- 1Bernstein Center Freiburg, University of Freiburg, Freiburg, Germany
- 2Faculty of Biology, University of Freiburg, Freiburg, Germany
- 3BrainLinks-BrainTools, University of Freiburg, Freiburg, Germany
- 4Centre National de la Recherche Scientifique, Université de Strasbourg, Institut des Neurosciences Cellulaires et Intégratives UPR3212, Strasbourg, France
- 5Department of Psychosomatic Medicine and Psychotherapy, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany
- 6Department of Psychiatry and Psychotherapy, Medical Center, University of Freiburg-Faculty of Medicine, Freiburg, Germany
- 7Center for Basics in Neuromodulation, Freiburg, Germany
Introduction: Transcranial direct current stimulation (tDCS) is increasingly used to modulate motor learning. Current polarity and intensity, electrode montage, and application before or during learning had mixed effects. Both Hebbian and homeostatic plasticity were proposed to account for the observed effects, but the explanatory power of these models is limited. In a previous modeling study, we showed that homeostatic structural plasticity (HSP) model can explain long-lasting after-effects of tDCS and transcranial magnetic stimulation (TMS). The interference between motor learning and tDCS, which are both based on HSP in our model, is a candidate mechanism to resolve complex and seemingly contradictory experimental observations.
Methods: We implemented motor learning and tDCS in a spiking neural network subject to HSP. The anatomical connectivity of the engram induced by motor learning was used to quantify the impact of tDCS on motor learning.
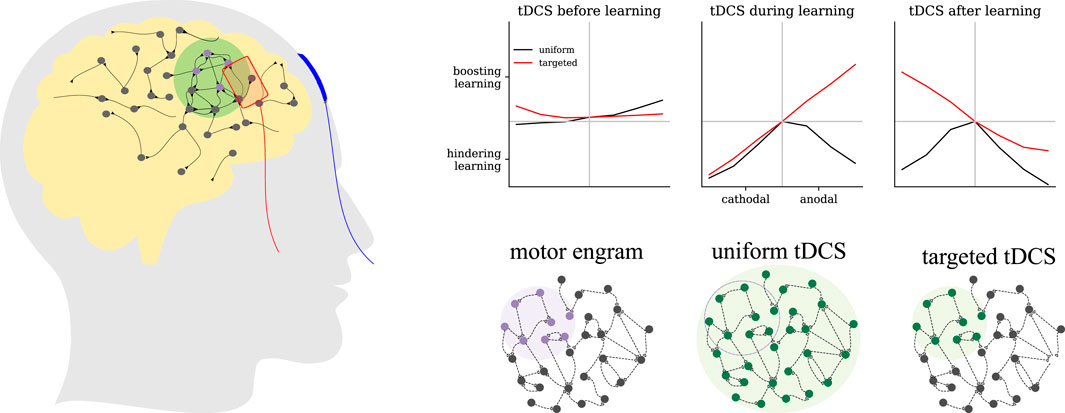
Results: Our modeling results demonstrated that transcranial direct current stimulation applied before learning had weak modulatory effects. It led to a small reduction in connectivity if it was applied uniformly. When applied during learning, targeted anodal stimulation significantly strengthened the engram, while targeted cathodal or uniform stimulation weakened it. Applied after learning, targeted cathodal, but not anodal, tDCS boosted engram connectivity. Strong tDCS would distort the engram structure if not applied in a targeted manner.
Discussion: Our model explained both Hebbian and homeostatic phenomena observed in human tDCS experiments by assuming memory strength positively correlates with engram connectivity. This includes applications with different polarity, intensity, electrode montage, and timing relative to motor learning. The HSP model provides a promising framework for unraveling the dynamic interaction between learning and transcranial DC stimulation.
Introduction
Transcranial direct current stimulation (tDCS) is a promising non-invasive brain stimulation with a long history (Sarmiento et al., 2016; Majdi et al., 2022; Burkhardt et al., 2023). By applying a weak direct current (1–
Motor learning is the paradigm most frequently studied in tDCS, as there are established protocols and effective tests for cortical excitability (Reis and Fritsch, 2011; Saucedo Marquez et al., 2013; Ammann et al., 2016; Buch et al., 2017), as well as its great potential in rehabilitating motor skills with low adverse events in patients with Parkinson’s disease or after stroke (Alsharidah et al., 2018; O’Brien et al., 2018; Ciechanski et al., 2020; Halakoo et al., 2020; Berrigan et al., 2021; Manto et al., 2021; Duan and Zhang, 2024), etc. Among these studies, the cerebellum, the primary motor cortex (M1), the premotor cortex (M2), the prefrontal cortex (PFC), and the posterior parietal cortex (PPC) are the most targeted areas, where parameters such as current polarity, current intensity, electrode montage, and stimulation timing are acknowledged to have a discernible influence on the outcome of stimulation (see a recent review in Qi et al. (2022)). For example, when applying tDCS to M1 concurrently with a motor learning task, it was reported that anodal tDCS improved learning (Soekadar et al., 2015; Debarnot et al., 2019), while cathodal tDCS did not produce comparable effects (Nitsche et al., 2003b; Ammann et al., 2016) or even reduced retention (Kim et al., 2024). However, some follow-up studies that used slightly different configurations or tasks failed to replicate these effects (Saucedo Marquez et al., 2013; Ambrus et al., 2016; Buch et al., 2017; Sevilla-Sanchez et al., 2022). In addition, the strength of the after-effects on cortical excitability did not correlate well with the current intensity (Batsikadze et al., 2013; Cuypers et al., 2013; Jamil et al., 2017). The focality of stimulation depends on the electrode montage, and this is known to also influence the effect of tDCS on motor learning. Traditionally, either unilateral (with the anode on the non-dominant M1 and the cathode over the contralateral supraorbital area) or bilateral (anode over the dominant and cathode over the non-dominant M1) electrode montages are used. Interestingly, these two configurations produce different results (Goodwill et al., 2013; Naros et al., 2016). To improve focality in a specific brain area, high definition tDCS (HD-tDCS) was proposed, e.g., employing a central anode surrounded by four cathodes. HD-tDCS induces a more focal electric field that was shown in recent pilot studies to facilitate motor learning (Cole et al., 2018; Iannone et al., 2022) and differentially recruits different pathways to M1 (Iannone et al., 2022). However, as more and more studies emerge, it becomes clear that the relation between motor learning and tDCS strongly depends on the learning phase. The same tDCS protocol applied before learning, during training blocks, or after learning leads to very different results (Tecchio et al., 2010; Stagg et al., 2011; Apolin et al., 2016; Parma et al., 2021; Rivera-Urbina et al., 2022). These effects appear to differ in brain area (Rivera-Urbina et al., 2022), subject age (Greeley et al., 2022), and health condition (Simpson and Mak, 2022). Assuming that tDCS is, in principle, a perturbation of the underlying neural activity, the outcome of tDCS should be brain state dependent. However, the neural correlate of this dependency has not yet been systematically elucidated.
Synaptic plasticity was recognized as the key mechanism underlying the modulatory effects of tDCS on motor learning. Animal studies focused primarily on M1 and suggested that M1 displays strong activity-dependent plasticity (Sanes and Donoghue, 2000), and dendrite-specific spine formation was observed in motor skills training (Xu et al., 2009; Yang et al., 2014). After 3 days of in vivo tDCS stimulation (
In our current study, we conceived motor learning as the formation of new structures corresponding to new memories (“engrams”) in a recurrent network. Transcranial stimulation was assumed to alter the equilibrium between neuronal activity and network structure, leading to additional network rewiring that interferes with learning. We analyzed the effect of different combinations of stimulation parameters, such as electrode montage, DC polarity and intensity, as well as the relation to the learning phase. In summary, our model can reconcile the observed impact of DC stimulation on motor learning and reconcile seemingly contradicting experimental results. We also made predictions that have not yet been reported by human experiments. The HSP model, linking structural changes and the homeostatic regulation of neural activity, provides a systematic framework for explaining and predicting the effects of tDCS on motor learning.
Methods
Neuron, synapse, and network models
Numerical simulations of neural networks with homeostatic structural plasticity were used to explore the interaction between tDCS and learning. We used the same neuron model, synapse model, and network model, as published in our previous work (Lu et al., 2019; Gallinaro et al., 2022; Lu et al., 2023). The NEST simulator (Linssen et al., 2018) with parallel MPI-based computation was used to perform the large-scale neural network simulations presented here.
We used a leaky integrate-and-fire (LIF) neuron model. The cortical area M1 was conceived as an inhibition-dominated recurrent neural network of
Motor learning protocol
In a previous work, learning and memory was simulated as an engram (Gallinaro et al., 2022). Here we followed the idea and simulated the motor learning training process in a motor engram comprising
Direct current stimulation protocol
A model of how tDCS affects single neurons was published and characterized in our previous paper (Lu et al., 2019). Thus, we assumed that the electric field induced by the administration of transcranial current polarizes the soma of neurons with extended nonisotropic morphology, such as pyramidal neurons (Radman et al., 2009b; Vöröslakos et al., 2018). The duration of the stimulus was
In all of our experiments, the E-E connections were grown during an initial
Electrode montage
We first devised three paradigmatic scenarios to explore the impact of the electrode montage. The traditional two-electrode montage may induce a diffuse electric field that covers a volume larger than the volume engaged in motor learning. In contrast, the HD-tDCS montage induces a focal electric field that targets only a subset of neurons. In our current study, we concentrated on three extreme scenarios: uniform, targeted, and unfocused. In the uniform scenario, DC stimulation was applied homogeneously to all excitatory neurons in the network. Targeted DC stimulation was administered exclusively to the motor engram. Unfocused stimulation was an intermediate scenario, in which DC stimulation covers only half of the engram cells and the same amount of non-engram excitatory neurons.
Relative timing of motor learning and transcranial DC stimulation
We tested three experimental conditions for each electrode montage. We applied tDCS immediately before, during, or immediately after learning input.
Polarity and intensity of transcranial DC stimulation
For each combination of electrode montage and relative timing, we systematically varied the amplitude of tDCS to polarize the membrane potential between
Measurement of firing rate and connectivity
To quantify the modulatory effect of tDCS on learning, we considered the temporal evolution of neural activity and the connectivity of the motor engram. The neural firing rate was calculated from the spike count in a recording window of
Data and code availability
All code and dataset are available here: https://github.com/ErbB4/tDCS-and-learning.git.
Results
Modeling motor learning with a motor engram
We modeled motor learning by introducing excitatory spike input to a subgroup of the excitatory neurons (Figures 1A,B, purple shading). The application and termination of motor learning input substantially altered the neural activity of stimulated neurons and induced homeostatic reorganization of synapses as previously described (Gallinaro and Rotter, 2018; Lu et al., 2019; Gallinaro et al., 2022). When the disrupted neural activity returned to the homeostatic level, the network architecture did not recover to the pre-learning state. Instead, neurons receiving motor learning input wired more with each other, but less with other non-stimulated excitatory neurons (Figure 1C). This cell assembly with elevated connectivity is referred to as a motor engram in the following.
