- 1Dipartimento di Fisica e Astronomia G. Galilei, Università degli Studi di Padova, Padova, Italy
- 2Dipartimento di Ingegneria, Università di Palermo, Palermo, Italy
- 3Department of Data Analysis, Ghent University, Ghent, Belgium
- 4Dipartimento Interateneo di Fisica, Università degli Studi di Bari Aldo Moro, Bari, Italy
- 5Center of Innovative Technologies for Signal Detection and Processing (TIRES), Università degli Studi di Bari Aldo Moro, Bari, Italy
The study of high order dependencies in complex systems has recently led to the introduction of statistical synergy, a novel quantity corresponding to a form of emergence in which patterns at large scales are not traceable from lower scales. As a consequence, several works in the last years dealt with the synergy and its counterpart, the redundancy. In particular, the O-information is a signed metric that measures the balance between redundant and synergistic statistical dependencies. In spite of its growing use, this metric does not provide insight about the role played by low-order scales in the formation of high order effects. To fill this gap, the framework for the computation of the O-information has been recently expanded introducing the so-called gradients of this metric, which measure the irreducible contribution of a variable (or a group of variables) to the high order informational circuits of a system. Here, we review the theory behind the O-information and its gradients and present the potential of these concepts in the field of network physiology, showing two new applications relevant to brain functional connectivity probed via functional resonance imaging and physiological interactions among the variability of heart rate, arterial pressure, respiration and cerebral blood flow.
1 Introduction
Two of the fields of complex systems science which are experiencing an increasing interest in the last years are (i) the analysis of high order interactions and (ii) the decomposition of multivariate information in redundant and synergistic contributions.
High order interactions represent the structural organization of couplings, in a complex system, where interactions may involve groups of three or more units. Indeed, high order structures, such as hypergraphs and simplicial complexes, are thought to be better tools than dyadic networks to map the real organization of many social, biological and man-made systems (Battiston et al., 2020; Battiston et al., 2021). On the other hand, the decomposition of multivariate information in redundant and synergistic contributions is related to the joint probability distribution of the system, evaluated exploiting samples from its dynamics, and speaks to the properties of the marginal probabilities of groups of variables. Since the pairwise description has been found to be insufficient for explaining the orchestrated information flow among multiple components of complex systems, the quantification of high order statistical dependencies attracted the attention of a large community (Crutchfield, 1994; Bettencourt et al., 2008; Stramaglia et al., 2012). In the language of information theory, redundancy occurs when multiple copies of the same information can be found in different parts of a group of variables, while synergy refers those information which is not stored in any specific element, but rather in the joint state of that group of variables.
These two lines of research are actually complementary and realize, at the level of high order phenomena, the dualism structure-function. The former focuses on the structural organization of a system in terms of structural hyperlinks, the latter on emergent properties related to what the system does, and characterises its high order behavior identifying an equivalent to functional hyperlinks from data sampled at nodes (Rosas et al., 2022). The present work deals with the second perspective, studying high order dependencies in data obtained from complex systems.
The emergence of new tools for the quantification of high order interactions is opening new possibilities in the field of network physiology, which aims to address the fundamental question of how physiological networks collectively behave to maintain human body in healthy conditions (Bashan et al., 2012; Lin et al., 2020; Ivanov 2021). Historically, the study of physiological time series has seen a shift from the univariate analysis of individual time series, where measures such as the approximate entropy (Pincus, 1991), the sample entropy (Richman and Moorman, 2000) and the corrected conditional entropy (Porta et al., 1998) have been introduced to characterize the predictable dynamics of a physiological system, to the bivariate analysis of two time series, where symmetric or causal measures based on cross-entropies (Porta et al., 1999; Faes et al., 2011), mutual information (Valderas, 2019) and its rate (Barà et al., 2023), directed information (Massey, 1990) or transfer entropy (Schreiber, 2000; Faes et al., 2014) have been used extensively to quantify the information shared and transferred between pairs of physiological systems. Multivariate analyses involving more than two physiological time series have been then introduced to quantify how the information transferred between processes is affected by the rest of the network. (Montalto et al., 2014; Wang et al., 2022). However, the multivariate approach has been implemented largely to analyze pairwise interactions between two systems while accounting for the presence of other systems, rather than to investigate how several systems interact collectively to shape the network dynamics. For this reason, high order interactions have not been studied explicitly in physiological networks until very recently (Stramaglia et al., 2021), and thus remain a tool whose potential in the field of network physiology is poorly addressed.
