- 1Exercise and Metabolism Research Center, College of Physical Education and Health Sciences, Zhejiang Normal University, Jinhua, China
- 2Key Laboratory of Intelligent Education Technology and Application of Zhejiang Province, Zhejiang Normal University, Jinhua, China
- 3Department of Sports Operation and Management, Jinhua Polytechnic, Jinhua, China
- 4Department of Microbiology, Yogi Vemana University, Kadapa, India
- 5Faculty of Agro Based Industry, Universiti Malaysia Kelantan, Kota Bharu, Malaysia
- 6School of Communication and Arts, Shanghai University of Sport, Shanghai, China
Background/Purpose: In this systematic review and meta-analysis, we assessed the effects of exercise (EX) combined with calorie restriction (CR) intervention on inflammatory biomarkers, and correlations between biomarkers and participants’ characteristics were calculated in overweight and obese adults.
Methods: An article search was conducted through PubMed, Web of Science, EMBASE, the Cochrane database, Scopus, and Google Scholar to identify articles published up to April 2021. Studies that examined the effect of EX + CR intervention on inflammatory biomarkers, including C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), and compared them with a CR trial in overweight and obese adults were included. We calculated the pooled effect by meta-analysis, identified the correlations (between inflammatory biomarkers and participants’ characteristics) through meta-regression, and explored the beneficial variable through subgroup analysis. The Cochrane risk of bias tool and Methodological Index for Non-randomized Studies were used to assess the risk of bias for the included trials.
Results: A total of 23 trials, including 1196 overweight and obese adults, were included in the meta-analysis. The pooled effect showed that EX + CR intervention significantly decreased CRP levels (P = 0.02), but had no effect on IL-6 (P = 0.62) and TNF-α (P = 0.11). Meta-regression analysis showed that the effect of EX + CR on CRP, IL-6, and TNF-α changes was correlated with lifestyle behavior of adults (Coef. = −0.380, P = 0.018; Coef. = −0.359, P = 0.031; Coef. = −0.424, P = 0.041, respectively), but not with age and BMI. The subgroup analysis results revealed that participants with sedentary lifestyle behavior did not respond to EX + CR intervention, as we found no changes in CRP, IL-6, and TNF-α concentrations (P = 0.84, P = 0.16, P = 0.92, respectively). However, EX + CR intervention significantly decreased CRP (P = 0.0003; SMD = −0.39; 95%CI: −0.60 to −0.18), IL-6 (P = 0.04; SMD = −0.21; 95%CI: −0.40 to −0.01) and TNF-α (P = 0.006; SMD = −0.40, 95%CI: −0.68 to −0.12) in adults without a sedentary lifestyle or with a normal lifestyle. Furthermore, the values between sedentary and normal lifestyle subgroups were statistically significant for CRP, IL-6, and TNF-α.
Conclusion: Our findings showed that combination EX + CR intervention effectively decreased CRP, IL-6, and TNF-α in overweight and obese adults with active lifestyles, but not with sedentary lifestyle behavior. We suggest that ‘lifestyle behavior’ is a considerable factor when designing new intervention programs for overweight or obese adults to improve their inflammatory response.
Introduction
According to the latest reports from the World Health Organization (WHO), worldwide prevalence of obesity has tripled since 1975. More than 1.9 billion adults (18 years or older) were overweight (39%) in 2016. Of these, more than 650 million adults (13%) were obese (WHO, 2021). The energy imbalance between calories consumption and calories expenditure is the fundamental cause of being overweight and obese. Increased intake of energy-dense foods (fat and sugar) over a period of time, and increased physical inactivity due to increased sedentary behavior are the primary contributors of being overweight and obese (Blüher, 2019; WHO, 2021). Weight gain has been associated with subclinical inflammation, which is mainly attributed to the secretion of various pro-inflammatory biomarkers (Saltiel and Olefsky, 2017). The inflammation caused by overnutrition or obesity is characterized by the activation of various immune cells, which release pro-inflammatory cytokines, such as tumor necrosis-alpha (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP). Studies have shown that increased production of these inflammatory biomarkers is closely associated with the prevalence of chronic diseases, such as type 2 diabetes (T2D), cardiovascular diseases (CVDs), chronic kidney disease, and cancer (Coussens and Werb, 2002; Hotamisligil, 2006). It is further stated that more than 50% of all deaths worldwide are directly or indirectly linked with the progression of inflammatory-related diseases (Furman et al., 2019).
Recent evidence identified a strong association between obesity and severity of the coronavirus disease-19 (COVID-19), the ongoing global pandemic (Albashir, 2020). The severity of COVID-19 is represented by an excessive production of IL-6 (Mehta et al., 2020), TNF-α (Mortaz et al., 2021), and CRP (Chen et al., 2020). The need of intensive care for obese COVID-19 patients was reportedly higher compared to patients with a normal weight (Lighter et al., 2020), and disease severity increased with body mass index (BMI) and existence of inflammatory diseases (Simonnet et al., 2020). A study demonstrated that CRP and BMI were significantly higher in critical COVID-19 patients. Among the non-survivors, 88.24% of patients reportedly had a higher BMI (>25 kg/m2) (Peng et al., 2020). Therefore, controlling the release of inflammatory biomarkers is crucial for prevention of chronic diseases and to enhance the treatment efficiently in COVID-19 patients. Since excessive calorie intake and adiposity cause systemic inflammation, calorie restriction (CR) without malnutrition is a potential strategy to treat the inflammation and inflammatory diseases (Kökten et al., 2021). On the other hand, any form of physical activity or exercise has been documented to promote weight loss and decrease chronic low-grade inflammation, which together can attenuate several complications caused by inflammation (Nimmo et al., 2013). There is an increasing interest on investigating the combined effect of exercise (EX) and CR on an inflammatory system (Tolkien et al., 2019;Zheng et al., 2019). CR intervention (4-week) has been shown to decrease bodyweight and fasting blood glucose and insulin levels of obese women. This beneficial effect of CR was accompanied by decreased systemic inflammation in obese women (Ott et al., 2017). A previous meta-analysis concluded that exercise can improve the inflammatory response in overweight and obese adults, in which aerobic exercise had a greater beneficial effect (Yu et al., 2017). Another recent meta-analysis on older adults demonstrated that exercise intervention decreased IL-6 and CRP levels, but not TNF-α. However, this study was unable to establish a dose-related response to exercise and chronic inflammation (Monteiro-Junior et al., 2018).
Owing to the independent positive effects of exercise and CR interventions on inflammatory biomarkers, researchers wondered whether a combination of EX plus CR would amplify the beneficial effects compared to CR alone. To address this, Fisher at al. studied the independent effect of energy restriction alone and also in combination with exercise on changes in inflammatory mediators in overweight premenopausal women. The results showed that weight loss was associated with a reduction of inflammatory mediators; however, combining exercise and energy restriction did not alter the response (Fisher et al., 2011). Contrarily, another study reported a greater reduction of subcutaneous fat (not intra-abdominal fat) with a combination of EX plus CR compared to CR alone in postmenopausal women (van Gemert et al., 2019). Another study on obese sedentary adults reported no significant additional benefit of exercise on inflammatory response in a CR plus exercise intervention group (Trussardi Fayh et al., 2013). Compared with CR alone, combination of EX plus CR intervention exerts only a minor influence on adipocytokines and inflammatory cytokines in postmenopausal women (Giannopoulou et al., 2005). A recent meta-analysis concluded that a combination of EX plus CR largely decreased the inflammatory cytokines and CRP than CR alone in overweight and obese adults (Khalafi et al., 2021).
These controversial results of research studies and meta-analyses may be due to the differences in participants’ characteristics and/or intervention protocol. From the perspective of participants’ characteristics, no meta-analysis has yet analyzed the effect of EX plus CR intervention on inflammatory biomarkers in overweight and obese adults. It is therefore necessary to pool the data from EX plus CR interventions and examine the influence of participant’s characteristics on improving the inflammatory cytokines. In this study, we conducted a systematic review and meta-analysis of trials and explored the effect of EX plus CR on the alterations of CRP, IL-6, and TNF-α in overweight and obese adults. We further examined the association between characteristics of participants (age, BMI, and lifestyle behavior) and inflammatory biomarkers to identify the influential and effective variable that decreases inflammation.
Methods
Search Strategy
A thorough article search was conducted primarily using PubMed, Web of Science, EMBASE, the Cochrane database, Scopus, and Google Scholar. Specific keywords, including “exercise” OR “physical activity” OR “training” OR “aerobic exercise” OR “resistance exercise” OR “strength training” AND “caloric restriction” OR “restricted diet” OR “low calorie diet” OR “weight loss” were independently used with “inflammation” OR “interleukin-6” OR “tumor necrosis factor-α” OR “C-reactive protein” to search for articles. Furthermore, a manual search from the reference list of the included articles was conducted to identify relevant articles. Studies published until April 2021 were systematically searched by a team of authors (YL, FH, and YZ), and the influence of calorie restriction with exercise on inflammatory biomarkers was analyzed in overweight and obese adults with different lifestyle behavior.
Inclusion and Exclusion Criteria
The article review and selection process was strictly followed according to the updated guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) as described before (Moher et al., 2009; Page et al., 2021a, b). The detailed selection process with the number of articles in each step is depicted as a flowchart in Figure 1.
