- 1Department of Exercise Physiology, School of Sport Science, Beijing Sport University, Beijing, China
- 2Chongqing Bishan Bashu Middle School, Chongqing, China
- 3China Volleyball Sport College, Beijing Sport University, Beijing, China
- 4Laboratory of Sports Stress and Adaptation of General Administration of Sport, Beijing, China
Introduction: This study examined the effects of probiotic supplementation alone or combined with aerobic exercise on antioxidant capacity and oxidative stress after high-intensity interval exercise (HIIE) in college students.
Methods: Thirty male college students were divided into three groups: control (C), probiotic (P), and combined probiotic and exercise (PE). The 6-week intervention involved moderate-intensity cycling three times a week. All participants underwent a single session of HIIE protocol. The tests for maximal oxygen uptake (VO2max), elimination rate of lactic acid (ER), blood oxidative stress markers, and blood rheology were performed.
Results: A decrease in superoxide dismutase (SOD) activity was observed at baseline in the P and PE groups (P < 0.01), while significantly increased glutathione peroxidase (GSH-Px) activity and reduced catalase activity were found in the PE group (P < 0.05). In the P and PE groups, SOD activity (P < 0.01) and total antioxidant capacity (T-AOC) level (P < 0.01) were significantly elevated after HIIE. The T-AOC level significantly increased from 0.47 ± 0.03 umol Trolox/mL to 0.78 ± 0.07 umol Trolox/mL in the P group and from 0.56 ± 0.04 umol Trolox/mL to 0.82 ± 0.05 umol Trolox/mL in the PE group. The 8-OHdG level increased significantly in both the C and P groups (P < 0.05), but remained unchanged in the PE group after the intervention. High shear rate whole blood viscosity was significantly decreased in the P and PE groups (P < 0.05). Additionally, a notable decline in plasma viscosity was observed in the PE group. After the intervention, medium and high shear rate whole blood viscosity levels (P < 0.05) were significantly lower in the PE group than in the C group, and plasma viscosity was dropped by 28.64% (P < 0.05). Following the intervention, a significant elevation in VO2max was only observed in the PE group from 38.14 ± 3.11 to 44.5 ± 2.94 mL/kg/min (P < 0.05), with a subsequent increase in ER detected after HIIE (P < 0.05).
Discussion: These findings indicate that combining probiotics with aerobic exercise enhances antioxidant and aerobic capacity more effectively than probiotics alone.
Introduction
Normally, the generation and clearance of reactive oxygen species within the human system are in dynamic equilibrium, which plays a positive role in sustaining normal body functions. Exercise serves as an important stressor of oxidative stress and plays a dual role in regulating the body’s redox system. After high-intensity strenuous exercise, especially high-intensity interval exercise (HIIE), The body’s overproduction of reactive oxygen species (ROS) induces a rise in oxidative stress (Sousa et al., 2025). Oxidative stress can lead to muscle damage, exercise-induced fatigue, and impaired athletic performance (Martinez-Canton et al., 2024). In young populations, oxidative stress induced by a single bout of high-intensity exercise (e.g., an acute session of HIIE) may impair recovery and hinder sustained exercise performance. (Cipryan, 2018; Lomiwes et al., 2024). In contrast, long-term HIIE has been shown to induce beneficial adaptations, including enhanced antioxidant enzyme activity and reduced oxidative stress levels, contributing to improved resilience and exercise capacity over time (Guo et al., 2025; Sarvasti et al., 2020). Consequently, numerous researchers have been exploring appropriate interventions to mitigate the level of oxidative stress and improve the body’s antioxidant capacity after high-intensity exercise (Souglis et al., 2023; Li et al., 2025; Wang et al., 2025).
When taken in adequate amounts, probiotics—living microorganisms—can positively influence overall health (Sarita et al., 2024). Common strains include Lactobacillus bulgaricus, Lactobacillus acidophilus, Bifidobacterium lactis, and Lactobacillus casei. Pro-biotics are proven to confer health benefits to the host, such as alleviating gastrointestinal discomfort and the duration/severity of upper respiratory tract infections (Bertuccioli et al., 2024; Damianos et al., 2025), enhancing the intestinal mucosal barrier defense (Li et al., 2025), and improving immune function (Aykut et al., 2024; Mafe et al., 2025). Studies in recent years have shown that probiotic supplementation contributes to improving antioxidant capacity and reduces serum malondialdehyde (MDA)level in individuals with regular physical exercise (Sánchez Macarro et al., 2021; Musazadeh et al., 2024), triathletes (Huang et al., 2019), and cyclists (Michalickova et al., 2018). However, these studies cannot exclude the effects of the exercise, studies on untrained individuals are limited.
In addition to the intake of probiotics, endurance training has been shown to enhance the antioxidant system. In older women, 8 weeks of aerobic exercise led to a notable decrease in 8-hydroxy-2′-deoxyguanosine (8-OHdG), a DNA oxidative damage biomarker, while boosting total antioxidant capacity (T-AOC) (Zhao et al., 2023). Eight weeks of aerobic exercise significantly reduces levels of 8-OHdG, a biomarker of DNA oxidative damage, and increases T-AOC level in older women (Zarrindast et al., 2021). Therefore, combining probiotic supplementation with aerobic exercise may have synergistic effects in enhancing antioxidant capacity and alleviating oxidative stress associated with high-intensity sessions. However, differences in the specific effects of probiotic supplementation alone or probiotics combined with aerobic exercise in these areas are not known. In addition, probiotic supplementation has been reported to improve aerobic capacity (Imanian et al., 2024). Our previous study also found that probiotic supplementation improved lactate metabolism after exhaustive exercise in college football players (Zhang et al., 2023). Therefore, this study focuses on the effects of probiotic supplementation alone or in combination with aerobic exercise on antioxidant capacity, oxidative stress induced by HIIE, and aerobic capacity. This study’s outcomes are projected to furnish experimental backing for the role of probiotics in alleviating oxidative stress induced by physical activity.
Study design and procedures
Participants
30 male undergraduate participants were selected from Beijing Sport University for this study. None of the participants had previous training experience. Given that aerobic exercise has been proven to significantly enhance antioxidant capacity (Zhao et al., 2023), this study did not establish a separate aerobic exercise group but instead focused on verifying the effects of probiotic supplementation alone or in combination with aerobic exercise. The subjects were randomly assigned to a placebo group (C), a probiotic group (P), and a probiotic combined with an aerobic exercise group (PE), ten persons per group. Throughout the experimental period, the participants continued with their usual diet.
Probiotic administration
Probiotic supplements were provided by Beijing Scitop Bio-tech Shareholding Co. Ltd. (Beijing, China). The probiotics, delivering 2 g per packet, came as a powder. The C group received a placebo consisting of maltodextrin, while the P group was given daily probiotic supplements containing three probiotic species: Lac-tobacillus casei Zhang (≥8 × 109CFU), Bifidobacterium lactis V9 (≥6 × 109CFU), and Lactoba-cillus plantarum P-8 (≥6 × 109CFU). The probiotic was consumed in cold water half an hour after lunch each day. During the experiment, the intake of other fermented foods was restricted, with a 2-h gap observed between taking antibiotics and supplements. Apart from consuming the same probiotics as the P group, the PE group participated in aerobic ex-ercise three times a week.