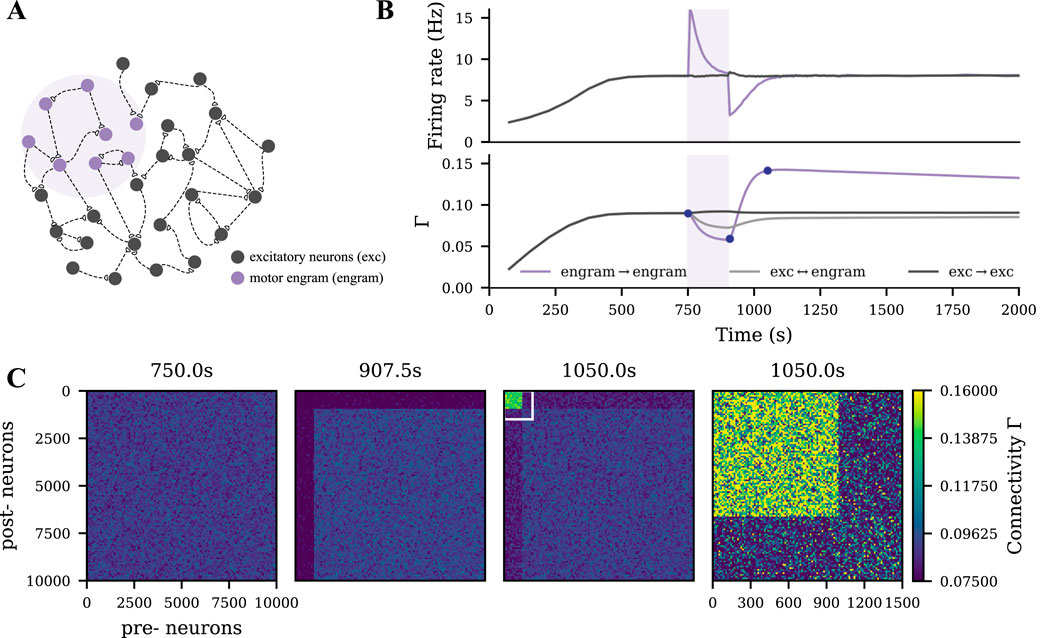
Figure 1. Strong motor engram formed during motor learning. (A) Schematic of the M1 neural network, part of which
tDCS triggers similar but weaker network remodeling than motor learning
We modeled tDCS by injecting subthreshold direct current into the soma as described in our previous study (Lu et al., 2019). When the membrane potential of a subpopulation was depolarized or hyperpolarized by DC (Figures 2A–C, green shading), a similar synapse reorganization process occurred. The DC input strength that we used in the current study was relatively weaker than the learning input, so the connectivity of the tDCS-stimulated cell assembly was accordingly weaker than the motor engram (Figures 2D,E). In contrast, no resulting cell assembly was triggered when tDCS was applied globally to all excitatory neurons.
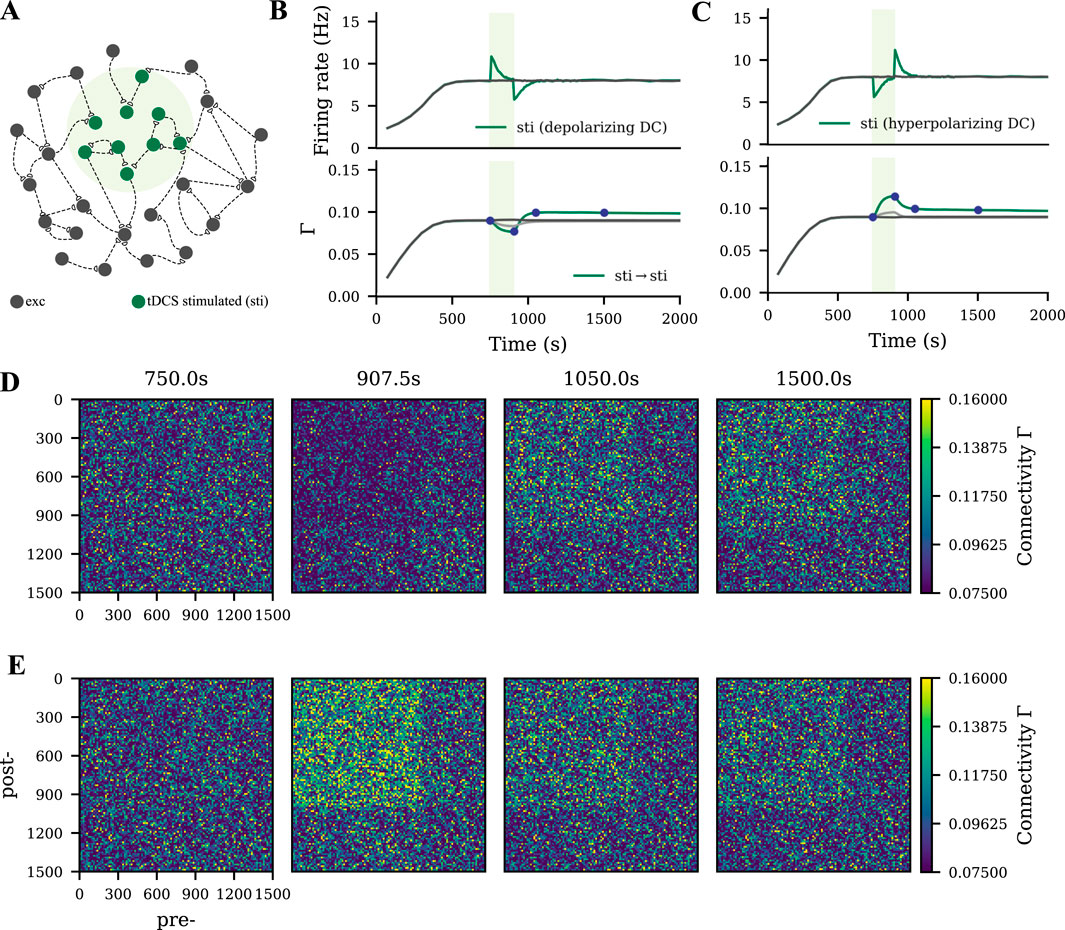
Figure 2. Weak cell assembly formed during tDCS. (A) Schematic of the M1 neural network, part of which
Uniform DC stimulation weakens the motor engram
To capture a rather diffusive tDCS scenario, uniform tDCS was administered to all excitatory neurons before, during, or after the learning process, with varying polarity and intensity (Figure 3A). In Figures 3B,C, we displayed the evolution of the firing rate and connectivity of the motor engram for all conditions. When applied before the learning process, uniform anodal tDCS slightly increased motor engram connectivity, while cathodal stimulation led to a reduction in connectivity. However, when applied concurrently with learning or post-learning, the uniform tDCS weakened the motor engram irrespective of the DC polarity. The weakening effects further depended on the DC intensity: Stronger tDCS resulted in lower connectivity of the motor engram. In summary, our simulation results demonstrated a divergent impact of uniform tDCS on learning, but with a strong tendency to negatively influence motor learning.
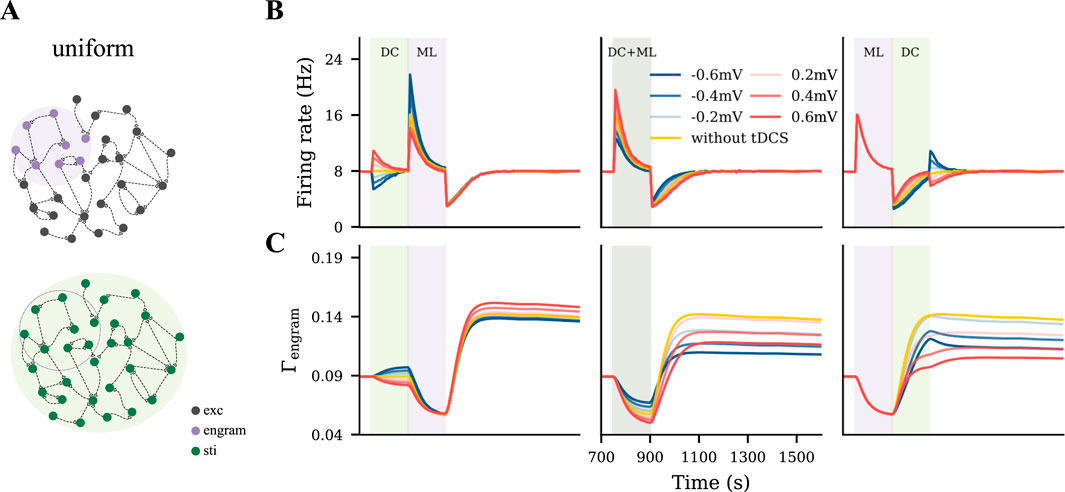
Figure 3. Uniform tDCS generally hinders motor learning. (A) Uniform tDCS was applied to the entire M1 network where the motor engram is embedded. (B, C) Temporal evolution of firing rate and connectivity under three different conditions: applying uniform tDCS immediately before, during, or immediately after learning input. The red and blue curves represent depolarizing and hyperpolarizing DCs, respectively. The yellow curve represents the condition without tDCS. The green and purple shaded areas represent the period of tDCS application (DC) and motor learning (ML), respectively.
Targeted tDCS influences motor engram in a phase- and polarity-dependent manner
To model a targeted focal stimulation, we applied tDCS exclusively to excitatory neurons within the motor engram before, during, and after motor learning with different polarities and intensities (Figure 4A). Our simulation results showed that the final connectivity of the motor engram strongly depended on the relative phase of motor learning and the polarity of tDCS (Figures 4B,C). When applied before motor learning, anodal and cathodal-centered tDCS slightly facilitated engram connectivity. However, when applied concurrently, anodal DC boosted the engram connectivity, whereas cathodal stimulation weakened it. Furthermore, when applied post-learning, the opposite effects were observed: cathodal DC increased the connectivity of the motor engram while the anodal DC reduced it. In summary, our simulation results suggested that the relative learning phase and polarity of tDCS should be chosen with care; targeted anodal and cathodal tDCS might exert opposite effects at different stages of motor learning.
