- 1IHU Liryc, INSERM, U1045, CRCTB, University Bordeaux, Bordeaux, France
- 2CHU de Bordeaux, Cardiology, INSERM, U1045, CRCTB, Bordeaux, France
Introduction
Cardiovascular disease remains the leading global cause of mortality, underscoring the urgent need to better understand the mechanisms driving cardiac dysfunction (Jagannathan et al., 2019). Ion channels are key regulators of cardiac excitability, and among them, the transient receptor potential (TRP) family has gained attention for its diverse physiological roles. TRP channels are ubiquitously expressed in mammalian hearts and function as essential “cellular switches” that respond to a wide range of chemical and physical stimuli, influencing sensory physiology (Hof et al., 2019; Zhang et al., 2023). Comprising 28 members across six subfamilies, TRP channels act as polymodal sensors responding to chemical, thermal, and mechanical cues. In the heart, these channels influence calcium (Ca2+) dynamics, electrical conduction, mechanical responsiveness, and stress adaptation (Hof et al., 2019).
Building on the foundational discoveries by Drs. David Julius and Ardem Patapoutian on temperature and mechanotransduction pathways—work that earned the 2021 Nobel Prize in Physiology or Medicine—there is growing recognition of TRP channels’ roles beyond sensory systems, including cardiovascular physiology and pathophysiology (Caterina et al., 1997; Peier et al., 2002). In particular, genetically modified knockout (KO) mice models have emerged as powerful tools to explore TRP channel functions in cardiac tissue, allowing for precise dissection of their mechanistic roles in disease progression and therapeutic potential. This opinion letter highlights key advances and future directions in further leveraging TRP KO models to unlock the therapeutic promise of TRP channels in cardiac health.
Cardiac TRP channels: multifaceted roles and research frontiers
TRP channels regulate numerous cardiac functions, including rhythm generation, Ca2+ handling, contractility, and responses to mechanical stress (Hof et al., 2016; Chaigne et al., 2023; Hu et al., 2023; Veteto et al., 2020). Several family members (e.g., TRPC6, TRPM4, TRPV4) have been implicated in arrhythmogenesis, hypertrophy, fibrosis, and ischemia-reperfusion injury. Their broad permeability to cations and ability to integrate diverse stimuli render them critical for both normal physiology and pathological remodeling (Numata et al., 2016; Guo et al., 2024). In physiological conditions, inward current through these channels is primarily due to the entry of Ca2+, sodium (Na+), or magnesium (Mg2+), while outward current results from potassium (K+) exiting the cell. Notably, very little is known about Mg2+ handling in relation to TRP channel activities and heart function, even though mutations in sub-families of these channels have been shown to be associated with disturbances in Mg2+, highlighting the need for further investigations (Gwanyanya et al., 2004; Jin et al., 2022).
Activation of TRP channels typically induces a depolarizing current because the main flux of cations is inward. This characteristic makes TRP channels particularly relevant in excitable cells like cardiomyocytes, where they can prolong action potential duration, as observed following activation or potentiation of TRPC3 (Ju et al., 2015), TRPM4 (permeable to Na+) (Hof et al., 2016; Simard et al., 2013), TRPM6-7 (Gwanyanya et al., 2021) and TRPV4 (Chaigne et al., 2023). However, many TRP channels do not function as classical mechanosensors. Recent studies show that while TRPs may not be directly gated by membrane stretch (O'Neil and Heller, 2005; Nikolaev et al., 2019), they can act as mechano-effectors downstream of primary mechanosensors, including PIEZO1 channels (Guo et al., 2024; Guo et al., 2021; Yu et al., 2022). For instance, TRPV4 interacts with volume-sensitive signalling molecules such as Src kinase (Zou et al., 2022) and phospholipase A2 (Gorelick and Nathanson, 2020). These emerging insights necessitate a more nuanced classification of TRP channels in mechanotransduction.
Importantly, the effector role of TRP channels—particularly their position downstream of primary sensors—may open opportunities for more progressive and targeted therapeutic interventions. Rather than blocking upstream mechanotransduction entirely, targeting TRPs may allow fine-tuning of maladaptive downstream signalling. This approach could offer graded, context-sensitive modulation in heart failure and hypertrophy, with lower risk of disrupting physiological homeostasis. Supporting this concept, TRPC3 and TRPC6, for example, have been shown to act as calcium-permeable effectors that activate the calcineurin–NFAT pathway downstream of neurohumoral and mechanical stimuli (Seo et al., 2014). Inhibiting such TRPs may thus interrupt pro-hypertrophic signalling while preserving upstream sensory mechanisms essential for baseline cardiac function. This positions TRP channels as viable and adaptable targets for therapeutic strategies focused on disease modification without systemic disruption.
However, selective agonists and antagonists for TRP channels are scarce, with only a few known agents such as capsaicin, an agonist of TRPV1 (Caterina et al., 1997; Wang and Wang, 2005), that have undergone comprehensive pharmacological evaluation. Hence, while this approach could offer graded, context-sensitive modulation in heart failure and hypertrophy, with lower risk of disrupting physiological homeostasis, further studies are required to identify selective modulators of these channels. The use of TRP KO mice offers a potent avenue to better understand the specific roles of this ion channel family and validate the effectiveness and safety of their targeted modulators.
Advantages of knockout models in TRP research
Given the limited availability and selectivity of pharmacological modulators for TRP channels, KO mice models provide an essential approach for understanding cardiac channel-specific functions. For example, TRPM4 KO mice have clarified this channel’s role in prolonging action potentials and promoting arrhythmia in stress models (Guinamard et al., 2015). Similarly, both TRPC6 and TRPV4 KO mice resist pressure-overload-induced hypertrophy (Jia et al., 2024; Kinoshita et al., 2010; Tang et al., 2022). Interestingly, TRPM4 has emerged as a critical integrator of distinct pathological stimuli. Studies have shown that both pressure overload and Angiotensin II (AngII) stimulation activate TRPM4 through different signalling pathways (Guo et al., 2021; Kecskes et al., 2015). This convergence highlights TRPM4’s central role in cardiac disease and underscores its promise as a therapeutic target across multiple disease contexts.
