- 1Division of Rheumatology, Johns Hopkins University, Baltimore, MD, United States
- 2Division of Rheumatology, Northwestern University, Chicago, IL, United States
- 3Department of Radiology, Johns Hopkins University, Baltimore, MD, United States
Background: Calcinosis is a morbid complication of dermatomyositis (DM) and systemic sclerosis (SSc) with no effective pharmacologic treatment or validated whole-body assessment modality. 18F-NaF PET/CT may help to quantify and characterize calcinosis.
Methods: In this pilot study, we enrolled three adults with DM and three with SSc, all with new calcinosis deposits. Each underwent 18F-NaF PET/CT and clinical examination with semi-quantitative scoring of calcinosis. We described the 18F-NaF PET/CT findings and compared these to CT imaging alone as well as to clinical examination.
Results: Calcinosis was noted on 18F-NaF PET/CT in the subcutaneous tissue in all patients and the muscle in three patients, including two with SSc. The average semi-quantitative score was 23.5 by 18F-NaF PET/CT and 20 by clinical exam. Wilcoxon signed rank test indicated greater scores by 18F-NaF PET/CT than by clinical exam (p = 0.0264). 18F-NaF uptake varied among calcinosis deposits and occurred without corresponding calcifications on CT.
Conclusions: 18F-NaF PET/CT appears to be a sensitive method of detecting and characterizing calcinosis that provides both quantitative and qualitative data beyond what can be obtained by physical examination or CT alone. 18F-NaF uptake occurs in muscle in both SSc and DM, suggesting the possibility that myositis may be driving calcinosis in a subset of patients with SSc.
Introduction
Calcinosis in dermatomyositis and systemic sclerosis (SSc; scleroderma) is ectopic soft tissue calcification that occurs in the absence of serum abnormalities of calcium and phosphorous. Calcinosis is common in dermatomyositis and SSc, affecting 30%–40% of patients, and causes substantial morbidity (1–4). There is no known effective pharmacologic intervention for calcinosis, and there is no established quantitative measure of whole-body disease burden.
18F-NaF PET/CT may be useful for identification, localization, and/or quantification of calcinosis burden or activity in dermatomyositis and SSc (5). 18F-NaF is a radiotracer used for detection of hydroxyapatite, which is the principal mineral component of bone as well as of dermatomyositis- and SSc- related calcinosis (6–8). For other disorders involving aberrant ossification or calcification, 18F-NaF PET/CT is highly sensitive. For example, in fibrodysplasia ossificans progressiva, which is characterized by heterotopic ossification at sites of trauma, 18F-NaF uptake on PET/CT identifies preclinical areas of ossification (9). Furthermore, in atherosclerosis, which is characterized by hydroxyapatite deposition within blood vessels, increased vascular 18F-NaF uptake is detectable in areas without radiographic coronary calcium and is an independent predictor of subsequent progressive coronary artery calcification (10, 11). In prostate cancer, 18F-NaF PET/CT is 97% sensitive and 94% specific for the detection of bony metastases (12). These studies demonstrate that 18F-NaF PET/CT accurately localizes and quantifies ectopic calcification in a variety of contexts and support the use of this imaging modality to assess calcinosis in dermatomyositis and SSc.
Development of effective therapies for calcinosis hinges upon being able to measure calcinosis disease burden. Image-based quantification of calcinosis with 18F-NaF PET/CT could provide an objective measure for use in clinical trials as well as a foundation for further mechanistic studies. To this end, we conducted a pilot study to demonstrate the feasibility of using this imaging modality and to compare characteristics of dermatomyositis- and SSc-related calcinosis on 18F-NaF PET/CT to expert clinical exam. We hypothesized that there may be areas of 18F-NaF uptake on PET without a CT correlate and that 18F-NaF PET/CT may be more sensitive than expert exam for the detection of calcinosis.
Methods
Patient selection
We recruited patients from the Johns Hopkins Myositis Center and the Johns Hopkins Scleroderma Center, which are both based out of a tertiary medical center in Baltimore, Maryland (USA). We included patients at least 18 years of age with a clinical diagnosis of dermatomyositis or SSc who had at least one new self-reported calcinosis deposit within the 6 months prior to enrollment. We excluded patients who had received more than 2.0 REM of radiation exposure over the previous year, were pregnant or were trying to become pregnant, had recent surgery, or had any metabolic disorder contributing to ectopic calcification. The Institutional Review Board of Johns Hopkins University approved this study, and we obtained written informed consent from all patients.