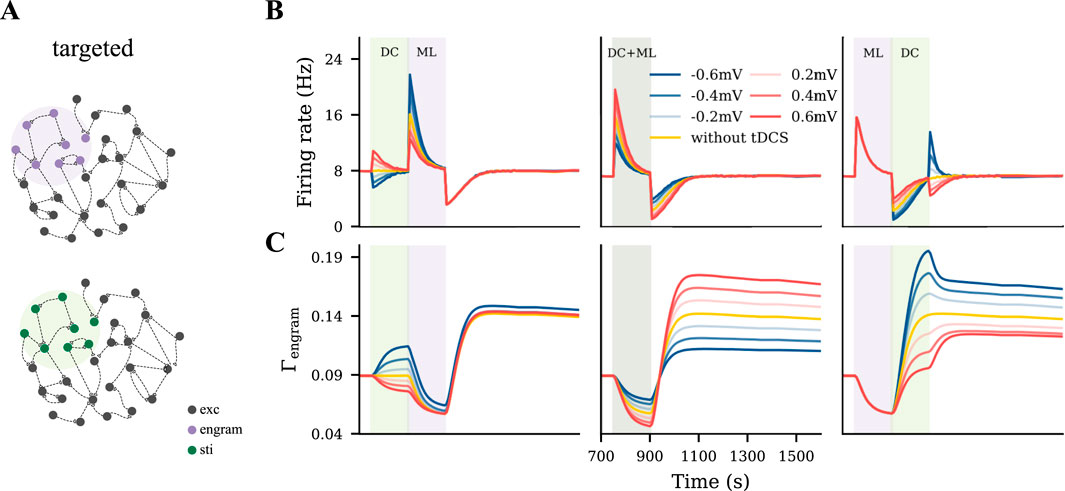
Figure 4. Targeted tDCS exerts opposite effects when applied at different stages of learning. (A) Targeted tDCS matched with the entire motor engram. (B, C) Temporal evolution of the motor engram’s firing rate and connectivity, when targeted tDCS was applied either immediately before, during, or immediately after the learning input.
Unfocused tDCS and the interaction of cell assemblies
The uniform and targeted tDCS represent two extreme conditions, in which tDCS either homogeneously covers the entire network or exclusively targets the motor engram. Neither holds in the practice of the application of tDCS in humans. So we proposed an intermediate condition, unfocused tDCS, by shifting the targeted tDCS to cover only half of the motor engram (Figure 5A). We expected the motor engram and the stimulated cell assembly to interact with each other during the dynamic stimulation process mediated by homeostatic structural plasticity. To analyze this effect, we presented two examples by applying a depolarizing tDCS at the same intensity before or after the motor learning process. In Figures 5B,D, we show the evolution of the firing rate and connectivity of four subpopulations: The overlapped half of the motor engram (orange), the non-overlapped half of the motor engram (purple), neurons receiving tDCS only (green), and the non-stimulated excitatory neurons (dark gray).
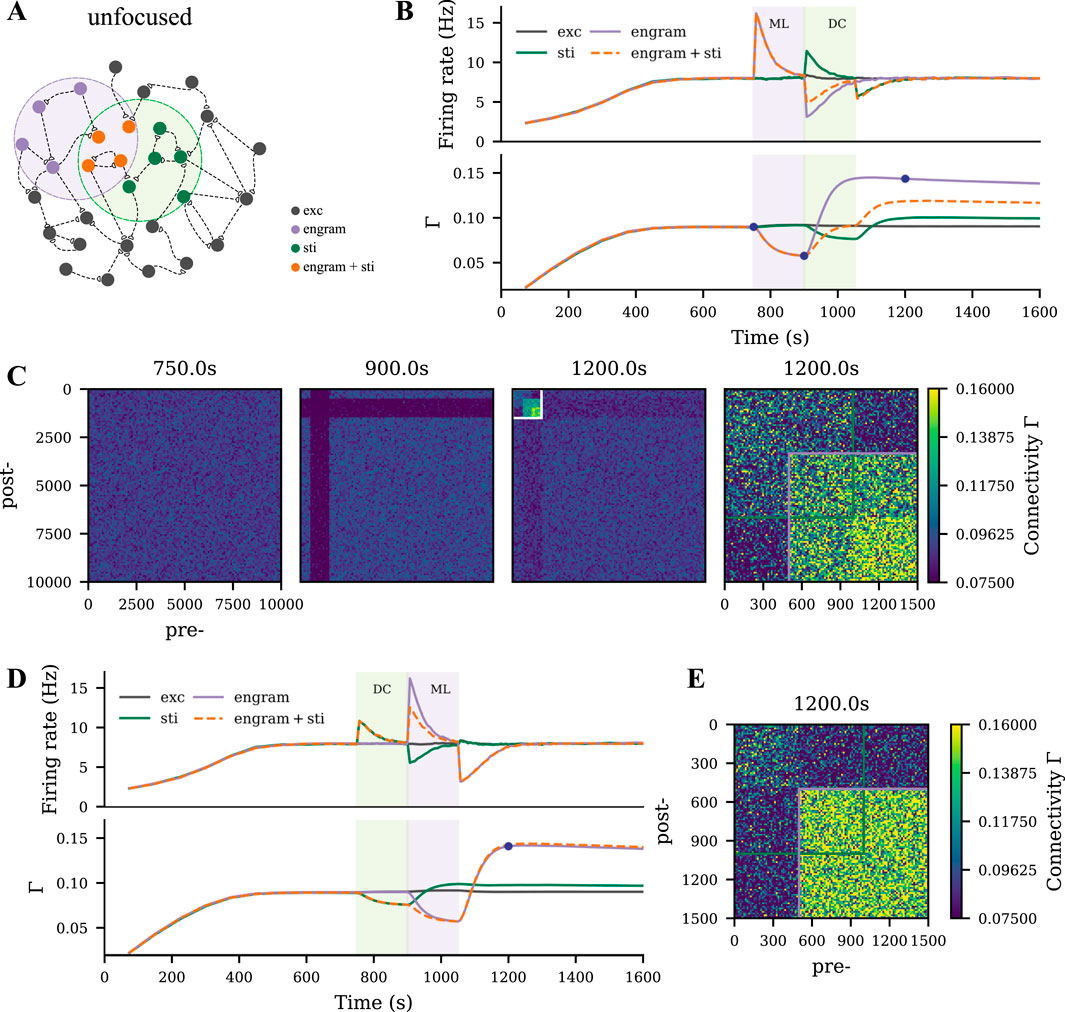
Figure 5. Motor engram and tDCS stimulated cell assembly interfere in case of overlap. (A) Schematic of the M1 neural network, where the motor learning engram and the tDCS stimulated cell assembly had
When applying the motor learning input prior to tDCS, substantial network remodeling took place to form a motor engram, while the following weak depolarizing tDCS posed the opposite dynamics to the overlapped motor engram. As a result, the connectivity of the overlapped half engram was lower than that of the non-overlapped half (Figure 5C, last panel). In contrast, when applying tDCS before learning, connectivity within the motor engram was not strongly affected, but the overlapped half ended up with higher connectivity than the non-overlapped half (Figure 5E).
To further explore the polarity, intensity, and phase-dependent effects of tDCS in the overlapping scenario, we also performed a systematic analysis. We plotted the temporal evolution of the firing rate and connectivity of the non-overlapped part of the motor engram, the overlapped part of the motor engram, and the tDCS-stimulated cell assembly separately in Figure 6. The non-overlapped half of the engram was slightly affected by tDCS (Figure 6A). The non-overlapped part of the tDCS-stimulated cell assembly was not much influenced by the learning process (Figure 6C). In contrast, the overlapped engram was modulated by tDCS in a contrary way compared to the non-overlapped half (Figures 6B,C). In general, our simulation results showed that weak and strong cell assemblies interacted with each other. The connectivity of the strong cell assembly (motor engram in this case) could be reduced by the following overlapping weak input, due to partial activation, but the connectivity of a weak cell assembly (the tDCS-stimulated cell assembly) is not drastically changed by the following strong input.
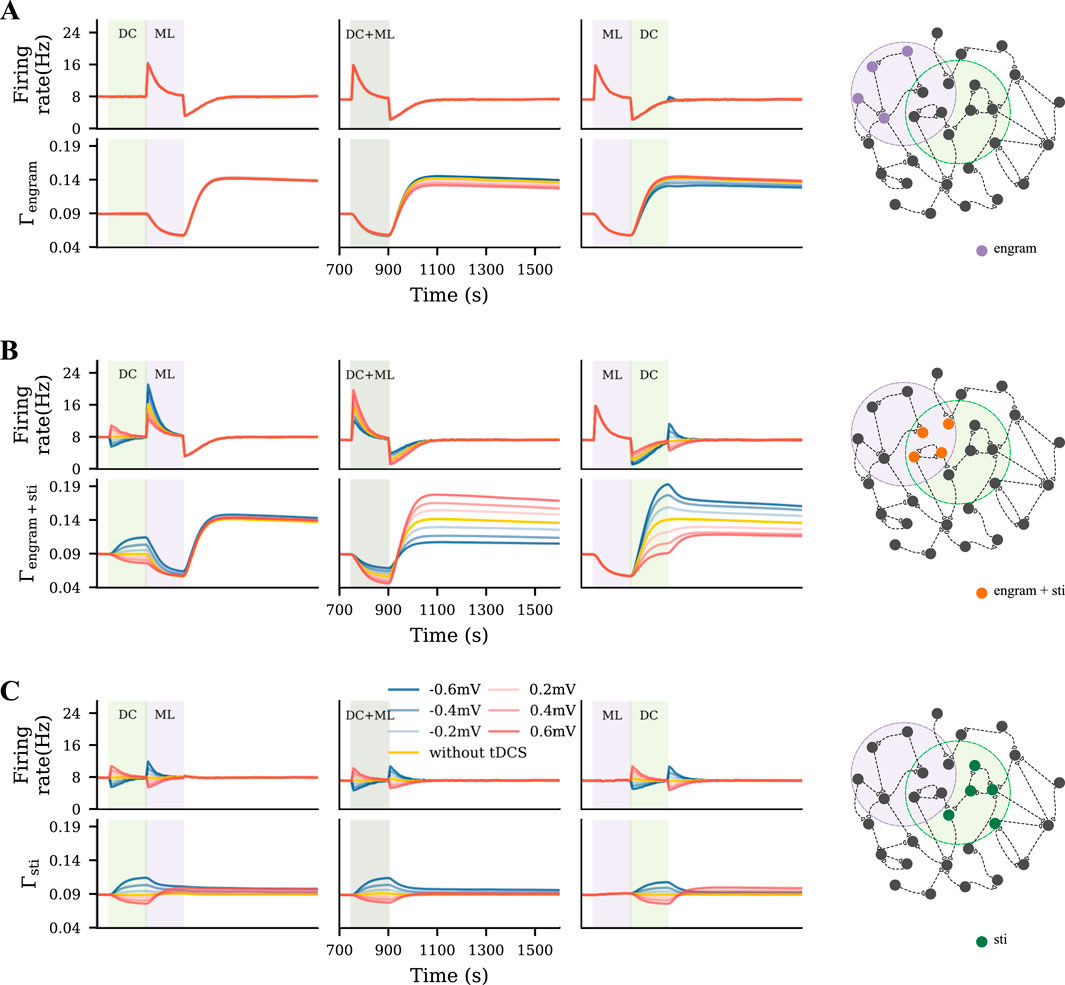
Figure 6. Unfocused tDCS of different intensities applied before, during, or after learning triggered different results. (A–C) Temporal evolution of firing rate and connectivity of the population that only sees motor learning input, the overlapped part of the motor engram that was also stimulated, and the neurons only subjected to tDCS, respectively.
