- 1Department of Surgery, The University of Auckland, Auckland, New Zealand
- 2Alimetry Ltd, Auckland, New Zealand
- 3Auckland Bioengineering Institute, The University of Auckland, Auckland, New Zealand
Background: Functional Gastrointestinal Disorders (FGID) are a group of symptom-based disorders that occur across the alimentary tract and have a high prevalence globally in both adults and children. These symptoms are chronic and/or recurrent and often have substantial effects on quality of life. Their incidence is tied to multiple factors, including gut-brain axis imbalance, which includes autonomic dysregulation related to a relative withdrawal of vagal activity. Heart rate variability biofeedback (HRVB) is a non-invasive intervention that can influence autonomic activity and has shown benefit for diverse conditions including depression and anxiety, however the evidence of its effect has not yet been systematically assessed in FGIDs. This scoping review aimed to collate and evaluate the available literature regarding HRVB and FGIDs.
Methods: We systematically searched four medical databases. Four interventional studies using HRVB in FGIDs met inclusion criteria.
Results: Studies were heterogeneous, including both paediatric and adult patients, as well different subtypes of FGID. Two of the four studies demonstrated significant symptom improvements from HRVB while the other two found no significant difference.
Discussion: Our findings suggested that at least 6 weeks of HRVB is required to observe an impact on FGID symptoms. We provide recommendations for future studies of HRVB in FGIDs, which are needed. Evidence on HRVB for FGID is still emerging, but appears promising when administered optimally.
1 Introduction
Functional Gastrointestinal Disorders (FGID), more recently termed Disorders of Gut-Brain Interactions (DGBIs), are a group of multiple symptom phenotypes that occur across the gastrointestinal (GI) tract. There are several subtypes and symptoms range from dysphagia to dyspepsia to abdominal pain and bloating (Black et al., 2020; Sperber et al., 2021). These disorders may have recurrent and potentially debilitating impacts, and an incomplete understanding of their pathophysiology means that clinical diagnosis and treatment often still rely upon trial and error. They are highly prevalent, affecting up to 40% of the global population (Black et al., 2020; Sperber et al., 2021; Andreasson et al., 2021).
FGIDs can be better understood through the Biopsychosocial model of disease (Black et al., 2020; Van Oudenhove et al., 2016). This model emphasises that the development and persistence of FGIDs are shaped by an interplay of physiological, psychological, and environmental factors. Recognising this complexity is essential for effective management, as it highlights the need for a holistic, multifactorial approach that goes beyond physiological symptoms to consider social and psychological influences as well.
Recently, there has been increasing evidence of a correlation between the prevalence of functional gut symptoms and an imbalance of autonomic nervous system activity, with greater relative sympathetic activity due to parasympathetic withdrawal or a decrease in cardiovagal modulatory ability (Aggarwal et al., 1994; Bharucha et al., 1993; Chelimsky et al., 2019; Mróz et al., 2022; Jung-Ho et al., 1999). This hypothesis is supported by the emerging efficacy of therapies proposed to enhance vagal tone, encompassing such diverse approaches as chewing gum, slow breathing exercises, moderate-pressure massage, or transcutaneous vagal electrical stimulation (Lunding et al., 2008; Zhang et al., 2023), accompanied by with evidence for improved antral, colonic and oesophageal motility and symptom reductions (Bonaz et al., 2016). This decrease in vagal modularity represents one possible physiological cause for FGID as per the biopsychosocial model.
Heart Rate Variability Biofeedback (HRVB) is a non-invasive technique that leverages the body’s physiology and autonomic regulation, using specific slow breathing rates to modulate heart rate and enhance baroreflex sensitivity (Lehrer and Gevirtz, 2014). HRVB is often stated to be carried out at one’s resonance frequency, the frequency of breathing where the oscillation of heart rate due to the respiratory sinus arrhythmia (produced by the slow breathing) resonates with the oscillation in heart rate due to the baroreflex (Figure 1). This results in a maximal heart rate variation, and a maximal increase in baroreflex gain with an increase in baroreflex gain at resting states after consistent practice as well (Lehrer et al., 2003; Shaffer, 2020; Vaschillo et al., 2006; Wheat and Larkin, 2010).
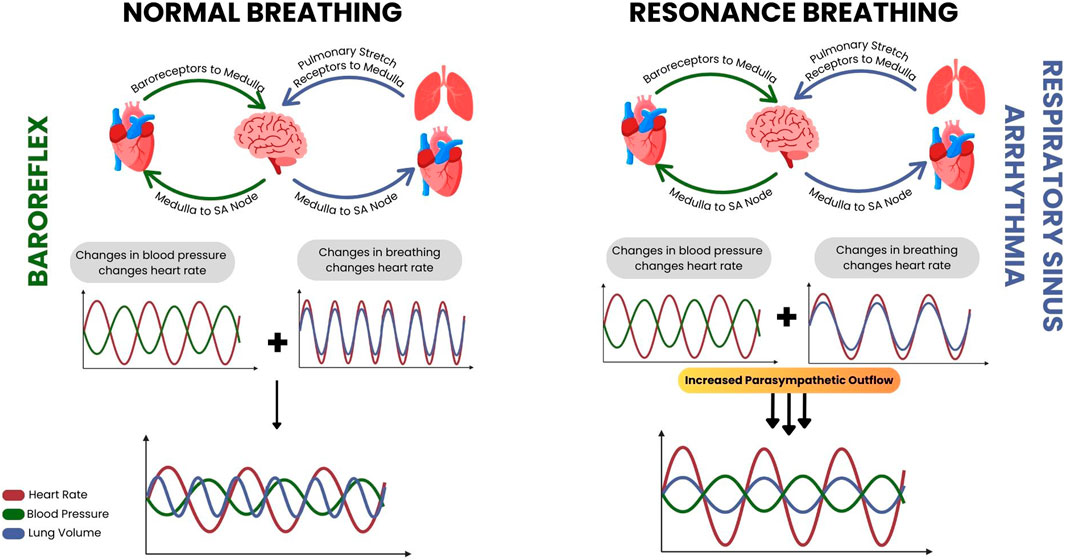
Figure 1. A diagrammatic resource developed by Pereira et al. to demonstrate the physiology underlying heart rate variability biofeedback. During normal breathing conditions (left) the resultant heart rate changes from the baroreflex and respiratory sinus arrhythmia are out of sync, but while conducting resonance breathing during biofeedback (right) the resultant heart rate changes from the respiratory sinus arrhythmia approaches that of the baroreflex and they resonate, along with the parasympathetic activity that occurs alongside them.
This ability to influence the activity of the autonomic nervous system could have therapeutic significance for FGIDs, but existing evidence on the effect of HRVB on the GI tract is limited. In addition, standardised protocols for performing HRVB in FGIDs have not been defined Although the most common protocol is that of Lehrer et al. (Lehrer et al., 2013), there still exists considerable variation.
The aim of this study was therefore to examine the role and effectiveness of HRVB as a therapy for individuals with FGIDs through a scoping review. The primary aim was to identify and assess relevant interventional clinical studies applying HRVB in populations with FGID and assessing their relevant symptoms. The secondary aims were to assess the protocols and measurement tools used by each study, while comparing the study’s outcomes, in order to develop a protocol for future studies to measure the effect of biofeedback on patients diagnosed with FGIDs, and to guide future research in this emerging area. As this is a scoping review in an emerging field, we aim to map the existing literature and identify gaps to guide further studies.
2 Methods
2.1 Study design
The scoping review was conducted and reported in accordance with the PRISMA 2020 guidelines and the scoping review extension (PRISMA-ScR), and thus, did not adhere strictly to PICOS guidelines (Page et al., 2021; Tricco et al., 2018).
