- 1Plastic Surgery & Burns Research Unit, Centre for Skin Sciences, University of Bradford, Bradford, United Kingdom
- 2St Andrew’s Anglia Ruskin (StAAR) Research Group, Anglia Ruskin University, Chelmsford, United Kingdom
- 3Department of Biology, College of Science, University of Sulaimani, Kurdistan Region, Iraq
- 4Charles Institute of Dermatology, School of Medicine, Dublin, Ireland
- 5Conway Institute, University College Dublin, Dublin, Ireland
Background: Hair plays a crucial role in social and sexual communication; hair disorders such as alopecia or hirsutism can therefore cause psychological distress. Current treatments are limited by unwanted side effects and a lack of understanding of hair follicle (HF) regulation, particularly in miniaturised intermediate or vellus-like follicles; the clinical targets in hair loss disorders. The discovery that bimatoprost, a prostamide F2α analogue, stimulates eyelash growth suggest a possible role for other prostanoids in hair growth.
Objectives: To evaluate the impact of the naturally occurring prostaglandin F2α (PGF2α) on human intermediate HF growth, comparing the effects on matched terminal and intermediate follicles using a pre-clinical ex vivo organ culture model. Furthermore, to determine the involvement of PGF2α receptors (FP) and their location within both these HF types.
Methods: Matched human female pre-auricular facelift skin HFs were incubated with PGF2α alone or in combination with an FP antagonist for 9 days in the gold-standard ex vivo HF organ culture model. To confirm FP gene expression in both terminal and intermediate lower HF bulbs, RT-PCR was performed using specific FP primers, confirmed by sequence analysis. Immunohistochemistry was conducted using frozen sections to locate the FP protein in HF components.
Results: PGF2α (100 nM) stimulated terminal HF fibre growth by 4.93% (p = 0.019) with a greater effect (10.03% (p < 0.001) stimulation) on intermediate HFs. PGF2α stimulation significantly prolonged anagen (the growth phase of the hair cycle) duration in both HF types and to similar extent. These increases in hair fibre elongation were blocked by the receptor (FP) antagonist in both terminal and intermediate follicles. RT-PCR confirmed FP gene expression and immunohistochemistry located FP protein in the dermal papilla and connective tissue sheath of both intermediate and terminal HFs.
Conclusion: We demonstrate, for the first time, that PGF2α stimulates human HF growth in organ culture via a receptor-driven mechanism, probably directly affecting the follicles’ regulatory dermal papilla function. The greater response of intermediate, compared to matched terminal, HFs suggests potential future clinical significance for medical conditions such as alopecia, or insufficient beard growth, and promoting hair growth in ‘relatively hairless’ donor graft skin or transplant follicles after elective, trauma or burn injury surgical reconstruction.
Introduction
Scalp hair plays an important role in human social and sexual communication, and disorders like alopecia or hirsutism can cause significant psychological distress and reduced quality of life (Randall, 2007; Randall, 2008; Aukerman and Jafferany, 2023). Non-pharmaceutical or non-surgical approaches for hair loss primarily involve concealing alopecic regions, e.g., comb-over hairstyles or utilising hairpieces (Beehner, 2013). Medical treatments have limited effectiveness and/or may be associated with side-effects. The three FDA- approved drugs for hypotrichosis have different origins and mechanisms (Devjani et al., 2023). Minoxidil, initially developed as a vasodilator, opens hair follicle (HF) associated potassium channels (Nusbaum et al., 2013; Shorter et al., 2008a; Randolph and Tosti, 2021), while finasteride, a 5α-reductase inhibitor evolved from benign prostatic hyperplasia treatment, reduces testosterone conversion to the more active form, 5α-dihydrotestosterone (Kaufman et al., 1998; Gupta et al., 2022). Lastly, bimatoprost, a prostamide F2α analogue, used for treatment of eyelash hypotrichosis, and initially a glaucoma therapy, can increase human scalp terminal HF growth in whole organ culture via direct action on the prostamide F2α receptor (Khidhir et al., 2013a; Subedi et al., 2022). Furthermore, some clinical reports have indicated effects of other medical treatments, such as platelet-rich-plasma (PRP), herbal extracts or their metabolites, on promoting hair growth and reducing hair loss (Wadstein et al., 2020; Gentile et al., 2017; Dhariwala and Ravikumar, 2019). Although time-consuming, HF micrografting enables delicate reconstruction, even in previously diseased areas as seen in scalp hair transplants; this is due to the unique nature of gene expression of single HFs, in part as they retain characteristics of their originating body site location (Rose, 2015; Saxena and Savant, 2017; Miranda et al., 2010; Miranda et al., 2011). More traditional surgical techniques, e.g., using rotation flaps or tissue expanders, remain important for reconstructing large areas (Krishna et al., 2023; Jayapaul et al., 2018).
New therapeutic approaches are hampered by our still-limited understanding of hair follicle bio-physiology. Specifically, human studies have largely been restricted to the large-calibre often pigmented terminal scalp HF that is much easier to microdissect and manipulate in whole-organ culture (Shorter et al., 2008a; Philpott et al., 1990; Anastassakis, 2022a; Anastassakis, 2022b). HFs are very prone to change in response to factors such as hormonal stimulation, ultimately altering the hair fibre produced (Randall, 2007). For example, under the influence of androgen stimulation during puberty, vellus HFs that produce the short and fine hair on a child’s face develop into intermediate-sized and eventually terminal HFs, producing longer, visible but low calibre and coarse hair fibres (e.g., man’s beard) respectively (Randall, 2008; Grymowicz et al., 2020). Paradoxically in genetically-predisposed patients with androgenetic and female-pattern alopecia, terminal HFs in androgen-sensitive scalp areas gradually respond to adult androgen levels by miniaturising to transform into intermediate and eventually tiny vellus-like HFs as seen on bald scalp skin (Hamilton, 1951; Whiting et al., 1999; Batrinos, 2014). HFs accomplish these transformative morphological changes during successive hair growth cycles (Saitoh et al., 1970). This cycle involves periods of active growth (anagen), regression (catagen), relative “quiescence” (telogen) and shedding (exogen) (Oh et al., 2016). In general, as the duration of anagen shortens, so too does the hair fibre length and calibre; HFs that remain in anagen for longer producing longer thicker hair fibres (Whiting, 2001).
Hair loss treatment in vivo is almost entirely targeted at small vellus-like or intermediate HFs, aiming to stimulate their transformation to terminal HFs. Despite that, there has been little research focus on these non-terminal human HF subtypes (Anastassakis, 2022b). Vellus-like and other miniaturised HFs are theoretically the ideal study model for hair loss investigations, as these represent the progressing or end point of the balding process. However, vellus-like HFs are so small, that their microdissection, manipulation and growth in organ culture is impractical. Intermediate HFs however, while still being technically extremely challenging to micro-dissect and culture as whole organs, have been morphometrically characterised and offer a more realistic model system (Miranda et al., 2010). Previous human studies have explored the relationship between HF morphologies and their associated hair fibre types; terminal anagen HFs extend deep into the skin’s subcutaneous fat layer and have significantly larger bulb diameters than vellus HFs (Vogt et al., 2007). Studies examining the relative size of HF components have demonstrated correlations between hair fibre calibre and length, volume and cellularity of the HF dermal papilla and hair bulb matrix size (Elliott et al., 1999). Intermediate HFs also exhibit morphological differences to terminal HFs, penetrating to a more shallow depth in the skin dermis, exhibiting lower volume HF cellularity, and importantly retain significant, biologically-relevant differences in vitro in organ culture (Miranda et al., 2010). Notably, intermediate HFs at the scalp/facial interface were the first human organ to show an appropriate response to a relevant hormone (testosterone), in organ culture (Miranda et al., 2018).
