- 1Medical University Varna, Department of Medical Genetics, Varna, Bulgaria
- 2Laboratory of Medical Genetics, University Hospital “Sveta Marina”, Varna, Bulgaria
- 3Medical University Varna, Department of Oncology, Varna, Bulgaria
This study presents a 5-year retrospective analysis of genetic counseling (GC) services for hereditary cancer syndromes (HCS) at a single center in Bulgaria. The aim is to describe the demographic and epidemiological characteristics of patients seeking GC, the uptake of genetic testing, and the spectrum of identified pathogenic variants. The results highlight an increasing trend in GC utilization. Key findings include differences in patient profiles between those seeking general HCS assessment and those undergoing tumor biomarker testing, the impact of financial accessibility on genetic testing uptake, and a pathogenic variant detection rate of 28% in tested individuals. The most frequently identified conditions were Hereditary Breast and Ovarian Cancer Syndrome and Lynch Syndrome, with pathogenic variants detected in genes such as BRCA1, MSH2, PALB2, and STK11. These findings underscore the need for enhanced awareness, improved financial access to testing, and the establishment of systematic cascade screening programs in Bulgaria.
1 Introduction
Hereditary cancer syndromes (HCS) are characterized by an increased risk of developing certain cancers due to inherited genetic mutations. Genetic counseling (GC) plays a crucial role in identifying individuals at risk, providing risk assessment, discussing genetic testing options, and facilitating informed decision-making regarding prevention and management strategies. The process involves multiple stages, including counseling that occurs prior to a decision on testing, known as pre-analytical GC, which is essential for obtaining informed consent. Understanding the characteristics of patients seeking GC and the outcomes of genetic testing in specific populations is essential for optimizing service delivery and improving patient care. This study aims to describe the 5-year experience of a single center in Bulgaria in providing GC for HCS, focusing on the demographic and epidemiological features of the patients undergoing GC and subsequent genetic testing.
2 Materials and methods
This was a retrospective observational study conducted at a single medical genetics center in Bulgaria. Data were collected from the records of patients who underwent genetic counseling between 2020 and 2024. The study included two main groups of patients: those who received GC for the evaluation of potential underlying hereditary tumor predisposition syndromes (HTPS) (n = 154) and those who underwent molecular genetic testing of tumor tissue with subsequent counseling for potential germline implications (n = 157). Data collected included patient demographics (age, gender, geographical location), referral source, risk factors (personal and family history of cancer), type of cancer, the scope of genetic tests performed (e.g., targeted panels, exome sequencing), and the identified genetic variants. Data analysis was descriptive, summarizing the characteristics of the study population and the outcomes of GC and genetic testing using frequencies and percentages.
3 Results
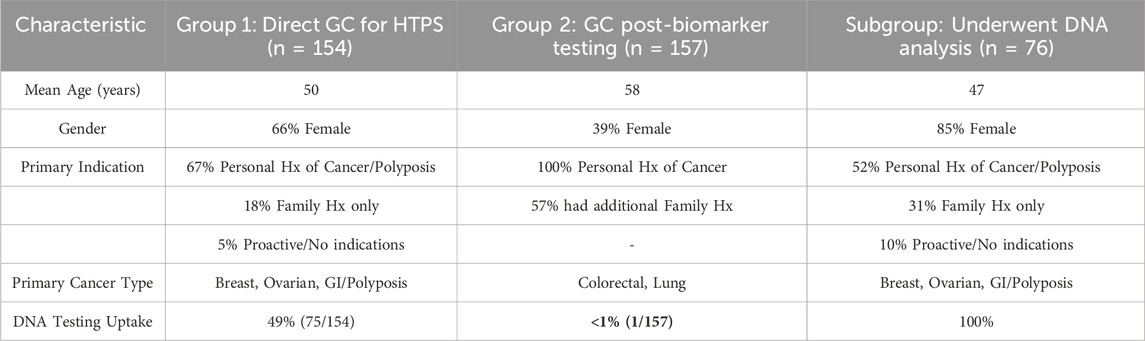
To provide a clearer overview of the patient populations, their demographic and clinical characteristics are summarized in Table 1.
3.1 Characteristics of patients undergoing GC for potential HTPS
3.1.1 Total number of patients
The number of patients undergoing GC for potential HTPS showed an overall increasing trend from 2020 to 2024, despite a doubling in the cost of services after October 2022 and a slight decrease observed in 2024. Approximately 80% of these patients were residents of Varna, with the rest residing within a 130-kilometer radius of the city. The proportion of GC sessions focused on oncogenetics ranged from 4.3% to 7.5% of all GC sessions per year.