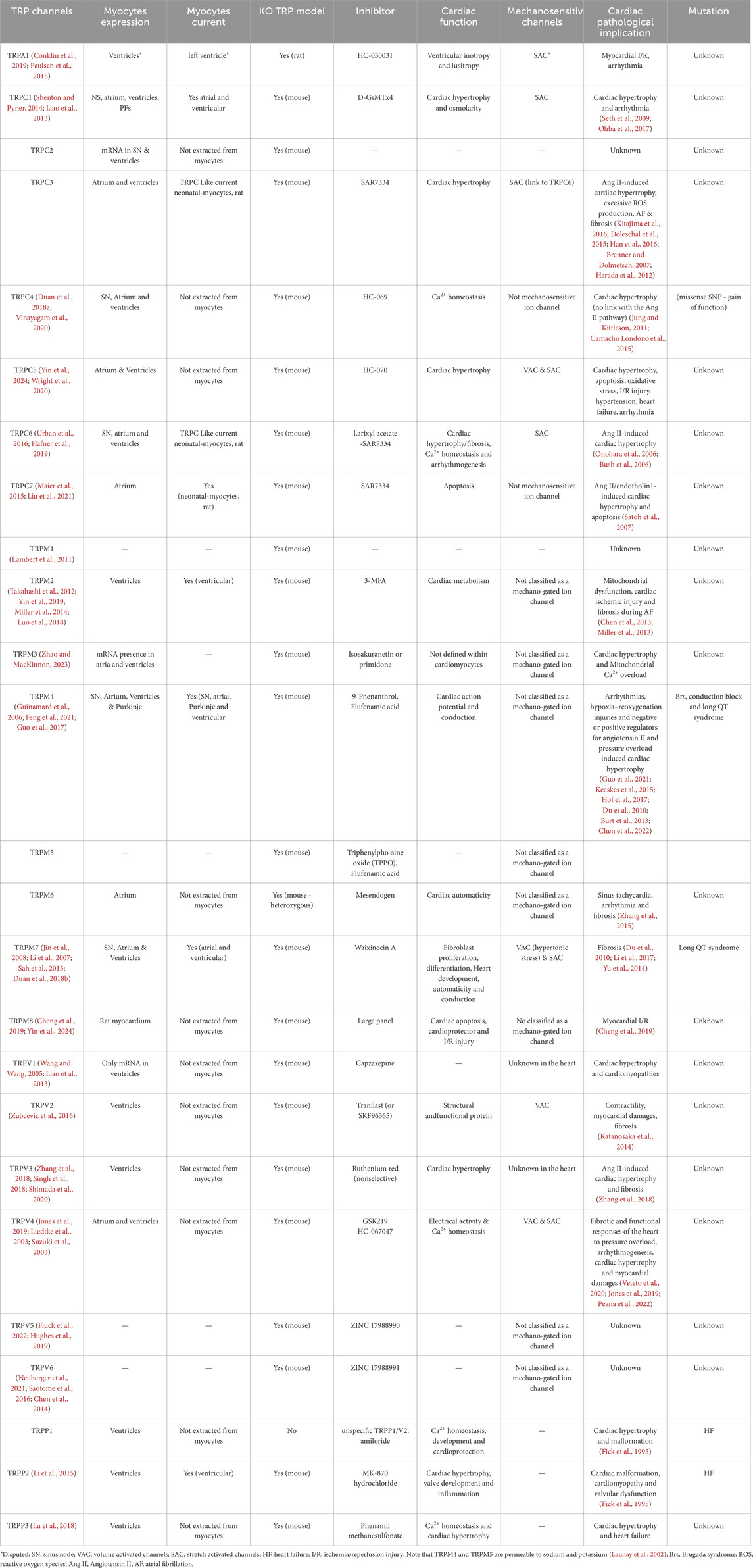
A comprehensive summary of current TRP KO mice models is presented in Table 1, demonstrating the scope and complexity of the roles of these channels in cardiovascular pathophysiology. KO models offer several advantages:
• Causality: Genetic deletion isolates the contribution of individual channels to disease phenotypes.
• Compensation Insight: TRP families exhibit overlapping roles. KO studies have revealed compensatory expression among TRPC channels (Vandewauw et al., 2018), highlighting complex redundancy.
• Therapeutic Discovery: By testing drugs in KO versus wild-type mice, researchers can assess target specificity and identify off-target effects.
These benefits make TRP KO models a cornerstone of mechanistic cardiovascular research.
KO mice models offer an invaluable tool for uncovering new components of cellular dysfunction and their translational impacts. While clinical electrocardiogram (ECG) features such as heart rate, PR interval, QRS complex, and QT/QTc interval, are crucial for diagnosing and monitoring physiological and various cardiac conditions, in-vitro parameters, offer focused, detailed insights into the cellular and molecular mechanisms underlying cardiac electrical activity. Unitary currents, measured through single-channel recordings, reveal the behaviour of individual ion channels that contribute to the macroscopic currents linked to cellular activation profiles (Guinamard et al., 2015). For example, TRP channels dysfunction detected through unitary current recordings, can be correlated with changes in both action potential and ECG parameters (Chaigne et al., 2021), indicating a risk of arrhythmias.
