- 1Department of Cell Growth and Differentiation, Center for iPS Cell Research and Application, Kyoto University, Kyoto, Japan
- 2Cardiomyopathy Project, Takeda-CiRA Joint Program (T-CiRA), Fujisawa, Kanagawa, Japan
- 3Department of Pancreatic Islet Cell Transplantation, National Institute of Global Health and Medicine, Japan Institute for Health Security, Tokyo, Japan
- 4T-CiRA Discovery, Takeda Pharmaceutical Company Limited, Fujisawa, Kanagawa, Japan
Numerous reports investigating channelopathies, including Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT), have successfully reproduced using cardiomyocytes (CMs) differentiated from human induced pluripotent stem cells (hiPSCs). However, the relationship between action potentials (AP) and calcium transient waveforms—especially after drug treatment—remains unclear. In this study, we simultaneously loaded a membrane potential dye FluoVolt and the new calcium indicator CalbryteTM 590 AM and optimized stimulation and detection of both dyes to successfully obtain a higher signal-to-noise (S/N) ratio than the conventional membrane potential dye-red fluorescence Ca2+ dye combination, thus enabling the simultaneous recording of both AP and calcium transient waveforms in single hiPSC-CMs, which continued even after gradual increases in drug concentration. In drug-loading experiments on CPVT1 (RyR2-I4587V) hiPSC-derived ventricular-like CMs, carvedilol and flecainide demonstrated some effectiveness, while JTV519 at 3 µM exhibited both efficacy and alterations in AP waveforms. The Ca2+/calmodulin-dependent serine-threonine protein kinase II (CaMKII) inhibitor KN-93 at 1 µM was highly effective (93%) at reducing Ca2+ transient abnormalities without altering AP waveforms.
Introduction
Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) have been successfully used to recapitulate the catecholaminergic polymorphic ventricular tachycardia (CPVT) (GeneReviews 2024) phenotype and evaluate drug effectiveness. However, the simultaneous recording of action potentials and calcium transient waves in single cells has not been routinely performed (Fatima et al., 2011; Itzhaki et al., 2012; Jung et al., 2012; Di Pasquale et al., 2013; Penttinen et al., 2015a; Penttinen et al., 2015b; Preininger et al., 2016; Sasaki et al., 2016; Maizels et al., 2017; Pölönen et al., 2018; Bueno-Levy et al., 2019; Zhang et al., 2021; Gao et al., 2023). We previously reported that a new membrane potential dye, FluoVolt (FV), enables CM subtype classification and early afterdepolarization (EAD) detection in long QT syndrome hiPSC-CMs following optimization (Takaki et al., 2019). Since CMs differentiated from CPVT-iPSCs likely include subtypes other than the ventricular subtype, we hypothesized that simultaneous imaging of membrane potentials and calcium transients would be necessary to analyze CPVT-iPSC-CMs. Before the development of the low phototoxicity membrane potential dye FV, RH237 or Di-8-ANEPPS was used in combination with the red Ca2+ dye Rhod-2 or Fura-4F, but it was difficult to capture single-cell images (Lang et al., 2011; Lee et al., 2012). To simultaneously measure membrane potential and Ca2+ transients in single cells, transfection using a viral vector such as ArcLight/R-GECO1 was necessary (Song et al., 2015). Although there have been reports of single-cell analysis using a combination of FV and Rhod-2 (Martinez-Hernandez et al., 2022; Yang et al., 2024), the conventional red calcium indicator exhibits higher mitochondrial affinity than cytosolic calcium ions, has a lower signal-to-noise (S/N) ratio, and requires the presence of an organic anion transporter inhibitor (Zhao et al., 2019; Yang et al., 2024). Therefore, we used the recently developed Ca2+ fluorescent indicator, CalbryteTM 590 AM (CB590), which is 10 times more sensitive than Rhod-2, in combination with FV and demonstrated the possibility of capturing single-cell images for long periods by reducing phototoxicity while optimizing the LED light intensity for each fluorophore and to observe the response of CPVT-iPSC-CMs to increasing drug concentrations.
Methods
Human iPS cells from type1 CPVT patient
This study was approved by the Ethics Committees of the Graduate School of Medicine Kyoto University and the Kyoto University Hospital. Written informed consent was obtained from the patient in accordance with the Declaration of Helsinki. The CPVT1 (RyR2-I4587V) hiPSC line was kindly gifted from Dr. Sasaki (Sasaki et al., 2016). The hiPSC line derived from a healthy donor, 201B7 (Takahashi et al., 2007), was used as a control hiPSC.
Human iPS cell culture
Human iPSCs were maintained on STO feeder layers cultured with primate ES cell medium (ReproCell, Yokohama, Kanagawa, Japan) as previously described (Takahashi et al., 2007). Please refer to Supplementary Methods for details.
Cardiac differentiation and fluorescence-activated cell sorting
Cardiac differentiation from hiPSCs was performed by embryoid body (EB) formation in 96-well plates, with reaggregation on day 3. EBs were subsequently transferred to 10 cm dishes on day 7, and FACS sorting was performed on day 30 using the surface marker SIRPA as in previous reports (Miki et al., 2015; Funakoshi et al., 2016; Takaki et al., 2019). Please refer to Supplementary Methods for details.
Thawing frozen hiPSC-CMs and cell seeding
hiPSC-CMs were thawing from frozen stocks and seeded as previously described (Takaki et al., 2019). Please refer to Supplementary Methods for details.
Loading of FV and CB590
When loading FluoVolt (Thermo Fisher Scientific) and CalbryteTM 590 AM (AAT Bioquest, Pleasanton, CA, United States), the medium was exchanged with Gey’s Balanced Salt Solution (GBSS, Sigma-Aldrich) containing FV (0.1% volume) and 10 µM CB590. The medium was replaced with GBSS at 40 min after loading.
Preincubation
One hour before imaging, the 35 mm glass bottom dish on which hiPSC-CMs were seeded was fixed in Stage Top Incubator® (Tokai Hit, Fujinomiya, Japan) and preincubated at 37°C with 5% CO2 supplied.
Setting up the imaging system
To simultaneously excite two wavelengths (490 nm for FV and 580 nm for CB590), the X-Cite Turbo Multi-wavelength LED Illumination System (Excelitas Technologies, Waltham, MA, United States) was used to optimize the fluorescence emission separately. In detail, 2 out of 6 wavelength LED lamps were used: LEDs 3 (460–495 nm, center 475 nm) and 5 (525–610 nm, center 575 nm) were set at 5% and 25%–100% light intensity, respectively, with ND filters to reduce the light by 16 times. The BrightLine® full-multiband filter set FITC/TxRed-A-NTE (Semrock, Rochester, NY, United States), consisting of FF01-479/585-25 (Dual Band Exciter), FF01-524/628-25 (Dual Band Exciter), and FF505/606-Di01-25x36 (Dual Band Dichroic), was used inside the ECLIPSE Ti-E inverted fluorescence microscope (Nikon). The objective lens used was Plan Apo Lambda 10x (Nikon) with a 0.45 numerical aperture. To simultaneously capture fluorescence emission at both wavelengths, an image splitting optics, W-VIEW GEMINI (Hamamatsu Photonics, Hamamatsu, Japan), was used. Filter settings inside were beamsplitter FF580-FDi01-25x36 (Semrock), 536/40 nm BrightLine® single-band bandpass filter FF01-536/40-25 (Semrock), and 631/36 nm BrightLine® single-band bandpass filter FF01-631/36-25 (Semrock).
Simultaneous dual optical recording of APs and calcium transients from single cells
Images were obtained using an electron multiplying CCD (EMCCD) camera, ImagEM X2 (Hamamatsu Photonics) at 37°C with 5% CO2 supplied. Subarray images at a resolution of 512 × 256 pixels were recorded every 16 ms using the AquaCosmos software (Hamamatsu Photonics) for 1 min each. The region of interest (ROI) was defined by manually enclosing the flashing area. Non-beating cells were excluded from the analysis. Data was plotted into graphs using Excel 2019 (Microsoft, Redmond, WA, United States).
