- 1Applied Sedimentary Geology, Technical University of Darmstadt, Darmstadt, Germany
- 2Bio-Inspired Energy Conversion, Technical University of Darmstadt, Darmstadt, Germany
Photosynthetic activity of cyanobacteria is a prominent driver of cell-surface catalysed extracellular calcium carbonate (CaCO3) precipitation. This natural process termed “biomineralization” occurs only under specific circumstances but has given rise to significant carbonate rock formation throughout geological time. Engineering cyanobacterial cell surfaces for enhanced and constitutive biomineralization of abundant ocean-water dissolved Ca2+ and flue-gas CO2 into CaCO3 may allow for the biotechnological re-capture of CO2 released by industrial processes such as thermal decarboxylation of CaCO3. This may both limit net greenhouse gas emissions and transform CaCO3 into a sustainable resource. Drawing from geological precedent and basic biological research, this perspective outlines promising synthetic biology strategies to convert cyanobacterial biomineralization into a cornerstone technology for a sustainable carbonate economy.
1 Introduction
1.1 Oxygenic photosynthesis and carbonate rocks: from deep time to climate solutions
Oxygenic photosynthesis may have evolved as early as 3.8–3.5 Gya (Rosing and Frei, 2004; Tice and Lowe, 2004; Oliver et al., 2021) and has shaped the Earth more than any other physiological process. Light-driven water splitting has not only resulted in the enrichment of the atmosphere with molecular oxygen (Luo et al., 2016) but also affected the geological record through, e.g., oxidation of ocean-water-dissolved iron, resulting in large-scale deposition of banded iron formations (Thompson et al., 2019). Beyond that, aquatic oxygenic photosynthesis is associated with the precipitation of carbonate minerals such as dolomite (MgCa(CO3)2) and calcite or aragonite (both CaCO3) in a process called “biomineralization” (Merz, 1992; Riding, 1992). Biomineralization has given rise to most extant carbonate rocks (Vasconcelos et al., 1995), which consist of >50% carbonate minerals and make up for 20%–25% of all sedimentary rocks and as much as 10% of all rocks exposed at the Earth’s surface (Parker, 1967). Such dolostones (dolomite) and limestones (aragonite and calcite) are estimated to store over 80% of the Earth’s carbon (Falkowski et al., 2000), but limestone is being extensively sourced as raw material for industry and agriculture. Upon mining, limestone is commonly converted into quicklime (CaO) through thermal decarboxylation (Niu et al., 2022; Comes et al., 2024), with CaO extraction for cement production alone causing around 7% of global CO2 emissions (Durastanti and Moretti, 2024). As less than half of this CO2 is subsequently re-sequestered through cement carbonation (Xi et al., 2016), CaO production contributes significantly to atmospheric CO2 enrichment and anthropogenic climate change (Callendar, 1938; Jones et al., 2023). Mitigating the latter through reduction of net CO2 emissions and opening up CaCO3 as a sustainable resource could be achieved by coupling CaCO3 thermolysis with microbial biomineralization that re-precipitates released CO2 and abundant ocean-water-dissolved Ca2+ into CaCO3, thus paving the way towards a more sustainable carbonate economy. While cyanobacterial biomineralization has been discussed as a potential means of cost-efficient CO2 capture and sequestration (CCS) for more than a decade (Jansson and Northen, 2010; Kamennaya et al., 2012) and some inherently productive calcifying species could be identified (Lee et al., 2004; Liang et al., 2013) little practical progress has been made in this field. In this perspective, we suggest a new approach to reason-guided enhancement of light-driven, cell-surface catalysed CaCO3 precipitation in planktonic cyanobacteria, allowing to harness this mechanism for future biotechnological applications.
1.2 Cyanobacterial cell-surface CaCO3 precipitation: passive yet engineerable
Cyanobacteria are photolithoautotrophic prokaryotes and the only recent bacteria known to perform oxygenic photosynthesis. Cyanobacterial photosynthetic activity is assumed to have given rise to significant limestone sediments (Kaźmierczak et al., 1996; Altermann et al., 2006; Banerjee et al., 2006) such as stromatolites (i.e., lithified laminated organosedimentary deposits) and micritic mudstones (Kaźmierczak et al., 1996; Suosaari et al., 2016). While in some cyanobacteria intracellular formation of CaCO3 granules has been documented (Benzerara et al., 2014; Moreira et al., 2017), extracellular CaCO3 precipitation is more commonplace and an arguably much more promising engineering target for light-driven biomineralization. This may technically allow to uncouple cell-surface catalysed carbonate precipitation from biomass production on which most approaches discussed for cyanobacterial CCS rely (Chen et al., 2012; Victoria et al., 2024).
Cyanobacterial CaCO3 precipitation is widely considered a passive byproduct of light-driven metabolic activity (Obst et al., 2009), with CaCO3 crystal formation being largely determined by alkaline conditions in the aqueous media, availability of Ca2+ cations, and presence of heterogenous crystallisation nuclei (Jroundi et al., 2022). Cyanobacteria in particular provide all these conditions in the microenvironment around their cells due to (i) media alkalization in the wake of photosynthetic carbon assimilation of CO2 from HCO3− releasing hydroxide ions (OH−) and thus increasing the extracellular pH to up to 10.5 (de Brito et al., 2022), and (ii) production of cell-surface components such as acidic exopolysaccharides (EPS) (Kamennaya et al., 2018; de Brito et al., 2022; Martinho De Brito et al., 2023) and negatively-charged surface-layer (S-layer) proteins (Schultze-Lam et al., 1992) attracting Ca2+ and nucleating CaCO3 crystallisation. Further contributing to (bi-) carbonate ion availability, some cyanobacteria produce active extracellular carbonic anhydrase (eCA) enzymes which catalyse the hydration of water-dissolved CO2 into HCO3−/H+, presumably as a means of re-capturing CO2 leaving the cell by diffusion (Soltes-Rak et al., 1997; Trimborn et al., 2009). While likely fostering extracellular CaCO3 mineralisation (Kupriyanova et al., 2007; Hazarika and Yadav, 2023), no direct benefits of cyanobacterial eCA for carbon assimilation have been documented in so far (Kupriyanova et al., 2024), rendering its physiological relevance elusive. Light-driven processes underlying passive cell-surface catalyzed biomineralization (Obst et al., 2009; Görgen et al., 2021) and its intersection with anthropogenic biogeochemical carbon cycle contributions (Friedlingstein et al., 2025) are schematically summarized in Figure 1. With all relevant components being mechanistically understood, cyanobacteria are uniquely suited as synthetic biology chassis for engineered CaCO3 production.
