- 1Department of Nephrology, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung, Taiwan
- 2Department of Rheumatology, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 3Department of Rheumatology, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung, Taiwan
Background: Sarcopenia has emerged as one of the major complications in end-stage kidney disease (ESKD), leading to greater disability and poor long-term outcomes. This study aimed to compare quadriceps muscle sonoelastographic parameters between ESKD patients with and without sarcopenia.
Materials and methods: We prospectively enrolled 50 ESKD patients with sarcopenia and 50 ESKD patients without sarcopenia as controls. All participants underwent clinical and laboratory evaluation, sonoelastography of the quadriceps muscle, and dual-energy X-ray absorptiometry (DXA) for muscle mass assessment. Sarcopenia was diagnosed according to the revised European Working Group on Sarcopenia in Older People (EWGSOP2, 2019), which emphasizes muscle strength as the principal determinant. Handgrip strength, gait speed, and appendicular skeletal muscle mass (ASM/height²) by DXA were assessed. The elastography ratio was calculated as the stiffness of the quadriceps muscle relative to the overlying subcutaneous tissue. Comparisons were made between the sarcopenia and non-sarcopenia groups.
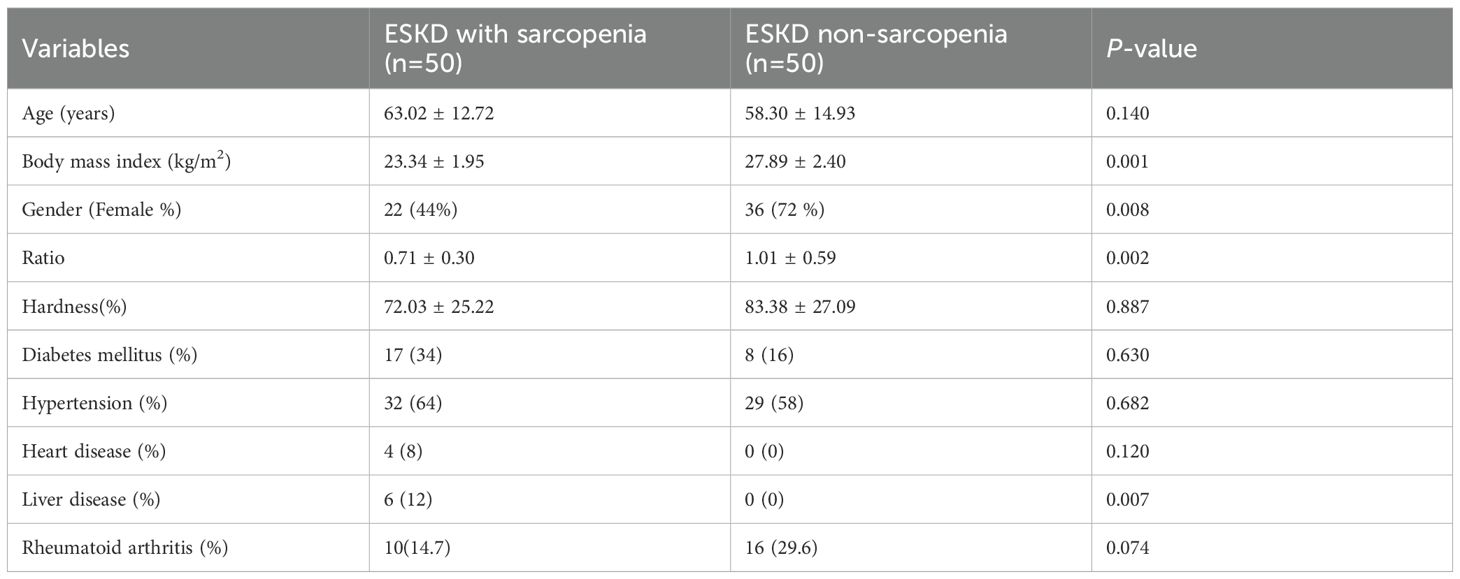
Results: A total of 100 ESKD patients were included: 50 with sarcopenia (mean age, 63.0 ± 12.7 years) and 50 without sarcopenia (mean age, 58.3 ± 14.9 years). The sarcopenia group demonstrated a lower quadriceps-to-subcutaneous tissue elastography ratio compared with the control group. Multivariate logistic regression identified the quadriceps-to-subcutaneous tissue ratio, muscle hardness, and body mass index (BMI) as independent predictors of sarcopenia (p < 0.05). Lower BMI was associated with an increased risk of sarcopenia. The optimal quadriceps-to-subcutaneous tissue elastography ratio cut-off value was 0.885 (sensitivity 82.4%; specificity 66.7%).