Addition of reagents or drugs
The stock solution was diluted, and 200 µL of the newly diluted solution was added to the top of the 35 mm glass-bottom dish to obtain the final concentration as previously described (Takaki et al., 2019). Please refer to Supplementary Methods for details.
Type of Ca2+ transient abnormalities and responses to drugs
Ca2+ transient abnormalities were classified into four types and listed in order of severity: oscillation, triple peaks, double peaks, and plateau abnormality. Cells selected for observing drug responses were ventricular-like cells with normal APD and had abnormal Ca2+ transients before drug additions. Normal APD was defined as <1 s as previously reported (Takaki et al., 2019). After drug addition, cells were determined as responders if they had normal Ca2+ transients, semi-responders if their Ca2+ transients changed to milder phenotypes, and non-responders if their Ca2+ transients remained unchanged. Responders and semi-responders were considered drug-responsive cells.
Statistical analysis
All statistical analyses were verified using Fisher’s exact test by Excel 2019. Values considered statistically significant are denoted as ∗p < 0.05 and ∗∗p < 0.01. When Fisher’s exact was performed multiple times, the significance of the difference was determined by the Bonferroni method. If more than half the hiPSC-CMs stopped beating, they were excluded from the analysis.
Results
Establishing a system to record membrane potentials and calcium transients simultaneously
We successfully captured the fluorescence emission from cells stained with the membrane potential dye FV and the calcium indicator CB590 using a single EMCCD camera equipped with separate band-pass filters (Figure 1A). Before setting up this system, we confirmed that the waveforms obtained from hiPSC-CMs stained with FV alone had no leakage into the calcium transient waveforms (Supplementary Figures S1a,b) and that the waveforms obtained from hiPSC-CMs stained with CB590 alone had no leakage into the membrane potential waveforms (Supplementary Figures S1c,d). An example of the image captured during recording is shown in Figure 1B. The sensor is divided into left and right halves. In this example, six hiPSC-CMs flash in response to excitation due to increases in intracellular calcium concentration and changes in membrane potentials on the left and right sides of the screen, respectively. The upper and lower panels of Supplementary Figure S1e show the waveforms of membrane potentials (i.e., AP) and Ca2+ transients, respectively. Figure 1C shows an example of normal ventricular APD and Ca2+ transient waveforms, and Figure 1D shows an example of a ventricular-like cell with long APD and an abnormal Ca2+ transient waveform. Other unusual waveforms from control hiPSC-CMs are shown in Supplementary Figures S1f–l.
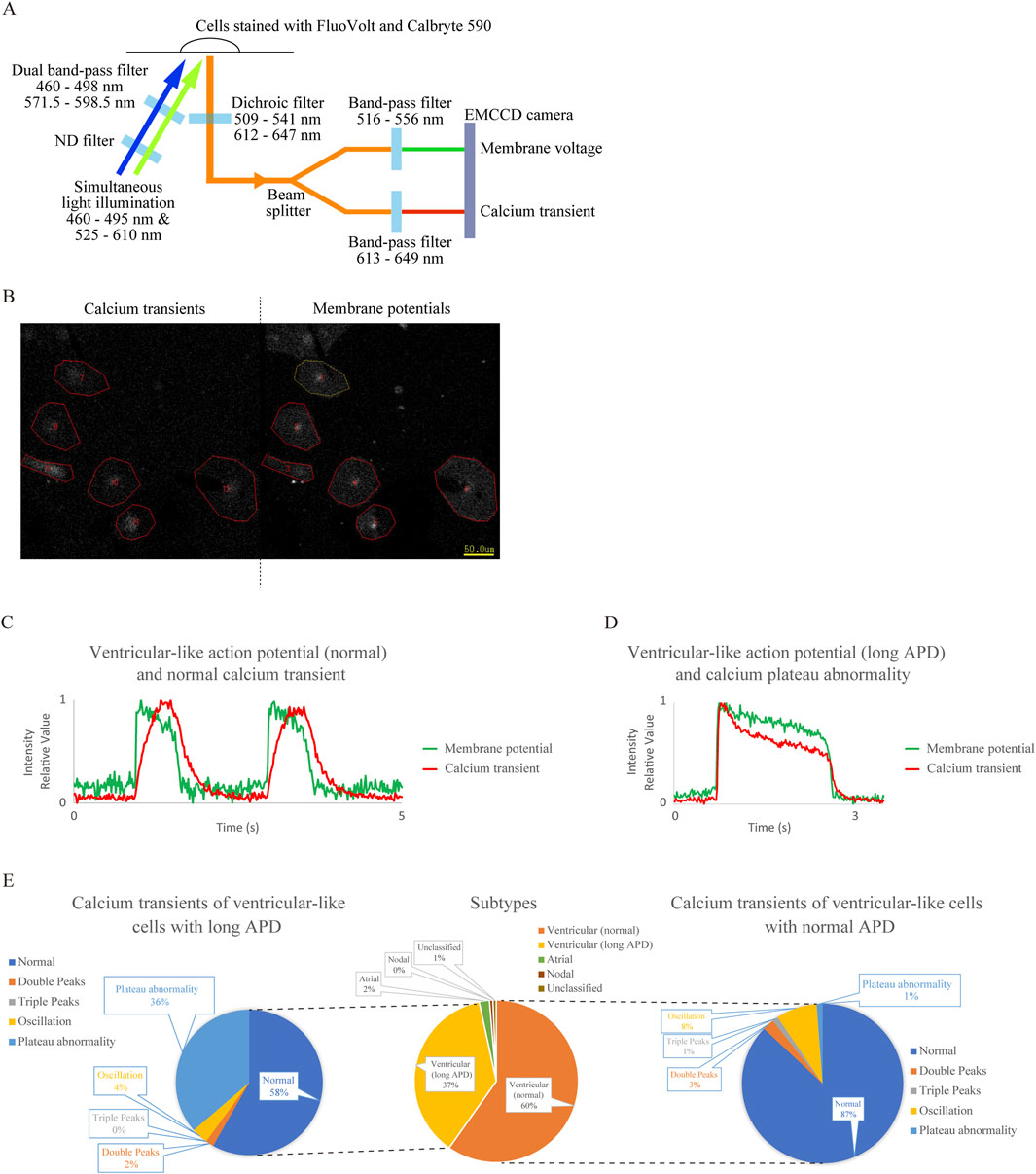
Figure 1. Establishment of a system for simultaneous recording of action potentials (AP) and Ca2+ transients using the membrane potential-sensitive dye FluoVolt (FV) and calcium indicator Calbryte 590 (CB590) and analysis of cardiomyocytes differentiated from control human induced pluripotent stem cells (hiPSC-CMs) using this system. (A) Schematic diagram of the simultaneous recording system. Two excitation wavelengths of LED light are attenuated to 1/16 intensity by an ND filter and wavelength-limited through a dual bandpass filter before being simultaneously irradiated onto cells stained with the fluorescent dyes. The emitted light at two wavelengths passes through a dichroic filter, is split into two by a beam splitter, and is simultaneously captured by a single electron multiplying CCD (EMCCD) camera through separate bandpass filters. (B) Screenshot during recording. Recorded CB590 and FV signals in hiPSC-CMs are on the left and right sides, respectively. Regions of interest (ROIs) were obtained by encircling blinking cells. (C) Representative waveform diagram showing membrane potential and Ca2+ transient simultaneously recorded from a ventricular-like cell with normal action potential duration (APD). The fluorescent intensity was expressed relative to the minimum value, 0, and the maximum value, 1. (D) Representative waveform diagram showing the simultaneous detection of membrane potential and Ca2+ transient in a ventricular-like cell with prolonged APD. The APD extended beyond 1 s, and calcium plateau abnormality was observed. The fluorescent intensity was expressed relative to the minimum value, 0, and the maximum value, 1. (E) The center panel shows subtype classification based on AP waveforms of control hiPSC-CMs. The right and left panels show classifications of Ca2+ transients in ventricular-like cells with normal and long APD, respectively (n = 149). Calcium plateau abnormalities were more frequent in long APD ventricular-like cells than in normal ventricular-like cells (p = 1.87 × 10−8).