The major approach for estimating synergy and redundancy from data is partial information decomposition (PID) (Williams and Beer, 2010; Wibral et al., 2017; Lizier et al., 2018): applications in neuroscience have shown that the description of the brain dynamics in terms of synergy and redundancy (Luppi et al., 2022; Varley et al., 2023) is particularly suited to the interplay between brain segregation and integration (Bassett and Sporns, 2017). Moreover, it has also been used in different physiological contexts, such as to dissects control mechanisms of heart rate variability at rest and during physiological stress (Krohova et al., 2019); however, the use of PID in many applications is greatly limited by the super-exponential growth of decomposition terms for large systems. To cope with the computational burden of PID, but giving up the possibility of independently evaluating synergy and redundancy, the O-information has been introduced in (Rosas et al., 2019) as a metric measuring the balance between synergy and redundancy and thus being capable of characterising synergy-dominated systems: its computational weight scales gracefully with the size of systems. In (Scagliarini et al., 2022) the local O-information has been proposed to study inter-dependencies on individual patterns. A new framework for the time- and frequency domain assessment of high order interactions in networks of random processes has been developed in (Faes et al., 2022).
To complement the global assessment provided by the O-information, it has been recently proposed to exploit the gradients of the O-information as low-order descriptors that can characterize how high order effects are localized across a system of interest (Scagliarini et al., 2023). Instead of focusing on the O-information of groups of variables, the attention here is focused on the variation of the O-information when variables are added to the rest of the system to form these groups. This provides a more nuanced description of synergistic or redundant informational circuits, in which the role of each variable can be disambiguated. In this work, we first review the approach for the computation of the O-information and its gradients, and then present two new applications related to network physiology: (i) fMRI data from healthy subjects in resting conditions and (ii) cardiovascular, cerebrovascular and respiratory oscillations in healthy subjects in the supine resting state and after head-up tilt.
2 Gradients of O-information
In this section, we recall the definition and the properties of the gradients of O-information. First of all, it is useful to introduce O-information, which measures the balance between redundancy and synergy, representing the two basic types of high order statistical dependencies. The two building blocks of O-information are the total correlation TC (Watanabe, 1960) and the dual total correlation DTC (Sun, 1975), defined as follows for a system described by n stochastic variables Xn = {X1, …, Xn}:
where
Consequently, the O-information of the system can be written as (Rosas et al., 2019)
If Ω > 0, the system is redundancy-dominated. On the other hand, when Ω < 0 the dependencies are better explained as patterns that can be observed in the joint state of multiple variables but not in subsets of these; in other words, the system is synergy-dominated. It is clear that the main drawback of the O-information is the fact it does not put in evidence multiplets of variables which are both redundant and synergistic with equal strength, whilst approaches like PID evaluate both quantities and may, in principle, deal with these cases. It is also worth mentioning that the O-information is connected to the Synergy-Redundancy Index (SRI) developed in (Gat and Tishby, 1998; Brenner et al., 2000; Reich et al., 2001; Puchalla et al., 2005); although the SRI does not provide separate quantifications of synergy and redundancy like PID does, it can consider the respective contributions of signal correlations and noise correlations to synergy and redundancy (Panzeri et al., 1999; Nirenberg and Latham, 2003; Latham and Nirenberg, 2005; Panzeri et al., 2022). Therefore, SRI should be regarded as complementary to PID/O-information approaches.
In order to measure how much a given variable Xi plays a role in the informational circuits contained in Xn, its “gradient of O-information” is calculated as follows (Scagliarini et al., 2023):
where I is the mutual information and
It has been shown in (Scagliarini et al., 2023) that the following bounds hold and are tight:
where
Following a similar rationale to the one that leads to Eq. 2, one can further introduce a second-order descriptor of high order interdependencies by considering gradients of gradients. In particular, the second-order gradient of a pair of variables Xi and Xj can be defined as
This second-order gradient captures how much the presence of the variable Xj alters the variation of O-information of the system due to the inclusion of Xi. It is direct to verify the symmetry ∂i∂jΩ(Xn) = ∂j∂iΩ(Xn); therefore, we simply denote this quantity as
In other words,
Successive gradients can be similarly introduced, resulting in a simple chain rule. If γ is a subset of {1, …, n} of cardinality |γ|, then:
the sum being over all the subsets α of γ. For example, for triplets of variables the gradient of the O-information reads:
and measures the irreducible contribution to the O-information by the triplet {i, j, k} which cannot be ascribed to the inclusion of pairs nor single variables of the triplet.
3 Application to physiological networks
This section reports the application of the measures defined in Section 2 to physiological networks where high order interactions are expected to play a role in the generation of the network dynamics, i.e., brain networks probed by functional magnetic resonance imaging (fMRI) and networks of cardiovascular and cerebrovascular interactions probed by the beat-to-beat variability series of cardiac, vascular, respiratory and cerebral blood flow parameters. Gradients of O-information were calculated using the Gaussian Copula approach described in (Ince et al., 2017) to estimate entropy terms.