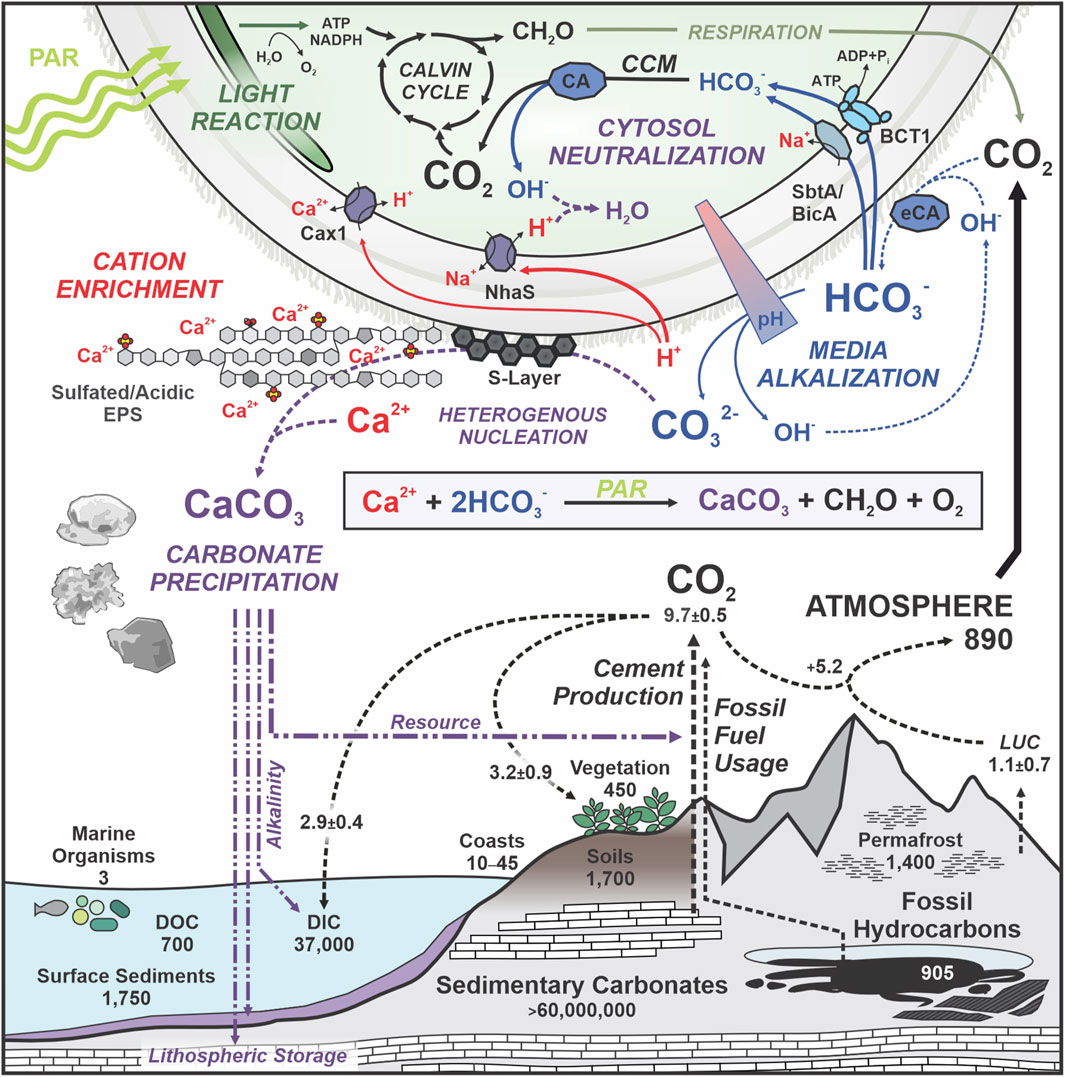
Figure 1. Cyanobacterial extracellular biomineralization and its connection to the biogeochemical carbon cycle. Top: metabolic processes and cellular components contributing to extracellular CaCO3 precipitation in cyanobacteria. PAR, photo-synthetically active radiation. CCM, carbon concentrating mechanism. (e) CA, (extracellular) carbonic anhydrase. EPS, extracellular polysaccharides. Plasma-membrane localized bicarbonate importers (BCT1, SbtA, BicA) and proton/cation antiporters NhaS (H+/Na+) and Cax1 (H+/Ca2+) are indicated. Relevant active and passive processes are indicated in italics. The sum reaction formula of photosynthesis-assisted carbonate precipitation is indicated within the inset box. Bottom: anthropogenic carbon cycle contributions (dashed lines) and carbon reservoirs in Gt. DOC, dissolved organic carbon; DIC, dissolved inorganic carbon; LUC, land use change. Natural destinations and possible applications of cyanobacterial CaCO3 precipitates are indicated (purple). Quantitative data adapted from (Falkowski et al., 2000; Friedlingstein et al., 2025).
2 Perspective
2.1 Seawater Ca2+ availability might enable scalable CO2 mineralization
Large-scale precipitation of water-dissolved CO2 as CaCO3 will require considerable amounts of Ca2+. With ocean water containing approximately 10 mM Ca2+ (Millero et al., 2008; Emmanuel et al., 2012) and total ocean volume ranging around 1.35 × 109 km3 (Charette and Smith, 2010) some 1.35 × 1020 mols of dissolved Ca2+ are available for CaCO3 precipitation, corresponding to approximately 1.351 × 1019 kg of CaCO3 or 4.985 × 106 km3 of limestone (density ∼2.71 g cm−3). For reference, precipitation of all anthropogenically emitted CO2 since the industrial revolution (i.e., ∼1.5 × 1015 kg) (Ritchie, 2019) as CaCO3 would result in 1,260 km3 of limestone equivalents, rendering ocean-water-dissolved Ca2+ a non-critical resource. Extracellular precipitation of seawater Ca2+ as CaCO3 using marine or euryhaline cyanobacterial cell surfaces may thus provide a powerful tool for light-driven re-capture of CO2 from CaCO3 thermolysis or other industrial processes. Since most modern cyanobacteria do not precipitate relevant amounts of CaCO3 for various reasons (Riding, 2006; Kamennaya et al., 2018), engineering their cell surface properties is likely required.
2.2 Promising engineering targets for enhanced CaCO3 biomineralization
2.2.1 Cyanobacterial EPS remodelling
Bacterial EPS have been shown to be potent inducers of CaCO3 precipitation (Ercole et al., 2007; Ercole et al., 2012). While cyanobacterial EPS production has been observed to be increased under elevated CO2 partial pressures (Kamennaya et al., 2018) or through supplementing pH buffer substances to the culture media (de Brito et al., 2022), anionic EPS production has not yet been the target of directed genetic engineering attempts. This is likely due to the complexity of the underlying biosynthesis and secretion pathways, with more than 20 unique proteins being associated with cyanobacterial EPS biosynthesis (Pereira et al., 2015). In Synechocystis, a minimum of 16 genetic components are involved in sulfated EPS biosynthesis alone (Maeda et al., 2021). The genetic complexity underlying anionic EPS biosynthesis hence obstructs reason-guided improvement attempts, rendering engineering of single gene encoded protein components a favourable target for altering the physicochemical properties of cyanobacterial cell surfaces.
2.2.2 Outer-membrane porins
Being Gram-negative bacteria, cells of cyanobacteria are enclosed by a second lipid bilayer membrane (i.e., the outer membrane) which is commonly equipped with pore-forming beta-barrel proteins (porins) facilitating the uptake of small molecules (Vergalli et al., 2020). Such outer membrane porins have been engineering targets to alter cell surface properties in both Escherichia coli (Hogervorst et al., 1990; Xu and Lee, 1999; Chen et al., 2019) and the cyanobacterial model species Synechococcus elongatus PCC 7942 (Fedeson and Ducat, 2017). As overexpression of E. coli porins OmpC, OmpF, and PhoE was found physiologically unproblematic, porins may present promising engineering targets for enhancing Ca2+ affinity of the cell surface in principle. However, cyanobacterial outer membranes have been found to naturally contain comparably few pore-forming proteins of low conductivity, likely facilitating the uptake of inorganic ions rather than small organic compounds (Hansel and Tadros, 1998; Kowata et al., 2017). Overexpression of modified porins may thus compromise cell viability as observed in Synechococcus elongatus PCC 7942 while also necessitating genetic removal of occluding factors such as EPS and S-layer proteins (Fedeson and Ducat, 2017), both of which serve as crystallization nuclei for CaCO3. Cyanobacterial porin engineering may thus not be an optimal strategy towards facilitating CaCO3 precipitation.