2.2 Search strategy and study selection
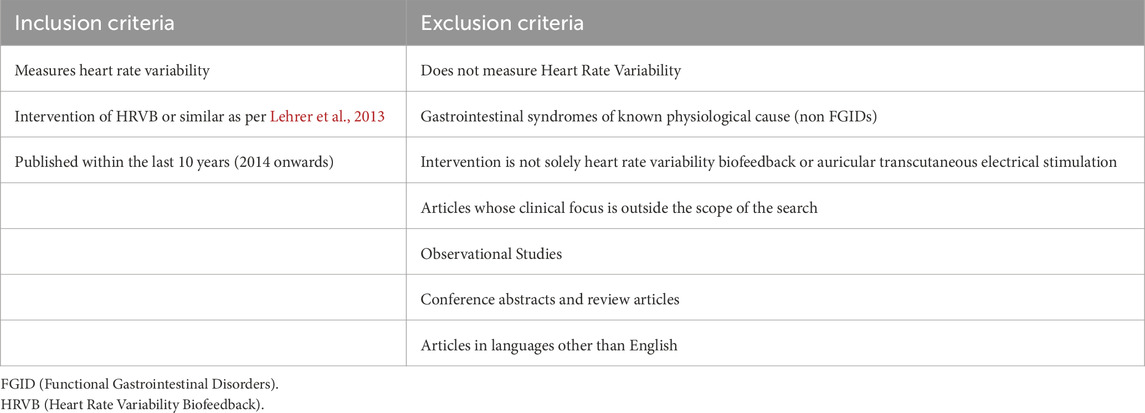
Four databases were searched: PubMed, Web of Science, ScienceDirect and Scopus; using the search term ‘Heart Rate Variability’ alongside terms to describe categories and terminologies for FGIDs as defined by ROME IV (Rome IV Criteria, 2020), including “Functional abdominal pain”, “Nausea and Vomiting Syndromes”, “Functional Dyspepsia”, “Gastroparesis”, “Irritable Bowel Syndrome”, “Functional Constipation”, “Functional Diarrhoea”, and “Functional abdominal bloating” (i.e., “Heart Rate Variability AND Functional Dyspepsia). The literature search was completed on 8 January 2024.
It was decided to use the search term ‘heart rate variability’ as opposed to ‘heart rate variability biofeedback’ as it was a broader search term, and many studies did not use this term in their work, instead opting for ‘slow deep breathing’ or similar phrases to describe a similar technique where heart rate variability (HRV) is measured and altered due to a breathing technique intervention. This consistent measurement of HRV across studies included, allowed for greater rigor when comparing interventions and their effect on autonomic activity. Individual searches were completed for each category of FGID, as opposed to simply searching FGID, so as to ensure adequate collection of studies in the search.
Two reviewers, who were not associated with Alimetry Ltd, independently screened the literature titles, abstracts and then the entire article according to the inclusion and exclusion criteria detailed in Table 1. Reviewers specifically confirmed that HRV was measured as part of the study so as to allow for a consistent comparison across literature, although there were no limits on the method by which HRV or gastrointestinal symptoms were measured. Studies that used electrocardiogram (ECG) and photoplethysmography (PPG). were both collected as both were proved to be equivalent to each other as justified by Plews et al. (2017), as well as studies that used both symptom questionnaires and concurrent clinical investigations. Literature that was published in languages other than English were excluded from this study due to limited translation resources and language proficiency within the research team. This ensured that accurate data extraction and synthesis was conducted on all included literature.
2.3 HRV metrics and biomarkers
There are several different metrics that can be used to assess HRV, and the following considerations were incorporated into the review. HRV metrics are primarily divided into Time Domain and Frequency Domain Measures (Shaffer and Ginsberg, 2017). The Time Domains largely focus on the interbeat intervals (IBI) defined as the time between each successive heartbeat, displaying the variance in these successive intervals. The two most commonly used metrics for this are SDNN (Standard Deviation of the N to N Intervals or normal R-R intervals without artefact) and RMSSD (Root Mean Square of the Successive Differences). There are other variables included within the time domain measurements which were not relevant to the scope of this review. The Frequency Domain Measures rely on the ability to conduct a Fast Fourier Transform (FFT) on the heart rate data, separating the data into three separate bands: high frequency, low frequency, and very low frequency (HF, LF and VLF, respectively). Each of these frequency bands cover a set range of frequencies: 0.15–0.40 Hz for HF, 0.04–0.15 Hz for the LF, and 0.0033–0.04 Hz for VLF, and they are expressed as a power within those frequency bands. Of these frequency metrics, LF originally is thought to represent the sympathetic arm of the baroreflex, and through its arterial oscillations (known as Mayer’s waves), stabilises blood flow (Julien, 2006). During slow breathing conditions however, the rhythm of respiration dependent modification decreases the range of LF, such that it closely aligns with the rhythm of respiratory sinus arrhythmia (Russo et al., 2017). The final metric that is sometimes used, which is simply a calculation, is the Baevsky Stress Index (SI) (refer to Figure 2 (Baevsky and Chernikova, 2017)). This is a geometric method to assess IBIs and represent the function of the sympathetic nervous system. Although HRV is often used as a measure of autonomic activity, its use comes with limitations. Variations in heart rate are controlled by both sympathetic and parasympathetic systems, and as a result, selecting biomarkers of solely one system from one’s HRV is difficult. There are, however, some HRV biomarkers that work best in different contexts. SDNN for instance is a reasonable approximation of parasympathetic activity at rest, while RMSSD and LF are better suited to measure parasympathetic activity during slow breathing conditions, such as during biofeedback (Thomas et al., 2019). Even so, there still remains contention if these biomarkers are truly accurate or not, especially due to the aforementioned co-existence of the sympathetic and parasympathetic systems (Shaffer and Ginsberg, 2017).
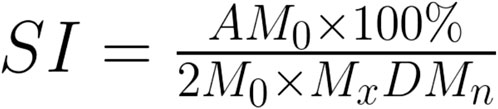
Figure 2. Baevsky Stress Index Calculation. M0 is the mode, AM0 is the mode amplitude calculated using a 50 m bin width, MxDMn is the difference between the longest (Mx) and the shortest (Mn) interval (Baevsky and Chernikova, 2017).
2.4 Data extraction and analysis
Records from each database search were screened for inclusion by two independent authors, with discrepancies being discussed and resolved by mediation by a third author as required. Relevant data from the full-text articles were extracted independently then compared. Due to the heterogenous design as well as the limited number of studies available to be statistically combined, meta analysis was not performed. A narrative scoping review was therefore conducted on the included studies, allowing the reviewers to assess the role of HRV Biofeedback as a potential therapy for FGID and examine the protocols employed by each of the studies per the study aims in order to guide future research in this field.
3 Results
3.1 Literature search results
This literature search had resulted in a total of 1,013 articles (including duplicates) with the following breakdown: 80 results for functional abdominal bloating, 104 results for functional abdominal pain, 90 for functional constipation, 38 for functional diarrhoea, 95 for functional dyspepsia, 71 for gastroparesis, 252 for irritable bowel syndrome and 283 for nausea and vomiting syndrome. The titles and abstracts of these articles were then screened independently by both reviewers according to the exclusion criteria as well as removing duplicates. This resulted in four total articles with some of them assessing multiple of the disorder subtypes that were included in the literature search (2 for functional abdominal pain, one for functional constipation, and three for irritable bowel syndrome). The data from these papers was then extracted and analysed. A graphical summary of the systematic literature review is presented in Figure 3.
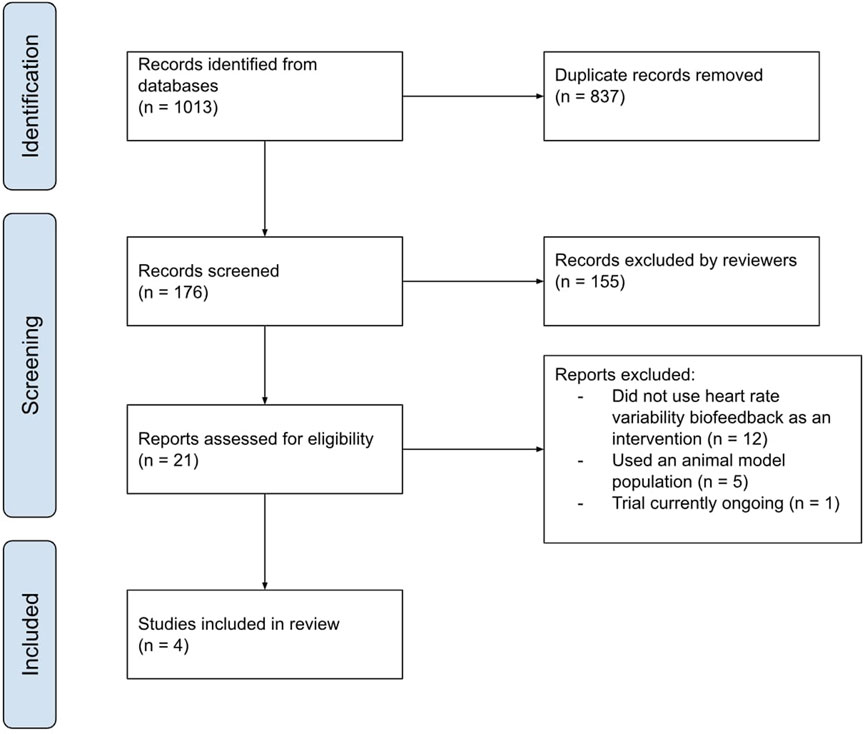
Figure 3. A graphical representation of the screening process of the articles retrieved for this review completed by both independent reviewers.