3.1 Brain networks: fMRI data
We first consider the data from the Human Connectome Project (Van Essen et al., 2012) corresponding to 1,083 healthy subjects whose organization of networks in the human cerebrum was explored using resting-state functional connectivity MRI (Yeo et al., 2011). The fMRI data acquisitions have been performed on a Siemens 3T Skyra scanner at Washington University (WashU). In order to construct a best-estimate parcellation of the human cerebral cortex to serve as a reference for future studies, a clustering algorithm was used to parcellate the cerebral cortex into networks of functionally coupled regions. Parcellations were examined for a coarse solution that organized the cortex into seven networks as well as a finer solution that identified 17 networks. The estimated networks were found to be consistent across the discovery and replication data samples and were confirmed by region-based functional connectivity MRI (fcMRI) analyses. Here we consider the parcellation in 7 clusters, each corresponding to the following connectivity networks: Default, Control, Limbic, Visual, Somatosensor, Ventral Attention, and Dorsal Attention. For each subject, we analyze the corresponding seven fMRI time series. The significance of the detected high order interactions is assessed using the statistics of subjects: gradients are considered significantly redundant (synergistic) when the 5-th (95-th) percentile of the distribution is higher (lower) than zero.
In bottom panel of Figure 1 we depict the first-order gradient computed for the seven intrinsic connectivity networks. Except for the Default, all the regions are significantly redundant. Going to the second order gradients (middle panel of Figure 1), four pairs of regions are significantly redundant: somatomotor—dorsal attention, somatomotor—visual, dorsal attention—ventral attention, dorsal attention—visual. Concerning third order gradients, we find a significantly redundant triplet, default—control—dorsal attention, and a significantly synergistic triplet: ventral attention-somatomotor—visual (see top panel of Figure 1). These results evidence peculiar intrinsic connectivity networks contributing to redundancy and synergy in the large-scale organization of the overall fMRI network, and confirm that gradients of increasing order tend to highlight less redundant/more synergistic interactions.
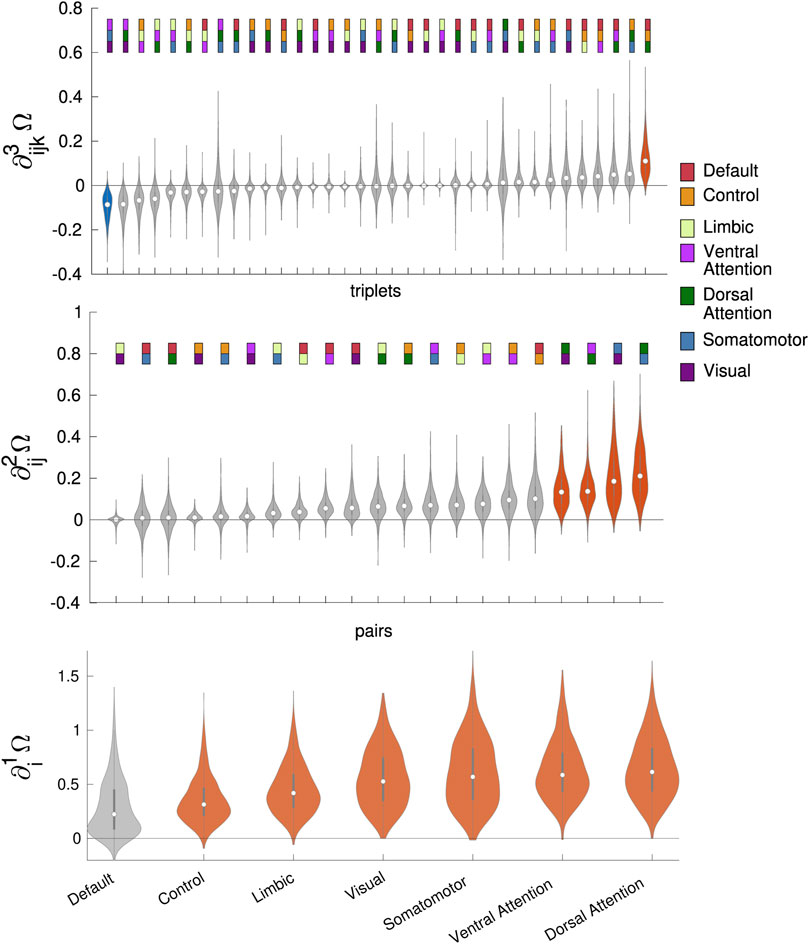
FIGURE 1. First order gradients of the O-information (bottom) for the seven fMRI time series of resting state brain networks. Six out of seven are significantly redundant. Signals from the Default Mode Network (DMN) are not significantly redundant, hence suggesting that the DMN it is the region for whom the balance synergy-redundancy is less leaning towards redundancy. The second-order (middle) and third-order (top) gradients of the O-information for the 21 pairs and the 35 triplets of fMRI time series of resting state brain networks. Colored rectangles represent the composition of the pairs and the triplets in terms of the resting state networks shown in legend. Redundant and synergistic violins are depicted in red and blue, respectively. Four pairs are significantly redundant. One triplet is significantly synergistic and one is significantly redundant.