2.2.3 Surface display of synthetic Ca2+-enriching polypeptides
Exposure of peptides on the surface of bacterial cells has been developed into a potent screening tool for affinity engineering (Rice and Daugherty, 2008; Kenrick and Daugherty, 2010). Relying on engineered variants of relatively small outer membrane proteins such as the beta-barrel proteins OmpX (Vogt and Schulz, 1999) and OmpA (Ruppert et al., 1994; Shi and Wen Su, 2001), these approaches are largely limited to extension of protein termini or exposed loops. This, however, bears the risk of compromising folding and insertion into the outer membrane. First successful engineering attempts of OmpA towards Ca2+ binding by insertion of an EF hand motif resulted in a binding capacity of one Ca2+ per OmpA (Johansson et al., 2007), which is likely insufficient for major enhancements of CaCO3 precipitation capacity. Meanwhile, targeting fully synthetic oligopeptides with Ca2+ binding capacity provided through, e.g., DXD, DXXD, DXDXDG, or DDXX (S/T) S motifs (Rigden and Galperin, 2004; Wu et al., 2008; Mishra et al., 2012) to the outer membrane via suitable secretion signal and transit peptides including palmitoylation sites for surface-exposed outer membrane anchoring (Wilson and Bernstein, 2016) may be a preferable alternative, but has not been achieved so far. Like porin engineering, synthetic peptide surface display likely requires genetic removal of obstructing EPS or S-Layer components, or the utilization of picoplanktonic strains inherently lacking such obstruction, like the emerging biotech chassis Picosynechococcus sp. PCC 7002 (Šmarda et al., 2002; Aikawa et al., 2014; Markley et al., 2015). Still, such an approach may prove fruitful and requires experimental validation.
2.2.4 Synthetic S-layers
Paracrystalline protein surface layers have been described in many phylogenetically distinct bacteria (Fagan and Fairweather, 2014) and all archaea (Rodrigues-Oliveira et al., 2017). While functionally similar, S-layer proteins are structurally highly diverse (Bahl et al., 1997; Hynönen and Palva, 2013) and thus likely products of multiple instances of convergent evolution. Many S-layer proteins have significant Ca2+ binding capacity due to aspartate-rich polypeptide sequences, and direct contribution of Ca2+ to protein lattice assembly and stability has been documented (Baranova et al., 2012; Bharat et al., 2017; Gambelli et al., 2019; Herdman et al., 2022). Being exposed on the very surface of the cell and known to facilitate CaCO3 crystal nucleation (Schultze-Lam et al., 1992; Schultze-Lam and Beveridge, 1994), S-layer proteins are promising targets to engineer cell surfaces for optimal biomineralization. Related S-layer proteins have already been successfully transferred between cyanobacterial model species (Zu et al., 2020), indicating sufficient modularity. Exchanging endogenous S-Layers for or assembling highly Ca2+-affine “synthetic” S-layers derived from, e.g., the RsaA S-layer of Caulobacter crescentus binding up to 19 Ca2+ cations per protein subunit (Bharat et al., 2017) on inherently S-Layer free cyanobacteria thus represents a promising avenue towards cell-surface-proximal Ca2+ enrichment and facilitation of CaCO3 crystal nucleation. As Ca2+ appears to be a structural component of many S-layer lattices, however, cell-surface recruited Ca2+ may not actually be available for CaCO3 mineralization. Identification of naturally occurring S-layer proteins with high levels of loosely associated Ca2+ cations or de novo engineering of non-structural low-affinity binding sites may thus be required to effectively foster S-layer-driven CaCO3 biomineralization.
2.2.5 Overexpression and surface-immobilization of eCA enzymes
In vitro, eCA activity has been shown to foster CaCO3 mineralization under high CO2 partial pressures (Srivastava et al., 2015; Heuer et al., 2022). As of now, precise localization studies on enzymatically active cyanobacterial eCAs distinguishing periplasmic and truly extracellular localization, e.g., in the glycocalyx, are sparse. Here, Cyanothece sp. ATCC 51142 EcaA and Sodalinema gerasimenkoae IPPAS B-353 CahB1 represent noteworthy exceptions (Kupriyanova et al., 2011; 2019; 2022), although the native secretion mechanism of the latter remains elusive (Minagawa and Dann, 2023). While likely not essential for CaCO3 mineralization per se, eCA activity near the extracellularly exposed surface of the outer membrane is likely to enhance CaCO3 precipitation in vivo. Overexpression of free eCA in S-layer harbouring strains, or eCA-anchoring to the outer membrane in strains expressing synthetic Ca2+-enriching polypeptide through, e.g., a palmitoylation motif (Wilson and Bernstein, 2016), are thus to be considered.
2.3 Drafting optimal light-driven CaCO3 biomineralization in cyanobacteria
Omitting the seemingly impractical engineering of cyanobacterial EPS, the previous considerations culminate in two promising strategies towards reason-guided enhancement of light-driven biomineralization. A schematic overview of engineered strains following both strategies, relying on modified S-layers and free eCA on the one hand, or synthetic Ca2+-enriching polypeptides and surface-immobilized eCA on the other hand, is provided in Figure 2.
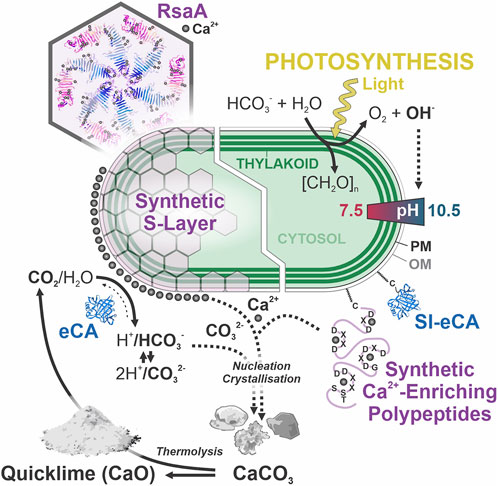
Figure 2. Enhancing light-driven CaCO3 precipitation in engineered cyanobacteria. Hexameric symmetry and Ca2+ binding sites of the S-layer-forming RsaA protein from Caulobacter crescentus, adapted from (Bharat et al., 2017). The formation and strength of the photosynthesis-associated pH gradient are outlined. [CH2O]n, photosynthesized carbohydrates; (SI-) eCA, (surface-immobilized) extracellular carbonic anhydrase; PM, plasma membrane; OM, outer membrane. OM-tethering of SI-eCA and synthetic Ca2+-enriching polypeptides with candidate Ca2+-binding motifs through S-palmitoylation via cysteine (C) is indicated. Curved arrows indicate relevant chemical reactions and fluxes involved in biomineralization. Separate cell engineering strategies resulting in enhanced micrite formation are indicated (white boundary).