Strong unfocused tDCS distorts the motor engram
As mentioned above, weak unfocused tDCS modulates motor learning in a phase-dependent manner. Then we wondered if a relatively stronger tDCS, compared to motor learning input, would yield similar results. We used tDCS to achieve a
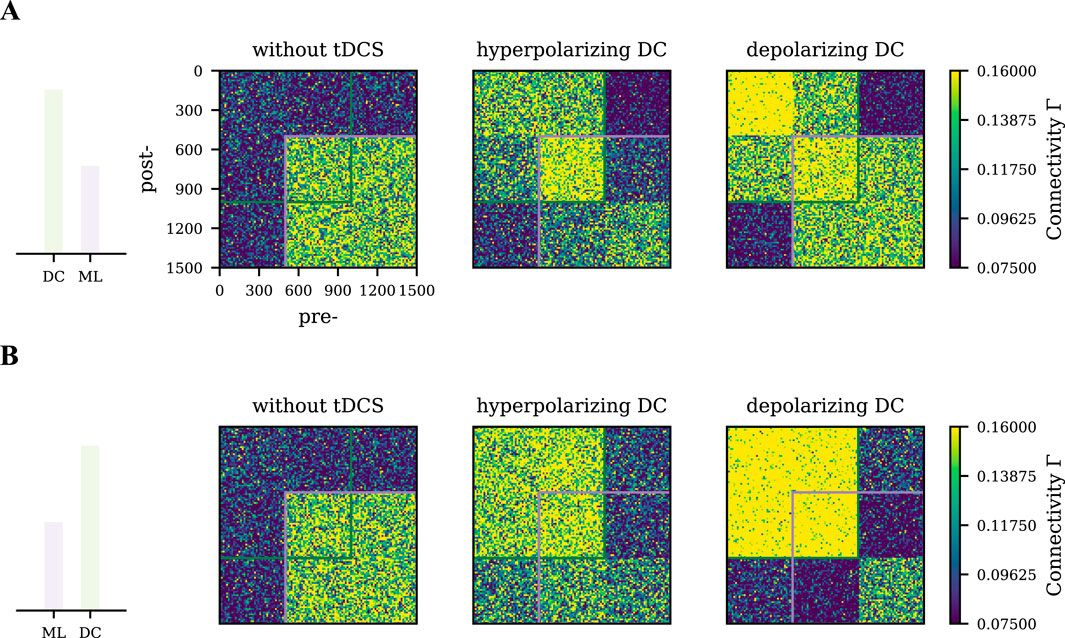
Figure 7. Differential effects of strong tDCS on the motor engram connectivity pattern. Network connectivity was captured at
Discussion
Motivated by the empirical observations that tDCS triggered changes in both synaptic transmission and dendritic spine density (Barbati et al., 2020; Jog et al., 2023), we used a neural network model that implements homeostatic structural plasticity in the present study to systematically explore the interference between tDCS and motor learning. Learning input to the network triggers specific remodeling and induces a memory engram that reflects an altered or entirely new motor program as modeled before (Gallinaro et al., 2022). Transcranial DC stimulation also alters the dynamics of the network and induces structural plasticity. However, when applied with different electrode montages and at different phases of motor learning, tDCS interferes with motor learning and modulates the connectivity of the motor engram in specific ways.
As summarized in Figure 8, both the timing with respect to learning and the focality of stimulation influenced the direction of the effects. Transcranial DC stimulation applied before learning showed generally weak modulatory effects. When administered concurrently with learning, the focality of stimulation played a critical role: Targeted anodal tDCS increased the engram’s connectivity, but targeted cathodal or uniform tDCS regardless of the polarity reduced it in an intensity-dependent manner. Given that the engram’s final connectivity is positively correlated with its evoked response (Gallinaro et al., 2022), such wide-sense “associative” effects of tDCS on motor learning have been observed before. We also predicted with our simulations that, when applied immediately after learning, uniform tDCS generally reduced the connectivity of the engram, but targeted tDCS showed reversed polarity-dependent modulatory effects: anodal tDCS weakened the memory engram, but cathodal stimulation strengthened it. Furthermore, unfocused tDCS affected the connectivity within the memory engram non-uniformly. In summary, our homeostatic plasticity model provides a systematic framework to account for all the confounding factors and mixed results regarding the interaction between tDCS and learning.
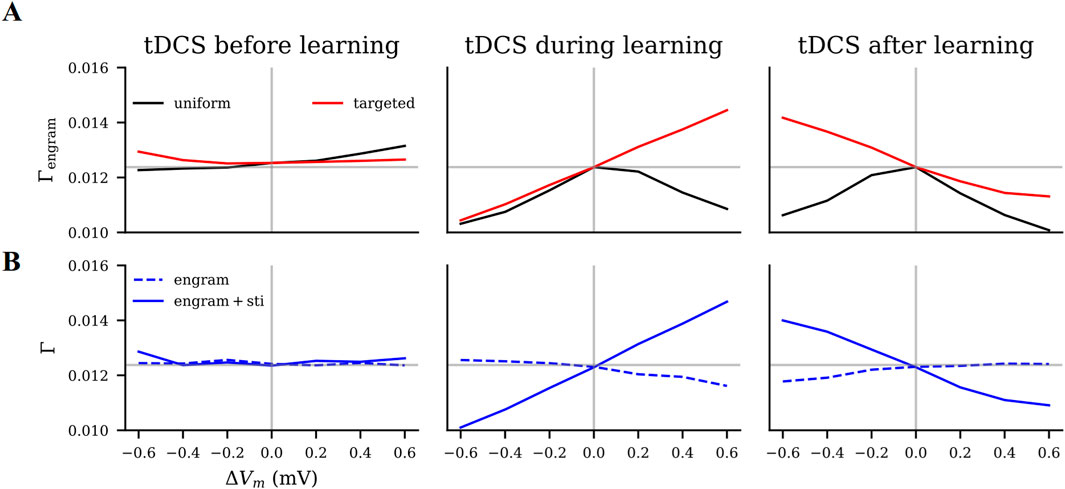
Figure 8. Modulatory effects of tDCS on motor learning as predicted by our model. We estimated the effects of tDCS by the connectivity of the motor engram
Importance of focality and electrode montage
Large electrodes were confirmed to induce less focal electric field distribution than small button-like electrodes used in the high-definition montage (Cole et al., 2018). However, the definition of focality that we discussed in the current study is a relevant concept with regard to the neural population engaged by a certain task. We classified it by the degree of overlap that the stimulated neuron population has with the neuron ensemble that encodes a motor engram. Our results confirm that the focality of the stimulus is critical to achieving a substantial modulatory effect of tDCS (Edwards et al., 2013; Kuo et al., 2013; Lu et al., 2019; Iannone et al., 2022). Clearly, the three classes of montage considered in our present work do not cover all variants used in motor learning experiments. Unilateral, bilateral, and high-definition electrode layouts are commonly used (Goodwill et al., 2013; Cole et al., 2018), and have been shown to result in mixed effects on motor tasks and learning phases.
We were able to show that uniform stimulation systematically reduces the effect of learning, if applied during or after learning, but will slightly boost learning if applied before learning as anodal stimulation. Notably, uniform stimulation simulated in the present work suggested a scenario in which the induced electric field covers more neurons or a larger area than the motor engram. This is a plausible situation in tDCS practice where large electrode pads induce diffuse electric fields in the brain (Bikson et al., 2010; Bikson et al., 2012). However, these hypothetical predictions do not always agree with the empirical observations. For example, unilateral or bilateral tDCS has been observed in experiments to increase the precision of motor tasks in a follow-up experiment, regardless of stimulation timing (Goodwill et al., 2013). Stimulation before learning using a bilateral montage facilitated learning more than in unilateral montages (Naros et al., 2016). This disagreement might be explained by how the learned skills are represented by the memory engram. The memory engram or motor engram has been demonstrated to be controlled by a group of neurons (Ramirez et al., 2013; Tonegawa et al., 2015) or even a specific population of synaptic spines (Hayashi-Takagi et al., 2015). Integration of new neurons into the memory engram has been proposed to degrade memory and explain infantile amnesia (Akers et al., 2014), and co-activation of neighboring neurons is considered to create false memories (Ramirez et al., 2013; Tonegawa et al., 2015). When uniform anodal tDCS is applied before motor learning, it “primes” the learning and eventually boosts the motor engram connectivity, which could explain the enhanced learning performance in practice. However, uniform tDCS applied during or after learning, regardless of polarity, interrupts motor engram formation by including more neurons than needed into the engram or by reducing motor engram connectivity. This may explain the reduced learning performance observed in practice.
In contrast, we also showed that targeted stimulation either boosts or reduces the impact of learning in a predictable way, depending on the current polarity and phase of the stimulus. Targeted stimulation could, for instance, be achieved by using focused stimulation such as HD-tDCS of the target region. In fact, HD-tDCS has been shown to have better effects in facilitating motor learning (Cole et al., 2018). Animal studies also confirmed that if tDCS precisely modulated the activity of the task-specific neural population, concurrent anodal tDCS restored motor learning in a task-specific manner (Wang et al., 2023). Alternatively, hypothetical engram-specific targeted stimulation would have the strongest effect. The distributed nature of engrams, however, renders this option unrealistic. Some studies have tried to modulate activity in specific neuronal populations when combined with neuroimaging techniques like functional MRI (fMRI), or functional near-infrared spectroscopy (fNIRS), or electroencephalography (EEG) in open-loop or closed-loop configurations (Gao et al., 2021; Martens et al., 2021), or in combination with numerical optimization approaches (Wagner et al., 2016).
Relative intensity of tDCS and motor learning
In human applications, tDCS is widely accepted as a weak and sub-threshold stimulation method (Jamil et al., 2017). This is the reason we assumed that tDCS provides a weaker stimulus than motor learning in our study. Our model suggests that the relative strength of tDCS compared to motor learning input is critical.