Clinical assessment of calcinosis
A single examiner (CR) assessed 26 body areas (bilateral fingers, hands, wrists, forearms, elbows, upper arms, shoulders, thighs, knees, lower legs, ankles, feet as well as head/neck, trunk, pelvic girdle within 1 week of each scan. A board-certified rheumatologist with expertise in the assessment of patients with dermatomyositis (LC-S) or SSc (AAS, LKH, or FMW) verified these examination findings during the same visit. We scored each body area as follows: 0 for no calcinosis, 1 for a <3 small deposits (light burden), or 2 for at least one large (≥3 cm) deposit and/or at least 3 deposits (heavy burden). The expert examiners were masked as to the results of the 18F-NaF PET/CET but were familiar with each patient's clinical history.
18NaF PET/CT imaging protocol
First, we administered 0.15 mCi/kg of 18F-NaF, followed by at least 10 ml of saline flush, intravenously to each patient. Second, each patient drank a 12-ounce bottle of water and voided 60 min after infusion of the radiotracer. Third, we positioned each patient on the PET/CT scanner bed for a vertex-to-toes PET/CT protocol in the arms down position. Fourth, we acquired a low dose CT from the vertex to the toes for attenuation correction. Following the CT, we performed a PET scan at 3 min per bed position. We estimated the total amount of radiation exposure at 2.14 REM per patient. All patients tolerated the scan well.
18F-NaF PET/CT reading
Two board-certified radiologists (MSJ, LBS) read the 18F-NaF PET/CT images and arrived at a consensus for each scan. Both readers were masked as to each patient's clinical history as well as to the physical examination scoring. We assessed the presence or absence of 18F-NaF uptake in each body area and recorded the maximum standardized uptake value (SUV) for each area with radiotracer uptake. We assessed calcinosis burden on 18F-NaF PET/CT for each of the 26 aforementioned body areas and scored each body area on 18F-NaF PET/CT as 0 for no calcinosis, 1 for <3 small deposits, and 2 for at least 1 large (≥3 cm) deposit or more than a few small deposits. Readers also commented on qualitative aspects of the scans as appropriate, including precise anatomical locations of deposits, qualitative description of the 18F-NaF uptake, and/or whether uptake was associated with CT evidence of calcification.
Statistical analysis
Comparisons between clinical examination and 18F-NaF PET/CET were made using two-tailed Student's t-test and Wilcoxon rank sum test as appropriate. A p value of <0.05 was considered significant. Statistical analyses were performed using Stata 14.1 (StataCorp, College Station, TX).
Results
Patient characteristics
Ages of the patients ranged from 32 to 65 years at the time of the study, and all of the patients were female. The demographic, clinical, and serologic characteristics of the patients are listed in Table 1. Of the three patients with dermatomyositis, one had clinically amyopathic dermatomyositis by modified Sontheimer criteria, and 2 patients with dermatomyositis met Bohan and Peter criteria for probable dermatomyositis (13, 14). All three patients with SSc met 2013 American College of Rheumatology Classification Criteria for SSc (15). None of the patients with SSc had a clinical diagnosis of dermatomyositis overlap. All three patients with SSc had diffuse cutaneous involvement, and all patients with dermatomyositis had characteristic rashes. Patient 1 had received anakinra for calcinosis, most recently 1 year prior to the scan. Patients 3 and 5 had received pamidronate for calcinosis more than 2 years prior to the scan, and patient 4 was receiving treatment with pamidronate for calcinosis at the time of the scan. Patient 2 had received denosumab for osteoporosis within the 6 months prior to the scan. All patients had been treated with immunosuppressive or immunomodulatory therapy in the past, as listed in the table.