3 Discussion
3.1 Small is beautiful – why cyanobacteria may outshine eukaryotic biomineralizers
Storing CO2 in the form of stable carbonate minerals faces fewer engineering and environmental challenges than other sequestration approaches like deep sea storage or deep ground injection, while maintaining minimal leakage risk as demonstrated by its natural counterpart (Ma et al., 2024). In natural systems, carbonate rocks are predominantly formed through biological and biologically induced processes, commonly summarized in the notion that carbonates are born, not made (James and Jones, 2016). Nowadays, photosynthetic plankton constitutes the most prolific carbonate factory, with recently evolved coccolithophore haptophyte algae producing around 50% of Holocene marine CaCO3 sedimentation (Broecker and Clark, 2009). Due to their large contribution to pelagic CaCO3 production in situ (Ziveri et al., 2023), these algae are being discussed as promising biotechnological CCS platforms. As these organisms remain hardly accessible to genetic engineering tools (Flavin and Chatterjee, 2024), any application remains largely limited to preexisting strains and their maximum productivity, however. A pronounced sensitivity to changes in carbonate chemistry and water acidification in most species (Beaufort et al., 2011; Meyer and Riebesell, 2015; Vázquez et al., 2023) and a general preference for growth temperatures below 30 °C (Gafar and Schulz, 2018; von Dassow et al., 2021) furthermore limit coccolithophore utility and application potential for CO2 capture from, e.g., hot cement industry flue gasses commonly containing 10%–20% CO2 (Camargo and Lombardi, 2018). Lastly, coccolithophore formation involves active transport and Ca2+ concentration within the cell (Sviben et al., 2016), rendering it more energetically taxing than passive extracellular CaCO3 precipitation. Cyanobacteria meanwhile are highly accessible to genetic engineering and tailoring to harsh growth conditions through adaptive laboratory evolution (Tillich et al., 2012; Dann et al., 2021), their photosynthetic activity results in more pronounced media alkalization than that of eukaryotic algae (Touloupakis et al., 2016; Zepernick et al., 2021; de Brito et al., 2022), and their smaller cells provide a favourable surface-to-volume ratio for cell-surface catalysed CaCO3 precipitation. This renders engineered cyanobacteria a likely superior platform for any future work on light-driven CaCO3 precipitation, and marks cyanobacterial cell-surface engineering a promising pathway towards flexible and scalable biotechnological CaCO3 recovery.
3.2 Towards geobiologically inspired carbon recovery and storage
In accordance with their large contribution to the geological record, the utilization of cyanobacterial biomineralization for CCS was suggested before (Jansson and Northen, 2010), but no significant upscaling or commercial application has been achieved so far. Owing to a focus on EPS and the practical inaccessibility of complex anionic EPS biosynthesis to genetic and metabolic engineering, previous studies have near-exclusively focused on the identification of inherently productive calcifying species (Lee et al., 2004; Liang et al., 2013) and conductive cultivation methods (McCutcheon et al., 2014). A single genetic engineering attempt to increase calcification capacities was limited to the knockout of cax1 (Ca2+/H+ antiporter) in the mesophilic freshwater model species Synechocystis sp. PCC 6803, resulting in enhanced BCT1 (Ca2+-dependent HCO3− transporter) activity, increased CCM activity, and thus increased CaCO3 precipitation (Jiang et al., 2013). Despite these first successes, biomineralization yields remain insufficient for large-scale applications. With documented rates of cyanobacterial Ca2+ precipitation from saltwater media corresponding to approximately 120–240 mg of CaCO3 per liter of batch culture over a 2-week cultivation cycle (Lee et al., 2004; Yang et al., 2023), precipitation of 1 metric ton (t) of CaCO3 would require the equivalent of two Olympic swimming pools (i.e., ∼5*106 L). This corresponds to approximately 5.8 t of CO2 sequestration capacity per Olympic swimming pool equivalent per year, valued around 430 € worth of CO2 certificates at current EU Emissions Trading System pricing. Hence, an increase in biomineralization capacity by several orders of magnitude is likely required to attain economic viability. Although no comprehensive understanding of the modulation of CaCO3 crystallization through biological agents has been achieved to date, mechanic deformation of heterogenous nucleation sites alone has been reported to increase CaCO3 nucleation rate by one order of magnitude (Taylor et al., 2020). Meanwhile, calcite and aragonite nuclei were found the only nuclei capable of markedly catalyzing CaCO3 precipitation in natural surface seawater (Pan et al., 2021), highlighting the crucial importance of crystallization nuclei surface properties for efficient CaCO3 mineralization. As crystallization rates in more complex biogenic systems such as supersaturated lysozyme solution have been found to increase by 8-10 orders of magnitude upon exposure to suitable heterogenous nuclei (Filobelo et al., 2005), ample room for major improvement of bio-mediated CaCO3 precipitation appears conceivable.
As opposed to previous attempts, engineering cyanobacterial cell surface properties through the introduction of modified or synthetic protein components and simultaneous genetic removal of obstructive features can be expected to allow for enhanced cell-surface catalysis of CaCO3 precipitation. Especially recent breakthroughs in protein structure prediction and engineering (Watson et al., 2023) render this new approach worth pursuing. Here, a two-pronged empirical approach of introducing a re-engineered S-layer or disorganized synthetic cell-surface peptides with Ca2+ binding capacity appears a reasonable choice to determine the most conductive strategy, while EPS engineering remains prohibitively complex and porin/OMP engineering likely too functionally constrained for large-scale Ca2+ attraction and nucleation site provision. Finally, utilization of thermophilic chassis strains accessible for genetic engineering such as Thermosynechococcus elongatus BP-1 which strives at cultivation temperatures as high as 55 °C (Yamaoka et al., 1978; Iwai et al., 2004), or adaptive laboratory evolution for enhanced thermotolerance of mesophilic strains (Tillich et al., 2012) can likely enhance CaCO3 precipitation efficiency due to reduced solubility of aragonite and calcite in warmer solutions, specifically enabling CO2 capture from hot thermolysis or flue gasses. This should eventually allow for efficient light-driven re-routing of CO2 emissions into carbonate resource production and lithospheric carbon storage.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MF: Writing – original draft, Conceptualization, Funding acquisition, Writing – review and editing. MD: Conceptualization, Funding acquisition, Visualization, Writing – review and editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Technical University of Darmstadt (FiF Project Grant 2024#17 to MF and MD).