3.1.2 Age and gender characteristics
The age distribution of these patients was 56% (n = 86) aged 50 years or younger and 44% (n = 68) aged over 50 years, with a mean age of 50 years. The patients were predominantly females (66%, n = 102) compared to males (34%, n = 52).
3.1.3 Characteristics by risk factor and referral source
The dominant risk factor was a personal history of cancer or multiple polyposis (67%, n = 103). An additional 12 (8%) had a history of more than one malignancy. Healthy individuals seeking consultation due to a positive family history constituted 18% (n = 28) of the group, while only 5% (n = 8), including three individuals from the same family, proactively sought testing without specific indications due to personal interest in preventative health. The majority of patients (80%, n = 123) were directly referred by a diagnostic hospital unit, an outpatient physician, or an oncology committee. Self-referrals accounted for 20% (n = 31). There was an observed increase in patients seeking outpatient genetic consultation via out-of-pocket payment (n = 100), while the number of patients referred during hospitalization remained relatively stable (n = 54).
3.2 Characteristics of patients undergoing GC following tumor biomarker testing
3.2.1 Total number of patients
The total number of patients undergoing targeted biomarker testing in tumor tissue increased significantly annually. Among these, 26% (n = 157) were additionally consulted for a potential underlying HTPS, with a threefold increase within the last 2 years (from 39 in 2023 to 118 in 2024).
3.2.2 Age and gender characteristics
The age distribution in this group showed 80% aged over 50 years, with a mean age of 58 years. Males predominated (61%) compared to females (39%), which is consistent with the dominant pathologies of colorectal and non-small cell lung carcinoma.
3.2.3 Characteristics by risk factor
Among these patients, the majority (57%, n = 90) had a positive family history in addition to their personal cancer diagnosis. Younger age at diagnosis (<50 years) was reported in 35% (n = 54), and 8% (n = 13) reported more than one malignancy.
3.3 Characteristics of patients undergoing DNA analysis
3.3.1 Total number of patients
Of all 311 patients who underwent GC, 24% (n = 76) proceeded with DNA analysis, with only one patient from the second analyzed group. Eighty-three percent of those who underwent DNA analysis were residents of Varna.
3.3.2 Age and gender characteristics
Statistically significantly more females (85%, n = 65) than males underwent DNA analysis, with a mean age of 47 years compared to 53 years for those who did not. A statistically significantly higher number of self-funded consultations (referred to as paid consultations, meaning they were paid out-of-pocket by the patient) (93%, n = 71) were followed by genetic analysis compared to inpatient consultations covered by the hospital (7%, n = 5 out of 54 patients).
3.3.3 Characteristics of performed analyses and detected pathology
Regarding the scope of testing, only 18% (n = 14) of patients chose a narrowly targeted gene panel based on their clinical presentation, while 78% (n = 59) preferred the broadest possible multi-gene panel offered within a similar price range. Three patients (4%) underwent whole-exome sequencing (WES).
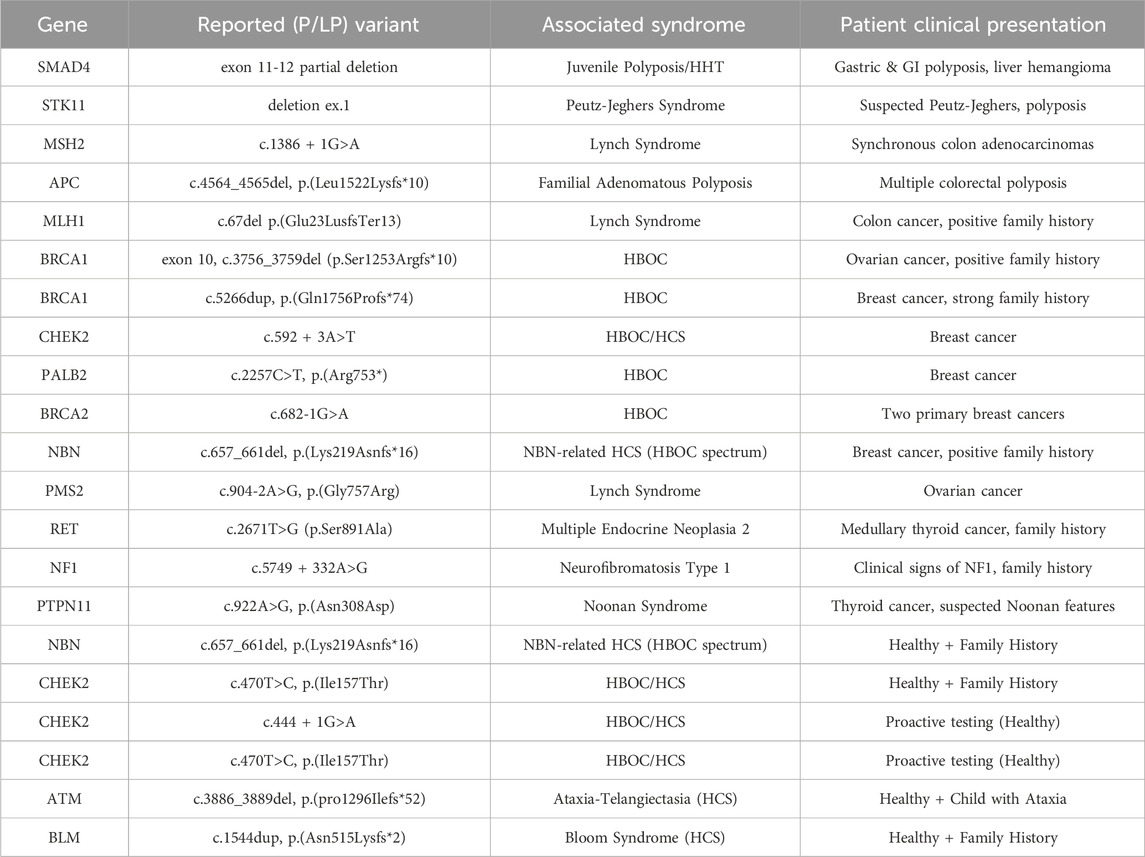