3.2 Article characteristics
From the four relevant studies identified in the literature search, three were conducted in the USA (Ebert, 2012; Katherine Jurek et al., 2022; Stern et al., 2014) and one in China (Liu et al., 2022). The majority of these addressed IBS, while some studies addressing functional abdominal pain, functional constipation, as well as other subtypes, were found to be lacking (Table 2).
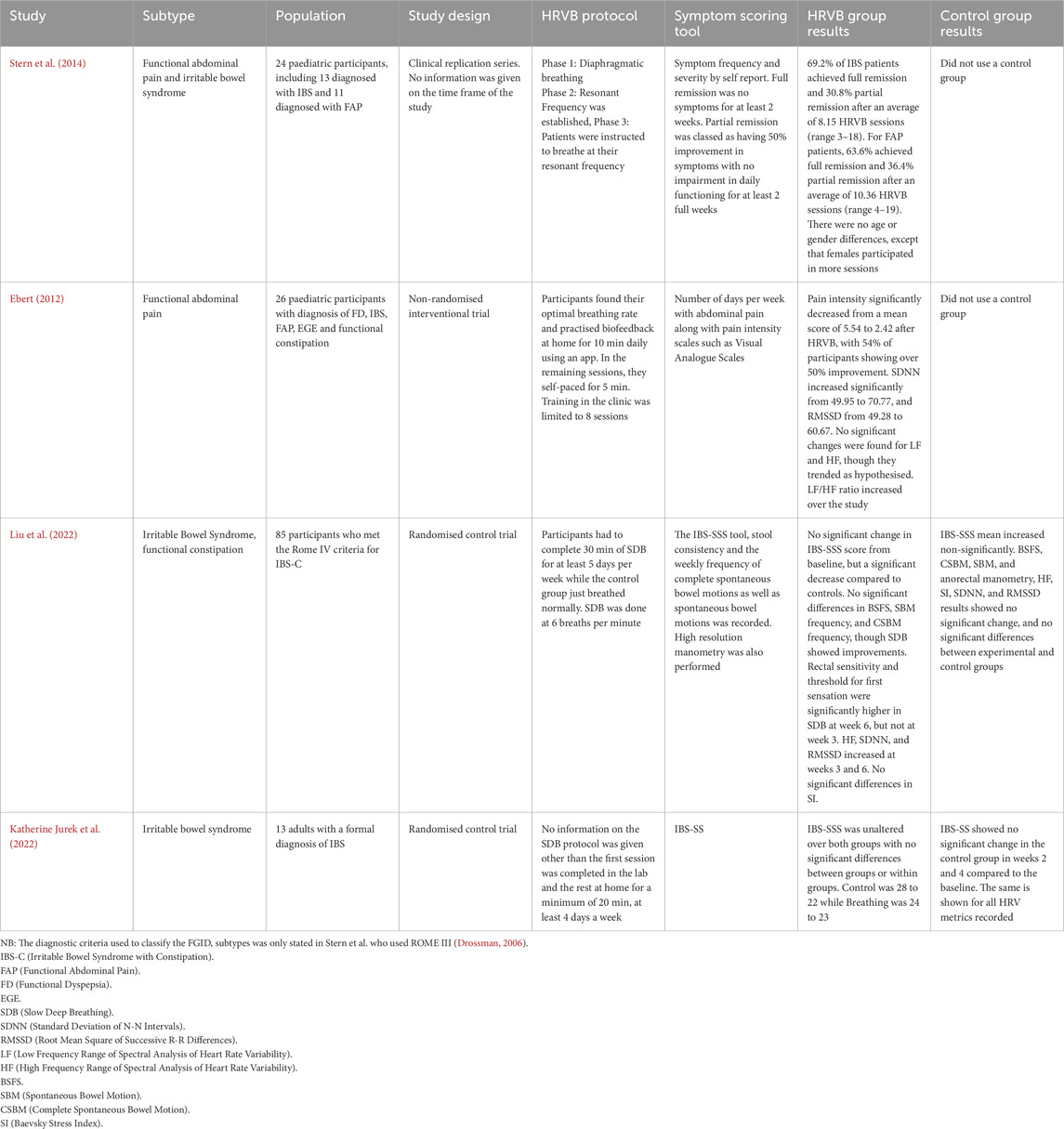
Table 2. Summary of the key data from the four studies that resulted from the literature search and inclusion and exclusion criteria.
Two of the studies were randomised control trials (Katherine Jurek et al., 2022; Liu et al., 2022) while the other two were interventional studies where HRV biofeedback was not compared to a sham control (Ebert, 2012; Stern et al., 2014). These four articles also varied in terms of the target population, with two focused on paediatric populations (Stern, Guiles, and Gevirtz, 2014; Ebert, 2012) and the other two on adult populations (Katherine Jurek et al., 2022; Liu et al., 2022) (Table 2)
All of the included studies used the same primary metrics when quantifying the HRV present, relying on time and frequency domain metrics, to make inferences of vagal and sympathetic tone (Katherine Jurek et al., 2022). All four studies also used ECG as the method of measuring HRV data while participants were in the research laboratory/clinic. For the at-home biofeedback training, Stern et al. used the StressEraser (Helicor Inc, New York, United States of America), a portable, handheld device, which had photoplethysmography (PPG) capabilities. Jurek et al. opted to use a video to guide participants through their biofeedback at home which did not collect heart rate data. Liu et al. and Ebert did not detail any at-home biofeedback practice. All of the four studies used different software to calculate and assess the HRV metrics stated above. Both Stern et al. and Ebert used the J and J Engineering I-330 C-2+ hardware and Stern et al. stated that they used the J and J Engineering USE3 software along with it, which is a combination of hardware and associated software to conduct biofeedback and measure HRV in the lab as well as calculate the metrics related to HRV (Table 3). Jurek et al. instead used a Polar Heart Rate Monitor (Polar Electro Oy, Kempele, Finland) and the raw ECG data from this was then fed through the Kubios software (Kubios Oy, Kuopio Finland), to analyse HRV data. Liu et al. did not state what system they used to analyse HRV data, nor how it was analysed. None of the articles mentioned how they removed any potential artefact from their data as part of their analysis.
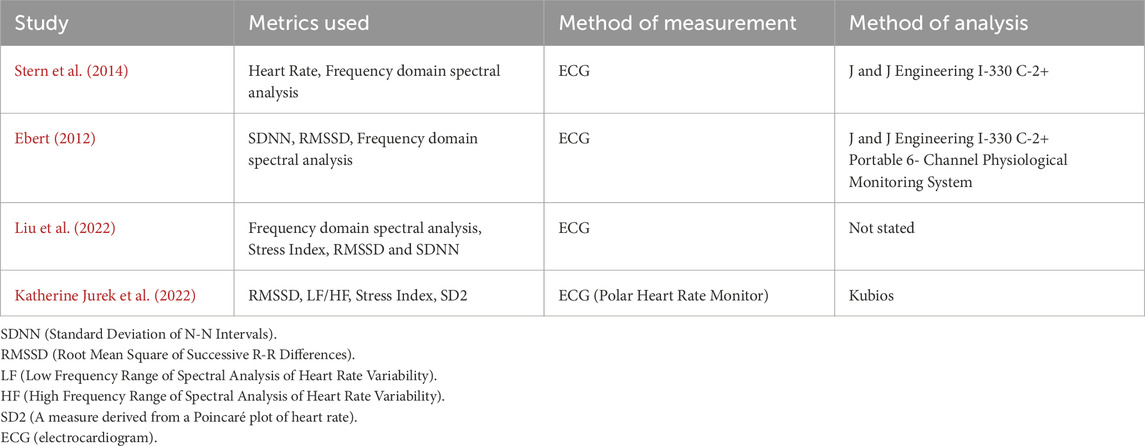
Table 3. Summary of the HRV metrics measured and used as part the respective analysis sections of each of the five studies.
3.3 HRV biofeedback protocols
All of these articles used HRV biofeedback as the form of intervention within their exposure groups. There is a considerable amount of variation present in the HRVB protocols being currently used, but the core structure is that it is initially started by providing some participant information to ensure participant buy-in. After this, participants are guided through a biofeedback session of slow, controlled breathing while their heart rate variability is being simultaneously measured. This occurs for a set period of time, while the participant attempts to maintain a mindful state. The remainder of the biofeedback sessions follow a similar format, but may differ if they occur within the clinic or home setting.