Acknowledgments
We thank Anne-Christin Pohland for critical reading of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aikawa, S., Nishida, A., Ho, S. H., Chang, J. S., Hasunuma, T., and Kondo, A. (2014). Glycogen production for biofuels by the euryhaline cyanobacteria Synechococcus sp. strain PCC 7002 from an oceanic environment. Biotechnol. Biofuels 7, 88. doi:10.1186/1754-6834-7-88
Altermann, W., Kazmierczak, J., Oren, A., and Wright, D. T. (2006). Cyanobacterial calcification and its rock-building potential during 3.5 billion years of Earth history. Geobiology 4, 147–166. doi:10.1111/j.1472-4669.2006.00076.x
Bahl, H., Scholz, H., Bayan, N., Chami, M., Leblon, G., Gulik-Krzywicki, T., et al. (1997). Molecular biology of S-layers. FEMS Microbiol. Rev. 20, 47–98. doi:10.1111/j.1574-6976.1997.tb00304.x
Banerjee, S., Bhattacharya, S. K., and Sarkar, S. (2006). Carbon and oxygen isotope compositions of the carbonate facies in the Vindhyan Supergroup, central India. J. Earth Syst. Sci. 115, 113–134. doi:10.1007/BF02703029
Baranova, E., Fronzes, R., Garcia-Pino, A., Gerven, N. V., Papapostolou, D., Péhau-Arnaudet, G., et al. (2012). SbsB structure and lattice reconstruction unveil Ca2+ triggered S-layer assembly. Nature 487, 119–122. doi:10.1038/nature11155
Beaufort, L., Probert, I., De Garidel-Thoron, T., Bendif, E. M., Ruiz-Pino, D., Metzl, N., et al. (2011). Sensitivity of coccolithophores to carbonate chemistry and ocean acidification. Nature 476, 80–83. doi:10.1038/nature10295
Benzerara, K., Skouri-Panet, F., Li, J., Férard, C., Gugger, M., Laurent, T., et al. (2014). Intracellular Ca-carbonate biomineralization is widespread in cyanobacteria. Proc. Natl. Acad. Sci. U. S. A. 111, 10933–10938. doi:10.1073/pnas.1403510111
Bharat, T. A. M., Kureisaite-Ciziene, D., Hardy, G. G., Yu, E. W., Devant, J. M., Hagen, W. J. H., et al. (2017). Structure of the hexagonal surface layer on Caulobacter crescentus cells. Nat. Microbiol. 2, 17059. doi:10.1038/nmicrobiol.2017.59
Broecker, W., and Clark, E. (2009). Ratio of coccolith CaCO3 to foraminifera CaCO3 in late Holocene deep sea sediments. Paleoceanography 24. doi:10.1029/2009PA001731
Callendar, G. S. (1938). The artificial production of carbon dioxide and its influence on temperature. Q. J. R. Meteorological Soc. 64, 223–240. doi:10.1002/qj.49706427503
Camargo, E. C., and Lombardi, A. T. (2018). Effect of cement industry flue gas simulation on the physiology and photosynthetic performance of Chlorella sorokiniana. J. Appl. Phycol. 30, 861–871. doi:10.1007/s10811-017-1291-3
Charette, M. A., and Smith, W. H. F. (2010). The volume of earth’s Ocean. Oceanography 23, 112–114. doi:10.5670/oceanog.2010.51
Chen, P. H., Liu, H. L., Chen, Y. J., Cheng, Y. H., Lin, W. L., Yeh, C. H., et al. (2012). Enhancing CO2 bio-mitigation by genetic engineering of cyanobacteria. Energy Environ. Sci. 5, 8318. doi:10.1039/c2ee21124f
Chen, T., Wang, K., Chi, X., Zhou, L., Li, J., Liu, L., et al. (2019). Construction of a bacterial surface display system based on outer membrane protein F. Microb. Cell Fact. 18, 70. doi:10.1186/s12934-019-1120-2
Comes, J., Islamovic, E., Lizandara-Pueyo, C., and Seto, J. (2024). Improvements in the utilization of calcium carbonate in promoting sustainability and environmental health. Front. Chem. 12, 1472284. doi:10.3389/fchem.2024.1472284
Dann, M., Ortiz, E. M., Thomas, M., Guljamow, A., Lehmann, M., Schaefer, H., et al. (2021). Enhancing photosynthesis at high light levels by adaptive laboratory evolution. Nat. Plants 7, 681–695. doi:10.1038/s41477-021-00904-2
de Brito, M. M., Bundeleva, I., Marin, F., Vennin, E., Wilmotte, A., Plasseraud, L., et al. (2022). Effect of culture pH on properties of exopolymeric substances from Synechococcus PCC7942: implications for carbonate precipitation. Geosci. Switz. 12, 210. doi:10.3390/geosciences12050210
Durastanti, C., and Moretti, L. (2024). Assessing the climate effects of clinker production: a statistical analysis to reduce its environmental impacts. Clean. Environ. Syst. 14, 100204. doi:10.1016/j.cesys.2024.100204
Emmanuel, A. O., Oladipo, F. A., and E, O. O. (2012). Investigation of salinity effect on compressive strength of reinforced concrete. J. Sustain Dev. 5. doi:10.5539/jsd.v5n6p74
Ercole, C., Bozzelli, P., Altieri, F., Cacchio, P., and Del Gallo, M. (2012). Calcium carbonate mineralization: involvement of extracellular polymeric materials isolated from calcifying bacteria. Microsc. Microanal. 18, 829–839. doi:10.1017/S1431927612000426
Ercole, C., Cacchio, P., Botta, A. L., Centi, V., and Lepidi, A. (2007). “Bacterially induced mineralization of calcium carbonate: the role of exopolysaccharides and capsular polysaccharides,” in Microscopy and microanalysis. doi:10.1017/S1431927607070122
Fagan, R. P., and Fairweather, N. F. (2014). Biogenesis and functions of bacterial S-layers. Nat. Rev. Microbiol. 12, 211–222. doi:10.1038/nrmicro3213
Falkowski, P., Scholes, R. J., Boyle, E., Canadell, J., Canfield, D., Elser, J., et al. (2000). The global carbon cycle: a test of our knowledge of earth as a system. Science 1979, 291–296. doi:10.1126/science.290.5490.291
Fedeson, D. T., and Ducat, D. C. (2017). Cyanobacterial surface display system mediates engineered interspecies and abiotic binding. ACS Synth. Biol. 6, 367–374. doi:10.1021/acssynbio.6b00254
Filobelo, L. F., Galkin, O., and Vekilov, P. G. (2005). Spinodal for the solution-to-crystal phase transformation. J. Chem. Phys. 123, 014904. doi:10.1063/1.1943413
Flavin, C., and Chatterjee, A. (2024). Cell-penetrating peptide delivery of nucleic acid cargo to emiliania huxleyi, a calcifying marine coccolithophore. ACS Synth. Biol. 13, 77–84. doi:10.1021/acssynbio.3c00670
Friedlingstein, P., O’Sullivan, M., Jones, M. W., Andrew, R. M., Hauck, J., Landschützer, P., et al. (2025). Global carbon budget 2024. Earth Syst. Sci. Data 17, 965–1039. doi:10.5194/essd-17-965-2025
Gafar, N. A., and Schulz, K. G. (2018). A niche comparison of Emiliania huxleyi and Gephyrocapsa oceanica and potential effects of climate change. Biogeosciences Discuss. 2100.