The role of prostanoids in HFs is not well understood. Eyelash HFs in vivo and scalp terminal HFs in ex vivo organ culture can be stimulated to produce more hair fibre by prostamide F2α (bimatoprost) (Khidhir et al., 2013a; Tauchi et al., 2010; Cohen, 2010). Scalp HFs are reported to express receptors for prostaglandin F2α (PGF2α) and prostamide F2α in vivo (Khidhir et al., 2013a). The aim of this study is to expand our understanding of prostanoid function in human skin by investigating whether the natural prostaglandin, PGF2α, affects the growth (as assessed by hair fibre elongation) of the more clinically-relevant human intermediate HF in organ culture. We also aim to determine whether such effects are mediated via PGF2α receptors (FP), investigating FP receptor location, and comparing findings between matched human intermediate and terminal HFs.
Materials and methods
Skin samples
Donor-matched terminal and intermediate HFs were micro-dissected from fresh human female (n = 6) pre-auricular facelift skin sourced from elective (cosmetic) plastic surgery (age range 49–65 years) (Figure 1). For organ culture investigations, samples were collected into sterile universal tubes (25 mL or 50 mL) containing basic culture medium: William’s E medium supplemented with 10μg/mL insulin, 10ng/mL hydrocortisone, 2 mM L-glutamine (Life Technologies, Paisley, UK), and 10U/mL penicillin. Unless specified, Sigma-Aldrich (Dorset, UK) supplied all materials. Skin specimens were transported on ice and stored at 4°C until HFs were isolated within 24 h of removal from the donor. For molecular biological investigations, small skin samples (about 1 cm3) were placed into sterile universal tubes (10 mL) containing RNA stabilization solution, RNAlater, to inhibit RNase activity. They were kept at 4°C overnight to allow tissue penetration by RNAlater before storage at −20°C until analysis. For immunohistochemical investigations, skin samples were similarly collected and cut into small pieces, embedded in optimal cutting temperature (OCT) compound (Raymond A. Lamb; ThermoFisher Scientific), and stored at −80°C until used.
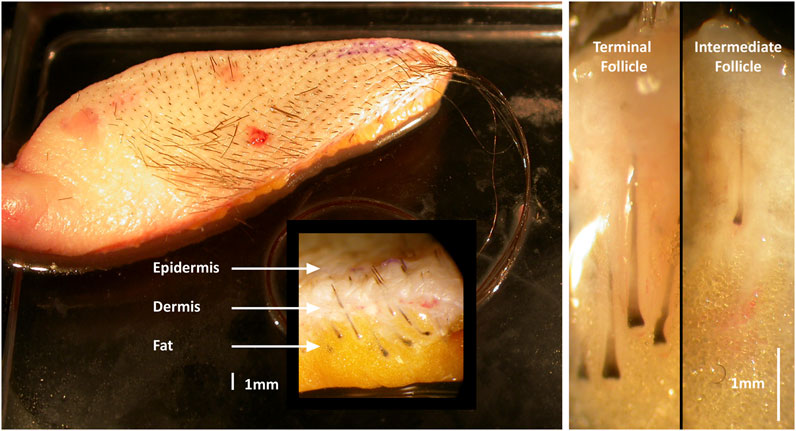
Figure 1. Human adult female pre-auricular facelift skin. Terminal hair follicles (HF) are larger and extend deeper into the sub-dermal fat than intermediate HFs.
Microdissection of terminal and intermediate hair follicles
Matched terminal and intermediate anagen HFs (from the same skin specimen) were micro-dissected, despite significant technical challenge, from each human pre-auricular facelift sample under sterile conditions (Leica MZ8 dissecting microscope with fibreoptic cool illumination, Leica Microsystems, Wetzlar, Germany). Each HF was transferred to a Petri dish containing sterile phosphate buffered saline (PBS; Oxoid, Basingstoke, UK) for organ culture or into RNAlater at 4°C for molecular biological studies. Isolated HFs were pooled into a fresh dish of cold PBS or RNAlater, and individually cleared of attached dermis or subcutaneous fat material using 27.5-gauge sterile syringe needles. Care was taken to ensure that HFs remained undamaged during isolation to protect their viability as is essential for successful culture (Figure 2) (Shorter et al., 2008b). Matched terminal (n = 60) and intermediate (n = 80) HFs were micro-dissected from each human pre-auricular facelift sample to investigate gene expression.
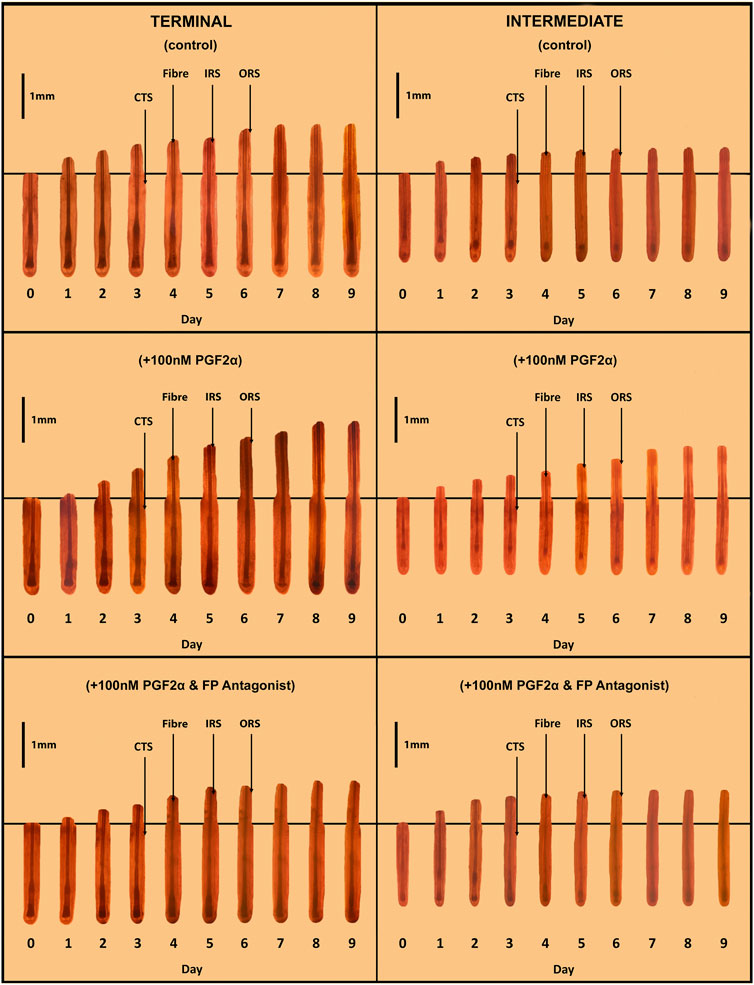
Figure 2. Sequential photo-micrographs of terminal and intermediate hair follicles (HF) in ex vivo whole organ culture under control, PGF2α-stimulated and PGF2α-inhibited conditions. Sequential photomicrographs, taken every 24h for 9 days, of representative individual terminal and intermediate HFs, demonstrate elongation/growth of the hair fibre (Fibre), inner (IRS) and outer root (ORS) sheaths. Note the connective tissue sheath (CTS) does not grow-out with the epithelial compartment of the HF, and so indicates the original length of the HF on isolation.