While it has been suggested that mammalian TRP channels are insensitive to membrane stretch, some TRP channels respond to mechanical forces (Liu and Montell, 2015) applied to the cell membrane from external influences (see Table 1). These forces can modulate the open probability of the channels, without involving a signalling cascade. This mechanosensitivity allows TRP channels to respond to various physical stimuli such as pressure, stretch, and shear stress, thereby playing a crucial role in various physiological processes (Liu and Montell, 2015; Moran et al., 2004). KO models have been effective in investigating the mechanosensitivity of TRP channels and their down-stream effects. Furthermore, using KO mice, researchers can also explore potential compensatory mechanisms that may arise due to the absence or inhibition of targeted TRP channels. This approach provides insights into the complex interplay between different ion channels and signalling pathways in maintaining physiological homeostasis.
Finally, integrative in-vivo models can help uncover systemic effects and potential off-target effects that might not be evident in isolated cardiomyocytes which lack the full spectrum of hormonal (Liu et al., 2022), immune (Wu et al., 2023), and nervous system regulation (Shanks et al., 2019). For instance, a drug might show promise in-vitro but could interact with other physiological systems in-vivo, leading to unforeseen side-effects. Comprehensive in-vivo testing can thus, identify early issues in the drug development process, saving time and resources. Through transesophageal stimulation, which enables the analysis of underlying mechanisms of cardiac rhythm disorders across the heart’s chambers, the use of KO models, helps to indirectly establish a link between the absence of a gene and its involvement in the development of arrhythmia. Note that this approach may not be ideal for mechanical ion channels that are sensitive to stretching. Ultimately, the absence of the gene of interest can indirectly enhance our understanding of the mechanisms underlying cardiac tissue hypertrophy and fibrosis (Guo et al., 2021; Zou et al., 2022; Watanabe et al., 2013).
Future directions and strategic areas for therapeutic insight
Several innovative approaches are currently under development and have the potential to pioneer change in cardiac research. A key strategy involves structure-based drug design, which aims to create highly specific inhibitors by targeting the three-dimensional TRP channels structure. Another promising technique, Surface Plasmon Resonance (SPR), provides real-time insights into how these inhibitors interact with TRP channels, enhancing our understanding of their binding mechanisms. Their thorough investigation could lead to new therapeutic approaches for treating cardiovascular abnormalities. Main takeaways that could influence future therapeutic strategies are outlined below:
1. Mechanotransduction and Heart Failure: Mechanosensitive TRP channels (e.g., TRPC6 (Onohara et al., 2006), TRPM7 (Yu et al., 2014), TRPV4 (Veteto et al., 2020)) are activated under pathological stretch, making them promising targets in heart failure. KO models subjected to pressure overload can dissect these channels’ contributions to maladaptive remodelling.
2. Sudden Cardiac Death and Arrhythmia: TRPA1 (Conklin et al., 2019) and TRPM4 (Vandewiele et al., 2022; Yang et al., 2006) KO mice exhibit resistance to arrhythmia under stress or ischemic conditions. TRP channels modulating action potential duration are ideal candidates for anti-arrhythmic drug development.
3. Inter-individual Variability in Drug Response: KO models help reveal how TRP channel expression variability influences therapeutic outcomes. For example, TRPC3 (Kitajima et al., 2016) and TRPM2 are stress-responsive and may contribute to variable responses to oxidative or hypertrophic stimuli (Onohara et al., 2006; Kitajima et al., 2016; Takahashi et al., 2012; Chen et al., 2013).
4. Brain-Heart Axis and Neurogenic Modulation: With expression in sensory neurons and cardiac tissue, channels like TRPM8 and TRPV1 (Yoshie et al., 2020) may mediate autonomic effects on the heart (Shanks et al., 2019; Yin et al., 2024). TRP KO models enable investigation into neuro-cardiac interactions and their therapeutic modulation.
5. Translation to Human Therapies: Though interspecies differences exist, KO mice remain critical in screening for TRP-targeted therapies, particularly where pharmacological tools are lacking or non-selective. Caution is needed when extrapolating findings, but the insights remain invaluable.
Limitations and integration with complementary models
While KO mice are indispensable, they have limitations. Genetic deletion can trigger compensatory mechanisms, masking functional deficits. Moreover, mouse cardiac electrophysiology differs from humans in ion channel expression and repolarization dynamics (Joukar, 2021). These differences, including variations in immune system responses (Gilbertson and Weinmann, 2021), can undeniably impact the relevance of findings to human clinical settings. In this case, human tissue or cellular models, offer promising alternatives. Additionally, while not all TRP channels can compensate for one another, functional redundancy within the TRP family has been documented. For example, research has shown that the presence of at least one of these channels (TRPA1, TRPM3 or TRPV1) helps preserve somatosensory heat responsiveness (Vandewauw et al., 2018). These compensatory effects must be carefully considered when interpreting KO model data.
To address these challenges, future studies could incorporate the following complementary strategies:
• Integrate human iPSC-derived cardiomyocytes and organoids
• Employ computational modelling of TRP-mediated currents
• Combine TRP KOs with spatial omics and functional imaging
These approaches could provide a comprehensive understanding of TRP channel function across species and contexts.
Conclusion
Cardiac TRP channels are emerging as pivotal regulators of cardiovascular physiology and pathology. KO mice models offer a unique opportunity to define their functions, explore disease mechanisms, and identify new therapeutic strategies. By refining our understanding of TRP channel biology, particularly through the lens of mechanotransduction and electrophysiology, the next-generation of targeted interventions may be realized. We advocate for a continued, strategic use of TRP KO models as a springboard for precision cardiovascular medicine.