Study protocol
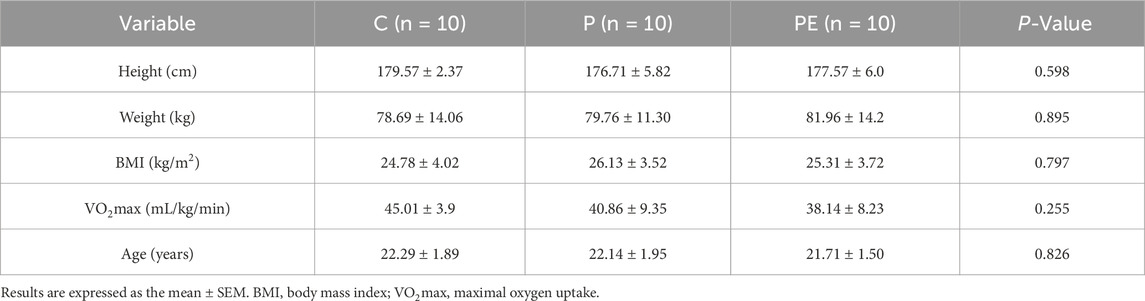
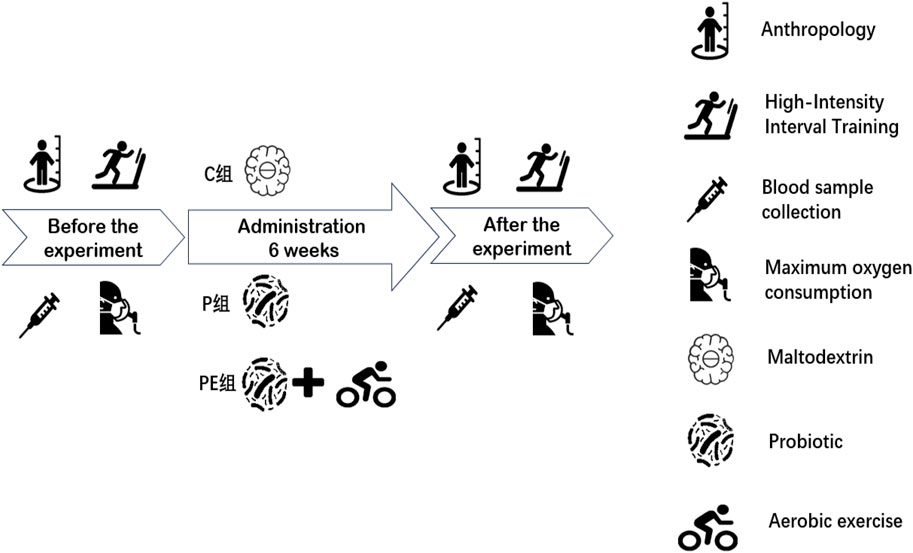
The primary objective of this experiment was to examine the impact of probiotic supplementation on antioxidant capacity and oxidative stress in college students post high-intensity intermittent exercise, and to assess if a combined effect with aerobic exercise exists. The qualifications for participant inclusion were as follows: (1). No consumption of microbial supplements, probiotics, synbiotics, or foods with fermentation (like yogurt). (2). No training experience. The exclusion criteria included sensitivity to probiotic components, use of probiotics, and antibiotics within 1 month before the start of the study. Prior to the experiment, all participants filled out an informed consent form, volunteered for the study, and confirmed they could undergo all procedures. The experimental protocol was approved by the Ethics Committee of Beijing Sport University (approval number: no.2022215H). There were no significant differences in basic information between the C, P, and PE sub-groups concerning height, weight, age, body fat, body mass index (BMI), or maximal oxygen consumption (VO2max). (Table 1). The assessment duration lasted 6 weeks, and the methodology remained unchanged for both the baseline and final tests (Figure 1). All 30 participants participated in the assessments and data gathering.
Anthropometry
The height of the participants was measured using a standardized stadiometer, and their fasting body weight was recorded in the morning with a GMCS RCS IV portable scale.
High-intensity interval exercise test
Two days before the high-intensity interval exercise experiment, participants first underwent an exercise test to determine their maximum treadmill speed (MTV). The participants warmed up by walking on the treadmill at a speed of 6 km/h for 5 min. Subsequently, an incremental test was conducted with the treadmill set at a constant 1% incline, running at 7 km/h for 1 min, followed by a fixed increment of 1 km/h per minute until exhaustion. The high-intensity interval exercise protocol consisted of a 5-min warm-up at 50% MTV on the treadmill, followed by eight sets of high-intensity interval exercise, each set comprising 1 min of running at 100% MTV, immediately followed by a 1-min rest interval, during which the participants recovered by walking at 30% MTV (Dantas, Farias Junior et al., 2017), running for 1 min, and resting for 1 min. This protocol was designed as a single-session high-intensity interval exercise test to evaluate acute exercise-induced oxidative stress responses.
Aerobic exercise protocol
The PE group performed 30 min of moderate-intensity aerobic exercise three times a week using a stationary bicycle, with all sessions conducted between 2:00 p.m. and 4:00 p.m. The workload was set at 60%–69% of the individual’s maximum heart rate, aiming for a heart rate range of 120–140 beats per minute. While participants were pedaling the stationary bicycle, their heart rate was assessed with a heart rate monitoring device. The timing of exercise session started when the participant’s heart rate reached the lower limit of the moderate-intensity aerobic exercise range.
Blood sampling and assay methods
Venous blood samples were collected from the anterior elbow vein of each participant at baseline and immediately after the high-intensity interval exercise test using standard venipuncture techniques. The blood was then centrifuged at high speed, and the plasma was stored at −80°C until further analysis. The activities of SOD, catalase (CAT), and glutathione peroxidase (GSH-Px), along with the level of 8-OHDG, were quantified using ELISA kits (Shanghai Jianglai Biotechnology, China). T-AOC was determined using a microplate meth-od (spectrophotometry).
Maximal oxygen uptake
The VO2max test followed the standard Bruce protocol, in which participants under-went an incremental treadmill exercise test. Prior to testing, they were fitted with a compact respiratory gas collection mask and a Polar V800 heart rate (HR) monitor belt. After completing the warm-up, the test commenced according to the study protocol (Vilcant and Zeltser, 2024). Participants engaged in continuous exercise, and the COSMED software was used to calculate real-time speed, gradient, and gas metabolism. The test was stopped when the participant became exhausted and could no longer continue. The operator assessed the participant’s Rating of Perceived Exertion (RPE) at each stage of the test and continuously tracked their heart rate to ensure the incremental exercise was completed safely.
To confirm the achievement of VO2max, a minimum of three criteria from the list had to be satisfied: (1) A plateau in oxygen uptake or a decline in oxygen consumption as intensity increases; (2) no increase in heart rate with increasing intensity; (3) a respiratory exchange ratio reaching or approaching 1.15; (4) the RPE scale indicating a level of fatigue where the participant could no longer sustain the current workload.
Lactic acid elimination rate
Following the high-intensity interval exercise test, samples were drawn from the par-ticipants’ fingertips at 0, 3, 5, 7, and 9 min post-exercise. The blood samples were analyzed using a portable blood lactate analyzer (Biosen S_line Lab, EKF Diagnostics Holdings Ltd., Germany). During blood collection, participants remained seated and were instructed to avoid activities like slow walking or stretching that could facilitate recovery. The lactic acid elimination rate (ER) was then estimated from the equation (Wang et al., 2019):
Here, ER represents the blood lactate elimination rate (mmol/Lmin); Lmax is the maximum blood lactate concentration after exercise (mmol/L); L9 is the blood lactate concentration at 9 min post-exercise (mmol/L); t9 is 9 min post-exercise (min); tmax is the time corresponding to the maximum blood lactate concentration (min).
Blood rheology
Venous blood samples were collected from the participants at baseline, and blood rheology tests were conducted using a fully automatic rheometer (LBY-N6C, China).
Statistical analysis
SPSS 25 software was employed to perform the analysis. Data are expressed as mean ± standard error (SEM). A one-way analysis of variance (ANOVA) was employed to compare the inter-group differences post-intervention and the rates of change. Paired sample t-tests were used to assess the changes within each group from pre-to post-intervention. Additionally, a two-way analysis of variance (ANOVA) was performed to examine the interaction effects between group and time. A statistical significance level of P < 0.05 was adopted.
Results
Effects of probiotics supplementation alone or in combination with aerobic exercise on oxidative stress
Effects on basal 8-OHdG level
This study assessed the impact of probiotic supplementation, either alone or combined with aerobic exercise, on 8-OHdG levels (an oxidative damage marker) at baseline. As shown in Figure 2A, 6 weeks of intervention resulted in no notable difference in 8-OHdG levels between the three groups (P = 0.63). Compared with pre-intervention levels, the rate of change in 8-OHdG levels showed no significant difference between groups post-intervention (P = 0.93, Figure 2B).
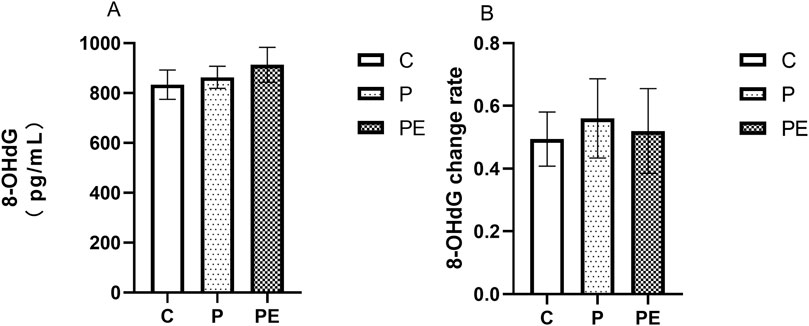
Figure 2. The effect of probiotics supplementation alone or in combination with aerobic exercise on 8-OHDG level at baseline in healthy college students. Results are reported as the mean ± SEM. C, control group (n = 10); P: probiotic group (n = 10); PE: probiotic and aerobic exercise group (n = 10) (A) 8-OHDG (B) 8-OHDG change rate. *: P < 0.05; **: P < 0.01.