Hair follicle organ culture
Ex vivo hair follicle organ culture is the gold standard in human hair growth research. Isolated terminal and intermediate HFs were transferred to individual wells of 24-well culture plates in 1 mL of appropriate medium as follows. Basic culture medium (see above) was supplemented with either PGF2α (100 nM; 900123P, Sigma-Aldrich) or PGF2α (100 nM) + PGF2α receptor (FP) antagonist (1μM; AL-8810, Sigma-Aldrich); both reagents were dissolved in 0.001% dimethyl sulfoxide (DMSO). Control (unstimulated) medium was supplemented only with 0.001% DMSO. Cultured HFs were maintained ‘free-floating’ at 37°C in an atmosphere of 5% CO2 and 95% air in a humidified incubator. Medium was refreshed every 3 days.
Statistical assessment of hair follicle growth in culture
Terminal and intermediate HFs were microscopically-assessed for maintenance of anagen HF bulb morphology and photographed (Nikon Coolpix 4,500 digital camera, Nikon, Tokyo, Japan). Hair fibre elongation (our marker of HF ‘growth’) was measured every 24h for 9 days, using an inverted microscope (Leitz Labovert FS; Leica Microsystems). HFs exhibiting failure to grow within the first 3 days of the culture period were deemed damaged and “nonviable” and were excluded. After confirming normal distribution by using Kolmogorov Smirnov’s test, growth data were analysed using the Student’s paired t-test, and the percentage of HFs remaining in anagen over 9 days were analysed using ANOVA (SPSS, Chicago, IL, United States) (Miranda et al., 2018).
FP mRNA expression in freshly-isolated terminal and intermediate hair follicles
Total RNA was isolated from fresh terminal and intermediate HFs (immediately after microdissection), as described previously (Shorter et al., 2008a) using a GenElute Mammalian Total RNA kit (Sigma-Aldrich, Dorset, England) or RNeasy Mini Kit (Qiagen, Hilden, Germany), and quality-checked by agarose gel electrophoresis (1.5%) before further purification to isolate poly (A+)RNA using a GenElute mRNA Miniprep kit (Sigma-Aldrich). RT-PCR was used to investigate FP mRNA expression. To remove any contaminating genomic DNA, mRNA samples were treated with DNase I (Invitrogen, Paisley, UK), and cDNAs were synthesised using the avian myeloblastosis virus (AMV) reverse-transcription system (Promega, Southampton, UK), as described previously (Shorter et al., 2008a). PCR amplification was performed using 10 μL of cDNA in a 50 μL reaction volume containing 0.3 μM concentrations of forward and reverse primers (Sigma-Genosys Ltd., Pamisford, UK), 200 μM concentrations of each dNTP (Promega), 5 μL of 10× reaction buffer (200 mM Tris-HCl, pH 8.4, and 500mM KCl; Invitrogen), 2.5 mM MgCl2 (Invitrogen), and 2.5U of recombinant TaqDNA polymerase (Invitrogen). Negative controls where nuclease-free water replaced cDNA were run with each PCR reaction. The primers were as follows: β-actin (GenBank NM_001101), forward 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′ and reverse 5′-CTCATACTCCT-GCTTGCTGATCCACATCTGC-3′; FP, forward 5′-CGATGCCATCATCACAGAAG-3′ and reverse 5′-CTGAGCAGCTTCTCTGGCTT-3′.
After initial cDNA denaturation at 95°C (5 min), β-actin cycling conditions were 35 cycles of 95°C (1 min), annealing at 55°C (1 min), and 72°C (1 min) (Shorter et al., 2008b) and FP cycling conditions were 35 cycles of 95°C (30 ), 53°C (30 ), and 72°C (30 ). A final 10 min extension period at 72°C completed the thermocycling before cooling at 4°C (Khidhir et al., 2013b). PCR products were analysed by gel electrophoresis on a 1.5% Tris Acetate-EDTA (TAE) agarose gel (Invitrogen), as described previously (Shorter et al., 2008a).
To confirm product identity, PCR was repeated in a thermocycler with heated lid and products separated on a low-melting-point 1.5% agarose gel, excised, purified using the MinElute Gel Extraction kit (Qiagen, Crawley, UK), and sequenced by Geneblitz (Sunderland, UK). Results were compared with known published sequences using the U.S. National Center for Biotechnology Information (NCBI) Basic Local Alignment Search Tool (BLAST) program (http://www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi).
Immunohistochemistry
Immunohistochemistry was performed to confirm FP protein expression in HFs. Seven μm longitudinal cryosections were taken from terminal and intermediate HFs (Leica cryostat, CM 1800) and collected on poly-l-lysine-coated slides to increase adherence (Shorter et al., 2008b). Immunohistochemistry was performed as described previously (Shorter et al., 2008b), using a goat anti-human FP polyclonal antibody (sc-33364, Santa Cruz Biotechnology, Santa Cruz, CA, United States), at 1:75 dilution at 4°C for 18h. Antibodies were diluted in 1.5% normal mouse serum in PBS.
Results
Hair follicle organ culture
A total of 594 matched terminal and intermediate pre-auricular HFs were micro-dissected from six female facelift patients; 18 terminal and 15 intermediate HFs from each donor were analysed and photographed daily in each of the three organ culture conditions (Figure 2).
Terminal and intermediate HFs were assessed for cumulative growth and duration of maintained anagen hair bulb morphology (to indicate true hair fibre growth capacity) over the 9-day duration of the whole-organ culture assay (Figure 3). Terminal HFs produced more hair fibre than intermediate HFs under control (unstimulated) culture conditions (0.561 ± 0.006 mm vs 0.387 ± 0.029mm; p < 0.001); when stimulated with PGF2α, both terminal (0.647 ± 0.010mm; p = 0.027) and intermediate (0.572 ± 0.013mm; p < 0.001) HFs produced more hair fibre versus control culture conditions (Figure 3). PGF2α–associated stimulated growth was inhibited when terminal (0.559 ± 0.007mm; p = 0.041) and intermediate (0.369 ± 0.043mm; p < 0.001) HFs were grown in PGF2α + FP antagonist conditions (Figure 3).