Ca2+ transient abnormalities are classified into four types based on the literature: double peaks (Supplementary Figure S1h), triple peaks (Supplementary Figure S1i), oscillations (Supplementary Figure S1j), and plateau abnormality (Figure 1D; Supplementary Figure S1g). Their frequencies were analyzed and shown in Figure 1E. Similar to our previous report (Takaki et al., 2019), CM subtypes were classified based on AP waveforms (n = 149), as shown in the center of Figure 1E. Next, Ca2+ transients were classified within both normal (right panel in Figure 1E) and long APD ventricular populations (left panel in Figure 1E). Whereas Ca2+ transient abnormalities were rare in the former, they were observed more frequently in the latter because calcium plateau abnormalities were more common in the ventricular (long APD) group (p = 1.87 × 10−8) (Supplementary Table S1).
Abnormal Ca2+ transients in CPVT1 hiPSC-derived ventricular-like CMs
Next, we also analyzed CPVT1 hiPSC-CMs (Supplementary Figure S2a). The upper and lower panels in Supplementary Figure S2b show AP and Ca2+ transient waveforms of the four cells, respectively. The membrane potential and Ca2+ transient waveforms of Cell 1 and Cell 3 in Supplementary Figure S2b are shown in Figures 2A,B, respectively. The former represents Ca2+ double peaks, while the latter is Ca2+ oscillation, both considered typical Ca2+ transient abnormalities. The waveforms of Cell 2, as shown in Supplementary Figure S2c, revealed that it is a nodal-like cell that beats very rapidly, indicating that Cell 2 did not exhibit Ca2+ oscillations. It has been reported that delayed afterdepolarization (DAD) is a transient inward current due to the exchange of Na+ and Ca2+ by the sodium-calcium exchanger (NCX) after Ca2+ leakage from the sarcoplasmic reticulum (Bers, 2014). In Supplementary Figure S2d, after the action potential ended, the dashed arrow indicated increased calcium intensity due to suspected calcium leakage, followed by an arrow indicating a small increase in membrane potential suggestive of DAD.
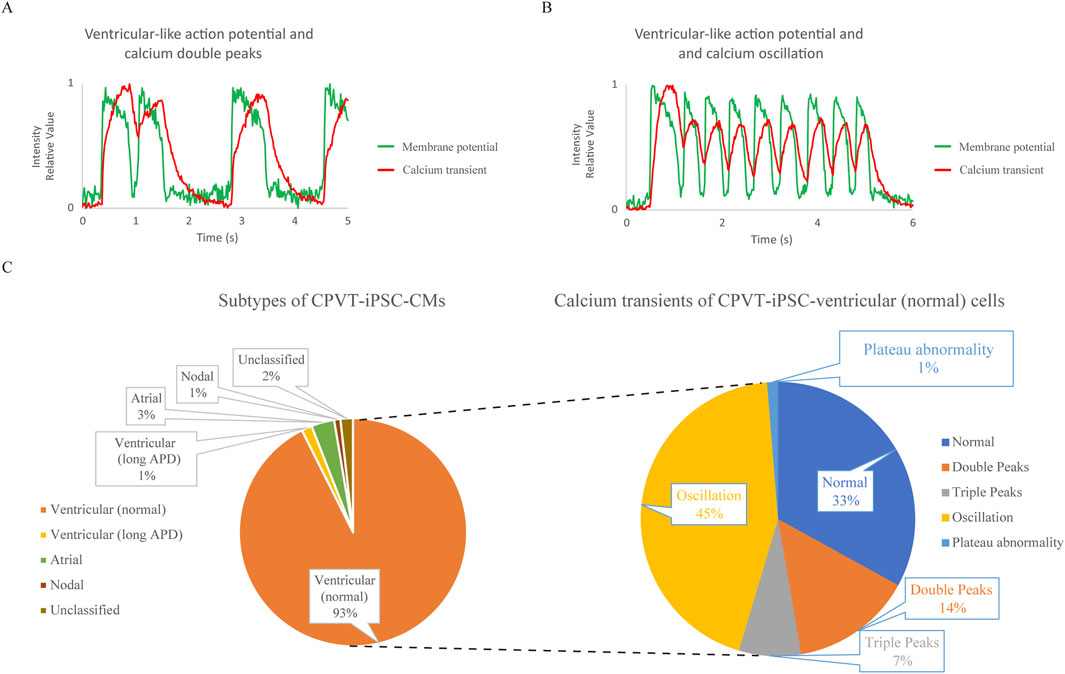
Figure 2. Analysis of cardiomyocytes differentiated from type 1 catecholaminergic polymorphic ventricular tachycardia (CPVT) patient-derived human induced pluripotent stem cells (CPVT1 hiPSC-CMs) using the system to record action potentials (APs) and Ca2+ transients simultaneously. (A) The waveform of Cell 1 in Supplementary Figure S2a,b. The fluorescence intensity was expressed relative to the minimum value, 0, and the maximum value, 1. Calcium double peaks and ventricular-like AP with normal AP duration (APD) are depicted. (B) The waveform of Cell 3 in Supplementary Figure S2a,b. The fluorescent intensity was expressed relative to the minimum value, 0, and the maximum value, 1. Calcium oscillation and ventricular-like AP with normal APD are depicted. (C) The left and right pie charts show the subtype classification based on the action potential waveforms of CPVT1 hiPSC-CMs and the classification of Ca2+ transients in ventricular-like cells with normal APD, respectively (n = 338). Among the subtypes, most were ventricular-like CMs with normal APD (93%). In their calcium transients, calcium oscillations accounted for a relatively large proportion, with calcium plateau abnormalities only accounting for 1%. Calcium abnormalities accounted for two-thirds of CPVT1 hiPSC-derived ventricular (normal) cells, which was significantly more frequent compared to control hiPSC-derived ventricular (normal) CMs (p = 7.68 × 10−21).
We also performed subtyping of CPVT1 hiPSC-CMs (n = 338) and classified Ca2+ transients within the normal ventricular-like cell population (Figure 2C). Cardiomyocyte subtyping showed that most cells were normal ventricular-like cells, with two-thirds exhibiting abnormal Ca2+ transients, which was significantly higher than those in control hiPSC-derived ventricular-like (normal) cells (p = 7.68 × 10−21) (Supplementary Table S2), even though calcium plateau abnormalities were observed in only 1%, consistent with control normal hiPSC-derived ventricular-like cells.
Response of CPVT1 hiPSC-CMs to existing clinical CPVT drugs
Next, we tested the response of CPVT hiPSC-CMs to approved clinical drugs, carvedilol (Figures 3A,B; Supplementary Tables S3, S4) and flecainide (Figures 3C,D; Supplementary Tables S5, S6), by increasing concentrations and observing simultaneous changes in membrane potential and Ca2+ transients.