The main points of variation across studies emerged when considering the protocols employed for each study. The first point of variation was whether study investigators instruct the participants to breathe at a standardised breathing frequency (i.e. 6 breaths per min) or at the participants’ resonance frequency (Table 4). It is important to acknowledge the distinction between SDB and HRVB used across the studies included in this review. Although they may appear similar, HRVB requires participants to be aware of aspects of their physiology and make conscious efforts to alter it. SDB on the other hand, does not imply this awareness and attempt to control a participant’s physiology. Thus, even if they achieve a similar result, the procedure involved, does have a distinct difference. Another point of variance in the protocol between the four included studies was the length of time during which they conducted the biofeedback training intervention. All four studies appeared to conduct studies of at least 4 weeks or more (excluding Stern et al. and Jurek et al. who did not state the timeframe of their study). The time for which the biofeedback training intervention or SDB intervention was implemented will be categorised by this study as either short-length (70 min weekly or less) or long-length (greater than 70 min weekly). Of the four studies, one falls within the short-length category, while two fall within the long-length category, the one remaining study did not state the weekly duration of training and so could not be categorised. This included time for at-home practice with a pacer device or a smartphone app that paced the individuals breathing along with a measurement of their heart rate via a PPG, averaging 120 min total per week (Table 4).
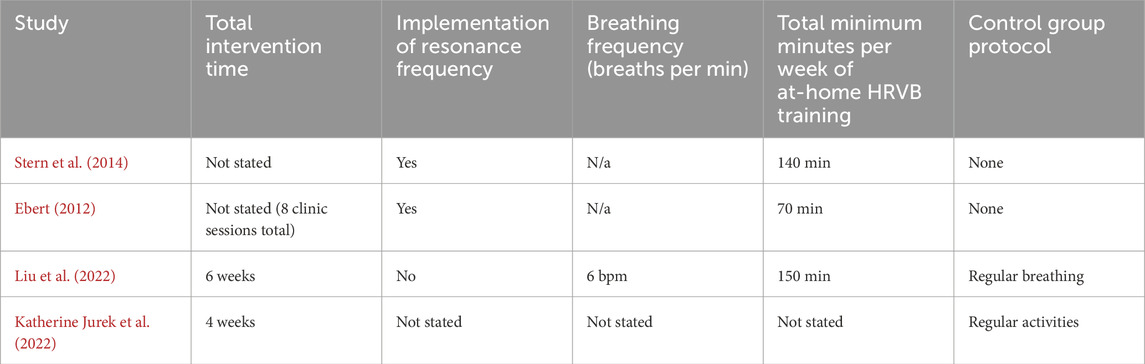
Table 4. Summary of the HRV Biofeedback and control protocols used as part the respective analysis sections of each of the five studies.
3.4 Gastrointestinal outcome measures
The primary outcome measured across all four studies was a change in GI symptoms. The most common symptom scoring tool used was the IBS-SSS (used by Liu et al. and Jurek et al.) (Spiegel et al., 2009), a validated questionnaire that assesses the severity of IBS according to four domains: pain intensity, frequency, location and relation to stool pattern. The remaining studies used symptom frequency and severity as common measures although there was no formal tool used other than a visual analogue scale (Table 5).
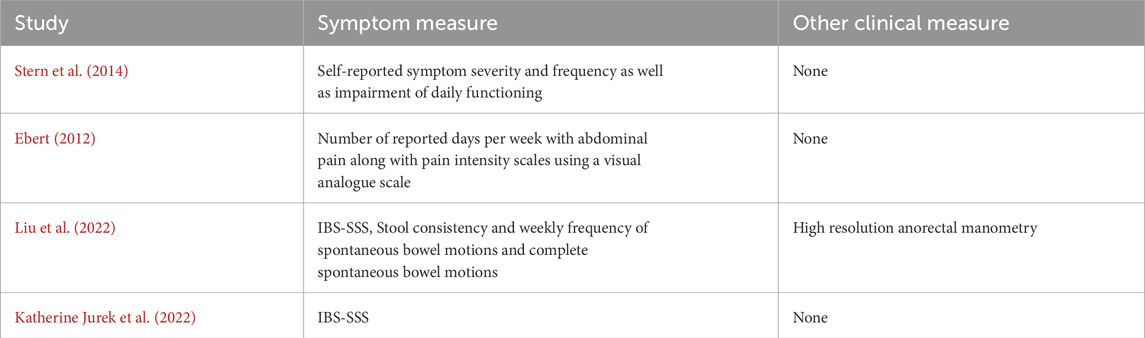
Table 5. Summary of the gastrointestinal symptoms measured and other clinical measures used as part the respective analysis sections of each of the four studies.
Out of the four studies, none used multiple physiological outcome measures. Liu et al. was the only study to include a singular physiological outcome measure of high resolution anorectal manometry.
3.5 Study outcomes
Only two of the four studies showed a significant improvement in patient outcomes. Stern et al. and Liu et al. were able to display evidence of the beneficial effects of biofeedback as an emerging therapy. There was a statistically significant decrease in symptom severity and frequency after the biofeedback trial had been completed, compared to baseline; with Stern demonstrating complete remission in 69.2% of participants and Liu et al. showing a statistically significant improvement in IBS-SSS and stool related measures. Both Ebert and Jurek et al. were unable to show symptom improvement, however, these studies were notably heterogeneous in their design and protocol. Due to small sample sizes (n = 24, 26 and 14, with the exception of Liu et al., n = 85), the power of these studies (although not otherwise stated) would be relatively low. In summary, the accumulated evidence from the reviewed papers indicates that biofeedback could be useful as a potential therapy for FGIDs, but more investigation is required to further assess its efficacy.
Jurek et al. stated the compliance of their participants to the SDB intervention, with six out of the seven participants completing at least 80% of their SDB sessions over the 4 weeks with an average of 19 sessions being completed (Katherine Jurek et al., 2022). Stern et al. did not give a measure of compliance to the HRVB intervention but rather stated the number of sessions completed over the study period, which ranged from three to 19 sessions, however they stated that all participants who returned to follow-up experienced some benefit (Stern et al., 2014). Neither Ebert or Liu et al. mentioned compliance to their study intervention. Of the two studies that included a control group (Liu et al. and Jurek et al.) within their protocol, both found no significant changes in the IBS-SSS/IBS-SS sores over the study, compared to baseline.
One of the studies (Liu et al., 2022) found a trend that could be indicative of such a period of time to find efficacy. Within their study, they completed follow ups at weeks three and six after the commencement of the slow, deep breathing exercise (SDB). During these follow-ups, a trend emerged where many of the GI based outcomes measured, only started showing a difference in the SDB group compared to the sham group at the 6 week follow-up and not the 3 week follow-up. This trend was present for the IBS-SSS, BSFS, weekly complete spontaneous bowel motions and weekly spontaneous bowel motions. The same was found for the HRV metrics, keeping in trend with what would be predicted from a sham group (Refer to Table 2). Jurek et al. also followed a similar trend with their study where they did not show a significant improvement in the recorded metrics at their 4 week follow-up mark. These same conclusions cannot be drawn for Stern et al. and Ebert as neither of these studies stated their follow-up periods for their participants.
Of the four studies, three of them provided a summary of the participants’ HRV profiles at various points of their studies. Ebert showed a marked improvement in their participants’ HRV profiles with increases in RMSSD and SDNN showing a statistically significant change at the end of the study, compared to the initiation (p < 0.05). Liu et al. also provided a summary of their participants HRV profiles throughout the study, and found that the interventional group experienced increases in their RMSSD, SDNN and HF compared to the control group, with the change in HF being statistically significant. Jurek et al. found no significant changes in the LF/HF, SNS index or PNS index in the interventional group compared to the control group across the course of the study. It is to be noted that none of the studies stated whether these measurements were taken at a participant’s baseline or during a HRVB/SDB session.
4 Discussion
This scoping review has systematically evaluated the current literature regarding HRVB and its potential use as a therapy for FGID, with a particular focus on the protocols and outcomes each study has employed. The studies identified had a heterogeneous design, with half being randomised controlled trials and the other being non-randomised interventional studies. Half of the studies identified, showed that HRVB had a beneficial effect on FGID symptoms, however, significant heterogeneity was identified across all studies. This review highlights the potential role for HRV biofeedback in FGIDs, while highlighting that duration of biofeedback training as a potential key parameter for treatment efficacy and providing guidance for advancing future studies based on the existing literature.
