies published in English or Indonesian, and additionally screened studies in other languages if an English abstract was available to minimize language bias.
Study selection
Two reviewers independently screened all titles and abstracts using Rayyan AI. Full-text screening was conducted for potentially eligible articles. Discrepancies were resolved by consensus or arbitration by a third senior reviewer. The screening process and study selection are summarized in the updated PRISMA 2020 flow diagram, which includes the number of records at each stage (identification, screening, eligibility, and inclusion).
Data extraction
After the literature selection process is completed, the next stage is data extraction from the selected articles. This process includes identifying and recording key information from each article relevant to the research objectives.
Search string
The literature search is conducted using various keywords relevant to the research topic. The keywords used are tailored to the databases accessed and include terms such as “chronic kidney disease”, “Septic Shock” and “Hypertension”.
Inclusion and exclusion criteria
Inclusion criteria
(1) articles published in reputable scientific journals; (2) publications from the last 20 years to ensure data relevance; and (3) articles available in either English or Indonesian.
Exclusion criteria
1) Articles that do not provide empirical data or concrete research findings.
2) Articles that are not fully accessible (only available as abstracts).
Results
Our initial search yielded 762 records across PubMed, Cochrane Library, and Google Scholar. After removing 104 duplicates, 658 unique records were screened by title and abstract. Of these, 617 were excluded because they did not meet inclusion criteria (unrelated topic, wrong population, or non-empirical articles). A total of 41 full-text articles were assessed for eligibility, and 32 were excluded for the following reasons: pediatric case reports (n=6), narrative reviews or editorials (n=9), incomplete or inaccessible full text (n=7), and wrong outcomes (n=10).
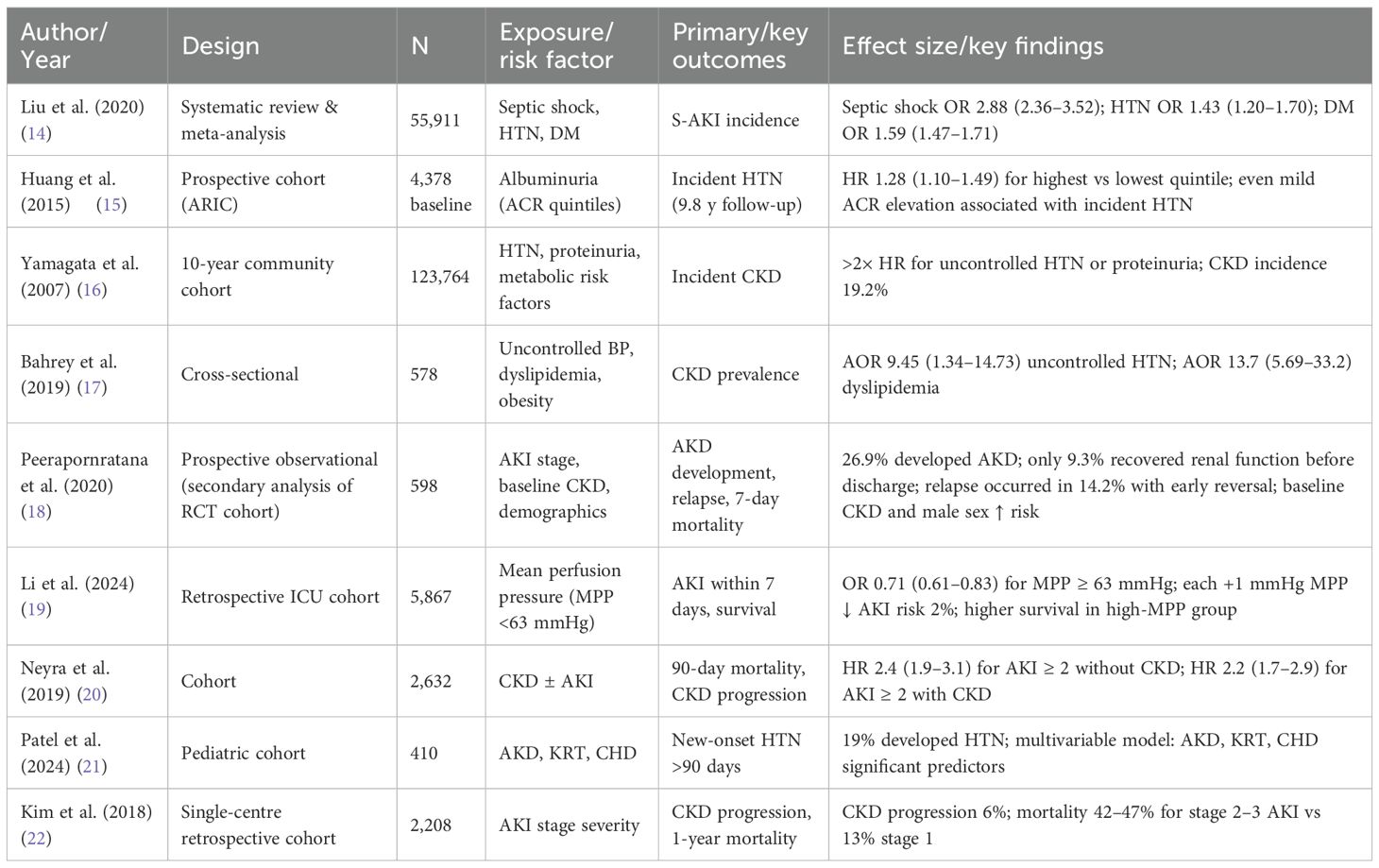
Ultimately, 9 studies met all inclusion criteria (Table 1) their summarized data and effect estimates are presented in Table 2, and were included in the final qualitative synthesis (Figure 1).