We started with weak transcranial DC stimulation (maximally at
Relative timing of tDCS and motor learning
Another factor confirmed in our model to mediate the modulatory effects of tDCS is the relative timing of tDCS applied to motor learning. Such results have been frequently observed in human experiments and have been summarized as state-dependent stimulation effects (Bradley et al., 2022; Qi et al., 2022; Lapenta et al., 2024). For example, in the case of pre-learning tDCS, anodal stimulation reduced the learning rate, while cathodal stimulation showed facilitation or impairment (Antal et al., 2008; Tecchio et al., 2010; Stagg et al., 2011; Apolin et al., 2016; Parma et al., 2021; Rivera-Urbina et al., 2022). Further studies showed that the impairment caused by pre-learning anodal tDCS was accompanied by a homeostatic increase of
Compared to other proposed homeostatic or Hebbian models, our HSP model can consistently explain both types of phenomena. Anodal pre-learning tDCS may hinder learning, when applied uniformly, or at an intensity that out-weights motor input. This is considered a homeostatic phenomenon. In contrast, it is considered a Hebbian phenomenon that concurrent anodal tDCS could facilitate learning. In addition, our model provides a systematic explanation for contradictory or confusing results in tDCS studies with different protocols. The electrode montage of tDCS, for instance, influences the relative coverage of the target region by the electric field, and this different focality can induce opposite effects, even if the polarity and intensity of the stimulus remain the same.
Memory encoding and consolidation
In addition to pre-learning and concurrent stimulation, our work also predicted the effects of post-learning tDCS on memory consolidation. Uniform stimulation applied post-learning or strong unfocused stimulation hindered memory consolidation, but targeted cathodal tDCS applied to the same brain region where motor learning occurs should boost memory consolidation.
Experimental results on the memory encoding and consolidation effects of post-learning tDCS are lacking. Some studies reported that tDCS applied to M1 facilitates memory encoding (Kim et al., 2024) or consolidation (Koyama et al., 2015; Rroji et al., 2015), where the stimulus was administered concurrently with learning, but not after learning. There are also preliminary results showing that the application of anodal tDCS
Model selection and limitations
Even though our model has the potential to explain inconsistent tDCS outcomes in the literature, certain choices made in the model require some attention in future work. The first issue concerns the choice of the growth curve. In our present study, we followed the same strategy as in our previous work (Lu et al., 2019) and applied the same linear growth rule for both dendritic spines and axonal boutons. However, the exact shape of the growth curve in a biological setting remains elusive. Computational studies have revealed the distinct properties of different rules, including the linear rule and the Gaussian rules (Butz et al., 2009; Butz and Van Ooyen, 2013; Diaz-Pier et al., 2016; Sinha et al., 2021; Lu et al., 2023). Compared to a linear rule, the Gaussian rules do not favor homeostatic synaptogenesis if the neuron is under the influence of strong chronic inhibition. In contrast, it does not imply any change or even allows for synapse loss, depending on whether the lower setpoint value is at zero or above zero [see Lu et al. (2023)] for comparison of three rules in a similar network setting). Our recent experimental data demonstrated the biological feasibility of a biphasic shape of the growth rule (Lu et al., 2023), which is well approximated by the Gaussian rule in simulations. Although the non-homeostatic property of the Gaussian HSP rule is critical to explain the divergent phenomena observed in chronic activity inhibition scenarios (Lu et al., 2023), it does not compromise the use of a linear rule in this study. Given the subthreshold property of tDCS treatment, namely, it excites or inhibits neuronal activity by slightly altering the membrane potential, a linear rule behaves qualitatively similar to a Gaussian rule in this range. Using the linear rule here further enables us to make a direct comparison with our previous tDCS modeling work (Lu et al., 2019).
The same principle applies when deciding whether different rules should be used to govern axonal boutons and dendritic spines. Asymmetric Gaussian-shaped rules—using different setpoint parameters for boutons and spines—in previous work have been considered “more physiological” in response to focal lesion (Butz et al., 2014; Sinha et al., 2021). Indeed, using asymmetric Gaussian rules in combination with distance-dependent connectivity is critical for natural re-wiring after focal lesion (Butz et al., 2014), but not necessarily essential for the after-effects of subthreshold stimulation and inhibition such as tDCS, which requires engram formation during network remodeling. In this regard, the linear rule and Gaussian rules display quite similar homeostatic behavior when modeling the long-term effects of tDCS and allows for engram formation. Therefore, one should not expect any important qualitative differences. We conclude that a model using identical linear rules for pre- and postsynaptic elements is able to capture all essential features.
A third concern is that we assumed the HSP rule and tDCS stimulation selectively to the excitatory, but not inhibitory neurons. This decision was mainly based on empirical evidence obtained from pyramidal cells. The rules of structural plasticity were mainly derived from studies of spine density [see reviews by Holtmaat and Svoboda (2009); Van Ooyen (2011); Caroni et al. (2012)]. Due to their spatially extended morphology, pyramidal cells were considered to be the main target cells of tDCS as well, as the polarization of their membrane is much more pronounced when placed in an electric field (Radman et al., 2009b; Vöröslakos et al., 2018). Nevertheless, computational studies demonstrate that applying HSP to both excitatory and inhibitory neurons can trigger interesting phenomena in response to external perturbations (Diaz-Pier et al., 2016). Studies have also shown that neural modulation can alter synaptic plasticity of inhibitory neurons (Lenz et al., 2016) or activate inhibitory circuits that modulate network dynamics (Anil et al., 2023). Such studies definitely suggest further modeling work to involve inhibitory neurons in plastic responses and neural plasticity.
It should be emphasized that we have only included one structural plasticity rule in the present study, while any forms of functional plasticity, such as spike-timing-dependent plasticity (STDP) and synaptic scaling, may alter the behavior of homeostatic structural plasticity itself. For example, a previous study has shown that the STDP rule could amplify the effects of stimulation and trigger further stronger engram formation (Manos et al., 2021). In contrast, homeostatic synaptic scaling could fine-tune recurrent synaptic weights when stimulated and inhibited, leading to a less prominent change in synapse numbers, which is considered as an indication of structural plasticity (Lu et al., 2023). Therefore, it is worth examining the impact of tDCS in a system with multiple activity-dependent plasticity rules, and the present study with a single plasticity rule can serve as an anchor point for bridging scientific observations.
Last but not least, the formation of engrams among excitatory neurons comes as a secondary effect of network remodeling when the excitatory population of the network is subject to the HSP rule. We use its connectivity to hypothetically represent the after-effects of tDCS or rTMS in this and previous studies (Lu et al., 2019; Anil et al., 2023) as inspired by empirical evidence of memory engram. In learning and memory tasks, for example, engram formation is considered to be elevated connectivity or increased spine density among a group of cells through coactivation (Tonegawa et al., 2015; Hwang et al., 2022). Activated pyramidal cells are confirmed to be the main components of an engram, since neural activation is easily confirmed by the expression of immediate early genes such as c-Fos (Tonegawa et al., 2015; Lu et al., 2021), dendritic morphology can be assessed using imaging techniques (Lu et al., 2021; Lu et al., 2023), and network connectivity can be probed by paired patch clamp (Lenz et al., 2016; Qi et al., 2020). In addition to those, it remains elusive whether inhibition of pyramidal cells leads to the formation of engrams, Furthermore, it has been recently proposed that inhibitory neurons can form a replica of the engram complementing their excitatory counterparts (Barron et al., 2017). Besides, although model parameters that result in increased engram connectivity seem to coincide with parameters that achieve lasting after-effects, there is still a lack of direct experimental evidence that tDCS works through engram formation. One of the reasons is that it is difficult to detect memory engrams experimentally. However, in similar studies, it has been shown that increased engram connectivity leads to more synchronized activity (Gallinaro et al., 2022; Lu et al., 2023), altered network dynamics may therefore serve as an experimental indicator of tDCS after-effects.
Conclusions and outlook
In the current study, we confirmed again that focal tDCS applied in a targeted manner leads to more predictable and reliable modulatory effects. We also showed that the HSP model can explain both the homeostatic and Hebbian plasticity phenomena observed in experiments using tDCS to modulate motor learning. When applied in a targeted manner or with high intensity, pre-learning anodal stimulation could impair the learning process, whereas concurrent anodal stimulation boosted learning. In addition, we predicted that post-learning cathodal tDCS might facilitate learning, which waits to be addressed in future experiments. Compared to previously proposed plasticity models, the HSP model, which is based on homeostatically controlled spine turnover dynamics, provides a more comprehensive explanation and leads to new predictions about the dynamic interaction between tDCS and motor learning.
But can the HSP rule also explain the tDCS effects observed in other forms of learning? Yes and no. The predictions of our model, such as improved learning for targeted concurrent anodal tDCS, match the results found in verbal learning (Nikolin et al., 2015) and in working memory tasks (Mancuso et al., 2016). Compared to motor tasks, however, verbal learning and working memory require the coordination of more brain regions and demand a model to reflect inter-network communication. Our present study is a first attempt to study the effect of transcranial brain stimulation on complex cognitive processes. To bring more insight to this field, more animal studies are still needed to calibrate the cellular mechanism of tDCS and optimize the homeostatic structural plasticity model.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. LF: Validation, Visualization, Writing – original draft, Writing – review and editing. CN: Validation, Visualization, Writing – original draft, Writing – review and editing. SR: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is funded by the Universitätsklinikum Freiburg and NEUREX.