4.1 Resonance frequency
One key point of variation emerging from this review is whether study investigators instructed the participants to breathe at a standardised frequency breathing as used in SDB protocols (i.e. 6 breaths per min) (Liu et al. and Juek et al.) or at the participants resonance frequency as used in the HRVB protocols (Stern et al. and Ebert). The research into the benefit of employing resonance frequency into biofeedback training is limited, although an analogue study conducted in 2017 found that using a resonant frequency compared to a standardised breathing frequency was associated with a higher positive mood and a significantly higher LF/HF HRV ratio, a theorised surrogate of parasympathetic/sympathetic activity (Steffen et al., 2017). There is a standardised methodology for finding one’s resonant frequency, which typically involves trialling several different breathing frequencies for a short period of time and assessing the resultant LF power, HRmax - HRmin, and participant comfort to find the optimum frequency (Lehrer et al., 2013; Shaffer and Meehan, 2020). Variations in resonant frequency can be influenced by one’s height and sex, with taller individuals and men having lower resonance frequencies than shorter individuals and women. However, most people tend to have resonant frequencies within a tight range of 5–6.5 breaths/min (Lehrer, 2013; Vaschillo et al., 2006). There is some evidence that one’s resonant frequency is not a stable metric, with one study finding a change in resonant frequency with 66.7% of its participants (Capdevila et al., 2021); however this was limited to a change in the mean of 0.2 breaths/min. When considering that most biofeedback systems are only able to adjust the breathing pacer in 0.5 breaths/min increments, this change in resonant frequency between tests may be clinically negligible. The results of this review do not, unfortunately, gleam the benefit of resonance frequency, with an equal distribution of resonant frequency breathing and standard frequency breathing between studies that showed improvements in gastrointestinal symptoms post biofeedback. However, the sample size of this review is small and previous research suggests that resonant frequency is a viable method to use to optimise biofeedback training. As such it is suggested that more research is required into the topic to further understand the extent of its benefit, particularly where it applies to gastrointestinal disorders.
4.2 HRV measurement and analysis
All the studies employed the use of ECG in the clinic/lab setting to monitor the HRV of participants during the biofeedback exercises. ECG (often measured over 24 h) is considered the gold standard of HRV measurement (Shaffer and Ginsberg, 2017). However, a question has emerged about the validity of other forms of HRV measurement. The main contender to the ECG is the PPG method which was used for HRV measurement during at-home biofeedback sessions in Stern et al. This method relies on a light source emitting into the participant’s peripheral artery (i.e., radial artery or digital artery), a proportion of this light is then absorbed according to the volume of blood in the artery at any one time, and the rest is reflected back towards the PPG device to be sensed as used in Stern et al. Because the volume of blood in the artery varies in accordance with the cardiac cycle, the PPG gives a reliable measure of the pulse rate and thus, by extension, the heart rate (Alian and Shelley, 2014). This method is much more portable and accessible than ECG, with its key drawback being that the ECG allows for better theoretical detection of ectopic beats with its ability to show the electrical activity of the heart. However, a recent study shows physicians were able to detect atrial fibrillation using PPG measurement with equivalent accuracy to single-lead ECG (Gruwez et al., 2021), and therefore PPG may be feasible for use during HRVB sessions. PPG also has the added benefit of being conducted with a participant’s smartphone using its inbuilt flash and camera, therefore being highly accessible, and that this method, when combined with applications that employ the biofeedback principles, is able to detect HRV to accurately (Plews et al., 2017; Vandenberk et al., 2017). This altogether, opens up the possibility of conducting HRVB sessions outside of the clinic/lab setting using PPG, increasing patient’s access to HRVB, and allowing further research to explore its use in different settings with comparable reliability. Further research into the use of HRVB using at-home PPG modalities is recommended.
In terms of how the studies chose to analyse their HRV data through their trials, there appears to be a lot of commonality between the studies in terms of how they measure, display and analyse HRV metrics. All of them calculated a spectral analysis to display the power of the different frequency domains; three of them measured SDNN and RMSSD, and two of them calculated some form of stress index, all of which have some relation to autonomic activity (Thomas et al., 2019). The studies also conducted a spectral analysis of an individual’s HRV data via a Fast Fourier Transform, which also provides useful information about one’s autonomic functions, with changes in power in certain frequency bands being related to changes in sympathetic and parasympathetic activity (Shaffer and Ginsberg, 2017). This spectral analysis is often expressed as a ratio of LF/HF to analyse the balance of the sympathetic and parasympathetic systems. The assumption behind this is that LF power and HF power both correspond to sympathetic and parasympathetic activity respectively (Pagani et al., 1984; Shaffer et al., 2014). However, as mentioned earlier, this has been challenged in the past as the SNS and PNS are not solely influenced by LF and HF power. There is often some cross-over between them along with confounding due to baroreflex activity and respiration mechanics (Billman, 2013). This is supported by the evidence that SDNN and RMSSD are commonly used metrics to describe the variation in heart rate with both of them being strongly correlated to autonomic activity and its influence on heart rate and respiratory sinus arrhythmia (Shaffer et al., 2014; Shaffer and Ginsberg, 2017). Both of these are also greatly correlated to the spectral analysis of heart rate, with SDNN being associated with changes in ULF, VLF and LF power and RMSSD being highly correlated to HF power.
The SI (Figure 2) is another measure that is used as an analogue of sympathetic activity. First developed by Baevsky, this metric is highly sensitive to changes in sympathetic tone both within emotional and physical stress situations (Baevsky and Chernikova, 2017). SI has been validated within its use in psychosomatic self-regulation, although evidence of its validation in biofeedback studies is scarce (Ognev et al., 2019).
In addition, although there are many time periods over which HRV is measured and these metrics can be calculated, the standard minimum period of time required to get a measurement of any of these is 5 minutes (Shaffer and Ginsberg, 2017). However, none of the studies specified whether measurements of HRV for participants were collected during the HRVB/SDB sessions or the periods of time outside this. The omission of this detail introduces a degree of confounding when comparing the effect of the interventions used on participant’s HRV, as one’s HRV can vary with the context in which it is being measured.
Overall, the measurement and analysis of HRV during HRVB sessions appears to be well explored and comparable across all studies, which aids interpretation across studies, despite differing HRVB protocols.
4.3 Length of heart rate variability biofeedback therapy
Across all studies included in this review, there emerges a trend between the length of HRVB therapy implementation and the improvement in GI symptoms, with improvements occurring at the minimum 6 weeks of therapy. This finding was demonstrated when examining both Jurek et al.‘s and Liu et al.‘s trials. Jurek et al. only had their participants practise biofeedback for 4 weeks while Liu et al. had their participants practise SDB for 6 weeks. Where Jurek et al. did not find any significant improvements in symptoms, Liu et al. did. And upon closer inspection into Liu et al.‘s findings, these differences only started to become significant after 4 weeks into Liu et al.‘s trial. Thus, it is possible that both consistency and duration of biofeedback training is an important factor predictive of improvement of clinical FGID symptoms. However, the evidence behind this theory is drawn from only two trials and thus more evidence is needed to support it. The remaining two studies (Ebert and Stern et al.) do not state how long their follow up periods are and so we are not able to draw this conclusion from them. There was noticeable protocol variance across studies that introduces confounding into this suggested correlation effect. Thus, even though it is possible that there is a 6 weeks minimum therapy period needed to observe improvements in symptoms, it is also possible that with improvements in overall protocol, this might be reduced. However, it holds true that consistency of HRVB practice improves its effectiveness outside of each session, although the extent to which this affects the gastrointestinal system is still yet to be explored (Lehrer et al., 2003; Wheat and Larkin, 2010). Due to this, it is recommended that HRVB therapies be used within a regular format rather than as a single session to improve their efficacy.
It is also to be stated that the two long-length studies (Stern et al. and Liu et al.) found statistically significant improvements with their protocol compared to the single short-length study (Ebert). This does suggest that practicing HRVB for over 70 min in a week could provide more of a benefit than a shorter length of training. This is however based on a small sample size of studies, and more research will be needed to confirm the accuracy of this conclusion.