Conclusion: Sonoelastography provides a reliable and non-invasive assessment of quadriceps muscle stiffness and demonstrates good predictive value for detecting sarcopenia in ESKD patients. Given its accessibility, low cost, and ease of use, sonoelastography may serve as a valuable adjunct to conventional DXA in evaluating muscle quality in this high-risk population.
Introduction
The global population is aging, highlighting the importance of understanding degenerative processes that accompany advancing age (1). One such process is sarcopenia, defined as the progressive, age-related loss of skeletal muscle mass, strength, and physical performance (2). Sarcopenia is associated with adverse health outcomes, including impaired mobility, increased risk of falls and fractures, difficulty performing activities of daily living, disability, loss of independence, and increased mortality death (3–5).
The etiology of sarcopenia is multifactorial, involving environmental influences, chronic diseases, systemic inflammation, physical inactivity, mitochondrial dysfunction, neuromuscular junction degeneration, depletion of satellite cells, and hormonal alterations (6). Sarcopenia is particularly prevalent in patients with chronic kidney disease (CKD) (7–9), with estimates suggesting that approximately 37% of dialysis patients are affected (10). In this population, muscle loss is strongly linked to increased morbidity and mortality, especially due to cardiovascular complications (11, 12). Early detection and assessment of modifiable risk factors are therefore crucial.
While most studies focus on sarcopenia in patients with CKD receiving dialysis, fewer have investigated sarcopenia in those with end-stage kidney disease (ESKD) who are not yet on dialysis (13, 14). Identifying reliable, non-invasive assessment tools in this population may help improve early diagnosis and intervention.
Sonoelastography—first introduced by Ophir et al.—is an imaging technique that measures the deformation of tissue in response to external force applied by an ultrasound transducer (13). Tissue stiffness varies depending on its structural composition: soft tissues deform more readily, whereas stiffer tissues deform less (14, 15). This mechanical property is displayed on the ultrasound monitor as a color spectrum, where blue typically denotes stiff regions, red represents soft regions, and green indicates intermediate stiffness (16). Initially applied to differentiate benign from malignant tumors, sonoelastography is most commonly used in breast and thyroid imaging. Its use in the musculoskeletal system is relatively recent but is gaining interest (17–19).
In musculoskeletal evaluation, sonographic assessment of the quadriceps muscle is often one of the first imaging approaches, complementing clinical examination and radiography. However, to date, no published studies have specifically examined quadriceps muscle stiffness using sonoelastography in ESKD patients with sarcopenia.
Therefore, the present study aimed to compare quadriceps muscle sonoelastographic findings between ESKD patients with and without sarcopenia, and to explore the potential role of sonoelastography in the early detection of sarcopenia in this high-risk population.
Materials and methods
Study design
This was a case–control study conducted over a one-year period. The case group consisted of patients diagnosed with end-stage kidney disease (ESKD) and sarcopenia, while the control group included ESKD patients without sarcopenia.
Patient selection
Diagnostic criteria for ESKD
ESKD was diagnosed according to established guidelines (22), based on evidence of kidney damage and/or reduced kidney function as determined by glomerular filtration rate (GFR), irrespective of the underlying cause.
Diagnostic criteria for sarcopenia (EWGSOP2, 2019)
Sarcopenia was diagnosed according to the updated EWGSOP2 guidelines. The diagnostic process followed a hierarchical approach emphasizing muscle strength as the key criterion:
1. Muscle Strength: Handgrip strength was measured using a calibrated Jamar dynamometer in the dominant hand. Low muscle strength was defined as <27 kg for men and <16 kg for women.