Quantitative comparisons of 18F-NaF PET/CT and clinical examination results
18F-NaF-PET/CT detected calcinosis in more body areas than clinical exam. Expert exam detected calcinosis clinically in 46.2% (72/156) of all of the body areas examined, compared to (89/156) body areas, or 57.1%, for 18F-NaF PET/CT (p = 0.0541). Calcinosis was detected more often by 18F-NaF PET/CT than clinical exam in the upper arms, feet, shoulder, leg, elbow, and knee. The average semi-quantitative score was 23.5 by 18F-NaF PET/CT and 20 by clinical exam. Wilcoxon signed rank test resulted in a p-value of 0.0264, indicating greater scores by 18F-NaF PET/CT than clinical exam.
Qualitative 18NaF PET/CT scan results
18F-NaF PET/CT demonstrated calcinosis in the subcutaneous layer in all 6 patients. However, 1 of 3 DM patient had calcinosis in the muscles as well as subcutaneous tissue, and 2 of 3 SSc patients had calcifications in the muscles as well as the subcutaneous tissue. 18F-NaF uptake was variable in areas of calcinosis that appeared uniform on CT. For example, there was intra-lesion variation in 18F-NaF uptake in sheets of calcification that appeared uniform on CT in and around the shoulder of Patient 1 (Figure 1). This patient also had calcifications of the rotator cuff as well as variable uptake in the anterior trunk. Patient 5 also had variable uptake in the hip girdle region, where there was intense 18F-NaF uptake overall. Patients 2 and 5 had calcification on CT without any 18F-NaF uptake on PET. Conversely, Patient 5 also had 18F-NaF uptake in the left leg without a CT correlate (Figure 2), and Patient 6 had multiple areas of non-calcified radiotracer uptake along with increased 18F-NaF uptake in the axial skeleton, indicative of increased skeletal bone turnover (Figure 3). As illustrated in Figure 3, 18F-NaF PET/CT also detected calcinosis in the elbows and foot/ankle regions, which was not detected on clinical exam for this patient.
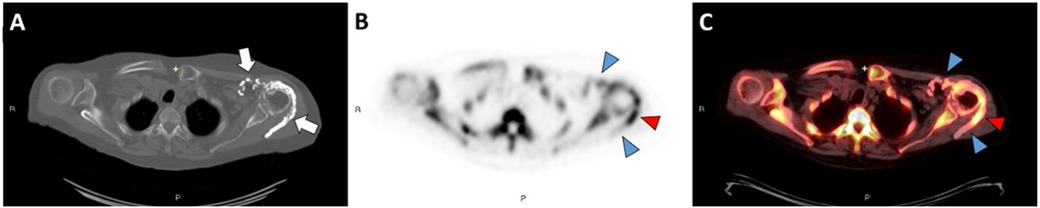
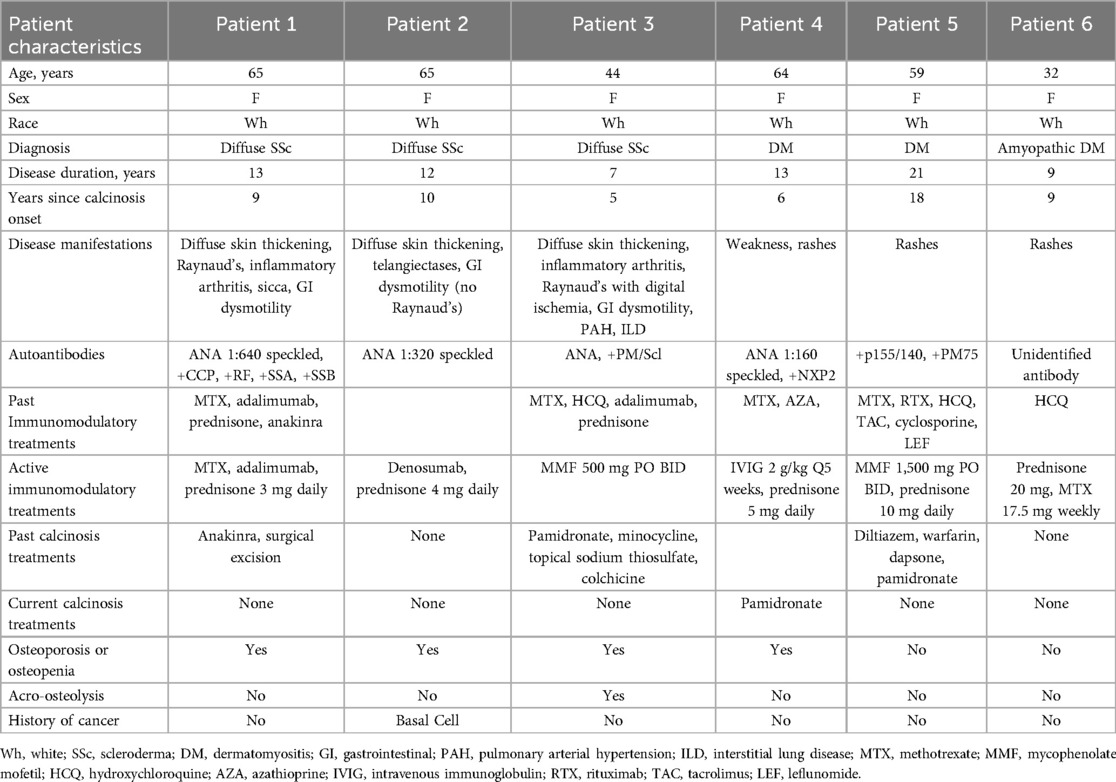
Figure 1. Axial CT image in bone windows (A) demonstrates periarticular calcinosis of the left shoulder involving the rotator cuff interposed between the deltoid and infraspinatus muscles (white arrows). Axial 18F-NaF PET/CT images (B,C) demonstrate intense activity at the mid/distal component of the calcification (red arrowheads) and mild activity along the proximal component and anterior calcifications (blue arrowheads). There is also increased 18F-NaF uptake at the left shoulder compared to the right, suggesting arthritic remodeling.