Impact on the 8-OhdG level immediately after high-intense interval training
Immediately after high-intensity interval exercise, the level of 8-OHdG was assessed in this study, both prior to the intervention and following 6 weeks of intervention. As shown in Figure 3A, the level of 8-OHdG increased significantly in the C and P groups after the intervention but remained unchanged in the PE group. The 8-OHdG level in the C group and P group was raised from 764.14 ± 75.69 pg/mL to 1,255.85 ± 75.75 pg/mL (P < 0.01) and from 799.77 ± 53.46 pg/mL to 992.93 ± 60.08 pg/mL (P < 0.05), respectively. As shown in Figure 3A, A significant increase in the 8-OHdG level was observed in the C group compared to both the P group and the PE group after the intervention. Additionally, as shown in Figure 3B, a significantly lower rate of change in 8-OHdG level was observed in the P and PE groups compared to the C group.
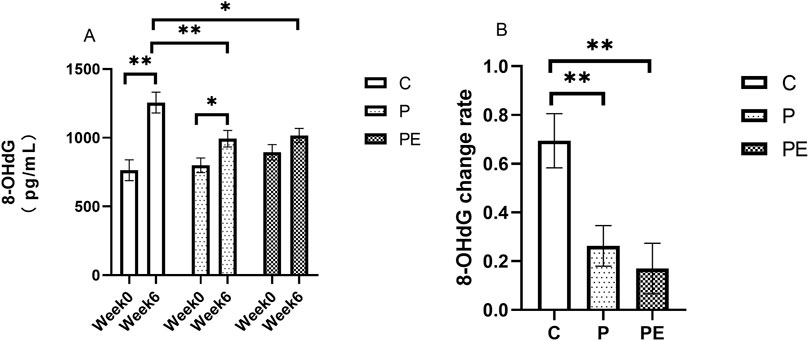
Figure 3. The effect of probiotics supplementation alone or in combination with aerobic exercise on 8-OHDG level immediately after high-intensity interval exercise in healthy college students. Results are reported as the mean ± SEM. C, control group (n = 10); P: probiotic group (n = 10); PE: probiotic and aerobic exercise group (n = 10) (A) 8-OHDG (B) 8-OHDG change rate. *: P < 0.05; **:P < 0.01.
Effects of probiotics supplementation alone or in combination with aerobic exercise on antioxidant capacity
Effects on antioxidant capacity at baseline
An assessment was made of the effect of 6 weeks of probiotic supplementation, either alone or combined with aerobic exercise, on antioxidant indicators at baseline. As shown in Figure 4A, the GSH-Px activity was increased in the PE group from 41.86 ± 2.27 to 50.21 ± 2.48 U/mL when compared to pre-intervention levels (P < 0.05). However, no substantial differences were found in the GSH-Px activity between the P and C groups. Additionally, the SOD activity was decreased from 4.37 ± 0.10 to 4.16 ± 0.92 ng/mL in the P group (P < 0.01), and was reduced by 4.46% in the PE group (P < 0.01, Figure 4B), while was no significant changed in the C group. Moreover, no significant changes in the activity of CAT and T-AOC level were observed among all groups before and after the intervention (P > 0.05, Figures 4C, D). Furthermore, the rates of change in the activities of GSH-Px, SOD, CAT, and T-AOC under basal conditions did not exhibit significant differences among the groups (Figure 5).
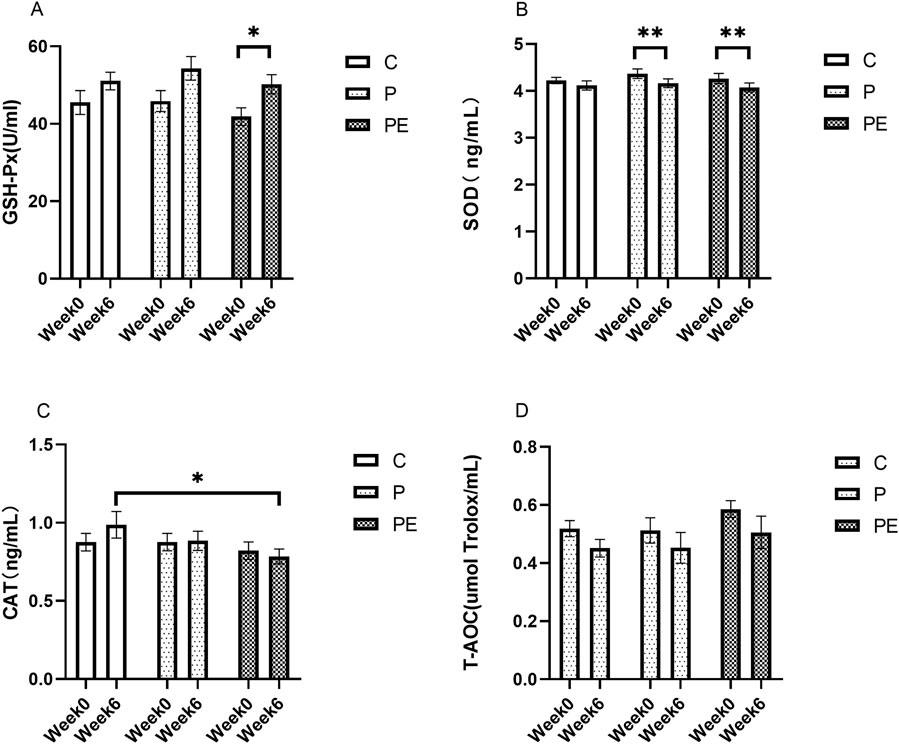
Figure 4. The impact of probiotics supplementation alone or in combination with aerobic exercise on antioxidant capacity at baseline in healthy college students. Results are reported as the mean ± SEM. C, control group (n = 10); P: probiotic group (n = 10); PE: probiotic and aerobic exercise group (n = 10). (A) GSH-Px. (B) SOD. (C) CAT. (D) T-AOC. *: P < 0.05; **: P < 0.01.
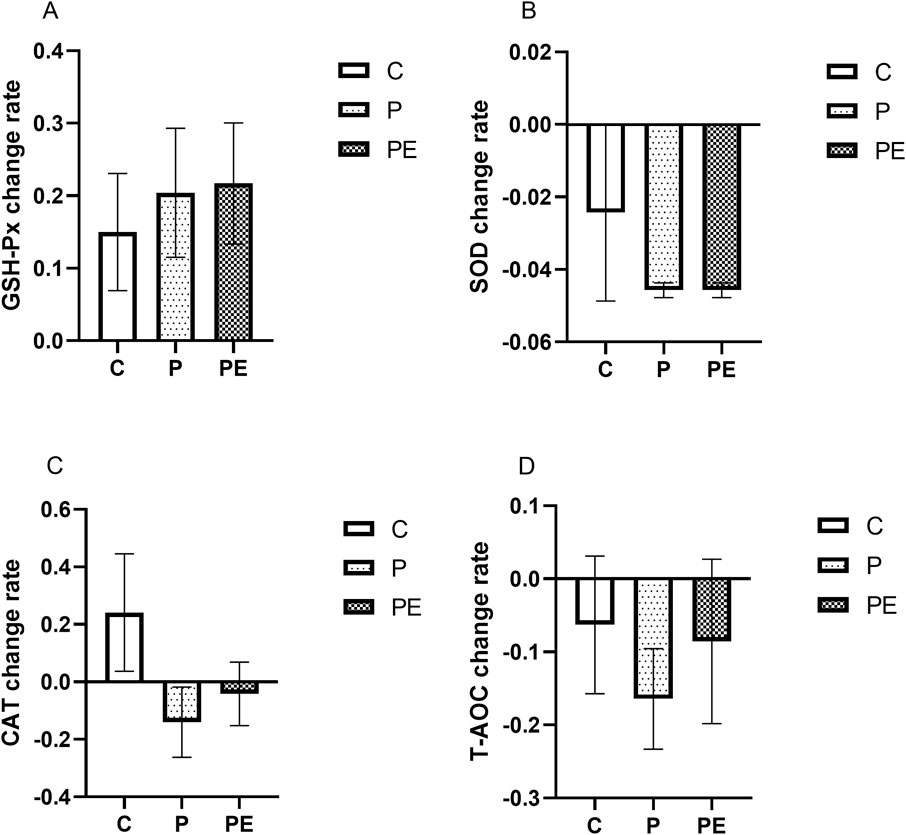
Figure 5. The impact of probiotics supplementation alone or in combination with exercise on the rate of change of antioxidant capacity at baseline in healthy college students. Results are reported as the mean ± SEM. C, control group (n = 10); P: probiotic group (n = 10); PE: probiotic and aerobic exercise group (n = 10). (A) GSH-Px change rate. (B) SOD change rate (C) CAT change rate. (D) T-AOC change rate. *:P < 0.05; **: P < 0.01.