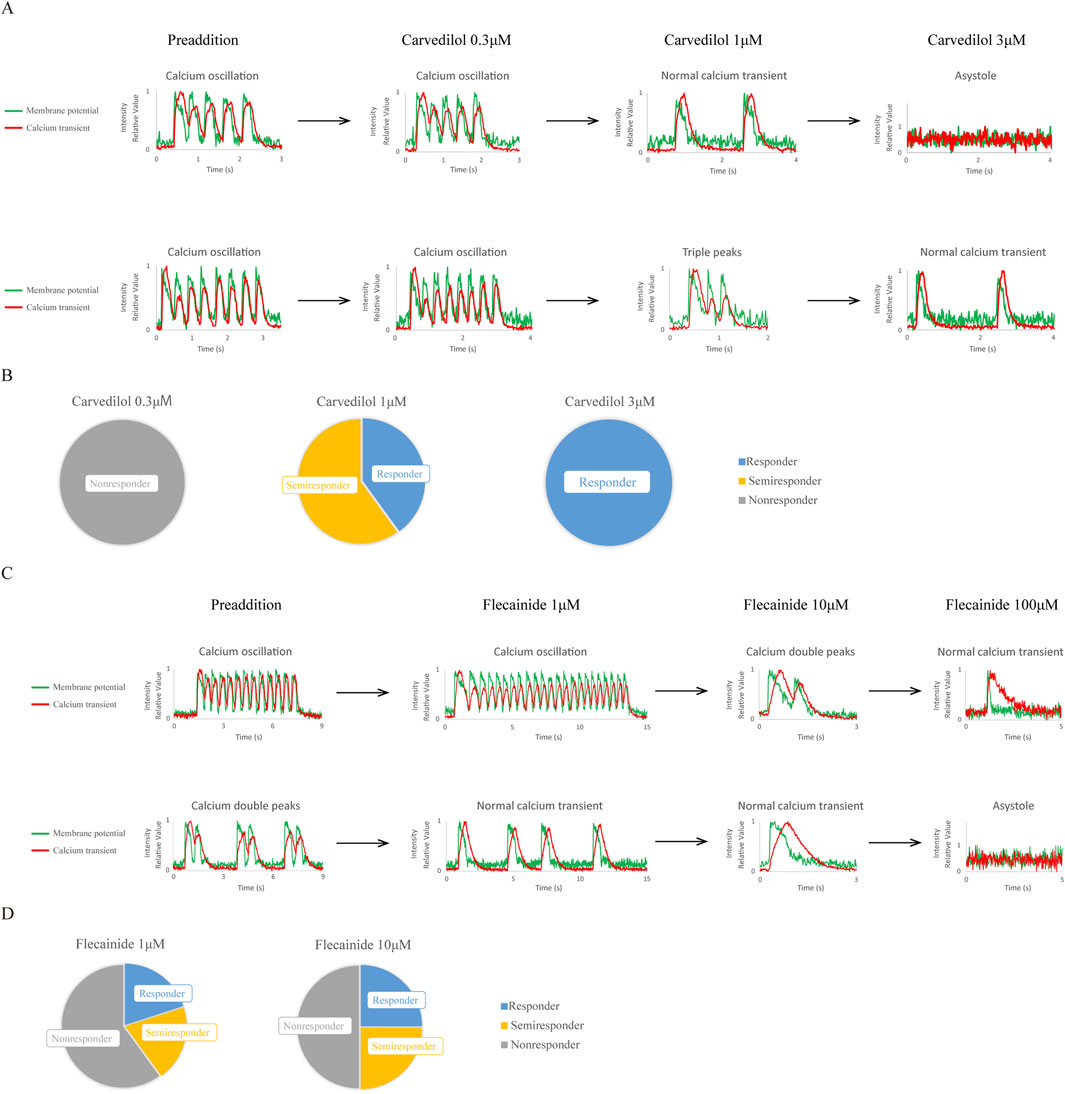
Figure 3. Effects of approved clinical drugs on CPVT1 hiPSC-CMs. (A) The upper and lower panels show concentration-dependent changes in action potentials and Ca2+ transients induced by nonselective β-blocker carvedilol in two CPVT1 hiPSC-derived ventricular-like cells exhibiting calcium oscillation. Carvedilol at 0.3 µM did not improve calcium abnormalities. At 1 μM, while calcium abnormalities tended to improve (p = 0.0152), some cells showed a shortening of the plateau phase. At 3 μM, while calcium abnormalities were improved (p = 0.0476), some cells stopped beating. (B) Analysis of the rate of improvement of Ca2+ transient abnormality by carvedilol (n = 5). (C) Concentration-dependent changes in action potentials and Ca2+ transients induced by a class 1C antiarrhythmic drug, flecainide, in two CPVT1 hiPSC-derived ventricular-like cells, exhibiting calcium oscillation in the upper panel and calcium double peaks in the lower panel. Some cells showed improvement at 1 μM, while another showed a semi-response at 10 µM. (D) Analysis of the rate of improvement of Ca2+ transient abnormality by flecainide at 1 µM (n = 5) (p = 0.545) and 10 µM (n = 4) (p = 0.545). Cells that stopped beating were excluded from the analysis. Because more than half the cells stopped beating at 100 μM, it was excluded from the analysis.
Figures 3A,C show the simultaneous recordings of changes in membrane potential and Ca2+ transients in two representative cells, with the resulting analysis for all observed cells in Supplementary Tables S3, S5. Figures 3B,D show the responsiveness of cells with calcium abnormalities, and Supplementary Tables S4, S6 show the percentage of cells with normal calcium transient and improved Ca2+ abnormalities. The results indicated that carvedilol at 1 and 3 µM improved Ca2+ abnormalities (p = 0.0152 and p = 0.0476, respectively), while flecainide at 1 and 10 µM tended to improve them without significant differences. It should be noted that atrial-like and non-beating cells appeared at 3 µM carvedilol and 10 µM flecainide, whereas 100 µM flecainide caused most cells to stop beating and was therefore excluded from the analysis.
Response of CPVT1 hiPSC-CMs to RyR2 modulator
JTV519 ((4-[3-(4-benzylpiperidin-1-yl)propionyl]-7-methoxy-2,3,4,5-tetrahydro-1,4-benzothiazepine monohydrochloride, K201) is a 1,4-benzothiazepine derivative developed as a cardioprotective drug, previously reported to reduce Ca2+ efflux from RyR and improve contractility in failing myocardium (Kohno et al., 2003; Toischer et al., 2010). We thus examined the response of CPVT1 hiPSC-CMs to JTV519 (Figures 4A,B; Supplementary Tables S7, S8). Ca2+ transient abnormalities improved in 20% of the cells at 1 μM and 60% at 3 µM. Although there was no significant difference at 0.3 µM (p = 0.350) and 1 µM (p = 0.350), a statistically significant difference was observed at 3 µM (p = 0.0230) (Supplementary Table S8). However, 80% of the cells at 3 µM also displayed atrial-like action potential waveforms (Supplementary Table S7).