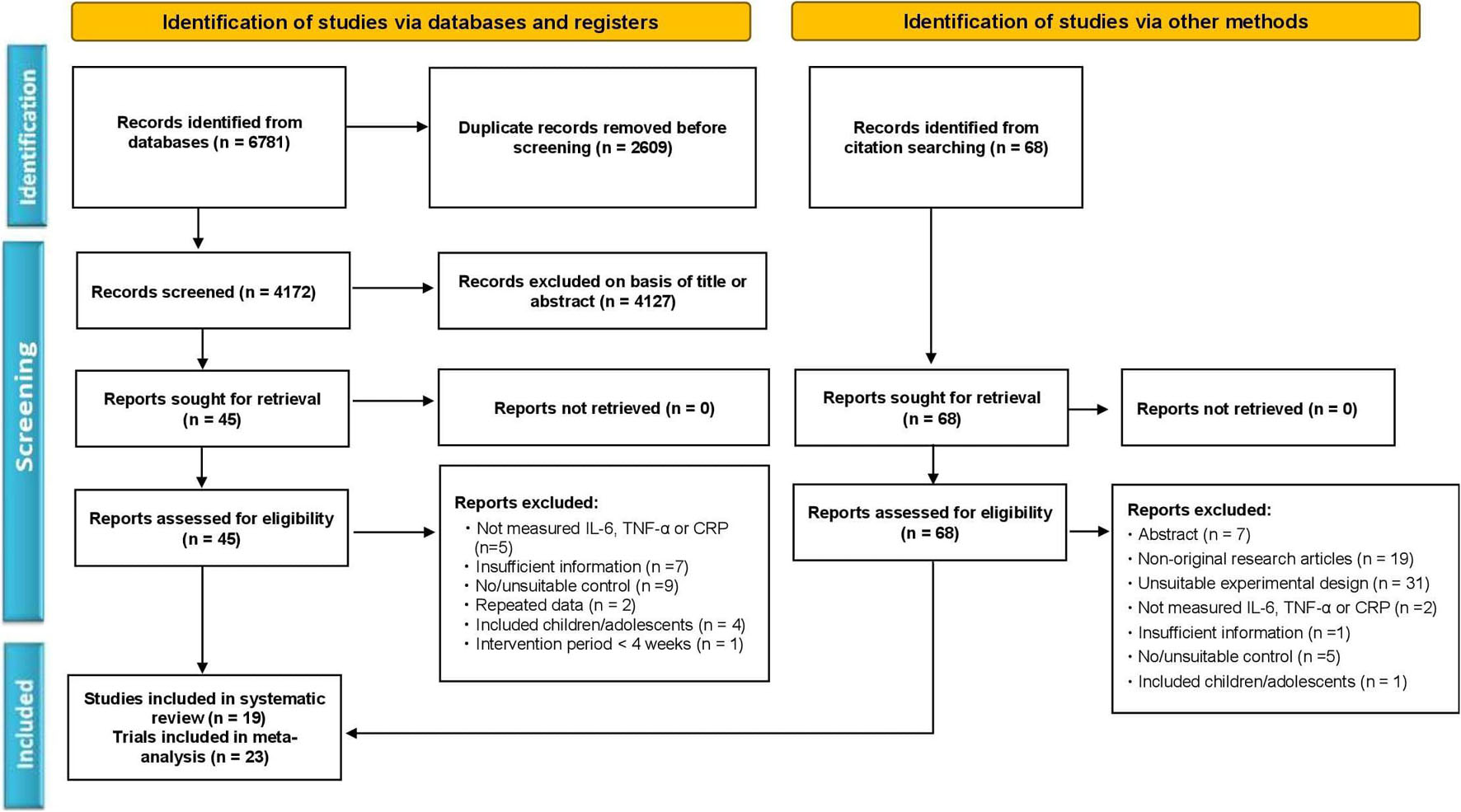
Figure 1. Flowchart of the study selection according to the Preferred Reporting Items for the Systematic Reviews and Meta-Analysis (PRISMA) method.
According to the selection criteria, a team of authors (YL, FH, and YZ) independently reviewed and assessed the relevant articles. Any differences in opinion among the authors on inclusion or exclusion of articles were solved by discussion with the corresponding author (MK). The inclusion criteria were: (1) All participants in the trials were overweight and obese adults; (2) the intervention group adopted calorie restriction with any type of therapeutic exercise (aerobic, resistance, mobility exercises, etc.), whilst the control group only underwent calorie restriction; (3) trials compared the effect of exercise and/or calorie restriction with respective controls; (4) trials reported inflammatory biomarkers (IL-6, TNF-α, or CRP) data as outcome measures after intervention, and (5) all studies were published in English as full-text articles. The exclusion criteria were: (1) Studies involving minors (<18 years) or animals; (2) articles with repeated data; (3) non-original research articles, such as protocols, case reports, meta-analyses, and systemic reviews, and (4) articles with inadequate information about the characteristics of the participants or intervention.
Outcome Measures and Data Extraction
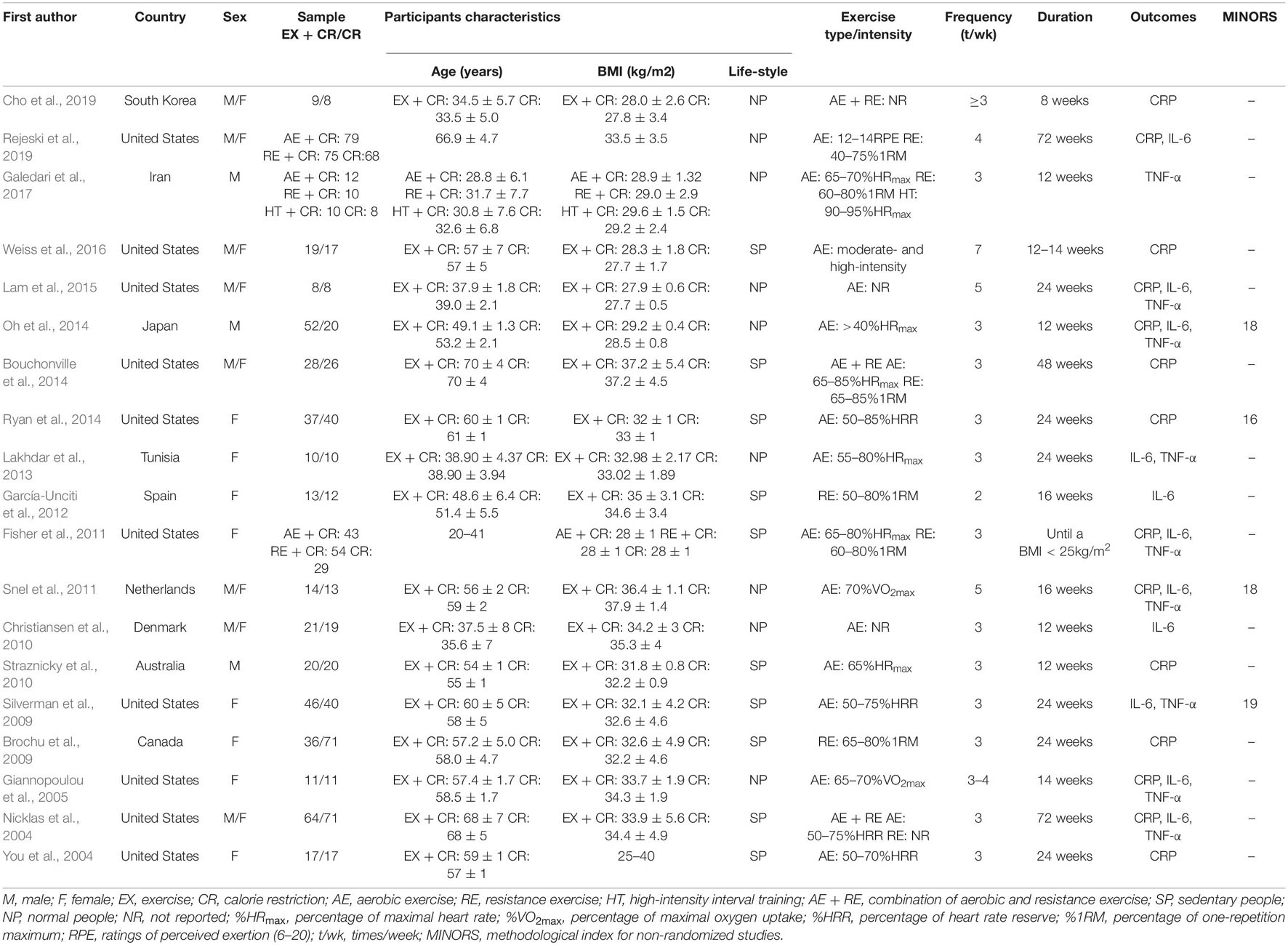
Two review authors, YL and FH, independently extracted the study characteristics, and the same was verified by another review author, MK. Three authors (VRL, AM, and LJ) provided in-depth analyses of the results and coordinated data extraction and interpretation. The information extracted from each study included values of inflammatory biomarkers (CRP, IL-6, and TNF-α), characteristics of participants (number, sex, baseline age, and baseline BMI), baseline lifestyle (sedentary or normal lifestyle), and details of intervention protocol (type of exercise, intensity, frequency, duration). Publication details, including name of authors, year of publication, and study conducted region were also extracted, and are described in Table 1.