4.4 Symptom measurement
All four studies identified had similarities in having a strong focus on the symptoms associated with the specific FGID subtype they were investigating. Only one of the studies conducted a clinical, specifically anorectal manometry, in order to identify the changes in the threshold of anorectal sensation, providing a more objective assessment of gastrointestinal physiology than symptoms alone (Liu et al., 2022). This focus on symptoms is likely due to the historic focus on the symptoms of FGID, such as in the Rome criteria of diagnosis, and relatively little is conclusively known about the physiology underlying these disorders (Rome IV Criteria, 2020). The most common symptom scoring used across studies was the IBS-SSS (used by Liu et al. and Jurek et al.). This scale is well tested psychometrically and is easy to use with a good reproducibility. However, the main drawback of this tool is that it lacks adequate correlation with other abdominal pain measurement tools (Mujagic et al., 2015). Only one of the studies also recorded the impact of these symptoms on the participant’s quality of life (Stern et al., 2014), an impact of these disorders which can sometimes be overlooked by clinicians (Rocque and Leanza, 2015). Overall, the focus on symptomatic effects of FGID is well observed across all studies, but the lack of co-existing physiological markers recorded is noticeable. Future research in the area would benefit from greater use of objective measure of gastrointestinal physiology as well as autonomic physiology, alongside symptom measures where appropriate, as this would allow for greater reproducibility and comparability across studies. There are several currently used methods that provide a more objective measure of physiological gastrointestinal changes such as Gastric Emptying Studies, Colonic Transit Studies and Electrogastrography; all of which can be used as potential outcome measures for similar future trials. There are also new methods that are being developed, giving researchers and clinicians an insight into the physiology of FGIDs and providing biomarkers for analysis. These techniques include Body Surface Gastric Mapping (BSGM) as well as High Resolution Manometry (HRM), both of which are a methods that measure either gastric electrical activity or pressure with high accuracy and correlate it with symptoms, providing a new understanding of the physiological basis of FGID symptoms (Schamberg et al., 2023; Shimamura et al., 2021). With future research being conducted, more focus can be applied to physiological biomarkers of FGID as outcome measures to better understand the effect of HRVB on the underlying physiology.
Across all four studies, it is clear that there exists substantial heterogeneity in HRVB implementation, which may affect its efficacy in FGID populations. Even with trends observed across the few studies included in this review, of a correlation between improvement in GI symptoms and duration of HRVB therapies, more studies need to be conducted using HRVB within FGID populations to better understand how differences in protocols can affect FGID symptom outcomes to inform optimal protocol design and facilitate standardisation.
4.5 Interventional and control group results
Out of the four studies, only two of them employed the use of a control group as a comparison for the biofeedback groups results (Katherine Jurek et al., 2022; Liu et al., 2022), while the other two did not employ a control or similar method (Ebert, 2012; Stern et al., 2014). The studies that did use a control group found no statistically significant improvements in any measurements within the control groups at follow-up compared to baseline for both gastrointestinal related outcomes as well as HRV biomarkers. Out of these two studies, one observed an improvement in gastrointestinal outcomes and HRV biomarkers compared to the control group (Lius et al.), while the other did not show an improvement (Jurek et al.). This difference in outcomes could likely be due to differences in protocol as well as therapy duration as Liu et al. conducted HRVB for 6 weeks, compared to Jurek et al. who conducted it for 4 weeks. However, in cases where HRVB has shown to improve measured outcomes, it does so significantly compared to controls.
Only two of the studies found a significant improvement in symptoms after partaking in biofeedback. This is likely due to the heterogeneity of the protocols exemplified earlier in the review. Only two of the studies employed the use of resonance frequency compared to a standardised breathing frequency, and the duration of the biofeedback intervention varies between each study, along with the time that is spent practising biofeedback while at home. One key finding that was found during this review was that it is likely that biofeedback will need to be consistently practised for at least 6 weeks for its effects to become evident. This finding was demonstrated when examining both Jurek et al.‘s and Liu et al.‘s trials. Jurek et al. only had their participants practise biofeedback for 4 weeks while Liu et al. had their participants practise SDB for 6 weeks. Where Jurek et al. did not find any significant improvements in symptoms, Liu et al. did. And upon closer inspection into Liu et al.‘s findings, these differences only started to become significant after 4 weeks into Liu et al.‘s trial. Thus, it is possible that both consistency and duration of biofeedback training is an important factor predictive of improvement of clinical FGID symptoms. However, the evidence behind this theory is drawn from only two trials and thus more evidence is needed to support it. The remaining two studies (Ebert and Stern et al.) do not state how long their follow up periods are and so we are not able to draw this conclusion from them.
Of the three studies that recorded their participants’ HRV profiles at various points of the study, two of them found a statistically significant change in these profiles, trending towards an improvement in HRV profiles for those that completed the intervention. This indicates that practicing HRVB/SDB regularly is able to alter one’s physiology, improving their parasympathetic/sympathetic tone balance. None of the studies stated whether these HRV measurements were taken during a participants baseline period or during HRVB/SDB sessions. This introduces confounding into the interpretation of these results, as HRVB/SDB in itself improves one’s HRV profile during the session, but without consistent measurements across groups, comparing HRV profiles becomes difficult to do. Future research investigating the effect of HRVB/SDB on longitudinal baseline HRV profiles could be beneficial as it would reinforce HRVB’s believed ability to alter one’s long term physiology.
4.6 Autonomic regulation techniques
HRVB/SDB is one specific technique that allows the regulation of the autonomic nervous system, shifting the balance of the system away from a consistent sympathetic prominence and allowing for a more flexible system. It does this by synchronising the activation of the vagus nerve during normal breathing with its cyclical activation during the regulation of peripheral blood pressure (Figure 1). Doing this practice frequently, takes advantage of one’s inherent potential capability for neuroplasticity and increases the adaptability and flexibility of the vagal system outside of when an individual is doing these exercises (Wheat and Larkin, 2010). This is important to the management of FGIDs as it targets one of the possible physiological determinants of FGIDs, vagal withdrawal. By “reawakening” the vagus nerve through techniques such as this, and improving one’s cardiovagal modulatory abilities, it opens up the possibility of addressing one of the key factors leading to the manifestation of FGID. Thus, HRVB/SDB becomes part of the puzzle for helping manage patients with these conditions.
There are other ways of activating this same vagal tone outside of solely breathing techniques. These include methods such as aerobic exercise, yoga, rhythmic muscle contractions and progressive muscle relaxation (Albinet et al., 2010; Shaffer et al., 2022; Tee et al., 2022; Tyagi and Cohen, 2016). Each of these techniques improve one’s HRV by improving their vagal tone. HRV is determined by autonomic activity, largely by the rhythmic pulses of the vagus nerve against sympathetic activity, which can be amplified through practices such as these (Baevsky and Chernikova, 2017; Gitler et al., 2022). Thus, during these practices as well as during HRVB/SDB, HRV acts as a measurement tool of autonomic flexibility. An autonomic system with greater inherent flexibility will be able to exhibit more variability in its heart rate when called upon, such as in these exercises. And thus, a greater HRV depicts greater autonomic flexibility, as well as a greater ability to adapt to changes in the environment (Lee et al., 2015).
4.7 Limitations of the review
There are several limitations to this review, including its focus on FGID without considering the potential for other disorders both within and outside the gastrointestinal system, such as within the urinary system (Zivkovic et al., 2017). This review also did not assess the variance in baseline HRV for those diagnosed with FGID compared to healthy controls as other reviews have done similar feats (Ali et al., 2023). The differences between SDB and HRVB described in the studies included in this review also provide another limitation. HRVB has a more in-depth learning process for participants as they receive, understand, and influence the real-time representation of their physiology, which can help facilitate better self-regulation as one learns to alter their physiology (Jafarova et al., 2020). It is possible that this improved self-regulation may relate to symptom improvement for FGIDs compared to SDB which does not promote self-regulation in the same way. Finally, we were not able to conduct a quantitative analysis of the studies identified due to the sample sizes of each study as well as the heterogeneity in participant population, FGID subtype, and methodology. These small sample sizes heterogeneity in protocols makes a robust comparison between studies difficult, and thus these results are tentative and hypothesis generating, reliant upon the publication of more research in the area to confirm or deny the trends identified in this review.
4.8 Future research
This scoping review, although small, demonstrates that the field of HRVB is promising yet still in its infancy, and thus, more research into its use within the FGID population is needed, particularly more randomised controlled trials to better assess the effect of biofeedback compared to controls. This review is unique to a similar review conducted by Goldenberg et al. which focused on biofeedback solely in the IBS population (Goldenberg et al., 2019). In comparison to Goldenberg et al., this review had a wider focus of FGID subtypes and a tighter focus on specifically HRVB rather than other forms of biofeedback. This review also had the aim of analysing the protocols used across studies to inform an optimal protocol, while Goldenberg et al. aimed to assess the efficacy and safety of biofeedback interventions in the IBS population.