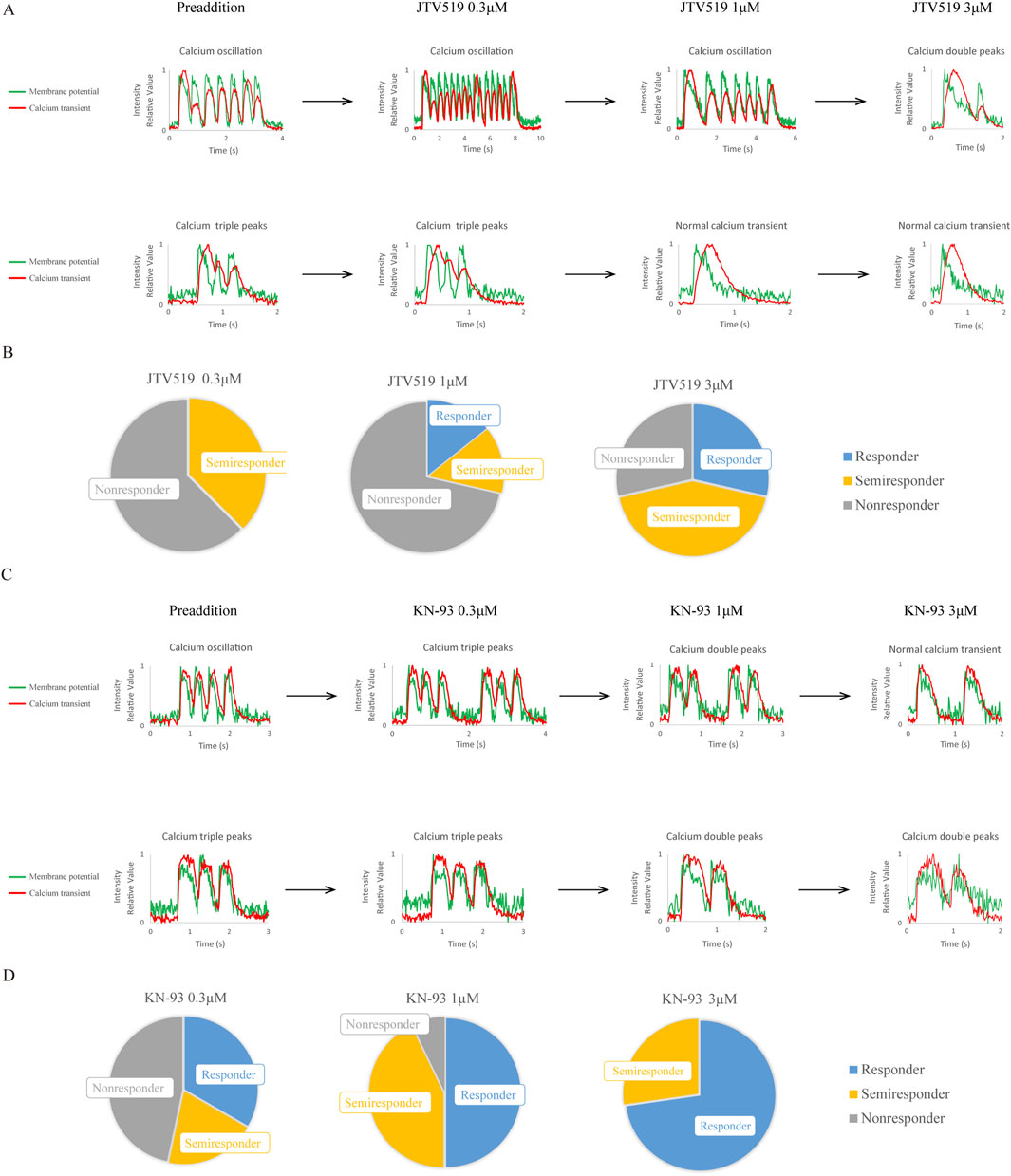
Figure 4. Effects of a RyR2 modulator, JTV519, and a Ca2+/calmodulin-dependent serine-threonine protein kinase II (CaMKII) inhibitor, KN-93, on CPVT1 hiPSC-CMs. (A) JTV519 (K201)-induced changes in action potentials and Ca2+ transients in two CPVT1 hiPSC-derived ventricular-like cells. The upper and lower panels show cells with calcium oscillation and triple peaks before drug additions, respectively. Although Ca2+ transient abnormalities tended to improve as the concentration increased, some cells showed a change in action potential to atrial-like at 3 µM. (B) Analysis of the rate of improvement of Ca2+ transient abnormality by JTV519 (K201) (n = 8). No significant difference at 0.3 (p = 0.350) or 1 µM (p = 0.350). Although a significant difference was observed at 3 µM (p = 0.0230), 80% of the cells displayed atrial-like action potential waveforms. (C) KN-93-induced changes in action potentials and Ca2+ transients in two CPVT1 hiPSC-derived ventricular-like cells. The upper and lower panels show cells with calcium oscillation and triple peaks before drug additions, respectively. Ca2+ transient abnormalities tended to improve as the concentration increased, but some cells showed asystole or changes to atrial-like action potential waveforms at 3 µM. (D) Analysis of the rate of improvement of Ca2+ transient abnormality by KN-93 at 1 (n = 14) and 3 µM (n = 11). Cells that stopped beating were excluded from the analysis. Ca2+ transient abnormalities improved at 0.3, 1, and 3 µM (p = 0.0201, 0.0000660, and 0.000280, respectively).
Response of CPVT1 hiPSC-CMs to CaMKII inhibitor
We observed the response of CPVT1 hiPSC-CMs to a CaMKII inhibitor, KN-93 (N-[2-[N-(4-chlorocinnamyl)-N-methylaminomethyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulfonamide) and found Ca2+ transient abnormalities to improve in a concentration-dependent manner at 0.3, 1, and 3 µM (Figures 4C,D; Supplementary Tables S9, S10) (p = 0.0201, 0.0000660, and 0.000280, respectively). KN-93 at 0.3 µM tended to be less effective on calcium double or triple peaks, but there was no significant difference between calcium oscillation and double or triple peaks (p = 0.277, Supplementary Table S11). Notably, KN-93 at 1 µM was effective in 93% (13/14) of cells without affecting action potentials.
Discussion
Many studies have demonstrated that single-cell calcium imaging of CPVT-hiPSC-CMs is highly effective in reproducing the pathological condition (Itzhaki et al., 2012; Jung et al., 2012; Penttinen et al., 2015a; Preininger et al., 2016; Bueno-Levy et al., 2019; Zhang et al., 2021; Gao et al., 2023). However, damage during single-cell dispersion, phototoxicity during recording, EAD associated with prolonged APD, and tachycardia caused by nodal or atrial subtypes could be misinterpreted as the CPVT phenotype. To address these issues, we stained hiPSC-CMs with the membrane potential dye FV and the calcium indicator CB590 and optimized two wavelengths of LED light to stimulate each simultaneously. This approach allowed us to classify subtypes and calcium transient abnormalities and observe the dose-dependent effects of several drugs on CPVT-iPSC-CMs.
The first-choice drug for CPVT is a nonselective β-blockers (e.g., propranolol and nadolol), not β1-selective β-blockers (Napolitano et al., 2022; Mazzanti et al., 2022; Peltenburg et al., 2022). Because the patient from which CPVT1 hiPSCs used in this study originated was initially treated with a β1-selective β blocker and the arrhythmia attacks were not controlled, the drug was switched to carvedilol, a nonselective β blocker, which showed some efficacy, however, she continued to experience episodes of ventricular tachycardia, which was prevented by flecainide treatment (Dochi et al., 2007; Sasaki et al., 2016).
Carvedilol is the only β-blocker known to inhibit SR Ca2+ store overload induced Ca2+ release (SOICR) in HEK293 cells expressing RyR2-R4496C regardless of its α-blocking and antioxidant properties and has been shown to reduce RyR2 mean open time and probability (Zhou et al., 2011). In our system, carvedilol at 1 µM induced response or semi-response to Ca2+ abnormalities, as in previous studies (Zhou et al., 2011; Maizels et al., 2017; Pölönen et al., 2018), but some cells showed asystole and atrial-like action potentials at 3 µM (Figures 3A,B; Supplementary Tables S3, S4). According to previous literature, the blood concentration of carvedilol is approximately 0.3 µM even when taken orally at a high dose of 25 mg per day (Yilmaz and Arslan, 2016), while the blood concentration has been reported to be approximately 0.06 µM in a patient in Japan (Kitakaze et al., 2012). The blood concentration of carvedilol in this patient did not reach 1 μM, and in vitro experiments also suggested that this drug alone could not suppress arrhythmia. However, carvedilol also inhibits phosphorylation of key Ca2+ handling proteins by blocking β-stimulatory signaling, thereby potentially preventing arrhythmias (Pölönen et al., 2018), suggesting that continuous oral administration of this drug is of great value. Flecainide binds to the open state of the cardiac sodium channel INa (Ramos and O’Leary, 2004) but is also known to inhibit ICaL, Ito, IKr, and IRyR (Yang et al., 2021). The proposed therapeutic mechanism of flecainide for CPVT1 involves a direct effect on RyR2 (Kryshtal et al., 2021), an increase in the threshold for triggered activity (TA) (Liu et al., 2011a), and a decrease in INa, followed by activation of the Na+/Ca2+ exchanger, thereby reducing the probability of RyR2 opening (Bannister et al., 2015). In our experimental system, flecainide at 1 µM was effective in approximately half the cells, consistent with a previous report on CPVT1 hiPSC-CMs (Pölönen et al., 2018; Zhang et al., 2021). According to the literature, the effective blood concentration of flecainide is about 1.5 µM (Anderson et al., 1981). However, increasing the concentration to 10 µM did not enhance efficacy significantly, suggesting the need for new drug candidates (Figures 3C,D; Supplementary Tables S5, S6). Given that it was effective in three-quarters of CPVT patients (van der Werf et al., 2011), its ability to suppress arrhythmias in about half of the cells in vitro may still reflect therapeutic benefit in vivo, likely due in part to Na+ channel blockade increasing the TA threshold (Liu et al., 2011a; Bannister et al., 2015).