Quality Assessment
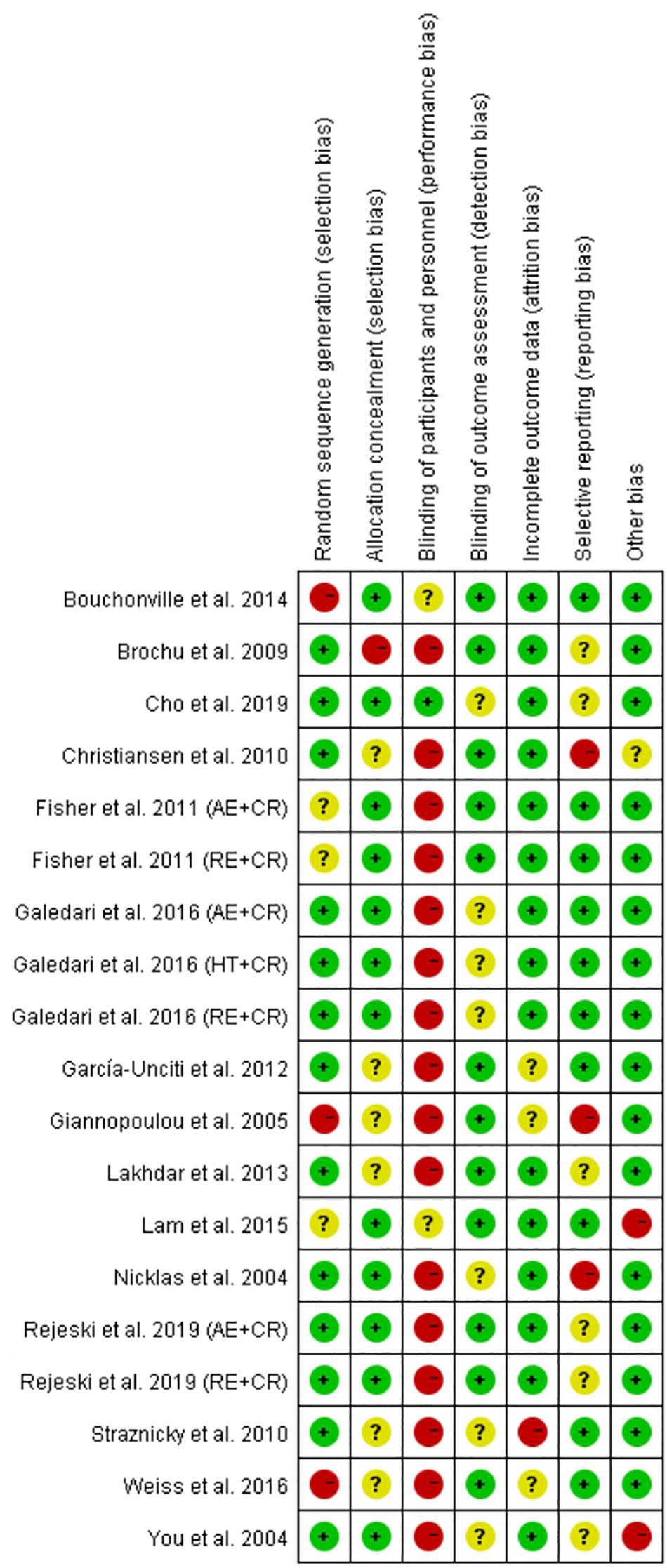
The quality of the included randomized controlled trials (RCTs) was assessed using the Cochrane risk of bias tool (Higgins et al., 2011). The Cochrane risk of bias assessment tool consists of seven domains, namely random sequence generation/allocation concealment (selection bias), blinding of participants/personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. The quality of each domain was rated as “low risk,” “high risk,” or “unclear,” and were indicated with green (+), red (−), and yellow (?) colors and symbols, respectively. The quality of trials was assessed by two authors (YL and FH), and discrepancies were resolved through discussion with a third reviewer (MK) to reach a consensus. The quality of the non-randomized controlled trials (non-RCTs) was assessed with the Methodological Index for Non-randomized Studies (MINORS) entry, and non-RCTs with MINORS scores > 12 were included in the study (Slim et al., 2003).
Statistical Analysis
In this study, Cochrane Collaboration’s Review Manager (RevMan 5.3., Copenhagen, Denmark) was employed to analyze the effect of intervention (EX + CR) and participants characteristics on inflammatory biomarkers (IL-6, TNF-α, and CRP) of overweight and obese adults. The outcome measures were summarized through standardized mean difference (SMD) and 95% confidence intervals (95% CI). Inter-study heterogeneity was tested using I2 statistic, when I2 ≥ 50%, this means strong heterogeneity. Then meta-regression analysis followed by subgroup analysis were performed to assess the sources of heterogeneity, and to explore the effective characteristics that promote the benefits of intervention. We used STATA version 12 (StataCorp., College Station, TX, United States) to run the meta-regression analysis, and examined the relationships between effect size estimates and the following covariates: (1) baseline age, (2) baseline BMI, and (3) baseline lifestyle behavior. We found baseline lifestyle behavior (sedentary or normal lifestyle) was correlated with the changes of inflammatory mediators. Sedentary behavior refers to any walking behavior that is characterized by energy expenditure ≤1.5 metabolic equivalents (METs), while in a sitting, reclining, or lying posture (Tremblay et al., 2017; Thivel et al., 2018). The included trials were subgrouped based on the lifestyle behavior (sedentary or normal) of adults, and the effective lifestyle behavior in response to intervention was determined. We then assessed the differences between subgroups using the test for heterogeneity in Review Manager version 5.3. The Egger’s test was conducted to examine potential publication bias. The level of statistical significance was set at P < 0.05.
Results
Search Results and Selection of Studies
We initially obtained a total of 6,849 articles, including 6781 from the electronic databases (PubMed, Web of Science, Scopus, EMBASE, SportDiscus, ScienceDirect, and Google Scholar) search, and 68 from the manual search. After removing duplicates (2,677), the remaining 4,172 articles were carefully screened by title and abstract, and a further 4,116 were excluded because of unsuitability. Then the full text of the 56 articles was thoroughly reviewed, and 37 of them were excluded due to the following reasons; not measuring IL-6, TNF-α, or CRP levels, insufficient information, no/unsuitable control, repeated data, included children or adolescents, and intervention period less than 4 weeks. Finally, 19 articles consisting of 23 trials met the inclusion criteria, and were included in the systematic review and meta-analysis. The informative flowchart (PRISMA) of article search, screening, and selection is presented in Figure 1.
Summary of the Included Studies
According to the inclusion and exclusion criteria, a total of 19 articles published between 2004 and 2019 were included for the systematic review, meta-analysis, and meta-regression analysis. These studies were from various parts of the world, most were from the United States (10), with one each from Australia, Canada, Denmark, Iran, Japan, Korea, Netherlands, Spain, and Tunisia. The total sample size was 1196 participants, consisting of 688 adults from the intervention group and 508 adults from the control group. The mean age of participants in the trials ranged from 18 to 75 years. The types of exercise intervention included aerobic (You et al., 2004; Giannopoulou et al., 2005; Silverman et al., 2009; Christiansen et al., 2010; Straznicky et al., 2010; Fisher et al., 2011; Snel et al., 2011; Lakhdar et al., 2013; Oh et al., 2014; Ryan et al., 2014; Lam et al., 2015; Weiss et al., 2016; Galedari et al., 2017; Rejeski et al., 2019), resistance (Brochu et al., 2009; Fisher et al., 2011; García-Unciti et al., 2012; Galedari et al., 2017; Rejeski et al., 2019), a combination of both aerobic and resistance (Nicklas et al., 2004; Bouchonville et al., 2014; Cho et al., 2019), and high-intensity interval training (Galedari et al., 2017). The exercise intervention duration ranged from 8 to 72 weeks with a frequency of 3 to 7 times per week. The characteristics of patients (sex, age, BMI, and life style behavior) and exercise intervention (type, intensity, frequency, and duration) are presented in Table 1.
Exercise Plus Calorie Restriction Decreases C-Reactive Protein Levels in Overweight and Obese Adults
Among the included trials (n = 23), 16 trials (981 participants) reported the effect of exercise plus CR intervention on alterations in CRP levels and compared the changes with a CR trial alone. The pooled outcome of meta-analysis revealed that exercise plus CR intervention significantly (P = 0.02) decreased CRP in overweight and obese adults. The standardized mean difference (SMD) of decreased CRP with EX + CR intervention was −0.16 with 95% CI: −0.29 to −0.03 (Figure 2 and Supplementary Figure 1). These findings revealed that overweight or obese adults required a combination of exercise and CR to decrease their CRP levels.