Impact on antioxidant capacity immediately after intense interval training
This study assessed the effects of 6 weeks of probiotic supplementation, with or without combined aerobic training, on the plasma antioxidant enzymes immediately after high-intensity interval exercise. As shown in Figure 6B, compared to pre-intervention, the SOD activity was significantly increased in both the P group and PE group immediately after high-intensity interval exercise (P < 0.05). The P group rose from 3.87 ± 0.18 ng/mL to 3.97 ± 0.16 ng/mL, and the PE group climbed from 3.88 ± 0.16 ng/mL to 3.98 ± 0.15 ng/mL. Similarly, as shown in Figure 6D, the T-AOC level in both the P group and PE group was increased immediately after high-intensity interval exercise from 0.47 ± 0.03 umol Trolox/mL to 0.78 ± 0.07 umol Trolox/mL (P < 0.05) and 0.56 ± 0.04 to 0.82 ± 0.05 (P < 0.05) in each case. Markedly elevated T-AOC levels were observed in the P and PE groups after 6 weeks of intervention, compared to the C group (P < 0.05, Figure 6D). Additionally, T-AOC levels showed a significant interaction effect between the group and time (F = 5.0, P = 0.019, η2 = 0.36). No significant changes were observed in the GSH-Px (Figures 6A, P > 0.05) and CAT activity (Figures 6C, P > 0.05) in any of the groups before and after the intervention. Furthermore, as shown in Figure 7D, the rate of change in T-AOC level in the P group and PE group was significantly higher than that in the C group (P < 0.01 and P < 0.05, respectively). No significant differences were noted in the rates of change for other indices.
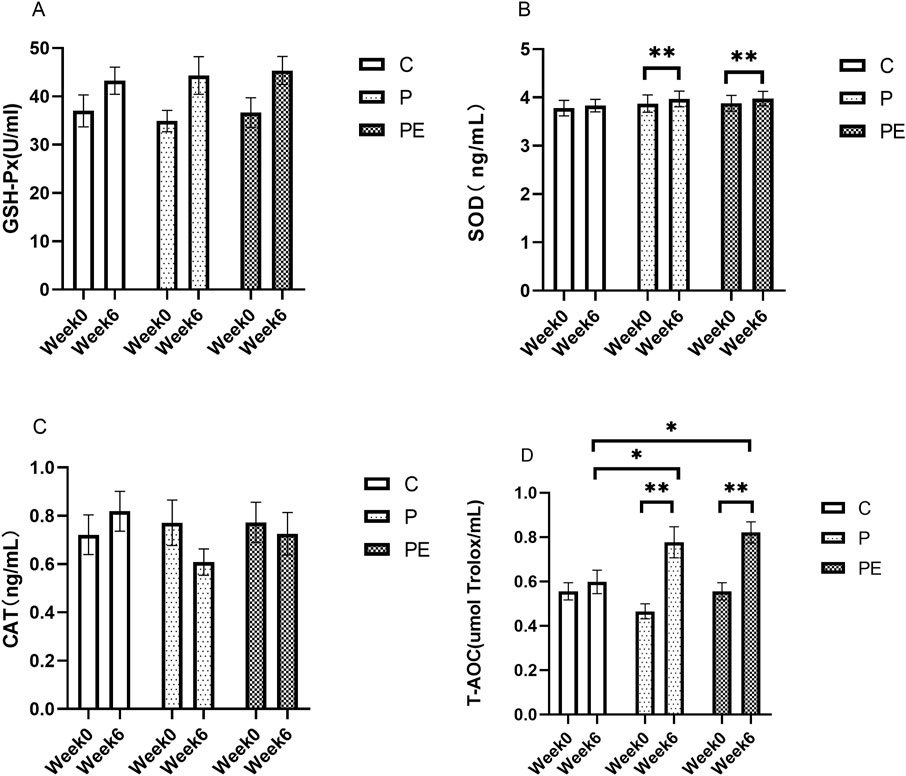
Figure 6. The impact of probiotics supplementation alone or in combination with exercise on the anti-oxidant capacity immediately after high-intensity interval exercise in healthy college students. Results are reported as the mean ± SEM. C, control group (n = 10); P: probiotic group (n = 10); PE: probiotic and aerobic exercise group (n = 10). (A) GSH-Px. (B) SOD. (C) CAT. (D) T-AOC. *:P < 0.05; **: P < 0.01.
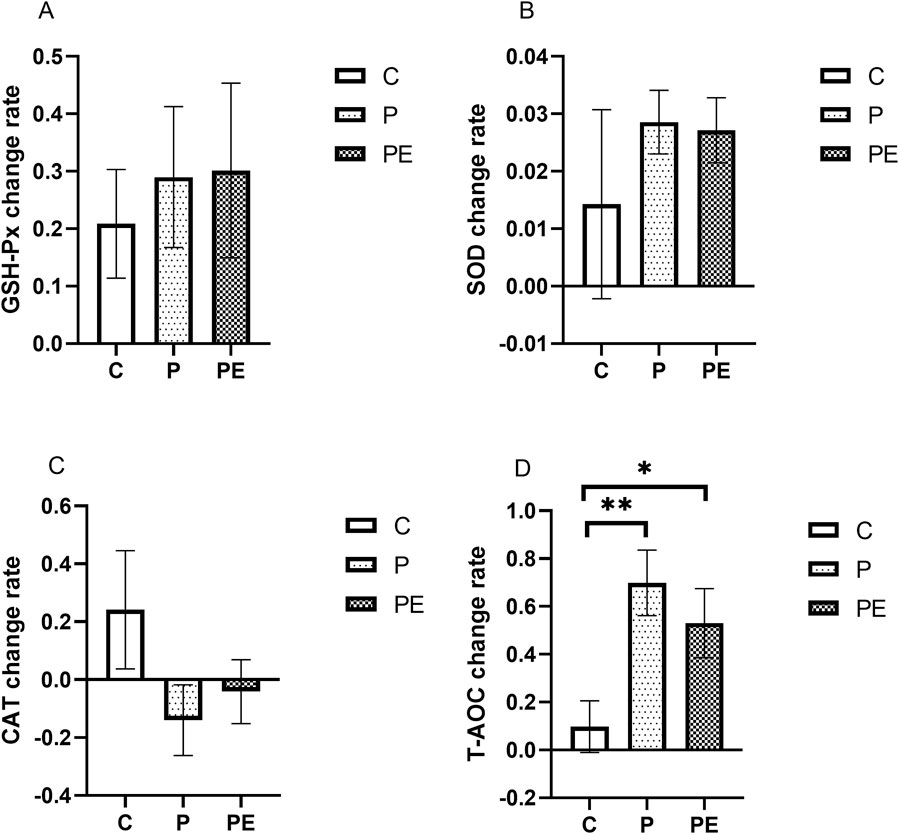
Figure 7. The impact of probiotics supplementation alone or in combination with aerobic exercise on the rate of change of antioxidant capacity immediately after high-intensity interval exercise of college students. Results are reported as the mean ± SEM. C, control group (n = 10); P: probiotic group (n = 10); PE: probiotic and exercise group (n = 10). (A) GSH-Px change rate. (B) SOD change rate. (C) CAT change rate. (D) T-AOC change rate. *: P < 0.05; **: P < 0.01.
Effects on blood rheology
After 6 weeks of probiotic intervention, either alone or in combination with aerobic exercise, its effects on blood rheology were examined before and after the intervention. As shown in Figure 8, at post-intervention, the whole blood viscosity at medium shear in the PE group was significantly lower than in the C group (P < 0.05). Compared to baseline, a significant reduction in whole blood viscosity at high shear rates was observed in both the P group and PE group (P < 0.05, Figure 8C) from 6.34 ± 0.26 to 5.05 ± 0.39 mPa s at 150 s-1, and decreased by 16.76%, respectively. Additionally, the PE group demonstrated a notable decrease relative to the C group in whole blood viscosity at high shear rates after the intervention (P < 0.05). Regarding hematocrit, the P group had a significant increase (P < 0.05, Figure 8D), while no notable variations were detected in the C and PE groups. Plasma viscosity in the PE group dropped by 28.64% after the intervention, from 2.06 ± 0.10 to 1.47 ± 0.02 mPa s at 150 s-1 (P < 0.05, Figure 8E). Moreover, the plasma viscosity of the PE group was significantly lower than that of both the C and P groups following the intervention, with a more pronounced difference compared to the C group (Figure 8E). There were no significant differences in whole blood viscosity at low shear rates among the groups (P > 0.05, Figure 8A). Furthermore, when compared to the C and P groups, significant differences were detected in the PE group in the rates of change of whole blood viscosity at high shear rates and plasma viscosity (P < 0.05, Figures 9C, E). In terms of blood viscosity, results showed a significant interaction effect (F = 5.89, P = 0.01, η2 = 0.40) and time effect (F = 7.04, P = 0.02, η2 = 0.28). No remarkable changes were detected in the rates of change for other indices.