2. Muscle Mass: Appendicular skeletal muscle mass (ASM) was measured by dual-energy X-ray absorptiometry (DXA). Low muscle mass was defined as ASM/height² <7.0 kg/m² for men and <5.5 kg/m² for women.
3. Physical Performance: Gait speed was evaluated over a 4-meter walk at usual pace. Low physical performance was defined as gait speed ≤0.8 m/s.
Sarcopenia was confirmed when both low muscle strength and low muscle mass were present. Severe sarcopenia was diagnosed when all three components were reduced.
Patients were reclassified according to EWGSOP2 criteria. After reanalysis, 48 patients fulfilled the definition of sarcopenia and 52 were classified as non-sarcopenic. The distribution was comparable to the previous EWGSOP (2010) classification due to consistent overlap of low strength and low mass in this advanced ESKD cohort.
According to EWGSOP2 (2019) criteria, 48 patients were classified as sarcopenic and 52 as non-sarcopenic. Baseline demographic and clinical characteristics were similar to the previous analysis. The sarcopenia group demonstrated significantly lower quadriceps-to-subcutaneous tissue elastography ratios compared with the control group. Multivariate logistic regression confirmed that lower elastography ratio, reduced muscle hardness, and lower BMI remained independent predictors of sarcopenia (p < 0.05). The optimal cut-off value for the quadriceps-to-subcutaneous ratio was 0.885 (sensitivity 82.4%; 1-specificity 33.3%).
Inclusion criteria
• Patients aged 20–70 years with a confirmed diagnosis of ESKD.
Exclusion criteria
Patients were excluded if they had any of the following:
1. Active infection.
2. History of primary intracerebral hemorrhage, myocardial infarction, unstable symptomatic peripheral vascular disease, major surgery, or systemic hemorrhage within the previous 3 months.
3. Known malignancy or hematologic disorders affecting thrombosis, platelet count, or function.
4. Severe systemic disease such as liver cirrhosis or congestive heart failure.
Clinical and laboratory assessment
Baseline demographic and clinical data, including age, sex, symptom duration, and current medications, were recorded. Laboratory assessments were performed according to standard protocols.
Muscle mass assessment by DXA
Muscle mass was measured using dual-energy X-ray absorptiometry (DXA). Low muscle mass was defined as an appendicular skeletal muscle mass index (ASM/height²) more than two standard deviations below the mean value of a healthy young reference population (<7.23 kg/m² for men and <5.67 kg/m² for women).
Ultrasound sonoelastography
Sonoelastographic measurements of the quadriceps muscle were performed using a high-frequency linear transducer. Quantitative values were obtained from circular regions of interest placed within the quadriceps muscle. Images were acquired in the longitudinal plane during the compression phase. Conventional B-mode images were displayed on the left side of the screen, and color-coded sonoelastographic maps on the right. Tissue stiffness was represented by a standardized color scale, and elastography ratios were calculated as the stiffness of the quadriceps muscle relative to the overlying subcutaneous tissue (20).
Statistical analysis
The statistical analysis was conducted by using SPSS version 22 software (IBM Corporation, Armonk, NY). Multivariate regressions and T-tests were utilized to compare difference and correlation of sonoelastography and dual energy X-ray absorptimetry assessments in ESKD with sarcopenia. Significance levels were set at P <.05.