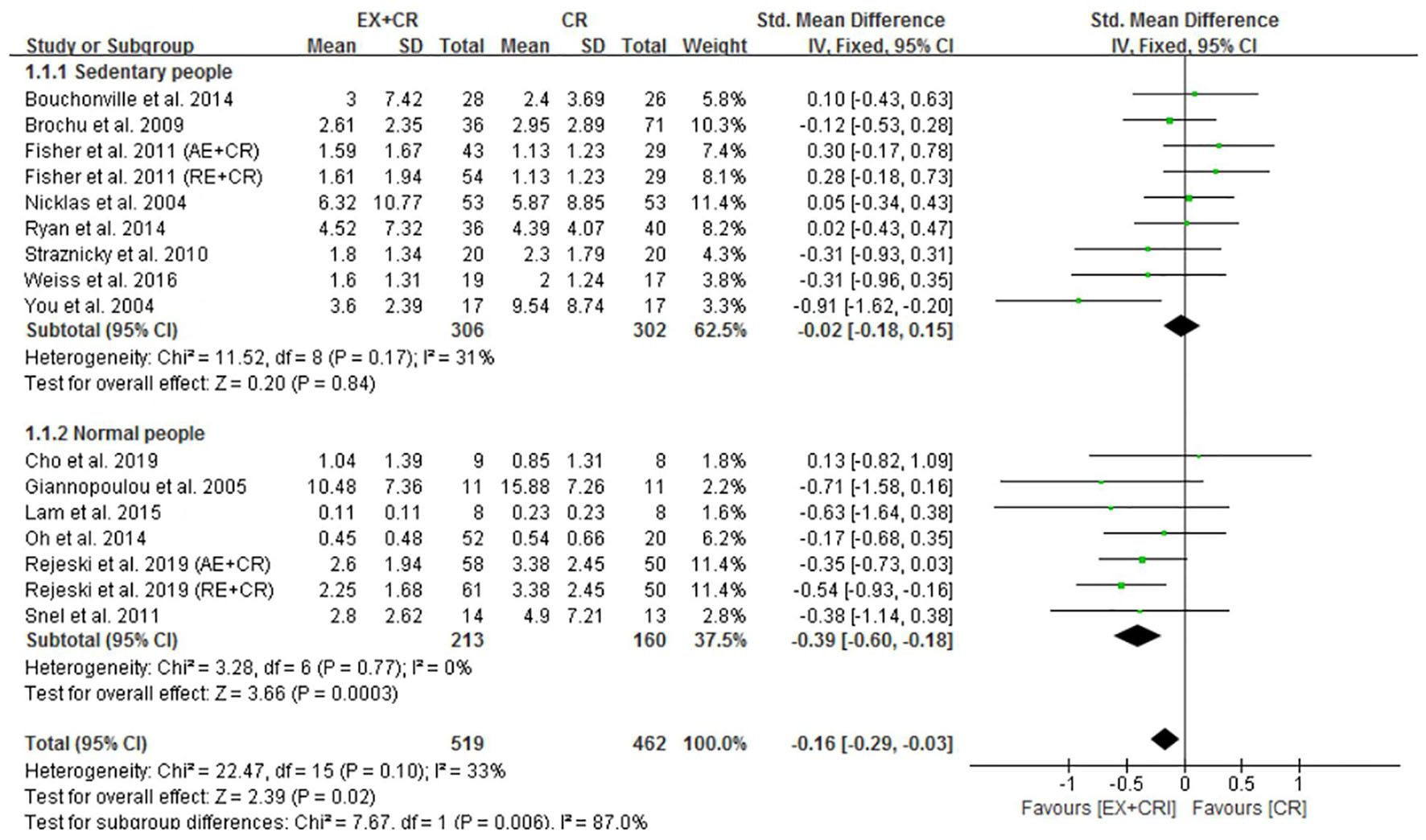
Figure 2. Forest plot of the effects of exercise plus calorie restriction (EX + CR) intervention on C-reactive protein (CRP) changes in overweight and obese adults with different (subgroup analysis) lifestyle behaviors.
Exercise Plus Calorie Restriction Does Not Affect IL-6 and TNF-α in Overweight and Obese Adults
The changes in IL-6 concentrations with EX + CR intervention were reported in 13 trials, and the differences were compared with CR trials. Results of the pooled outcome showed that an EX + CR intervention for a period of 12–72 weeks did not influence IL-6 concentrations in overweight and obese adults (P = 0.62; SMD = −0.04; 95% CI: −0.18 to 0.11) (Figure 3 and Supplementary Figure 2). Besides, the effect of exercise plus CR on TNF-α concentration was evaluated in 12 trials of 560 overweight and obese adults. Similar to the IL-6 response, EX + CR-mediated changes in TNF-α were also not statistically significant (P = 0.11; SMD = −0.14; 95% CI: −0.31 to 0.03). Although TNF-α tended to decrease with EX + CR, such a change was not favorable to experimental intervention (Figure 4 and Supplementary Figure 3).
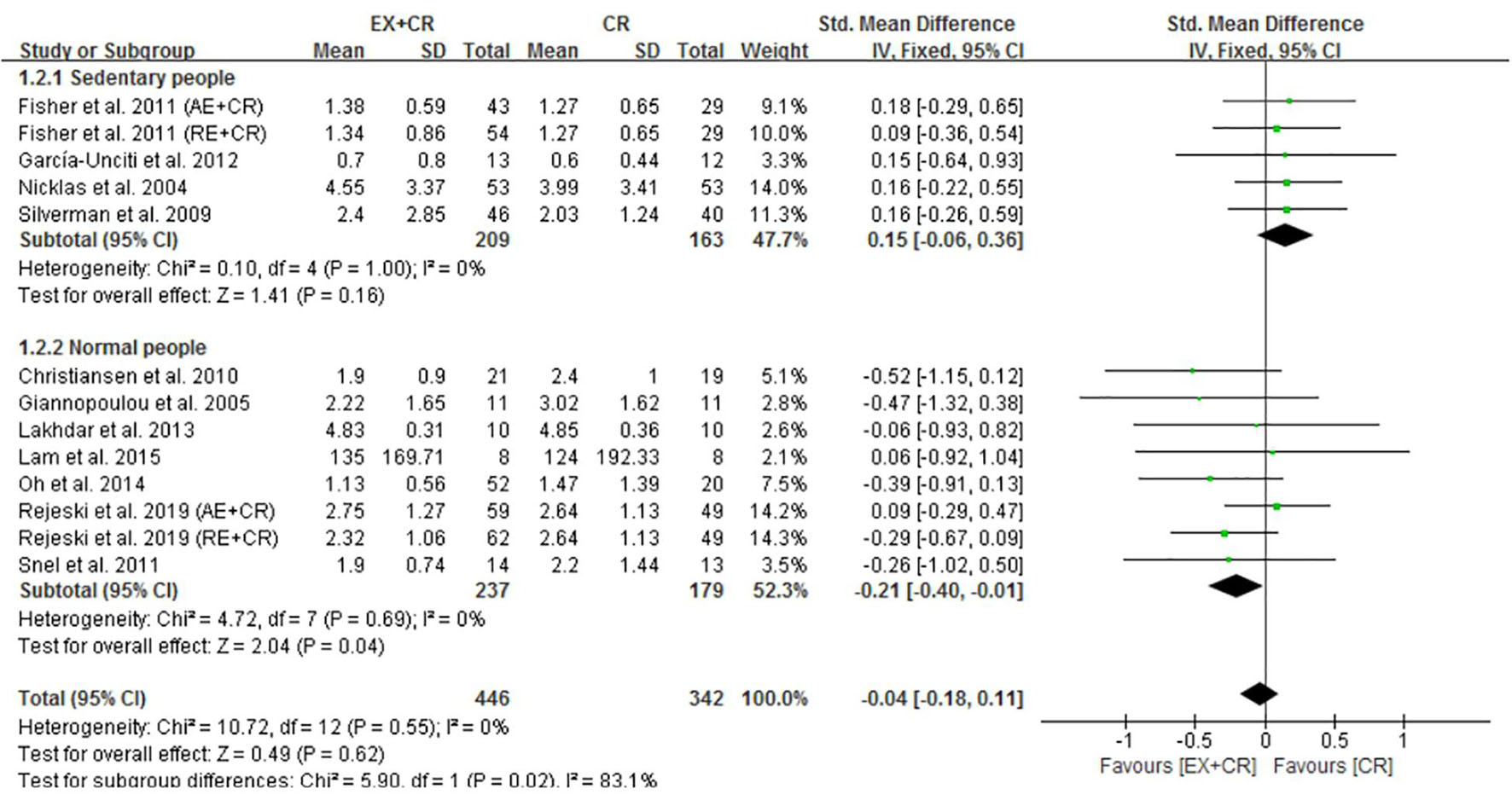
Figure 3. Forest plot of the effects of exercise plus calorie restriction (EX + CR) intervention on IL-6 changes in overweight and obese adults with different (subgroup analysis) lifestyle behaviors.