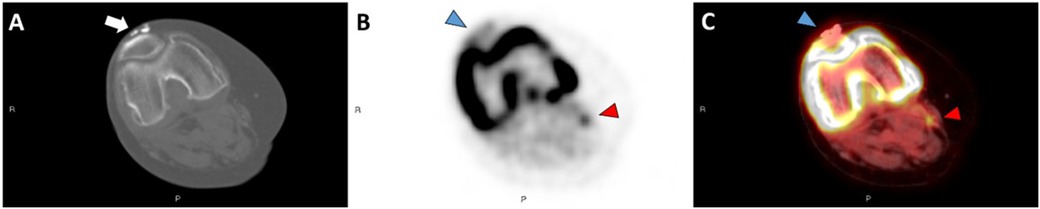
Figure 2. Axial CT image in bone windows (A) demonstrates calcinosis involving the quadriceps and patellar tendon complex overlying the left patella in patient 5. Axial 18F-NaF PET/CT images (B,C) demonstrate mild activity along this calcified complex (blue arrowheads). However, there is focal PET signal activity located along the semimembranosus muscle without associated calcification on CT (red arrowheads).
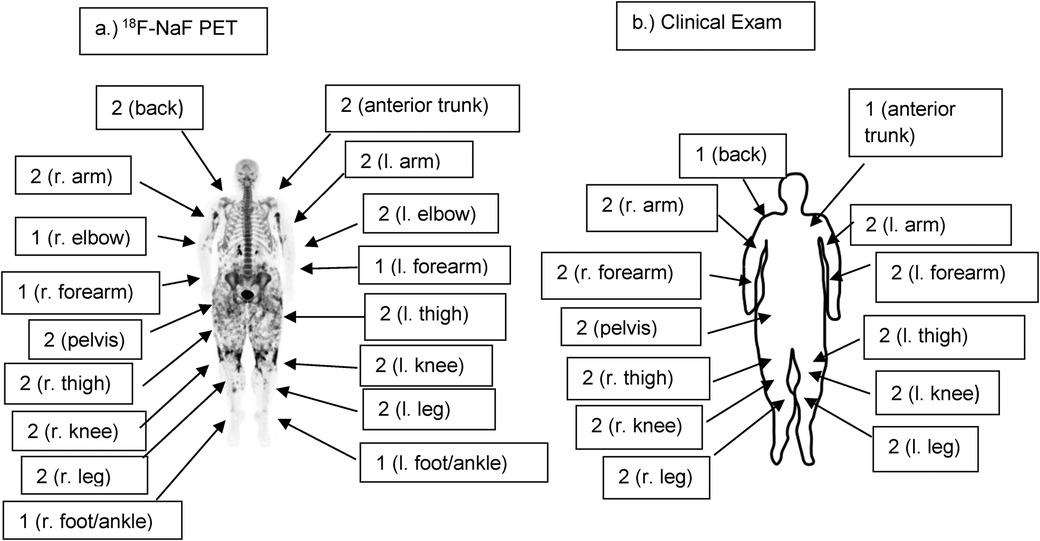
Figure 3. (a) Full-body coronal 18F-NaF PET imageof patient 6 demonstrating the distribution and intensity of 18F-NaF uptake. Note the intense activity in the upper arms and around the knees as well as widespread uptake in the thighs and pelvic girdle. Semi-quantitative radiographic scores are noted, with scores of 0 where not noted (head/neck, shoulders, fingers, hands). (b) Semi-quantitative clinical exam scores of Patient 6 are similar to radiographic scores. However, calcinosis was not noted clinically in the feet/ankles or elbows on clinical exam, whereas it was present in these areas radiographically. Clinical scores are 0 where not noted (head/neck, shoulders, fingers, hands, elbows, feet/ankles).
Discussion
In this pilot study, we found that 18F-NaF PET/CT offered additional quantitative and qualitative data beyond what could be obtained from expert exam or CT alone in dermatomyositis- and SSc-related calcinosis. Consistent with the findings of Atzeni et al. (5), 18F-NaF PET/CT identified more calcinosis than appreciated on expert examination, and there was 18F-NaF uptake in areas where calcification was not apparent on CT. Based on previous studies demonstrating that 18F-NaF uptake predicts the development of ossification in fibrodysplasia ossificans progressiva and the progression of calcification in atherosclerosis, we hypothesize that these areas of 18F-NaF uptake may ultimately progress to radiographic calcification. We also found calcification in the muscles in 3 of 6 patients, including 2 with SSc, suggesting that calcifications may originate in the muscular layer due to active myositis. We also found that markedly increased skeletal bone turnover accompanied calcinosis in one patient. In light of the association between calcinosis and osteoporosis (16), this suggests that accelerated bone loss may accompany calcinosis in some patients.