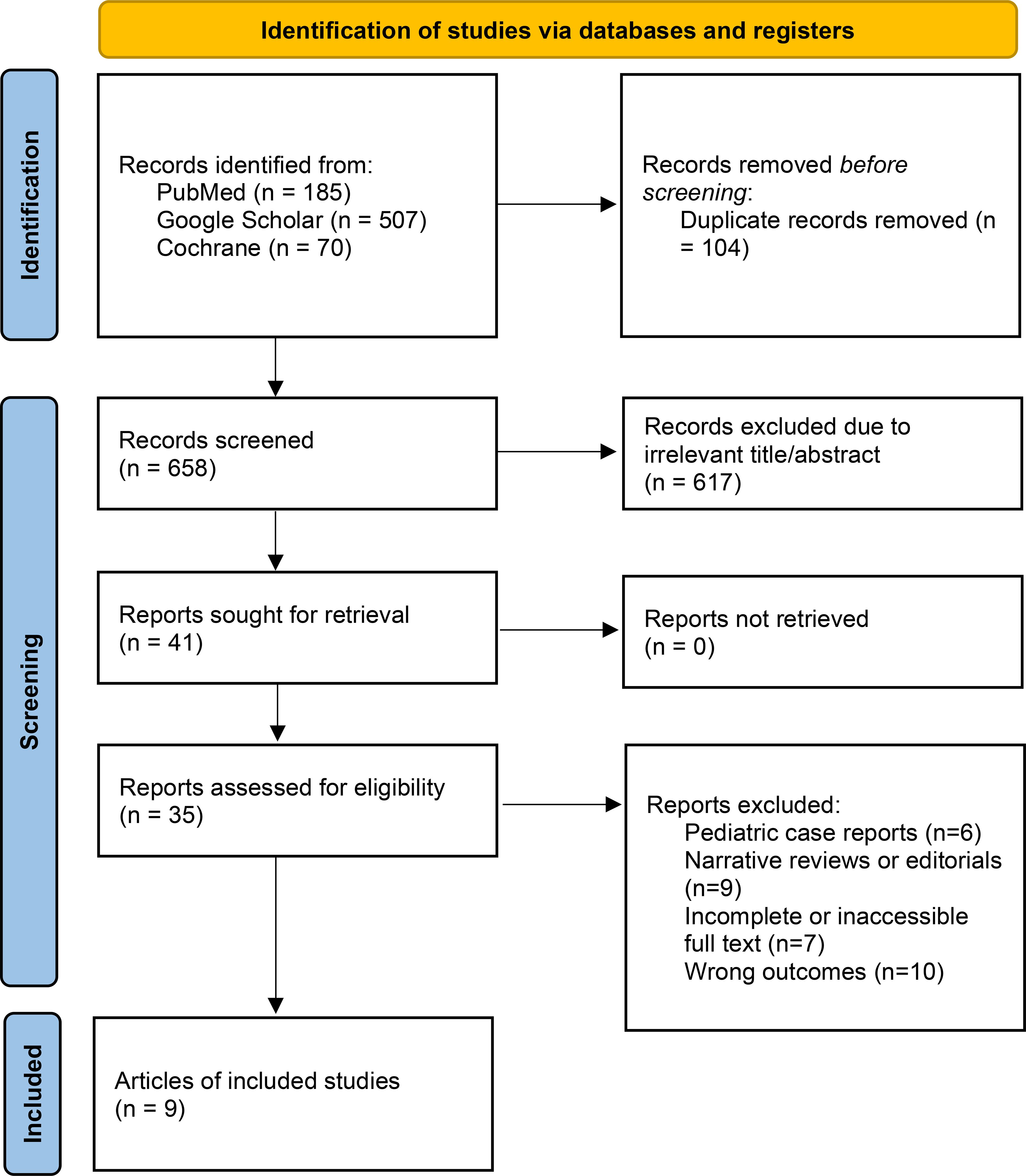
Figure 1. Systematic review diagram based on PRISMA (1).