3.2 Multi-organ networks: cardiovascular, respiratory and cerebral blood flow variability
In the second application, we analyze a database of physiological time series collected to study the effect of postural stress on cardiovascular, cerebrovascular and respiratory variability (Faes et al., 2013; Bari et al., 2016). The original dataset is comprised of 13 healthy subjects (age: 27 ± 8 years; 5 males), enrolled at the Neurology Division of Sacro Cuore Hospital, Negrar, Italy. Electrocardiogram (ECG, lead II) was acquired together with arterial pressure (AP) measured at the level of middle finger through a photopletysmographic device (Finapres Medical Systems, Ohmenda, Netherlands). Cerebral blood flow velocity (CBFV) and respiration were measured at the level of the middle cerebral artery by means of a transcranial Doppler ultrasonographic device (Multi-Dop T2, Dwl, San Juan Capistrano, CA) and through a thoracic impedance belt, respectively. Signals were synchronously acquired at a sampling rate of 1 kHz. From the raw signals, the physiological beat-to-beat variability series of heart period (H), systolic AP (S), mean AP (M), mean CBFV (F) and respiration (R) were measured as detailed in (Faes et al., 2013; Bari et al., 2016) during two stationary time windows of length 250 beats in the following physiological conditions: (i) supine rest (REST) and (ii) head-up tilt test with table inclination of 60° (TILT). Prior to network analysis, each series was high-pass filtered to remove slow trends and normalized to zero mean and unit variance.
The first and second order gradients evaluated for the physiologic network constituted by the five time series {H, S, M, F, R} (average over subjects) are reported in Figure 2. Bootstrap data analysis was applied to assess the statistical significance of the computed measures for each subject: gradients are considered significantly redundant (synergistic) when the 5-th (95-th) percentile of the bootstrap distribution is higher (lower) than zero. Looking at the first order gradients (Figure 2, top row), we see that the heart plays a synergistic role in the resting state, whilst in the orthostatic position the system becomes dominated by redundancy with a disconnection of respiration. The analysis of the second order gradients provided similar results (Figure 2, bottom row), with the cardiovascular link between H and S showing a synergistic character during the supine rest, and with increasing redundant behavior of the whole network after head-up tilt.
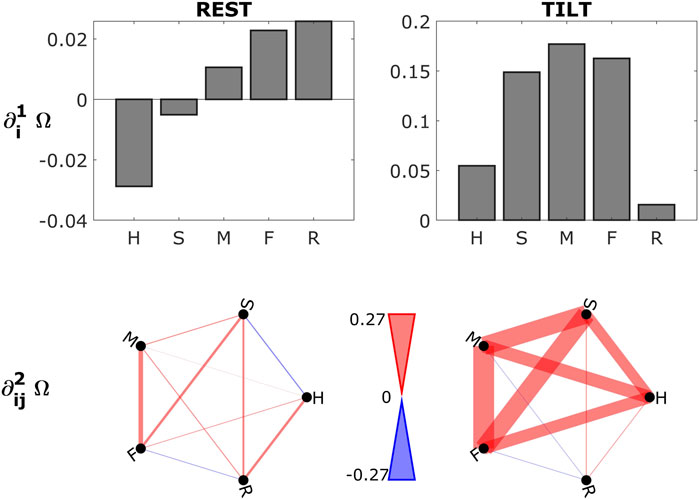
FIGURE 2. First (top row) and second (bottom row) order gradients for the physiological system composed of the five time series {H, S, M, F, R}, averaged over subjects and computed in the two experimental conditions: supine resting state (REST, left plots) and head-up tilt (TILT, right plots). Colours indicate redundant (red) and synergistic (blue) characters of interaction. Width of the links indicates the strength of the gradients. Statistical significance was assessed via bootstrap data analysis for each subject. As regards the first order gradients in the resting state, one out of the five showed significant synergy in 50% of subjects (H), while in TILT significant redundancy was found for three of the five (S, M, F). Going to the second order gradients, only in the TILT condition redundancy was significant in more than 50% of subjects for the pairs H-M, H-F, S-M, S-F, M-F. This suggests an important role of the sympathetic activation led by head-up tilt in increasing redundancy in physiologic networks.
4 Discussion
In this work, we have shown that recently proposed computational techniques show the ability to find multiplets of synergistic variables in physiological applications without requiring a huge amount of data.