Author contributions
KK: Conceptualization, Writing – review and editing. RW: Conceptualization, Writing – review and editing. SC: Conceptualization, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the French Government as part of the “Investments of the Future,” National Research Agency (ANR), Grant reference ANR-10-IAHU-04.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Brenner J. S., Dolmetsch R. E. (2007). TrpC3 regulates hypertrophy-associated gene expression without affecting myocyte beating or cell size. PLoS One 2, e802. doi:10.1371/journal.pone.0000802
Burt R., Graves B. M., Gao M., Li C., Williams D. L., Fregoso S. P., et al. (2013). 9-Phenanthrol and flufenamic acid inhibit calcium oscillations in HL-1 mouse cardiomyocytes. Cell calcium 54, 193–201. doi:10.1016/j.ceca.2013.06.003
Bush E. W., Hood D. B., Papst P. J., Chapo J. A., Minobe W., Bristow M. R., et al. (2006). Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J. Biol. Chem. 281, 33487–33496. doi:10.1074/jbc.M605536200
Camacho Londono J. E., Tian Q., Hammer K., Schröder L., Reil J. C., Oberhofer M., et al. (2015). A background Ca2+ entry pathway mediated by TRPC1/TRPC4 is critical for development of pathological cardiac remodelling. Eur. Heart J. 36, 2257–2266. doi:10.1093/eurheartj/ehv250
Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. doi:10.1038/39807
Chaigne S., Barbeau S., Ducret T., Guinamard R., Benoist D. (2023). Pathophysiological roles of the TRPV4 channel in the heart. Cells 12, 1654. doi:10.3390/cells12121654
Chaigne S., Cardouat G., Louradour J., Vaillant F., Charron S., Sacher F., et al. (2021). Transient receptor potential vanilloid 4 channel participates in mouse ventricular electrical activity. Am. J. Physiol. Heart Circ. Physiol. 320, H1156–H1169. doi:10.1152/ajpheart.00497.2020
Chen F., Ni B., Yang Y. O., Ye T., Chen A. (2014). Knockout of TRPV6 causes osteopenia in mice by increasing osteoclastic differentiation and activity. Cell Physiol. Biochem. 33, 796–809. doi:10.1159/000358653
Chen J., Chang Y., Zhu J., Peng Y., Li Z., Zhang K., et al. (2022). Flufenamic acid improves survival and neurologic outcome after successful cardiopulmonary resuscitation in mice. J. Neuroinflammation 19, 214. doi:10.1186/s12974-022-02571-2
Chen S. J., Zhang W., Tong Q., Conrad K., Hirschler-Laszkiewicz I., Bayerl M., et al. (2013). Role of TRPM2 in cell proliferation and susceptibility to oxidative stress. Am. J. Physiol. Cell Physiol. 304, C548–C560. doi:10.1152/ajpcell.00069.2012
Cheng Q. Y., Yang M. C., Wu J., Jia X. L., Xiao C., Lian T., et al. (2019). Reduced cardiac ischemia/reperfusion injury by hypothermic reperfusion via activation of transient receptor potential M8 channel. Life Sci. 232, 116658. doi:10.1016/j.lfs.2019.116658
Conklin D. J., Guo Y., Nystoriak M. A., Jagatheesan G., Obal D., Kilfoil P. J., et al. (2019). TRPA1 channel contributes to myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 316, H889-H899–H899. doi:10.1152/ajpheart.00106.2018
Doleschal B., Primessnig U., Wölkart G., Wolf S., Schernthaner M., Lichtenegger M., et al. (2015). TRPC3 contributes to regulation of cardiac contractility and arrhythmogenesis by dynamic interaction with NCX1. Cardiovasc Res. 106, 163–173. doi:10.1093/cvr/cvv022
Du J., Xie J., Zhang Z., Tsujikawa H., Fusco D., Silverman D., et al. (2010). TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ. Res. 106, 992–1003. doi:10.1161/CIRCRESAHA.109.206771
Duan J., Li J., Zeng B., Chen G. L., Peng X., Zhang Y., et al. (2018a). Structure of the mouse TRPC4 ion channel. Nat. Commun. 9, 3102. doi:10.1038/s41467-018-05247-9
Duan J., Li Z., Li J., Hulse R. E., Santa-Cruz A., Valinsky W. C., et al. (2018b). Structure of the mammalian TRPM7, a magnesium channel required during embryonic development. Proc. Natl. Acad. Sci. U. S. A. 115, E8201-E8210–E8210. doi:10.1073/pnas.1810719115
Feng J., Zong P., Yan J., Yue Z., Li X., Smith C., et al. (2021). Upregulation of transient receptor potential melastatin 4 (TRPM4) in ventricular fibroblasts from heart failure patients. Pflugers Arch. 473, 521–531. doi:10.1007/s00424-021-02525-2
Fick G. M., Johnson A. M., Hammond W. S., Gabow P. A. (1995). Causes of death in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 5, 2048–2056. doi:10.1681/ASN.V5122048
Fluck E. C., Yazici A. T., Rohacs T., Moiseenkova-Bell V. Y. (2022). Structural basis of TRPV5 regulation by physiological and pathophysiological modulators. Cell Rep. 39, 110737. doi:10.1016/j.celrep.2022.110737
Gilbertson S. E., Weinmann A. S. (2021). Conservation and divergence in gene regulation between mouse and human immune cells deserves equal emphasis. Trends Immunol. 42, 1077–1087. doi:10.1016/j.it.2021.10.007
Gorelick F., Nathanson M. H. (2020). TRPV4 helps Piezo1 put the squeeze on pancreatic acinar cells. J. Clin. Investigation 130, 2199–2201. doi:10.1172/JCI136525