Bias assessment
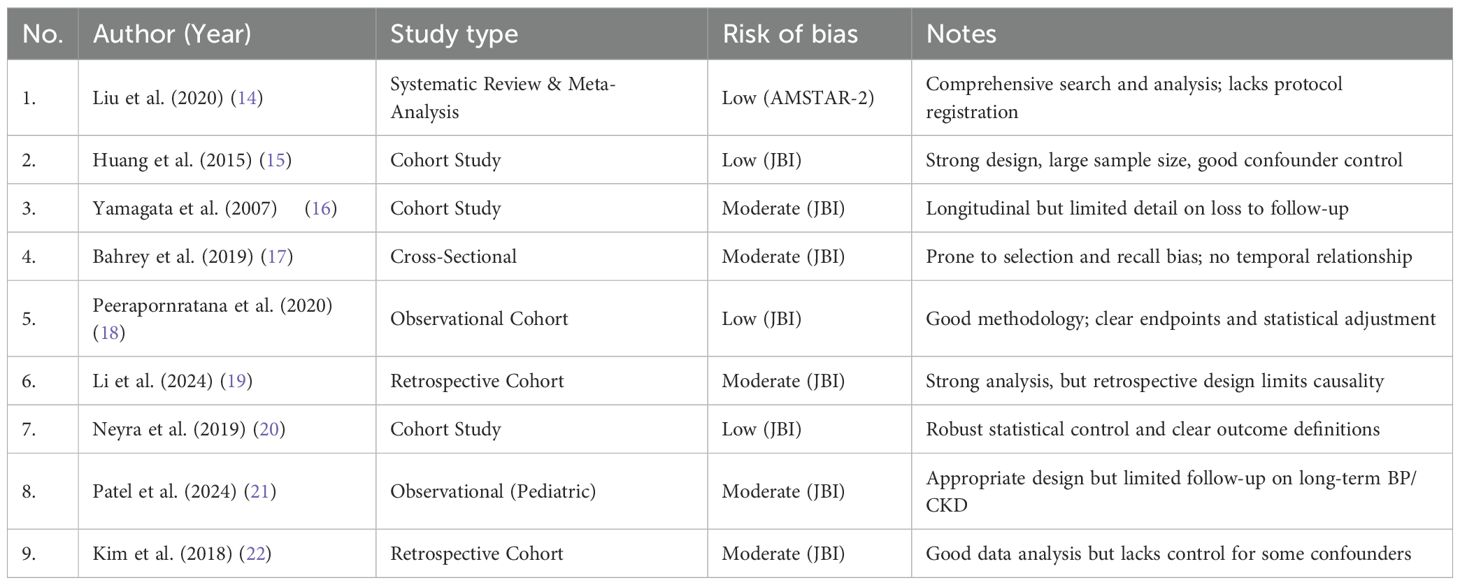
A risk of bias assessment was conducted for all nine included studies using appropriate tools according to study design: JBI Critical Appraisal Tools for observational studies and AMSTAR 2 for the systematic review and meta-analysis. Most of the studies were rated as low to moderate risk of bias. Common strengths across studies included clearly defined inclusion criteria, appropriate statistical analyses, and clinically relevant outcomes. However, several cohort and cross-sectional studies demonstrated potential limitations such as lack of adjustment for confounders, unclear follow-up duration, or insufficient detail on blinding and selection procedures (Table 3).
Study characteristics
Nine studies published between 2007 and 2024 were included, encompassing more than 185,000 participants across various settings, including community-based cohorts, retrospective ICU analyses, and one prospective observational study nested within a randomized trial (14–22). Six studies focused exclusively on adult populations, one included both adults and children, one was a pediatric cohort, and one was a systematic review and meta-analysis synthesizing 47 observational studies (14).