Results
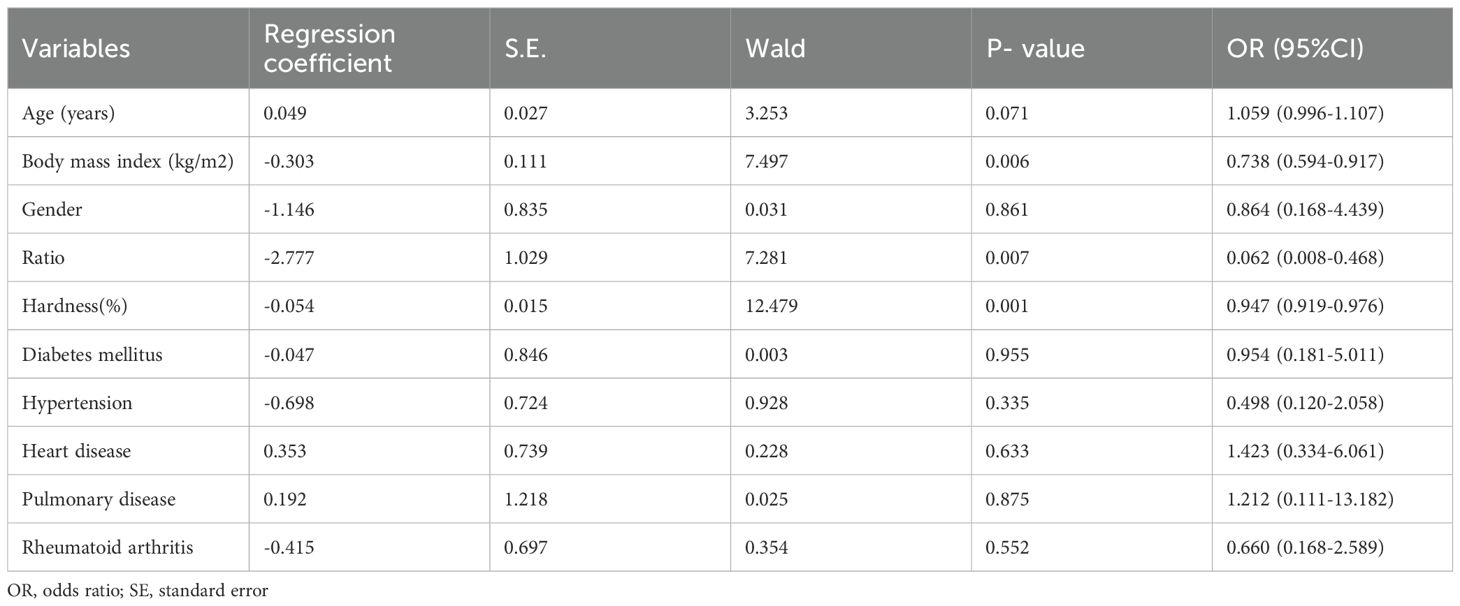
A total of 100 ESKD patients including 50 with sarcopenia (mean age 63.02 ± 12.72 years) and 50 without sarcopenia (mean age 58.30 ± 14.936 years) were enrolled. Sonoelastography showed quadriceps hardness did not differ significantly between groups (p = 0.887) and a lower elastography ratio of quadriceps over subcutaneous tissue than the non-sarcopenia group (Table 1; Figure 1). Multivariate logistical regression analysis showed that the quadriceps to subcutaneous ratio, hardness and BMI could predict sarcopenia (p<0.05) (Table 2), while a lower BMI increased the risk of sarcopenia. The optimal quadriceps-to-subcutaneous tissue elastography ratio cut-off value was 0.885 (sensitivity 82.4%; specificity 66.7%).
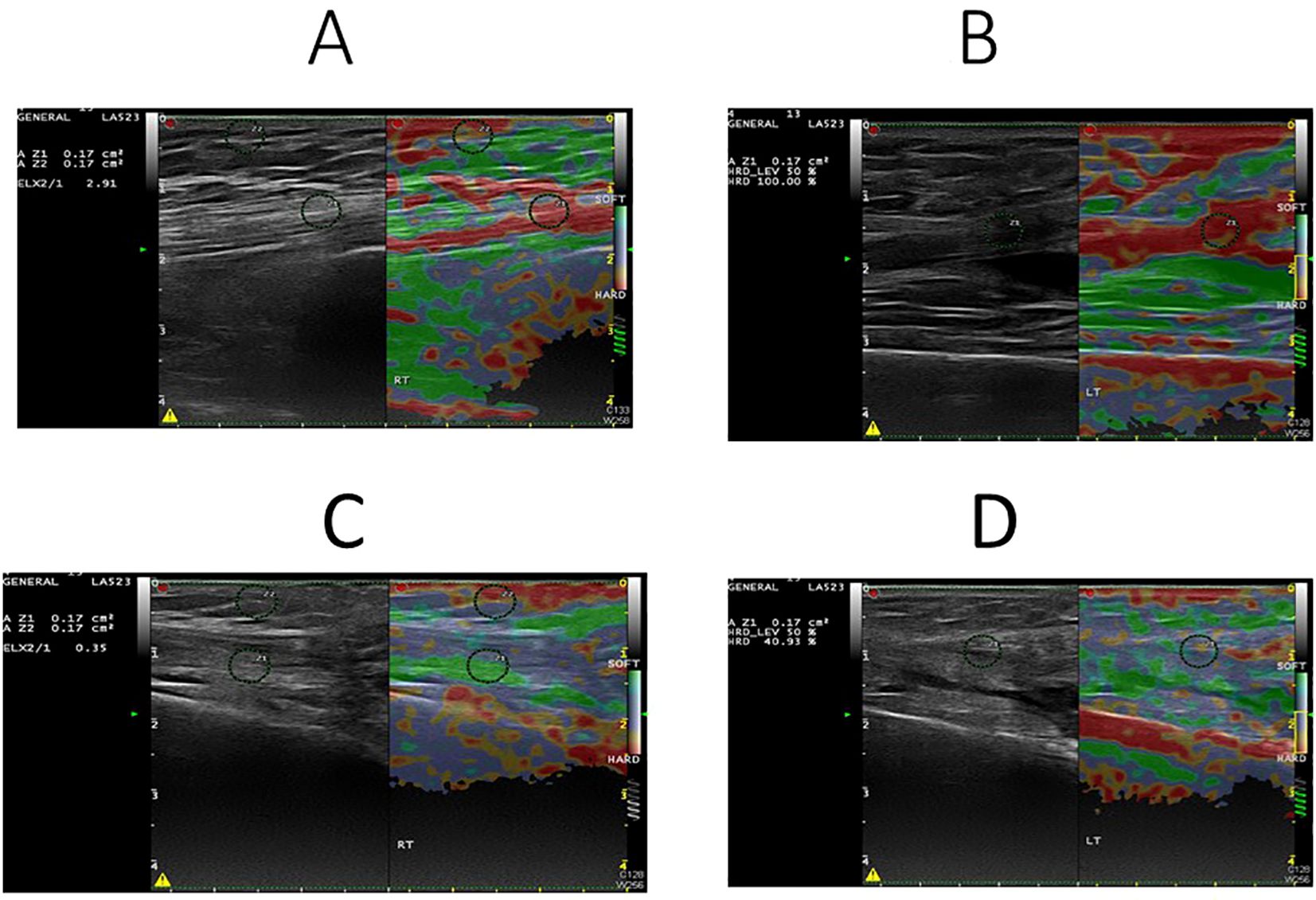
Figure 1. Sonoelastography images of quadriceps muscle in normal (A, B) and sarcopenic (C, D) patients. The elasticity ratio of quadriceps muscle to subcutaneous fat was 2.91 in the normal patient (A) and 0.35 in the sarcopenic patient (C). Correspondingly, muscle hardness was 100% in the normal patient (B) and 40.93% in the sarcopenic patient (D).
Discussion
This study demonstrates that sonoelastography of the quadriceps muscle is a valuable and non-invasive tool for predicting sarcopenia in patients with end-stage kidney disease (ESKD). By utilizing the quadriceps-to-subcutaneous tissue elastography ratio and muscle hardness, we found that sonoelastography provides strong predictive power for sarcopenia. The quadriceps muscle plays a critical role in daily functional activities, and its structural and mechanical properties are closely linked to mobility and independence in older and chronically ill populations (24). Our findings establish a feasible protocol for obtaining reproducible sonoelastographic measurements of the quadriceps muscle.