Guinamard R., Bouvagnet P., Hof T., Liu H., Simard C., Sallé L. (2015). TRPM4 in cardiac electrical activity. Cardiovasc Res. 108, 21–30. doi:10.1093/cvr/cvv213
Guinamard R., Demion M., Magaud C., Potreau D., Bois P. (2006). Functional expression of the TRPM4 cationic current in ventricular cardiomyocytes from spontaneously hypertensive rats. Hypertension 48, 587–594. doi:10.1161/01.HYP.0000237864.65019.a5
Guo J., She J., Zeng W., Chen Q., Bai X. C., Jiang Y. (2017). Structures of the calcium-activated, non-selective cation channel TRPM4. Nature 552, 205–209. doi:10.1038/nature24997
Guo Y., Cheng D., Yu Z. Y., Schiatti T., Chan A. Y., Hill A. P., et al. (2024). Functional coupling between Piezo1 and TRPM4 influences the electrical activity of HL-1 atrial myocytes. J. Physiol. 602, 4363–4386. doi:10.1113/JP284474
Guo Y., Yu Z. Y., Wu J., Gong H., Kesteven S., Iismaa S. E., et al. (2021). The Ca(2+)-activated cation channel TRPM4 is a positive regulator of pressure overload-induced cardiac hypertrophy. Elife 10, e66582. doi:10.7554/eLife.66582
Gwanyanya A., Amuzescu B., Zakharov S. I., Macianskiene R., Sipido K. R., Bolotina V. M., et al. (2004). Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: permeation of divalent cations and pH-mediated regulation. J. physiology 559, 761–776. doi:10.1113/jphysiol.2004.067637
Gwanyanya A., Andriulė I., Istrate B. M., Easmin F., Mubagwa K., Mačianskienė R. (2021). Modulation of the cardiac myocyte action potential by the magnesium-sensitive TRPM6 and TRPM7-like current. Int. J. Mol. Sci. 22, 8744. doi:10.3390/ijms22168744
Hafner S., Urban N., Schaefer M. (2019). Discovery and characterization of a positive allosteric modulator of transient receptor potential canonical 6 (TRPC6) channels. Cell Calcium 78, 26–34. doi:10.1016/j.ceca.2018.12.009
Han J. W., Lee Y. H., Yoen S. I., Abramowitz J., Birnbaumer L., Lee M. G., et al. (2016). Resistance to pathologic cardiac hypertrophy and reduced expression of CaV1.2 in Trpc3-depleted mice. Mol. Cell Biochem. 421, 55–65. doi:10.1007/s11010-016-2784-0
Harada M., Luo X., Qi X. Y., Tadevosyan A., Maguy A., Ordog B., et al. (2012). Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 126, 2051–2064. doi:10.1161/CIRCULATIONAHA.112.121830
Hof T., Chaigne S., Récalde A., Sallé L., Brette F., Guinamard R. (2019). Transient receptor potential channels in cardiac health and disease. Nat. Rev. Cardiol. 16, 344–360. doi:10.1038/s41569-018-0145-2
Hof T., Liu H., Sallé L., Schott J. J., Ducreux C., Millat G., et al. (2017). TRPM4 non-selective cation channel variants in long QT syndrome. BMC Med. Genet. 18, 31. doi:10.1186/s12881-017-0397-4
Hof T., Sallé L., Coulbault L., Richer R., Alexandre J., Rouet R., et al. (2016). TRPM4 non-selective cation channels influence action potentials in rabbit Purkinje fibres. J. Physiol. 594, 295–306. doi:10.1113/JP271347
Hu Y., Cang J., Hiraishi K., Fujita T., Inoue R. (2023). The role of TRPM4 in cardiac electrophysiology and arrhythmogenesis. Int. J. Mol. Sci. 24, 11798. doi:10.3390/ijms241411798
Hughes T. E., Del Rosario J. S., Kapoor A., Yazici A. T., Yudin Y., Fluck E. C., et al. (2019). Structure-based characterization of novel TRPV5 inhibitors. Elife 8, e49572. doi:10.7554/eLife.49572
Jagannathan R., Patel S. A., Ali M. K., Narayan K. V. (2019). Global updates on cardiovascular disease mortality trends and attribution of traditional risk factors. Curr. diabetes Rep. 19, 44–12. doi:10.1007/s11892-019-1161-2
Jia M., Liu W., Zhang K., Wang Z., Li R., Pan J., et al. (2024). Larixyl acetate, a TRPC6 inhibitor, attenuates pressure overload-induced heart failure in mice. Mol. Med. Rep. 29, 49. doi:10.3892/mmr.2024.13174
Jin F., Huang Y., Hattori M. (2022). Recent advances in the structural biology of Mg(2+) channels and transporters. J. Mol. Biol. 434, 167729. doi:10.1016/j.jmb.2022.167729
Jin J., Desai B. N., Navarro B., Donovan A., Andrews N. C., Clapham D. E. (2008). Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322, 756–760. doi:10.1126/science.1163493
Jones J. L., Peana D., Veteto A. B., Lambert M. D., Nourian Z., Karasseva N. G., et al. (2019). TRPV4 increases cardiomyocyte calcium cycling and contractility yet contributes to damage in the aged heart following hypoosmotic stress. Cardiovasc Res. 115, 46–56. doi:10.1093/cvr/cvy156
Joukar S. (2021). A comparative review on heart ion channels, action potentials and electrocardiogram in rodents and human: extrapolation of experimental insights to clinic. Lab. Anim. Res. 37, 25. doi:10.1186/s42826-021-00102-3
Ju Y. K., Lee B. H., Trajanovska S., Hao G., Allen D. G., Lei M., et al. (2015). The involvement of TRPC3 channels in sinoatrial arrhythmias. Front. Physiol. 6, 86. doi:10.3389/fphys.2015.00086