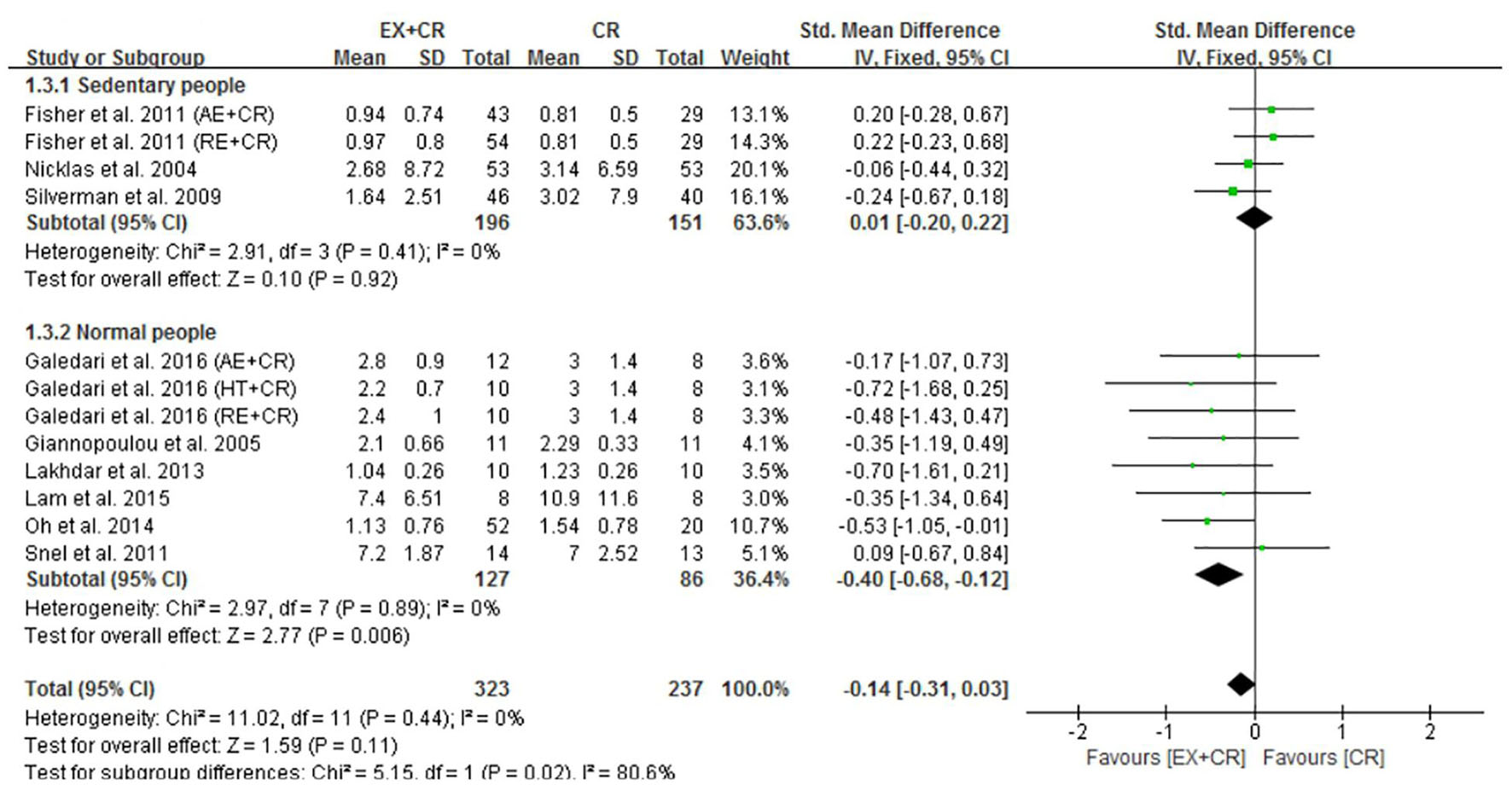
Figure 4. Forest plot of the effects of exercise plus calorie restriction (EX + CR) intervention on TNF-α changes in overweight and obese adults with different (subgroup analysis) lifestyle behaviors.
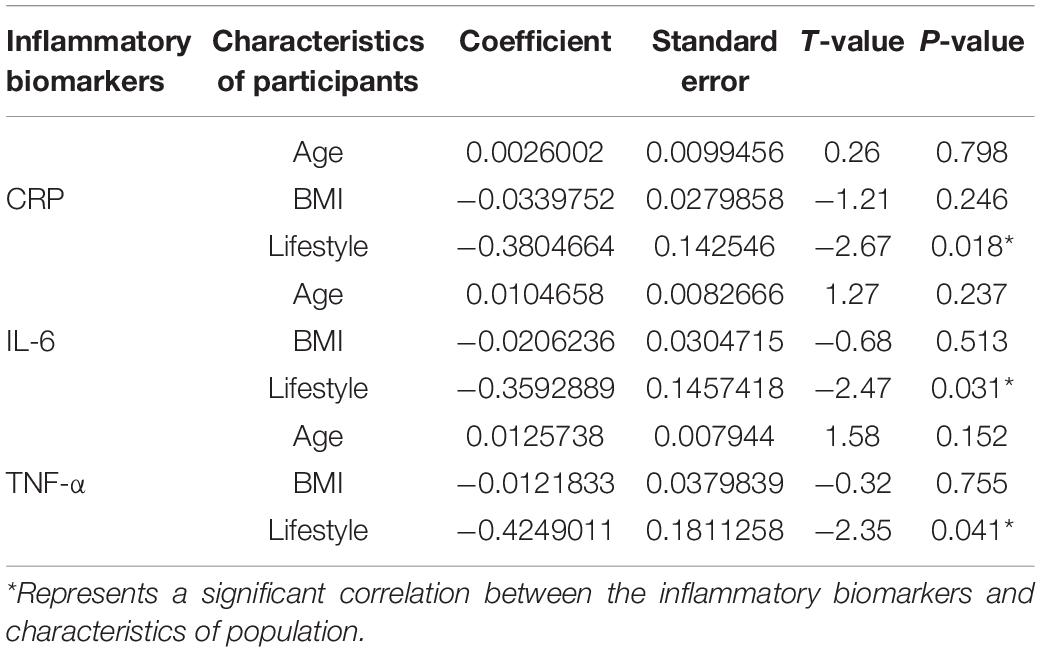
Meta-Regression Analysis: Correlations Between Participants’ Characteristics and Inflammatory Biomarkers
Meta-regression analysis was conducted to identify the correlations between characteristics of participants (age, BMI, and lifestyle behavior) and inflammatory biomarkers (CRP, IL-6, and TNF-α). One of the key findings in our analysis is that ‘lifestyle behavior’ of participants was significantly correlated with the changes of CRP (Coef. = −0.380; P = 0.018), IL-6 (Coef. = −0.359; P = 0.031), and TNF-α (Coef. = −0.424; P = 0.041) after exercise plus CR intervention. In contrast, age and BMI of participants were not correlated with the changes of any inflammatory biomarkers (Table 2). These results indicate that lifestyle behavior, either sedentary or normal, is the key variable that is involved in improving the inflammatory mediators following EX + CR intervention.
Subgroup Analysis Results
Normal Lifestyle With EX + CR Intervention Decreases C-Reactive Protein, Not Sedentary Lifestyle
Subgroup analysis was performed to identify the effective lifestyle (sedentary or normal) behavior of adults that could influence the beneficial effects of EX + CR intervention on inflammatory mediators. As shown in Figure 2, participants (213 from 7 trials) without sedentary behavior (normal lifestyle) showed greater beneficial effects of EX + CR intervention. This was evidenced by a substantial decrease of CRP levels (P = 0.0003; SMD = −0.39; 95%CI: −0.60 to −0.18) in normal-lifestyle adults after EX + CR intervention. However, CRP levels in participants with sedentary lifestyle behavior did not respond to EX + CR intervention. The heterogeneity of the subgroup analysis explained that the intervention effect of EX + CR on CRP levels was influenced by lifestyle behavior of adults (I2in–subgroup < 50%; I2between–subgroups = 87%) (Figure 2).
EX + CR Intervention Decreases IL-6 and TNF-α in Adults Without Sedentary Lifestyle
It is worth pointing out that EX + CR intervention had no effect on IL-6 in overweight and obese adults. Nevertheless, when trials separated participants based on lifestyle behavior, the IL-6 concentrations were significantly decreased in normal-lifestyle participants (237 from 8 trials) after EX + CR intervention (P = 0.04, SMD = −0.21; 95%CI: −0.40 to −0.01). Contrary to this, participants (209 from 5 trials) with sedentary lifestyle behavior did not show any positive response to EX + CR intervention (Figure 3). The heterogeneity (I2 = 83.1%) between the subgroups was strong, while the heterogeneity (I2 = 0%) within the subgroups was negligible, which reveals that the results of this study were relatively reliable (Figure 3).
Next, we found that a combination of exercise and CR intervention remarkably decreased TNF-α concentrations in participants with normal lifestyle behavior (P = 0.006; SMD = −0.40, 95%CI: −0.68 to −0.12), but not in adults with sedentary lifestyle (Figure 4). We further identified that there was no significant heterogeneity within the subgroups (I2 = 0%), but there was a significant heterogeneity between the subgroups (I2 = 80.6%), indicating the influential role of lifestyle behavior of participants on improving TNF-α (Figure 4).
Summary of Risk of Bias
Risk of bias in the included studies (19) was assessed according to the Cochrane risk of bias tool, and the judgment is presented in Figure 5. For the selection bias, more studies (13 trials) reported a low risk of random sequence generation, and 12 trials were judged to have a low risk of allocation concealment. Physical exercise is the main intervention among all the trials, and therefore it may not be feasible to adopt the blind method. As a result, several studies were judged as having a high risk of performance bias (16 trials). However, reporting of such a high risk of performance bias does not compromise the quality of the study (Hong et al., 2019; Liu et al., 2019). No studies were judged to have a detection bias. We identified one trial with an attrition bias, three trials with reporting bias, and two trials with other bias. The quality of the four non-RCT studies was evaluated using the MINORS evaluation criteria. One trial scored 19 points, two trials scored 18 points, and one trial scored 16 points.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to compare the combination effect of exercise and CR with CR alone on inflammatory biomarkers in obese and overweight adults. We included 23 trials, which reported the changes in CRP, IL-6, and TNF-α concentrations with response to EX + CR and CR alone treatments. The pooled outcome results showed that combination of EX + CR intervention significantly decreased CRP levels in overweight and obese adults, while IL-6 and TNF-α did not respond to EX + CR intervention. Meta-regression analyses revealed that the changes in CRP, IL-6, and TNF-α were correlated with lifestyle behavior, but not correlated with age and BMI of participants. Subgroup analysis results further explored that normal lifestyle with EX + CR intervention effectively decreased CRP, IL-6, and TNF-α concentrations. However, baseline sedentary lifestyle behavior with EX + CR intervention could not decrease the inflammatory mediators in overweight and obese adults. These findings imply that participants’ lifestyle behavior, especially active lifestyle is important to achieve the beneficial effects of EX + CR intervention on improving the inflammatory biomarkers in overweight and obese adults.