Primary outcome: incidence of sepsis-associated AKI
The incidence of S-AKI among septic patients ranged from 30% to 57%, with the highest rates observed in septic shock (14, 20, 22). In the meta-analysis including 55,911 patients, septic shock was the most potent risk factor for S-AKI (OR 2.88, 95% CI 2.36–3.52) (14). Prevalent CKD was reported in 46% of critically ill patients, and the combination of CKD and AKI significantly increased 90-day mortality (HR 2.2, 95% CI 1.7–2.9) (20).
Hemodynamic targets (MAP and MPP)
One large retrospective cohort of 5,867 septic ICU patients demonstrated that lower MPP (<63 mmHg) was associated with a significantly higher incidence of AKI (87.6% vs. 78.3%, p<0.001) and higher in-hospital mortality (19). Each 1 mmHg increase in MPP conferred a 2% reduction in AKI risk (OR 0.98, 95% CI 0.97–0.99), supporting MPP as a potentially modifiable target in sepsis management (19).
CKD and hypertension as risk factors
Several consistent risk factors for CKD emerged across studies, including age, hypertension, diabetes mellitus, proteinuria/albuminuria, obesity, and dyslipidemia (2–4). Yamagata et al. reported that treated hypertension and systolic BP >160 mmHg were associated with more than a twofold increase in CKD risk in females (3). Similarly, albuminuria was shown to be a strong predictor of both prevalent and incident hypertension, even at mildly elevated levels (2). Among hypertensive patients, uncontrolled blood pressure, overweight/obesity, and dyslipidemia were strongly associated with CKD (AOR 7.42–13.74) (4).
Progression to AKD and CKD
The continuum from AKI to AKD and CKD was highlighted in several studies (18, 20, 22). Among patients with septic shock and AKI, only 27% regained baseline renal function within one year, while 6–10% progressed to CKD despite mild initial AKI stages (22). In the study of AKD, 26.9% of patients developed persistent renal dysfunction, and fewer than 10% of those recovered before discharge (18). These findings underscore the need for vigilant follow-up after discharge, particularly in patients with pre-existing CKD, diabetes, or high creatinine levels at discharge (22).
Mortality and long-term outcomes
Mortality among patients with sepsis-associated AKI (S-AKI) remained high across the included studies, ranging from 13% in stage 1 AKI to >40% in stage 2–3 AKI at one year (22). The presence of both CKD and AKI amplified the risk of death, with adjusted hazard ratios for 90-day mortality reaching 2.2 (95% CI 1.7–2.9) in patients with CKD and stage ≥2 AKI compared to those without kidney disease (20). Patients with persistent AKD after sepsis had particularly poor outcomes, with in-hospital mortality of 19.9% and low rates of renal recovery (9.3%) before discharge (18).
Long-term outcomes showed that renal function recovery was incomplete in a substantial proportion of patients. Only about 27% of patients with septic shock–associated AKI achieved full renal recovery by 12 months, while 6–10% developed CKD regardless of initial AKI severity (22). These findings suggest that the risk of progression to CKD persists even after apparent clinical improvement.
Pediatric survivors of AKI also face long-term consequences, including new-onset hypertension (19%) and increased risk for CKD progression (21). These data reinforce the need for structured post-discharge follow-up programs, early nephrology referral, and surveillance of kidney function and blood pressure in both adult and pediatric populations recovering from septic AKI.
Pediatric findings
Pediatric data remain limited but suggest that children surviving AKI are at substantial risk of developing new-onset hypertension within 90 days (19%) (21). Risk factors included AKD, need for kidney replacement therapy, congenital heart disease, and prior solid organ transplantation (21). These findings highlight the importance of early blood pressure surveillance and nephrology follow-up in pediatric AKI survivors.
Discussion
This systematic review provides comprehensive evidence that CKD, hypertension, and sepsis form an interconnected pathophysiological triad that substantially impacts morbidity and mortality in critically ill patients. Across the nine included studies, pre-existing CKD and hypertension consistently emerged as independent predictors for S-AKI, adverse hemodynamic profiles, and worse survival outcomes (14–16, 20, 22, 23).
Several large-scale cohort studies and meta-analyses confirmed that patients with CKD are highly susceptible to developing S-AKI, and that failure to fully recover renal function after the acute episode may lead to AKD or progression to more advanced stages of CKD (14, 18, 22, 24). Importantly, Liu et al. demonstrated that nearly half of patients with sepsis developed AKI, and both CKD and hypertension were among the strongest predictors of adverse outcomes (14). This underscores the synergistic effect of CKD and hypertension in reducing renal reserve and amplifying vulnerability to septic insults (25).