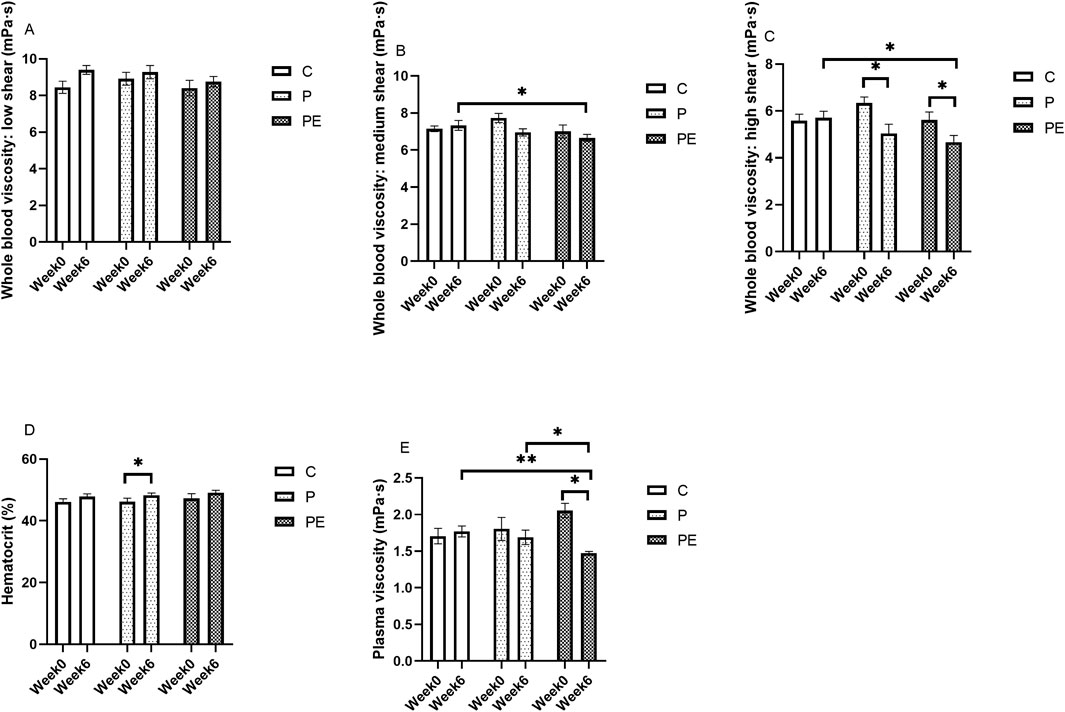
Figure 8. The impact of probiotics supplementation alone or in combination with aerobic exercise on hemorheological parameters at baseline in healthy college students. Results are reported as the mean ± SEM. C, control group (n = 10); P: probiotic group (n = 10); PE: probiotic and aerobic exercise group (n = 10) (A) Whole Blood Viscosity: Low Shear. (B) Whole Blood Viscosity: Medium Shear. (C) Whole Blood Viscosity: High Shear. (D) Hematocrit. (E) Plasma Viscosity. *:P < 0.05; **: P < 0.01.

Figure 9. The Influence of Probiotics supplementation alone or in combination with aerobic exercise on the variation rate of hemorheological parameters in healthy university students at baseline. Results are reported as the mean ± SEM. C, control group (n = 10); P: probiotic group (n = 10); PE: probiotic and aerobic exercise group (n = 10). (A) Whole Blood Viscosity: Low-Shear Rate Variation. (B) Whole blood viscosity: medium shear Rate Variation. (C) Whole Blood Viscosity: High-Shear Rate Variation. (D) Hematocrit Variation Rate. (E) Plasma Viscosity Variation Rate. *:P < 0.05; **: P < 0.01.
Effects on aerobic capacity
An assessment was made of the effect of 6 weeks of probiotic supplementation, either alone or combined with aerobic exercise, on participants’ aerobic capacity. As shown in Figure 10, compared to pre-intervention, the VO2max demonstrated a marked rise in the PE group, but not in the P group, rising from 38.14 ± 3.11 to 44.5 ± 2.94 mL/kg/min (P < 0.01). Meanwhile, the PE group showed a notably greater increase in VO2max compared to the C group (P < 0.05, Figure 11). The VO2max of the C group remained unchanged before and after the intervention. A significant decrease in HRmax was observed in the P group during HIIE (P < 0.05, Figure 10B), while the C and PE groups showed no significant changes. Compared to pre-intervention, ER was markedly improved in the PE group, increasing from 0.46% ± 0.03% to 0.62% ± 0.06% (P < 0.05, Figure 10C). However, no notable alteration in ER was found in the C or P groups. Additionally, no statistically significant variations were observed in the rates of change for HRmax and ER between the groups (Figures 11B, C).
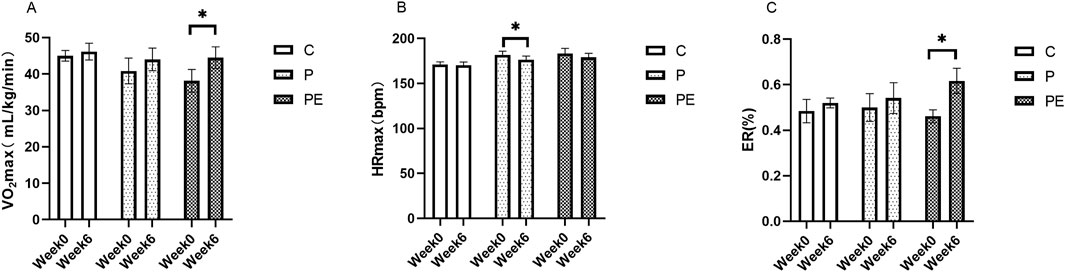
Figure 10. The influence of probiotics supplementation alone or in combination with aerobic exercise on the aerobic capacity of college students. Results are reported as the mean ± SEM. C, control group (n = 10); P: probiotic group (n = 10); PE: probiotic and aerobic exercise group (n = 10). (A) VO2max. (B) HRmax. (C) ER. *: P < 0.05; **: P < 0.01.
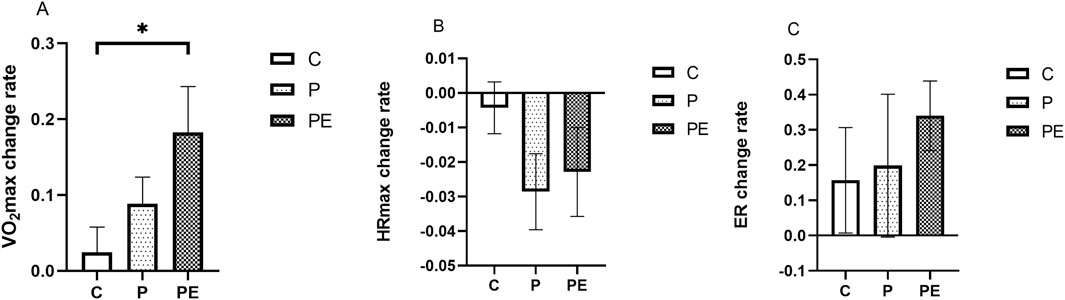
Figure 11. The influence of probiotics supplementation alone or in combination with aerobic exercise on the change rate of aerobic capacity in healthy university students. Results are reported as the mean ± SEM. C, control group (n = 10); P: probiotic group (n = 10); PE: probiotic and aerobic exercise group (n = 10). (A) VO2max variation rate. (B) HRmax change rate. (C) ER Variation Rate. *:P < 0.05; **: P < 0.01.
Discussion
In summary, our results suggest that probiotic supplementation combined with aerobic exercise is more effective than probiotic supplementation alone in enhancing the antioxidant capacity of the body, but both have the same role in ameliorating the oxidative stress caused by HIIE. These results emphasize the potential of combined strategies to enhance antioxidant capacity, while also demonstrating the notable efficacy of standalone probiotic supplementation in managing oxidative stress after vigorous exercise. Furthermore, increases in VO2max and ER following HIIE were observed only in the group that combined probiotics with aerobic exercise, not in the probiotic-only group. This research offers novel scientific insights into the role of probiotics in exercise nutrition and recovery, recommending the integration of probiotics with aerobic exercise in daily training regimens to optimize post-exercise recovery.