A pathogenic/likely pathogenic (P/LP) variant associated with the clinical picture was identified in 28% (n = 21) of the 76 tested patients. The diagnostic yield varied by clinical indication: it was 32% for patients with breast and/or ovarian cancer, 28% for patients with gastrointestinal cancer and/or polyposis, 25% for patients with other cancers, and 25% for clinically healthy individuals.
The spectrum of identified pathogenic variants and their associated syndromes is detailed in Table 2. The most common findings were related to Hereditary Breast and Ovarian Cancer (HBOC) and Lynch Syndrome. A variant of uncertain significance (VUS) was reported in 25% (n = 19) of patients.
Based on the 21 patients with a diagnosed P/LP variant, 36 first-degree relatives were identified as being at risk. Of these, only 19% (n = 7) underwent GC and subsequent targeted testing as a form of cascade screening. This resulted in the identification of 4 additional healthy carriers of pathogenic variants in the BRCA1, CHEK2, MSH2, and NBN genes, enabling access to preventative care.
4 Discussion
This single-center study in Bulgaria provides valuable insights into the uptake and characteristics of individuals seeking GC for HCS. While the increasing number of patients suggests growing awareness, individuals undergoing oncogenetic counseling represent a small proportion of all counseled patients, with growth primarily in self-funded services. This highlights a critical disparity in access, likely driven by financial barriers.
Our finding that younger women predominate among those seeking GC aligns with previous studies [1]. Conversely, the predominance of older men in the tumor-to-germline testing group highlights an important opportunity to reach an otherwise under-accessed population.
A key finding is the significantly higher rate of genetic testing among individuals who self-funded GC (93%) compared to those receiving it through the hospital system (7%). This stark contrast strongly suggests that cost is a major barrier. This discrepancy underscores the need for policies promoting equitable access.
The 28% pathogenic variant detection rate is comparable to other studies [2]. The identification of variants in clinically actionable genes such as BRCA1, BRCA2, MSH2, MLH1, APC, and STK11 reinforces the clinical utility of genetic testing in identifying high-risk individuals and guiding personalized management [3–5]. However, the geographical concentration of tested individuals in Varna (83%) underscores the impact of accessibility.
The preference for broader gene panels indicates a patient desire for comprehensive genetic information [6, 7]. The VUS rate (25%), similar to the P/LP detection rate, emphasizes the ongoing need for variant re-analysis, a crucial component of responsible genomic medicine.
The low percentage (5%) of proactive counseling (defined as counseling for healthy individuals without specific high-risk family history, sought for preventative purposes) underscores the need for increased public awareness campaigns [8, 9]. A significant limitation of this study is its retrospective nature, which precluded the collection of data on the clinical impact of genetic testing results, such as the uptake of enhanced surveillance or prophylactic surgeries. Furthermore, the small sample size for certain subgroups limits the generalizability of some findings. These represent important areas for future prospective research.