Acknowledgments
Additional support by the German Research Foundation (DFG) through EXC 1086, and by the state of Baden-Württemberg through bwHPC and the German Research Foundation (DFG) through INST 39/963–1 FUGG is acknowledged. The authors thank Júlia V. Gallinaro for establishing the fundamental HSP model. We also thank Uwe Grauer from the Bernstein Center Freiburg, as well as Bernd Wiebelt and Michael Janczyk from the Freiburg University Computing Center for their assistance with HPC applications.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnetp.2025.1565802/full#supplementary-material
References
Akers, K. G., Martinez-Canabal, A., Restivo, L., Yiu, A. P., De Cristofaro, A., Hsiang, H. L., et al. (2014). Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344 (6184), 598–602. doi:10.1126/science.1248903
Alsharidah, M., Al-Hussain, F., Iqbal, M., Hamza, A., Yoo, W., and Bashir, S. (2018). The effect of transcranial direct current stimulation combined with functional task training on motor recovery in stroke patients. Eur. Rev. Med. Pharmacol. Sci. 22 (21), 7385–7392. doi:10.26355/eurrev_201811_16277
Amadi, U., Allman, C., Johansen-Berg, H., and Stagg, C. J. (2015). The homeostatic interaction between anodal transcranial direct current stimulation and motor learning in humans is related to GABAA activity. Brain Stimul. 8 (5), 898–905. doi:10.1016/j.brs.2015.04.010
Ambrus, G. G., Chaieb, L., Stilling, R., Rothkegel, H., Antal, A., and Paulus, W. (2016). Monitoring transcranial direct current stimulation induced changes in cortical excitability during the serial reaction time task. Neurosci. Lett. 616, 98–104. doi:10.1016/j.neulet.2016.01.039
Ammann, C., Spampinato, D., and Márquez-Ruiz, J. (2016). Modulating motor learning through transcranial direct-current stimulation: an integrative view. Front. Psychol. 7, 1981. doi:10.3389/fpsyg.2016.01981
Anil, S., Lu, H., Rotter, S., and Vlachos, A. (2023). Repetitive transcranial magnetic stimulation (rTMS) triggers dose-dependent homeostatic rewiring in recurrent neuronal networks. PLOS Comput. Biol. 19 (11), e1011027. doi:10.1371/journal.pcbi.1011027
Antal, A., Begemeier, S., Nitsche, M. A., and Paulus, W. (2008). Prior state of cortical activity influences subsequent practicing of a visuomotor coordination task. Neuropsychologia 46 (13), 3157–3161. doi:10.1016/j.neuropsychologia.2008.07.007
Apolinário-Souza, T., Romano-Silva, M. A., de Miranda, D. M., Malloy-Diniz, L. F., Benda, R. N., Ugrinowitsch, H., et al. (2016). The primary motor cortex is associated with learning the absolute, but not relative, timing dimension of a task: a tDCS study. Physiology and Behav. 160, 18–25. doi:10.1016/j.physbeh.2016.03.025
Baeken, C., Brunelin, J., Duprat, R., and Vanderhasselt, M. A. (2016). The application of tDCS in psychiatric disorders: a brain imaging view. Socioaffective Neurosci. and Psychol. 6 (1), 29588. doi:10.3402/snp.v6.29588
Balzan, P., Tattersall, C., and Palmer, R. (2022). Non-invasive brain stimulation for treating neurogenic dysarthria: a systematic review. Ann. Phys. Rehabilitation Med. 65 (5), 101580. doi:10.1016/j.rehab.2021.101580
Barbati, S. A., Cocco, S., Longo, V., Spinelli, M., Gironi, K., Mattera, A., et al. (2020). Enhancing plasticity mechanisms in the mouse motor cortex by anodal transcranial direct-current stimulation: the contribution of nitric oxide signaling. Cereb. Cortex 30 (5), 2972–2985. doi:10.1093/cercor/bhz288
Barron, H. C., Vogels, T. P., Behrens, T. E., and Ramaswami, M. (2017). Inhibitory engrams in perception and memory. Proc. Natl. Acad. Sci. 114 (26), 6666–6674. doi:10.1073/pnas.1701812114
Batsikadze, G., Moliadze, V., Paulus, W., Kuo, M. F., and Nitsche, M. (2013). Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiology 591 (7), 1987–2000. doi:10.1113/jphysiol.2012.249730
Berrigan, P., Hodge, J., Kirton, A., Moretti, M. E., Ungar, W. J., and Zwicker, J. D. (2021). Protocol for a cost–utility analysis of neurostimulation and intensive camp-based therapy for children with perinatal stroke and hemiparesis based on a multicentre clinical trial. BMJ Open 11 (1), e041444. doi:10.1136/bmjopen-2020-041444
Bikson, M., Datta, A., Rahman, A., and Scaturro, J. (2010). Electrode montages for tDCS and weak transcranial electrical stimulation: role of “return” electrode’s position and size. Clin. neurophysiology official J. Int. Fed. Clin. Neurophysiology 121 (12), 1976–1978. doi:10.1016/j.clinph.2010.05.020
Bikson, M., Inoue, M., Akiyama, H., Deans, J. K., Fox, J. E., Miyakawa, H., et al. (2004). Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J. Physiology 557 (1), 175–190. doi:10.1113/jphysiol.2003.055772
Bikson, M., Rahman, A., and Datta, A. (2012). Computational models of transcranial direct current stimulation. Clin. EEG Neurosci. 43 (3), 176–183. doi:10.1177/1550059412445138
Bradley, C., Nydam, A. S., Dux, P. E., and Mattingley, J. B. (2022). State-dependent effects of neural stimulation on brain function and cognition. Natature Rev. Neurosci. 23, 459–475. doi:10.1038/s41583-022-00598-1
Brunel, N. (2000). Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. J. Comput. Neurosci. 8 (3), 183–208. doi:10.1023/a:1008925309027
Buch, E. R., Santarnecchi, E., Antal, A., Born, J., Celnik, P. A., Classen, J., et al. (2017). Effects of tDCS on motor learning and memory formation: a consensus and critical position paper. Clin. Neurophysiol. 128 (4), 589–603. doi:10.1016/j.clinph.2017.01.004
Burkhardt, G., Kumpf, U., Crispin, A., Goerigk, S., Andre, E., Plewnia, C., et al. (2023). Transcranial direct current stimulation as an additional treatment to selective serotonin reuptake inhibitors in adults with major depressive disorder in Germany (DepressionDC): a triple-blind, randomised, sham-controlled, multicentre trial. Lancet 402 (10401), 545–554. doi:10.1016/S0140-6736(23)00640-2
Butz, M., Steenbuck, I. D., and van Ooyen, A. (2014). Homeostatic structural plasticity can account for topology changes following deafferentation and focal stroke. Front. Neuroanat. 8, 115. doi:10.3389/fnana.2014.00115
Butz, M., and Van Ooyen, A. (2013). A simple rule for dendritic spine and axonal bouton formation can account for cortical reorganization after focal retinal lesions. PLOS Comput. Biol. 9 (10), e1003259. doi:10.1371/journal.pcbi.1003259
Butz, M., Van Ooyen, A., and Wörgötter, F. (2009). A model for cortical rewiring following deafferentation and focal stroke. Front. Comput. Neurosci. 3, 330. doi:10.3389/neuro.10.010.2009
Caroni, P., Donato, F., and Muller, D. (2012). Structural plasticity upon learning: regulation and functions. Nat. Rev. Neurosci. 13 (7), 478–490. doi:10.1038/nrn3258
Chakraborty, D., Truong, D. Q., Bikson, M., and Kaphzan, H. (2018). Neuromodulation of axon terminals. Cereb. Cortex 28 (8), 2786–2794. doi:10.1093/cercor/bhx158
Ciechanski, P., Carlson, H., Herrero, M., Lane, C., MacMaster, F., and Kirton, A. (2020). A case of transcranial direct-current stimulation for childhood stroke hemiparesis: a brief report. Dev. Neurorehabilitation 23 (2), 133–136. doi:10.1080/17518423.2019.1655678
Clark, V. P., Coffman, B. A., Mayer, A. R., Weisend, M. P., Lane, T. D., Calhoun, V. D., et al. (2012). TDCS guided using fMRI significantly accelerates learning to identify concealed objects. NeuroImage 59 (1), 117–128. doi:10.1016/j.neuroimage.2010.11.036
Coffman, B. A., Clark, V. P., and Parasuraman, R. (2014). Battery powered thought: enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. NeuroImage. 85, 895–908. doi:10.1016/j.neuroimage.2013.07.083
Cole, L., Giuffre, A., Ciechanski, P., Carlson, H. L., Zewdie, E., Kuo, H. C., et al. (2018). Effects of high-definition and conventional transcranial direct-current stimulation on motor learning in children. Front. Neurosci. 12, 787. doi:10.3389/fnins.2018.00787
Cuypers, K., Leenus, D. J., van den Berg, F. E., Nitsche, M. A., Thijs, H., Wenderoth, N., et al. (2013). Is motor learning mediated by tDCS intensity? PlOS ONE 8 (6), e67344. doi:10.1371/journal.pone.0067344
Debarnot, U., Neveu, R., Samaha, Y., Saruco, E., Macintyre, T., and Guillot, A. (2019). Acquisition and consolidation of implicit motor learning with physical and mental practice across multiple days of anodal tDCS. Neurobiol. Learn. Mem. 164, 107062. doi:10.1016/j.nlm.2019.107062
Diaz-Pier, S., Naveau, M., Ostendorf, M., and Morrison, A. (2016). Automatic generation of connectivity for large-scale neuronal network models through structural plasticity. Front. Neuroanat. 10, 57. doi:10.3389/fnana.2016.00057
Duan, Z., and Zhang, C. (2024). Transcranial direct current stimulation for Parkinson’s disease: systematic review and meta-analysis of motor and cognitive effects. npj Parkinson’s Dis. 10 (1), 214. doi:10.1038/s41531-024-00821-z
Edwards, D., Cortes, M., Datta, A., Minhas, P., Wassermann, E. M., and Bikson, M. (2013). Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. NeuroImage 74, 266–275. doi:10.1016/j.neuroimage.2013.01.042
Flöel, A. (2014). tDCS-enhanced motor and cognitive function in neurological diseases. NeuroImage 85, 934–947. doi:10.1016/j.neuroimage.2013.05.098
Frase, L., Jahn, F., Tsodor, S., Krone, L., Selhausen, P., Feige, B., et al. (2020). Offline Bi-frontal anodal transcranial direct current stimulation decreases total sleep time without disturbing overnight memory consolidation. Neuromodulation Technol. A. T. Neural Interface 24, 910–915. doi:10.1111/ner.13163
Fregni, F., and Pascual-Leone, A. (2007). Technology insight: noninvasive brain stimulation in neurology—perspectives on the therapeutic potential of rTMS and tDCS. Nat. Clin. Pract. Neurol. 3 (7), 383–393. doi:10.1038/ncpneuro0530
Gallinaro, J. V., Gašparović, N., and Rotter, S. (2022). Homeostatic control of synaptic rewiring in recurrent networks induces the formation of stable memory engrams. PLOS Comput. Biol. 18 (2), e1009836. doi:10.1371/journal.pcbi.1009836