Future studies should also continue to evaluate the use of PPG as a method to measure HRV compared to a single lead ECG, especially when participants are outside of the clinic or lab. PPG has a high level of accessibility and has an ability to be used alongside a smartphone application for biofeedback exercises. And although the benefit of ectopic beat detection is reduced with PPG compared to ECG, the benefit of accessibility could far outweigh the limitations of artefact removal. This makes it a tool that can potentially improve the way that biofeedback is conducted in clinical trials and opens the door to assess how HRV metrics change with each session completed with an almost similar accuracy compared to ECG. This finding, although not unique to this study, is important to recognise in this review, as it improves the accessibility to at-home HRVB interventions, improving methodological guidance for further research.
The utilisation of more clinical tools can further assess the underlying physiology beyond just the symptoms of FGIDs. This could result in a more objective measurement of how an individual’s gastrointestinal physiology changes during the biofeedback intervention, thus allowing for greater advancements in FGID diagnosis and treatment options. Although the size of this scoping review is small, we believe it will act as a valuable resource, highlighting gaps, and informing methodological improvements for further research. The review will likely act to encourage further research in this field, providing guidance to a growing field.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review and editing. LF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review and editing. WX: Methodology, Supervision, Writing – review and editing. AG: Conceptualization, Supervision, Writing – review and editing. GO: Conceptualization, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding for this research and it’s publication was received as a grant from the Health Research Council of New Zealand.
Conflict of interest
GOG and AG are co-founders and shareholders in Alimetry Ltd. GOG is a co-founder and shareholder in the The Insides Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal A., Cutts T. F., Abell T. L., Cardoso S., Familoni B., Bremer J., et al. (1994). Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology 106 (4), 945–950. doi:10.1016/0016-5085(94)90753-6
Albinet C. T., Boucard G., Bouquet C. A., Audiffren M. (2010). Increased heart rate variability and executive performance after aerobic training in the elderly. Eur. J. Appl. Physiology 109 (4), 617–624. doi:10.1007/s00421-010-1393-y
Ali M. K., Gong S., Nojkov B., Burnett C., Chen J. D. Z. (2023). Best parameters of heart rate variability for assessing autonomic responses to brief rectal distention in patients with irritable bowel syndrome. Sensors 23 (19), 8128. doi:10.3390/s23198128
Alian A. A., Shelley K. H. (2014). Photoplethysmography. Clin. Anaesthesiol. 28 (4), 395–406. doi:10.1016/j.bpa.2014.08.006
Andreasson A., Talley N. J., Walker M. M., Jones M. P., Platts L. G., Wallner B., et al. (2021). An increasing incidence of upper gastrointestinal disorders over 23 Years: a prospective population-based study in Sweden. Am. J. Gastroenterology 116 (1), 210–213. doi:10.14309/ajg.0000000000000972
Baevsky R. М., Chernikova A. G. (2017). Heart rate variability analysis: physiological foundations and main methods. Cardiometry 10, 66–76. doi:10.12710/cardiometry.2017.10.6676
Bharucha A. E., Camilleri M., Low P. A., Zinsmeister A. R. (1993). Autonomic dysfunction in gastrointestinal motility disorders. Gut 34 (3), 397–401. doi:10.1136/gut.34.3.397
Billman G. E. (2013). The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiology 4, 26. doi:10.3389/fphys.2013.00026
Black C. J., Drossman D. A., Talley N. J., Ruddy J., Ford A. C. (2020). Functional gastrointestinal disorders: advances in understanding and management. Lancet 396 (10263), 1664–1674. doi:10.1016/S0140-6736(20)32115-2
Bonaz B., Sinniger V., Pellissier S. (2016). Vagal tone: effects on sensitivity, motility, and inflammation. Neurogastroenterol. Motil. Official J. Eur. Gastrointest. Motil. Soc. 28 (4), 455–462. doi:10.1111/nmo.12817
Capdevila L., Parrado E., Ramos-Castro J., Zapata-Lamana R., Lalanza J. F. (2021). Resonance frequency is not always stable over time and could be related to the inter-beat interval. Sci. Rep. 11 (1), 8400. doi:10.1038/s41598-021-87867-8
Chelimsky G., Rausch S., Bierer D., Feng M., Simpson P., Awe E., et al. (2019). Cardiovagal modulation in pediatric functional gastrointestinal disorders. Neurogastroenterol. Motil. Official J. Eur. Gastrointest. Motil. Soc. 31 (5), e13564. doi:10.1111/nmo.13564
Drossman D. A. (2006). Rome III: the new criteria. Chin. J. Dig. Dis. 7 (4), 181–185. doi:10.1111/j.1443-9573.2006.00265.x
Ebert C. G. (2012). The use of heart rate variability biofeedback for the treatment of functional gastrointestinal disorders in children and adolescents. Available online at: https://www.proquest.com/openview/c121e3abf9580a39343232ecceb1aa4b/1?casa_token=6Z4ghXVZZ6YAAAAA:GhCHHMqew7lHhwUVOFdX_0EmZeOqy7KLWvxo0HcDDG2FO1YNSs-6roHx9AEHZFPQFo1aABl1TNU&cbl=18750&pq-origsite=gscholar&parentSessionId=hfla1AEZuIJ%2F39YRkmXMxHBPlkTAdFsOQV4u3V7865Y%3D.
Gitler A., Vanacker L., De Couck M., De Leeuw I., Gidron Y. (2022). Neuromodulation applied to diseases: the case of HRV biofeedback. J. Clin. Med. Res. 11 (19), 5927. doi:10.3390/jcm11195927
Goldenberg J. Z., Brignall M., Hamilton M., Beardsley J., Batson R. D., Hawrelak J., et al. (2019). Biofeedback for treatment of irritable bowel syndrome. Cochrane Database Syst. Rev. 2019 (11). doi:10.1002/14651858.CD012530.pub2
Gruwez H., Evens S., Proesmans T., Duncker D., Linz D., Heidbuchel H., et al. (2021). Accuracy of physicians interpreting photoplethysmography and electrocardiography tracings to detect atrial fibrillation: INTERPRET-AF. Front. Cardiovasc. Med. 8, 734737. doi:10.3389/fcvm.2021.734737
Jafarova O., Mazhirina K., Sokhadze E., Shtark M. (2020). Self-regulation strategies and heart rate biofeedback training. Appl. Psychophysiol. Biofeedback 45 (2), 87–98. doi:10.1007/s10484-020-09460-5
Julien C. (2006). The enigma of Mayer waves: facts and models. Cardiovasc. Res. 70 (1), 12–21. doi:10.1016/j.cardiores.2005.11.008
Jung-Ho L. E. E., Song J.-Y., Whang E.-W., Chung D.-W., Young-Mee K. I. M. (1999). The autonomic nervous function and the yin-yang constitutional characteristics of the patients with functional gastrointestinal disorders. J. Korean Neuropsychiatric Assoc., 723–737.
Katherine Jurek M., Seavey H., Guidry M., Slomka E., Hunter S. D. (2022). The effects of slow deep breathing on microvascular and autonomic function and symptoms in adults with irritable bowel syndrome: a pilot study. Neurogastroenterol. Motil. Official J. Eur. Gastrointest. Motil. Soc. 34 (5), e14275. doi:10.1111/nmo.14275
Lee J., Kim J. K., Wachholtz A. (2015). The benefit of heart rate variability biofeedback and relaxation training in reducing trait anxiety. Han’guk Simni Hakhoe Chi. Kon'gang = Korean J. Health Psychol. 20 (2), 391–408. doi:10.17315/kjhp.2015.20.2.002
Lehrer P. (2013). How does heart rate variability biofeedback work? Resonance, the baroreflex, and other mechanisms. Biofeedback Self-Regulation 41 (1), 26–31. doi:10.5298/1081-5937-41.1.02
Lehrer P., Vaschillo B., Zucker T., Graves J., Wamboldt F., Aviles M., et al. (2013). Protocol for heart rate variability biofeedback training. Biofeedback Self-Regulation 41 (3), 98–109. doi:10.5298/1081-5937-41.3.08
Lehrer P. M., Gevirtz R. (2014). Heart rate variability biofeedback: how and why does it work? Front. Psychol. 5, 756. doi:10.3389/fpsyg.2014.00756
Lehrer P. M., Vaschillo E., Vaschillo B., Lu S.-E., Eckberg D. L., Edelberg R., et al. (2003). Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosom. Med. 65 (5), 796–805. doi:10.1097/01.psy.0000089200.81962.19
Liu J., Lv C., Wang W., Huang Y., Wang B., Tian J., et al. (2022). Slow, deep breathing intervention improved symptoms and altered rectal sensitivity in patients with constipation-predominant irritable bowel syndrome. Front. Neurosci. 16, 1034547. doi:10.3389/fnins.2022.1034547
Lunding J. A., Nordström L. M., Haukelid A.-O., Gilja O. H., Berstad A., Hausken T. (2008). Vagal activation by sham feeding improves gastric motility in functional dyspepsia. Neurogastroenterol. Motil. Official J. Eur. Gastrointest. Motil. Soc. 20 (6), 618–624. doi:10.1111/j.1365-2982.2007.01076.x
Mróz M., Czub M., Brytek-Matera A. (2022). Heart rate variability-an index of the efficacy of complementary therapies in irritable bowel syndrome: a systematic review. Nutrients 14 (16), 3447. doi:10.3390/nu14163447
Mujagic Z., Keszthelyi D., Aziz Q., Reinisch W., Quetglas E. G., De Leonardis F., et al. (2015). Systematic review: instruments to assess abdominal pain in irritable bowel syndrome. Alimentary Pharmacol. and Ther. 42 (9), 1064–1081. doi:10.1111/apt.13378
Ognev A. S., Zernov V. A., Likhacheva E. V., Nikolaeva L. P., Rudenko M. Y., Kagonyan R. S., et al. (2019). Validity of cardiometric performance data: an integral part of complex assessment of training session effectiveness. Cardiometry 14 (14), 96–100. doi:10.12710/cardiometry.2019.14.96100
Pagani M., Lombardi F., Guzzetti S., Sandrone G., Rimoldi O., Malfatto G., et al. (1984). Power spectral density of heart rate variability as an index of sympatho-vagal interaction in normal and hypertensive subjects. J. Hypertens. 2 (3), S383–S385.
Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Plews D. J., Scott B., Altini M., Wood M., Kilding A. E., Laursen P. B. (2017). Comparison of heart-rate-variability recording with smartphone photoplethysmography, polar H7 chest strap, and electrocardiography. Int. J. Sports Physiology Perform. 12 (10), 1324–1328. doi:10.1123/ijspp.2016-0668
Rocque R., Leanza Y. (2015). A systematic review of patients’ experiences in communicating with primary care physicians: intercultural encounters and a balance between vulnerability and integrity. PloS One 10 (10), e0139577. doi:10.1371/journal.pone.0139577
Rome IV Criteria (2020). Rome IV criteria. Available online at: https://theromefoundation.org/rome-iv/rome-iv-criteria/.
Russo M. A., Santarelli D. M., O’Rourke D. (2017). The physiological effects of slow breathing in the healthy human. Breathe Sheff. Engl. 13 (4), 298–309. doi:10.1183/20734735.009817
Schamberg G., Calder S., Varghese C., Xu W., Wang W. J., Ho V., et al. (2023). Comparison of Gastric Alimetry® body surface gastric mapping versus electrogastrography spectral analysis. Sci. Rep. 13 (1), 14987. doi:10.1038/s41598-023-41645-w
Shaffer F. (2020). Resonance frequency assessment: the challenge of standardizing heart rate variability biofeedback research. Biofeedback (Online); Lawrence 48 (1), 7–15. doi:10.5298/1081-5937-48.01.06
Shaffer F., Ginsberg J. P. (2017). An overview of heart rate variability metrics and norms. Front. Public Health 5, 258. doi:10.3389/fpubh.2017.00258
Shaffer F., McCraty R., Zerr C. L. (2014). A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 5, 1040. doi:10.3389/fpsyg.2014.01040
Shaffer F., Meehan Z. M. (2020). A practical guide to resonance frequency assessment for heart rate variability biofeedback. Front. Neurosci. 14, 570400. doi:10.3389/fnins.2020.570400
Shaffer F., Moss D., Meehan Z. M. (2022). Rhythmic skeletal muscle tension increases heart rate variability at 1 and 6 contractions per minute. Appl. Psychophysiol. Biofeedback 47 (3), 183–192. doi:10.1007/s10484-022-09541-7
Shimamura Y., Inoue H., Rodriguez de Santiago E., Abad M. R. A., Fujiyoshi Y., Toshimori A., et al. (2021). Characterization of intragastric pressure waveform in endoscopic pressure study integrated system: novel diagnostic device for gastroesophageal reflux disease. Dig. Endosc. Official J. Jpn. Gastroenterological Endosc. Soc. 33 (5), 780–787. doi:10.1111/den.13867
Sperber A. D., Bangdiwala S. I., Drossman D. A., Ghoshal U. C., Simren M., Tack J., et al. (2021). Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology 160 (1), 99–114.e3. doi:10.1053/j.gastro.2020.04.014
Spiegel B., Bolus R., Harris L. A., Lucak S., Naliboff B., Esrailian E., et al. (2009). Measuring irritable bowel syndrome patient-reported outcomes with an abdominal pain numeric rating scale. Alimentary Pharmacol. and Ther. 30 (11-12), 1159–1170. doi:10.1111/j.1365-2036.2009.04144.x
Steffen P. R., Austin T., DeBarros A., Brown T. (2017). The impact of resonance frequency breathing on measures of heart rate variability, blood pressure, and mood. Front. Public Health 5, 222. doi:10.3389/fpubh.2017.00222
Stern M. J., Guiles R. A. F., Gevirtz R. (2014). HRV biofeedback for pediatric irritable bowel syndrome and functional abdominal pain: a clinical replication series. Appl. Psychophysiol. Biofeedback 39 (3-4), 287–291. doi:10.1007/s10484-014-9261-x
Tee V., Kuan G., Kueh Y. C., Abdullah N., Sabran K., Tagiling N., et al. (2022). Development and validation of audio-based guided imagery and progressive muscle relaxation tools for functional bloating. PloS One 17 (9), e0268491. doi:10.1371/journal.pone.0268491
Thomas B. L., Claassen N., Becker P., Viljoen M. (2019). Validity of commonly used heart rate variability markers of autonomic nervous system function. Neuropsychobiology 78 (1), 14–26. doi:10.1159/000495519
Tricco A. C., Lillie E., Zarin W., O’Brien K. K., Colquhoun H., Levac D., et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 169 (7), 467–473. doi:10.7326/M18-0850
Tyagi A., Cohen M. (2016). Yoga and heart rate variability: a comprehensive review of the literature. Int. J. Yoga 9 (2), 97–113. doi:10.4103/0973-6131.183712
Vandenberk T., Stans J., Mortelmans C., Van Haelst R., Van Schelvergem G., Pelckmans C., et al. (2017). Clinical validation of heart rate apps: mixed-methods evaluation study. JMIR mHealth uHealth 5 (8), e129. doi:10.2196/mhealth.7254
Van Oudenhove L., Crowell M. D., Drossman D. A., Halpert A. D., Keefer L., Lackner J. M., et al. (2016). Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology 150 (6), 1355–1367.e2. doi:10.1053/j.gastro.2016.02.027
Vaschillo E. G., Vaschillo B., Lehrer P. M. (2006). Characteristics of resonance in heart rate variability stimulated by biofeedback. Appl. Psychophysiol. Biofeedback 31 (2), 129–142. doi:10.1007/s10484-006-9009-3
Wheat A. L., Larkin K. T. (2010). Biofeedback of heart rate variability and related physiology: a critical review. Appl. Psychophysiol. Biofeedback 35 (3), 229–242. doi:10.1007/s10484-010-9133-y
Zhang S., Zhang C., Fan M., Chen T., Yan H., Shi N., et al. (2023). Neuromodulation and functional gastrointestinal disease. Neuromodulation J. Int. Neuromodulation Soc. 27, 243–255. doi:10.1016/j.neurom.2023.08.001
Keywords: heart rate variability, functional gastrointestinal disorder, biofeedback, disorder of gut brain interaction, resonance breathing
Citation: Pereira AG, Fu L, Xu W, Gharibans AA and O’Grady G (2025) The effects of heart rate variability biofeedback on functional gastrointestinal disorders: a scoping review. Front. Physiol. 16:1511391. doi: 10.3389/fphys.2025.1511391
Received: 14 October 2024; Accepted: 24 April 2025;
Published: 22 May 2025.
Edited by:
Karin Meissner, Hochschule Coburg, GermanyReviewed by:
Zheng Yu, Chengdu University of Traditional Chinese Medicine, ChinaMaisiyiti Alimujiang, People’s Hospital of Xinjiang Uygur Autonomous Region, China
Vincent Tee, Universiti Sains Malaysia Health Campus, Malaysia
Copyright © 2025 Pereira, Fu, Xu, Gharibans and O’Grady. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Greg O’Grady, Z3JlZy5vZ3JhZHlAYXVja2xhbmQuYWMubno=
 Ashley G. Pereira
Ashley G. Pereira Lily Fu1
Lily Fu1 Armen A. Gharibans
Armen A. Gharibans Greg O’Grady
Greg O’Grady