Degenerative changes in the quadriceps muscle can arise from lipid infiltration, repetitive microtrauma, and hypoxia, leading to microscopic structural alterations. Although the clinical diagnosis can be challenging, both ultrasound and magnetic resonance imaging have proven useful in detecting muscle abnormalities (19, 21). While muscle rupture is rare in healthy individuals, degenerative processes from aging, fatty infiltration, steroid use, and sarcopenia can predispose the muscle to rupture under relatively low mechanical stress (22, 23). High-frequency ultrasound transducers allow detailed visualization of the quadriceps muscle due to its superficial location. When imaged perpendicularly, the muscle exhibits high echogenicity, with longitudinal collagen fibrils surrounded by a peritendinous sheath, visible as parallel echogenic lines on ultrasound. Ultrasound has been reported to have high sensitivity and specificity for detecting quadriceps muscle rupture (24). Sonoelastography extends these capabilities by providing information on tissue stiffness and mechanical integrity (14, 25). In this study, we applied compression elastography, which measures tissue strain in response to controlled pressure (26). After reclassification using EWGSOP2, 48 patients were identified as sarcopenic and 52 as non-sarcopenic. Although the sample distribution remained comparable to that under EWGSOP (2010), this consistency is attributable to the clinical characteristics of our advanced ESKD cohort, in which reduced muscle mass was almost invariably accompanied by impaired muscle strength. Consequently, patients classified as sarcopenic by EWGSOP (2010) largely overlapped with those meeting EWGSOP2 criteria, resulting in similar statistical outcomes. This reanalysis confirms that our findings are robust across contemporary diagnostic standards and supports the external validity of quadriceps sonoelastography as a predictor of sarcopenia.
Previous studies have shown that diseased muscle appear softer on sonoelastography compared to healthy ones, with color mapping revealing a transition from red (stiff) to green (soft) (27). Consistent with these findings, our sarcopenia group exhibited significantly reduced muscle stiffness and increased heterogeneity in elastographic patterns. Given that muscle quality is influenced by adipose and fibrous tissue infiltration, sonoelastography may provide indirect insights into muscle composition and functional capacity (28, 29).
To our knowledge, this is the first study to assess quadriceps muscle elasticity using sonoelastography in ESKD patients. The observed decrease in muscle stiffness among sarcopenic patients suggests that sonoelastography could enhance diagnostic accuracy for muscle quality assessment. Furthermore, our results showed that lower BMI was associated with sarcopenia, aligning with prior reports linking low BMI to increased long-term mortality risk. Although our study did not include mortality data, future longitudinal research is warranted to explore prognostic implications.
This study has limitations. First, the sample size was relatively small. Second, sonoelastography is highly operator-dependent, and we did not assess inter- or intra-observer variability. Lastly, standardized protocols for quadriceps muscle sonoelastography are currently lacking, limiting cross-study comparisons. This study did not include inflammatory markers (e.g., CRP, TNF-α, IL-6), nutritional indicators (albumin), or functional performance tests (grip strength, gait speed). These are important confounders. We also lacked longitudinal follow-up for falls, hospitalization, and mortality. Operator variability and medication effects (steroids, immunosuppressants) were not analyzed. Future studies should address these aspects.
In conclusion, sonoelastography is a reliable, accurate, and accessible imaging modality for evaluating muscle stiffness and quality. It holds potential for early detection of sarcopenia-related muscle changes, guiding preventive and therapeutic strategies in ESKD patients.
Sarcopenia was diagnosed according to the revised European Working Group on Sarcopenia in Older People (EWGSOP2, 2019), which emphasizes muscle strength as the principal determinant. Handgrip strength, gait speed, and appendicular skeletal muscle mass (ASM/height²) by DXA were assessed. Sarcopenia was defined as low muscle strength combined with low muscle mass, and severe sarcopenia was defined as low muscle strength, low muscle mass, and low gait speed.