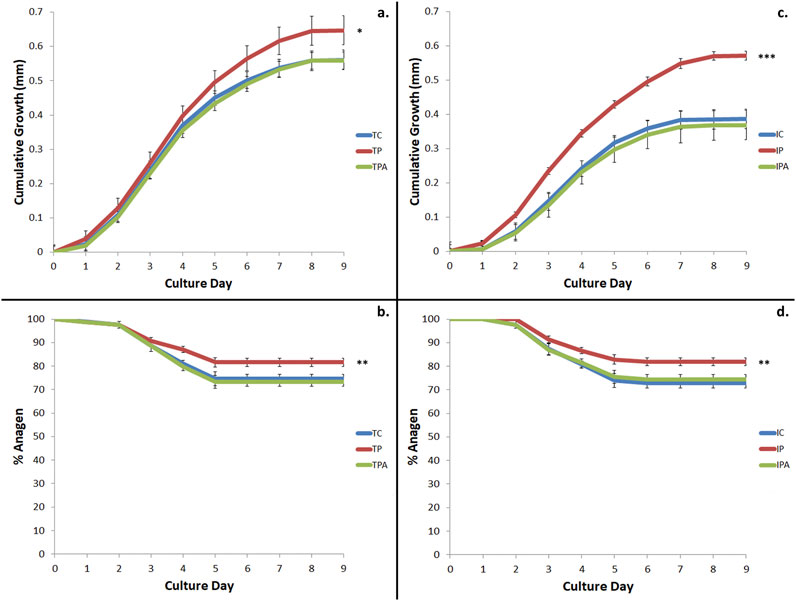
Figure 3. Cumulative hair growth and duration of anagen over 9 days in ex vivo hair follicle (HF) organ culture. (a) Terminal HF growth was stimulated by the addition of 100 nM PGF2α (TP) versus control culture conditions (TC) (p = 0.027); this growth was inhibited by the addition of FP antagonist (TPA) (p = 0.041). (b) Increased terminal hair growth occurred due to a longer time HFs spent in anagen when stimulated by 100 nM PGF2α (TP) versus control (TC) culture conditions (p < 0.01). This was inhibited by the addition of FP antagonist (TPA) (p < 0.01). (c) Intermediate HF growth was stimulated with the addition of 100 nM PGF2α (IP) versus control culture conditions (IC) (p < 0.001); this stimulated growth was inhibited by the addition of FP antagonist (TPA). (d) Increased intermediate HF growth occurred due to a longer time spent in anagen when stimulated by 100 nM PGF2α (IP) versus control (IC) culture conditions (p = 0.001); this was inhibited by the addition of FP antagonist (IPA) (p < 0.001). Of note, intermediate HFs stimulated by 100 nM PGF2α (IP) grew a similar amount as hair fibre as terminal HFs under control culture conditions (TC) (p = 0.708). * = p < 0.05; ** = p < 0.01; *** = p < 0.001.
These above findings concurred with our observation that anagen duration was extended for both terminal (81.59% ± 1.21% vs 74.53% ± 1.92%; p < 0.01) and intermediate (81.86% ± 1.70% vs 72.82% ± 2.07%; p = 0.001) HFs when stimulated with PGF2α versus control culture conditions (Figure 3). Importantly, anagen duration was subsequently reduced when terminal (73.34% ± 1.78%; p < 0.01) and intermediate (74.48% ± 1.91%; p < 0.001) HFs were grown under PGF2α + FP antagonist culture conditions, versus PGF2α culture conditions (Figure 3).
When hair growth was expressed as a percentage of starting HF length, terminal HFs produced more hair fibre than intermediate HFs when cultured under control culture conditions (29.57% ± 0.70% vs 22.42% ± 0.75% respectively; p < 0.001). When stimulated by PGF2α, both terminal (34.50% ± 1.05%; p = 0.019) and intermediate (32.45% ± 0.64%; p < 0.001) HFs exhibited greater percentage fibre growth versus control culture conditions (Figure 4). This PGF2α-stimulated fibre growth effect was inhibited when HFs were grown under PGF2α + FP antagonist culture conditions with terminal HFs showing a 29.22% ± 0.99% (p = 0.016) change and intermediate HFs showing an even lower change of 21.53% ± 0.43% (p < 0.001). Of note, intermediate HFs grown under PGF2α culture conditions exhibited a broadly similar percentage change in new hair fibre growth as terminal HFs grown in control culture conditions (32.45% ± 0.64% vs 29.57% ± 0.70%; p = 0.055) (Figure 4).
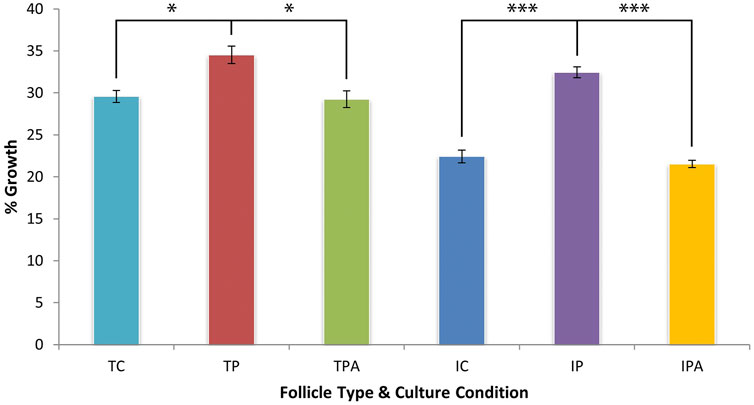
Figure 4. Hair fibre production over 9 days of organ culture, expressed as a percentage of initial hair fibre length. PGF2α stimulated terminal HFs (TP) to increase growth significantly versus control (TC) culture conditions (p = 0.019). This stimulation by 4.93% was less compared to that of intermediate HFs; the increased growth effect was inhibited when terminal HFs were grown in PGF2α + FP antagonist (TPA) culture conditions (p = 0.016). PGF2α also stimulated intermediate HFs (IP) to increase growth significantly by 10.03% versus control (IC) culture conditions (p < 0.001); this increased growth effect was inhibited when intermediate HFs were grown in PGF2α + FP antagonist (IPA) culture conditions (p < 0.001). Of note, intermediate HFs grown in PGF2α culture (IP) conditions were stimulated to produce a similar percentage of hair fibre to terminal HFs grown in control (TC) culture conditions (p = 0.055). * = p < 0.05; *** = p < 0.001.
Prostaglandin receptor expression in anagen hair follicles
RT-PCR and immunohistochemistry was conducted on matched terminal and intermediate pre-auricular HFs micro-dissected from six female facelift patients (Figures 5, 6). RT-PCR confirmed PGF2α receptor gene expression (1,080 bp) (Figure 5), and immunohistochemical analysis located the PGF2α receptor to the dermal papilla and connective sheath in both HF types (Figure 6).
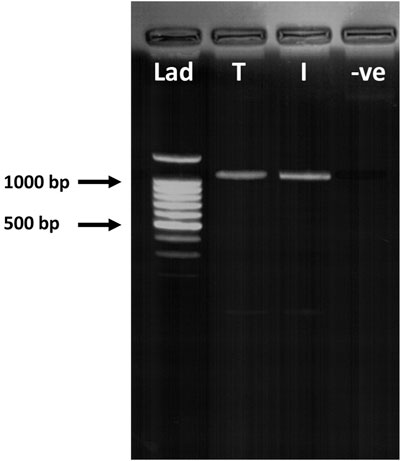
Figure 5. RT-PCR of terminal and intermediate hair follicles (HF). RT-PCR confirmed PGF2α receptor (FP) gene expression in both terminal (T) and intermediate (I) HFs (1,080 bp). Lad = DNA ladder; -ve = negative control.