Jung S. W., Kittleson M. D. (2011). The effect of atenolol on NT-proBNP and troponin in asymptomatic cats with severe left ventricular hypertrophy because of hypertrophic cardiomyopathy: a pilot study. J. Vet. Intern Med. 25, 1044–1049. doi:10.1111/j.1939-1676.2011.0754.x
Katanosaka Y., Iwasaki K., Ujihara Y., Takatsu S., Nishitsuji K., Kanagawa M., et al. (2014). TRPV2 is critical for the maintenance of cardiac structure and function in mice. Nat. Commun. 5, 3932. doi:10.1038/ncomms4932
Kecskes M., Jacobs G., Kerselaers S., Syam N., Menigoz A., Vangheluwe P., et al. (2015). The Ca(2+)-activated cation channel TRPM4 is a negative regulator of angiotensin II-induced cardiac hypertrophy. Basic Res. Cardiol. 110, 43. doi:10.1007/s00395-015-0501-x
Kinoshita H., Kuwahara K., Nishida M., Jian Z., Rong X., Kiyonaka S., et al. (2010). Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-A signaling in the heart. Circulation Res. 106, 1849–1860. doi:10.1161/CIRCRESAHA.109.208314
Kitajima N., Numaga-Tomita T., Watanabe M., Kuroda T., Nishimura A., Miyano K., et al. (2016). TRPC3 positively regulates reactive oxygen species driving maladaptive cardiac remodeling. Sci. Rep. 6, 37001. doi:10.1038/srep37001
Lambert S., Drews A., Rizun O., Wagner T. F. J., Lis A., Mannebach S., et al. (2011). Transient receptor potential melastatin 1 (TRPM1) is an ion-conducting plasma membrane channel inhibited by zinc ions. J. Biol. Chem. 286, 12221–12233. doi:10.1074/jbc.M110.202945
Launay P., Fleig A., Perraud A. L., Scharenberg A. M., Penner R., Kinet J. P. (2002). TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109, 397–407. doi:10.1016/s0092-8674(02)00719-5
Li A., Tian X., Zhang X., Huang S., Ma Y., Wu D., et al. (2015). Human polycystin-2 transgene dose-dependently rescues ADPKD phenotypes in Pkd2 mutant mice. Am. J. Pathol. 185, 2843–2860. doi:10.1016/j.ajpath.2015.06.014
Li M., Du J., Jiang J., Ratzan W., Su L. T., Runnels L. W., et al. (2007). Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J. Biol. Chem. 282, 25817–25830. doi:10.1074/jbc.M608972200
Li S., Li M., Yi X., Guo F., Zhou Y., Chen S., et al. (2017). TRPM7 channels mediate the functional changes in cardiac fibroblasts induced by angiotensin II. Int. J. Mol. Med. 39, 1291–1298. doi:10.3892/ijmm.2017.2943
Liao M., Cao E., Julius D., Cheng Y. (2013). Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112. doi:10.1038/nature12822
Liedtke W., Tobin D. M., Bargmann C. I., Friedman J. M. (2003). Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 100 (Suppl. 2), 14531–14536. doi:10.1073/pnas.2235619100
Liu C., Montell C. (2015). Forcing open TRP channels: mechanical gating as a unifying activation mechanism. Biochem. Biophys. Res. Commun. 460, 22–25. doi:10.1016/j.bbrc.2015.02.067
Liu X., Zhao R., Ding Q., Yao X., Tsang S. Y. (2021). TRPC7 regulates the electrophysiological functions of embryonic stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 12, 262. doi:10.1186/s13287-021-02308-7
Liu Y., Lyu Y., Wang H. (2022). TRP channels as molecular targets to relieve endocrine-related diseases. Front. Mol. Biosci. 9, 895814. doi:10.3389/fmolb.2022.895814
Lu Z., Cui Y., Wei X., Gao P., Zhang H., Wei X., et al. (2018). Deficiency of PKD2L1 (TRPP3) exacerbates pathological cardiac hypertrophy by augmenting NCX1-mediated mitochondrial calcium overload. Cell Rep. 24, 1639–1652. doi:10.1016/j.celrep.2018.07.022
Luo X., Li M., Zhan K., Yang W., Zhang L., Wang K., et al. (2018). Selective inhibition of TRPM2 channel by two novel synthesized ADPR analogues. Chem. Biol. Drug Des. 91, 552–566. doi:10.1111/cbdd.13119
Maier T., Follmann M., Hessler G., Kleemann H. W., Hachtel S., Fuchs B., et al. (2015). Discovery and pharmacological characterization of a novel potent inhibitor of diacylglycerol-sensitive TRPC cation channels. Br. J. Pharmacol. 172, 3650–3660. doi:10.1111/bph.13151
Miller B. A., Hoffman N. E., Merali S., Zhang X. Q., Wang J., Rajan S., et al. (2014). TRPM2 channels protect against cardiac ischemia-reperfusion injury: role of mitochondria. J. Biol. Chem. 289, 7615–7629. doi:10.1074/jbc.M113.533851
Miller B. A., Wang J., Hirschler-Laszkiewicz I., Gao E., Song J., Zhang X. Q., et al. (2013). The second member of transient receptor potential-melastatin channel family protects hearts from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 304, H1010–H1022. doi:10.1152/ajpheart.00906.2012
Moran M. M., Xu H., Clapham D. E. (2004). TRP ion channels in the nervous system. Curr. Opin. Neurobiol. 14, 362–369. doi:10.1016/j.conb.2004.05.003
Neuberger A., Nadezhdin K. D., Sobolevsky A. I. (2021). Structural mechanisms of TRPV6 inhibition by ruthenium red and econazole. Nat. Commun. 12, 6284. doi:10.1038/s41467-021-26608-x