Strenuous exercise causes ROS accumulation and instigates oxidative stress in skeletal muscle, which plays a critical role in muscle damage and performance degradation (García-Giménez et al., 2024). Oxidative stress is an adverse physiological response, and the antioxidant defense system in the blood is activated to maintain and restore the dynamic balance between oxidative stress and antioxidant responses. The antioxidant system consists of an enzymatic defense system containing GSH-Px, SOD, and CAT, and a nonenzymatic antioxidant system (Zhao et al., 2023). 8-OHdG is the most common biomarker of DNA damage in urine and blood samples and is recognized as a biomarker of oxidative DNA damage (Spoto et al., 2025). This study revealed that SOD activity and T-AOC levels exhibited a marked elevation in the P group immediately after HIIE compared to pre-intervention. Moreover, post-intervention, the level of 8-OHDG and its rate of change immediately after HIIE were notably diminished in the P group compared to the C group, suggesting that probiotics supplementation may help reduce oxidative damage caused by exercise. Published investigations support our findings. Kola et al. (Kayacan et al., 2022) found that probiotic supplementation by itself had no effect on oxidative stress levels in the basal state of rats. However, when combined with chronic exercise, probiotics signif-icantly reduced the oxidative damage caused by the exercise. Two weeks of probiotic yogurt supplementation in healthy subjects significantly increased the levels of GSH-Px, SOD, and T-AOC, and decreased the MDA level after sub-exhaustive exercise (Mazani et al., 2018). The most recent study also found that supplementation with probiotics (including Bacillus longum CECT7347, Lactobacillus casei CECT9104, and Lactobacillus rhamnosus CECT8361) significantly reduced the levels of serum MDA and urinary 8-OHDG in healthy men after high-intensity cycling (Sánchez Macarro et al., 2021). However, in our study, there were no changes in basal state GSH-Px and T-AOC activities after probiotic supplementation, which is inconsistent with the results of previous studies (Mazani et al., 2018), possibly because the probiotic species and subjects were different. It is important to note that our results reflect the acute response to a single bout of high-intensity exercise, which tends to increase oxidative stress in the short term. However, previous studies have shown that long-term HIIE can reduce oxidative stress through adaptation mechanisms, including the upregulation of antioxidant enzymes such as SOD and CAT (Sarvasti et al., 2020; Guo et al., 2025). In addition, studies have shown that long-term HIIE combined with supplements such as green tea or caffeine can have beneficial effects in mitigating oxidative stress. (Ghasemi et al., 2020; Alkhatib et al., 2020). Nevertheless, whether long-term HIIE combined with probiotic supplementation can similarly improve systemic antioxidant capacity remains unclear and warrants further investigation.
Our data showed a significant increase in basal GSH-Px activity in the PE group compared to pre-intervention levels. No significant changes were observed in the P group. It is suggested that probiotics combined with exercise are more effective in enhancing basal antioxidant capacity. Aerobic exercise has been shown to increase T-AOC levels, enhance the activities of CAT, GSH-Px, and SOD (Zarrindast et al., 2021), and decrease MDA level (Sun et al., 2023). However, given the lack of a separate aerobic exercise group in this study, it is not possible to determine whether the increase in basal state GSH-Px activity was a result of aerobic exercise or a synergistic effect of probiotics and aerobic exercise. Notably, no significant difference was observed between the P and PE groups in terms of improving oxidative stress caused by HIIE in this study. Suggesting that probiotics may help regulate exercise-induced oxidative stress by enhancing SOD and T-AOC, while reducing 8-OHdG during the acute recovery phase following HII. Additionally, in terms of T-AOC levels, the results showed a significant interaction effect between the group and time. Post-hoc comparisons revealed that the combination of probiotics and aerobic exercise led to a more significant increase in T-AOC levels than probiotic supplementation alone, particularly at the post-intervention time point. The effectiveness of probiotics suggests that they can be used as a convenient and effective stand-alone strategy, particularly for those who do not regularly participate in aerobic exercise but can mitigate oxidative stress through probiotic supplementation. Given its convenience and accessibility, probiotic supplementation may be particularly valuable for college students and younger individuals who lack regular aerobic training.
This study found that after the intervention, the high shear rate blood viscosity in the P group significantly decreased, indicating that probiotic supplementation may reduce blood viscosity. This outcome aligns with previous research, which suggests that probiotic supplementation can regulate vascular endothelial function and inflammatory responses (Hofeld et al., 2021; Zhai et al., 2022). Probiotics likely achieve this by modulating the gut microbiota, which reduces systemic inflammation and improves endothelial function. This decrease in inflammation can enhance vascular health and lower blood viscosity (Liu et al., 2025). Additionally, the reduction in blood viscosity in the PE group may be partly due to aerobic exercise, which improves endothelial function and promotes vasodilation, resulting in smoother blood flow. Aerobic exercise boosts nitric oxide production, relaxing blood vessels, improving circulation, and lowering blood viscosity (Shing et al., 2024; Meier et al., 2023). This enhanced endothelial function helps regulate blood flow more efficiently. Furthermore, after the intervention, both the high shear rate whole blood viscosity and plasma viscosity were significantly decreased in the PE group. The levels of high shear rate whole blood viscosity and plasma viscosity were significantly lower in the PE group than in the C group, and plasma viscosity was significantly lower than in the P group. These results suggest that the combination of probiotics and aerobic exercise may be more effective in improving blood rheology than probiotics supplementation alone. Thus, the improvement in blood rheology may be partly attributed to the effects of aerobic exercise. The synergy between probiotics and aerobic exercise likely enhances the beneficial effects on blood viscosity and circulation, as probiotics reduce systemic inflammation while aerobic exercise improves vascular function and blood flow. Similarly, aerobic training has been shown to significantly improve cardiovascular function and oxygen uptake capacity (Collins et al., 2024; Mardyła et al., 2023). Regular interval aerobic training can significantly improve blood rheological parameters (Mougin et al., 2024). Mardyła et al. found that regular training can improve blood rheological functions, such as reducing fibrinogen concentration, plasma viscosity, and aggregation index in the rowers (Mardyła et al., 2023). Additionally, 30 minutes of aerobic exercise three times a week also can improve hematocrit and plasma viscosity (Marchewka et al., 2015). Considering the changes in blood viscosity at high shear rates and viscosity of blood plasma, smoother blood flow in the blood vessels is facilitated, improving circulation, enhancing the fluidity of blood, and increasing the capacity for oxygen and nutrient transport. The improvement of blood rheology in the PE group may contribute to the improvement of aerobic capacity to some extent. This study also found that the VO2max was significantly higher in the PE group compared to pre-intervention, which may be related to the improvement of blood rheology.
VO2max is one of the key indicators of an individual’s aerobic capacity, reflecting the ability of the human body to inhale, transport, and utilize oxygen (Zhang et al., 2023). Evidence from earlier studies indicates that probiotic supplementation may boost the VO2max of participants. A study involving 66 long-distance runners found that a 12-week probiotic supplementation led to an increase in VO2max in both male and female participants (Smarkusz-Zarzecka et al., 2020). Additionally, VO2max was also significantly elevated in probiotic-supplemented male soccer players (Imanian et al., 2024) and badminton players (Salleh et al., 2021). The improvement of aerobic capacity by probiotics supplementation may be because probiotics can balance gut microbiota (Zhang et al., 2024), thereby promoting iron absorption and hemoglobin synthesis, and short-chain fatty acids, metabolites produced by the gut microbiota, directly enhance hemoglobin synthesis (Takahashi et al., 1975; Li et al., 2023). However, the subjects of the above studies were athletes, and the effect of exercise on VO2max was not excluded. Conversely, some studies have shown no change in VO2max after probiotic supplementation (Michalickova et al., 2016), possibly due to differences in probiotic formulations, dosages, duration of intervention, and experimental designs. The study results showed an upward trend in VO2max in the P group after the intervention period, though no substantial change was found. In contrast, the VO2max increased significantly in the PE group, and the change in VO2max was significantly more pronounced in the PE group compared to the C group. It is suggested that the results may be due to the positive effect of aerobic exercise. Additionally, this study examined the HRmax levels during high-intensity interval training. A notable decrease in HRmax was found in the P group, which is consistent with the findings of Paulina et al. (Mazur-Kurach et al., 2022) who found that probiotic supplementa-tion could significantly reduce the HRmax in road cyclists. However, the PE group showed no significant difference. Thus, further studies should explore the optimization of combined probiotic and aerobic exercise interventions. Furthermore, this study found a significant increase in ER after probiotics combined with aerobic training intervention, whereas no notable change was observed in the group receiving probiotic supplementation alone. Suggesting that the improvement in ER may be caused by aerobic exercise, this result supports the key role of exercise training in regulating ER (Fukuba et al., 1999; Rahimi and Beaven, 2024). The enhancement in ER may contribute to post-exercise recovery and also reflect the body’s aerobic capacity. In summary, probiotics combined with aerobic exercise are more helpful for aerobic capacity than supplementing with probiotics alone.