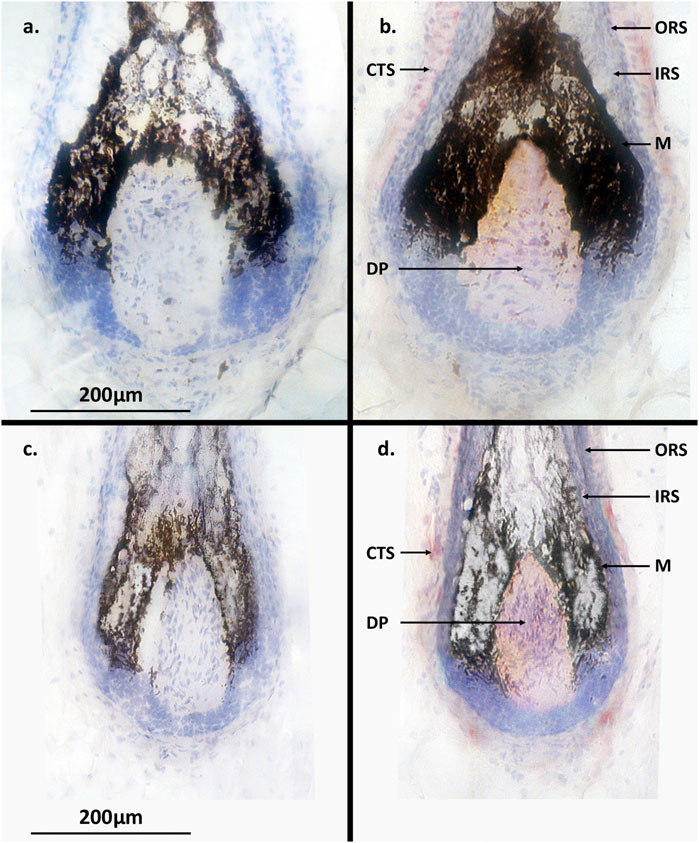
Figure 6. Terminal and intermediate hair follicle (HF) immunohistochemistry. (a) No PGF2α receptor (FP) expression was detected in terminal HFs without incubation with anti-FP antibody. (b) Expression (pink-red staining) of FP was located in the dermal papilla and connective tissue sheath of terminal HFs but not in the epithelial cell hair matrix. (c) No PGF2α receptor (FP) expression was detected in the control intermediate HFs. (d) Expression of FP (pink-red staining) was located in the dermal papilla and connective tissue sheath of intermediate HFs. ORS = outer root sheath; IRS = inner root sheath; CTS = connective tissue sheath; M = melanocytes; DP = dermal papilla.
Discussion
We present the first investigation of the effects of the naturally-occurring PGF2α on human intermediate HF growth in ex vivo organ culture. Ex vivo organ culture of terminal scalp HFs is the gold standard in human hair growth research and is now considered a pre-clinical assay (Miranda et al., 2010; Philpott et al., 1990; Miranda et al., 2018). The development of human HF ex vivo organ culture techniques was a very significant milestone in cutaneous biology research (Philpott et al., 1990). Importantly, these cultured HFs, despite being isolated from their circulation and innervation, retain for several days (typically around 7 days) their capacity to produce hair fiber at approximately in vivo rates. The smaller, finer intermediate HFs are relatively much more difficult to isolate and grow undamaged compared to their larger terminal scalp HF counterparts. This was evidenced by our observation that as many as one in four isolated intermediate HFs failed to exhibit hair fibre elongation during the first few days after microdissection, despite having anagen hair bulb morphology.
We demonstrate the capacity of intermediate HFs, isolated from adult human female pre-auricular skin, to respond to PGF2α stimulation with increased hair growth versus their behaviour under control culture conditions; however, the degree of this stimulated growth was similar to that observed with unstimulated terminal HFs grown under control culture conditions. This provides support to the concept that intermediate HF growth is less compared to terminal HF growth, however may be stimulated to a similar rate. Moreover, antagonising FP receptors in both HF types (adding PGF2α receptor antagonist) reduced PGF2α-associated growth stimulation to control (unstimulated) levels. Thus, it was evident that the enhanced growth effect was primarily the result of PGF2α stimulation.
FP gene expression in the lower hair follicle bulb and FP protein expression in the dermal papilla and connective tissue sheath were confirmed by RT-PCR and immunohistochemistry, respectively. In that context, our findings support the view that this ex vivo stimulation was mediated via FP receptors located in the HF mesenchyme, particularly those in the hair-growth regulatory centre, the dermal papilla, but also in the lower connective tissue sheath (also known as the HF dermal sheath) in both terminal and intermediate HFs.
The implication of FP receptor-mediated mechanism(s) in vivo remain unclear, as other paracrine signalling scenarios may also occur in vivo (Chen et al., 2020; Kim et al., 2019; Lü et al., 2006; Bassino et al., 2015). Still, our findings align with previous studies that identified the expression of FP receptors and receptors for other stimulatory hormones within the nuclei of dermal papilla cells in terminal HFs but not in other HF anatomical (typically epithelial) components (Randall, 2007; Ami et al., 1995). The expression of FP receptors in connective tissue sheath cells aligns with our previous research (Khidhir et al., 2013a) and with the reported role of these HF mesenchymal cells in initiating new HF development (McElwee et al., 2003; Wang et al., 2014).
The dermal papilla is regarded as the organising centre of the HF, regulating HF growth and likely also pigmentation (Lei et al., 2017; Ng et al., 2022; Kwack et al., 2019). Other studies also localised key prostanoid receptors in the HF, especially within the dermal papilla, including FP, prostaglandin E2 receptors (EP2, EP3, EP4), prostaglandin D2 receptor (DP2), prostanoid thromboxane A2 receptor (TP), and prostaglandin I2 receptor (IP) (Khidhir et al., 2013a; Colombe et al., 2008; Xu and Chen, 2018). Furthermore, several studies have reported a role for prostaglandins in modulating hair growth (Johnstone and Albert, 2002; Colombe et al., 2007; Shin, 2022; Chovarda et al., 2021). For example, PGF2α exhibits hair growth-promoting properties, while reduced PGE2 levels have been observed in androgenetic alopecia scalp tissue (Shin, 2022; Chovarda et al., 2021; Choi et al., 2015). Conversely, the prostaglandin PGD2 exerts hair growth-inhibitory effects and is found in elevated levels in androgenetic alopecia scalp tissue (Garza et al., 2012; Nieves and Garza, 2014). Meanwhile, bimatoprost, a synthetic structural analogue of PGF2α-ethanolamide, can stimulate growth (length and caliber) and pigmentation of terminal eyebrow and eyelash HFs (Cohen, 2010; Beer et al., 2013; Suwanchatchai et al., 2012; Carruthers et al., 2016; Fagien, 2010). This molecule is used widely for treating glaucoma and eyelash hypotrichosis (Khidhir et al., 2013a; Woodward et al., 2013; Wall et al., 2022; Law, 2010; Schweiger et al., 2012). Similarly, latanoprost, another PGF2α analogue, is reported to induce the anagen (growth) phase and to some limited extent augment hair density in scalps affected by androgenetic alopecia (Johnstone and Albert, 2002; Choi et al., 2015; Blume-Peytavi et al., 2012; Rafati et al., 2022).