IL-6 and TNF-α are the two important pro-inflammatory cytokines that mediate the process of inflammation and healing. However, excessive production of IL-6 and TNF-α over a period of time causes systemic inflammation, which is eventually involved in developing metabolic diseases, including CVDs, diabetes, various cancer types, chronic kidney disease, non-alcoholic fatty liver disease (NAFLD), and neurodegenerative conditions (Furman et al., 2019). Both cytokines, IL-6 and TNF-α, are responsible for the production and release of CRP, an acute phase protein that induces the inflammatory stage (Popko et al., 2010). Elevated levels of IL-6 and CRP are strongly associated with development of type 2 diabetes in healthy middle-aged women (Pradhan et al., 2001). Several lifestyle factors, including physical inactivity, poor diet, nighttime blue light exposure, smoking, and psychological stress are reported to promote systemic inflammation. Therefore, lifestyle modifications may reduce inflammatory proteins and improve metabolic health (Furman et al., 2019; Kökten et al., 2021). Studies have targeted these modifiable risk factors to decrease the inflammatory response, however, the corresponding reduction of inflammatory biomarkers is limited due to various reasons. Here, we addressed the influential role of lifestyle behavior on improving CRP, IL-6, and TNF-α with exercise plus CR intervention.
Exercise and dietary intake are important lifestyle interventions that are reported to orchestrate inflammatory biomarkers independently or together. A study with a very low calorie diet (CR) for 4 weeks has been shown to reduce inflammatory biomarkers in obese women, and this beneficial effect of CR was accompanied by a significant weight loss (Ott et al., 2017). In a randomized clinical trial, a modified alternate-day fasting diet was reported to be more effective than CR on weight loss and reduction of CRP levels in patients with metabolic syndrome. Despite this, the changes in IL-6 and TNF-α were not statistically different between the diets (Razavi et al., 2021). On the other hand, a meta-analysis reported positive effects of aerobic exercise intervention on reduction of CRP, IL-6, and TNF-α in middle-aged and older adults, but not on IL-4 (Zheng et al., 2019). The anti-inflammatory effect of exercise may be attributed to a decrease of adipose tissue; however, its independent effect on inflammation has not yet been elucidated. Evidence suggests that exercise can directly affect the immune cells by regulating systemic inflammatory mediators without relying on the loss of bodyweight (Gleeson et al., 2011). The anti-inflammatory effect of exercise is independent of weight loss and it can inhibit pro-inflammatory mediators, stimulate anti-inflammatory pathways, and thereby regulate insulin sensitivity. Nevertheless, it is not clear whether a combination of exercise and CR has a greater beneficial effect than that of CR alone on inflammatory cytokines in obese adults.
One of the key findings of our meta-analysis is that exercise plus CR has greater beneficial effects than CR alone in decreasing inflammatory mediators when participants are subgrouped based on their lifestyle behavior. Lifestyle modification with the Mediterranean diet (CR) and exercise intervention for 2 years induced significant weight loss and reduced CRP, IL-6, and TNF-α in healthy obese women. Nevertheless, the deteriorated adipokine profile was not improved with combination of CR and exercise intervention (Gomez-Huelgas et al., 2019). In a recent meta-analysis, exercise plus CR intervention significantly decreased IL-6 and TNF-α levels and marginally decreased CRP in overweight and obese adults (Khalafi et al., 2021). In contrast to our findings, subgroup analysis for BMI showed a significant decrease of CRP and TNF-α with higher BMI values, but not IL-6 (Khalafi et al., 2021). We performed meta-regression analysis for participants’ characteristics (age, BMI, and lifestyle), and found that lifestyle behavior is significantly correlated with decreased CRP, IL-6, and TNF-α after exercise plus CR intervention. BMI and age variables were not correlated with decreased inflammatory biomarkers in overweight and obese adults. Here, our findings emphasize the importance of lifestyle behavior in enhancing the beneficial effects of exercise plus CR intervention. The differences with previous findings may be due to the differences in population characteristics. Our study included only overweight or obese adults, and children below 18 years were excluded. Therefore, our conclusions are convincing to construct an intervention program for overweight or obese adults to improve their inflammatory response.
The mechanism of improving inflammatory markers by exercise intervention is not completely clear, which may be achieved by improving the hypoxia state of adipose tissue (Dumitriu et al., 2007), promoting the phenotype transformation of macrophages in adipose tissue (Kawanishi et al., 2010), and regulating the function of peripheral blood cells (Yeh et al., 2009). Although changes in body weight and composition are not necessarily factors that affect inflammatory markers, changes in body composition (muscle and fat content) caused by exercise may indirectly affect the inflammatory response of an individual (Beasley et al., 2009). A meta-analysis compared the effects of exercise and CR on body weight and composition, and found that CR has a larger impact on total body weight loss, but exercise has a superior effect in reducing visceral adipose tissue (VAT) (Verheggen et al., 2016), which is an important source of pro-inflammatory factors, such as IL-6 and TNF-α. In addition, skeletal muscle is the key source of inflammatory mediators involved in systemic inflammation. A meta-analysis of 13 articles showed that resistance exercise-induced increased muscle mass contributes to control of inflammatory biomarkers in older adults (Sardeli et al., 2018). The positive effect of exercise on muscle mass is not limited to resistance exercise or high-intensity exercise. In a study, overweight to obese adults were randomly assigned into diet-induced weight loss alone or diet-induced weight loss combined with exercise intervention groups for 4 months. The results showed that diet-induced weight loss (intentional CR) significantly decreased skeletal muscle mass in overweight to obese adults. Interestingly, moderate aerobic exercise combined with an intentional weight loss trial attenuated the loss of skeletal muscle mass (Chomentowski et al., 2009).
Although exercise and CR are important strategies to curb the level of inflammation, paying attention to the different characteristics of participants is the primary concern to achieve the goal. This study emphasizes that individual lifestyle behavior (sedentary or non-sedentary) is the key factor to achieve an intervention effect. Sedentary behavior refers to any walking behavior characterized by energy expenditure ≤ 1.5 metabolic equivalents (METs), while in a sitting, reclining, or lying posture (Tremblay et al., 2017; Thivel et al., 2018). To the best of our knowledge, this is the first meta-analysis and subgroup analysis to evaluate the combination effect of EX + CR on inflammatory mediators in overweight and obese adults with sedentary behavior. We found that exercise plus CR intervention could not improve the CRP, IL-6, and TNF-α response in overweight and obese adults with long-term sedentary behavior. An existing meta-analysis mainly revealed the combination effect of EX + CR on inflammatory mediators from the aspects of intervention duration, exercise type, and BMI, but did not disclose the influence of sedentary behavior of participants on the effectiveness of the intervention (Khalafi et al., 2021).
It is well known that sedentary lifestyle behavior is the main cause of obesity, and both obesity and sedentary behavior are strongly associated with developing inflammatory-related diseases (Park et al., 2020). Apart from the fact that obesity can cause chronic inflammation, sedentary behavior itself also contributes to chronic inflammation and inflammatory diseases in the course of life (Burini et al., 2020). Adults who have sedentary behavior for a long time may have impaired metabolic health and cannot benefit from exercise or other interventions. Previous studies have reported that long-term sedentary behavior can lead to inflammation of subcutaneous adipose tissue, and negative health consequences, including type 2 diabetes, obesity, hypertension, and CVDs (Grøntved and Hu, 2011; Biswas et al., 2015). A multi-ethnic cross-sectional study with a large sample has shown that sedentary behavior is associated with high levels of TNF-α and leptin and low adiponectin-to-leptin ratios. The degree of these associations does not vary with ethnic groups, and is independent of related co-variables (including moderate to high intensity exercise) (Allison et al., 2012). Moreover, exercise combined with nutrient intake or new exercise methods seems to improve the inflammatory response. A study on sedentary men showed that high intensity exercise plus honey intake significantly decreased IL-6 concentrations, but had no effect on other inflammatory mediators (Bakhtyar et al., 2018). Another study on sedentary middle-aged men reported that cycling training decreased only CRP levels, while small-sided games decreased both CRP and IL-6 concentrations and increased muscle mass (Mendham et al., 2014). Therefore, it is suggested that we should further investigate the effect of exercise combined with nutrient intake or a new exercise protocol on inflammatory factors.
Limitations
The intervention duration of the included trials ranged between 8 and 72 weeks. Although the duration range appears to be wide, this may not influence the final outcome of our study. Meta-regression analysis results also showed no significant correlation between intervention duration and inflammatory mediators. In addition, our study did not provide the statistical evidence to demonstrate the influence of exercise type, intensity, or frequency on inflammatory biomarkers. We included trials that performed any type of exercise, and therefore it is inconclusive which exercise type has greater beneficial effects in combination with CR. Further studies with meta-regression analysis are necessary to identify the influential role of exercise variables on changes in inflammatory mediators in overweight and obese adults.
Conclusion
Our findings demonstrated that a combination of exercise with calorie restriction could improve the CRP, IL-6, and TNF-α response in overweight and obese adults with normal lifestyle behavior, but not in adults with sedentary behavior. Therefore, lifestyle behavior is the key variable that influences the beneficial effects of exercise plus CR intervention on inflammatory biomarkers. Whenever designing interventional programs for obese or overweight adults, ‘lifestyle behavior’ should be considered as an important characteristic of adults to achieve the goal of intervention on the inflammatory system.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
YL, FH, and MK designed the study. YL, FH, and YZ performed the article search and screening. YL and FH performed statistical analyses and drafted the manuscript. VL, AM, and LJ assisted in interpretation of data and provided additional suggestions. YL, FH, YZ, and MK reviewed the full-text articles and extracted the data. YZ and MK revised and finalized the manuscript. All authors read and approved the submission.