According to EWGSOP2 (2019) criteria, 48 patients were classified as sarcopenic and 52 as non-sarcopenic. Baseline demographic and clinical characteristics were similar to the previous analysis. The sarcopenia group demonstrated significantly lower quadriceps-to-subcutaneous tissue elastography ratios compared with the control group. Multivariate logistic regression confirmed that lower elastography ratio, reduced muscle hardness, and lower BMI remained independent predictors of sarcopenia (p < 0.05). The optimal cut-off value for the quadriceps-to-subcutaneous ratio was 0.885 (sensitivity 82.4%; 1-specificity 33.3%).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the local Institutional Review Board of Chang Gung Memorial Hospital in Kaohsiung City NiaoSong. IRB No: 201700882A3. All participants had signed a written informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Y-CC: Project administration, Writing – original draft, Validation, Resources, Visualization, Data curation, Investigation, Methodology, Conceptualization, Supervision, Funding acquisition, Software, Formal Analysis, Writing – review & editing. C-HC: Validation, Data curation, Conceptualization, Formal Analysis, Methodology, Project administration, Supervision, Writing – review & editing, Writing – original draft, Resources, Investigation, Visualization, Software, Funding acquisition. J-FC: Data curation, Writing – review & editing, Project administration, Formal Analysis, Validation, Writing – original draft, Methodology, Software, Resources, Investigation, Visualization, Conceptualization, Funding acquisition, Supervision. S-FY: Investigation, Resources, Writing – original draft, Funding acquisition, Software, Formal Analysis, Visualization, Conceptualization, Project administration, Data curation, Validation, Methodology, Writing – review & editing, Supervision. C-YH: Visualization, Investigation, Software, Resources, Conceptualization, Validation, Funding acquisition, Supervision, Data curation, Project administration, Writing – review & editing, Formal Analysis, Methodology, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung grant CMRPG8G1491. The funding body played no role in the study design, writing of the manuscript, or decision to submit the manuscript for publication.
Acknowledgments
The authors are grateful to the Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. (1997) 127:990S–1S. doi: 10.1093/jn/127.5.990S
2. Reiss J, Iglseder B, Alzner R, Mayr-Pirker B, Pirich C, Kassmann H, et al. Consequences of applying the new EWGSOP2 guideline instead of the former EWGSOP guideline for sarcopenia case finding in older patients. Age Ageing. (2019) 48:719–24. doi: 10.1093/ageing/afz035
3. Janssen I, Heymsfield SB, and Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. (2002) 50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x
4. Metter EJ, Talbot LA, Schrager M, and Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. (2002) 57:B359–65. doi: 10.1093/gerona/57.10.B359
5. Lino VT, Rodrigues NC, O’Dwyer G, Andrade MK, Mattos IE, and Portela MC. Handgrip Strength and Factors Associated in Poor Elderly Assisted at a Primary Care Unit in Rio de Janeiro, Brazil. PloS One. (2016) 11:e0166373. doi: 10.1371/journal.pone.0166373
6. Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. (2012) 24:623–7. doi: 10.1097/BOR.0b013e328358d59b
7. Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care. (2010) 33:1497–9. doi: 10.2337/dc09-2310
8. Dogan MH, Karadag B, Ozyigit T, Kayaoglu S, Ozturk AO, and Altuntas Y. Correlations between sarcopenia and hypertensive target organ damage in a Turkish cohort. Acta Clin Belg. (2012) 67:328–32.
9. Souza VA, Oliveira D, Mansur HN, Fernandes NM, and Bastos MG. Sarcopenia in chronic kidney disease. J Bras Nefrol. (2015) 37:98–105. doi: 10.5935/0101-2800.20150014
10. Kim JK, Choi SR, Choi MJ, Kim SG, Lee YK, Noh JW, et al. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin Nutr. (2014) 33:64–8. doi: 10.1016/j.clnu.2013.04.002
11. Fahal IH. Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant. (2014) 29:1655–65. doi: 10.1093/ndt/gft070
12. Macedo C, Amaral TF, Rodrigues J, Santin F, and Avesani CM. Malnutrition and sarcopenia combined increases the risk for mortality in older adults on hemodialysis. Front Nutr. (2021) 8:721941. doi: 10.3389/fnut.2021.721941
13. Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, and Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. (1991) 13:111–34. doi: 10.1177/016173469101300201
14. Wadugodapitiya S, Sakamoto M, Suzuki S, Morise Y, and Kobayashi K. In vivo stiffness assessment of patellar and quadriceps tendons by strain ultrasound elastography. BioMed Mater Eng. (2021) 32:257–66. doi: 10.3233/BME-206016
15. Moon SW, Lee SH, Woo A, Leem AY, Lee SH, Chung KS, et al. Reference values of skeletal muscle area for diagnosis of sarcopenia using chest computed tomography in Asian general population. J Cachexia Sarcopenia Muscle. (2022) 13:955–65. doi: 10.1002/jcsm.12946
16. Lalitha P, Reddy M, and Reddy KJ. Musculoskeletal applications of elastography: a pictorial essay of our initial experience. Korean J Radiol. (2011) 12:365–75. doi: 10.3348/kjr.2011.12.3.365
17. Klauser AS and Peetrons P. Developments in musculoskeletal ultrasound and clinical applications. Skeletal Radiol. (2010) 39:1061–71. doi: 10.1007/s00256-009-0782-y
18. De Zordo T, Lill SR, Fink C, Feuchtner GM, Jaschke W, Bellmann-Weiler R, et al. Real-time sonoelastography of lateral epicondylitis: comparison of findings between patients and healthy volunteers. AJR Am J Roentgenol. (2009) 193:180–5. doi: 10.2214/AJR.08.2020
19. Klauser AS, Faschingbauer R, and Jaschke WR. Is sonoelastography of value in assessing tendons? Semin Musculoskelet Radiol. (2010) 14:323–33.