Gallinaro, J. V., and Rotter, S. (2018). Associative properties of structural plasticity based on firing rate homeostasis in recurrent neuronal networks. Sci. Rep. 8 (1), 3754–13. doi:10.1038/s41598-018-22077-3
Gao, Y., Cavuoto, L., Dutta, A., Kruger, U., Yan, P., Nemani, A., et al. (2021). Decreasing the surgical errors by neurostimulation of primary motor cortex and the associated brain activation via neuroimaging. Front. Neurosci. 15, 651192. doi:10.3389/fnins.2021.651192
Ghasemian-Shirvan, E., Farnad, L., Mosayebi-Samani, M., Verstraelen, S., Meesen, R. L., Kuo, M. F., et al. (2020). Age-related differences of motor cortex plasticity in adults: a transcranial direct current stimulation study. Brain Stimul. 13 (6), 1588–1599. doi:10.1016/j.brs.2020.09.004
Goodwill, A. M., Reynolds, J., Daly, R. M., and Kidgell, D. J. (2013). Formation of cortical plasticity in older adults following tDCS and motor training. Front. Aging Neurosci. 5, 87. doi:10.3389/fnagi.2013.00087
Greeley, B., Barnhoorn, J. S., Verwey, W. B., and Seidler, R. D. (2022). Anodal transcranial direct current stimulation over prefrontal cortex slows sequence learning in older adults. Front. Hum. Neurosci. 16, 814204. doi:10.3389/fnhum.2022.814204
Halakoo, S., Ehsani, F., Hosnian, M., Zoghi, M., and Jaberzadeh, S. (2020). The comparative effects of unilateral and bilateral transcranial direct current stimulation on motor learning and motor performance: a systematic review of literature and meta-analysis. J. Clin. Neurosci. 72, 8–14. doi:10.1016/j.jocn.2019.12.022
Hayashi-Takagi, A., Yagishita, S., Nakamura, M., Shirai, F., Wu, Y. I., Loshbaugh, A. L., et al. (2015). Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525 (7569), 333–338. doi:10.1038/nature15257
Holtmaat, A., and Svoboda, K. (2009). Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 10 (9), 647–658. doi:10.1038/nrn2699
Hwang, F. J., Roth, R. H., Wu, Y. W., Sun, Y., Kwon, D. K., Liu, Y., et al. (2022). Motor learning selectively strengthens cortical and striatal synapses of motor engram neurons. Neuron 110 (17), 2790–2801.e5. doi:10.1016/j.neuron.2022.06.006
Iannone, A., Santiago, I., Ajao, S. T., Brasil-Neto, J., Rothwell, J. C., and Spampinato, D. A. (2022). Comparing the effects of focal and conventional tDCS on motor skill learning: a proof of principle study. Neurosci. Res. 178, 83–86. doi:10.1016/j.neures.2022.01.006
Jamil, A., Batsikadze, G., Kuo, H. I., Labruna, L., Hasan, A., Paulus, W., et al. (2017). Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J. Physiology 595 (4), 1273–1288. doi:10.1113/JP272738
Jog, M. A., Anderson, C., Kubicki, A., Boucher, M., Leaver, A., Hellemann, G., et al. (2023). Transcranial direct current stimulation (tDCS) in depression induces structural plasticity. Sci. Rep. 13 (1), 2841. doi:10.1038/s41598-023-29792-6
Karabanov, A., Ziemann, U., Hamada, M., George, M. S., Quartarone, A., Classen, J., et al. (2015). Consensus paper: probing homeostatic plasticity of human cortex with non-invasive transcranial brain stimulation. Brain Stimul. 8 (5), 442–454. doi:10.1016/j.brs.2015.01.404
Kim, M. S., Koo, H., Han, S. W., Paulus, W., Nitsche, M. A., Kim, Y. H., et al. (2017). Repeated anodal transcranial direct current stimulation induces neural plasticity-associated gene expression in the rat cortex and hippocampus. Restor. Neurology Neurosci. 35 (2), 137–146. doi:10.3233/RNN-160689
Kim, T., Kim, H., Philip, B. A., and Wright, D. L. (2024). M1 recruitment during interleaved practice is important for encoding, not just consolidation, of skill memory. npj Sci. Learn. 9 (1), 77. doi:10.1038/s41539-024-00290-2
Koyama, S., Tanaka, S., Tanabe, S., and Sadato, N. (2015). Dual-hemisphere transcranial direct current stimulation over primary motor cortex enhances consolidation of a ballistic thumb movement. Neurosci. Lett. 588, 49–53. doi:10.1016/j.neulet.2014.11.043
Kronberg, G., Bridi, M., Abel, T., Bikson, M., and Parra, L. C. (2017). Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimul. 10 (1), 51–58. doi:10.1016/j.brs.2016.10.001
Kronberg, G., Rahman, A., Sharma, M., Bikson, M., and Parra, L. C. (2020). Direct current stimulation boosts Hebbian plasticity in vitro. Brain Stimul. 13 (2), 287–301. doi:10.1016/j.brs.2019.10.014
Kuo, H. I., Bikson, M., Datta, A., Minhas, P., Paulus, W., Kuo, M. F., et al. (2013). Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul. 6 (4), 644–648. doi:10.1016/j.brs.2012.09.010
Kuo, M. F., Paulus, W., and Nitsche, M. A. (2014). Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage. 85, 948–960. doi:10.1016/j.neuroimage.2013.05.117
Kuo, M. F., Unger, M., Liebetanz, D., Lang, N., Tergau, F., Paulus, W., et al. (2008). Limited impact of homeostatic plasticity on motor learning in humans. Neuropsychologia 46 (8), 2122–2128. doi:10.1016/j.neuropsychologia.2008.02.023
Lang, N., Siebner, H. R., Ward, N. S., Lee, L., Nitsche, M. A., Paulus, W., et al. (2005). How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur. J. Neurosci. 22 (2), 495–504. doi:10.1111/j.1460-9568.2005.04233.x
Lapenta, O. M., Rego, G. G., and Boggio, P. S. (2024). Transcranial electrical stimulation for procedural learning and rehabilitation. Neurobiol. Learn. Mem. 213, 107958. doi:10.1016/j.nlm.2024.107958
Laste, G., Caumo, W., Adachi, L. N. S., Rozisky, J. R., de Macedo, I. C., Filho, P. R. M., et al. (2012). After-effects of consecutive sessions of transcranial direct current stimulation (tDCS) in a rat model of chronic inflammation. Exp. Brain Res. 221, 75–83. doi:10.1007/s00221-012-3149-x
Latchoumane, C. F. V., Jackson, L., Sendi, M. S. E., Tehrani, K. F., Mortensen, L. J., Stice, S. L., et al. (2018). Chronic electrical stimulation promotes the excitability and plasticity of ESC-derived neurons following glutamate-induced inhibition in vitro. Scitific Rep. 8 (1), 10957–16. doi:10.1038/s41598-018-29069-3
Lenz, M., Galanis, C., Müller-Dahlhaus, F., Opitz, A., Wierenga, C. J., Szabó, G., et al. (2016). Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nat. Commun. 7 (1), 10020. doi:10.1038/ncomms10020
Linssen, C., Lepperød, M. E., Mitchell, J., Pronold, J., Eppler, J. M., Keup, C., et al. (2018). NEST 2.16.0. zenodo. doi:10.5281/zenodo.1400175
Lu, H., Diaz, S., Lenz, M., and Vlachos, A. (2023). The interplay between homeostatic synaptic scaling and homeostatic structural plasticity maintains the robust firing rate of neural networks. eLife, 2023–03. doi:10.7554/eLife.88376.2
Lu, H., Gallinaro, J. V., Normann, C., Rotter, S., and Yalcin, I. (2021). Time course of homeostatic structural plasticity in response to optogenetic stimulation in mouse anterior cingulate cortex. Cereb. Cortex, 2020–09. doi:10.1093/cercor/bhab281
Lu, H., Gallinaro, J. V., and Rotter, S. (2019). Network remodeling induced by transcranial brain stimulation: a computational model of tDCS-triggered cell assembly formation. Netw. Neurosci. 3 (4), 924–943. doi:10.1162/netn_a_00097
Majdi, A., van Boekholdt, L., Sadigh-Eteghad, S., and Mc Laughlin, M. (2022). A systematic review and meta-analysis of transcranial direct-current stimulation effects on cognitive function in patients with Alzheimer’s disease. Mol. Psychiatry 27 (4), 2000–2009. doi:10.1038/s41380-022-01444-7
Mancuso, L. E., Ilieva, I. P., Hamilton, R. H., and Farah, M. J. (2016). Does transcranial direct current stimulation improve healthy working memory? a meta-analytic review. J. Cognitive Neurosci. 28 (8), 1063–1089. doi:10.1162/jocn_a_00956
Manos, T., Diaz-Pier, S., and Tass, P. A. (2021). Long-term desynchronization by coordinated reset stimulation in a neural network model with synaptic and structural plasticity. Front. Physiology 12, 716556. doi:10.3389/fphys.2021.716556
Manto, M., Argyropoulos, G. P., Bocci, T., Celnik, P. A., Corben, L. A., Guidetti, M., et al. (2021). Consensus paper: novel directions and next steps of non-invasive brain stimulation of the cerebellum in health and disease. Cerebellum 21, 1092–1122. doi:10.1007/s12311-021-01344-6
Martens, G., Ibáñez-Soria, D., Barra, A., Soria-Frisch, A., Piarulli, A., Gosseries, O., et al. (2021). A novel closed-loop EEG-tDCS approach to promote responsiveness of patients in minimally conscious state: a study protocol. Behav. Brain Res. 409, 113311. doi:10.1016/j.bbr.2021.113311
Morya, E., Monte-Silva, K., Bikson, M., Esmaeilpour, Z., Biazoli, C. E., Fonseca, A., et al. (2019). Beyond the target area: an integrative view of tDCS-induced motor cortex modulation in patients and athletes. J. NeuroEngineering Rehabilitation 16 (1), 141–29. doi:10.1186/s12984-019-0581-1
Naros, G., Geyer, M., Koch, S., Mayr, L., Ellinger, T., Grimm, F., et al. (2016). Enhanced motor learning with bilateral transcranial direct current stimulation: impact of polarity or current flow direction? Clin. Neurophysiol. 127 (4), 2119–2126. doi:10.1016/j.clinph.2015.12.020
Nikolin, S., Loo, C. K., Bai, S., Dokos, S., and Martin, D. M. (2015). Focalised stimulation using high definition transcranial direct current stimulation (HD-tDCS) to investigate declarative verbal learning and memory functioning. NeuroImage. 117, 11–19. doi:10.1016/j.neuroimage.2015.05.019
Nitsche, M. A., Fricke, K., Henschke, U., Schlitterlau, A., Liebetanz, D., Lang, N., et al. (2003a). Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiology 553 (1), 293–301. doi:10.1113/jphysiol.2003.049916
Nitsche, M. A., Jakoubkova, M., Thirugnanasambandam, N., Schmalfuss, L., Hullemann, S., Sonka, K., et al. (2010). Contribution of the premotor cortex to consolidation of motor sequence learning in humans during sleep. J. Neurophysiology 104 (5), 2603–2614. doi:10.1152/jn.00611.2010
Nitsche, M. A., and Paulus, W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57 (10), 1899–1901. doi:10.1212/wnl.57.10.1899