Our study has limitations. Most notably, this was a pilot study with a small sample size of 6 patients, all of whom were white females between the ages of 30 and 65. Because of the small sample size, the power of our study was limited. Notably, our study was not able to detect a statistically significant difference between the number of calcified body areas detected by 18F-NaF PET/CT and by expert exam. Future studies would ideally include larger groups of patients to improve power as well as a more diverse patient population to improve generalizability. Furthermore, while there is a validated scoring system for hand calcinosis in systemic sclerosis (17), there is no validated scoring system for full-body calcinosis in either systemic sclerosis or dermatomyositis. Therefore, our study used a semi-quantitative scoring system to assess calcinosis burden as none (0), light (1), or heavy (2) in 26 body areas. Our study demonstrated that this scoring system is feasible in a small cohort, but this scoring system needs to be validated in a future study.
While 18F-NaF PET/CT involves greater time, expense, and radiation exposure than other imaging modalities for assessment of calcinosis, its unique characteristics make it a superior imaging modality. In contrast to computed tomography alone, the use of 18F-NaF PET/CT provides a functional assessment of hydroxyapatite deposition, as animal studies have demonstrated that 18F-NaF uptake is closely related to bone turnover (18). Compared to 99mTc based bone imaging, 18F-NaF PET/CT has more robust attenuation correction and scatter correction and higher spatial and contrast resolution (19). Furthermore, 18F-NaF PET/CT is amenable to quantitative analysis including kinetic modeling and can provide a quantitative assessment of bone perfusion and bone turnover (20). These characteristics make 18F-NaF PET/CT a particularly promising modality for use in clinical trials of calcinosis, as it provides granular, quantitative assessments of whole-body calcinosis and may be able to identify actively forming calcinosis deposits amenable to pharmacologic therapy. Our study demonstrates that 18F-NaF PET/CT is well-tolerated by patients and feasible as an assessment modality for calcinosis in future studies. Subsequent longitudinal studies should examine the sensitivity of this imaging modality to changes in calcinosis burden over time as well as whether 18F-NaF uptake predicts progression of calcinosis in DM and SSc. Further studies should also assess and validate clinically meaningful thresholds of 18F-NaF uptake and whole-body quantitative calcinosis burden on 18F-NaF PET/CT, incorporating patient-reported outcome measures.