Adult female facial intermediate HFs represent an innovative, hormone-responsive human organ model that can be cultured and manipulated in the standard laboratory setting. While these intermediate HFs hold promise for fundamental scientific research investigations and drug testing, there are inherent challenges associated with their sourcing and in-lab manipulation due to their diminutive size. The relatively enormous rodent vibrissae HF has frequently been the preferred model to study developmental processes and stem cell niches (Jahoda et al., 1984; Whitehouse et al., 2002). This is because elaboration of the lower HF during the anagen phase of the hair growth cycle recapitulates many of the same molecular processes that underpin hair follicle morphogenesis including tissue regeneration from epithelial and melanocyte stem cells, under the guiding influence of the dermal papilla (Deng et al., 2021; Zhang et al., 2021; Fu et al., 2021). Still, research utilising human intermediate HFs may have significant relevance in understanding mesenchymal-epithelial biological systems in general, including cell signalling, development, stem cell manipulation, and tissue engineering. We emphasise here the importance of leveraging human HF models for hair growth studies like these. Rodent models by contrast, are highly limiting, not only because of their HFs’ miniature size, but also because these mammals do not exhibit the common hair loss disorders affecting humans (e.g., androgenetic alopecia, frontal fibrosing alopecia, etc.). Similarly, implanting individual human HFs into the skin of rodent hosts for assessment of drug effects is fraught with several confounding issues. The attempt to integrate the relatively ‘enormous’ human scalp HF into fragile and thin mouse epidermis is likely to be highly disruptive, artefactual and importantly inconsistent with retaining the human HFs in the anagen (i.e., growth) state.
In summary, our findings offer the first evidence for the impact of the natural prostaglandin, PGF2α, on human intermediate hair follicle growth using an ex vivo whole organ culture model; this seemingly occurs via action on the PGF2α receptor FP via direct modulation of dermal papilla function. The potential clinical significance of these findings relates to their potential use in dermatological conditions such as alopecia and insufficient beard growth. It is unknown whether prolonged use of PGF2α may lead to tolerance or other changes to the hair growth pattern, but experience with the FDA-approved and highly-effective prostaglandin-related bimatoprost, for hair growth of the eyelashes, provides a helpful context for likely continued benefit. Furthermore, extended application to surgical reconstruction may also be beneficial, for example, after trauma or burn injury, to promote hair growth in relatively ‘hairless’ donor graft skin or transplant follicles.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Plastic Surgery & Burns Unit, Centre for Skin Sciences, University of Bradford. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review and editing. KK: Data curation, Formal Analysis, Investigation, Validation, Writing – original draft, Writing – review and editing. DT: Formal Analysis, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Plastic Surgery & Burns Unit, Centre for Skin Sciences, University of Bradford, United Kingdom.
Acknowledgments
We thank Professor Valerie A Randall and the late Professor David T Sharpe for their support with this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ami S., Rata S., Noda T., Kayasu S. (1995). Interaction between dermal papilla cells and follicular epithelial cells in vitro: effect of androgen. Br. J. Dermatology 132 (4), 527–532. doi:10.1111/j.1365-2133.1995.tb08706.x
Anastassakis K. (2022a). “Types of hair follicles in humans,” in Androgenetic alopecia from A to Z: vol 1 basic science, diagnosis, etiology, and related disorders, 77–81.
Anastassakis K. (2022b). The mission of hair follicles and hair. Androg. Alopecia A Z Vol 1 Basic Sci. Diagnosis, Etiology, Relat. Disord., 15–21. doi:10.1007/978-3-030-76111-0_2
Aukerman E. L., Jafferany M. (2023). The psychological consequences of androgenetic alopecia: a systematic review. J. Cosmet. Dermatology 22 (1), 89–95. doi:10.1111/jocd.14983
Bassino E., Gasparri F., Giannini V., Munaron L. (2015). Paracrine crosstalk between human hair follicle dermal papilla cells and microvascular endothelial cells. Exp. Dermatol. 24 (5), 388–390. doi:10.1111/exd.12670
Beehner M. L. (2013). Management of advanced hair loss patterns. Facial Plast. Surg. Clin. 21 (3), 385–395. doi:10.1016/j.fsc.2013.05.008
Beer K. R., Julius H., Dunn M., Wilson F. (2013). Treatment of eyebrow hypotrichosis using bimatoprost: a randomized, double-blind, vehicle-controlled pilot study. Dermatol Surg. 39 (7), 1079–1087. doi:10.1111/dsu.12199
Blume-Peytavi U., Lönnfors S., Hillmann K., Bartels N. G. (2012). A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J. Am. Acad. Dermatology 66 (5), 794–800. doi:10.1016/j.jaad.2011.05.026
Carruthers J., Beer K., Carruthers A., Coleman W. P., Draelos Z. D., Jones D., et al. (2016). Bimatoprost 0.03% for the treatment of eyebrow hypotrichosis. Dermatol Surg. 42 (5), 608–617. doi:10.1097/DSS.0000000000000755
Chen Y., Huang J., Chen R., Yang L., Wang J., Liu B., et al. (2020). Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth. Theranostics 10 (3), 1454–1478. doi:10.7150/thno.39566
Choi Y. M., Diehl J., Levins P. C. (2015). Promising alternative clinical uses of prostaglandin F2α analogs: beyond the eyelashes. J. Am. Acad. Dermatology 72 (4), 712–716. doi:10.1016/j.jaad.2014.10.012
Chovarda E., Sotiriou E., Lazaridou E., Vakirlis E., Ioannides D. (2021). The role of prostaglandins in androgenetic alopecia. Int. J. Dermatology 60 (6), 730–735. doi:10.1111/ijd.15378
Cohen J. L. (2010). Enhancing the growth of natural eyelashes: the mechanism of bimatoprost-induced eyelash growth. Dermatol Surg. 36 (9), 1361–1371. doi:10.1111/j.1524-4725.2010.01522.x
Colombe L., Michelet J. F., Bernard B. A. (2008). Prostanoid receptors in anagen human hair follicles. Exp. Dermatol. 17 (1), 63–72. doi:10.1111/j.1600-0625.2007.00639.x
Colombe L., Vindrios A., Michelet J. F., Bernard B. A. (2007). Prostaglandin metabolism in human hair follicle. Exp. Dermatol. 16 (9), 762–769. doi:10.1111/j.1600-0625.2007.00586.x
Deng W., Zhang Y., Wang W., Song A., Mukama O., Huang J., et al. (2021). Hair follicle-derived mesenchymal stem cells decrease alopecia areata mouse hair loss and reduce inflammation around the hair follicle. Stem Cell Res. and Ther. 12, 548.