JTV519 (K201) is a 1,4-benzothiazepine derivative that was developed as a cardioprotective drug and was reported to have cardioprotective effects through its inhibitory effect on contractions in a myofibrillar overcontraction model of isolated rat hearts (Kaneko, 1994). It was subsequently shown to block fast Na+ current (INa), ICaL, and IK1 in guinea pig ventricular myocardium (Kimura et al., 1999), as well as inhibit IKr (Kiriyama et al., 2000), and was reported to reduce Ca2+ efflux from RyR and improve contractility in failing myocardium (Kohno et al., 2003; Toischer et al., 2010). By contrast, no inhibitory effect on ventricular arrhythmias was observed in Ryr2R4496C+/− mice (Liu et al., 2006). Recently, a report showed that JTV519 at 2 µM reduced Ca2+ sparks in CPVT1 hiPSC-CMs, although changes in APs were not investigated (Zhang et al., 2021). In our system, Ca2+ abnormalities were improved in 60% of the cells at 3 µM (Figures 4A,B; Supplementary Tables S7, S8), but 80% of the cells exhibited a transition from ventricular-like to atrial-like AP waveform at this concentration possibly due to ICaL and SERCA inhibition by JTV519 (Loughrey et al., 2007; Sacherer et al., 2012; Darcy et al., 2016). Notably, agents such as carvedilol, flecainide, and JTV519 induced asystole or a change to atrial-like AP at higher concentration, underscoring the clinical need for RyR2 inhibitors that are effective at lower, safer doses (Takenaka et al., 2023; Kurebayashi et al., 2024). In addition, dual-action drugs have been developed that inhibit Ca2+ leak from cardiac ryanodine receptors while activating Ca2+ uptake via SERCA2 (Wegener et al., 2024).
KN-93, a CaMKII inhibitor, was shown to improve Ca2+ abnormalities in the Ryr2+/R4496C KI mouse model of CPVT1 and CPVT1 (RyR2-E2311D)-iPSC-CMs (Liu et al., 2011b; Di Pasquale et al., 2013). We also confirmed that KN-93 was effective even in CPVT-iPSC-CMs with RYR2 mutation in the transmembrane domain (Figures 4C,D; Supplementary Tables S9, S10). The reason why KN-93, even at low concentrations, had a superior antiarrhythmic effect compared to the three drugs mentioned above is thought to be that it reduces the opening probability by inhibiting RyR2 receptor phosphorylation and CaMKII autophosphorylation, thereby suppressing positive feedback and exerting a sustained effect (Wehrens et al., 2004; Swaminathan et al., 2012). KN-93 at 0.3 µM tended to be less effective on calcium double or triple peaks than oscillation (Supplementary Table S11), likely because it does not directly inhibit RyR2 receptors and is thus incapable of rescuing all calcium abnormalities. Although KN-93 at 1 µM improved all types of calcium abnormalities, CaMKII inhibitors have the disadvantage of acting on other organs (Sałaciak et al., 2021; Chacar et al., 2024; Sun et al., 2024). Therefore, to avoid inhibition of extracardiac CAMKII, attempts have been made to develop peptide or nucleotide drugs and drugs targeting enzymes that act on CaMKII (Reyes Gaido et al., 2023).
This study has some limitations. First, we analyzed hiPSC-CMs with only one RYR2 mutation. Second, cells were relatively immature. Third, the analysis was performed on single cells without pacing. Thus, there may be variability of condition across cells. Finally, suppressive effects on arrhythmia and side effects at the tissue level were not considered.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Ethics Committees of the Graduate School of Medicine Kyoto University and the Kyoto University Hospital. Written informed consent was obtained from the patient in accordance with the Declaration of Helsinki.
Author contributions
TT: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review and editing. NT: Conceptualization, Writing – review and editing. KI: Conceptualization, Writing – review and editing. TN: Conceptualization, Writing – review and editing. YY: Conceptualization, Funding acquisition, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Takeda-CiRA collaboration program and by grants from Takeda Pharmaceutical Co., Ltd. This work was also partially supported by a grant from the Leducq Foundation (18CVD05); Research Center Network for Realization of Regenerative Medicine, Japan Agency for Medical Research and Development (AMED) (JP20bm0104001, JP21bm0804022); Acceleration Program of R&D and implementation for Regenerative Medicine and Cell and Gene Therapy, AMED (JP23bm1423011 and JP23bm1323001, JP24bm1123054); Research on Regulatory Science of Pharmaceuticals and Medical Devices, AMED (JP22mk0101241, JP24mk0121280); Program for Creating Deep Tech Startups with Global Reach, Japan Science and Technology Agency (JPMJSF2323), and the iPS Cell Research Fund.
Acknowledgments
We thank Shinya Yamanaka, Seigo Izumo, and Yasushi Kajii for supporting this project, Tomomi Gibson for her administrative support, and Kelvin Hui for critical reading of the manuscript.
Conflict of interest
Authors NT, KI, and TN were employed by Takeda Pharmaceutical Company Limited. YY is a scientific advisor of Orizuru Therapeutics, Inc., owns stocks in iPS Portal, Inc., and received research funding from Takeda Pharmaceutical Co., Ltd. and Altos Labs, Inc. Takeda Pharmaceutical Co., Ltd had the following involvement in the study: the decision to submit it for publication. Funding received from Altos Labs, Inc. was unrelated to the present study.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. We used chatGPT for proofreading the manuscript. The authors used ChatGPT to proofread the manuscript. After using this tool, the authors reviewed and edited the content as needed and will take full responsibility for the content of this publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1579815/full#supplementary-material
References
Anderson J. L., Stewart J. R., Perry B. A., Van Hamersveld D. D., Johnson T. A., Conard G. J., et al. (1981). Oral flecainide acetate for the treatment of ventricular arrhythmias. N. Engl. J. Med. 305 (9), 473–477. doi:10.1056/nejm198108273050901
Bannister M. L., Thomas N. L., Sikkel M. B., Mukherjee S., Maxwell C., MacLeod K. T., et al. (2015). The mechanism of flecainide action in CPVT does not involve a direct effect on RyR2. Circ. Res. 116(8), 1324–1335. doi:10.1161/CIRCRESAHA.116.305347
Bers D. M. (2014). Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu. Rev. Physiol. 76 (76), 107–127. doi:10.1146/annurev-physiol-020911-153308
Bueno-Levy H., Weisbrod D., Yadin D., Haron-Khun S., Peretz A., Hochhauser E., et al. (2019). The hyperpolarization-activated cyclic-nucleotide-gated channel blocker ivabradine does not prevent arrhythmias in catecholaminergic polymorphic ventricular tachycardia. Front. Pharmacol. 10, 1566. doi:10.3389/fphar.2019.01566
Chacar S., Abdi A., Almansoori K., Alshamsi J., Al Hageh C., Zalloua P., et al. (2024). Role of CaMKII in diabetes induced vascular injury and its interaction with anti-diabetes therapy. Rev. Endocr. Metab. Disord. 25 (2), 369–382. doi:10.1007/s11154-023-09855-9
Darcy Y. L., Diaz-Sylvester P. L., Copello J. A. (2016). K201 (JTV519) is a Ca2+-dependent blocker of SERCA and a partial agonist of ryanodine receptors in striated muscle. Mol. Pharmacol. 90 (2), 106–115. doi:10.1124/mol.115.102277
Di Pasquale E., Lodola F., Miragoli M., Denegri M., Avelino-Cruz J. E., Buonocore M., et al. (2013). CaMKII inhibition rectifies arrhythmic phenotype in a patient-specific model of catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis. 4, e843. doi:10.1038/cddis.2013.369
Dochi K., Matsumoto Y., Nagaoka I., Ito M., Ashihara T., Ito H., et al. (2007). A novel missense mutation in the human cardiac ryanodine receptor gene (I4587V) in a patient with catecholaminergic polymorphic ventricular tachycardia. Jpn. J. Electrocardiol. 27 (3), 246–252. doi:10.5105/jse.27.246
Fatima A., Xu G., Shao K., Papadopoulos S., Lehmann M., Arnaiz-Cot J. J., et al. (2011). In vitro modeling of ryanodine receptor 2 dysfunction using human induced pluripotent stem cells. Cell Physiol. Biochem. 28 (4), 579–592. doi:10.1159/000335753
Funakoshi S., Miki K., Takaki T., Okubo C., Hatani T., Chonabayashi K., et al. (2016). Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 6, 19111. doi:10.1038/srep19111
Gao J., Makiyama T., Yamamoto Y., Kobayashi T., Aoki H., Maurissen T. L., et al. (2023). Novel calmodulin variant p.E46K associated with severe catecholaminergic polymorphic ventricular tachycardia produces robust arrhythmogenicity in human induced pluripotent stem cell-derived cardiomyocytes. Circ. Arrhythm. Electrophysiol. 16 (3), e011387. doi:10.1161/circep.122.011387
GeneReviews (2024). GeneReviews is a registered trademark of the University of Washington, Seattle. University of Washington, Seattle: All Rights Reserved.