Key risk factors for CKD development and progression—such as uncontrolled blood pressure, albuminuria, diabetes, dyslipidemia, and obesity—were consistently reported across studies (15–17). Hypertension was identified as the most potent modifiable risk factor, directly driving glomerular injury through intraglomerular hypertension and hyperfiltration. Even mildly elevated albuminuria was associated with future hypertension and CKD, emphasizing its utility as a screening biomarker for early intervention (15, 26). Metabolic comorbidities and advanced age further contribute to microvascular dysfunction, oxidative stress, and reduced nephron reserve, which together increase susceptibility to S-AKI and poor recovery following sepsis (16, 17, 22, 27).
Hemodynamic optimization plays a central role in mitigating S-AKI risk. The retrospective cohort by Li et al. reported that patients with MPP ≥63 mmHg had significantly lower AKI incidence and improved survival (19). These findings support individualized perfusion targets, particularly for patients with chronic hypertension, in whom higher MAP goals may be beneficial. Nevertheless, causality cannot be inferred, and randomized trials are needed to determine optimal hemodynamic thresholds (29).
Mortality remained unacceptably high among patients with S-AKI, particularly in those with advanced AKI stages or concurrent CKD (20, 22). Long-term follow-up data revealed that fewer than one-third of patients achieved full renal recovery by 12 months, and 6–10% progressed to CKD despite apparent initial improvement (22, 30). Pediatric survivors also demonstrated a 19% incidence of new-onset hypertension following AKI, highlighting the need for post-discharge surveillance and early nephrology referral in this population (21, 30).
Several clinical implications emerge from these findings. First, early identification of high-risk patients using baseline kidney function and albuminuria screening is critical for risk stratification (15, 26). Second, individualized hemodynamic targets, including consideration of higher MAP or MPP goals for hypertensive patients, may help preserve renal function (19, 28). Third, structured follow-up programs should be implemented to monitor renal recovery, blood pressure control, and CKD progression in both adults and children following sepsis (21, 30).
This review has several limitations. Most included studies were observational, introducing potential confounding and precluding causal inference. Definitions of AKI, AKD, and sepsis varied across studies, and significant heterogeneity was present in study designs, settings, and outcome reporting (23). The small number of pediatric-focused studies limits generalizability to younger populations, and no pooled meta-analysis could be performed due to heterogeneity. Despite these limitations, the consistency of associations across diverse populations strengthens the reliability of our conclusions (24, 30).
Future research should focus on well-designed prospective studies to define optimal blood pressure and perfusion targets, identify early biomarkers for S-AKI risk stratification, and evaluate interventions to improve renal recovery after sepsis (23, 29). Pediatric-specific trials are urgently needed to establish evidence-based guidelines for this vulnerable group (21, 31).
In conclusion, CKD and hypertension markedly increase the risk and severity of sepsis-related renal complications. Early recognition of at-risk patients, individualized hemodynamic management, and vigilant long-term follow-up represent key strategies to improve outcomes. A multidisciplinary approach involving nephrology, critical care, and pediatrics is essential to mitigate preventable morbidity and mortality in this high-risk population.
Strengths and limitations
This review adhered to PRISMA 2020 guidelines, specified a PECO framework, and extracted effect sizes from all included studies. However, most of the included studies were observational, which introduces residual confounding. Significant heterogeneity exists in defining AKI, AKD, and fluid management protocols, precluding formal meta-analysis. Although we expanded the search to multiple databases, language bias remains possible as we primarily included English and Indonesian full texts.
Future directions
Future research should focus on prospective, multicenter studies to establish optimal hemodynamic targets (MAP and MPP) and fluid strategies in patients with CKD and hypertension. Additionally, standardized definitions for AKD and CKD progression are needed to enable pooling of data across studies. Dedicated pediatric cohorts are urgently required to guide best practices for this vulnerable population.