In fMRI, data motion and physiological noise contribute substantially to the overall system variance (Liu et al., 2017; Colenbier et al., 2020). Redundancy is thus the first quantity to be naturally reduced at lower orders. In (Luppi et al., 2022) the synergistic and redundant districts of the resting brain have been explored, and it has been found that redundant interactions are especially prominent in the primary sensory, primary motor and insular cortices, corresponding to the brain’s somatomotor and salience subnetworks. In contrast, regions with higher relative importance for synergy predominate in high order association cortex, and are affiliated with the default mode (DMN) and fronto-parietal executive control (FPN) subnetworks. We note that in Varley et al. (2023) an analogous synergy-redundancy gradient as in Luppi et al. (2022) has been found using partial entropy decomposition.
It is worth mentioning that in (Luppi et al., 2022) dynamical synergy and redundancy (from the double redundancy lattice, (Mediano et al., 2021), have been explored for each pair of the 232 regions of the augmented Schaefer atlas. In agreement with (Luppi et al., 2022), we find that the default network has the minimum first order gradient, i.e., it is the less redundant; moreover a major redundant role is played by the somatosensor network. However our results refer to a different spatial scale and, come from a static analysis, are not expected to be fully reproducing with the results in (Luppi et al., 2022): notice that the emergence of a synergistic circuit made of visual, somatomotor and ventral attention has not been observed in previous studies. We remark that in a recent paper it has been observed that ventral attention and motor network connectivity are relevant to functional impairment in spatial neglect after right brain stroke (Barrett et al., 2019); moreover higher functional connectivity of ventral attention and visual network has been found to play a role to maintain cognitive performance in white matter hyperintensity (Zhu et al., 2023). These findings renders even more interesting our results, i.e., these three networks belonging to a synergistic informational circuit in the resting brain.
Our results also document a well known fact in physiology, i.e., that cardiovascular, cerebrovascular and respiratory interactions are highly redundant. On the other hand, we also show an interesting aspect of cardiovascular oscillations, i.e., that the heart rate plays a synergistic role in the resting state analyzed with our static analysis; synergy could result from the fact that heart rate variability is the target for several neuro-autonomic mechanisms including the cardiac baroreflex and the respiratory sinus arrhythmia (Cohen and Taylor, 2002). We also find that redundancy is strongly enhanced by the entrainment of cardiovascular and cerebrovascular oscillations and by sympathetic activation; in particular, in the upright position all the series are highly redundant, except for the respiration signal, which is out of the redundant circuits in tilt conditions. These results agree with the tilt-induced shift of the sympatho-vagal balance towards increased sympathetic activity and decreased parasympathetic activity (Montano et al., 1994), also previously documented via information-theoretic analyses (Faes et al., 2011). Overall, the redundancy showed also a tendency to increase with tilt, documenting an effect of sympathetic activation on the redundant interactions among cardiovascular and cerebrovascular oscillations (Faes et al., 2022). Bootstrap data analysis (Politis, 2003) confirmed these findings, suggesting that interactions involving respiratory, arterial pressure and blood flow variabilities are more shifted to synergistic rather than redundant modes of interplay, as well as that significance increases moving from the supine to the upright position, confirming that redundancy is significantly strongly enhanced during the orthostatic stress.
Summarizing, gradients of O-information constitute a promising tool to analyze many-body effects in complex systems, with the advantage of providing a description of high order phenomena which can be tuned and can even be at the level of single variables or pairs. The applications here described show the effectiveness of this approach for multivariate data in physiology. In the big-data setting, evaluation of gradients of O-information remains an heavy computational burden, indeed for n variables even first order gradients require the estimation of entropy terms or order up to n: further work will be devoted to develop approximate approaches for the evaluation of gradients so as to make it feasible also for a large number of variables n.