Itzhaki I., Maizels L., Huber I., Gepstein A., Arbel G., Caspi O., et al. (2012). Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. J. Am. Coll. Cardiol. 60 (11), 990–1000. doi:10.1016/j.jacc.2012.02.066
Jung C. B., Moretti A., Mederos y Schnitzler M., Iop L., Storch U., Bellin M., et al. (2012). Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol. Med. 4 (3), 180–191. doi:10.1002/emmm.201100194
Kaneko N. (1994). New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug Dev. Res. 33 (4), 429–438. doi:10.1002/ddr.430330406
Kimura J., Kawahara M., Sakai E., Yatabe J., Nakanishi H. (1999). Effects of a novel cardioprotective drug, JTV-519, on membrane currents of Guinea pig ventricular myocytes. Jpn. J. Pharmacol. 79 (3), 275–281. doi:10.1254/jjp.79.275
Kiriyama K., Kiyosue T., Wang J. C., Dohi K., Arita M. (2000). Effects of JTV-519, a novel anti-ischaemic drug, on the delayed rectifier K+ current in Guinea-pig ventricular myocytes. Naunyn Schmiedeb. Arch. Pharmacol. 361 (6), 646–653. doi:10.1007/s002100000230
Kitakaze M., Sarai N., Ando H., Sakamoto T., Nakajima H. (2012). Safety and tolerability of once-daily controlled-release carvedilol 10-80mg in Japanese patients with chronic heart failure. Circ. J. 76 (3), 668–674. doi:10.1253/circj.CJ-11-0210
Kohno M., Yano M., Kobayashi S., Doi M., Oda T., Tokuhisa T., et al. (2003). A new cardioprotective agent, JTV519, improves defective channel gating of ryanodine receptor in heart failure. Am. J. Physiol. Heart Circ. Physiol. 284 (3), H1035–H1042. doi:10.1152/ajpheart.00722.2002
Kryshtal D. O., Blackwell D. J., Egly C. L., Smith A. N., Batiste S. M., Johnston J. N., et al. (2021). RYR2 channel inhibition is the principal mechanism of flecainide action in CPVT. Circ. Res. 128 (3), 321–331. doi:10.1161/circresaha.120.316819
Kurebayashi N., Kodama M., Murayama T., Ishida R., Mori S., Sugihara M., et al. (2024). Effects of a high-affinity and selective RyR2 inhibitor on Ca2+ signals in cardiomyocytes and arrhythmias in CPVT model mice. Biophys. J. 123 (3), 23a. doi:10.1016/j.bpj.2023.11.247
Lang D., Sulkin M., Lou Q., Efimov I. R. (2011). Optical mapping of action potentials and calcium transients in the mouse heart. J. Vis. Exp. 55, 3275. doi:10.3791/3275
Lee P., Yan P., Ewart P., Kohl P., Loew L. M., Bollensdorff C. (2012). Simultaneous measurement and modulation of multiple physiological parameters in the isolated heart using optical techniques. Pflugers Arch. 464 (4), 403–414. doi:10.1007/s00424-012-1135-6
Liu N., Colombi B., Memmi M., Zissimopoulos S., Rizzi N., Negri S., et al. (2006). Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ. Res. 99 (3), 292–298. doi:10.1161/01.RES.0000235869.50747.e1
Liu N., Denegri M., Ruan Y., Avelino-Cruz J. E., Perissi A., Negri S., et al. (2011a). Short communication: flecainide exerts an antiarrhythmic effect in a mouse model of catecholaminergic polymorphic ventricular tachycardia by increasing the threshold for triggered activity. Circ. Res. 109 (3), 291–295. doi:10.1161/circresaha.111.247338
Liu N., Ruan Y., Denegri M., Bachetti T., Li Y., Colombi B., et al. (2011b). Calmodulin kinase II inhibition prevents arrhythmias in RyR2(R4496C+/-) mice with catecholaminergic polymorphic ventricular tachycardia. J. Mol. Cell Cardiol. 50 (1), 214–222. doi:10.1016/j.yjmcc.2010.10.001
Loughrey C. M., Otani N., Seidler T., Craig M. A., Matsuda R., Kaneko N., et al. (2007). K201 modulates excitation-contraction coupling and spontaneous Ca2+ release in normal adult rabbit ventricular cardiomyocytes. Cardiovasc Res. 76 (2), 236–246. doi:10.1016/j.cardiores.2007.06.014
Maizels L., Huber I., Arbel G., Tijsen A. J., Gepstein A., Khoury A., et al. (2017). Patient-Specific drug screening using a human induced pluripotent stem cell model of catecholaminergic polymorphic ventricular tachycardia type 2. Circ. Arrhythm. Electrophysiol. 10 (6), e004725. doi:10.1161/circep.116.004725
Martinez-Hernandez E., Kanaporis G., Blatter L. A. (2022). Mechanism of carvedilol induced action potential and calcium alternans. Channels 16 (1), 97–112. doi:10.1080/19336950.2022.2055521
Mazzanti A., Kukavica D., Trancuccio A., Memmi M., Bloise R., Gambelli P., et al. (2022). Outcomes of patients with catecholaminergic polymorphic ventricular tachycardia treated with β-blockers. JAMA Cardiol. 7 (5), 504–512. doi:10.1001/jamacardio.2022.0219
Miki K., Endo K., Takahashi S., Funakoshi S., Takei I., Katayama S., et al. (2015). Efficient detection and purification of cell populations using synthetic MicroRNA switches. Cell Stem Cell 16 (6), 699–711. doi:10.1016/j.stem.2015.04.005
Napolitano C., Mazzanti A., Bloise R., Priori S. G. (2022). “Catecholaminergic Polymorphic Ventricular Tachycardia,” in GeneReviews(®). 2004 Oct 14 [Updated 2022 Jun 23]. Editors M. P. Adam, J. Feldman, G. M. Mirzaaet al. (Seattle (WA): University of Washington, Seattle). 1993–2025. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK1289/.