Conclusion
This systematic review highlights the complex interplay between CKD, hypertension, and septic shock in both adults and pediatric populations. The evidence consistently demonstrates that pre-existing hypertension and CKD are not only significant risk factors for (S-AKI, but also contribute to poor renal recovery and increased mortality during septic episodes. Key predictors such as albuminuria, advanced age, diabetes mellitus, and uncontrolled blood pressure were repeatedly identified across studies as drivers of both CKD progression and sepsis vulnerability. Additionally, impaired hemodynamic regulation—particularly reduced MPP—was shown to exacerbate renal injury in septic patients. Careful fluid management, individualized blood pressure targets, and early identification of high-risk patients using clinical markers like albuminuria are essential to improving outcomes. In pediatric patients, early-onset AKI and hypertension require long-term surveillance due to their potential to trigger lifelong renal compromise. Overall, integrated, multidisciplinary strategies are critical for optimizing care in this high-risk population, minimizing the progression to end-stage kidney disease, and reducing sepsis-related mortality.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
RJ: Investigation, Validation, Software, Methodology, Writing – review & editing, Supervision, Formal Analysis, Resources, Data curation, Visualization, Funding acquisition, Project administration, Conceptualization, Writing – original draft. JS: Conceptualization, Supervision, Investigation, Validation, Writing – review & editing, Writing – original draft, Formal Analysis. BA: Methodology, Conceptualization, Investigation, Writing – review & editing, Validation, Writing – original draft, Visualization. BP: Supervision, Formal Analysis, Methodology, Writing – original draft, Conceptualization, Resources, Investigation, Writing – review & editing, Project administration. CN: Writing – original draft, Writing – review & editing, Investigation, Methodology, Validation, Supervision. IM: Methodology, Conceptualization, Investigation, Writing – review & editing, Resources, Writing – original draft, Formal Analysis, Project administration. LA: Writing – review & editing, Writing – original draft, Project administration, Visualization, Funding acquisition, Methodology, Data curation, Investigation, Conceptualization. IR: Writing – review & editing, Investigation, Writing – original draft, Supervision, Resources, Data curation, Validation, Project administration. CA: Investigation, Writing – review & editing, Methodology, Project administration, Writing – original draft, Formal Analysis. YK: Methodology, Investigation, Supervision, Writing – review & editing, Project administration, Writing – original draft, Data curation. AF: Methodology, Conceptualization, Visualization, Project administration, Writing – review & editing, Writing – original draft, Data curation. NS: Writing – review & editing, Supervision, Writing – original draft, Formal Analysis, Funding acquisition, Investigation, Resources, Conceptualization, Project administration. FS: Formal Analysis, Writing – review & editing, Investigation, Project administration, Conceptualization, Writing – original draft, Methodology, Visualization. MN: Writing – review & editing, Funding acquisition, Supervision, Writing – original draft, Resources, Project administration, Conceptualization. NC: Resources, Methodology, Formal Analysis, Data curation, Investigation, Writing – review & editing, Funding acquisition, Writing – original draft. AP: Resources, Conceptualization, Project administration, Formal Analysis, Methodology, Writing – review & editing, Supervision, Investigation, Writing – original draft. DM: Project administration, Resources, Writing – original draft, Conceptualization, Supervision, Writing – review & editing, Formal Analysis, Investigation, Methodology. MS: Methodology, Formal Analysis, Conceptualization, Writing – original draft, Investigation, Writing – review & editing, Resources. IW: Formal Analysis, Project administration, Resources, Methodology, Writing – review & editing, Conceptualization, Writing – original draft. AB: Conceptualization, Writing – review & editing, Methodology, Project administration, Writing – original draft, Resources, Visualization. MZ: Resources, Writing – original draft, Validation, Writing – review & editing, Supervision, Data curation, Conceptualization. RL: Project administration, Writing – review & editing, Validation, Writing – original draft, Methodology, Data curation, Investigation. MA: Methodology, Writing – review & editing, Investigation, Writing – original draft, Resources, Data curation, Conceptualization, Project administration. HA: Writing – original draft, Supervision, Writing – review & editing, Investigation, Project administration, Methodology, Validation. KK: Formal Analysis, Writing – review & editing, Data curation, Validation, Writing – original draft, Conceptualization, Investigation, Supervision, Visualization, Resources.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Rayyan AI was used to assist in screening articles during the systematic review process.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease,1990e 2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2020) 395:709e33. doi: 10.1016/S0140-6736(20)30045-3
2. Stevens PE, Ahmed SB, Carrero JJ, Foster B, Francis A, Hall RK, et al. KDIGO 2024 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. (2024) 105:S117e314. doi: 10.1016/j.kint.2023.10.018
3. Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders H-J, et al. Acute kidney injury. Nat Rev Dis Primers. (2021) 7:52. doi: 10.1038/s41572-021-00284-z
4. Zarbock A, Nadim MK, Pickkers P, Gomez H, Bell S, Joannidis M, et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. (2023) 19:1–21. doi: 10.1038/s41581-023-00683-3
5. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47:1181–247. doi: 10.1007/s00134-021-06506-y
6. Tang Y, Sorenson J, Lanspa M, Grissom CK, Mathews VJ, Brown SM, et al. Systolic blood pressure variability in patients with early severe sepsis or septic shock: a prospective cohort study. MC Anesthesiology. (2017) 17:82. doi: 10.1186/s12871-017-0377-4
7. Kato R and Pinsky M. Personalizing blood pressure management in septic shock. Ann Intensive Care. (2015) 5:41. doi: 10.1186/s13613-015-0085-5
8. Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. (2015) 41:1411–23. doi: 10.1007/s00134-015-3934-7
9. Pedro Tda C, Morcillo AM, and Baracat EC. Etiology and prognostic factors of sepsis among children and adolescents admitted to the intensive care unit. Rev Bras Ter Intensiva. (2015) 27:240–6. doi: 10.5935/0103-507X.20150044
10. Garcia PCR, Tonial CT, and Piva JP. Septic shock in pediatrics: the state-of-the-art. J Pediatr (Rio J). (2020) 96 Suppl 1:87–98. doi: 10.1016/j.jped.2019.10.007
11. He G, Li C, Zhong X, Wang F, Wang H, Shi Y, et al. Risk factors for progression of chronic kidney disease with glomerular etiology in hospitalized children. Front Pediatr. (2021) 9:752717. doi: 10.3389/fped.2021.752717
12. Zarbock A and Kellum JA. Sepsis-associated AKI: from mechanisms to management. Nat Rev Nephrol. (2023) 19:401–17. doi: 10.1038/s41581-023-00683-3
13. Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, and Anders HJ. Acute kidney injury. In: Nature Reviews Disease Primers, London, UK: Nature Portfolio vol. 7. (2021). doi: 10.1038/s41572-021-00284-z
14. Liu J, Xie H, Ye Z, Li F, and Wang L. Rates, predictors, and mortality of sepsis-associated acute kidney injury: A systematic review and meta-analysis. BMC Nephrol. (2020) 21:318. doi: 10.1186/s12882-020-01974-8
15. Huang M, Matsushita K, Sang Y, Ballew SH, Astor BC, and Coresh J. Association of kidney function and albuminuria with prevalent and incident hypertension: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. (2015) 65:58–66. doi: 10.1053/j.ajkd.2014.06.025
16. Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, Shiigai T, et al. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int. (2007) 71:159–66. doi: 10.1038/sj.ki.5002017
17. Bahrey D, Gebremedhn G, Mariye T, Girmay A, Aberhe W, Hika A, et al. Prevalence and associated factors of chronic kidney disease among adult hypertensive patients in Tigray teaching hospitals: a cross-sectional study. BMC Res Notes. (2019) 12:562. doi: 10.1186/s13104-019-4610-8
18. Peerapornratana S, Priyanka P, Wang S, Smith A, Singbartl K, Palevsky PM, et al. ProCESS and proGReSS-AKI investigators. Sepsis-associated acute kidney disease. Kidney Int Rep. (2020) 5:839–50. doi: 10.1016/j.ekir.2020.03.005