Gambelli, L., Meyer, B. H., McLaren, M., Sanders, K., Quax, T. E. F., Gold, V. A. M., et al. (2019). Architecture and modular assembly of Sulfolobus S-layers revealed by electron cryotomography. Proc. Natl. Acad. Sci. U. S. A. 116, 25278–25286. doi:10.1073/pnas.1911262116
Görgen, S., Benzerara, K., Skouri-Panet, F., Gugger, M., Chauvat, F., and Cassier-Chauvat, C. (2021). The diversity of molecular mechanisms of carbonate biomineralization by bacteria. Discov. Mater 1, 2. doi:10.1007/s43939-020-00001-9
Hansel, A., and Tadros, M. H. (1998). Characterization of two pore-forming proteins isolated from the outer membrane of Synechococcus PCC 6301. Curr. Microbiol. 36, 321–326. doi:10.1007/s002849900316
Hazarika, A., and Yadav, M. (2023). Biomineralization of carbon dioxide by carbonic anhydrase. Biocatal. Agric. Biotechnol. 51, 102755. doi:10.1016/j.bcab.2023.102755
Herdman, M., von Kügelgen, A., Kureisaite-Ciziene, D., Duman, R., El Omari, K., Garman, E. F., et al. (2022). High-resolution mapping of metal ions reveals principles of surface layer assembly in Caulobacter crescentus cells. Structure 30, 215–228.e5. doi:10.1016/j.str.2021.10.012
Heuer, J., Kraus, Y., Vučak, M., and Zeng, A. P. (2022). Enhanced sequestration of carbon dioxide into calcium carbonate using pressure and a carbonic anhydrase from alkaliphilic Coleofasciculus chthonoplastes. Eng. Life Sci. 22, 178–191. doi:10.1002/elsc.202100033
Hogervorst, E. J. M., Agterberg, M., Wagenaar, P. A., Adriaanse, J., Boog, C. J. P., Zee, R. V. D., et al. (1990). Efficient recognition by rat T cell clones of an epitope of mycobacterial hsp 65 inserted in Escherichia coli outer membrane protein PhoE. Eur. J. Immunol. 20, 2763–2768. doi:10.1002/eji.1830201234
Hynönen, U., and Palva, A. (2013). Lactobacillus surface layer proteins: structure, function and applications. Appl. Microbiol. Biotechnol. 97, 5225–5243. doi:10.1007/s00253-013-4962-2
Iwai, M., Katoh, H., Katayama, M., and Ikeuchi, M. (2004). Improved genetic transformation of the thermophilic cyanobacterium, Thermosynechococcus elongatus BP-1. Plant Cell Physiol. 45, 171–175. doi:10.1093/pcp/pch015
Jansson, C., and Northen, T. (2010). Calcifying cyanobacteria-the potential of biomineralization for carbon capture and storage. Curr. Opin. Biotechnol. 21, 365–371. doi:10.1016/j.copbio.2010.03.017
Jiang, H. B., Cheng, H. M., Gao, K. S., and Qiu, B. S. (2013). Inactivation of Ca2+/H+ exchanger in Synechocystis sp. strain PCC 6803 promotes cyanobacterial calcification by upregulating CO2-concentrating mechanisms. Appl. Environ. Microbiol. 79, 4048–4055. doi:10.1128/AEM.00681-13
Johansson, M. U., Alioth, S., Hu, K., Walser, R., Koebnik, R., and Pervushin, K. (2007). A minimal transmembrane β-barrel platform protein studied by nuclear magnetic resonance. Biochemistry 46, 1128–1140. doi:10.1021/bi061265e
Jones, M. W., Peters, G. P., Gasser, T., Andrew, R. M., Schwingshackl, C., Gütschow, J., et al. (2023). National contributions to climate change due to historical emissions of carbon dioxide, methane, and nitrous oxide since 1850. Sci. Data 10, 155. doi:10.1038/s41597-023-02041-1
Jroundi, F., Merroun, M. L., Martínez-Ruiz, F., and González-Muñoz, M. T. (2022). Intracellular and extracellular bacterial biomineralization. doi:10.1007/978-3-030-80807-5_2
Kamennaya, N. A., Ajo-Franklin, C. M., Northen, T., and Jansson, C. (2012). Cyanobacteria as biocatalysts for carbonate mineralization. Minerals 2, 338–364. doi:10.3390/min2040338
Kamennaya, N. A., Zemla, M., Mahoney, L., Chen, L., Holman, E., Holman, H. Y., et al. (2018). High pCO2-induced exopolysaccharide-rich ballasted aggregates of planktonic cyanobacteria could explain Paleoproterozoic carbon burial. Nat. Commun. 9, 2116. doi:10.1038/s41467-018-04588-9
Kaźmierczak, J., Coleman, M. L., Gruszczyński, M., and Kempe, S. (1996). Cyanobacterial key to the genesis of micritic and peloidal limestones in ancient seas. Acta Palaeontol. Pol. 41.
Kenrick, S. A., and Daugherty, P. S. (2010). Bacterial display enables efficient and quantitative peptide affinity maturation. Protein Eng. Des. Sel. 23, 9–17. doi:10.1093/protein/gzp065
Kowata, H., Tochigi, S., Takahashi, H., and Kojima, S. (2017). Outer membrane permeability of cyanobacterium Synechocystis sp. strain PCC 6803: studies of passive diffusion of small organic nutrients reveal the absence of classical porins and intrinsically low permeability. J. Bacteriol. 199, e00371-17. doi:10.1128/JB.00371-17
Kupriyanova, E., Villarejo, A., Markelova, A., Gerasimenko, L., Zavarzin, G., Samuelsson, G., et al. (2007). Extracellular carbonic anhydrases of the stromatolite-forming cyanobacterium Microcoleus chthonoplastes. Microbiol. (N Y) 153, 1149–1156. doi:10.1099/mic.0.2006/003905-0
Kupriyanova, E. V., Sinetova, M. A., Gabrielyan, D. A., and Los, D. A. (2024). The freshwater cyanobacterium Synechococcus elongatus PCC 7942 does not require an active external carbonic anhydrase. Plants 13, 2323. doi:10.3390/plants13162323
Kupriyanova, E. V., Sinetova, M. A., Leusenko, A. V., Voronkov, A. S., and Los, D. A. (2022). A leader peptide of the extracellular cyanobacterial carbonic anhydrase ensures the efficient secretion of recombinant proteins in Escherichia coli. J. Biotechnol. 344, 11–23. doi:10.1016/j.jbiotec.2021.12.006
Kupriyanova, E. V., Sinetova, M. A., Markelova, A. G., Allakhverdiev, S. I., Los, D. A., and Pronina, N. A. (2011). Extracellular β-class carbonic anhydrase of the alkaliphilic cyanobacterium Microcoleus chthonoplastes. J. Photochem Photobiol. B 103, 78–86. doi:10.1016/j.jphotobiol.2011.01.021
Kupriyanova, E. V., Sinetova, M. A., Mironov, K. S., Novikova, G. V., Dykman, L. A., Rodionova, M. V., et al. (2019). Highly active extracellular α-class carbonic anhydrase of Cyanothece sp. Biochimie 160, 51142. doi:10.1016/j.biochi.2019.03.009
Lee, B. D., Apel, W. A., and Walton, M. R. (2004). Screening of cyanobacterial species for calcification. Biotechnol. Prog. 20, 1345–1351. doi:10.1021/bp0343561
Liang, A., Paulo, C., Zhu, Y., and Dittrich, M. (2013). CaCO3 biomineralization on cyanobacterial surfaces: insights from experiments with three Synechococcus strains. Colloids Surf. B Biointerfaces 111, 600–608. doi:10.1016/j.colsurfb.2013.07.012
Luo, G., Ono, S., Beukes, N. J., Wang, D. T., Xie, S., and Summons, R. E. (2016). Rapid oxygenation of Earth’s atmosphere 2.33 billion years ago. Sci. Adv. 2, e1600134. doi:10.1126/sciadv.1600134