Funding
This study was partially supported by a grant (no. jykf20069) from the Open Research Fund of College of Teacher Education, Zhejiang Normal University, Zhejiang, China.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.754731/full#supplementary-material
References
Albashir, A. A. D. (2020). The potential impacts of obesity on COVID-19. Clin. Med. (Lond) 20, e109–e113. doi: 10.7861/clinmed.2020-0239
Allison, M. A., Jensky, N. E., Marshall, S. J., Bertoni, A. G., and Cushman, M. (2012). Sedentary behavior and adiposity-associated inflammation: the multi-ethnic study of atherosclerosis. Am. J. Prev. Med. 42, 8–13. doi: 10.1016/j.amepre.2011.09.023
Bakhtyar, T., Naser, A., and Khayat, S. M. A. (2018). IL-6 and IL-12 levels in response to honey supplementation combined with HIIT training in sedentary individuals. Iran. J. Allergy Asthma Immunol. 17:232.
Beasley, L. E., Koster, A., Newman, A. B., Javaid, M. K., Ferrucci, L., Kritchevsky, S. B., et al. (2009). Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring) 17, 1062–1069. doi: 10.1038/oby.2008.627
Biswas, A., Oh, P. I., Faulkner, G. E., Bajaj, R. R., Silver, M. A., Mitchell, M. S., et al. (2015). Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults. Ann. Intern. Med. 162, 123–132. doi: 10.7326/M14-1651
Blüher, M. (2019). Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298. doi: 10.1038/s41574-019-0176-8
Bouchonville, M., Armamento-Villareal, R., Shah, K., Napoli, N., Sinacore, D. R., Qualls, C., et al. (2014). Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int. J. Obes. (Lond) 38, 423–431. doi: 10.1038/ijo.2013.122
Brochu, M., Malita, M. F., Messier, V., Doucet, E., Strychar, I., Lavoie, J., et al. (2009). Resistance training does not contribute to improving the metabolic profile after a 6-month weight loss program in overweight and obese postmenopausal women. J. Clin. Endocrinol. Metab. 94, 3226–3233. doi: 10.1210/jc.2008-2706
Burini, R. C., Anderson, E., Durstine, J. L., and Carson, J. A. (2020). Inflammation, physical activity, and chronic disease: an evolutionary perspective. Sports Med. Health Sci. 2, 1–6. doi: 10.1016/j.smhs.2020.03.004
Chen, W., Zheng, K. I., Liu, S., Yan, Z., Xu, C., and Qiao, Z. (2020). Plasma CRP level is positively associated with the severity of COVID-19. Ann. Clin. Microbiol. Antimicrob. 19:18. doi: 10.1186/s12941-020-00362-2
Cho, A. R., Moon, J.-Y., Kim, S., An, K.-Y., Oh, M., Jeon, J. Y., et al. (2019). Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: a pilot randomized controlled trial. Metabolism 93, 52–60. doi: 10.1016/j.metabol.2019.01.002
Chomentowski, P., Dubé, J. J., Amati, F., Stefanovic-Racic, M., Zhu, S., Toledo, F. G. S., et al. (2009). Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. J. Gerontol. A Biol. Sci. Med. Sci. 64, 575–580. doi: 10.1093/gerona/glp007
Christiansen, T., Paulsen, S. K., Bruun, J. M., Pedersen, S. B., and Richelsen, B. (2010). Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. Am. J. Physiol. Endocrinol. Metab. 298, E824–E831. doi: 10.1152/ajpendo.00574.2009
Coussens, L. M., and Werb, Z. (2002). Inflammation and cancer. Nature 420, 860–867. doi: 10.1038/nature01322
Dumitriu, I. E., Bianchi, M. E., Bacci, M., Manfredi, A. A., and Rovere-Querini, P. (2007). The secretion of HMGB1 is required for the migration of maturing dendritic cells. J. Leukoc. Biol. 81, 84–91. doi: 10.1189/jlb.0306171
Fisher, G., Hyatt, T. C., Hunter, G. R., Oster, R. A., Desmond, R. A., and Gower, B. A. (2011). Effect of diet with and without exercise training on markers of inflammation and fat distribution in overweight women. Obesity (Silver Spring) 19, 1131–1136. doi: 10.1038/oby.2010.310
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832. doi: 10.1038/s41591-019-0675-0
Galedari, M., Azarbayjani, M. A., and Peeri, M. (2017). Effects of type of exercise along with caloric restriction on plasma apelin 36 and HOMA-IR in overweight men. Sci. Sport 32, e137–e145. doi: 10.1016/j.scispo.2016.12.002
García-Unciti, M., Izquierdo, M., Idoate, F., Gorostiaga, E., Grijalba, A., Ortega-Delgado, F., et al. (2012). Weight-loss diet alone or combined with progressive resistance training induces changes in association between the cardiometabolic risk profile and abdominal fat depots. Ann. Nutr. Metab. 61, 296–304. doi: 10.1159/000342467
Giannopoulou, I., Fernhall, B., Carhart, R., Weinstock, R. S., Baynard, T., Figueroa, A., et al. (2005). Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism 54, 866–875. doi: 10.1016/j.metabol.2005.01.033
Gleeson, M., Bishop, N. C., Stensel, D. J., Lindley, M. R., Mastana, S. S., and Nimmo, M. A. (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–615. doi: 10.1038/nri3041
Gomez-Huelgas, R., Ruiz-Nava, J., Santamaria-Fernandez, S., Vargas-Candela, A., Alarcon-Martin, A. V., Tinahones, F. J., et al. (2019). Impact of intensive lifestyle modification on levels of adipokines and inflammatory biomarkers in metabolically healthy obese women. Mediators Inflamm. 2019:4165260. doi: 10.1155/2019/4165260
Grøntved, A., and Hu, F. B. (2011). Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA 305, 2448–2455. doi: 10.1001/jama.2011.812
Higgins, J. P. T., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. doi: 10.1136/bmj.d5928
Hong, F., Ye, W., Kuo, C. H., Zhang, Y., Qian, Y., and Korivi, M. (2019). Exercise intervention improves clinical outcomes, but the “time of session” is crucial for better quality of life in breast cancer survivors: a systematic review and meta-analysis. Cancers (Basel) 11:706. doi: 10.3390/cancers11050706
Hotamisligil, G. S. (2006). Inflammation and metabolic disorders. Nature 444, 860–867. doi: 10.1038/nature05485
Kawanishi, N., Yano, H., Yokogawa, Y., and Suzuki, K. (2010). Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 16, 105–118.
Khalafi, M., Symonds, M. E., and Akbari, A. (2021). The impact of exercise training versus caloric restriction on inflammation markers: a systemic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 1–16. doi: 10.1080/10408398.2021.1873732
Kökten, T., Hansmannel, F., Ndiaye, N. C., Heba, A., Quilliot, D., Dreumont, N., et al. (2021). Calorie restriction as a new treatment of inflammatory diseases. Adv. Nutr. 12, 1558–1570. doi: 10.1093/advances/nmaa179
Lakhdar, N., Denguezli, M., Zaouali, M., Zbidi, A., Tabka, Z., and Bouassida, A. (2013). Diet and diet combined with chronic aerobic exercise decreases body fat mass and alters plasma and adipose tissue inflammatory markers in obese women. Inflammation 36, 1239–1247. doi: 10.1007/s10753-013-9661-8
Lam, Y. Y., Ghosh, S., Civitarese, A. E., and Ravussin, E. (2015). Six-month calorie restriction in overweight individuals elicits transcriptomic response in subcutaneous adipose tissue that is distinct from effects of energy deficit. J. Gerontol. A Biol. Sci. Med. Sci. 71, 1258–1265. doi: 10.1093/gerona/glv194
Lighter, J., Phillips, M., Hochman, S., Sterling, S., Johnson, D., Francois, F., et al. (2020). Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin. Infect. Dis. 71, 896–897. doi: 10.1093/cid/ciaa415
Liu, Y., Ye, W., Chen, Q., Zhang, Y., Kuo, C. H., and Korivi, M. (2019). Resistance exercise intensity is correlated with attenuation of HbA1c and insulin in patients with type 2 diabetes: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health 16:140. doi: 10.3390/ijerph16010140
Mehta, P., Mcauley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., Manson, J. J., et al. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034. doi: 10.1016/S0140-6736(20)30628-0
Mendham, A. E., Duffield, R., Marino, F., and Coutts, A. J. (2014). Small-sided games training reduces CRP, IL-6 and leptin in sedentary, middle-aged men. Eur. J. Appl. Physiol. 114, 2289–2297. doi: 10.1007/s00421-014-2953-3
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Monteiro-Junior, R. S., De Tarso Maciel-Pinheiro, P., Da Matta Mello Portugal, E., Da Silva, Figueiredo, L. F., Terra, R., et al. (2018). Effect of exercise on inflammatory profile of older persons: systematic review and meta-analyses. J. Phys. Act. Health 15, 64–71. doi: 10.1123/jpah.2016-0735
Mortaz, E., Tabarsi, P., Jamaati, H., Dalil Roofchayee, N., Dezfuli, N. K., Hashemian, S. M., et al. (2021). Increased serum levels of soluble TNF-α receptor is associated with ICU mortality in COVID-19 patients. Front. Immunol. 12:592727. doi: 10.3389/fimmu.2021.592727
Nicklas, B. J., Ambrosius, W., Messier, S. P., Miller, G. D., Penninx, B. W. J. H., Loeser, R. F., et al. (2004). Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am. J. Clin. Nutr. 79, 544–551. doi: 10.1093/ajcn/79.4.544
Nimmo, M. A., Leggate, M., Viana, J. L., and King, J. A. (2013). The effect of physical activity on mediators of inflammation. Diabetes Obes. Metab. 15(Suppl. 3), 51–60. doi: 10.1111/dom.12156
Oh, S., Tanaka, K., Tsujimoto, T., So, R., Shida, T., and Shoda, J. (2014). Regular exercise coupled to diet regimen accelerates reduction of hepatic steatosis and associated pathological conditions in nonalcoholic fatty liver disease. Metab. Syndr. Relat. Disord. 12, 290–298. doi: 10.1089/met.2013.0143
Ott, B., Skurk, T., Hastreiter, L., Lagkouvardos, I., Fischer, S., Büttner, J., et al. (2017). Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci. Rep. 7:11955. doi: 10.1038/s41598-017-12109-9
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021a). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 134, 178–189.