Nitsche, M. A., Schauenburg, A., Lang, N., Liebetanz, D., Exner, C., Paulus, W., et al. (2003b). Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J. Cognitive Neurosci. 15 (4), 619–626. doi:10.1162/089892903321662994
O’Brien, A., Bertolucci, F., Torrealba-Acosta, G., Huerta, R., Fregni, F., and Thibaut, A. (2018). Non-invasive brain stimulation for fine motor improvement after stroke: a meta-analysis. Eur. J. Neurology 25 (8), 1017–1026. doi:10.1111/ene.13643
Orban de Xivry, J. J., and Shadmehr, R. (2014). Electrifying the motor engram: effects of tDCS on motor learning and control. Exp. Brain Res. 232 (11), 3379–3395. doi:10.1007/s00221-014-4087-6
Palm, U., Hasan, A., Strube, W., and Padberg, F. (2016). tDCS for the treatment of depression: a comprehensive review. Eur. Archives Psychiatry Clin. Neurosci. 266 (8), 681–694. doi:10.1007/s00406-016-0674-9
Parma, J. O., Profeta, V. L. S., Andrade, A. G. P., Lage, G. M., and Apolinário-Souza, T. (2021). TDCS of the primary motor cortex: learning the absolute dimension of a complex motor task. J. Mot. Behav. 53 (4), 431–444. doi:10.1080/00222895.2020.1792823
Pergher, V., Au, J., Shalchy, M. A., Santarnecchi, E., Seitz, A., Jaeggi, S. M., et al. (2022). The benefits of simultaneous tDCS and working memory training on transfer outcomes: a systematic review and meta-analysis. Brain Stimul. 15 (6), 1541–1551. doi:10.1016/j.brs.2022.11.008
Qi, G., Yang, D., Ding, C., and Feldmeyer, D. (2020). Unveiling the synaptic function and structure using paired recordings from synaptically coupled neurons. Front. Synaptic Neurosci. 12, 5. doi:10.3389/fnsyn.2020.00005
Qi, S., Liang, Z., Wei, Z., Liu, Y., and Wang, X. (2022). Effects of transcranial direct current stimulation on motor skills learning in healthy adults through the activation of different brain regions: a systematic review. Front. Hum. Neurosci. 16, 1021375. doi:10.3389/fnhum.2022.1021375
Radman, T., Datta, A., Ramos, R. L., Brumberg, J. C., and Bikson, M. (2009a). One-dimensional representation of a neuron in a uniform electric field. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 6481–6484. doi:10.1109/IEMBS.2009.5333586
Radman, T., Parra, L., and Bikson, M. (2006). Amplification of small electric fields by neurons; implications for spike timing. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 4949–4952. doi:10.1109/IEMBS.2006.259636
Radman, T., Ramos, R. L., Brumberg, J. C., and Bikson, M. (2009b). Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2 (4), 215–228. doi:10.1016/j.brs.2009.03.007
Radman, T., Su, Y., An, J. H., Parra, L. C., and Bikson, M. (2007). Spike timing amplifies the effect of electric fields on neurons: implications for endogenous field effects. J. Neurosci. 27 (11), 3030–3036. doi:10.1523/JNEUROSCI.0095-07.2007
Rahman, A., Reato, D., Arlotti, M., Gasca, F., Datta, A., Parra, L. C., et al. (2013). Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J. Physiology 591 (10), 2563–2578. doi:10.1113/jphysiol.2012.247171
Ramirez, S., Liu, X., Lin, P. A., Suh, J., Pignatelli, M., Redondo, R. L., et al. (2013). Creating a false memory in the hippocampus. Science 341 (6144), 387–391. doi:10.1126/science.1239073
Reis, J., and Fritsch, B. (2011). Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr. Opin. Neurology 24 (6), 590–596. doi:10.1097/WCO.0b013e32834c3db0
Reis, J., Robertson, E., Krakauer, J. W., Rothwell, J., Marshall, L., Gerloff, C., et al. (2008). Consensus: “Can tDCS and TMS enhance motor learning and memory formation?”. Brain Stimul. 1 (4), 363–369. doi:10.1016/j.brs.2008.08.001
Rivera-Urbina, G. N., Molero-Chamizo, A., and Nitsche, M. A. (2022). Discernible effects of tDCS over the primary motor and posterior parietal cortex on different stages of motor learning. Brain Struct. Funct. 227, 1115–1131. doi:10.1007/s00429-021-02451-0
Rroji, O., van Kuyck, K., Nuttin, B., and Wenderoth, N. (2015). Anodal tDCS over the primary motor cortex facilitates long-term memory formation reflecting use-dependent plasticity. PLOS ONE 10 (5), e0127270. doi:10.1371/journal.pone.0127270
Sanes, J. N., and Donoghue, J. P. (2000). Plasticity and primary motor cortex. Annu. Rev. Neurosci. 23 (1), 393–415. doi:10.1146/annurev.neuro.23.1.393
Sarmiento, C., San-Juan, D., and Prasath, V. (2016). Letter to the Editor: brief history of transcranial direct current stimulation (tDCS): from electric fishes to microcontrollers. Psychol. Med. 46 (15), 3259–3261. doi:10.1017/S0033291716001926
Saucedo Marquez, C. M., Zhang, X., Swinnen, S. P., Meesen, R., and Wenderoth, N. (2013). Task-specific effect of transcranial direct current stimulation on motor learning. Front. Hum. Neurosci. 7, 333. doi:10.3389/fnhum.2013.00333
Sevilla-Sanchez, M., Hortobágyi, T., Carballeira, E., Fogelson, N., and Fernandez-del Olmo, M. (2022). A lack of timing-dependent effects of transcranial direct current stimulation (tDCS) on the performance of a choice reaction time task. Neurosci. Lett. 782, 136691. doi:10.1016/j.neulet.2022.136691
Shaner, S., Lu, H., Lenz, M., Garg, S., Vlachos, A., and Asplund, M. (2023). Brain stimulation-on-a-chip: a neuromodulation platform for brain slices. Lab a Chip 23 (23), 4967–4985. doi:10.1039/d3lc00492a
Simpson, M. W., and Mak, M. (2022). Single session transcranial direct current stimulation to the primary motor cortex fails to enhance early motor sequence learning in Parkinson’s disease. Behav. Brain Res. 418, 113624. doi:10.1016/j.bbr.2021.113624
Sinha, A., Metzner, C., Davey, N., Adams, R., Schmuker, M., and Steuber, V. (2021). Growth rules for the repair of Asynchronous Irregular neuronal networks after peripheral lesions. PLOS Comput. Biol. 17 (6), e1008996. doi:10.1371/journal.pcbi.1008996
Soekadar, S. R., Witkowski, M., Birbaumer, N., and Cohen, L. G. (2015). Enhancing Hebbian learning to control brain oscillatory activity. Cereb. Cortex 25 (9), 2409–2415. doi:10.1093/cercor/bhu043
Stagg, C., Jayaram, G., Pastor, D., Kincses, Z., Matthews, P., and Johansen-Berg, H. (2011). Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 49 (5), 800–804. doi:10.1016/j.neuropsychologia.2011.02.009
Tecchio, F., Zappasodi, F., Assenza, G., Tombini, M., Vollaro, S., Barbati, G., et al. (2010). Anodal transcranial direct current stimulation enhances procedural consolidation. J. Neurophysiology 104 (2), 1134–1140. doi:10.1152/jn.00661.2009
Thibaut, A., Russo, C., Morales-Quezada, L., Hurtado-Puerto, A., Deitos, A., Freedman, S., et al. (2017). Neural signature of tDCS, tPCS and their combination: comparing the effects on neural plasticity. Neurosci. Lett. 637, 207–214. doi:10.1016/j.neulet.2016.10.026
Tonegawa, S., Pignatelli, M., Roy, D. S., and Ryan, T. J. (2015). Memory engram storage and retrieval. Curr. Opin. Neurobiol. 35, 101–109. doi:10.1016/j.conb.2015.07.009
Van Ooyen, A. (2011). Using theoretical models to analyse neural development. Nat. Rev. Neurosci. 12 (6), 311–326. doi:10.1038/nrn3031
Vasu, S. O., and Kaphzan, H. (2021). The role of sodium channels in direct current stimulation—axonal perspective. Cell Rep. 37 (2), 109832. doi:10.1016/j.celrep.2021.109832
Vasu, S. O., and Kaphzan, H. (2022). Calcium channels control tDCS-induced spontaneous vesicle release from axon terminals. Brain Stimul. 15, 270–282. doi:10.1016/j.brs.2022.01.005
Vöröslakos, M., Takeuchi, Y., Brinyiczki, K., Zombori, T., Oliva, A., Fernández-Ruiz, A., et al. (2018). Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat. Commun. 9 (1), 483. doi:10.1038/s41467-018-02928-3
Wade, S., and Hammond, G. (2015). Anodal transcranial direct current stimulation over premotor cortex facilitates observational learning of a motor sequence. Eur. J. Neurosci. 41 (12), 1597–1602. doi:10.1111/ejn.12916
Wagner, S., Burger, M., and Wolters, C. H. (2016). An optimization approach for well-targeted transcranial direct current stimulation. SIAM J. Appl. Math. 76 (6), 2154–2174. doi:10.1137/15m1026481
Wang, S. M., Chan, Y. W., Tsui, Y. O., and Chu, F. Y. (2021). Effects of anodal cerebellar transcranial direct current stimulation on movements in patients with cerebellar ataxias: a systematic review. Int. J. Environ. Res. Public Health 18 (20), 10690. doi:10.3390/ijerph182010690
Wang, Y., Wang, J., Zhang, Q. F., Xiao, K. W., Wang, L., Yu, Q. P., et al. (2023). Neural mechanism underlying task-specific enhancement of motor learning by concurrent transcranial direct current stimulation. Neurosci. Bull. 39 (1), 69–82. doi:10.1007/s12264-022-00901-1
Xu, T., Yu, X., Perlik, A. J., Tobin, W. F., Zweig, J. A., Tennant, K., et al. (2009). Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462 (7275), 915–919. doi:10.1038/nature08389
Yang, G., Lai, C. S. W., Cichon, J., Ma, L., Li, W., and Gan, W. B. (2014). Sleep promotes branch-specific formation of dendritic spines after learning. Science 344 (6188), 1173–1178. doi:10.1126/science.1249098
Keywords: tDCS, motor learning, homeostatic structural plasticity, spiking neural network, cell assembly
Citation: Lu H, Frase L, Normann C and Rotter S (2025) Resolving inconsistent effects of tDCS on learning using a homeostatic structural plasticity model. Front. Netw. Physiol. 5:1565802. doi: 10.3389/fnetp.2025.1565802
Received: 23 January 2025; Accepted: 12 June 2025;
Published: 07 July 2025.
Edited by:
Mojtaba Madadi Asl, Institute for Research in Fundamental Sciences (IPM), IranReviewed by:
Ankur Sinha, University College London, United KingdomMihaly Voroslakos, New York University, United States
Copyright © 2025 Lu, Frase, Normann and Rotter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Rotter, c3RlZmFuLnJvdHRlckBiaW8udW5pLWZyZWlidXJnLmRl
†Present address: Han Lu, Institute for Advanced Simulation (IAS), Jülich Supercomputing Center (JSC), Forschungszentrum Jülich, Jülich, Germany
 Han Lu
Han Lu Lukas Frase5
Lukas Frase5 Claus Normann
Claus Normann