Data and code availability
fMRI data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. The code for the evaluation of gradients of O-information can be found in https://github.com/tomscag/GOI/.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Neurology Division of Sacro Cuore Hospital, Negrar, Italy. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
TS: Conceptualization, Data curation, Investigation, Software, Writing–original draft, Writing–review and editing. LS: Conceptualization, Data curation, Investigation, Software, Writing–original draft, Writing–review and editing. LF: Conceptualization, Investigation, Supervision, Writing–original draft, Writing–review and editing. DM: Conceptualization, Investigation, Supervision, Writing–original draft, Writing–review and editing. SS: Conceptualization, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. LF and SS were supported by Ministero dell’Istruzione, dell’Università e della Ricerca, Award ID: PRIN 2022YMHNPY. This research was supported by the project “HONEST - High-Order Dynamical Networks in Computational Neuroscience and Physiology: an Information-Theoretic Framework”, Italian Ministry of University and Research (funded by MUR, PRIN 2022, code 2022YMHNPY, CUP: B53D23003020006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barà, C., Sparacino, L., Pernice, R., Antonacci, Y., Porta, A., Kugiumtzis, D., et al. (2023). Comparison of discretization strategies for the model-free information-theoretic assessment of short-term physiological interactions. Chaos Interdiscip. J. Nonlinear Sci. 33, 033127. doi:10.1063/5.0140641
Bari, V., Marchi, A., Maria, B. D., Rossato, G., Nollo, G., Faes, L., et al. (2016). Nonlinear effects of respiration on the crosstalk between cardiovascular and cerebrovascular control systems. Phil. Trans. R. Soc. A 374, 20150179. doi:10.1098/rsta.2015.0179
Barrett, A., Boukrina, O., and Saleh, S. (2019). Ventral attention and motor network connectivity is relevant to functional impairment in spatial neglect after right brain stroke. Brain Cogn. 129, 16–24. doi:10.1016/j.bandc.2018.11.013
Bashan, A., Bartsch, R. P., Kantelhardt, J. W., Havlin, S., and Ivanov, P. C. (2012). Network physiology reveals relations between network topology and physiological function. Nat. Commun. 3, 702–709. doi:10.1038/ncomms1705
Bassett, D., and Sporns, O. (2017). Network neuroscience. Nat. Neurosci. 20, 353–364. doi:10.1038/nn.4502
Battiston, F., Amico, E., Barrat, A., Bianconi, G., Ferraz de Arruda, G., Franceschiello, B., et al. (2021). The physics of higher-order interactions in complex systems. Nat. Phys. 17, 1093–1098. doi:10.1038/s41567-021-01371-4
Battiston, F., Cencetti, G., Iacopini, I., Latora, V., Lucas, M., Patania, A., et al. (2020). Networks beyond pairwise interactions: structure and dynamics. Phys. Rep. 874, 1–92. doi:10.1016/j.physrep.2020.05.004
Bettencourt, L. M. A., Gintautas, V., and Ham, M. I. (2008). Identification of functional information subgraphs in complex networks. Phys. Rev. Lett. 100, 238701. doi:10.1103/PhysRevLett.100.238701
Brenner, N., Strong, S. P., Koberle, R., Bialek, W., and RrdRv, S. (2000). Synergy in a neural code. Neural Comput. 12, 1531–1552. doi:10.1162/089976600300015259
Cohen, M. A., and Taylor, J. A. (2002). Short-term cardiovascular oscillations in man: measuring and modelling the physiologies. J. physiology 542, 669–683. doi:10.1113/jphysiol.2002.017483
Colenbier, N., Van de Steen, F., Uddin, L. Q., Poldrack, R. A., Calhoun, V. D., and Marinazzo, D. (2020). Disambiguating the role of blood flow and global signal with partial information decomposition. NeuroImage 213, 116699. doi:10.1016/j.neuroimage.2020.116699
Crutchfield, J. P. (1994). The calculi of emergence: computation, dynamics and induction. Phys. D. Nonlinear Phenom. 75, 11–54. doi:10.1016/0167-2789(94)90273-9
Faes, L., Marinazzo, D., Montalto, A., and Nollo, G. (2014). Lag-specific transfer entropy as a tool to assess cardiovascular and cardiorespiratory information transfer. IEEE Trans. Biomed. Eng. 61, 2556–2568. doi:10.1109/TBME.2014.2323131
Faes, L., Mijatovic, G., Antonacci, Y., Pernice, R., Barà, C., Sparacino, L., et al. (2022). A new framework for the time- and frequency-domain assessment of high-order interactions in networks of random processes. IEEE Trans. Signal Process. 70, 5766–5777. doi:10.1109/TSP.2022.3221892
Faes, L., Nollo, G., and Porta, A. (2011). Information domain approach to the investigation of cardio-vascular, cardio-pulmonary, and vasculo-pulmonary causal couplings. Front. physiology 2, 80. doi:10.3389/fphys.2011.00080
Faes, L., Porta, A., Rossato, G., Adami, A., Tonon, D., Corica, A., et al. (2013). Investigating the mechanisms of cardiovascular and cerebrovascular regulation in orthostatic syncope through an information decomposition strategy. Aut. Neurosci. 178, 76–82. doi:10.1016/j.autneu.2013.02.013
Gat, I., and Tishby, N. (1998). “Synergy and redundancy among brain cells of behaving monkeys,” in Advances in neural information processing systems vol. 11. Editors M. Kearns, S. Solla, and D. Cohn (Massachusetts, United States: MIT Press).