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021b). Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J. Clin. Epidemiol. 134, 103–112. doi: 10.31222/osf.io/jb4dx
Park, J. H., Moon, J. H., Kim, H. J., Kong, M. H., and Oh, Y. H. (2020). Sedentary Lifestyle: overview of Updated Evidence of Potential Health Risks. Korean J. Fam. Med. 41, 365–373. doi: 10.4082/kjfm.20.0165
Peng, Y. D., Meng, K., Guan, H. Q., Leng, L., Zhu, R. R., Wang, B. Y., et al. (2020). [Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV]. Zhonghua Xin Xue Guan Bing Za Zhi 48, 450–455.
Popko, K., Gorska, E., Stelmaszczyk-Emmel, A., Plywaczewski, R., Stoklosa, A., Gorecka, D., et al. (2010). Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 15(Suppl. 2), 120–122. doi: 10.1186/2047-783X-15-S2-120
Pradhan, A. D., Manson, J. E., Rifai, N., Buring, J. E., and Ridker, P. M. (2001). C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286, 327–334. doi: 10.1001/jama.286.3.327
Razavi, R., Parvaresh, A., Abbasi, B., Yaghoobloo, K., Hassanzadeh, A., Mohammadifard, N., et al. (2021). The alternate-day fasting diet is a more effective approach than a calorie restriction diet on weight loss and hs-CRP levels. Int. J. Vitam. Nutr. Res. 91, 242–250. doi: 10.1024/0300-9831/a000623
Rejeski, W. J., Marsh, A. P., Fanning, J., Ambrosius, W. T., Walkup, M. P., and Nicklas, B. J. (2019). Dietary weight loss, exercise, and inflammation in older adults with overweight or obesity and cardiometabolic disease. Obesity (Silver Spring) 27, 1805–1811. doi: 10.1002/oby.22600
Ryan, A. S., Ge, S., Blumenthal, J. B., Serra, M. C., Prior, S. J., and Goldberg, A. P. (2014). Aerobic exercise and weight loss reduce vascular markers of inflammation and improve insulin sensitivity in obese women. J. Am. Geriatr. Soc. 62, 607–614. doi: 10.1111/jgs.12749
Saltiel, A. R., and Olefsky, J. M. (2017). Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 127, 1–4. doi: 10.1172/JCI92035
Sardeli, A. V., Tomeleri, C. M., Cyrino, E. S., Fernhall, B., Cavaglieri, C. R., and Chacon-Mikahil, M. P. T. (2018). Effect of resistance training on inflammatory markers of older adults: a meta-analysis. Exp. Gerontol. 111, 188–196. doi: 10.1016/j.exger.2018.07.021
Silverman, N. E., Nicklas, B. J., and Ryan, A. S. (2009). Addition of aerobic exercise to a weight loss program increases BMD, with an associated reduction in inflammation in overweight postmenopausal women. Calcif. Tissue Int. 84, 257–265. doi: 10.1007/s00223-009-9232-z
Simonnet, A., Chetboun, M., Poissy, J., Raverdy, V., Noulette, J., Duhamel, A., et al. (2020). High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 28, 1195–1199. doi: 10.1002/oby.22831
Slim, K., Nini, E., Forestier, D., Kwiatkowski, F., Panis, Y., and Chipponi, J. (2003). Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 73, 712–716. doi: 10.1046/j.1445-2197.2003.02748.x
Snel, M., Van Diepen, J. A., Stijnen, T., Pijl, H., Romijn, J. A., Meinders, A. E., et al. (2011). Immediate and long-term effects of addition of exercise to a 16-week very low calorie diet on low-grade inflammation in obese, insulin-dependent type 2 diabetic patients. Food Chem. Toxicol. 49, 3104–3111. doi: 10.1016/j.fct.2011.09.032
Straznicky, N. E., Lambert, E. A., Nestel, P. J., Mcgrane, M. T., Dawood, T., Schlaich, M. P., et al. (2010). Sympathetic neural adaptation to hypocaloric diet with or without exercise training in obese metabolic syndrome subjects. Diabetes 59, 71–79. doi: 10.2337/db09-0934
Thivel, D., Tremblay, A., Genin, P. M., Panahi, S., Rivière, D., and Duclos, M. (2018). Physical activity, inactivity, and sedentary behaviors: definitions and implications in occupational health. Front. Public Health 6:288. doi: 10.3389/fpubh.2018.00288
Tolkien, K., Bradburn, S., and Murgatroyd, C. (2019). An anti-inflammatory diet as a potential intervention for depressive disorders: a systematic review and meta-analysis. Clin. Nutr. 38, 2045–2052. doi: 10.1016/j.clnu.2018.11.007
Tremblay, M. S., Aubert, S., Barnes, J. D., Saunders, T. J., Carson, V., Latimer-Cheung, A. E., et al. (2017). Sedentary behavior research network (SBRN) – terminology consensus project process and outcome. Int. J. Behav. Nutr. Phys. Act. 14:75. doi: 10.1186/s12966-017-0525-8
Trussardi Fayh, A. P., Lopes, A. L., Fernandes, P. R., Reischak-Oliveira, A., and Friedman, R. (2013). Impact of weight loss with or without exercise on abdominal fat and insulin resistance in obese individuals: a randomised clinical trial. Br. J. Nutr. 110, 486–492. doi: 10.1017/S0007114512005442
van Gemert, W. A., Peeters, P. H., May, A. M., Doornbos, A. J. H., Elias, S. G., Van Der Palen, J., et al. (2019). Effect of diet with or without exercise on abdominal fat in postmenopausal women – a randomised trial. BMC Public Health 19:174. doi: 10.1186/s12889-019-6510-1
Verheggen, R. J. H. M., Maessen, M. F. H., Green, D. J., Hermus, A. R. M. M., Hopman, M. T. E., and Thijssen, D. H. T. (2016). A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes. Rev. 17, 664–690. doi: 10.1111/obr.12406
Weiss, E. P., Albert, S. G., Reeds, D. N., Kress, K. S., Mcdaniel, J. L., Klein, S., et al. (2016). Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: a randomized intervention trial. Am. J. Clin. Nutr. 104, 576–586. doi: 10.3945/ajcn.116.131391
WHO (2021). Fact sheet: Obesity and Overweight. 9 June, 2021 Edn. Geneva: Wordl Health Organization.
Yeh, S. H., Chuang, H., Lin, L. W., Hsiao, C. Y., Wang, P. W., Liu, R. T., et al. (2009). Regular Tai Chi Chuan exercise improves T cell helper function of patients with type 2 diabetes mellitus with an increase in T-bet transcription factor and IL-12 production. Br. J. Sports Med. 43, 845–850. doi: 10.1136/bjsm.2007.043562
You, T., Berman, D. M., Ryan, A. S., and Nicklas, B. J. (2004). Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J. Clin. Endocrinol. Metab. 89, 1739–1746. doi: 10.1210/jc.2003-031310
Yu, N., Ruan, Y., Gao, X., and Sun, J. (2017). Systematic review and meta-analysis of randomized, controlled trials on the effect of exercise on serum leptin and adiponectin in overweight and obese individuals. Horm. Metab. Res. 49, 164–173. doi: 10.1055/s-0042-121605
Keywords: physical exercise, diet, C-reacting protein, meta-regression, inflammation, obesity
Citation: Liu Y, Hong F, Lebaka VR, Mohammed A, Ji L, Zhang Y and Korivi M (2021) Calorie Restriction With Exercise Intervention Improves Inflammatory Response in Overweight and Obese Adults: A Systematic Review and Meta-Analysis. Front. Physiol. 12:754731. doi: 10.3389/fphys.2021.754731
Received: 06 August 2021; Accepted: 05 October 2021;
Published: 15 November 2021.
Edited by:
Leonardo Alexandre Peyré-Tartaruga, Federal University of Rio Grande do Sul, BrazilReviewed by:
Guido Gembillo, University of Messina, ItalyMarli Maria Knorst, Federal University of Rio Grande do Sul, Brazil
Copyright © 2021 Liu, Hong, Lebaka, Mohammed, Ji, Zhang and Korivi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yean Zhang, YW50b255NzlAc3VzLmVkdS5jbg==; Mallikarjuna Korivi, bWFsbGlrQHpqbnUuZWR1LmNu; bWFsbGlrLms1QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Yubo Liu
Yubo Liu Feng Hong
Feng Hong Veeranjaneya Reddy Lebaka
Veeranjaneya Reddy Lebaka Arifullah Mohammed
Arifullah Mohammed Lei Ji
Lei Ji Yean Zhang
Yean Zhang Mallikarjuna Korivi
Mallikarjuna Korivi