Calcinosis is a painful, disfiguring and debilitating complication of SSc and DM, and efforts to find an effective therapy have been stymied by a lack of quantitative outcome measures. Our study suggests that 18F-NaF PET/CT may have advantages over expert clinical exam or CT alone for detection and quantification of calcinosis. We suggest that 18F-NaF PET/CT is a useful imaging modality for identification and quantification of calcinosis activity.
Data availability statement
The datasets presented in this article are not readily available because this dataset involves a small number of participants. The raw dataset will not be made available due to possible breach of privacy associated with transfer of data. Requests to access the datasets should be directed to Lisa Christopher-Stine,bGNocmlzdDRAamhtaS5lZHU=.
Ethics statement
The studies involving humans were approved by Johns Hopkins Medicine Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CR: Investigation, Conceptualization, Methodology, Formal analysis, Writing – review & editing, Data curation, Writing – original draft. MJ: Methodology, Resources, Writing – review & editing, Investigation, Writing – original draft, Conceptualization. AS: Methodology, Writing – review & editing, Writing – original draft. CC: Writing – review & editing, Writing – original draft, Data curation. LS: Writing – original draft, Data curation, Writing – review & editing, Investigation, Conceptualization. FW: Conceptualization, Writing – review & editing, Methodology, Writing – original draft. LH: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. LC-S: Methodology, Writing – original draft, Funding acquisition, Investigation, Data curation, Conceptualization, Writing – review & editing, Resources, Project administration, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Jerome L. Greene Foundation Award, the Scleroderma Research Foundation, the Martha McCrory Professorship, the Chresanthe Stauralakis Memorial Discovery Fund, the Lerman Family Fund, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (T32AR048522, K24 AR080217). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Valenzuela A, Chung L. Calcinosis: pathophysiology and management. Curr Opin Rheumatol. (2015) 27:542–8. doi: 10.1097/BOR.0000000000000220
2. Fredi M, Bartoli F, Cavazzana I, Ceribelli A, Carabellese N, Tincani A, et al. Calcinosis in poly-dermatomyositis: clinical and laboratory predictors and treatment options. Clin Exp Rheumatol. (2017) 35:303–8.27908312
3. Simeon-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C, Espinosa-Garriga G, Ramos-Casals M, Campillo-Grau M, et al. Registry of the Spanish network for systemic sclerosis: clinical pattern according to cutaneous subsets and immunological status. Semin Arthritis Rheum. (2012) 41:789–800. doi: 10.1016/j.semarthrit.2011.10.004
4. Cruz-Dominguez MP, García-Collinot G, Saavedra MA, Medina G, Carranza-Muleiro RA, Vera-Lastra OL, et al. Clinical, biochemical, and radiological characterization of the calcinosis in a cohort of Mexican patients with systemic sclerosis. Clin Rheumatol. (2017) 36:111–7. doi: 10.1007/s10067-016-3412-9
5. Atzeni IM, Hogervorst EM, Stel AJ, de Leeuw K, Bijl M, Bos R, et al. [(18)F]Sodium fluoride PET has the potential to identify active formation of calcinosis cutis in limited cutaneous systemic sclerosis. Semin Arthritis Rheum. (2022) 55:152027. doi: 10.1016/j.semarthrit.2022.152027
6. Kawakami T, Nakamura C, Hasegawa H, Eda S, Akahane S, Yamazaki T, et al. Ultrastructural study of calcinosis universalis with dermatomyositis. J Cutan Pathol. (1986) 13:135–43. doi: 10.1111/j.1600-0560.1986.tb01514.x