Ma, Y., Yi, S., and Wang, M. (2024). Biomimetic mineralization for carbon capture and sequestration. Carbon Capture Sci. and Technol. 13, 100257. doi:10.1016/j.ccst.2024.100257
Maeda, K., Okuda, Y., Enomoto, G., Watanabe, S., and Ikeuchi, M. (2021). Biosynthesis of a sulfated exopolysaccharide, synechan, and bloom formation in the model cyanobacterium synechocystis sp. Strain pcc 6803. Elife 10, e66538. doi:10.7554/eLife.66538
Markley, A. L., Begemann, M. B., Clarke, R. E., Gordon, G. C., and Pfleger, B. F. (2015). Synthetic biology toolbox for controlling gene expression in the cyanobacterium Synechococcus sp. strain PCC 7002. ACS Synth. Biol. 4, 595–603. doi:10.1021/sb500260k
Martinho De Brito, M., Bundeleva, I., Marin, F., Vennin, E., Wilmotte, A., Plasseraud, L., et al. (2023). Properties of exopolymeric substances (EPSs) produced during cyanobacterial growth: potential role in whiting events. Biogeosciences 20, 3165–3183. doi:10.5194/bg-20-3165-2023
McCutcheon, J., Power, I. M., Harrison, A. L., Dipple, G. M., and Southam, G. (2014). A greenhouse-scale photosynthetic microbial bioreactor for carbon sequestration in magnesium carbonate minerals. Environ. Sci. Technol. 48, 9142–9151. doi:10.1021/es500344s
Merz, M. U. E. (1992). The biology of carbonate precipitation by cyanobacteria. Facies 26, 81–101. doi:10.1007/BF02539795
Meyer, J., and Riebesell, U. (2015). Reviews and syntheses: responses of coccolithophores to ocean acidification: a meta-analysis. Biogeosciences 12, 1671–1682. doi:10.5194/bg-12-1671-2015
Millero, F. J., Feistel, R., Wright, D. G., and McDougall, T. J. (2008). The composition of standard seawater and the definition of the reference-composition salinity scale. Deep Sea Res. 1 Oceanogr. Res. Pap. 55, 50–72. doi:10.1016/j.dsr.2007.10.001
Minagawa, J., and Dann, M. (2023). Extracellular CahB1 from Sodalinema gerasimenkoae IPPAS B-353 acts as a functional carboxysomal β-carbonic anhydrase in synechocystis sp. PCC6803. Plants 12, 265. doi:10.3390/plants12020265
Mishra, A., Suman, S. K., Srivastava, S. S., Sankaranarayanan, R., and Sharma, Y. (2012). Decoding the molecular design principles underlying Ca2+ binding to βγ-crystallin motifs. J. Mol. Biol. 415, 75–91. doi:10.1016/j.jmb.2011.10.037
Moreira, D., Tavera, R., Benzerara, K., Skouri-Panet, F., Couradeau, E., Gérard, E., et al. (2017). Description of Gloeomargarita lithophora gen. nov., sp. nov., a thylakoid-bearing, basal-branching cyanobacterium with intracellular carbonates, and proposal for Gloeomargaritales ord. nov. Int. J. Syst. Evol. Microbiol. 67, 653–658. doi:10.1099/ijsem.0.001679
Niu, Y. Q., Liu, J. H., Aymonier, C., Fermani, S., Kralj, D., Falini, G., et al. (2022). Calcium carbonate: controlled synthesis, surface functionalization, and nanostructured materials. Chem. Soc. Rev. 51, 7883–7943. doi:10.1039/d1cs00519g
Obst, M., Wehrli, B., and Dittrich, M. (2009). CaCO3 nucleation by cyanobacteria: laboratory evidence for a passive, surface-induced mechanism. Geobiology 7, 324–347. doi:10.1111/j.1472-4669.2009.00200.x
Oliver, T., Sánchez-Baracaldo, P., Larkum, A. W., Rutherford, A. W., and Cardona, T. (2021). Time-resolved comparative molecular evolution of oxygenic photosynthesis. Biochim. Biophys. Acta Bioenerg. 1862, 148400. doi:10.1016/j.bbabio.2021.148400
Pan, Y., Li, Y., Ma, Q., He, H., Wang, S., Sun, Z., et al. (2021). The role of Mg2+ in inhibiting CaCO3 precipitation from seawater. Mar. Chem. 237 237, 104036. doi:10.1016/j.marchem.2021.104036
Parker, R. L. (1967). Composition of the Earth’s crust. Washington, DC: U.S. Government Printing Office.
Pereira, S. B., Mota, R., Vieira, C. P., Vieira, J., and Tamagnini, P. (2015). Phylum-wide analysis of genes/proteins related to the last steps of assembly and export of extracellular polymeric substances (EPS) in cyanobacteria. Sci. Rep. 5, 14835. doi:10.1038/srep14835
Rice, J. J., and Daugherty, P. S. (2008). Directed evolution of a biterminal bacterial display scaffold enhances the display of diverse peptides. Protein Eng. Des. Sel. 21, 435–442. doi:10.1093/protein/gzn020
Riding, R. (1992). Temporal variation in calcification in marine cyanobacteria. J. - Geol. Soc. Lond. 149, 979–989. doi:10.1144/gsjgs.149.6.0979
Riding, R. (2006). Cyanobacterial calcification, carbon dioxide concentrating mechanisms, and Proterozoic-Cambrian changes in atmospheric composition. Geobiology 4, 299–316. doi:10.1111/j.1472-4669.2006.00087.x
Rigden, D. J., and Galperin, M. Y. (2004). The DxDxDG motif for calcium binding: multiple structural contexts and implications for evolution. J. Mol. Biol. 343, 971–984. doi:10.1016/j.jmb.2004.08.077
Ritchie, H. (2019). Who has contributed most to global CO2 emissions? - our World in Data. Our World in Data. Available online at: https://ourworldindata.org/contributed-most-global-co2 [Online Resource].
Rodrigues-Oliveira, T., Belmok, A., Vasconcellos, D., Schuster, B., and Kyaw, C. M. (2017). Archaeal S-layers: overview and current state of the art. Front. Microbiol. 8, 2597. doi:10.3389/fmicb.2017.02597
Rosing, M. T., and Frei, R. (2004). U-rich Archaean sea-floor sediments from Greenland – indications of >3700 Ma oxygenic photosynthesis. Earth Planet Sci. Lett. 217, 237–244. doi:10.1016/S0012-821X(03)00609-5
Ruppert, A., Arnold, N., and Hobom, G. (1994). OmpA-FMDV VP1 fusion proteins: production, cell surface exposure and immune responses to the major antigenic domain of foot-and-mouth disease virus. Vaccine 12, 492–498. doi:10.1016/0264-410X(94)90305-0
Schultze-Lam, S., and Beveridge, T. J. (1994). Physicochemical characteristics of the mineral-forming S-layer from the cyanobacterium Synechococcus strain GL24. Can. J. Microbiol. 40, 216–223. doi:10.1139/m94-035
Schultze-Lam, S., Harauz, G., and Beveridge, T. J. (1992). Participation of a cyanobacterial S layer in fine-grain mineral formation. J. Bacteriol. 174, 7971–7981. doi:10.1128/jb.174.24.7971-7981.1992
Shi, H., and Wen Su, W. (2001). Display of green fluorescent protein on Escherichia coli cell surface. Enzyme Microb. Technol. 28, 25–34. doi:10.1016/S0141-0229(00)00281-7
Šmarda, J., Šmajs, D., Komrska, J., and Krzyžánek, V. (2002). S-layers on cell walls of cyanobacteria. Micron 33, 257–277. doi:10.1016/S0968-4328(01)00031-2
Soltes-Rak, E., Mulligan, M. E., and Coleman, J. R. (1997). Identification and characterization of a gene encoding a vertebrate-type carbonic anhydrase in cyanobacteria. J. Bacteriol. 179, 769–774. doi:10.1128/jb.179.3.769-774.1997