20. Teber MA, Ogur T, Bozkurt A, Er B, Turan A, Gulbay M, et al. Real-time sonoelastography of the quadriceps tendon in patients undergoing chronic hemodialysis. J Ultrasound Med. (2015) 34:671–7. doi: 10.7863/ultra.34.4.671
21. Nallamshetty L, Nazarian LN, Schweitzer ME, Morrison WB, Parellada JA, Articolo GA, et al. Evaluation of posterior tibial pathology: comparison of sonography and MR imaging. Skeletal Radiol. (2005) 34:375–80. doi: 10.1007/s00256-005-0903-1
22. Kaplan PA, Matamoros A Jr, and Anderson JC. Sonography of the musculoskeletal system. AJR Am J Roentgenol. (1990) 155:237–45. doi: 10.2214/ajr.155.2.2115246
23. Fornage BD. The hypoechoic normal tendon. A pitfall. J Ultrasound Med. (1987) 6:19–22. doi: 10.7863/jum.1987.6.1.19
24. Rai V, Dietz NE, Dilisio MF, Radwan MM, and Agrawal DK. Vitamin D attenuates inflammation, fatty infiltration, and cartilage loss in the knee of hyperlipidemic microswine. Arthritis Res Ther. (2016) 18:203. doi: 10.1186/s13075-016-1099-6
25. Bianchi S, Zwass A, Abdelwahab IF, and Banderali A. Diagnosis of tears of the quadriceps tendon of the knee: value of sonography. AJR Am J Roentgenol. (1994) 162:1137–40. doi: 10.2214/ajr.162.5.8165998
26. Drakonaki EE, Allen GM, and Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol. (2012) 85:1435–45. doi: 10.1259/bjr/93042867
27. Sharma P and Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. (2006) 6:181–90.
28. Wilhelm EN, Rech A, Minozzo F, Radaelli R, Botton CE, and Pinto RS. Relationship between quadriceps femoris echo intensity, muscle power, and functional capacity of older men. Age (Dordr). (2014) 36:9625. doi: 10.1007/s11357-014-9625-4
Keywords: end-stage kidney disease (ESKD), sarcopenia, ultrasound sonoelastography, dual-energy X-ray absorptiometry (DXA), quadriceps
Citation: Chiu C-H, Chen J-F, Yu S-F, Hsu C-Y and Chen Y-C (2025) Decreased quadriceps muscle stiffness on ultrasound elastography is associated with sarcopenia in end-stage kidney disease. Front. Nephrol. 5:1682826. doi: 10.3389/fneph.2025.1682826
Received: 09 August 2025; Accepted: 27 October 2025;
Published: 11 December 2025.
Edited by:
Heitor S. Ribeiro, University of Brasilia, BrazilReviewed by:
Alessandro Domenico Quercia, Nephrology and Dialysis ASLCN1, ItalyFatemeh Pourteymour Fard Tabrizi, Tabriz University of Medical Sciences, Iran
Copyright © 2025 Chiu, Chen, Yu, Hsu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-Chou Chen, cjgyMDcxM0BtczEzLmhpbmV0Lm5ldA==
 Chien-Hua Chiu
Chien-Hua Chiu Jia-Feng Chen2
Jia-Feng Chen2 Ying-Chou Chen
Ying-Chou Chen