7. Hsu VM, Emge T, Schlesinger N. X-ray diffraction analysis of spontaneously draining calcinosis in scleroderma patients. Scand J Rheumatol. (2017) 46:118–21. doi: 10.1080/03009742.2016.1219766
8. Hawkins RA, Choi Y, Huang SC, Hoh CK, Dahlbom M, Schiepers C, et al. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med. (1992) 33:633–42.1569473
9. Eekhoff EMW, Botman E, Coen Netelenbos J, de Graaf P, Bravenboer N, Micha D, et al. [18F]NaF PET/CT scan as an early marker of heterotopic ossification in fibrodysplasia ossificans progressiva. Bone. (2018) 109:143–6. doi: 10.1016/j.bone.2017.08.012
10. Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. (2012) 59:1539–48. doi: 10.1016/j.jacc.2011.12.037
11. Bellinge JW, Francis RJ, Lee SC, Phillips M, Rajwani A, Lewis JR, et al. (18)F-Sodium fluoride positron emission tomography activity predicts the development of new coronary artery calcifications. Arterioscler Thromb Vasc Biol. (2021) 41:534–41. doi: 10.1161/ATVBAHA.120.315364
12. Sheikhbahaei S, Jones KM, Werner RA, Salas-Fragomeni RA, Marcus CV, Higuchi T, et al. (18)F-NaF-PET/CT for the detection of bone metastasis in prostate cancer: a meta-analysis of diagnostic accuracy studies. Ann Nucl Med. (2019) 33:351–61. doi: 10.1007/s12149-019-01343-y
13. Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol. (2002) 46:626–36. doi: 10.1067/mjd.2002.120621
14. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. (1975) 292:344–7. doi: 10.1056/NEJM197502132920706
15. van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 Classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. (2013) 72:1747–55. doi: 10.1136/annrheumdis-2013-204424
16. Valenzuela A, Baron M, Canadian Scleroderma Research Group, Herrick AL, Proudman S, Stevens W, et al. Calcinosis is associated with digital ulcers and osteoporosis in patients with systemic sclerosis: a Scleroderma clinical trials consortium study. Semin Arthritis Rheum. (2016) 46:344–9. doi: 10.1016/j.semarthrit.2016.05.008
17. Chung L, Valenzuela A, Fiorentino D, Stevens K, Li S, Harris J, et al. Validation of a novel radiographic scoring system for calcinosis affecting the hands of patients with systemic sclerosis. Arthritis Care Res. (2015) 67:425–30. doi: 10.1002/acr.22434
18. Piert M, Zittel TT, Becker GA, Jahn M, Stahlschmidt A, Maier G, et al. Assessment of porcine bone metabolism by dynamic. J Nucl Med. (2001) 42:1091–100.11438633
19. Vaquero JJ, Kinahan P. Positron emission tomography: current challenges and opportunities for technological advances in clinical and preclinical imaging systems. Annu Rev Biomed Eng. (2015) 17:385–414. doi: 10.1146/annurev-bioeng-071114-040723
Keywords: calcinosis, scleroderma (or systemic sclerosis), dermatomyositis, 18F-NaF PET/CT, imaging
Citation: Richardson C, Javadi MS, Shah AA, Connolly C, Solnes LB, Wigley FM, Hummers LK and Christopher-Stine L (2025) 18F-NaF PET/CT identifies muscular and subcutaneous calcifications in both dermatomyositis- and systemic sclerosis-related calcinosis. Front. Nucl. Med. 5:1593825. doi: 10.3389/fnume.2025.1593825
Received: 21 March 2025; Accepted: 8 May 2025;
Published: 30 May 2025.
Edited by:
Mario Petretta, University of Naples Federico II, ItalyReviewed by:
Aslihan Avanoglu Guler, Ufuk University Faculty of Medicine, TürkiyeOmer Aras, Memorial Sloan Kettering Cancer Center, United States
Copyright: © 2025 Richardson, Javadi, Shah, Connolly, Solnes, Wigley, Hummers and Christopher-Stine. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa Christopher-Stine, bGNocmlzdDRAamhtaS5lZHU=
 Carrie Richardson
Carrie Richardson Mehrbod S. Javadi3
Mehrbod S. Javadi3 Lilja B. Solnes
Lilja B. Solnes Lisa Christopher-Stine
Lisa Christopher-Stine