Nikolaev Y. A., Cox C. D., Ridone P., Rohde P. R., Cordero-Morales J. F., Vásquez V., et al. (2019). Mammalian TRP ion channels are insensitive to membrane stretch. J. Cell Sci. 132, jcs238360. doi:10.1242/jcs.238360
Numata T., Takahashi K., Inoue R. (2016). TRP inflammation relationship in cardiovascular system. Semin. Immunopathol. 38, 339–356. doi:10.1007/s00281-015-0536-y
Ohba T., Watanabe H., Murakami M., Iino K., Adachi T., Baba Y., et al. (2017). Stromal interaction molecule 1 haploinsufficiency causes maladaptive response to pressure overload. PLoS One 12, e0187950. doi:10.1371/journal.pone.0187950
O'Neil R. G., Heller S. (2005). The mechanosensitive nature of TRPV channels. Pflugers Arch. 451, 193–203. doi:10.1007/s00424-005-1424-4
Onohara N., Nishida M., Inoue R., Kobayashi H., Sumimoto H., Sato Y., et al. (2006). TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 25, 5305–5316. doi:10.1038/sj.emboj.7601417
Paulsen C. E., Armache J. P., Gao Y., Cheng Y., Julius D. (2015). Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520, 511–517. doi:10.1038/nature14367
Peana D., Polo-Parada L., Domeier T. L. (2022). Arrhythmogenesis in the aged heart following ischaemia-reperfusion: role of transient receptor potential vanilloid 4. Cardiovasc Res. 118, 1126–1137. doi:10.1093/cvr/cvab141
Peier A. M., Moqrich A., Hergarden A. C., Reeve A. J., Andersson D. A., Story G. M., et al. (2002). A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715. doi:10.1016/s0092-8674(02)00652-9
Sah R., Mesirca P., Mason X., Gibson W., Bates-Withers C., Van den Boogert M., et al. (2013). Timing of myocardial trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation 128, 101–114. doi:10.1161/CIRCULATIONAHA.112.000768
Saotome K., Singh A. K., Yelshanskaya M. V., Sobolevsky A. I. (2016). Crystal structure of the epithelial calcium channel TRPV6. Nature 534, 506–511. doi:10.1038/nature17975
Satoh S., Tanaka H., Ueda Y., Oyama J. I., Sugano M., Sumimoto H., et al. (2007). Transient receptor potential (TRP) protein 7 acts as a G protein-activated Ca2+ channel mediating angiotensin II-induced myocardial apoptosis. Mol. Cell Biochem. 294, 205–215. doi:10.1007/s11010-006-9261-0
Seo K., Rainer P. P., Shalkey Hahn V., Lee D. I., Jo S. H., Andersen A., et al. (2014) “Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy,”, 111. 1551–1556. doi:10.1073/pnas.1308963111Proc. Natl. Acad. Sci. U. S. A.
Seth M., Zhang Z. S., Mao L., Graham V., Burch J., Stiber J., et al. (2009). TRPC1 channels are critical for hypertrophic signaling in the heart. Circ. Res. 105, 1023–1030. doi:10.1161/CIRCRESAHA.109.206581
Shanks J., de Morais S. D. B., Gao L., Zucker I. H., Wang H. J. (2019). TRPV1 (transient receptor potential vanilloid 1) cardiac spinal afferents contribute to hypertension in spontaneous hypertensive rat. Hypertension 74, 910–920. doi:10.1161/HYPERTENSIONAHA.119.13285
Shenton F. C., Pyner S. (2014). Expression of transient receptor potential channels TRPC1 and TRPV4 in venoatrial endocardium of the rat heart. Neuroscience 267, 195–204. doi:10.1016/j.neuroscience.2014.02.047
Shimada H., Kusakizako T., Dung Nguyen T. H., Nishizawa T., Hino T., Tominaga M., et al. (2020). The structure of lipid nanodisc-reconstituted TRPV3 reveals the gating mechanism. Nat. Struct. Mol. Biol. 27, 645–652. doi:10.1038/s41594-020-0439-z
Simard C., Hof T., Keddache Z., Launay P., Guinamard R. (2013). The TRPM4 non-selective cation channel contributes to the mammalian atrial action potential. J. Mol. Cell Cardiol. 59, 11–19. doi:10.1016/j.yjmcc.2013.01.019
Singh A. K., McGoldrick L. L., Sobolevsky A. I. (2018). Structure and gating mechanism of the transient receptor potential channel TRPV3. Nat. Struct. Mol. Biol. 25, 805–813. doi:10.1038/s41594-018-0108-7
Suzuki M., Mizuno A., Kodaira K., Imai M. (2003). Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 278, 22664–22668. doi:10.1074/jbc.M302561200
Takahashi K., Sakamoto K., Kimura J. (2012). Hypoxic stress induces transient receptor potential melastatin 2 (TRPM2) channel expression in adult rat cardiac fibroblasts. J. Pharmacol. Sci. 118, 186–197. doi:10.1254/jphs.11128fp
Tang N., Tian W., Ma G. Y., Xiao X., Zhou L., Li Z. Z., et al. (2022). TRPC channels blockade abolishes endotoxemic cardiac dysfunction by hampering intracellular inflammation and Ca2+ leakage. Nat. Commun. 13, 7455. doi:10.1038/s41467-022-35242-0
Urban N., Wang L., Kwiek S., Rademann J., Kuebler W. M., Schaefer M. (2016). Identification and validation of larixyl acetate as a potent TRPC6 inhibitor. Mol. Pharmacol. 89, 197–213. doi:10.1124/mol.115.100792
Vandewauw I., De Clercq K., Mulier M., Held K., Pinto S., Van Ranst N., et al. (2018). A TRP channel trio mediates acute noxious heat sensing. Nature 555, 662–666. doi:10.1038/nature26137