Srivastava, S., Bharti, R. K., Verma, P. K., and Thakur, I. S. (2015). Cloning and expression of gamma carbonic anhydrase from Serratia sp. ISTD04 for sequestration of carbon dioxide and formation of calcite. Bioresour. Technol. 188, 209–213. doi:10.1016/j.biortech.2015.01.108
Suosaari, E. P., Reid, R. P., Playford, P. E., Foster, J. S., Stolz, J. F., Casaburi, G., et al. (2016). New multi-scale perspectives on the stromatolites of shark bay, western Australia. Sci. Rep. 6, 20557. doi:10.1038/srep20557
Sviben, S., Gal, A., Hood, M. A., Bertinetti, L., Politi, Y., Bennet, M., et al. (2016). A vacuole-like compartment concentrates a disordered calcium phase in a key coccolithophorid alga. Nat. Commun. 7, 11228. doi:10.1038/ncomms11228
Taylor, J. M., Konda, A., and Morin, S. A. (2020). Spatiotemporal control of calcium carbonate nucleation using mechanical deformations of elastic surfaces. Soft Matter 16, 6038–6043. doi:10.1039/d0sm00734j
Thompson, K. J., Kenward, P. A., Bauer, K. W., Warchola, T., Gauger, T., Martinez, R., et al. (2019). Photoferrotrophy, deposition of banded iron formations, and methane production in Archean oceans. Sci. Adv. 5, eaav2869. doi:10.1126/sciadv.aav2869
Tice, M. M., and Lowe, D. R. (2004). Photosynthetic microbial mats in the 3,416-Myr-old ocean. Nature 431, 549–552. doi:10.1038/nature02888
Tillich, U. M., Lehmann, S., Schulze, K., Dühring, U., and Frohme, M. (2012). The optimal mutagen dosage to induce point-mutations in synechocystis sp. PCC6803 and its application to promote temperature tolerance. PLoS One 7, e49467. doi:10.1371/journal.pone.0049467
Touloupakis, E., Cicchi, B., Benavides, A. M. S., and Torzillo, G. (2016). Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.). Appl. Microbiol. Biotechnol. 100, 1333–1341. doi:10.1007/s00253-015-7024-0
Trimborn, S., Wolf-Gladrow, D., Richter, K. U., and Rost, B. (2009). The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms. J. Exp. Mar. Biol. Ecol. 376, 26–36. doi:10.1016/j.jembe.2009.05.017
Vasconcelos, C., McKenzie, J. A., Bernasconi, S., Grujic, D., and Tiens, A. J. (1995). Microbial mediation as a possible mechanism for natural dolomite formation at low temperatures. Nature 377, 220–222. doi:10.1038/377220a0
Vázquez, V., León, P., Gordillo, F. J. L., Jiménez, C., Concepción, I., Mackenzie, K., et al. (2023). High-CO2 levels rather than acidification restrict emiliania huxleyi growth and performance. Microb. Ecol. 86, 127–143. doi:10.1007/s00248-022-02035-3
Vergalli, J., Bodrenko, I. V., Masi, M., Moynié, L., Acosta-Gutiérrez, S., Naismith, J. H., et al. (2020). Porins and small-molecule translocation across the outer membrane of Gram-negative bacteria. Nat. Rev. Microbiol. 18, 164–176. doi:10.1038/s41579-019-0294-2
Victoria, A. J., Astbury, M. J., and McCormick, A. J. (2024). Engineering highly productive cyanobacteria towards carbon negative emissions technologies. Curr. Opin. Biotechnol. 87, 103141. doi:10.1016/j.copbio.2024.103141
Vogt, J., and Schulz, G. E. (1999). The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence. Structure 7, 1301–1309. doi:10.1016/S0969-2126(00)80063-5
von Dassow, P., Muñoz Farías, P. V., Pinon, S., Velasco-Senovilla, E., and Anguita-Salinas, S. (2021). Do differences in latitudinal distributions of species and organelle haplotypes reflect thermal reaction norms within the emiliania/gephyrocapsa complex? Front. Mar. Sci. 8. doi:10.3389/fmars.2021.785763
Watson, J. L., Juergens, D., Bennett, N. R., Trippe, B. L., Yim, J., Eisenach, H. E., et al. (2023). De novo design of protein structure and function with RFdiffusion. Nature 620, 1089–1100. doi:10.1038/s41586-023-06415-8
Wilson, M. M., and Bernstein, H. D. (2016). Surface-exposed lipoproteins: an emerging secretion phenomenon in gram-negative bacteria. Trends Microbiol. 24, 198–208. doi:10.1016/j.tim.2015.11.006
Wu, Y. M., Hsu, C. H., Wang, C. H., Liu, W., Chang, W. H., and Lin, C. S. (2008). Role of the DxxDxD motif in the assembly and stability of betanodavirus particles. Arch. Virol. 153, 1633–1642. doi:10.1007/s00705-008-0150-6
Xi, F., Davis, S. J., Ciais, P., Crawford-Brown, D., Guan, D., Pade, C., et al. (2016). Substantial global carbon uptake by cement carbonation. Nat. Geosci. 9, 880–883. doi:10.1038/ngeo2840
Xu, Z., and Lee, S. Y. (1999). Display of polyhistidine peptides on the Escherichia coli cell surface by using outer membrane protein C as an anchoring motif. Appl. Environ. Microbiol. 65, 5142–5147. doi:10.1128/aem.65.11.5142-5147.1999
Yamaoka, T., Satoh, K., and Katoh, S. (1978). Photosynthetic activities of a thermophilic blue-green alga. Plant Cell Physiol. 19, 943–954. doi:10.1093/oxfordjournals.pcp.a075684
Yang, G., Li, F., Deng, Z., Wang, Y., Su, Z., Huang, L., et al. (2023). Abnormal crystallization sequence of calcium carbonate in the presence of Synechococcus sp. PCC 7942. Geomicrobiol. J. 40, 34–45. doi:10.1080/01490451.2022.2100948
Zepernick, B. N., Gann, E. R., Martin, R. M., Pound, H. L., Krausfeldt, L. E., Chaffin, J. D., et al. (2021). Elevated pH conditions associated with microcystis spp. blooms decrease viability of the cultured diatom fragilaria crotonensis and natural diatoms in lake erie. Front. Microbiol. 12, 598736. doi:10.3389/fmicb.2021.598736
Ziveri, P., Gray, W. R., Anglada-Ortiz, G., Manno, C., Grelaud, M., Incarbona, A., et al. (2023). Pelagic calcium carbonate production and shallow dissolution in the North Pacific Ocean. Nat. Commun. 14, 805. doi:10.1038/s41467-023-36177-w
Zu, Y., Hong, S., Xu, C., Li, W., Chen, S., and Li, J. (2020). Cell wall surface layer (S-layer) promotes colony formation in Microcystis: comparison of S-layer characteristics between colonial and unicellular forms of Microcystis and function conformation. Environ. Sci. Pollut. Res. 27, 42254–42263. doi:10.1007/s11356-020-08254-w
Keywords: biomineralization and calcification, cyanobacteria, photosynthesis, cell surface engineering, CaCO3
Citation: Falkenroth M and Dann M (2025) Engineering light-driven biomineralization for a sustainable carbonate economy. Front. Photobiol. 3:1619812. doi: 10.3389/fphbi.2025.1619812
Received: 30 April 2025; Accepted: 11 July 2025;
Published: 21 July 2025.
Edited by:
Alberto Mezzetti, Sorbonne Universités, FranceReviewed by:
José Bonomi-Barufi, Federal University of Santa Catarina, BrazilCopyright © 2025 Falkenroth and Dann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcel Dann, bWFyY2VsLmRhbm5AdHUtZGFybXN0YWR0LmRl
 Michaela Falkenroth1
Michaela Falkenroth1 Marcel Dann
Marcel Dann