Ince, R. A., Giordano, B. L., Kayser, C., Rousselet, G. A., Gross, J., and Schyns, P. G. (2017). A statistical framework for neuroimaging data analysis based on mutual information estimated via a Gaussian copula. Hum. Brain Mapp. 38, 1541–1573. doi:10.1002/hbm.23471
Ivanov, P. C. (2021). The new field of network physiology: building the human physiolome. Front. Netw. Physiology 1, 711778. doi:10.3389/fnetp.2021.711778
Krohova, J., Faes, L., Czippelova, B., Turianikova, Z., Mazgutova, N., Pernice, R., et al. (2019). Multiscale information decomposition dissects control mechanisms of heart rate variability at rest and during physiological stress. Entropy 21, 526. doi:10.3390/e21050526
Latham, P. E., and Nirenberg, S. (2005). Synergy, redundancy, and independence in population codes, revisited. J. Neurosci. 25, 5195–5206. doi:10.1523/JNEUROSCI.5319-04.2005
Lin, A., Liu, K. K., Bartsch, R. P., and Ivanov, P. C. (2020). Dynamic network interactions among distinct brain rhythms as a hallmark of physiologic state and function. Commun. Biol. 3 (1), 197. doi:10.1038/s42003-020-0878-4
Liu, T. T., Nalci, A., and Falahpour, M. (2017). The global signal in fmri: nuisance or information? Neuroimage 150, 213–229. doi:10.1016/j.neuroimage.2017.02.036
Lizier, J., Bertschinger, N., Jost, J., and Wibral, M. (2018). Information decomposition of target effects from multi-source interactions: perspectives on previous, current and future work. Entropy 20, 307–316. doi:10.3390/e20040307
Luppi, A., Mediano, P., Rosas, F., Holland, N., Fryer, T., O’Brien, J., et al. (2022). A synergistic core for human brain evolution and cognition. Nat. Neurosci. 25, 771–782. doi:10.1038/s41593-022-01070-0
Massey, J. (1990). Causality, feedback and directed information. Proc. 1990 Intl. Symp. Info. Th. its Appl. Waikiki, Hawaii, Nov. 27-30, 1990.
Mediano, P. A., Rosas, F. E., Luppi, A. I., Carhart-Harris, R. L., Bor, D., Seth, A. K., et al. (2021). Towards an extended taxonomy of information dynamics via integrated information decomposition. Available at: https://arxiv.org/abs/2109.13186.
Montalto, A., Faes, L., and Marinazzo, D. (2014). Mute: a matlab toolbox to compare established and novel estimators of the multivariate transfer entropy. PloS one 9, e109462. doi:10.1371/journal.pone.0109462
Montano, N., Ruscone, T. G., Porta, A., Lombardi, F., Pagani, M., and Malliani, A. (1994). Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 90, 1826–1831. doi:10.1161/01.cir.90.4.1826
Nirenberg, S., and Latham, P. (2003). Decoding neuronal spike trains: how important are correlations? PNAS 100, 7348–7353. doi:10.1073/pnas.1131895100
Panzeri, S., Moroni, M., Safaai, H., and Harvey, C. (2022). The structures and functions of correlations in neural population codes. Nat. Rev. Neurosci. 23, 551–567. doi:10.1038/s41583-022-00606-4
Panzeri, S., Schultz, S. R., Treves, A., and Rolls, E. T. (1999). Correlations and the encoding of information in the nervous system. Proc. Biol. Sci. 266, 1001–1012. doi:10.1098/rspb.1999.0736
Pincus, S. M. (1991). Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. 88, 2297–2301. doi:10.1073/pnas.88.6.2297
Politis, D. (2003). The impact of bootstrap methods on time series analysis. Stat. Sci. 18, 219–230. doi:10.1214/ss/1063994977
Porta, A., Baselli, G., Liberati, D., Montano, N., Cogliati, C., Gnecchi-Ruscone, T., et al. (1998). Measuring regularity by means of a corrected conditional entropy in sympathetic outflow. Biol. Cybern. 78, 71–78. doi:10.1007/s004220050414
Porta, A., Baselli, G., Lombardi, F., Montano, N., Malliani, A., and Cerutti, S. (1999). Conditional entropy approach for the evaluation of the coupling strength. Biol. Cybern. 81, 119–129. doi:10.1007/s004220050549
Puchalla, J., Schneidman, E., Harris, R., and Mj, B. (2005). Redundancy in the population code of the retina. Neuron 46, 493–504. doi:10.1016/j.neuron.2005.03.026
Reich, D. S., Mechler, F., and Victor, J. D. (2001). Independent and redundant information in nearby cortical neurons. Science 294, 2566–2568. doi:10.1126/science.1065839
Richman, J. S., and Moorman, J. R. (2000). Physiological time-series analysis using approximate entropy and sample entropy. Am. J. physiology-heart circulatory physiology 278, H2039–H2049. doi:10.1152/ajpheart.2000.278.6.H2039