Peltenburg P. J., Kallas D., Bos J. M., Lieve K. V. V., Franciosi S., Roston T. M., et al. (2022). An international multicenter cohort study on β-blockers for the treatment of symptomatic children with catecholaminergic polymorphic ventricular tachycardia. Circulation 145 (5), 333–344. doi:10.1161/CIRCULATIONAHA.121.056018
Penttinen K., Siirtola H., Avalos-Salguero J., Vainio T., Juhola M., Aalto-Setala K. (2015a). Novel analysis software for detecting and classifying Ca2+ transient abnormalities in stem cell-derived cardiomyocytes. PLoS One 10 (8), e0135806. doi:10.1371/journal.pone.0135806
Penttinen K., Swan H., Vanninen S., Paavola J., Lahtinen A. M., Kontula K., et al. (2015b). Antiarrhythmic effects of dantrolene in patients with catecholaminergic polymorphic ventricular tachycardia and replication of the responses using iPSC models. PLoS One 10 (5), e0125366. doi:10.1371/journal.pone.0125366
Pölönen R. P., Penttinen K., Swan H., Aalto-Setälä K. (2018). Antiarrhythmic effects of carvedilol and flecainide in cardiomyocytes derived from catecholaminergic polymorphic ventricular tachycardia patients. Stem Cells Int. 2018, 9109503. doi:10.1155/2018/9109503
Preininger M. K., Jha R., Maxwell J. T., Wu Q., Singh M., Wang B., et al. (2016). A human pluripotent stem cell model of catecholaminergic polymorphic ventricular tachycardia recapitulates patient-specific drug responses. Dis. Model Mech. 9 (9), 927–939. doi:10.1242/dmm.026823
Ramos E., O’Leary M. E. (2004). State-dependent trapping of flecainide in the cardiac sodium channel. J. Physiol. 560 (Pt 1), 37–49. doi:10.1113/jphysiol.2004.065003
Reyes Gaido O. E., Nkashama L. J., Schole K. L., Wang Q., Umapathi P., Mesubi O. O., et al. (2023). CaMKII as a therapeutic target in cardiovascular disease. Annu. Rev. Pharmacol. Toxicol. 63, 249–272. doi:10.1146/annurev-pharmtox-051421-111814
Sacherer M., Sedej S., Wakuła P., Wallner M., Vos M. A., Kockskämper J., et al. (2012). JTV519 (K201) reduces sarcoplasmic reticulum Ca2+ leak and improves diastolic function in vitro in murine and human non-failing myocardium. Br. J. Pharmacol. 167 (3), 493–504. doi:10.1111/j.1476-5381.2012.01995.x
Sałaciak K., Koszałka A., Żmudzka E., Pytka K. (2021). The calcium/calmodulin-dependent kinases II and IV as therapeutic targets in neurodegenerative and neuropsychiatric disorders. Int. J. Mol. Sci. 22 (9), 4307. doi:10.3390/ijms22094307
Sasaki K., Makiyama T., Yoshida Y., Wuriyanghai Y., Kamakura T., Nishiuchi S., et al. (2016). Patient-Specific human induced pluripotent stem cell model assessed with electrical pacing validates S107 as a potential therapeutic agent for catecholaminergic polymorphic ventricular tachycardia. PLoS One 11 (10), e0164795. doi:10.1371/journal.pone.0164795
Song L., Awari D. W., Han E. Y., Uche-Anya E., Park S. H., Yabe Y. A., et al. (2015). Dual optical recordings for action potentials and calcium handling in induced pluripotent stem cell models of cardiac arrhythmias using genetically encoded fluorescent indicators. Stem Cells Transl. Med. 4 (5), 468–475. doi:10.5966/sctm.2014-0245
Sun Y., Hao M., Wu H., Zhang C., Wei D., Li S., et al. (2024). Unveiling the role of CaMKII in retinal degeneration: from biological mechanism to therapeutic strategies. Cell Biosci. 14 (1), 59. doi:10.1186/s13578-024-01236-2
Swaminathan P. D., Purohit A., Hund T. J., Anderson M. E. (2012). Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ. Res. 110(12), 1661–1677. doi:10.1161/CIRCRESAHA.111.243956
Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131 (5), 861–872. doi:10.1016/j.cell.2007.11.019
Takaki T., Inagaki A., Chonabayashi K., Inoue K., Miki K., Ohno S., et al. (2019). Optical recording of action potentials in human induced pluripotent stem cell-derived cardiac single cells and monolayers generated from long QT syndrome type 1 patients. Stem Cells Int. 2019, 7532657. doi:10.1155/2019/7532657
Takenaka M., Kodama M., Murayama T., Ishigami-Yuasa M., Mori S., Ishida R., et al. (2023). Screening for novel type 2 ryanodine receptor inhibitors by endoplasmic reticulum Ca(2+) monitoring. Mol. Pharmacol. 104 (6), 275–286. doi:10.1124/molpharm.123.000720
Toischer K., Lehnart S. E., Tenderich G., Milting H., Körfer R., Schmitto J. D., et al. (2010). K201 improves aspects of the contractile performance of human failing myocardium via reduction in Ca2+ leak from the sarcoplasmic reticulum. Basic Res. Cardiol. 105 (2), 279–287. doi:10.1007/s00395-009-0057-8
van der Werf C., Kannankeril P. J., Sacher F., Krahn A. D., Viskin S., Leenhardt A., et al. (2011). Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J. Am. Coll. Cardiol. 57 (22), 2244–2254. doi:10.1016/j.jacc.2011.01.026
Wegener J. W., Mitronova G. Y., ElShareif L., Quentin C., Belov V., Pochechueva T., et al. (2024). A dual-targeted drug inhibits cardiac ryanodine receptor Ca(2+) leak but activates SERCA2a Ca(2+) uptake. Life Sci. Alliance 7 (2), e202302278. doi:10.26508/lsa.202302278
Wehrens X. H., Lehnart S. E., Reiken S. R., Marks A. R. (2004). Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ. Res. 94 (6), e61–e70. doi:10.1161/01.res.0000125626.33738.e2
Yang H., Yang Y., Lu Z., Zhang J. Z. (2024). Simultaneous optical imaging of action potentials and calcium transients in human induced pluripotent stem cell-derived cardiomyocytes. Curr. Protoc. 4 (7), e1101. doi:10.1002/cpz1.1101
Yang P. C., Giles W. R., Belardinelli L., Clancy C. E. (2021). Mechanisms of flecainide induced negative inotropy: an in silico study. J. Mol. Cell Cardiol. 158, 26–37. doi:10.1016/j.yjmcc.2021.05.007
Yilmaz B., Arslan S. (2016). HPLC/Fluorometric detection of carvedilol in real human plasma samples using liquid–liquid extraction. J. Chromatogr. Sci. 54 (3), 413–418. doi:10.1093/chromsci/bmv157
Zhang X. H., Wei H., Xia Y., Morad M. (2021). Calcium signaling consequences of RyR2 mutations associated with CPVT1 introduced via CRISPR/Cas9 gene editing in human-induced pluripotent stem cell-derived cardiomyocytes: comparison of RyR2-R420Q, F2483I, and Q4201R. Heart Rhythm 18 (2), 250–260. doi:10.1016/j.hrthm.2020.09.007
Zhao Q., Guo H., Ruogu P., Jixiang L., Liao J., Diwu Z. (2019). New red fluorescent calcium indicators for functional analysis of GPCRs and Ca2+ channel targets. Biophys. J. 116 (3), 240a. doi:10.1016/j.bpj.2018.11.1315
Keywords: FluoVolt, membrane potential dye, Calbryte 590, calcium transient, catecholaminergic polymorphic ventricular tachycardia, induced pluripotent stem cells, JTV519, KN-93
Citation: Takaki T, Tamura N, Imahashi K, Nishimoto T and Yoshida Y (2025) Simultaneous optical recording of action potentials and calcium transients in cardiac single cells differentiated from type 1 CPVT-iPS cells. Front. Physiol. 16:1579815. doi: 10.3389/fphys.2025.1579815
Received: 19 February 2025; Accepted: 12 May 2025;
Published: 04 June 2025.
Edited by:
Jong-Kook Lee, Osaka University, JapanReviewed by:
Luigi Venetucci, The University of Manchester, United KingdomRoman Y. Medvedev, University of Wisconsin-Madison, United States
Copyright © 2025 Takaki, Tamura, Imahashi, Nishimoto and Yoshida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshinori Yoshida, eW9zaGlub3JAY2lyYS5reW90by11LmFjLmpw
†Former affiliations
 Tadashi Takaki
Tadashi Takaki Norihisa Tamura2,4†
Norihisa Tamura2,4† Yoshinori Yoshida
Yoshinori Yoshida