19. Li L, Qin S, Lu X, Huang L, Xie M, and Huang D. Association between the mean perfusion pressure and the risk of acute kidney injury in critically ill patients with sepsis: a retrospective cohort study. BMC Infect Dis. (2024) 24:806. doi: 10.1186/s12879-024-09706-1
20. Neyra JA, Mescia F, Li X, Adams-Huet B, Yessayan L, Yee J, et al. Impact of acute kidney injury and CKD on adverse outcomes in critically ill septic patients. Kidney Int Rep. (2018) 3:1344–53. doi: 10.1016/j.ekir.2018.07.016
21. Patel M, Hornik C, Diamantidis C, Selewski DT, and Gbadegesin R. A reappraisal of risk factors for hypertension after pediatric acute kidney injury. Pediatr Nephrol. (2024) 39:1599–605. doi: 10.1007/s00467-023-06222-3
22. Kim JS, Kim YJ, Ryoo SM, Sohn CH, Seo DW, Ahn S, et al. One–year progression and risk factors for the development of chronic kidney disease in septic shock patients with acute kidney injury: A single-centre retrospective cohort study. J Clin Med. (2018) 7:554. doi: 10.3390/jcm7120554
23. De Backer D, Deutschman CS, Hellman J, Myatra SN, Ostermann M, Prescott HC, et al. Surviving Sepsis Campaign Research Committee — update and research priorities for kidney outcomes in sepsis. Intensive Care Med. (2024) 52:2021–3. doi: 10.1097/CCM.0000000000006135
24. Georgianos PI and Agarwal R. Hypertension in chronic kidney disease — treatment standard 2023. Clin Kidney J. (2023) 17:ii36–50. doi: 10.1093/ckj/sfad110
25. Thomas J, Stonebrook E, and Kallash M. Pediatric hypertension: Review of the definition, diagnosis, and initial management. Int J Pediatr Adolesc Med. (2022) 9:1–6. doi: 10.1016/j.ijpam.2020.09.005
26. Uber AM and Sutherland SM. Acute kidney injury in hospitalized children: consequences and outcomes. In: Pediatric Nephrology, vol. 35. Berlin, Germany: Springer (2020). p. 213–20. doi: 10.1007/s00467-018-4128-7
27. Spandidos Publications Team. Risk factors of acute kidney injury, septic shock and acute respiratory distress syndrome in sepsis patients with positive blood cultures. Exp Ther Med. (2024) 28:12792. doi: 10.3892/etm.2024.12792
28. Park CH, Kim HW, Park JT, Chang TI, Yoo T-H, Oh K-H, et al. CRIC and KNOW-CKD Investigators. BP and kidney disease progression in advanced CKD: findings from CRIC and KNOW-CKD. Clin J Am Soc Nephrol. (2025) 20:1179–89. doi: 10.2215/CJN.0000000760
29. Wang D, Sun T, and Liu Z. Sepsis-associated acute kidney injury. Intensive Care Res. (2023) 3:251–8. doi: 10.1007/s44231-023-00049-0
30. Yong Y, Dong J, Chen X, Chen R, and Wang H. Incidence, risk factors and clinical outcomes of septic acute renal injury in cancer patients with sepsis admitted to the ICU: A retrospective study. Front Med (Lausanne). (2022) 9:1015735. doi: 10.3389/fmed.2022.1015735
Keywords: chronic kidney disease (CKD), hypertension, sepsis, septic shock, fluid management, central venous pressure (CVP)
Citation: Javier RM, Salim J, Aji BL, Pradipta BPA, Nur C, Muhammad I, Aflah LN, Rettauli IAB, Audrey C, Wijayaningtyas I, Kosen YD, Fajriyadi A, Sabila N, Salipadang FP, Nugraha MA, Cheda NY, Putra APY, Mahasin DF, Subiyanto MD, Berlian AZ, Hadiaturahman MZ, Luthfi RK, Aditya MR, Arif HC and Kurniawan K (2025) Risk factors for chronic kidney disease and septic shock with hypertension in adults and children. Front. Nephrol. 5:1671763. doi: 10.3389/fneph.2025.1671763
Received: 23 July 2025; Accepted: 30 September 2025;
Published: 22 October 2025.
Edited by:
Lei Yin, Shanghai Jiaotong University School of Medicine, ChinaReviewed by:
Ramona Stroescu, Victor Babes University of Medicine and Pharmacy, RomaniaYaman Walid Kassab, National University of Science and Technology, Oman
Copyright © 2025 Javier, Salim, Aji, Pradipta, Nur, Muhammad, Aflah, Rettauli, Audrey, Wijayaningtyas, Kosen, Fajriyadi, Sabila, Salipadang, Nugraha, Cheda, Putra, Mahasin, Subiyanto, Berlian, Hadiaturahman, Luthfi, Aditya, Arif and Kurniawan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. Mohamad Javier, ampmb3J3b3JrOThAZ21haWwuY29t
 R. Mohamad Javier
R. Mohamad Javier Jonathan Salim
Jonathan Salim Bethari Lekso Aji3
Bethari Lekso Aji3 Choirin Nur
Choirin Nur Yosua Darmadi Kosen
Yosua Darmadi Kosen Fernando Pangruruk Salipadang
Fernando Pangruruk Salipadang Mahardika Adhitya Nugraha
Mahardika Adhitya Nugraha Andra Purwanto Yogatama Putra
Andra Purwanto Yogatama Putra Muhamad Zulfikar Hadiaturahman
Muhamad Zulfikar Hadiaturahman Kristian Kurniawan
Kristian Kurniawan