Rosas, F., Mediano, P., Luppi, A., Varley, T., Lizier, J., Stramaglia, S., et al. (2022). Disentangling high-order mechanisms and high-order behaviours in complex systems. Nat. Phys. 18, 476–477. doi:10.1038/s41567-022-01548-5
Rosas, F. E., Mediano, P. A., Gastpar, M., and Jensen, H. J. (2019). Quantifying high-order interdependencies via multivariate extensions of the mutual information. Phys. Rev. E 100, 032305. Publisher: APS. doi:10.1103/PhysRevE.100.032305
Scagliarini, T., Marinazzo, D., Guo, Y., Stramaglia, S., and Rosas, F. E. (2022). Quantifying high-order interdependencies on individual patterns via the local o-information: theory and applications to music analysis. Phys. Rev. Res. 4, 013184. doi:10.1103/PhysRevResearch.4.013184
Scagliarini, T., Nuzzi, D., Antonacci, Y., Faes, L., Rosas, F. E., Marinazzo, D., et al. (2023). Gradients of o-information: low-order descriptors of high-order dependencies. Phys. Rev. Res. 5, 013025. doi:10.1103/physrevresearch.5.013025
Schreiber, T. (2000). Measuring information transfer. Phys. Rev. Lett. 85, 461–464. doi:10.1103/PhysRevLett.85.461
Stramaglia, S., Scagliarini, T., Daniels, B. C., and Marinazzo, D. (2021). Quantifying dynamical high-order interdependencies from the o-information: an application to neural spiking dynamics. Front. Physiology 11, 595736. Publisher: Frontiers. doi:10.3389/fphys.2020.595736
Stramaglia, S., Wu, G. R., Pellicoro, M., and Marinazzo, D. (2012). Expanding the transfer entropy to identify information circuits in complex systems. Phys. Rev. E 86, 066211. doi:10.1103/PhysRevE.86.066211
Sun, T. (1975). Linear dependence structure of the entropy space. Inf. Control 29, 337–368. doi:10.1016/s0019-9958(75)80004-0
Valderas, M. T., Bolea, J., Laguna, P., Bailón, R., and Vallverdú, M. (2019). Mutual information between heart rate variability and respiration for emotion characterization. Physiol. Meas. 40, 084001. doi:10.1088/1361-6579/ab310a
Van Essen, D. C., Ugurbil, K., Auerbach, E., Barch, D., Behrens, T. E., Bucholz, R., et al. (2012). The human connectome project: a data acquisition perspective. Neuroimage 62, 2222–2231. doi:10.1016/j.neuroimage.2012.02.018
Varley, T., Pope, M., Puxeddu, M., and Sporns, O. (2023). Partial entropy decomposition reveals higher-order information structures in human brain activity. PNAS 120, e2300888120. doi:10.1073/pnas.2300888120
Wang, Y., Chen, C., and Chen, W. (2022). Nonlinear directed information flow estimation for fnirs brain network analysis based on the modified multivariate transfer entropy. Biomed. Signal Process. Control 74, 103422. doi:10.1016/j.bspc.2021.103422
Watanabe, S. (1960). Information theoretical analysis of multivariate correlation. IBM J. Res. Dev. 4, 66–82. doi:10.1147/rd.41.0066
Wibral, M., Priesemann, V., Kay, J. W., Lizier, J. T., and Phillips, W. A. (2017). Partial information decomposition as a unified approach to the specification of neural goal functions. Brain cognition 112, 25–38. Publisher: Elsevier. doi:10.1016/j.bandc.2015.09.004
Yeo, B., Krienen, F., Sepulcre, J., Sabuncu, M., Lashkari, D., Hollinshead, M., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165. doi:10.1152/jn.00338.2011
Keywords: information theory, high-order interactions, network science, functional brain connectivity, cardiovascular interactions, cardiorespiratory interactions, cerebrovascular interactions
Citation: Scagliarini T, Sparacino L, Faes L, Marinazzo D and Stramaglia S (2024) Gradients of O-information highlight synergy and redundancy in physiological applications. Front. Netw. Physiol. 3:1335808. doi: 10.3389/fnetp.2023.1335808
Received: 09 November 2023; Accepted: 21 December 2023;
Published: 09 January 2024.
Edited by:
Dante Mantini, KU Leuven, BelgiumReviewed by:
Klaus Lehnertz, University of Bonn, GermanyAndrea I. Luppi, University of Cambridge, United Kingdom
Copyright © 2024 Scagliarini, Sparacino, Faes, Marinazzo and Stramaglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastiano Stramaglia, c2ViYXN0aWFuby5zdHJhbWFnbGlhQGJhLmluZm4uaXQ=
†These authors share first authorship
 Tomas Scagliarini1†
Tomas Scagliarini1† Laura Sparacino
Laura Sparacino Luca Faes
Luca Faes Daniele Marinazzo
Daniele Marinazzo Sebastiano Stramaglia
Sebastiano Stramaglia