5 Conclusion
This 5-year single-center experience provides valuable insights into the landscape of GC for HCS in Bulgaria. The increasing demand is encouraging, but challenges related to awareness, financial accessibility, and the interpretation of results remain. The diagnostic yield of 28% and the spectrum of identified mutations, particularly in HBOC and Lynch syndrome-associated genes, confirm the urgent need for a national strategy. Future efforts should focus on enhancing public and professional education, improving the affordability of genetic testing, and most critically, implementing a systematic cascade screening program to maximize the preventative benefits of cancer genetic services in the Bulgarian population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Approval for this research, including the use of anonymized patient data, was granted by the Medical University Varna Ethics Committee (Ethics Approval Number 9/30.01.2025), and patient consent has been secured. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MH: Writing – original draft. ML: Writing – review and editing. DY: Writing – review and editing. MS: Writing – original draft. ED: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study is financed by the European Union-NextGenerationEU through the National Recovery and Resilience Plan of the Republic of Bulgaria, project no BG-RRP-2.004-0009-C02.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Gemini was used to revise and refine the language and clarity of the manuscript, ensuring it effectively conveyed the research’s significance and clinical relevance.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ek, S. Gender differences in health information behaviour: a Finnish population-based survey. Health Promotion Int (2013) 30(3):736–45. doi:10.1093/heapro/dat063
2. Kurian, AW, Abrahamse, P, Furgal, A, Ward, KC, Hamilton, AS, Hodan, R, et al. Germline genetic testing after cancer diagnosis. Jama (2023) 330(1):43–51. doi:10.1001/jama.2023.9526
3. Kuzbari, Z, Bandlamudi, C, Loveday, C, Garrett, A, Mehine, M, George, A, et al. Germline-focused analysis of tumour-detected variants in 49,264 cancer patients: ESMO Precision Medicine Working Group recommendations. Ann Oncol (2023) 34(3):215–27. doi:10.1016/j.annonc.2022.12.003
4. LaDuca, H, Polley, EC, Yussuf, A, Hoang, L, Gutierrez, S, Hart, SN, et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med (2020) 22(2):407–15. doi:10.1038/s41436-019-0633-8
5. Miller, DT, Lee, K, Abul-Husn, NS, Amendola, LM, Brothers, K, Chung, WK, et al. ACMG SF v3.2 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med (2023) 25(8):100866. doi:10.1016/j.gim.2023.100866
6. Tung, N, Ricker, C, Messersmith, H, Balmaña, J, Domchek, S, Stoffel, EM, et al. Selection of germline genetic testing panels in patients with cancer: ASCO guideline. J Clin Oncol (2024) 42:2599–615. doi:10.1200/jco.24.00662
7. National Academies of Sciences, E., and Medicine; Health and Medicine Division; Board on Health Care Services; National Cancer Policy Forum; Board on Health Sciences Policy; Roundtable on Genomics and Precision Health. In: S Beachy, M Hackmann, and K Asalone, editors. Realizing the potential of genomics across the continuum of precision health care: proceedings of a workshop. Washington (DC): National Academies Press US (2023).
8. Finney, J, Fargas, V, Gonzalez, T, Taylor, N, Wakefield, CE, Tucker, K, et al. Cancer genetic counseling via telegenetics and telephone: a qualitative study exploring the experience of patients and genetic counselors in an Australian cancer genetics context. J Genet Couns (2024) 34. doi:10.1002/jgc4.1982
Keywords: hereditary cancer, tumor predisposition syndromes, genetic counseling, genetic testing, HBOC, Lynch Syndrome
Citation: Hachmeriyan M, Levkova M, Yahya D, Stoyanova M and Dimitrova E (2025) Genetic counseling for hereditary cancer syndromes: a 5-year experience from a single center in Bulgaria. Oncol. Rev. 19:1605606. doi: 10.3389/or.2025.1605606
Received: 03 April 2025; Accepted: 03 July 2025;
Published: 25 July 2025.
Edited by:
Hikmat Abdel-Razeq, King Hussein Cancer Center, JordanReviewed by:
Akemi J. Tanaka, University of California, San Diego, United StatesMoon Ley Tung, The University of Iowa, United States
Copyright © 2025 Hachmeriyan, Levkova, Yahya, Stoyanova and Dimitrova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mari Hachmeriyan, Z2VuZXRpa2F2YXJuYUBnbWFpbC5jb20=
 Mari Hachmeriyan
Mari Hachmeriyan Mariya Levkova
Mariya Levkova Dinnar Yahya
Dinnar Yahya Milena Stoyanova1,2
Milena Stoyanova1,2