Devjani S., Ezemma O., Kelley K. J., Stratton E., Senna M. (2023). Androgenetic alopecia: therapy update. Drugs 83, 701–715. doi:10.1007/s40265-023-01880-x
Dhariwala M. Y., Ravikumar P. (2019). An overview of herbal alternatives in androgenetic alopecia. J. Cosmet. Dermatology 18 (4), 966–975. doi:10.1111/jocd.12930
Elliott K., Messenger A. G., Stephenson T. J. (1999). Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: implications for the control of hair follicle size and androgen responses. J. investigative dermatology 113 (6), 873–877. doi:10.1046/j.1523-1747.1999.00797.x
Fagien S. (2010). Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin. Cosmet. Investig. Dermatol 3, 39–48. doi:10.2147/ccid.s5488
Fu D., Huang J., Li K., Chen Y., He Y., Sun Y., et al. (2021). Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed. and Pharmacother. 137, 111247. doi:10.1016/j.biopha.2021.111247
Garza L. A., Liu Y., Yang Z., Alagesan B., Lawson J. A., Norberg S. M., et al. (2012). Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci. Transl. Med. 4 (126), 126ra34–ra34. doi:10.1126/scitranslmed.3003122
Gentile P., Cole J. P., Cole M. A., Garcovich S., Bielli A., Scioli M. G., et al. (2017). Evaluation of not-activated and activated PRP in hair loss treatment: role of growth factor and cytokine concentrations obtained by different collection systems. Int. J. Mol. Sci. 18 (2), 408. doi:10.3390/ijms18020408
Grymowicz M., Rudnicka E., Podfigurna A., Napierala P., Smolarczyk R., Smolarczyk K., et al. (2020). Hormonal effects on hair follicles. Int. J. Mol. Sci. 21 (15), 5342. doi:10.3390/ijms21155342
Gupta A. K., Talukder M., Williams G. (2022). Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia. J. Dermatological Treat. 33 (7), 2946–2962. doi:10.1080/09546634.2022.2109567
Hamilton J. B. (1951). Patterned loss of hair in man: types and incidence. Ann. N. Y. Acad. Sci. 53 (3), 708–728. doi:10.1111/j.1749-6632.1951.tb31971.x
Jahoda C. A., Horne K. A., Oliver R. F. (1984). Induction of hair growth by implantation of cultured dermal papilla cells. Nature 311 (5986), 560–562. doi:10.1038/311560a0
Jayapaul P., Lee J. H., Park I. S. (2018). Large scalp defect repair with flap reconstruction using tissue expander after combined bypass in case of Moyamoya disease. World Neurosurg. 120, 185–189. doi:10.1016/j.wneu.2018.08.221
Johnstone M. A., Albert D. M. (2002). Prostaglandin-induced hair growth. Surv. Ophthalmol. 47, S185–S202. doi:10.1016/s0039-6257(02)00307-7
Kaufman K. D., Olsen E. A., Whiting D., Savin R., DeVillez R., Bergfeld W., et al. (1998). Finasteride in the treatment of men with androgenetic alopecia. Finasteride male pattern hair loss study group. J. Am. Acad. Dermatology 39 (4), 578–589. doi:10.1016/s0190-9622(98)70007-6
Khidhir K. G., Woodward D. F., Farjo N. P., Farjo B. K., Tang E. S., Wang J. W., et al. (2013a). The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 27 (2), 557–567. doi:10.1096/fj.12-21856
Khidhir K. G., Woodward D. F., Farjo N. P., Farjo B. K., Tang E. S., Wang J. W., et al. (2013b). The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 27 (2), 557–567. doi:10.1096/fj.12-218156
Kim Y. E., Choi H. C., Nam G., Choi B. Y. (2019). Costunolide promotes the proliferation of human hair follicle dermal papilla cells and induces hair growth in C57 BL/6 mice. J. Cosmet. Dermatology 18 (1), 414–421. doi:10.1111/jocd.12674
Krishna D., Khan M. M., Dubepuria R., Cheruvu V. P. R., Chaturvedi G. (2023). Reconstruction of scalp and forehead defects: options and strategies. Cureus 15 (7), e41479. doi:10.7759/cureus.41479
Kwack M. H., Seo C. H., Gangadaran P., Ahn B. C., Kim M. K., Kim J. C., et al. (2019). Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp. Dermatol. 28 (7), 854–857. doi:10.1111/exd.13927
Law S. K. (2010). Bimatoprost in the treatment of eyelash hypotrichosis. Clin. Ophthalmol. 4, 349–358. doi:10.2147/opth.s6480
Lei M., Yang L., Chuong C.-M. (2017). Getting to the core of the dermal papilla. J. investigative dermatology 137 (11), 2250–2253. doi:10.1016/j.jid.2017.07.824
Lü Z.-f., Cai S.-q., Wu J.-j., Zheng M. (2006). Biological characterization of cultured dermal papilla cells and hair follicle regeneration in vitro and in vivo. Chin. Med. J. 119 (04), 275–281. doi:10.1097/00029330-200602020-00002
McElwee K. J., Kissling S., Wenzel E., Huth A., Hoffmann R. (2003). Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J. investigative dermatology 121 (6), 1267–1275. doi:10.1111/j.1523-1747.2003.12568.x
Miranda B. H., Charlesworth M. R., Tobin D. J., Sharpe D. T., Randall V. A. (2018). Androgens trigger different growth responses in genetically identical human hair follicles in organ culture that reflect their epigenetic diversity in life. FASEB J. 32 (2), 795–806. doi:10.1096/fj.201700260RR
Miranda B. H., Farjo N., Farjo B. (2011). Eyebrow reconstruction in dormant keratosis pilaris atrophicans. J. Plast. Reconstr. Aesthet. Surg. 64 (12), e303–e305. doi:10.1016/j.bjps.2011.06.009
Miranda B. H., Tobin D. J., Sharpe D. T., Randall V. A. (2010). Intermediate hair follicles: a new more clinically relevant model for hair growth investigations. Br. J. Dermatology 163 (2), 287–295. doi:10.1111/j.1365-2133.2010.09867.x
Ng K. J., Lim J., Tan Y. N., Quek D., Lim Z., Pantelireis N., et al. (2022). Sox2 in the dermal papilla regulates hair follicle pigmentation. Cell Rep. 40 (3), 111100. doi:10.1016/j.celrep.2022.111100
Nieves A., Garza L. A. (2014). Does prostaglandin D2 hold the cure to male pattern baldness? Exp. Dermatol. 23 (4), 224–227. doi:10.1111/exd.12348
Nusbaum A. G., Rose P. T., Nusbaum B. P. (2013). Nonsurgical therapy for hair loss. Facial Plast. Surg. Clin. 21 (3), 335–342. doi:10.1016/j.fsc.2013.04.003
Oh J. W., Kloepper J., Langan E. A., Kim Y., Yeo J., Kim M. J., et al. (2016). A guide to studying human hair follicle cycling in vivo. J. investigative dermatology 136 (1), 34–44. doi:10.1038/JID.2015.354
Philpott M. P., Green M. R., Kealey T. (1990). Human hair growth in vitro. J. cell Sci. 97 (3), 463–471. doi:10.1242/jcs.97.3.463
Rafati M., Mahmoudian R., Golpour M., Kazeminejad A., Saeedi M., Nekoukar Z. (2022). The effect of latanoprost 0.005% solution in the management of scalp alopecia areata, a randomized double-blind placebo-controlled trial. Dermatol. Ther. 35 (6), e15450. doi:10.1111/dth.15450
Randall V. A. (2007). Hormonal regulation of hair follicles exhibits a biological paradox. Semin. Cell Dev. Biol. 18 (2), 274–285. doi:10.1016/j.semcdb.2007.02.004
Randall V. A. (2008). Androgens and hair growth. Dermatol Ther. 21 (5), 314–328. doi:10.1111/j.1529-8019.2008.00214.x
Randolph M., Tosti A. (2021). Oral minoxidil treatment for hair loss: a review of efficacy and safety. J. Am. Acad. Dermatology 84 (3), 737–746. doi:10.1016/j.jaad.2020.06.1009
Rose P. T. (2015). Hair restoration surgery: challenges and solutions. Clin. Cosmet. Investigational Dermatology 8, 361–370. doi:10.2147/CCID.S53980
Saitoh M., Uzuka M., Sakamoto M. (1970). Human hair cycle. J. investigative dermatology 54 (1), 65–81. doi:10.1111/1523-1747.ep12551679
Saxena K., Savant S. S. (2017). Body to scalp: evolving trends in body hair transplantation. Indian Dermatology Online J. 8 (3), 167–175. doi:10.4103/idoj.IDOJ_283_16
Schweiger E. S., Pinchover L., Bernstein R. M. (2012). Topical bimatoprost for the treatment of eyebrow hypotrichosis. J. Drugs Dermatol 11 (1), 106–108.