The probiotics investigated in this study have been shown to modulate gut microbiome composition. Supplementation with Lactobacillus zhang significantly altered the gut microbiota composition of healthy volunteers (Zhang et al., 2014), increasing the abundance of beneficial bacteria (Lactobacillus and Bifidobacterium), short-chain fatty acids-producing bacteria (such as Prevotella), and anti-inflammatory bacteria (Faecalibacterium), while significantly reducing the number of opportunistic pathogens (Clostridium and Enterococcus faecalis). Similarly, Lactobacillus plantarum P-8 (Wang et al., 2014) also increased the abundance of Bifidobacterium in the gut of healthy volunteers and decreased the abundance of Clostridium. Unfortunately, gut microbiota was not measured in the study, which should be taken into account in future studies. In addition, factors limiting this study included a relatively small sample size and the lack of a separate aerobic exercise group. Future studies should explore the long-term influence of probiotics and aerobic recovery processes on oxidative stress and offer evidence-based recommendations for athletes and young individuals.
Conclusion
In conclusion, combining probiotics with aerobic exercise not only enhances antioxidant capacity but also improves aerobic performance, making it a promising strategy for optimizing exercise recovery and performance in college students.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Beijing Sport University Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TW: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review and editing. YC: Conceptualization, Validation, Writing – original draft. KZ: Project administration, Writing – original draft. CL: Data curation, Methodology, Project administration, Supervision, Writing – review and editing. WJ: Methodology, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The present study was funded by the National Key R&D Program of China (2022YFC3600201) and the Fundamental Research Funds for the Central Universities, Beijing Sport University (2024YJSY004).
Acknowledgments
The experiments comply with the current laws of the country in which they were performed. The authors have no conflict of interest to declare. The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request, who was an organizer of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alkhatib A., Hsieh M. J., Kuo C. H., Hou C. W. (2020). Caffeine optimizes HIIT benefits on obesity-associated metabolic adversity in women. Med. Sci. Sports Exerc 52 (8), 1793–1800. doi:10.1249/MSS.0000000000002311
Aykut M. N., Erdoğan E. N., Çelik M. N., Gürbüz M. (2024). An updated view of the effect of probiotic supplement on sports performance: a detailed review. Curr. Nutr. Rep. 13 (2), 251–263. doi:10.1007/s13668-024-00527-x
Bertuccioli A., Cardinali M., Micucci M., Rocchi M. B. L., Palazzi C. M., Zonzini G. B., et al. (2024). Efficacy of Streptococcus salivarius blis K12 in the prevention of upper respiratory tract infections in physically active individuals: a randomized controlled trial. Microorganisms 12 (11), 2164. doi:10.3390/microorganisms12112164
Cipryan L. (2018). The effect of fitness level on cardiac autonomic regulation, IL-6, total antioxidant capacity, and muscle damage responses to a single bout of high-intensity interval training. J. Sport Health Sci. 7 (3), 363–371. doi:10.1016/j.jshs.2016.11.001
Collins B. E. G., Hartmann T. E., Marino F. E., Skein M. (2024). The effect of a 12 Week mixed-modality training intervention on the cardio-metabolic health of rotational shift workers. J. Sci. Sport Exerc. 6 (2), 120–130. doi:10.1007/s42978-022-00207-8
Damianos J., Abdelnaem N., Camilleri M. (2025). Gut goo: physiology, diet, and therapy of intestinal mucus and biofilms in gastrointestinal health and disease. Clin. Gastroenterol. Hepatol. 23 (2), 205–215. doi:10.1016/j.cgh.2024.09.007
Dantas T. C. B., Farias Junior L. F., Frazão D. T., Silva P. H. M., Sousa Junior A. E., Costa I. B. B., et al. (2017). A single session of low-volume high-intensity interval exercise reduces ambulatory blood pressure in normotensive men. J. Strength Cond. Res. 31 (8), 2263–2269. doi:10.1519/JSC.0000000000001688
Fukuba Y., Walsh M. L., Morton R. H., Cameron B. J., Kenny C. T., Banister E. W. (1999). Effect of endurance training on blood lactate clearance after maximal exercise. J. Sports Sci. 17 (3), 239–248. doi:10.1080/026404199366145
García-Giménez J. L., Cánovas-Cervera I., Pallardó F. V. (2024). Oxidative stress and metabolism meet epigenetic modulation in physical exercise. Free Radic. Biol. Med. 213, 123–137. doi:10.1016/j.freeradbiomed.2024.01.008
Ghasemi E., Afzalpour M. E., Nayebifar S. (2020). Combined high-intensity interval training and green tea supplementation enhance metabolic and antioxidant status in response to acute exercise in overweight women. J. Physiol. Sci. 70 (1), 31. doi:10.1186/s12576-020-00756-z
Guo D., Sun J., Feng S. (2025). Comparative analysis of the effects of high-intensity interval training and traditional aerobic training on improving physical fitness and biochemical indicators in patients with non-alcoholic fatty liver disease. J. Sports Med. Phys. Fit. 65 (1), 132–139. doi:10.23736/S0022-4707.24.16206-8
Hofeld B. C., Puppala V. K., Tyagi S., Ahn K. W., Anger A., Jia S., et al. (2021). Lactobacillus plantarum 299v probiotic supplementation in men with stable coronary artery disease suppresses systemic inflammation. Sci. Rep. 11 (1), 3972. doi:10.1038/s41598-021-83252-7
Huang W. C., Wei C. C., Huang C. C., Chen W. L., Huang H. Y. (2019). The beneficial effects of Lactobacillus plantarum PS128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients 11 (2), 353. doi:10.3390/nu11020353
Imanian B., Hemmatinafar M., Daryanoosh F., Koureshfard N., Sadeghi R., Niknam A., et al. (2024). The effect of probiotics and casein supplementation on aerobic capacity parameters of male soccer players. J. Int. Soc. Sports Nutr. 21 (1), 2382165. doi:10.1080/15502783.2024.2382165
Kayacan Y., Kola A. Z., Guandalini S., Yazar H., Söğüt M. (2022). The use of probiotics combined with exercise affects thiol/disulfide homeostasis, an oxidative stress parameter. Nutrients 14 (17), 3555. doi:10.3390/nu14173555
Li J., Fan R., Zhang Z., Zhao L., Han Y., Zhu Y., et al. (2025). Role of gut microbiota in rheumatoid arthritis: potential cellular mechanisms regulated by prebiotic, probiotic, and pharmacological interventions. Microbiol. Res. 290, 127973. doi:10.1016/j.micres.2024.127973
Li T., Rui Z., Mao L., Chang Y., Shao J., Chen Y., et al. (2023). Eight weeks of Bifidobacterium lactis BL-99 supplementation improves lipid metabolism and sports performance through short-chain fatty acids in cross-country skiers: a preliminary study. Nutrients 15 (21), 4554. doi:10.3390/nu15214554
Liu Y., Bai Z., Yan R., Ma J., Wang L., Li Y., et al. (2025). Lactobacillus rhamnosus GG ameliorates atherosclerosis via suppression of oxidative stress and inflammation by reshaping the gut microbiota. Biochem. Biophys. Res. Commun. 751, 151417. doi:10.1016/j.bbrc.2025.151417
Lomiwes D., Barnes M., Shaw O. M., Ngametua N., Sawyer G. M., Burr N. S., et al. (2024). Characterising the cytokine and circulating immune cell response after a single bout of eccentric stepping exercise in healthy untrained males. J. Sci. Sport Exerc. 6 (4), 332–344. doi:10.1007/s42978-023-00227-y
Mafe A. N., Iruoghene Edo G., Akpoghelie P. O., Gaaz T. S., Yousif E., Zainulabdeen K., et al. (2025). Probiotics and food bioactives: unraveling their impact on gut microbiome, inflammation, and metabolic health. Probiotics Antimicrob. Proteins. doi:10.1007/s12602-025-10452-2
Marchewka A., Filar-Mierzwa K., Dąbrowski Z., Teległó A. (2015). Effects of rhythmic exercise performed to music on the rheological properties of blood in women over 60 years of age. Clin. Hemorheol. Microcirc. 60 (4), 363–373. doi:10.3233/CH-131793
Mardyła M., Teległów A., Ptaszek B., Jekiełek M., Mańko G., Marchewka J. (2023). Effects of rowing on rheological properties of blood. Int. J. Environ. Res. Public Health 20 (6), 5159. doi:10.3390/ijerph20065159
Martinez-Canton M., Galvan-Alvarez V., Martin-Rincon M., Calbet J. A. L., Gallego-Selles A. (2024). Unlocking peak performance: the role of Nrf2 in enhancing exercise outcomes and training adaptation in humans. Free Radic. Biol. Med. 224, 168–181. doi:10.1016/j.freeradbiomed.2024.08.011
Mazani M., Nemati A., Amani M., Haedari K., Mogadam R. A., Baghi A. N. (2018). The effect of probiotic yoghurt consumption on oxidative stress and inflammatory factors in young females after exhaustive exercise. J. Pak Med. Assoc. 68 (12), 1748–1754.