Vandewiele F., Pironet A., Jacobs G., Kecskés M., Wegener J., Kerselaers S., et al. (2022). TRPM4 inhibition by meclofenamate suppresses Ca2+-dependent triggered arrhythmias. Eur. Heart J. 43, 4195–4207. doi:10.1093/eurheartj/ehac354
Veteto A. B., Peana D., Lambert M. D., McDonald K. S., Domeier T. L. (2020). Transient receptor potential vanilloid-4 contributes to stretch-induced hypercontractility and time-dependent dysfunction in the aged heart. Cardiovasc Res. 116, 1887–1896. doi:10.1093/cvr/cvz287
Vinayagam D., Quentin D., Yu-Strzelczyk J., Sitsel O., Merino F., Stabrin M., et al. (2020). Structural basis of TRPC4 regulation by calmodulin and pharmacological agents. Elife 9, e60603. doi:10.7554/eLife.60603
Wang L., Wang D. H. (2005). TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation 112, 3617–3623. doi:10.1161/CIRCULATIONAHA.105.556274
Watanabe H., Iino K., Ohba T., Ito H. (2013). Possible involvement of TRP channels in cardiac hypertrophy and arrhythmia. Curr. Top. Med. Chem. 13, 283–294. doi:10.2174/1568026611313030006
Wright D. J., Simmons K. J., Johnson R. M., Beech D. J., Muench S. P., Bon R. S. (2020). Human TRPC5 structures reveal interaction of a xanthine-based TRPC1/4/5 inhibitor with a conserved lipid binding site. Commun. Biol. 3, 704. doi:10.1038/s42003-020-01437-8
Wu Y., Lu K., Lu Y., Liao J., Zhang S., Yang S., et al. (2023). Transient receptor potential vanilloid 4 (TRPV4) in neutrophils enhances myocardial ischemia/reperfusion injury. J. Leukoc. Biol. 114, 266–279. doi:10.1093/jleuko/qiad063
Yang K., Chang W. L., Yang P. C., Chien C. L., Lai M. S., Su M. J., et al. (2006). Activation of the transient receptor potential M2 channel and poly (ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death. Cell Death & Differ. 13, 1815–1826. doi:10.1038/sj.cdd.4401813
Yin Y., Park C. G., Zhang F., G Fedor J., Feng S., Suo Y., et al. (2024). Mechanisms of sensory adaptation and inhibition of the cold and menthol receptor TRPM8. Sci. Adv. 10, eadp2211. doi:10.1126/sciadv.adp2211
Yin Y., Wu M., Hsu A. L., Borschel W. F., Borgnia M. J., Lander G. C., et al. (2019). Visualizing structural transitions of ligand-dependent gating of the TRPM2 channel. Nat. Commun. 10, 3740. doi:10.1038/s41467-019-11733-5
Yoshie K., Rajendran P. S., Massoud L., Mistry J., Swid M. A., Wu X., et al. (2020). Cardiac TRPV1 afferent signaling promotes arrhythmogenic ventricular remodeling after myocardial infarction. JCI insight 5, e124477. doi:10.1172/jci.insight.124477
Yu Y., Chen S., Xiao C., Jia Y., Guo J., Jiang J., et al. (2014). TRPM7 is involved in angiotensin II induced cardiac fibrosis development by mediating calcium and magnesium influx. Cell Calcium 55, 252–260. doi:10.1016/j.ceca.2014.02.019
Yu Z.-Y., Gong H., Kesteven S., Guo Y., Wu J., Li J. V., et al. (2022). Piezo1 is the cardiac mechanosensor that initiates the cardiomyocyte hypertrophic response to pressure overload in adult mice. Nat. Cardiovasc. Res. 1, 577–591. doi:10.1038/s44161-022-00082-0
Zhang M., Ma Y., Ye X., Zhang N., Pan L., Wang B. (2023). TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target Ther. 8, 261. doi:10.1038/s41392-023-01464-x
Zhang Q., Qi H., Cao Y., Shi P., Song C., Ba L., et al. (2018). Activation of transient receptor potential vanilloid 3 channel (TRPV3) aggravated pathological cardiac hypertrophy via calcineurin/NFATc3 pathway in rats. J. Cell Mol. Med. 22, 6055–6067. doi:10.1111/jcmm.13880
Zhang Y. J., Ma N., Su F., Liu H., Mei J. (2015). Increased TRPM6 expression in atrial fibrillation patients contribute to atrial fibrosis. Exp. Mol. Pathol. 98, 486–490. doi:10.1016/j.yexmp.2015.03.025
Zhao C., MacKinnon R. (2023). Structural and functional analyses of a GPCR-inhibited ion channel TRPM3. Neuron 111, 81–91.e7. doi:10.1016/j.neuron.2022.10.002
Zou Y., Zhang M., Wu Q., Zhao N., Chen M., Yang C., et al. (2022). Activation of transient receptor potential vanilloid 4 is involved in pressure overload-induced cardiac hypertrophy. Elife 11, e74519. doi:10.7554/eLife.74519
Keywords: TRP channels, knockout (KO) mice, cardiac physiology, cardiac pathology, calcium homeostasis
Citation: Kulkarni K, Walton RD and Chaigne S (2025) Unlocking the potential of cardiac TRP channels using knockout mice models. Front. Physiol. 16:1585356. doi: 10.3389/fphys.2025.1585356
Received: 28 February 2025; Accepted: 07 April 2025;
Published: 17 April 2025.
Edited by:
Deniz Yilmazer-Hanke, University of Ulm, GermanyReviewed by:
James Henry Peters, Washington State University, United StatesPraghalathan Kanthakumar, University of Missouri, United States
Yang Guo, Victor Chang Cardiac Research Institute, Australia
Copyright © 2025 Kulkarni, Walton and Chaigne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastien Chaigne, c2ViYXN0aWVuLmNoYWlnbmVAaWh1LWxpcnljLmZy
 Kanchan Kulkarni
Kanchan Kulkarni Richard D. Walton
Richard D. Walton Sebastien Chaigne
Sebastien Chaigne