Shin D. W. (2022). The physiological and pharmacological roles of prostaglandins in hair growth. Korean J. Physiology and Pharmacol. Official J. Korean Physiological Soc. Korean Soc. Pharmacol. 26 (6), 405–413. doi:10.4196/kjpp.2022.26.6.405
Shorter K., Farjo N. P., Picksley S. M., Randall V. A. (2008a). Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 22 (6), 1725–1736. doi:10.1096/fj.7-099424
Shorter K., Farjo N. P., Picksley S. M., Randall V. A. (2008b). Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 22 (6), 1725–1736. doi:10.1096/fj.07-099424
Subedi L., Pandey P., Shim J.-H., Kim K.-T., Cho S.-S., Koo K.-T., et al. (2022). Preparation of topical bimatoprost with enhanced skin infiltration and in vivo hair regrowth efficacy in androgenic alopecia. Drug Deliv. 29 (1), 328–341. doi:10.1080/10717544.2022.2027046
Suwanchatchai W., Tanglertsampan C., Pengsalae N., Makornwattana M. (2012). Efficacy and safety of bimatoprost 0.03% versus minoxidil 3% in enhancement of eyebrows: a randomized, double-blind, split-face comparative study. J. Dermatol 39 (10), 865–866. doi:10.1111/j.1346-8138.2012.01579.x
Tauchi M., Fuchs T. A., Kellenberger A. J., Woodward D. F., Paus R., Lutjen-Drecoll E. (2010). Characterization of an in vivo model for the study of eyelash biology and trichomegaly: mouse eyelash morphology, development, growth cycle, and anagen prolongation by bimatoprost. Br. J. Dermatol 162 (6), 1186–1197. doi:10.1111/j.1365-2133.2010.09685.x
Vogt A., Hadam S., Heiderhoff M., Audring H., Lademann J., Sterry W., et al. (2007). Morphometry of human terminal and vellus hair follicles. Exp. Dermatol. 16 (11), 946–950. doi:10.1111/j.1600-0625.2007.00602.x
Wadstein J., Thom E., Gadzhigoroeva A. (2020). Integral roles of specific proteoglycans in hair growth and hair loss: mechanisms behind the bioactivity of proteoglycan replacement therapy with Nourkrin® with Marilex® in pattern hair loss and telogen Effluvium. Dermatology Res. Pract. 2020, 8125081. doi:10.1155/2020/8125081
Wall D., Meah N., Fagan N., York K., Sinclair R. (2022) “Advances in hair growth,” Fac. Rev. 11. 1. doi:10.12703/r/11-1
Wang X., Marr A. K., Breitkopf T., Leung G., Hao J., Wang E., et al. (2014). Hair follicle mesenchyme-associated PD-L1 regulates T-cell activation induced apoptosis: a potential mechanism of immune privilege. J. investigative dermatology 134 (3), 736–745. doi:10.1038/jid.2013.368
Whitehouse C. J., Huckle J. W., Demarchez M., Reynolds A. J., Jahoda C. A. (2002). Genes that are differentially expressed in rat vibrissa follicle germinative epithelium in vivo show altered expression patterns after extended organ culture. Exp. Dermatol 11 (6), 542–555. doi:10.1034/j.1600-0625.2002.110607.x
Whiting D. A. (2001). Possible mechanisms of miniaturization during androgenetic alopecia or pattern hair loss. J. Am. Acad. Dermatology 45 (3), S81–S86. doi:10.1067/mjd.2001.117428
D. A. Whiting, J. Waldstreicher, M. Sanchex, and K. D. Kaufman (1999). “Measuring reversal of hair miniaturization in androgenetic alopecia by follicular counts in horizontal sections of serial scalp biopsies: results of finasteride 1 mg treatment of men and postmenopausal women,”.J. Investigative Dermatology Symposium Proc. doi:10.1038/sj.jidsp.5640230
Woodward D., Wang J., Poloso N. (2013). Recent progress in prostaglandin F2α ethanolamide (prostamide F2α) research and therapeutics. Pharmacol. Rev. 65 (4), 1135–1147. doi:10.1124/pr.112.007088
Xu X.-G., Chen H.-D. (2018). Prostanoids and hair follicles: implications for therapy of hair disorders. Acta Dermato-Venereologica 98 (3), 318–323. doi:10.2340/00015555-2843
Keywords: hair loss (alopecia), prostaglandin (PGF2a), intermediate hair follicles, balding or thinning hair, plastic surgery & cosmetic surgery, hair regeneration, hair loss treatment, dermatology
Citation: Miranda BH, Khidhir KG and Tobin DJ (2025) Prostaglandin F2α stimulates the growth of human intermediate hair follicles in ex vivo organ culture with potential clinical relevance. Front. Physiol. 16:1556431. doi: 10.3389/fphys.2025.1556431
Received: 06 January 2025; Accepted: 20 May 2025;
Published: 18 June 2025.
Edited by:
Majid Alam, Hamad Medical Corporation, QatarReviewed by:
Ang Li, University of Texas at Arlington, United StatesHan Jimin, Northwestern University, United States
Copyright © 2025 Miranda, Khidhir and Tobin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ben H. Miranda, YmVuLm1pcmFuZGFAbmhzLm5ldA==, QmVuLk1pcmFuZGFAYXJ1LmFjLnVr
 Ben H. Miranda
Ben H. Miranda Karzan G. Khidhir
Karzan G. Khidhir Desmond J. Tobin
Desmond J. Tobin