Mazur-Kurach P., Frączek B., Klimek A. T. (2022). Does multi-strain probiotic supplementation impact the effort capacity of competitive road cyclists? Int. J. Environ. Res. Public Health 19 (19), 12205. doi:10.3390/ijerph191912205
Meier N., Sietmann D., Schmidt A. (2023). Comparison of cardiovascular parameters and internal training load of different 1-h training sessions in non-elite CrossFit® athletes. J. Sci. Sport Exerc. 5 (2), 130–141. doi:10.1007/s42978-022-00169-x
Michalickova D., Kotur-Stevuljevic J., Miljkovic M., Dikic N., Kostic-Vucicevic M., Andjelkovic M., et al. (2018). Effects of probiotic supplementation on selected parameters of blood prooxidant-antioxidant balance in elite athletes: a double-blind randomized placebo-controlled study. J. Hum. Kinet. 64, 111–122. doi:10.1515/hukin-2017-0203
Michalickova D., Minic R., Dikic N., Andjelkovic M., Kostic-Vucicevic M., Stojmenovic T., et al. (2016). Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: a randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 41 (7), 782–789. doi:10.1139/apnm-2015-0541
Mougin L., Riccetti M., Merlet A. N., Bartolucci P., Gellen B., Blervaque L., et al. (2024). Endurance training improves oxygen uptake/demand mismatch, metabolic flexibility and recovery in patients with sickle cell disease. Haematologica 109 (8), 2628–2638. doi:10.3324/haematol.2023.284474
Musazadeh V., Faghfouri A. H., Zarezadeh M., Pakmehr A., Moghaddam P. T., Hamedi-Kalajahi F., et al. (2024). Corrigendum: remarkable impacts of probiotics supplementation in enhancing of the antioxidant status: results of an umbrella meta-analysis. Front. Nutr. 11, 1371746. doi:10.3389/fnut.2024.1371746
Rahimi M. R., Beaven C. M. (2024). Caffeine modifies the immune and anti-inflammatory responses to short incremental cycling exercise until exhaustion in humans: a pilot study. J. Sci. Sport Exerc. 6 (4), 404–408. doi:10.1007/s42978-023-00226-z
Salleh R. M., Kuan G., Aziz M. N. A., Rahim M. R. A., Rahayu T., Sulaiman S., et al. (2021). Effects of probiotics on anxiety, stress, mood and fitness of badminton players. Nutrients 13 (6), 1783. doi:10.3390/nu13061783
Sánchez Macarro M., Ávila-Gandía V., Pérez-Piñero S., Cánovas F., García-Muñoz A. M., Abellán-Ruiz M. S., et al. (2021). Antioxidant effect of a probiotic product on a model of oxidative stress induced by high-intensity and duration physical exercise. Antioxidants (Basel) 10 (2), 323. doi:10.3390/antiox10020323
Sarita B., Samadhan D., Hassan M. Z., Kovaleva E. G. (2024). A comprehensive review of probiotics and human health-current prospective and applications. Front. Microbiol. 15, 1487641. doi:10.3389/fmicb.2024.1487641
Sarvasti D., Lalenoh I., Oepangat E., Purwowiyoto B. S., Santoso A., Romdoni R. (2020). Cardiovascular protection variables based on exercise intensity in stable coronary heart disease patients after coronary stenting: a comparative study. Vasc. Health Risk Manag. 16, 257–270. doi:10.2147/VHRM.S259190
Shing C. L. H., Bond B., Moreau K. L., Coombes J. S., Taylor J. L. (2024). The therapeutic role of exercise training during menopause for reducing vascular disease. Exp. Physiol. doi:10.1113/EP092191
Smarkusz-Zarzecka J., Ostrowska L., Leszczyńska J., Orywal K., Cwalina U., Pogodziński D. (2020). Analysis of the impact of a multi-strain probiotic on body composition and cardiorespiratory fitness in long-distance runners. Nutrients 12 (12), 3758. doi:10.3390/nu12123758
Souglis A., Bourdas D. I., Gioldasis A., Ispirlidis I., Philippou A., Zacharakis E., et al. (2023). Time course of performance indexes, oxidative stress, inflammation, and muscle damage markers after a female futsal match. Sports (Basel) 11 (7), 127. doi:10.3390/sports11070127
Sousa A., Chambion-Diaz M., Pialoux V., Carin R., Viana J. L., Milheiro J., et al. (2025). Dietary nitrate supplementation very slightly mitigates the oxidative stress induced by high-intensity training performed in normobaric hypoxia. Biol. Sport 42 (1), 243–251. doi:10.5114/biolsport.2025.139851
Spoto B., Politi C., Pizzini P., Parlongo R. M., Testa A., Mobrici M., et al. (2025). 8-hydroxy-2'-deoxyguanosine, a biomarker of oxidative DNA injury, in diabetic kidney disease. Nutr. Metab. Cardiovasc Dis. 35 (2), 103722. doi:10.1016/j.numecd.2024.08.015
Sun M., Zhao X., Li X., Wang C., Lin L., Wang K., et al. (2023). Aerobic exercise ameliorates liver injury in Db/Db mice by attenuating oxidative stress, apoptosis and inflammation through the Nrf2 and JAK2/STAT3 signalling pathways. J. Inflamm. Res. 16, 4805–4819. doi:10.2147/JIR.S426581
Takahashi E., Yamada M., Saito M., Kuboyama M., Ogasa K. (1975). Differentiation of cultured Friend leukemia cells induced by short-chain fatty acids. Gan 66 (5), 577–580.
Vilcant V., Zeltser R. (2024). Treadmill stress testing. Treasure Island (FL): StatPearls Publishing LLC.
Wang C., Liu H., Zhang S., Ren C., Xu J., Chen J., et al. (2025). Spirulina supplementation alleviates intense exercise-induced damage and modulates gut microbiota in mice. Nutrients 17 (2), 355. doi:10.3390/nu17020355
Wang J., Qiu J., Yi L., Hou Z., Benardot D., Cao W. (2019). Effect of sodium bicarbonate ingestion during 6 weeks of HIIT on anaerobic performance of college students. J. Int. Soc. Sports Nutr. 16 (1), 18. doi:10.1186/s12970-019-0285-8
Wang L., Zhang J., Guo Z., Kwok L., Ma C., Zhang W., et al. (2014). Effect of oral consumption of probiotic Lactobacillus planatarum P-8 on fecal microbiota, SIgA, SCFAs, and TBAs of adults of different ages. Nutrition 30 (7-8), 776–783. doi:10.1016/j.nut.2013.11.018
Zarrindast S., Ramezanpour M. R., Moghaddam M. G. (2021). Effects of eight weeks of moderate intensity aerobic training and training in water on DNA damage, lipid peroxidation and total antioxidant capacity in sixty years sedentary women. Sci. and Sports 36 (3), e81–e85. doi:10.1016/j.scispo.2020.04.005
Zhai T., Ren W., Wang P., Zheng L. (2022). Lactobacillus rhamnosus GG protects against atherosclerosis by improving ketone body synthesis. Appl. Microbiol. Biotechnol. 106 (24), 8233–8243. doi:10.1007/s00253-022-12265-7
Zhang J., Wang L., Guo Z., Sun Z., Gesudu Q., Kwok L., et al. (2014). 454 pyrosequencing reveals changes in the faecal microbiota of adults consuming Lactobacillus casei Zhang. FEMS Microbiol. Ecol. 88 (3), 612–622. doi:10.1111/1574-6941.12328
Zhang L., Li H., Song Z., Liu Y., Zhang X. (2024). Dietary strategies to improve exercise performance by modulating the gut microbiota. Foods 13 (11), 1680. doi:10.3390/foods13111680
Zhang L., Xiao H., Zhao L., Liu Z., Chen L., Liu C. (2023). Comparison of the effects of prebiotics and synbiotics supplementation on the immune function of male university football players. Nutrients 15 (5), 1158. doi:10.3390/nu15051158
Keywords: probiotics, aerobic exercise, oxidative stress, antioxidant capacity, high-intensity interval training
Citation: Wu T, Chen Y, Zhao K, Liu C and Jiang W (2025) The effect of probiotic supplementation combined with aerobic exercise on the antioxidant capacity of college students. Front. Physiol. 16:1586888. doi: 10.3389/fphys.2025.1586888
Received: 03 March 2025; Accepted: 19 May 2025;
Published: 02 June 2025.
Edited by:
Ahmad Alkhatib, Birmingham City University, United KingdomReviewed by:
Mohamed Elloumi, Prince Sultan University, Saudi ArabiaPiyawan Bunpo, Chiang Mai University, Thailand
Copyright © 2025 Wu, Chen, Zhao, Liu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenzhe Liu, bGl1Y2hlbnpoZUBic3UuZWR1LmNu; Wei Jiang, MjQzMEBic3UuZWR1LmNu
 Tong Wu
Tong Wu Yingfeng Chen1
Yingfeng Chen1